Abstract
Background.
To calculate the 1-year prevalence of schizophrenia and related disorders in a catchment area of Malaga (Spain) and determine the prevalence by gender, dwelling (rural or urban) and socioeconomic area (deprived or non-deprived area).
Method.
This cross-sectional study comprised the mental health area covered by Carlos Haya Hospital. We used multiple large clinical databases and key informants to identify cases.
Results.
The mean 1-year prevalence of schizophrenia and related disorders was 6.27 per 1000. It was nearly double in men (8.45 per 1000) than in women (4.26 per 1000) (p < 0.001), with a male-to-female ratio of 1.98. The rate was higher in urban (6.64 per 1000) than rural areas (3.95 per 1000) (p < 0.0001) and in socioeconomic deprived areas (7.56 per 1000) than non-deprived areas (6.12 per 1000) (p = 0.005). For the subgroup of schizophrenia, the rates were: men, 5.88 per 1000 and women, 2.2 per 1000 (p < 0.0001), with a male-to-female ratio of 2.67. The rate was also higher in urban (4.2 per 1000) than rural areas (2.49 per 1000) (p < 0.0001) and in socioeconomic deprived areas (4.49 per 1000) than non-deprived areas (3.9 per 1000) (p = 0.149).
Conclusions.
The use of multiple clinical sources of information not only from mental health services, but also from emergency departments, primary care and private settings revealed high prevalence rates of schizophrenia and related disorders. This diagnosis is more common in men and in cities. Such precise estimates of the prevalence of schizophrenia have important repercussions for resource allocation and policy planning.
Key words: Case register, clinical databases, prevalence, schizophrenia
Introduction
Data from epidemiological studies suggest that schizophrenia cannot be considered a disorder with a similar distribution in different parts of the world (McGrath, 2005, 2007). This variable prevalence of schizophrenia is influenced not only by aetiological and environmental factors, but also by factors related with the characteristics of the studies. Indeed, there is increasing agreement that the variation in the results of different epidemiological studies can be explained by the variation in the methodology and that use of the case-finding strategy is one of the reasons for these different results (Përalä et al. 2007; Moreno-Küstner, 2014).
The case-finding strategy to detect the prevalence of schizophrenia can be divided into three types. The first relies on clinical databases recording the patients' contacts with mental health services to estimate the prevalence or incidence of cases treated by these services (Jörgensen et al. 2010). The second, the key informant method, can be used to expand information derived from the mental healthcare facilities. The key informant method involves establishing a list of services and agencies in a defined area that are likely sites of contact for potential cases, so the case-finding strategy in this particular method covers an extensive network of both mental and non-mental health services. This method was used several times in studies of the prevalence of schizophrenia during the 1990s (Bamrah et al. 1991; Youssef et al. 1991; Harvey et al. 1996; Jeffreys et al. 1997; McCreadie et al. 1997; Widerlöv et al. 1997; Thornicroft et al. 1998). Finally, the third type concerns population surveys undertaken through household interviews to identify all cases of a disorder within an entire population or a representative random sample.
The relationship between gender and the prevalence of schizophrenia has been analysed frequently in population-based studies, but fewer studies have been undertaken in samples of persons in contact with health services and the results are controversial (Iniesta et al. 2012). Saha et al. (2005), in a systematic review, found no differences in the prevalence of schizophrenia between urban and rural dwellers. However, other studies have found a higher prevalence in urban areas (Harvey et al. 1996; Widerlöv et al. 1997). Measures of social deprivation are usually included as another factor associated with the incidence and prevalence of schizophrenia. Socioeconomic deprivation in urban areas has been associated with higher incidence (Drukker et al. 2006, Kirkbride et al. 2008; March et al. 2008) and prevalence rates (Jörgensen et al. 2014).
In this study, we attempted to overcome many of the case-finding problems inherent to general population studies calculating the prevalence of schizophrenic disorders. Accordingly, we screened multiple large healthcare registries, as well as using information from case notes and key informants, which increases the case detection substantially.
The primary aim of this study was to calculate the 1-year prevalence of schizophrenia and related disorders in one hospital catchment area of Malaga (Spain), in 2008. The secondary aim was to determine the prevalence of schizophrenia and related disorders in relation to gender, dwelling (rural or urban) and socioeconomic area (deprived or non-deprived area).
Methods
Study area and health services
This cross-sectional study was carried out in Malaga, a province in Andalusia, Southern Spain, with a population of nearly 1 million inhabitants. Specifically, the study involved one of the two mental health areas in Malaga, the Mental Health Department of Carlos Haya Hospital, which is an administrative and geographically defined area with a population of 315 159 inhabitants. The total population aged 14 years or older was 265 229 persons in 2008. The study area includes not only the capital city but also villages, with a rural population of about 14%. Of the population involved, 9.7% were living in socioeconomic deprived areas, all located in the urban zone and defined by the Andalusian Government as ‘places with extreme poverty and social marginalisation’. For measurement purposes, homes where the family income was less than 50% of the mean Andalusian income were considered to be experiencing ‘social exclusion’ and the geographic location of these homes was identified as belonging to socioeconomic deprived areas (Junta de Andalucía, 2004).
This Mental Health Department of Carlos Haya Hospital comprises two community mental health centres, one day centre, one general-hospital psychiatric unit (with 45 beds), one medium and long-stay ward (30 beds), one child and adolescent mental health unit, and a mental health unit attending the homeless (the only one in Andalusia). In Malaga, there is a network of public residential shelters used mainly by persons with severe mental illness. The province of Malaga has 120 beds in a private psychiatric hospital supported by public funding (San Juan de Dios hospital). Private psychiatry is well developed in Malaga although we do not know the official figures.
Mental health emergencies are covered by a call phone centre attended by medical staff and by ambulance medical services that assess the patients and transport them to hospital if they need specialised care. At the hospital there is a psychiatrist on duty 24 h/day integrated in the general emergency services. Primary care physicians act as gatekeepers to the health services in Spain. Patients attended by the community mental health services are referred there by their general practitioner or by the emergency services, or after discharge from an acute ward at the general hospital. All these settings are included in the case finding procedure.
Patient identification
All patients older than 14 years of age with a clinical diagnosis of schizophrenia or related disorders attended by health services in the catchment area in the index year (2008) were identified. The clinical diagnosis of a patient identified for the study was checked against the mental health team case notes, where possible, before inclusion of that patient in the study. Thus, diagnoses were confirmed by senior psychiatrists who knew the patients as the majority of cases were extracted from psychiatric case notes. In cases obtained from the primary care setting or emergency services we confirmed that the diagnoses were made by a psychiatrist before including the patients in the study.
For the purposes of this study we confirmed that patients were living in the catchment area by consulting the address in the clinical databases and if this information was missing we asked the patients directly. Concerning patients living in the psychiatric hospitals, we only included those who were resident in the study area before hospitalisation.
Patients included were those living in the catchment study area, with diagnoses of schizophrenia or related disorders and who had any contact with health services during 2008.
Case-finding procedure
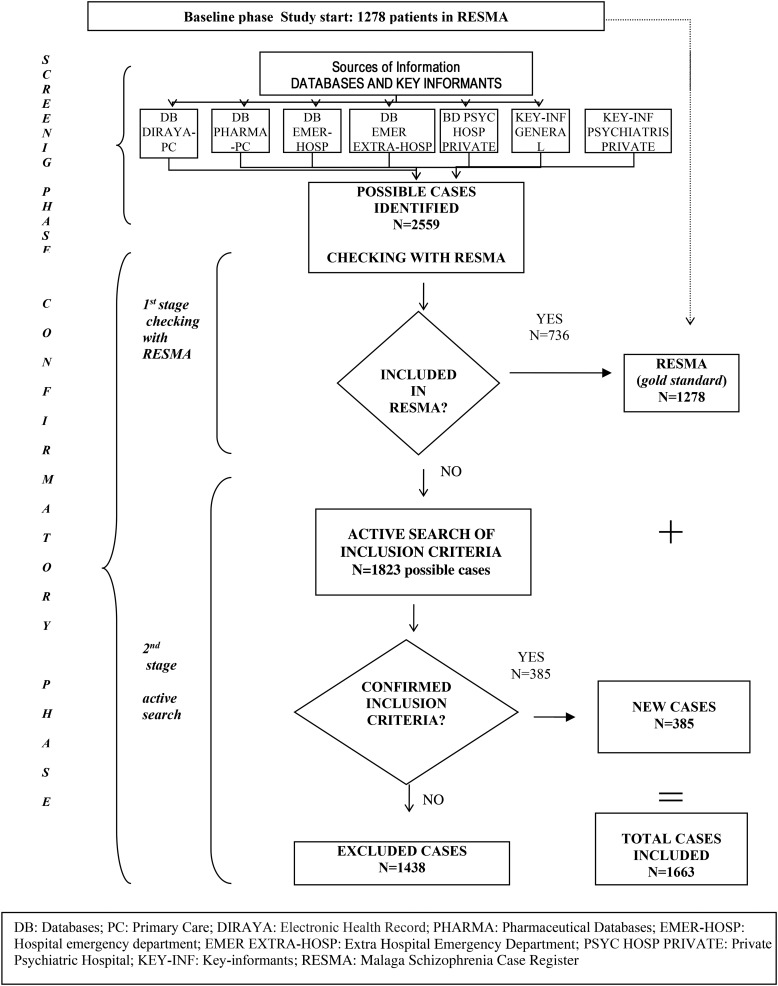
The case-finding procedure was divided into three phases (Fig. 1).
Fig. 1.
Flow-chart for the inclusion of patients in the study. Screening and confirmatory phases.
Baseline phase, RESMA
The Mental Health Department of Carlos Haya Hospital was created in 2003 and one of its main objectives was to improve the care of patients with severe mental illness. The strategy included the development of a case register of patients with a diagnosis of schizophrenia or other related disorders attending in this area: the Malaga Schizophrenia Case Register (RESMA). The RESMA is based on health care data routinely collected from several psychiatric record systems merged into one database, as described previously (Moreno-Küstner et al. 2009, 2011).
The main source of information for the present study was therefore RESMA. Thus, information (e.g., diagnoses and personal information) about patients who contacted the mental health services during 2008 was revised and those patients for whom the study inclusion criteria were confirmed were considered cases for this study.
Screening phase: identification of possible cases
In order to identify the maximum number of possible cases this phase involved a census of all individuals who had contact with any of the health departments at which patients with schizophrenia or related disorders might be seen during 2008. Here and subsequently, as Jablensky (2000) did, we use the term ‘mainstream’ to denote the predominantly public psychiatric services (inpatient, outpatient, and day patient services, mental health unit for homeless), general practice centres, emergency department (in-hospital and extra-hospital) and private care (hospital and ambulatory). Complementary to this procedure, and for the purpose of possible case identification, the key informants (GPs and private psychiatrists) were asked to provide a list of patients who fulfilled the diagnostic inclusion criteria (Table 1).
Table 1.
Information sources and search criteria
| Information sources | Search criteria |
|---|---|
| 1. Public psychiatric services | |
| ⇒DATABASES: RESMA | Patients with diagnosis of schizophrenia in contact with community mental health services |
| ⇒KEY-INFORMANT: mental health professionals | |
| 2. Primary care | |
| ⇒ DATABASES: DIRAYA primary care | Patients treated in Primary Care with a diagnosis of schizophrenia or related disorders: Codes 295, 297 and 298 of ICD-9 |
| ⇒DATABASES: PHARMA-PSYCHIATRIC | Patients with antipsychotic medication prescription: Group NO5A of the ATC classification |
| ⇒KEY-INFORMANT: primary care practitioners | Any patients with an established diagnosis of schizophrenia seen solely by General Practitioners |
| 3. Emergency departments | |
| ⇒DATABASES: HOSPITAL EMERGENCY DEPARTMENT | Patients with diagnoses of schizophrenia and related disorders |
| ⇒DATABASES: EXTRA-HOSPITAL EMERGENCY DEPARTMENT | Patients with diagnoses of schizophrenia and related disorders |
| 4. Private care | |
| ⇒DATABASES: PHARMA-PSYCHIATRIC HOSPITAL | Patients with antipsychotic medication prescription: Group NO5A of the ATC classification |
| ⇒KEY-INFORMANT: psychiatrists | Patients with diagnoses of schizophrenia and related disorders |
ATC – Anatomical, Therapeutic, Chemical Classification System.
Confirmatory phase of inclusion criteria
We compared possible cases from all agencies with the RESMA, the latter considered to represent gold standard patients (stage 1). Record linkages between the various data sources were enabled using common information, including such data as health care identification number, identity card number and names. After checking, those patients already included in RESMA were considered as study cases.
Concerning the cases not included in RESMA (possible cases) we made an active search for information about diagnoses and place of residence with mental health services teams, and amended the results as necessary (stage 2).
The field work took place from 2009 to 2010. An introductory letter detailing the project aims, methodology and diagnostic inclusion criteria was sent to all information providers.
This study was approval by the Malaga Health District and Carlos Haya Hospital Ethics Committees. Data confidentiality was guaranteed under Organic Law 15/1999 on the Protection of Personal Data.
Variables included in the analysis
The sociodemographic information gathered for each patient included the following variables: gender, year of birth, marital status, type of living arrangement, educational level and employment status. Data were also recorded on place of residence, considering rural v. urban classified as in our previous study, with Malaga city considered an urban area and all the other municipalities considered as rural (Moreno-Küstner et al. 2011). Patient residence was considered to be in a socioeconomic deprived area (yes/no) according to the classification of the Andalusian Goverment (Junta de Andalucía, 2004). The clinical diagnoses corresponded to F20–F29 codes of the 10th version of the International Classification of Diseases (ICD-10) (World Health Organization, 1993): schizophrenia (F20), schizotypal disorder (F21), persistent delusional disorder (F22), acute or transient psychotic disorder (F23), schizoaffective disorders (F25), or other and unspecified non-organic psychotic disorder (F28, F29).
Statistical analysis
We calculated the 1-year prevalence rate per 1000 inhabitants for the Mental Health Department of Carlos Haya Hospital in Malaga (Spain). We adjusted rates for population age older than 14 years and gender, which attempts to account for different population age structures (e.g., large numbers of children will lower the prevalence) (Fletcher et al. 2002). The 95% confidence intervals were calculated by the exact method, showing the standard error. The χ2 test was used to examine for potential differences in the prevalence of schizophrenia and related disorders between gender, dwelling (rural or urban) and socioeconomic area (deprived area or otherwise). A probability level of p < 0.0001 was used to indicate significant differences between groups. Data were analysed using STATA (Stata Corporation, 2009).
Missing data for sociodemographic variables were imputed using the method of multiple imputation based on discrete statistical distribution. For the imputation of the sociodemographic variables (except age and gender, which were not imputed) we assumed discrete statistical distributions defined by the original database values and based on basic statistics (means and standard deviations).
Results
Case-finding procedure
From the RESMA and after revising the diagnoses, place of residence, and contact with services in 2008, 1278 patients were included in the study as gold standard cases.
As the result of the screening phase in the different sources of information (Table 1), a total of 2559 individuals were identified by key informants or from clinical databases as fulfilling the criteria of possible study cases. After cross-checking these with the RESMA we found that 736 were already included in the RESMA while the remaining 1823 were identified as possible cases for whom we had to actively seek information in order to confirm the diagnosis and place of residence. After the active search 1438 were excluded and 385 were considered as new cases for the study and therefore added to the RESMA (n = 1278), giving a total of 1663 (n = 1663) cases included in this study (Fig. 1). The reasons for exclusion were: 974 with no diagnosis of schizophrenia or related disorders, 187 living outside the study catchment area, six had died and 271 lacked sufficient information to be included in the study.
Concerning the key informants, 76 of 165 general practitioners responded to the invitation to participate, giving a responses rate of 46%. Of the 30 private psychiatrists contacted only four (13%) responded, providing information about possible cases. In order to maintain the confidentiality of their patients, they only gave initials rather than full names.
Diagnoses and sociodemographic profile
Schizophrenia was the most frequent diagnosis, accounting for 1052 (63.3%) of the 1-year census sample. A further 268 (16.1%) cases were diagnosed with persistent delusional disorder, and 155 (9.3%) had acute or transient psychotic disorder. Another 108 (6.5%) had schizoaffective disorders. The remaining 80 (4.8%) did not meet the ICD-10 criteria for any of the above diagnoses and were retained in the study sample as a heterogeneous category that included schizotypal disorder (because of the low number of cases), induced delusional disorder, other non-organic psychotic disorders or unspecified non-organic psychosis.
The demographic characteristics of the 1663 patients with schizophrenia or related disorders were: mean age 45.5 years (standard deviation 13.5; range 15–88); 64.7% male; 64.3% single, 25.34% married/cohabiting and 10.4% separated, divorced or widowed. The majority of patients (51.4%) were living with their parents or other relatives, 23.4% with their own family in independent accommodation, 23.4% in sheltered accommodation, 8.8% living alone and 1.2% were homeless. Concerning education, 47.9% had not completed secondary schooling and 19.6% had not attained any school qualification. However, 22.1% had completed secondary schooling and 10.4% had a tertiary education diploma or degree. Concerning employment status, 38.5% were unable to work (receiving benefits), 16.7% were working, 14.2% without work, 9.2% studying, and 6.1% housekeeping; other categories comprised the remaining 15.3%.
Prevalence of schizophrenia and related disorders
In the catchment study area, the number of prevalent cases in 2008 aged 14 years or older was 1663 for schizophrenia and related disorders and 1052 for the subgroup of schizophrenia only, corresponding to 1-year prevalence rates of 6.27 per 1000 (95% CI, 5.97–6.57) and 3.97 per 1000 (95% CI, 3.73–4.21), respectively (Table 2).
Table 2.
The 1-year prevalence of schizophrenia and related disorders by gender, urban/rural areas and deprived and non-deprived zones
| Number of persons | Rate per 1000 inhabitants | 95% CI | Standard error | χ2 | p value | |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Schizophrenia and related disorders‡ | 186.4 | <0.0001 | ||||
| Male | 1076 | 8.45 | 8.53–8.85 | 0.83 | ||
| Female | 587 | 4.26 | 3.92–4.61 | 0.18 | ||
| Total | 1663 | 6.27 | 5.97–6.57 | 1.54 | ||
| Schizophrenia† | 226.7 | <0.0001 | ||||
| Male | 749 | 5.88 | 5.46–6.31 | 0.21 | ||
| Female | 303 | 2.20 | 1.95–2.24 | 0.13 | ||
| Total | 1052 | 3.97 | 3.73–4.21 | 0.12 | ||
| Urban and rural areas | ||||||
| Schizophrenia and related disorders‡ | 36.75 | <0.0001 | ||||
| Urban | 1518 | 6.64 | 6.31–6.98 | 0.17 | ||
| Rural | 145 | 3.95 | 3.33–4.65 | 0.32 | ||
| Schizophrenia† | 23.8 | <0.0001 | ||||
| Urban | 959 | 4.20 | 3.94–4.48 | 0.13 | ||
| Rural | 93 | 2.49 | 2.01–3.05 | 0.25 | ||
| Socioeconomic deprived zone | ||||||
| Schizophrenia and related disorders‡ | 7.83 | 0.005 | ||||
| Non-deprived | 1466 | 6.12 | 5.81–6.45 | 0.16 | ||
| Deprived | 197 | 7.56 | 6.54–8.70 | 0.53 | ||
| Schizophrenia† | 2.08 | 0.149 | ||||
| Non-deprived | 935 | 3.90 | 3.66–4.16 | 0.12 | ||
| Deprived | 117 | 4.49 | 3.71–5.38 | 0.41 |
CI, confidence interval.
‡ICD-10 F20–F29 codes or corresponding group of diagnoses.
†ICD-10 F20 codes or corresponding group of diagnoses.
The estimates for schizophrenia and related disorders were higher for men than women: men, 8.45 per 1000 (95% CI, 8.53–8.85) and women, 4.26 per 1000 (95% CI, 3.92–4.61), with a male-to-female ratio of 1.98. For the subgroup of schizophrenia, the rates were: men, 5.88 per 1000 (95% CI, 5.46–6.31) and women, 2.20 per 1000 (95% CI, 1.95–2.24), with a male-to-female ratio of 2.67. The prevalence rate in males was significantly higher than in females, for both schizophrenia and related disorders (p < 0.0001) and for the schizophrenia only subgroup (p < 0.0001).
In the urban area, the prevalence of schizophrenia and related disorders was 6.64 per 1000 (95% CI, 6.31–6.98) while in the rural area the figure was almost half (3.95 [95% CI, 3.33–4.65]) (p < 0.0001). The same pattern was found for the subgroup of schizophrenia, with a prevalence of 4.20 per 1000 (95% CI, 3.94–4.2) in the urban area and 2.49 per 1000 (95% CI, 2.01–3.05) in the rural area (p < 0.0001).
The prevalence of schizophrenia and related disorders was higher in socioeconomic deprived areas, 7.56 per 1000 (95% CI, 6.54–8.7), compared with non-deprived areas, 6.12 per 1000 (95% CI, 5.81–6.45) (p = 0.005) For the subgroup of schizophrenia the respective figures were 3.9 per 1000 (95% CI, 3.66–4.16) and 4.49 per 1000 (95% CI, 3.71–5.38) in non-deprived and deprived areas, respectively (p = 0.149), although the differences were not large enough to reach statistical significance (Table 2).
Discussion
Methodological issues
This study has a number of strengths. First of all, in order to provide the most reliable and valid estimates of the rates of schizophrenia and related disorders we used a two-stage procedure for case identification, gathering extensive information from case registers, clinical databases, case notes and key informants. A sampling procedure on this scale involved a large amount of information resources, a great number of patients included in clinical databases, and a high number of key informants. We therefore considered this an accurate methodology for the identification of a district-based population of people with broadly defined schizophrenia. In addition, although, as it is known that about 0.3 per 1000 patients with schizophrenia or related disorders are not treated by psychiatric services (Jablensky, 2000) and assuming that some individuals with severe mental illness might never be treated, or have had just intermittent contact with psychiatric services (Katschnig, 2011), we also included in our study a range of other agencies besides the mental health services. Consequently, this study represents a complete screening of persons with schizophrenia and related disorders as we included all the care organisations considered to have information about these individuals. Thus, users of the mainstream services involved different groups: (i) patients treated in mental health services, (ii) patients under the care of general practitioners, (iii) patients attended by private psychiatrists, (iv) patients in contact only with the emergency departments, (v) patients attended by private psychiatrists and (vi) homeless persons, as in this area there is a mental health team which attends persons living on the street. As far as we know, this is the first study in Spain in which a method including large clinical databases complemented by key informants has been used to determine the prevalence of schizophrenia. Furthermore, we have found no study in the international literature that includes patients with schizophrenia or related disorders from the emergency services (either hospital or extra-hospital) in their calculations of the prevalence rates.
The results, although, should be viewed in light of some possible limitations. Using multiple sources often has the potential to introduce diagnostic misclassifications (reliability) (Byrne et al. 2005; Morgan & Jablensky, 2010). However, we tried our best to confirm that all patients included in the study had a diagnosis confirmed by a psychiatrist. Several authors have demonstrated the validity of the clinical diagnoses made by professionals and included in case notes, concluding that the reliability of this diagnostic procedure is satisfactory (Ruggeri et al. 2000; Aräjarvi et al. 2005; Oiesvold et al. 2013). Another limitation of our study is the classification of a socieconomic deprived area according to the Andalusian Government, which is based on administrative criteria; although as it is used at the local level to implement more health services it could still be useful in our case. Finally, we are aware that in the statistical analysis we did not take into account possible confounders using a regression analysis to examine the effects of one variable while the others are controlled for. However, future studies will include this type of analysis.
Findings
Global prevalence
As far as we know, the 1-year prevalence rates for schizophrenia and related disorders of 6.27 per 1000, and 3.97 per 1000 for the subgroup of schizophrenia, for persons aged over 14 years in one catchment area of Malaga are the highest yet found in Spain (Ayuso et al. 2006; Tizón et al. 2007), but are within the limits of past studies when compared with international studies (Table 3, appendix 1).
To place these findings in a broader perspective comparisons with similar studies conducted elsewhere would be of interest, with the proviso that any such comparisons must take into account the design and methodology of each study, as well as differences related to the background characteristics of the populations, the health care context, the point in time of data collection and the methods of case ascertainment (Anderson, 2013). Despite these methodological aspects, we have attempted to compare our data with those derived from studies published since 1990, especially those most similar to ours in that the patients were selected from a varied range of health services, including mental and primary care or social services and using databases and key informants.
As can be seen in Table 3 (appendix 1), for studies reporting figures for the subgroup of schizophrenia only, de Salvia et al. (1993) reported the lowest prevalence rate (2.7 per 1000) using the Case Registry of Portogruaro (Italy). The highest figure was that reported by Bamrah et al. (1991) in an inner-city population, who found a prevalence rate in Salford (UK) of 7 per 1000. Analysis of those studies that give figures for the whole group of schizophrenia and related disorders shows that the rates range from 3.4 per 1000, offered by Ruggeri et al. (2000) from the South Verona Psychiatric Case Register, to the 6.7 per 1000 (the rate most similar to ours, which was 6.9 per 1000), recently reported by Jörgensen et al. (2014), although it was extracted from clinical databases of psychiatric services only (Table 3, appendix 1).
Gender prevalence
We found a higher prevalence in males than females. Although recent studies on the prevalence of schizophrenia in the general population found no gender difference (Përalä et al. 2007; McGrath et al. 2008), our results are similar to those of other studies, especially of persons in contact with mental health services (Aleman et al. 2003; Usall et al. 2007; Morgan et al. 2008; Iniesta et al. 2012; Ochoa et al. 2012; Jörgensen et al. 2014) and also in agreement with those of McGrath & Susser (2009), who concluded that schizophrenia does not affect men and women equally; specifically, for every three affected men there are two affected women. The male-to-female ratio found in our study is higher than that in most studies of the prevalence of schizophrenia but it is consistent with previous studies in Andalusia (Moreno-Küstner et al. 2007, 2009). A possible explanation for the differences is that the social course of schizophrenia is less favourable in men than in women (Häfner et al. 2013) and the disorder affects men more seriously, reflected in their greater utilisation of health care services. Thus, when examining treatment prevalence, men are overrepresented because they contact services more often than women over time.
Urban and rural prevalence
The 1-year prevalence of schizophrenia and related disorders in one catchment area of Malaga showed a higher rate in the city than in the rural area, with the same occurring for the subgroup with just schizophrenia. Our data are compatible with other results showing higher prevalence rates in urban v. rural areas (Harvey et al. 1996; Widerlöv et al. 1997). However, our results are in disagreement with two systematic reviews by Saha et al. (2005) and Kirkbride et al. (2012), who found similar prevalence rates in both dwelling areas. We need to better understand what these patterns of distribution of schizophrenia are telling us (Tandon et al. 2008).
Socioeconomic deprived and non-deprived area and prevalence
Our results showing a higher rate of schizophrenia in socioeconomic deprived areas failed to reach statistical significance. This might be because this classification, done by the Andalusian Government, is based on administrative parameters. However, our results are in accordance with those of Jablensky et al. (2000) in that the majority of psychotic persons live in extreme social isolation and adverse socioeconomic circumstances or tend to reside in the most deprived areas (Lix et al. 2007) and are more likely to stay in or migrate to the most socially or materially deprived territories (Ngamini Ngui et al. 2013). Similarly to us, Jörgensen et al. (2014) found a correlation between the 1-year prevalence of persons with non-affective psychoses and persons receiving social welfare benefits in the geographical area of Stockholm, and Kirkbride et al. found that greater levels of neighbourhood income inequality and absolute deprivation (neighbourhood social composition) were associated with the incidence of non-affective psychosis (Kirkbride et al. 2014). Further studies are needed in our local area to better explain the differences between the socioeconomic deprived and non-deprived areas in relation with the distribution of the schizophrenia figures.
Conclusion
The prevalence of schizophrenia and related disorders is not uniform in terms of gender or place (urban/rural). We found high figures for the prevalence of schizophrenia, supporting the idea that multiple sources of information are essential to estimate accurate rates. Such precise estimates of the prevalence of schizophrenia have important repercussions concerning resource allocation and policy planning, because these rates reflect the true burden of the disease in the mental health services. The results also provide an extensive database on persons with schizophrenic disorders as a reference framework for future epidemiological studies.
Acknowledgements
The authors acknowledge the Primary Care District of Malaga, particularly Maximiliano Vilaseca, Francisco Javier Navarro and Patricia Moreno, for their support and also Carlos García (ETEA, Córdoba) for his collaboration. We are also grateful to all the agencies which collaborated with this study offering their databases, especially, from Malaga Primary Care Sanitary District (Pharmaceutical Department, Carmen Vela, Users Department, Ma Dolores Domínguez and Emergency Department, Ana María Martínez), also, from Carlos Haya Hospital (Information System Department) and from San Juan de Dios Hospital (Diego Arenas), and finally, general practices directors and general practitioners and also the private psychiatrists who collaborated with this study.
Appendix 1
Table 3.
(Appendix 1). Epidemiological studies (since 1990) reporting prevalence figures of schizophrenia and related disorders in patients seen by health services
| Rate per 1000 inhabitants | Information sources | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Year | First author | Country | Schizophrenia | Schizophrenia and related disorders | Diagnostic procedure | Agencies | Data bases | Age lower– upper | Classification | Type of prevalence |
| 1991 | Youssef | Ireland | 4.6 | Clinical | MHS + PC | CDB + KI | 15–90 | DSM-III-R | Point | |
| 1991 | Bamrah | UK | 7 | 7.5 (5) | PSE | MHS + PC | CDB + KI | 15–90 | ICD-9 | 1-year |
| 1993 | de Salvia | Italy | 2.7 | Clinical | MHS | CDB | 15–90 | ICD-9 | 1-year | |
| 1996 | Harvey | UK | 3.3 | 5.3 (4) | MSP | MHS + PC + SS | CDB + KI | 18–90 | DSM III-R | Point |
| 1997 | McCreadie | UK | 3.5 | OPCRIT | MHS + PC + SS | CDB + KI | 18–90 | ICD-10 | Point | |
| 1997 | Widerlöv | Sweden | 4.2 | 7.3 (1) | Clinical | MHS + PC + SS | CDB + KI | 18–90 | DSM-III-R | 1-year |
| 1997 | Jeffreys | UK | 5.9 (3) | Clinical | MHS + PC + SS + PS | CDB + KI | 15–54 | DSM III-R | Point | |
| 1998 | Thornicroft | UK | 7.7 (1) | SCAN | MHS + PC + SS + PR | CDB + KI | 15–85 | ICD-10 | 1-year | |
| 2000 | Ruggeri | Italy | 3.4 (1) | Clinical | MHS | CDB | 18–90 | ICD-10 | 1-year | |
| 2000 | Jablensky | Australia | 4.7 (1) | OPCRIT + SCAN | MHS + PC + SS | CDB | 18–64 | ICD-10 | Point | |
| 2007 | Tizón | Spain | 4.6 | Clinical | MHS + PC | CDB | 15–90 | DSM-IV | 1-year | |
| 2014 | Jörgensen | Sweden | 3.7 | 6.7 (2) | Clinical | MHS | CDB | 18–64 | ICD-10 | 1-year |
| 2014 | Moreno-Küstner | Spain | 4.3 | 6.9 (2) | Clinical | MHS + PC + EMERG + PR | CDB + KI | 15–90 | ICD-10 | 1-year |
Agencies as information sources. MHS: Public Mental Health Services; PC: Primary Care; SS: Social Services; PR: Private psychiatrist; EMERG: Emergency Services; PS: Prison.
Social Services also include hostels, persons in social welfare, church, among others. CDB, Clinical Databases; KI, Key-informant.
OPCRIT, operational criteria checklist for psychotic illness; SCAN, Schedules for Clinical Assessment in Neuropsychiatry; PSE, Present State Examination.
1. – Affective and no affective psychosis: F20–F22; F24, F25, F28–F31, F32.3, F33.3 of ICD-10.
2. – Schizophrenia and related disorders: F20–F29 of ICD-10.
3. – Schizoaffective psychosis, paranoid psychosis and possible schizophrenia.
4. – Schizophrenia, schizophreniform psychosis, schizoaffective psychosis, paranoid psychosis, chronic psychosis or query schizophrenia.
5. – Reactive and unspecified forms of schizophrenia: 295.9, 297.9, 298.3, 298.4, 298.8, 298.9 of ICD-9.
Financial Support
This study was supported by the Andalusian Government (grant references, 05/353, PI-0338/08, PI-0332/08, P10-CTS-5862, CTS-945) and the Spanish Ministry of Health (RedIAPP, RD06/0018/0039).
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Standards
The author asserts that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional Committees on human experimentation and with the Helsinki declaration of 1975, as revised in 2008.
References
- Anderson KK (2013). Health service registry data in psychiatric epidemiology: challenges for definition and interpretation. Acta Psychiatrica Scandinavica 127, 9–10. [DOI] [PubMed] [Google Scholar]
- Aleman A, Kahn RS, Selten JP (2003). Sex differences in the risk of schizophrenia, evidence from meta-analysis. Archives of General Psychiatry 60, 565–571. [DOI] [PubMed] [Google Scholar]
- Aräjarvi R, Suvisaari J, Suokas J, Schreck M, Haukka J, Hintikka J, Partonen T, Lönnqvist J (2005). Prevalence and diagnosis of schizophrenia based on register, case record and interview data in an isolated Finnish birth cohort born 1940–1969. Social Psychiatry and Psychiatric Epidemiology 40, 808–816. [DOI] [PubMed] [Google Scholar]
- Ayuso-Mateos JL, Gutierrez-Recacha P, Haro JM, Chisholm D (2006). Estimating the prevalece of schizophrenia in Spain using a disease model. Schizophrenia Research 86, 194–201. [DOI] [PubMed] [Google Scholar]
- Bamrah JS, Freeman HL, Goldberg DP (1991). Epidemiology of schizophrenia in Salford, 1974–84. Changes in an urban community over ten years. British Journal of Psychiatry 159, 802–810. [DOI] [PubMed] [Google Scholar]
- Byrne N, Regan C, Howard L (2005). Administrative registers in psychiatric research, a systematic review of validity studies. Acta Psychiatrica Scandinavica 112, 409–414. [DOI] [PubMed] [Google Scholar]
- de Salvia D, Barbato A, Salvo P, Zadro F (1993). Prevalence and incidencre of schizophreic disorders in Portogruaro. An Italian Case Register Study. Journal of Nervous and Mental Disease 181, 275–282. [DOI] [PubMed] [Google Scholar]
- Drukker M, Krabbendam L, Driessen G, vas Os J (2006). Social disadvantage and schizophrenia. A combined neighbourhood and individual-level analysis. Social Psychiatry and Psychiatric Epidemiology 41, 595–604. [DOI] [PubMed] [Google Scholar]
- Fletcher RH, Fletcher SW, Wagner EH (2002). Epidemiología Clínica. Masson: Barcelona. [Google Scholar]
- Häfner H, Maurer K, Heiden W (2013). ABC Schizophrenia study: an overview of results since 1996. Social Psychiatry and Psychiatric Epidemiology 48, 1021–1031. [DOI] [PubMed] [Google Scholar]
- Harvey CA, Pantelis C, Taylor J, Mccabe PJ, Lefevre K, Campbell PG, Hirsch SR (1996). The Camden schizophrenia surveys. II. High prevalence of schizophrenia in an inner London borough and its relationship to socio-demographic factors. British Journal of Psychiatry 168, 418–426. [DOI] [PubMed] [Google Scholar]
- Iniesta R, Ochoa S, Usall J (2012). Gender differences in service use in a sample of people with schizophrenia and other psychoses. Schizophrenia Research Treatment, 365452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablensky A (2000). Prevalence and incidence of schizophrenia spectrum disorders, implications for prevention. Australian New Zeland Journal of Psychiatry 34(Suppl.), S26–S34. [DOI] [PubMed] [Google Scholar]
- Jablensky A, McGrath J, Herrman H, Castle D, Gureje O, Evans M, Carr V, Morgan V, Koerten A, Harvey C (2000). Psychotic disorders in urban areas: an overview of the study on low prevalence disorders. Australian and New Zealand Journal of Psychiatry 34, 221–236. [DOI] [PubMed] [Google Scholar]
- Jeffreys SE, Harvey CA, Mcnaught AS, Quayle AS, King MB, Bird AS (1997). The Hampstead Schizophrenia Survey 1991. I, Prevalence and service use comparisons in an inner London health authority, 1986–1991. British Journal of Psychiatry 170, 301–306. [DOI] [PubMed] [Google Scholar]
- Jörgensen L, Ahlbom A, Allebeck P, Dalman C (2010). The Stockholm non-affective psychoses study (SNAPS), the importance of including out-patient data in incidence studies. Acta Psychiatrica Scandinavica 121, 389–392. [DOI] [PubMed] [Google Scholar]
- Jörgensen L, Dalman C, Allebeck P (2014). Prevalence of psychoses in Stockholm County. A population-based study using comprehensive healthcare registers. Nordic Journal of Psychiatry 68, 60–65. [DOI] [PubMed] [Google Scholar]
- Junta de Andalucía (2004). Atención a la Salud en las Zonas con Necesidades de Transformación Social. Consejería para la Igualdad y el Bienestar Social de la Junta de Andalucía: Sevilla. [Google Scholar]
- Katschnig H (2011). Monitoring service utilization of persons with mental disorders-a case for mapping pathways of care. Epidemiology and Psychiatric Sciences 20, 7–13. [DOI] [PubMed] [Google Scholar]
- Kirkbride JB, Boydell J, Ploubidis GB, Morga C, Dazzan P, McKenzie K, Murray RM and Jones PB (2008). Testing the association between the incidence of schizophrenia and social capital in an urban area. Psychological Medicine 38, 1083–1094. [DOI] [PubMed] [Google Scholar]
- Kirkbride JB, Errazuriz A, Croudace TJ, Morgan C, Jackson D, McCrone P, Murray RM, Jones PB (2012). Department of Health report on prevalence (Chapter 5). Retrieved 13 June 2014 from http://www.psychiatry.cam.ac.uk/wp-content/uploads/2012/05/Final-report-v1.05-Jan-12.pdf
- Kirkbride J, Jones PB, Ullrich S, Coid JW (2014). Social Deprivation, inequality, and the neihborhood-level incidence of psychotic syndromes in East London. Schizophrenia Bulletin 40, 169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lix LM, Deverteuil G, Walker JR, Robinson RJ, Hinds AM, Roos LL (2007). Residential mobility of individuals with diagnoses schizophrenia: a comparison of single and multiple movers. Social Psychiatry and Psychiatric Epidemiology 42, 221–228. [DOI] [PubMed] [Google Scholar]
- March D, Hatch SL, Morgan C, Kirkbride JB, Bresnahan M, Fearon P, Susser E (2008). Psychosis and Place. Epidemiologic Reviews 30, 84–100. [DOI] [PubMed] [Google Scholar]
- McCreadie RG, Leese M, Tilak-Singh D, Loftus L, Macewan T, Thornicroft G (1997). Nithsdale, Nunhead and Norwood, similarities and differences in prevalence of schizophrenia and utilisation of services in rural and urban areas. British Journal of Psychiatry 170, 31–36. [DOI] [PubMed] [Google Scholar]
- McGrath JJ (2005). Myths and plain truths about schizophrenia epidemiology. The NAPE lecture 2004. Acta Psychiatrica Scandinavica 111, 4–11. [DOI] [PubMed] [Google Scholar]
- McGrath JJ (2007). The surprisingly rich contours of schizophrenia epidemiology. Archives of General Psychiatry 64, 14–16. [DOI] [PubMed] [Google Scholar]
- McGrath JJ, Susser E (2009). New directions in the epidemiology of schizophrenia. Medical Journal of Australia 190, S7–S9. [DOI] [PubMed] [Google Scholar]
- McGrath JJ, Saha S, Chant D, Welham J (2008). Schizophrenia, A concise overview of incidence, prevalence and mortality. Epidemiological Review 30, 67–76. [DOI] [PubMed] [Google Scholar]
- Moreno B, Arroyo B, Torres F, Luna JD, Cervilla J (2007). Social Predictors of out-patient mental health contact in schizophrenia patients. Social Psychiatry and Psychiatric Epidemiology 42, 452–456. [DOI] [PubMed] [Google Scholar]
- Moreno B, Mayoral F, Pérez O, García-Herrera JM, Algarra J, Rivas F, Pérez R, Becerra F, Gornemann I (2009). The Málaga Schizophrenia Case-Register (RESMA). Overview of methodology and patient cohort. International Journal of Social Psychiatry 55, 5–15. [DOI] [PubMed] [Google Scholar]
- Moreno-Küstner B, Mayoral F, Rivas F, Angona P, Requena J, García-Herrera JM, Navas D, Moreno P, Serrano-Blanco A, Bellón JA (2011). Factors associated with use of community mental health services by schizophrenia patients using multilevel analysis. BMC Health Services Research 11, 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Küstner B, Martín C, Almenara J (2014). Revisión crítica de las fuentes de variabilidad en la medición de la prevalencia de esquizofrenia. Salud Mental 37, 127–138. [Google Scholar]
- Morgan VA, Jablensky A (2010). From inventory to benchmark: quality of psychiatric case registers in research. British Journal of Psychiatry 197, 8–10. [DOI] [PubMed] [Google Scholar]
- Morgan VA, Castle DJ, Jablensky AV (2008). Do women express and experience psychosis differently from men? Epidemiological evidence from the Australian National Study of Low Prevalence (Psychotic) Disorders. Australian and New Zealand Journal of Psychiatry 42, 74–82. [DOI] [PubMed] [Google Scholar]
- Ngamini Ngui A, Cohen AA, Courteau J, Lesage A, Fleury MJ, Grégoire JP, Moisan J, Vanesse A (2013). Does elapsed time between first diagnosis of schizophrenia and migration between health territories vary by place of residence? A survival analysis approach. Health and Place 20, 66–74. [DOI] [PubMed] [Google Scholar]
- Ochoa S, Usall J, Cobo J, Labad X, Kulkarni J (2012). Gender differences in schizophrenia and first-episode psychosis. A comprehensive literature review. Schizophrenia Research Treatment, 916198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oiesvold T, Nivison M, Hansen V, Skre I, Ostensen L, Sorgaard KW (2013). Diagnosing comorbidity in psychiatric hospital: challenging the validity of administrative registers. BMC Psychiatry 13, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Përalä J, Suvisaari J, Saarni SI, Kuoppasalmi K, Isometsä E, Pirkola S, Partonen T, Tuulio-Henriksson A, Hintikka J, Kieseppä T, Härkänen T, Koskinen S, Lönnqvist J (2007). Lifetime prevalence of psychotic and bipolar I disorders in a general population. Archives of General Psychiatry 64, 19–28. [DOI] [PubMed] [Google Scholar]
- Ruggeri M, Leese M, Thornicroft G, Bisoffi G, Tansella M (2000). Definition and prevalence of severe and persistent mental illness. British Journal of Psychiatry 177, 149–155. [DOI] [PubMed] [Google Scholar]
- Saha S, Chant D, Welham J, McGrath JJ (2005). A systematic review of the prevalence of schizophrenia. PLoS Medicine 2, 413–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stata Corporation LP (2009). Stata, Release 11. Statistical Software: College Station, Texas. [Google Scholar]
- Tandon R, Keshavan MS, Nasrallah HA (2008). Schizophrenia, “Just the Facts” What we know in 2008. 2. Epidemiology and etiology. Schizophrenia Research 102, 1–18. [DOI] [PubMed] [Google Scholar]
- Thornicroft G, Strathdee G, Phelan M, Holloway F, Wykes T, Dunn G, McCrone P, Leese M, Johnson S, Szmukler G (1998). Rationale and design. PRiSM Psychosis Study I. British Journal of Psychiatry 173, 363–370. [DOI] [PubMed] [Google Scholar]
- Tizón J, Ferrando J, Parés A, Artigué J, Parra B, Pérez C (2007). Schizophrenic disorders in primary care mental health. Atención Primaria 39, 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usall J, Haro JM, Araya S, Moreno B, Muñoz PE, Martínez A, Salvador L, Psicost Group. (2007). Social functioning in schizophrenia, what is the influence of gender? European Journal of Psychiatry 21, 199–205. [Google Scholar]
- Widerlöv B, Lindström E, Von Knorring L (1997). One-year prevalence of long-term functional psychosis in three different areas of Uppsala. Acta Psychiatrica Scandinavica 96, 452–458. [DOI] [PubMed] [Google Scholar]
- World Health Organization (1993). International Classification of Mental and Behavioural Disorders, Diagnostic Criteria for Research, 10th revision. World Health Organization: Geneva. [Google Scholar]
- Youssef HA, Kinsella A, Waddington JL (1991). Evidence for geographical variations in the prevalence of schizophrenia in rural Ireland. Archives of General Psychiatry 48, 254–258. [DOI] [PubMed] [Google Scholar]