Abstract
Background.
Data from the general population show higher prevalence of different anxiety disorders in women as compared with men. We analysed gender differences in a naturalistic sample of outpatients with anxiety disorders in a mental healthcare setting.
Method.
Routine outcome monitoring data were collected from 1333 patients (age: 18–65; 63.3% women) fulfilling Diagnostic and Statistical Manual of Mental Disorders IV criteria of current anxiety disorder according to the Mini-International Neuropsychiatric Interview between 2004 through 2006. Data included Comprehensive Psychopathological Rating Scale, Brief Symptom Inventory (BSI), Short Form Health Survey (SF-36), Mood and Anxiety Symptom Questionnaire (MASQ). Chi-squared test and t-test were used to compare women with men for variables with parametric distributions, and Mann–Whitney test for non-parametric distribution. Adjustments for potential confounders (age, level of education, ethnicity and comorbidites) were made by logistic regression models (for discrete variables) or analysis of covariance.
Results.
The female-to-male ratio (i.e., 844 women, 489 men) for any anxiety disorder was 1.73 : 1 (95% confidence interval [CI]: 1.63–1.83), with the strongest skewness for post-traumatic stress disorder (2.80 : 1) and the smallest one for social phobia (1.18 : 1). Compared with men, women reported more severe self-rating scores on the BSI (on average, the scores were 12.3% higher on 3 of 9 subscales: somatisation, interpersonal sensitivity and anxiety), SF-36 (self-reported generic health status was lower on 5 of 8 subscales: physical functioning, social functioning, physical problems, vitality and bodily pain) and MASQ (on average, the scores were 6.6% higher on 4 of 5 subscales: anxious arousal, general distress, general distress depression, general distress anxiety). On the contrary, no gender difference was found in the severity of anxiety symptoms measured by the Brief Anxiety Scale. Women were more likely to suffer from comorbid depression and bulimia nervosa, and less likely from substance abuse.
Conclusions.
In a treatment-seeking population the prevalence rate of anxiety disorders was 1.7 times higher in female compared with men. Female outpatients were more severely affected on self-rated but not on observer-rated scales.
Key words: Epidemiology, gender differences, outpatient psychiatry, panic disorder, post-traumatic stress disorder
Introduction
Nearly all anxiety disorders are substantially more prevalent in women than in men (Breslau et al. 1997; Pigott, 1999; Altemus, 2006; Vesga-Lopez et al. 2008; McLean et al. 2011). Two large epidemiologic surveys conducted in the USA, the National Comorbidity Survey (NCS) and the Epidemiological Catchment Area (ECA) study, have compared the lifetime prevalence rates for anxiety disorders between the sexes. The NCS, conducted from 1990 to 1992, found that lifetime prevalence rates for any anxiety disorder were 30.5% for women and 19.2% for men; prevalence rates were higher in women than men for each separate anxiety disorder (Kessler et al. 1994). Gender ratios were 2.5 : 1 for panic disorder (PD), 2 : 1 for agoraphobia, 1.4 : 1 for social phobia, 2.3 : 1 for simple phobia and 1.8 : 1 for generalised anxiety disorder (GAD). Similar findings were found in the ECA study, with 1-month prevalence rates of 9.7% in women and 4.7% in men (Regier et al. 1990). These initial US community studies, have been widely replicated in the USA (National Comorbidity Survey-replication (NCS-R)) (Kessler et al. 2005a; Gum et al. 2009) as well as in Europe. In the Netherlands, the first and the second Netherlands Mental Health Survey and Incidence Study (NEMESIS 1 and 2) were conducted (Bijl et al. 1998; de Graaf et al. 2012). The NEMESIS 1, which dated from 1996, found that gender ratio was 3 : 1 for PD, 2.6 : 1 for agoraphobia, 2 : 1 for simple phobia, 1.6 : 1 for social phobia, 1.8 : 1 for GAD, and 0.9 : 1 for obsessive-compulsive disorder (OCD).
The available data on PD, agoraphobia and post-traumatic stress disorder (PTSD) indicate that women are at higher risks for these disorders than men (Katerndahl & Realini, 1993; Kessler et al. 1994, 2005a). Gender differences in prevalence proportions were less pronounced for social phobia and OCD, but findings are mixed (Bogetto et al. 1999; Pigott, 1999; Bekker & van Mens-Verhulst, 2007). For example, the Cross National Collaborative Group conducted a community survey in seven countries and found prevalence of OCD to be moderately higher in females than males (Weissman et al. 1994).
The reasons for gender differences in prevalence proportions and in clinical profiles are not well understood. Various biological, social and demographic influences have been suggested to explain these gender differences (Klose & Jacobi, 2004; McLean & Anderson, 2009), but also effects of response bias, symptoms severity, treatment and service utilisation rates may explain these findings. Since it is well known that many patients with psychiatric disorders do not seek treatment, and therefore if anxious men would be less likely than women to seek professional treatment, this may result in a biased female preponderance in the prevalence of anxiety disorders. However, it is largely unknown whether gender differences in anxiety disorders are similar between epidemiologic surveys and treatment-seeking populations of outpatient department (Burger & Neeleman, 2007; Kessler, 2007), as the latter population has been less often studied with regard to gender differences.
Moreover not only naturalistic prevalence data are scarce, but much is yet to be learned about gender differences in disease severity and symptoms profile across anxiety disorders. We are not aware of previous studies that examined gender differences in these parameters in large groups of outpatients with anxiety disorders.
We conducted a study in a naturalistic population of outpatients assessed with Routine outcome monitoring (ROM) and diagnosed with Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV) anxiety disorders to: (1) study gender ratios in prevalence for separate and combined anxiety disorders in treatment-seeking populations; (2) evaluate gender differences in anxiety severity scores; and (3) evaluate gender differences in anxiety-related symptom profiles and comorbidity patterns.
Methods
Patients
Patients with tentative mood-, anxiety- or somatoform (MAS) disorder were referred to the Dutch Regional Mental Health Provider (RMHP) Rivierduinen (RD) or the psychiatric outpatient department of the Leiden University Medical Center (LUMC) in the Western part of The Netherlands, where they enrolled in the standard ROM procedure.
About 80% of the patients referred to the LUMC or RD for treatment of a MAS disorder were enrolled in ROM. Subjects who declined participation were not different in terms of demographic or clinical characteristics compared with those who were willing to participate.
ROM is a method devised to systematically collect diagnostic data and data on the effectiveness of treatments in everyday clinical practice. It provides information on type and severity of psychopathology before starting treatment, feedback to therapists and patients on progress during treatment and databases for effectiveness research (de Beurs et al. 2011). ROM was performed by well-trained and supervised psychiatric research nurses, who were not involved in the clinical management.
Patients who entered the clinics between January 2004 and December 2006 were screened for inclusion as part of the usual ROM procedure. All literate patients with a good mastery of the Dutch language who are referred to RD or LUMC for treatment of a MAS disorder are routinely assessed with an extensive psychometric battery at baseline and prospectively during treatment. We used a dataset of 3798 adults (age range 18–65) who had a baseline ROM assessment between 2004 and 2006. Only ROM data collected during their first visit were used for the present study. We selected patients with a current DSM-IV-TR anxiety disorder (i.e., PTSD, agoraphobia, specific phobia, PD, GAD, OCD, social phobia) according to the Mini-International Neuropsychiatric Interview (MINI-Plus; 1386 patients, (36.5%) were identified. Patients with incomplete data were excluded; they did not differ significantly from the patients with complete data coverage on demographic and clinical variables (data not shown). Complete data on all variables of interest were available for 1333 (96.2%).
The use of anonymised data for research purposes has been approved by the Medical Ethical Committee of the Leiden University Medical Hospital.
As ROM data were primarily used for diagnosis and to inform clinicians and patients about treatment progress, informed consent was not required.
Covariates
Demographic variables were obtained using a self-report questionnaire. A Dutch ethnicity was assumed when the patient and both parents were born in the Netherlands. Marital status was categorised in ‘married’ (which included living together in a relationship) and ‘unmarried’ (which included divorced or widowed). Housing situation was categorised in ‘living alone’, ‘living with partner’ and ‘living with family’. Educational situation was categorised in ‘lower education’, defined as having completed elementary school, ‘high school – low’, defined as having completed lower secondary education, ‘high school – high’ and ‘college/university’. Employment situation was categorised in ‘employed full-time’, ‘employed part-time’, ‘unemployed/retired’ and ‘work-related disability’.
Psychiatric diagnoses
Diagnostic status according to the DSM-IV -TR was assessed with a standardised diagnostic interview (Dutch version of the MINI- Plus, version 5.00-R) (Sheehan et al. 1998; Van Vliet et al. 2000). The interview consists of 23 modules in which the presence or absence of DSM-IV criteria for the main psychiatric disorders was examined. The MINI-plus has good psychometric properties: it has been validated with Composite International Diagnostic Interview diagnoses (World Health Organization, 1990), and a good reliability has been shown (Lecrubier, 1997).
Dedicated Web-based computer software has been developed for the administration of the MINI-Plus diagnostic interview, completion of rating scales and administration of self-report measures. The software presents each question of the MINI-Plus on the screen of the interviewer together with the response options. The computer software is able to deal with the sometimes complicated scoring rules in this interview and is ‘intelligent’: if sufficient symptoms are answered as absent to preclude a diagnosis or if sufficient symptoms are rated present to establish a positive diagnosis, no additional questions are asked; after which, the module is closed and the next module is started.
The time needed for the MINI-Plus is about 30 min.
Clinical assessments
Psychosocial functioning was assessed with the Short Form Health Survey 36 (SF-36) (McHorney et al. 1993; Ware, 2000; Schulte-van Maaren et al. 2012). Psychopathological symptoms were assessed with the Brief Symptom Inventory (BSI) (Derogatis & Melisaratos, 1983; Endermann, 2005), the Mood and Anxiety Symptom Questionnaire (MASQ; Clark & Watson, 1991; de Beurs et al. 2011) and the Abbreviated Comprehensive Psychopathological Rating Scale (CPRS) (Goekoop et al. 2007). The CPRS scale was completed during a face-to-face interview by independent assessors, whereas the self-report questionnaires were filled out by the patient using a touch-screen computer.
The BSI is a self-rated questionnaire with 53 items to be answered on a five-point Likert-type scale (0–4 ranging from ‘not at all’ to ‘extremely’) selected from the Symptom Checklist 90 Revised (SCL-90-R). Its items define a broad spectrum of perceived restrictions due to physical and psychological symptoms occurring in the preceding 7-day period. The nine subscales represent domains of psychopathology: somatisation, obsessive–compulsive, interpersonal sensitivity, depression, anxiety, anger–hostility, phobic anxiety, paranoid ideation and psychoticism (Derogatis & Melisaratos, 1983; Endermann, 2005).
The MASQ is a self-rated 90-item self-report scale, designed to measure the three dimensions of Clark and Watson's tripartite model. It consists of five subscales: anhedonia specific to depression, anxious arousal specific to panic and anxiety, two general distress dimensions specific to depression and to panic and anxiety and a non-specific general distress dimension (Clark & Watson, 1991).
The CPRS is an observer-rated scale of 21 items, divided over three subscales: Brief Anxiety Scale (BAS), Montgomery–Asberg Depression Rating Scale (MADRS) and Motivational Inhibition (Goekoop et al. 2007). The BAS assesses pathological anxiety alone or anxiety occurring in the setting of other psychological or medical disorder (Tyrer et al. 1984).
The observer-rated abbreviated CPRS consists of the MADRS, the BAS and a scale that assesses psychomotor inhibition (INH; Goekoop et al. 1992). The MADRS has an internal consistency (Cronbach's alpha) of 0.86, and an inter-rater reliability coefficient of 0.65–0.97 (Montgomery et al. 1979).
The SF-36 is a self-rated generic outcome measure which yields eight multi-item scales measuring physical functioning, role limitations due to physical health problems, bodily pain, general health perceptions, vitality, social functioning, role limitations due to emotional health problems, mental health, a single-item evaluation of change in health and physical and emotional summary component scales. Raw scores are transformed to scale scores ranging from 0 to 100, with higher scores indicating better levels of functioning (McHorney et al. 1993; Ware, 2000; Schulte-van Maaren et al. 2012).
The total assessment took about 120 min.
Statistical analysis
Data are presented as mean (standard deviation (s.d.)) or number (percentage). Data were analysed for the whole group of patients with anxiety disorders (n = 1333), and for the separate groups of various anxiety disorders (GAD [n = 181], PD with or without agoraphobia [n = 587], agoraphobia with or without PD [n = 435], OCD [n = 152], PTSD [n = 308], social phobia [n = 382] and specific phobia [n = 77]). To compare men with women, χ2 tests were used for categorical variables, t-tests for variables with parametric distributions and Mann–Whitney test for variables with non-parametric distributions. Potential confounding variables were age, level of education (with four categories), ethnicity (with two categories) and comorbidity (using five dichotomous variables; major depression, alcohol abuse/dependence, drug abuse/dependence, bulimia, somatoform disorder). We did not adjust for multiple comparisons. Adjustments for confounders were made by logistic regression models or analysis of covariance (ANCOVA), when appropriate. Significance level was set at p < 0.05. Data were analysed using SPSS 20.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient characteristics, comorbidity and prevalence of disorders
The characteristics of 1333 patients are presented in Table 1. Out of this 63% of patients were female (n = 844). Compared with males, women were more likely to be younger (mean age difference 1.4 years; 95% confidence interval (CI): 0.13–2.67), to have a partner, to work part-time (as opposed to full-time) and to have a lower level of education. Ethnic background did not differ significantly between the sexes.
Table 1.
Clinical characteristics according to gender in the 1333 outpatients suffering from DSM-IV-TR anxiety disorders according to the MINI-Plus
| Total | Male | Female | |
|---|---|---|---|
| (n = 1333) | (n = 489; 36.7%) | (n = 844; 63.3%) | |
| Age (mean, s.d.) | 36.2 (11.4) | 37.0 (11.3) | 35.6 (11.5) |
| Ethnic background (n, %) | |||
| Dutch | 1186 (89.0) | 439 (89.8) | 747 (88.5) |
| Other ethnicity | 147 (11.0) | 50 (10.2) | 97 (11.5) |
| Marital status (n, %) | |||
| Married/living with partner | 655 (49.1) | 217 (44.4) | 438 (51.9) |
| Unmarried | 678 (50.9) | 272 (55.6) | 406 (48.1) |
| Housing situation (n, %) | |||
| Living alone | 321 (24.1) | 153 (31.3) | 168 (19.9) |
| Living with partner | 676 (50.7) | 224 (45.8) | 452 (53.6) |
| Living with family | 336 (25.2) | 112 (22.9) | 224 (26.5) |
| Educational status (n, %) | |||
| Lower education | 127 (9.5) | 54 (11.0) | 73 (8.6) |
| High school (lower) | 437 (32.8) | 140 (28.6) | 297 (35.2) |
| High school (higher) | 543 (40.7) | 203 (41.5) | 340 (40.3) |
| College/university | 226 (17.0) | 92 (18.8) | 134 (15.9) |
| Employment status (n, %) | |||
| Employed – part-time | 284 (21.3) | 47 (9.6) | 237 (28.1) |
| Employed – full-time | 314 (23.6) | 195 (39.9) | 119 (14.1) |
| Unemployed/retired | 349 (26.2) | 101 (20.7) | 248 (29.4) |
| Work-related disability | 386 (29.0) | 146 (29.9) | 240 (28.4) |
| Comorbidity (n, %) | |||
| Major depression | 574 (43.1) | 198 (40.5) | 376 (44.5) |
| Alcohol abuse/dependence | 75 (5.6) | 46 (9.4) | 29 (3.4) |
| Drug abuse/dependence | 65 (4.9) | 33 (6.7) | 32 (3.8) |
| Bulimia | 19 (1.4) | 3 (0.6) | 16 (1.9) |
| Somatoform disorder | 177 (13.3) | 69 (14.1) | 108 (12.8) |
| Comorbid anxiety disorder | 568 (43) | 181 (37) | 387 (46) |
The gender ratio for outpatients anxiety disorders was a strongly skewed towards females. There were 844 women and 489 men with any anxiety disorder, resulting in a gender ratio of 1.73 : 1 (95% CI: 1.63–1.83). Of the individual disorders, PTSD, agoraphobia, specific phobia and PD were clearly more prevalent in women; whereas GAD and OCD showed a less pronounced gender skewness (Fig. 1).
Fig. 1.
Forest plot of gender ratio of the prevalence of anxiety disorders in 1333 outpatients with current DSM IV-TR anxiety disorder. The area of the diamonds is proportional to the sample size. Error bars are 95% confidence intervals.
Forty-three percent of patients were affected by comorbid anxiety disorders, the 37% of the male sample and the 46% of the female sample.
Gender differences in self-report scores
All comparisons from self-report scales showed a higher subjective severity in women compared with men (Table 2).
Table 2.
Severity scores according to gender in 1333 outpatients suffering from anxiety disorders
| Male | Female | p-value* | Adjusted p-value** | |
|---|---|---|---|---|
| (n = 496; 36.7%) | (n = 857; 63.3%) | |||
| BSI subscales and total score | ||||
| Somatisation | 0.96 ± 0.03 | 1.10 ± 0.03 | 0.004 | 0.02 |
| Obsession–compulsion | 1.60 ± 0.04 | 1.65 ± 0.03 | 0.48 | 0.84 |
| Interpersonal sensitivity | 1.50 ± 0.05 | 1.70 ± 0.04 | 0.002 | 0.007 |
| Depression | 1.54 ± 0.04 | 1.67 ± 0.03 | 0.03 | 0.07 |
| Anxiety | 1.57 ± 0.04 | 1.71 ± 0.03 | 0.01 | 0.02 |
| Anger–hostility | 0.92 ± 0.04 | 0.95 ± 0.03 | 0.58 | 0.74 |
| Phobic anxiety | 1.30 ± 0.04 | 1.40 ± 0.03 | 0.07 | 0.20 |
| Paranoid ideation | 1.12 ± 0.04 | 1.15 ± 0.03 | 0.61 | 0.84 |
| Psychoticism | 1.24 ± 0.04 | 1.26 ± 0.03 | 0.71 | 0.78 |
| Total score | 1.30 ± 0.03 | 1.40 ± 0.02 | 0.02 | 0.07 |
| MASQ subscales | ||||
| Anhedonic depression | 75.6 ± 0.74 | 77.48 ± 0.58 | 0.05 | 0.12 |
| Anxious arousal | 33.1 ± 0.58 | 35.34 ± 0.47 | 0.004 | 0.008 |
| General distress | 38.8 ± 0.55 | 41.10 ± 0.42 | 0.001 | 0.003 |
| General distress depression | 30.7 ± 0.53 | 33.13 ± 0.43 | <0.001 | 0.001 |
| General distress anxiety | 26.6 ± 0.38 | 28.10 ± 0.30 | 0.003 | 0.007 |
| vCPRS subscales | ||||
| BAS | 16.17 ± 0.32 | 17.1 ± 0.23 | 0.19 | 0.27 |
| MADRS | 18.5 ± 0.46 | 19.6 ± 0.32 | 0.39 | 0.051 |
| Psychomotor inhibition | 4.10 ± 0.16 | 3.69 ± 0.11 | 0.33 | 0.007 |
BSI, Brief Symptom Inventory; MASQ, Mood and Anxiety Symptoms Questionnaire: self-reported scales; BAS, Brief Anxiety Scale; MADRS, Montgomery–Asberg Depression Rating Scale; Psychomotor inhibition: observer-rated scales.
Data are mean ± s.e.
*p-values were calculated by t-test for independent samples.
**Adjusted for age, education level, ethnicity and comorbidity using analysis of covariance (ANCOVA).
BSI
Women displayed higher mean scores than men on the BSI total score and on subscales for symptoms of somatisation, interpersonal sensitivity, depression and anxiety dimensions. After adjustment for age, level of education, ethnicity and comorbidities, the gender differences for somatisation, interpersonal sensitivity and anxiety remained statistically significant. On average the scores were 12.3% higher on these three BSI subscales in women compared with men.
MASQ
Women displayed higher scores than men on four of five subscales of MASQ (anxious arousal, general distress, general distress depression, general distress anxiety). These results persisted after adjustments for covariates. On average, the scores were 6.6% higher on these four MASQ subscales in women compared with men.
SF-36 (Table 3)
Table 3.
Psychosocial functioning according to gender in 1333 outpatients suffering from anxiety disorders
| Male vs. Female | |||||
|---|---|---|---|---|---|
| Male | Female | ||||
| (n = 489; 36.7%) | (n = 844; 63.3%) | ||||
| SF-36 | <50 | <50 | Adjusted OR* | 95% CI | Adjusted p-value* |
| Physical functioning | 47 (9.6%) | 123 (14.6%) | 1.66 | 1.14–2.43 | 0.008 |
| Social functioning | 207 (42.3%) | 413 (48.9%) | 1.30 | 1.01–1.64 | 0.03 |
| Physical problems | 235 (48.1%) | 473 (56.0%) | 1.40 | 1.10–1.76 | 0.006 |
| Emotional problems | 339 (69.3%) | 626 (74.2%) | 1.26 | 0.97–1.64 | 0.08 |
| Mental health | 316 (64.6%) | 586 (69.4%) | 1.17 | 0.90–1.53 | 0.23 |
| Vitality | 325 (66.5%) | 650 (77.0%) | 1.72 | 1.32–2.26 | <0.001 |
| Bodily pain | 115 (23.5%) | 254 (30.1%) | 1.41 | 1.08–1.85 | 0.01 |
| General health | 196 (40.1%) | 367 (43.5%) | 1.15 | 0.91–1.46 | 0.23 |
OR, odds ratio; CI, confidence interval.
*Adjusted for age, education level, ethnicity and comorbidity.
After adjustment for confounders, self-reported generic health status was significantly lower (cut-off value <50) in women than in men on five of eight subscales of the SF-36: physical functioning, social functioning, physical problems, vitality and bodily pain. No gender differences were found in the subscales concerning emotional problems, mental health and general health.
Gender differences in observer-rated scores
When focusing on the three CPRS subscales, no gender difference was found in the severity of anxiety symptoms measured by the observer-based BAS, while men were more severely affected by psychomotor inhibition than women. Using the MADRS, relatively mild depressive symptoms (mean score <20) were observed on average inour patients with anxiety disorders. MADRS mean scores were higher in women than in men, which approached statistical significance after adjustment for covariates (p = 0.051).
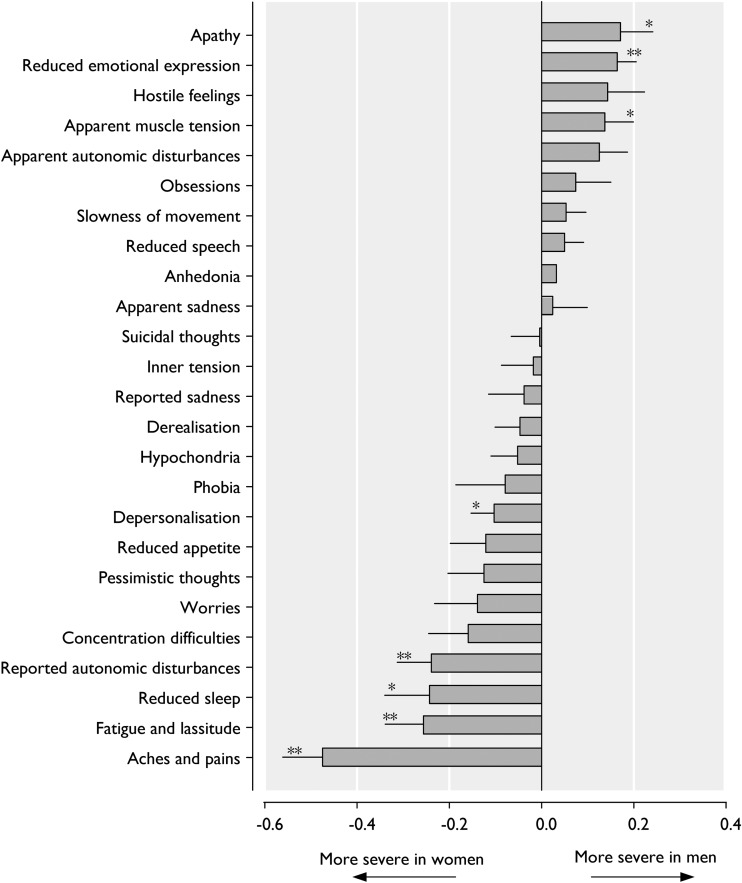
Figure 2 shows the relative severity in women compared with men for individual symptoms assessed by the CPRS. Women showed significantly more pronounced symptoms of depersonalisation, reported autonomic disturbances, reduced sleep, fatigue/lassitude and aches/pains. In contrast, men showed more pronounced symptoms of apathy, reduced emotional expression and apparent muscle tension. Thus, in our sample, women expressed more (severe) somatic symptoms, while men expressed more (severe) symptoms related to withdrawal and internalisation.
Fig. 2.
Proportional severity of individual symptoms of vCPRS in 1333 outpatients with current DSM IV-TR anxiety disorder. Error bars are standard errors. *p-value <0.05 for gender difference, adjusted for age, ethnic background, educational status and comorbidities by logistic regression analysis. **p-value <0.005 for gender difference, adjusted for age, ethnic background, educational level and comorbidity by logistic regression analysis.
Discussion
Gender differences were studied in a large naturalistic sample of patients with anxiety disorders. The sex difference in prevalence rates of different anxiety disorders has been well-established in population-based samples, with higher prevalence of anxiety disorders in women than in men (Regier et al. 1990; Kessler et al. 1994; Bijl et al. 1998; Kringlen et al. 2001; Gum et al. 2009; de Graaf et al. 2012). When comparing our gender ratios to the average one's derived from these six large population based studies, our results yielded very similar estimates (agoraphobia 2.4 in the previous studies v. 2.2 in our cohort; specific phobia 1.8 v. 2.1; PD 1.9 v. 2.0; GAD 1.9 v. 1.7; OCD 1.8 v. 1.4; and social phobia 1.5 v. 1.2). The overall gender ratio derived from population-based studies was 1.8 : 1 which was also very close to our estimate of 1.7 : 1. The epidemiology of PTSD is more difficult to compare as the exposure to assaultive violence and severe trauma may differ among genders. Nevertheless, the gender ratio from previous studies (e.g., 2.1 : 1 for the NCS (Kessler et al. 1994) 2.8 : 1 for the NCS-R (Kessler et al. 2005b), and 2.1 : 1 for the Detroit Area Survey of Trauma (Breslau et al. 1998; Carballo et al. 2010)) was rather similar to our estimate of 2.8 : 1. This suggests that there are no gender-specific referral rates and service utilisation rates, as the sex differences in prevalence found in population-based studies seem to persist in treatment-seeking populations.
Few studies have been conducted in treatment-seeking populations. Most of these have focused on prevalence without taking gender differences into account, or have focused on specific anxiety disorders or on specific age ranges. Carballo et al. (2010) studied anxiety disorders with regard to gender in a population of children and adolescents. Alnaes & Torgersen (1988) conducted a study among 298 psychiatric outpatients in which the gender ratio showed a female preponderance only for PD (gender ratio of 2 : 1), but not for the other anxiety disorders that were studied (agoraphobia, OCD, GAD, social phobia, specific phobia). As only 63 female and 29 male outpatients with anxiety disorders were included (representing a ratio of 2.2 : 1) effect estimates were not very precise.
There is limited data on gender differences in severity ratings in patients with anxiety disorders. Based on Turk et al. (1998), women exhibited more severe social phobia than men, assessed by several instruments, as well as more fear in specific situations. Other studies using clinical samples also found that female patients had more severe anxiety than male patients, assessed with the BSI (Kennedy et al. 1995; Kim et al. 2000; Moser et al. 2003) and the MASQ (Casper et al. 1996). Likewise, we demonstrated that women showed higher severity scores in a wide range of scales (i.e., BSI, MASQ, SF-36), indicating a higher level of subjective suffering. Yet, these gender differences were not apparent on our observer-rated scale (BAS), where the severity of anxiety did not show gender differences. Nevertheless, on the one hand, observer-rated depressive symptoms (MADRS) were slightly more severe in women, approaching statistical significance. On the other hand, psychomotor inhibition was more severe in men than women.
Self-report and observer-ratings scales may differ. Cuijpers et al. (2010) in a meta-analysis showed that clinician-rated instruments resulted in a significantly higher effect size than self-report instruments from the same studies. This meta-analysis has made it clear that clinician-rated and self-report measures of improvement following psychotherapy for depression are not equivalent. Different symptoms may be more suitable for self-report or ratings by clinicians and in clinical trials it is probably best to include both.
When zooming in on the symptom profile using the observer-rated data we found some remarkable differences among the genders, with fatigue, lassitude, autonomic disturbances, sleep reduction and pain being significantly more severe in women, while reduced emotional expression, apathy and muscle tension being more severe in men. Women with anxiety disorders seemed to have more suffering physical complaints, as reflected in significantly higher scores (see Fig. 2).
Besides, self-reported health status was worse in women than in men, measured with the SF-36.
Previous studies on sex differences in the symptom profiles of anxiety disorders in treatment-seeking population only analysed separate anxiety disorders. Studies in PTSD suggest that the manifestations of this disorder among male and female patients were rather similar (Zlotnick et al. 2001). Studies in OCD showed that women exhibited more cleaning compulsions and aggressive obsessions, whereas men more commonly showed obsessive slowness, symmetry obsessions and compulsions, touching rituals and sexual symptoms (Castle et al. 1995).
Our findings may have some clinical implication. First, physicians should be aware of gender differences in prevalence proportions of anxiety disorders since such epidemiological data may aid them when diagnosing and staging such patients. Second, the gender differences in anxiety symptoms, with a more severe subjective suffering in women but accompanied by similar observer-rated scale severity symptoms, suggest that women and men may benefit from more specifically targeted measurement tools as well as treatment strategies.
Strengths and limitations
The strength of our study is that we were able to collect a well-characterised large naturalistic sample of outpatients affected by anxiety disorders, in whom testing of severity scores and symptom profile was complete. The population available for this study was large, yielding rather precise estimations of the prevalence rates of these conditions in a treatment-seeking population. The current study was sufficiently powered to detect small but clinically relevant gender differences in severity and symptoms. Finally, we could combine and weigh both self-reported and observer-rated scales.
Our study also has potential limitations. No information about somatic comorbidity, potentially affecting the outcome of psychiatric illness, was collected. However, because we studied a large young population, it is unlikely that this has biased our results.
Information about concomitant and previous pharmacological or psychotherapeutical treatments was not ascertained. When such treatments were already initiated by the general practitioner, it may have affected our findings, especially for the severity of reported symptoms. However, it is unlikely that our results are affected to a large extend by gender differences in such treatments.
Patients with incomplete data were excluded. However, they did not differ significantly from patients with complete data and it was a small percentage.
Finally, despite we included many variables in our analysis, stressors related to socioeconomic factors, life events and trauma were not available, and may explain some of the gender differences found.
With respect to the many statistical tests that were done, our findings need to be interpreted cautiously, and need to be replicated in other cohorts.
Further studies are warranted as it is important to better understand the markers of disease and underlying risk factors that contribute to gender differences in anxiety disorders. Both biological differences that exist between the sexes and socio-cultural/behavioural differences between men and women may be involved. Female-specific research in anxiety disorders may help to uncover aetiological factors.
Acknowledgements
None.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
References
- Alnaes R, Torgersen S (1988). DSM-III symptom disorders (Axis I) and personality disorders (Axis II) in an outpatient population. Acta Psychiatrica Scandinavica 78, 348–355. [DOI] [PubMed] [Google Scholar]
- Altemus M (2006). Sex differences in depression and anxiety disorders: potential biological determinants. Hormones and Behavior 50, 534–538. [DOI] [PubMed] [Google Scholar]
- Bekker MH, Van Mens-Verhulst J (2007). Anxiety disorders: sex differences in prevalence, degree, and background, but gender-neutral treatment. Gender Medecine 4(Suppl. B), S178–S193. [DOI] [PubMed] [Google Scholar]
- Bijl RV, Ravelli A, Van Zessen G (1998). Prevalence of psychiatric disorder in the general population: results of The Netherlands Mental Health Survey and Incidence Study (NEMESIS). Social Psychiatry and Psychiatric Epidemiology 33, 587–595. [DOI] [PubMed] [Google Scholar]
- Bogetto F, Venturello S, Albert U, Maina G, Ravizza L (1999). Gender-related clinical differences in obsessive-compulsive disorder. European Psychiatry 14, 434–441. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Andreski P, Peterson EL, Schultz LR (1997). Sex differences in posttraumatic stress disorder. Archives of General Psychiatry 54, 1044–1048. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P (1998). Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Archives of General Psychiatry 55, 626–632. [DOI] [PubMed] [Google Scholar]
- Burger H, Neeleman J (2007). A glossary on psychiatric epidemiology. Journal of Epidemiology and Community Health 61, 185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carballo JJ, Baca-Garcia E, Blanco C, Perez-Rodriguez MM, Arriero MA, Artes-Rodriguez A, Rynn M, Shaffer D, Oquendo MA (2010). Stability of childhood anxiety disorder diagnoses: a follow-up naturalistic study in psychiatric care. European Child and Adolescent Psychiatry 19, 395–403. [DOI] [PubMed] [Google Scholar]
- Casper RC, Belanoff J, Offer D (1996). Gender differences, but no racial group differences, in self-reported psychiatric symptoms in adolescents. Journal of the American Academy of Child and Adolescent Psychiatry 35, 500–508. [DOI] [PubMed] [Google Scholar]
- Castle DJ, Deale A, Marks IM (1995). Gender differences in obsessive compulsive disorder. Australian and New Zealand Journal of Psychiatry 29, 114–117. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D (1991). Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. Journal of Abnormal Psychology 100, 316–336. [DOI] [PubMed] [Google Scholar]
- Cuijpers P, Li J, et al. (2010) Self-reported versus clinician-rated symptoms of depression as outcome measures in psychotherapy research on depression: a meta-analysis. Clin Psychol Rev. 30(6), 768–78. [DOI] [PubMed] [Google Scholar]
- De Beurs E, Den Hollander-Gijsman ME, Van Rood YR, Van Der Wee NJ, Giltay EJ, Van Noorden MS, Van Der Lem R, Van Fenema E, Zitman FG (2011). Routine outcome monitoring in the Netherlands: practical experiences with a web-based strategy for the assessment of treatment outcome in clinical practice. Clinical Psychology and Psychotherapy 18, 1–12. [DOI] [PubMed] [Google Scholar]
- De Graaf R, Ten Have M, Van Gool C, Van Dorsellaer S (2012). Prevalence of mental disorders and trends from 1996 to 2009. Results from the Netherlands Mental Health Survey and Incidence Study-2. Social Psychiatry and Psychiatric Epidemiology 47, 203–213. [DOI] [PubMed] [Google Scholar]
- Derogatis LR, Melisaratos N (1983). The Brief Symptom Inventory: an introductory report. Psychological Medicine 13, 595–605. [PubMed] [Google Scholar]
- Endermann M (2005). The Brief Symptom Inventory (BSI) as a screening tool for psychological disorders in patients with epilepsy and mild intellectual disabilities in residential care. Epilepsy and Behavior 7, 85–94. [DOI] [PubMed] [Google Scholar]
- Goekoop JG, et al. (1992). Multidimensional ordering of psychopathology. A factor-analytic study using the Comprehensive Psychopathological Rating Scale. Acta Psychiatrica Scandinavica 86(4), 306–312. [DOI] [PubMed] [Google Scholar]
- Goekoop JG, De Beurs E, Zitman FG (2007). Four-dimensional structure underlying scales for depression anxiety and retardation: emergence of trapped anger and scale improvements. Comprehensive Psychiatry 48(2), 192–198. [DOI] [PubMed] [Google Scholar]
- Gum AM, King-Kallimanis B, Kohn R (2009). Prevalence of mood, anxiety, and substance-abuse disorders for older Americans in the national comorbidity survey-replication. American Journal of Geriatric Psychiatry 17, 769–781. [DOI] [PubMed] [Google Scholar]
- Katerndahl DA, Realini JP (1993). Lifetime prevalence of panic states. American Journal of Psychiatry 150, 246–249. [DOI] [PubMed] [Google Scholar]
- Kennedy CA, Skurnick JH, Foley M, Louria DB (1995) Gender differences in HIV-related psychological distress in heterosexual couples. AIDS Care 7(Suppl. 1), S33–S38. [DOI] [PubMed] [Google Scholar]
- Kessler RC (2007). Psychiatric epidemiology: challenges and opportunities. International Review of Psychiatry 19, 509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS (1994). Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Archives of General Psychiatry 51, 8–19. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE (2005a). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry 62, 593–602. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Demler O, Frank RG, Olfson M, Pincus HA, Walters EE, Wang P, Wells KB, Zaslavsky AM (2005b). Prevalence and treatment of mental disorders, 1990 to 2003. New England Journal of Medicine 352, 2515–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KA, Moser DK, Garvin BJ, Riegel BJ, Doering LV, Jadack RA, Mckinley S, Schueler AL, Underman L, Mcerlean E (2000). Differences between men and women in anxiety early after acute myocardial infarction. American Journal of Critical Care 9, 245–253. [PubMed] [Google Scholar]
- Klose M, Jacobi F (2004). Can gender differences in the prevalence of mental disorders be explained by sociodemographic factors? Archives of Women's Mental Health 7, 133–148. [DOI] [PubMed] [Google Scholar]
- Kringlen E, Torgersen S, Cramer V (2001). A Norwegian psychiatric epidemiological study. American Journal of Psychiatry 158, 1091–1098. [DOI] [PubMed] [Google Scholar]
- Lecrubier Y, SD, Weiller E, et al. (1997). The mini international neuropsychiatric interview (mini). A short diagnostic structured interview: Reliability and validity according to the cidi. European Journal of Psychiatry 12, 224–231. [Google Scholar]
- Mchorney CA, Ware JE Jr., Raczek AE (1993). The MOS 36-item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Medical Care 31, 247–263. [DOI] [PubMed] [Google Scholar]
- McLean CP, Anderson ER (2009). Brave men and timid women? A review of the gender differences in fear and anxiety. Clinical Psychology Review 29, 496–505. [DOI] [PubMed] [Google Scholar]
- McLean CP, Asnaani A, Litz BT, Hofmann SG (2011). Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. Journal of Psychiatric Research 45, 1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A. R. I. E. (1979). A new depression scale designed to be sensitive to change. The British Journal of Psychiatry, 134(4), 382–389. [DOI] [PubMed] [Google Scholar]
- Moser DK, Dracup K, Mckinley S, Yamasaki K, Kim CJ, Riegel B, Ball C, Doering LV, An K, Barnett M (2003). An international perspective on gender differences in anxiety early after acute myocardial infarction. Psychosomatic Medicine 65, 511–516. [DOI] [PubMed] [Google Scholar]
- Pigott TA (1999). Gender differences in the epidemiology and treatment of anxiety disorders. Journal of Clinical Psychiatry 60(Suppl. 18), 4–15. [PubMed] [Google Scholar]
- Regier DA, Narrow WE, Rae DS (1990). The epidemiology of anxiety disorders: the Epidemiologic Catchment Area (ECA) experience. Journal of Psychiatric Research 24(Suppl. 2), 3–14. [DOI] [PubMed] [Google Scholar]
- Schulte-Van Maaren YWM, Carlier IVE, Zitman FG, Van Hemert AM, De Waal MWM, Van Noorden MS, Giltay EJ (2012). Reference values for generic instruments used in routine outcome monitoring: the leiden routine outcome monitoring study. BMC Psychiatry 12, 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry 59(Suppl. 20), 22–33; quiz 34–57. [PubMed] [Google Scholar]
- Turk CL, Heimberg RG, Orsillo SM, Holt CS, Gitow A, Street LL, Schneier FR, Liebowitz MR (1998). An investigation of gender differences in social phobia. Journal of Anxiety Disorders 12, 209–223. [DOI] [PubMed] [Google Scholar]
- Tyrer P, Owen RT, Cicchetti DV (1984). The brief scale for anxiety: a subdivision of the comprehensive psychopathological rating scale. Journal of Neurology Neurosurgery and Psychiatry 47, 970–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vliet IM, Leroy H, Van Megen HJGM (2000). M.I.N.I. Plus: M.I.N.I. Internationaal Neuropsychiatrisch Interview: Nederlandse versie 5.0.0.
- Vesga-Lopez O, Schneier FR, Wang S, Heimberg RG, Liu SM, Hasin DS, Blanco C (2008). Gender differences in generalized anxiety disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Journal of Clinical Psychiatry 69, 1606–1616. [PMC free article] [PubMed] [Google Scholar]
- Ware JE Jr. (2000). SF-36 health survey update. Spine (Phila Pa 1976) 25, 3130–3139. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Bland RC, Canino GJ, Greenwald S, Hwu HG, Lee CK, Newman SC, Oakley-Browne MA, Rubio-Stipec M, Wickramaratne PJ (1994). The cross national epidemiology of obsessive compulsive disorder. The Cross National Collaborative Group. Journal of Clinical Psychiatry 55(Suppl.), 5–10. [PubMed] [Google Scholar]
- World Health Organisation (1990). The Composite International Diagnostic Interview (CIDI) Authorized Core Version 1.O. Geneva: World Health Organisation. [Google Scholar]
- Zlotnick C, Zimmerman M, Wolfsdorf BA, Mattia JI (2001). Gender differences in patients with posttraumatic stress disorder in a general psychiatric practice. American Journal of Psychiatry 158, 1923–1925. [DOI] [PubMed] [Google Scholar]