Abstract
Patient: Male, 61-year-old
Final Diagnosis: Corneal ulcer
Symptoms: Pain • redness • watering • photophobia
Medication: —
Clinical Procedure: IODIM therapy
Specialty: Ophthalmology
Objective:
Unusual clinical course
Background:
Corneal ulceration is caused by various corneal diseases, including infection, inflammatory disease, neurotrophic keratitis, dry-eye, autoimmune disease, and blepharitis. Treatment should be based on the etiology. In cases of infection, corneal scraping and pathogen culture should be carried out before treatment. Bacterial pathogens are the most common etiology, but it can be caused also by viruses, fungi, and protozoa. Quinolones are the first-line drug for bacterial keratitis, but the treatment should be changed according to the culture and drug sensitivity test results.
The purpose of this case report is to show the resolution of a corneal ulcer case unusually treated with 0.66% povidone-iodine (PVP-I).
Case Report:
A 61-year-old man showed signs of pain, redness, watering, and photophobia in the left eye (oculus sinister; OS) over a 5-month period, starting as conjunctivitis and degenerating into keratitis. Clinical examination revealed an ulcer in the inferior cornea and biomicroscopy analysis confirmed this diagnosis.
Previous therapies, starting with antibiotics first and then antiviral medications, were unable to control the signs and symptoms. Therefore, treatment with 0.66% PVP-I, based on its antiseptic activity, was administered 3 times a day for 4 weeks.
Conclusions:
PVP-I 0.66%, an antiseptic with broad-spectrum activity against bacteria, fungi, viruses, and protozoa, was found to be effective in treating the signs and symptoms of the ulcer until its complete closure and resolution. It could be a useful therapeutic tool when the pathogen is unknown, as in this case. Its use for treatment of corneal ulcers warrants further investigation.
MeSH Keywords: Cornea, Eye Infections, Povidone-Iodine
Background
A corneal ulcer is a corneal epithelial defect, often involving the underlying stroma, usually caused by an inflammatory process or by infection, frequently diagnosed and treated by ophthalmologists [1]. This clinical condition can have vision-threatening consequences if not treated appropriately and promptly during the acute phase [2]. Patients can have significant complications such as corneal perforation, which can lead to development of severe ocular morbidities like glaucoma, cataracts, or synechiae, leading to vision loss [3]. Endophthalmitis and related loss of vision can also be a consequence of an untreated corneal ulcer [4].
Infection is the most common cause of corneal ulcers, with bacterial pathogens responsible in most cases [5]. Abrasions start as keratitis, which, by causing a break in the epithelium, allows penetration of bacteria into the cornea. Epithelial breaks are frequently caused by use of contact lenses, corneal abrasions, ophthalmic surgery, and other ocular trauma [5]. Systemic diseases, chronic ocular surface diseases, and the use of corticosteroids are considered other risk factors [6]. Staphylococci, particularly Staphylococcus aureus and coagulase-negative Staphylococcus, and Pseudomonas aeruginosa are the most commonly occurring bacterial pathogens associated with keratitis [6].
In contact lens wearers, keratitis and corneal ulcers are primarily caused by Acanthamoeba, a free-living protozoan found mainly in freshwater and in soil. Fungi and virus are responsible for only 5–10% of all corneal infections [5].
Topical antibiotics are the first-choice treatment for bacterial keratitis and corneal ulcers. The most commonly used are fluoroquinolones (ciprofloxacin or ofloxacin), but for severe infections, systemic antibiotics are also used [5]. Indiscriminate use of antibiotics, however, leads to the danger of antibiotic resistance [7]. Therefore, corneal culture and sensitivity tests should be part of the management of all corneal ulcers and should be based on microbiologically-proven results [8].
Povidone-iodine (PVP-I) is a potent antiseptic that exhibits rapid broad-spectrum activity against bacteria, fungi, viruses, and protozoa. The literature supports its use in ophthalmology for treatment of the ocular surface, especially as pre-treatment prior to invasive surgical procedures [9]. In vitro studies showed that PVP-I kills bacteria quickly at dilute concentrations (0.05–1.0%). In many instances, these dilute concentrations of PVP-I kill bacteria more quickly than more conventional concentrations such as 5–10% [10,11]. This report describes the use of a nanoemulsion formulation based on 0.66% PVP-I for the treatment of a corneal ulcer resistant to traditional antibiotic and antiviral therapy and its unexpected resolution.
Case Report
The patient signed informed consent to authorize the publication of this case report.
On 14 Feb 2018 a 61-year-old male non-smoker, with no coexisting disease and no concomitant medication or treatment, came to our clinic due to a deep ulcer (about half of the stroma) located in the inferior sector of the cornea.
The patient stated that the pathology had started about 5 months before as conjunctivitis and had developed into a keratitis. He complained of pain, burning, and severe photophobia, requiring him to wear sunglasses even at home. The slit lamp examination confirmed in the oculus sinister (OS) a deep predesmetical ulcer with neo-vessels in the inferior sector of the cornea (Figure 1). The visual acuity was 20/70 with +0.75=–1.5 and 180. The intraocular pressure was normal.
Figure 1.
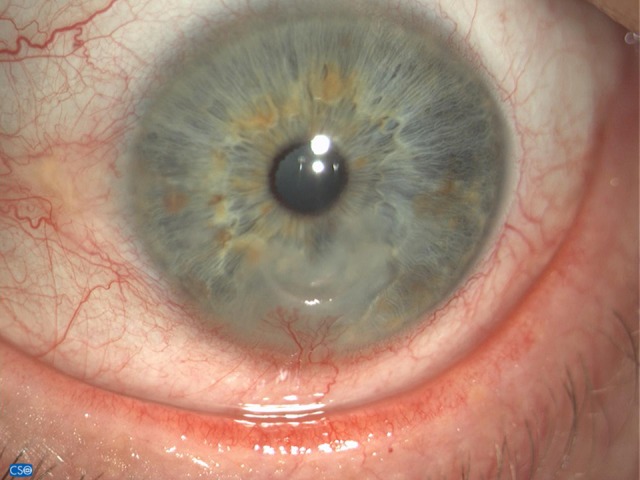
Corneal ulcer before starting therapy (14 Feb 2018).
Before presenting at our clinic, this patient had been previously treated with various local topical therapies: antibiotic-corticosteroid combinations, standard antibiotics, and, finally, antivirals. Conjunctival swabs performed during those therapies gave negative results, as no microbial growth was revealed, probably due to the ongoing antimicrobial therapy. The patient reported that for a few days, in the previous 5 months, the various therapies in use had been suspended, maintaining only the tear substitute. However, rather than improvement, there was a worsening of the symptoms.
A conjunctival swab collected with the Copan ESwab™ device (a tube with 1 ml liquid Amies medium and a FLOQSwab®) was performed again at our clinic, and the corneal scraping was added to the analysis. However, both were negative, probably due to the antibiotic therapy being used. Based on this result, it was not possible identify the etiology of the ulcer. Differential diagnosis resulted in the hypothesis of a viral ulcer, probably herpetic. In view of the progressive deepening of the ulcer, he had been offered the possibility of a keratoplasty.
Before planning surgery, the antibiotic therapy was substituted with IODIM®, a nanoparticular solution containing 0.66% PVP-I, hyaluronic acid, and medium-chain triglycerides, administered 3 times a day for 4 weeks, before changing to a tear substitute only.
IODIM® therapy was well tolerated, and after 12 days of treatment (on 26 Feb) a reduction in the depth of the ulcer and corneal neo-vessels was visible (Figure 2). During the same visit the patient reported a reduction in the symptomatology and said that he no longer needed to wear sunglasses at home.
Figure 2.
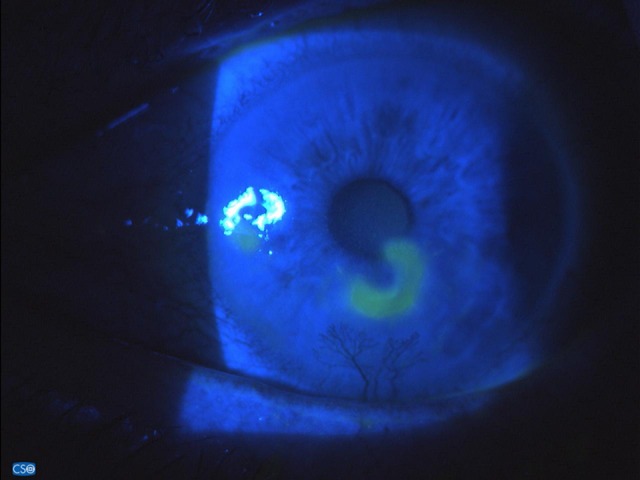
Fluoresceine staining after 10 days of IODIM therapy (26 Feb 2018).
Photographic evidence shows gradual and continuous reduction of the corneal ulcer and neo-vessels that occurred in less than 4 weeks (from 14 Feb to 12 Mar) (Figures 1–4) with IODIM® therapy alone. The reduction of the extent and the depth of the corneal ulcer can be observed in Figure 4.
Figure 3.
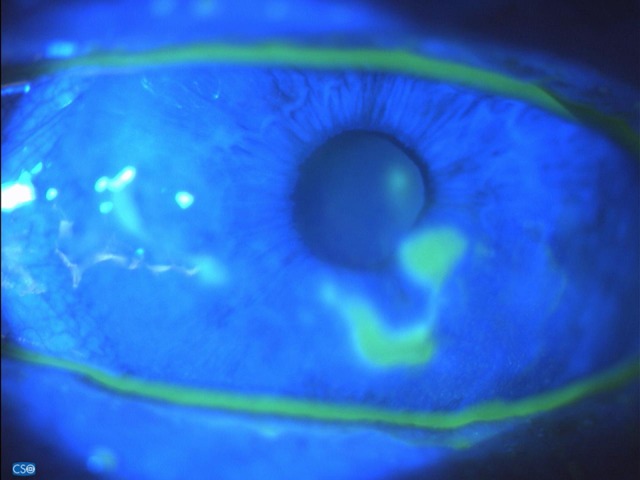
Fluoresceine staining after 18 days of IODIM therapy (5 Mar 2018).
Figure 4.
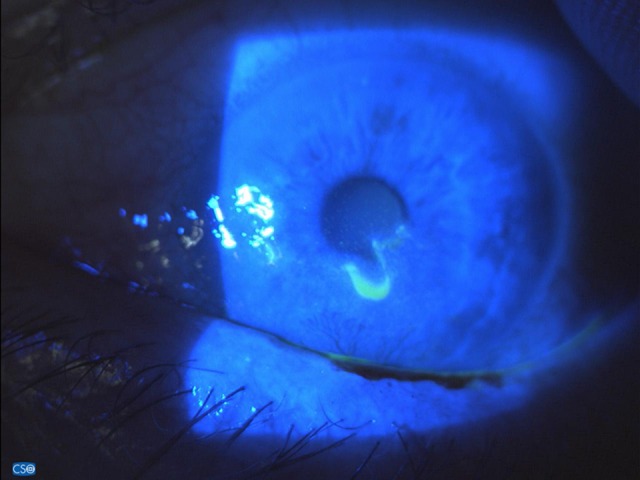
Fluoresceine staining after 25 days of IODIM therapy (12 Mar 2018).
Thereafter, only hyaluronic acid eye drops, 4/5 times a day, were used. The result was a complete return to integrum, which has remained stable over time (Figures 5, 6). This is particularly evident in Figure 6, which shows the condition of the cornea 45 days after the start of therapy. To date, after 8 months (Figures 7, 8), there has been no recurrence and the visual acuity is 20/20 with +0.25=–1.5 and 180.
Figure 5.
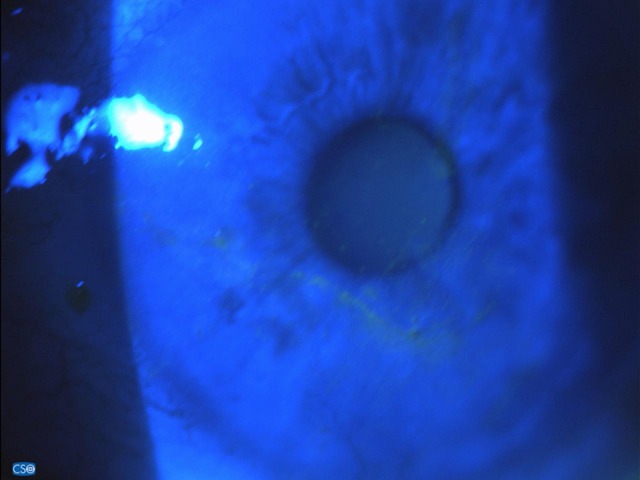
Fluoresceine staining 10 days after stopping IODIM therapy (26 Mar 2018).
Figure 6.
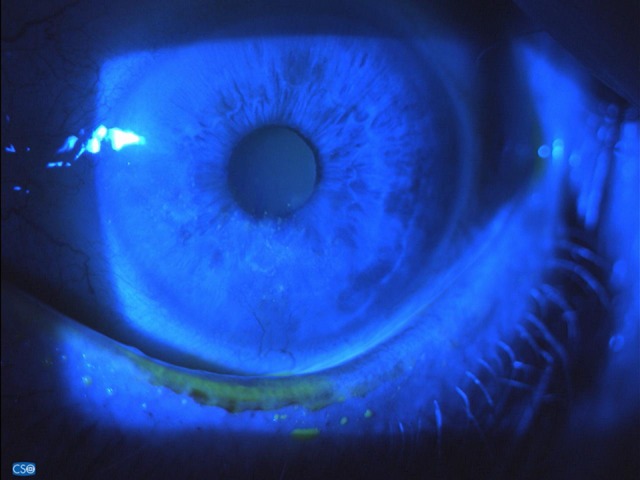
Fluoresceine staining 15 days after stopping IODIM therapy (complete recovery) (30 Mar 2018).
Figure 7.
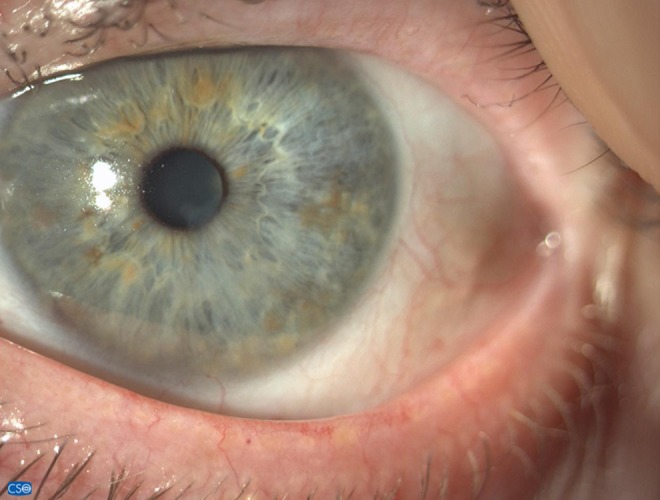
Corneal appearance 8 months after stopping IODIM therapy (6 Nov 2018).
Figure 8.
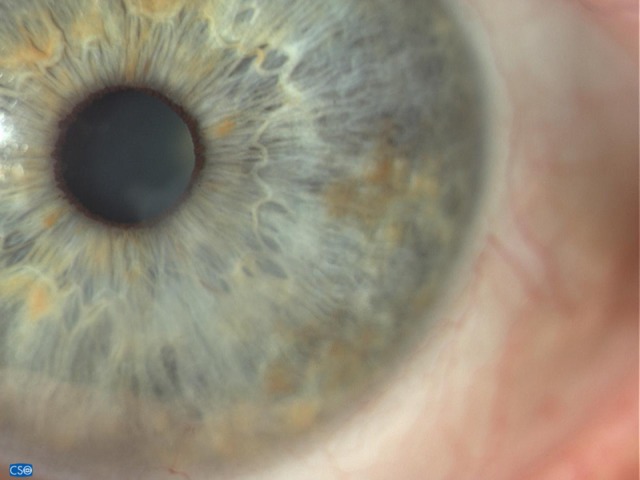
Corneal appearance 8 months after stopping IODIM therapy (6 Nov 2018).
Discussion
Corneal ulcer is often a consequence of infective keratitis. Treatment consists of topical and sometimes systemic antibiotics, often fluoroquinolones [7]. Topical antivirals are also added: trifluridine being the most common in the USA, while acyclovir is the first-line antiviral therapy in Europe. Adjuvant topical steroids can be also used. Ulcers caused by fungal infections often have worse outcomes than those caused by bacteria, as the treatment options are fewer. Natamycin, a polyene for topical use, is the primary treatment, introduced in the 1960s. An alternative treatment option is amphotericin B 0.3–0.5%, although its use is limited due to its toxicity. Prognosis of the corneal ulcer depends on its etiology, its size, and its location, as well as by the response to treatment [4].
This report describes the successful treatment of a corneal ulcer with a novel formulation consisting of a nanoparticular solution containing hyaluronic acid, medium-chain triglycerides, and 0.66% PVP-I.
PVP-I is a disinfectant and antiseptic agent with a broad spectrum of microbiological activity. It is effective against multidrug-resistant bacteria, viruses, fungi, and protozoa [9]. Moreover, it is active against bacterial biofilms and against Acanthamoeba [12]. Povidone acts as a reservoir of “free” iodine; it is hydrophilic and therefore acts as a carrier for transferring iodine through cell membranes to the target cells. It seems that the delivery of diatomic free iodine (I2) to the target cell is the critical event making PVP-I an antimicrobial agent [10]. This results in rapid bactericidal effects through its action on the cytoplasm and cytoplasmic membrane by inactivating the key cytosolic proteins, fatty acids, and nucleotides that immediately destroy the prokaryotic cells [9,13].
PVP-I is still an important antiseptic measure in ocular surgery [12]. For this reason, it is used widely, primarily for pre-operative preparation of the skin and mucous membranes, as well as the eyelids, eyelashes, and conjunctiva, prior to intraocular surgery [14].
The efficacy of PVP-I for treatment of corneal ulcers has been assessed in some studies.
In the 1960s, PVP-I was reported to be effective in the treatment of corneal ulcers when applied as an adjunct to Neosporin in patients with rapid destruction of ocular tissues related to Pseudomonas corneal ulcers [15]. Several years later, Katz et al. conducted a study on patients with corneal ulcers in rural Nepal; the addition of 2.5% PVP-I to standard antibiotic therapy did not improve visual outcomes, although the design of the study did not allow assessment of whether PVP-I alone would have resulted in comparable visual outcomes to that of standard therapy [16].
Isemberg et al. conducted a prospective randomized trial comparing the use of 1.25% PVP-I with antibiotics that are commonly available in the developing world. In the treatment of bacterial keratitis, PVP-I was compared with neomycin-polymyxin B-gramicidin in the Philippines and with 0.3% ciprofloxacin in India. Children and adults were involved and hospitalized for 7 days. There was no significant difference between the effect of topical 1.25% PVP-I and the aforementioned antibiotics [17]. Another recent paper has also shown clinical resolution and adenoviral eradication in patients with acute adenoviral conjunctivitis treated with a combination of Dexamethasone and PVP-I 0.6% [18].
The present case report shows the effect on a corneal ulcer of a new formulation containing 0.66% PVP-I, which has never been tested before. The greater antimicrobial effect of dilute PVP-I concentrations (0.05–1.0%) compared to higher concentrations (5–10%) has been demonstrated [10].
In fact, in many instances PVP-I kills bacteria more quickly at a dilute concentration (0.05–1.0%) than more conventional (5–10%) concentrations [19]. In particular, in vitro exposure of bacteria isolated from corneal ulcers to a dilute 0.25% PVP-I solution resulted in no growth after 30 s, whereas 10% and 5% solutions took longer to kill several of the isolated bacteria [20]. Likewise, 0.66% PVP-I was demonstrated to act faster than 5% PVP-I preparation in killing gram-positive and gram-negative bacteria [11]. This effect is probably caused by the higher availability of diatomic free iodine present in the dilute solution, as it is the bactericidal part of PVP-I. This fact is crucial, because the in vitro and in vivo toxicity of PVP-I is relative to its concentration in ophthalmic preparations [19], and it is critically important to administer PVP-I at a concentration that is both safe and effectives.
Successful results of a single case report are, of course, limited, and it is necessary to confirm the present results with more findings from additional cases. Controls are also lacking, as well as a study design that includes more rigorous analysis of the therapy. However, this clinical experience with its novel formulation based on 0.66% PVP-I and also containing hyaluronic acid and medium-chain triglycerides, which are well-accepted substances for nourishing the ocular surface, may be a promising start to increasing knowledge in subsequent controlled clinical trials.
PVP-I is mainly used as an antiseptic in surgical prophylaxis. However, it could be used as an adjunct therapy for treating stubborn infections, for reducing the duration of antibiotic use, or as a primary treatment for infections. Expanded applications in situations where antibiotics are heavily used could focus on decreasing exposure to antibiotics and in situations where an adjunct therapy for multi-and pan-drug-resistant infections is required. Resistance to PVP-I has not been reported in conjunctival cultures, and even repeated exposure to PVP-I does not produce resistance or cross-resistance [14,21].
Conclusions
Because PVP-I has broad-spectrum activity against bacteria, fungi, viruses and protozoa, it could be a useful therapeutic tool, especially in case of unknown pathogens where the use of antibiotics might merely result in increased antibiotic exposure without clinical success. The use of PVP-I for the treatment of corneal ulcers appears to be a promising area of investigation.
Acknowledgments
Thanks to Dr Gabriele Vizzari of the Ophthalmic Surgery Department, Legnago Hospital, for technical assistance.
References:
- 1.Ahmed F, House RJ, Feldman BH. Corneal abrasions and corneal foreign bodies. Prim Care. 2015;42:363–75. doi: 10.1016/j.pop.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Fusco N, Stead TG, Lebowitz D, Ganti L. Traumatic corneal abrasion. Cureus. 2019;11(4):e4396. doi: 10.7759/cureus.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stamate AC, Tătaru CP, Zemba M. Update on surgical management of corneal ulceration and perforation. Rom J Ophthalmol. 2019;63(2):166–73. [PMC free article] [PubMed] [Google Scholar]
- 4.Byrd LB, Martin N. StatPearls. Treasure Island (FL): StatPearls Publishing; 2019. Corneal ulcer. [Google Scholar]
- 5.Farahani M, Patel R, Dwarakanathan S. Infectious corneal ulcers. Dis Mon. 2017;63:33–37. doi: 10.1016/j.disamonth.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Gilani CJ, Yang A, Yonkers M, Boysen-Osborn M. Differentiating urgent and emergent causes of acute red eye for the emergency physician. West J Emerg Med. 2017;18:509–17. doi: 10.5811/westjem.2016.12.31798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Austin A, Lietman T, Rose-Nussbaumer J. Update on the management of infectious keratitis. Ophthalmology. 2017;124:1678–89. doi: 10.1016/j.ophtha.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Acharya M, Farooqui JH, Jain S, Mathur U. Pearls and paradigms in Infective Keratitis. Rom J Ophthalmol. 2019;63(2):119–27. [PMC free article] [PubMed] [Google Scholar]
- 9.Zamora JL. Chemical and microbiologic characteristics and toxicity of povidone-iodine solutions. Am J Surg. 1986;151:400–6. doi: 10.1016/0002-9610(86)90477-0. [DOI] [PubMed] [Google Scholar]
- 10.Berkelman RL, Holland BW, Anderson RL. Increased bactericidal activity of dilute preparations of povidoneiodine solutions. J Clin Microbiol. 1982;15:635–39. doi: 10.1128/jcm.15.4.635-639.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Musumeci R, Bandello F, Martinelli M, et al. In vitro bactericidal activity of 0.6% povidone-iodine eye drops formulation. Eur J Ophthalmol. 2019;29(6):673–77. doi: 10.1177/1120672118802541. [DOI] [PubMed] [Google Scholar]
- 12.Grzybowski A, Kanclerz P, Myers WG. The use of povidone-iodine in ophthalmology. Curr Opin Ophthalmol. 2018;29:19–32. doi: 10.1097/ICU.0000000000000437. [DOI] [PubMed] [Google Scholar]
- 13.Saggers BA, Stewart GT. Polyvinyl-pyrrolidone-iodine: An assessment of antibacterial activity. J Hyg (Lond) 1964;62:509–18. doi: 10.1017/s0022172400040225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grzybowski A, Brona P. Povidone-iodine is still a premium antiseptic measure in ocular surgery. Acta Ophthalmol. 2017;95:e253–e4. doi: 10.1111/aos.13144. [DOI] [PubMed] [Google Scholar]
- 15.Hale LM. The treatment of corneal ulcer with povidone-iodine (Betadine) N C Med J. 1969;30:54–56. [PubMed] [Google Scholar]
- 16.Katz J, Khatry SK, Thapa MD, et al. A randomised trial of povidone-iodine to reduce visual impairment from corneal ulcers in rural Nepal. Br J Ophthalmol. 2004;88:1487–92. doi: 10.1136/bjo.2004.044412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isenberg SJ, Apt L, Valenton M, et al. Prospective, randomized clinical trial of povidone-iodine 1.25% solution versus topical antibiotics for treatment of bacterial keratitis. Am J Ophthalmol. 2017;176:244–53. doi: 10.1016/j.ajo.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Pepose JS, Ahuja A, Liu W, Narvekar A, Haque R. Randomized, controlled, phase 2 trial of povidone-iodine/dexamethasone ophthalmic suspension for treatment of adenoviral conjunctivitis. Am J Ophthalmol. 2018;194:7–15. doi: 10.1016/j.ajo.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Koerner JC, George MJ, Meyer DR, et al. Povidone-iodine concentration and dosing in cataract surgery. Surv Ophthalmol. 2018;63:862–68. doi: 10.1016/j.survophthal.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Koerner JC, George MJ, Kissam EA, Rosco MG. Povidone-iodine concentration and in vitro killing time of bacterial corneal ulcer isolates. Digit J Ophthalmol. 2018;24:24–26. doi: 10.5693/djo.01.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houang ET, Gilmore OJ, Reid C, Shaw EJ. Absence of bacterial resistance to povidone iodine. J Clin Pathol. 1976;29:752–55. doi: 10.1136/jcp.29.8.752. [DOI] [PMC free article] [PubMed] [Google Scholar]


