Abstract
Patient: Female, 66-year-old
Final Diagnosis: Choriocarcinoma
Symptoms: Vaginal bleeding
Medication: —
Clinical Procedure: —
Specialty: Obstetrics and Gynecology
Objective:
Unusual clinical course
Background:
Choriocarcinoma is the most aggressive form of gestational trophoblastic disease and usually occurs in women of childbearing age, most commonly within 1 year after an abnormal pregnancy. Postmenopausal chorio-carcinoma is exceptionally rare and few cases have been described in the literature.
Case Report:
We present the case of a 66-year-old woman who presented to the Emergency Department with sudden onset of left upper- and lower-extremity weakness. She was found to have a brain mass, which was excised by neurosurgery and found to be a choriocarcinoma. She was then started on standard first-line therapy of EMACO, but was subsequently lost to follow-up.
Conclusions:
Postmenopausal choriocarcinoma is rare and there are few case reports in the literature. It is a rare but possibly under-diagnosed metastatic disease in women. At present, a postmenopausal woman without a clear primary tumor should have a pregnancy test performed to rule out choriocarcinoma, as it is readily responsive to therapy.
MeSH Keywords: Choriocarcinoma, Neoplasm Metastasis, Postmenopause
Background
Choriocarcinomas are tumors that result from abnormal proliferation of trophoblastic and syncytiotrophoblastic cells and are associated with secretion of beta-human chorionic gonadotropin (β-hCG) [1]. Choriocarcinoma is most commonly seen in women of childbearing age within 1 year following a pregnancy; approximately 50% of choriocarcinomas have an antecedent molar pregnancy [2]. They are rapidly invasive and widely meta-static, most often to the lung, vagina, brain, liver, and kidney [2].
The incidence of choriocarcinoma varies by location, but has been estimated to occur in between 1 in 20 000 to 1 in 25 000 women between the ages of 15 and 49. The incidence in post-menopausal women is extremely low and an estimate is not available [3].
Here, we describe a rare case of postmenopausal metastatic choriocarcinoma to the brain in a 66-year-old woman presenting with neurologic symptoms 41 years after her last pregnancy and 17 years after menopause.
Case Report
A 66-year-old white woman, gravida 3 para 2, with history of 1 elective abortion, presented with symptoms of left upper-and lower-extremity weakness and focal seizure. Her medical history was significant for insulin-dependent diabetes mellitus, dyslipidemia, hypertension, hypothyroidism, bipolar disorder, and morbid obesity. Her obstetric history was significant for 2 uncomplicated spontaneous vaginal deliveries, a first-trimester elective abortion, and menopause at 49 years of age.
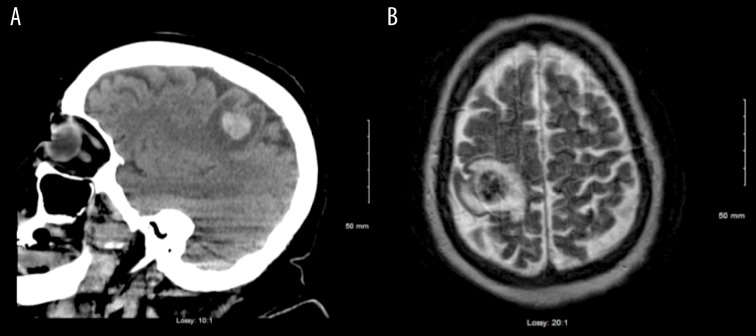
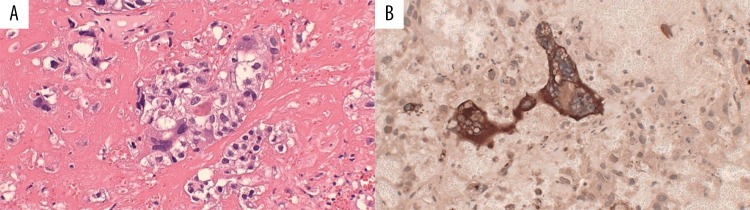
The patient was seen in the Emergency Department, where computed tomography (CT) and magnetic resonance imaging (MRI) of the brain revealed a 2-cm right parietal lobe hemorrhagic brain mass (Figure 1A, 1B). She was admitted to the Neurosurgery Service, where she underwent craniotomy with excision of the mass. The pathology report stated that the tumor was composed of malignant syncytiotrophoblastic and cytotrophoblastic cells, consistent with choriocarcinoma, and staining for immunohistochemical expression of β-hCG was positive (Figure 2A, 2B).
Figure 1.
(A) Head computed tomography at initial diagnosis showing a hemorrhagic lesion of the right parietal lobe. (B) Brain magnetic resonance imaging showing a tumor of the right parietal lobe of the brain.
Figure 2.
(A) The brain tumor was composed of large multi-nucleated syncytiotrophoblastic cells and medium-sized cytotrophoblastic cells lying in a hemorrhagic and necrotic stroma (hematoxylin and eosin staining). (B) Staining for the intracerebral metastatic choriocarcinoma shows strong immunohistochemical expression of B-hCG (dark brown).
During further interview, the patient admitted to a few sporadic episodes of postmenopausal vaginal bleeding over the past month. She denied seeking any medical attention for these symptoms. A pelvic exam revealed scant vaginal blood with no cervical or vaginal masses or lesions seen. The uterus was palpated to be approximately 20-week size.
Laboratory results showed an elevated quantitative β-hCG level of 9023 mIU/mL. Of note, the β-hCG was drawn 7 days after removal of the brain mass. Cancer antigen 125 (CA-125), alpha fetoprotein (AFP), and carcinoembryonic antigen (CEA) levels were normal.
CT imaging of the chest, abdomen, and pelvis was significant for right upper-lobe lung nodules, the largest measuring 2 cm, and an enlarged, irregularly-shaped uterus containing an intrauterine device (Figure 3). A pelvic sonogram demonstrated an enlarged, irregularly contoured uterus measuring 12.8×19.7 cm, with an intrauterine device seen in the endometrial cavity. No adnexal masses were identified. The study was limited secondary to the patient’s obesity.
Figure 3.
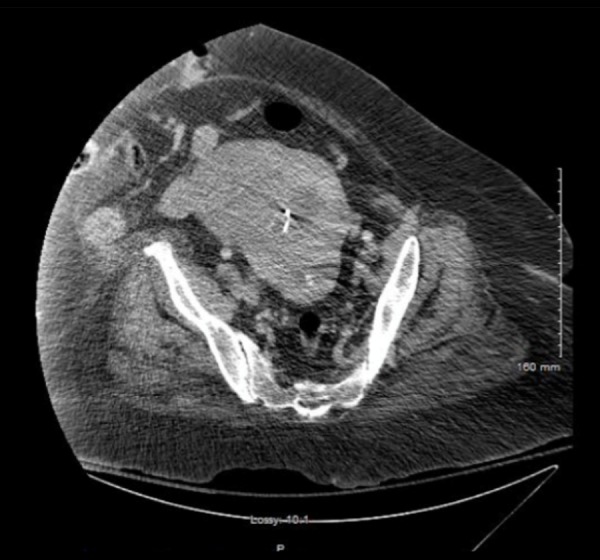
Pelvis computed tomography showing an enlarged, irregularly-shaped uterus with intrauterine device.
An endometrial biopsy was performed. Histologic examination was conclusive for endocervix accompanied by fragments of choriocarcinoma (Figure 4).
Figure 4.
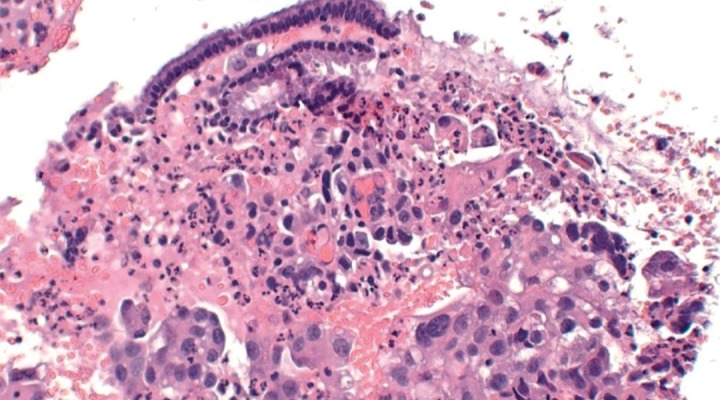
Histology of the endometrial biopsy revealed malignant cells with some multi-nucleation and hyper-chromatic nuclei, consistent with choriocarcinoma (hematoxylin and eosin staining).
According to the World Health Organization (WHO) prognostic score for gestational trophoblastic disease, our patient had a score of 13, making her high risk for resistance to monotherapy and characterized her as FIGO stage IV gestational trophoblastic neoplasia (Table 1).
Table 1.
Modified WHO prognostic scoring system as adapted by FIGO.
| Risk factor | 0 | 1 | 2 | 4 |
|---|---|---|---|---|
| Age | <40 years old | >40 years old | – | – |
| Antecedent Pregnancy | Mole | Abortion | Term | – |
| Interval months from pregnancy | <4 | 4–6 | 7–12 | >12 |
| Pretreatment serum hCG level | <103 | 103–104 | 104–105 | >105 |
| Largest tumor size (including uterus) | <3 cm | 3–4 cm | >5 cm | – |
| Size of metastases | Lung | Spleen, kidney | Gastrointestinal system | Liver, brain |
| Number of metastases | – | 1–4 | 5–8 | >8 |
| Previous failed chemotherapy | – | – | Single drug | 2 or more drugs |
The patient was scheduled for an appointment with Gynecologic Oncology and Medical Oncology for discussion of treatment options, including chemotherapy. Given her metastatic disease, she was offered combination chemotherapy with the EMACO regimen (etoposide, methotrexate, and dactinomycin, followed by cyclophosphamide and vincristine), which is considered first-line therapy [4]. Previous case reports of post-menopausal choriocarcinoma have reported good response and cure with this regimen [5]. Our patient is currently in a skilled nursing facility where she will receive interdisciplinary care to allow for improved quality of life and was scheduled to begin EMACO therapy. Due to multiple co-morbidities and immobility, our patient has not been able to follow-up with our facility; therefore, we were unable to determine the treatment effect.
Discussion
The presentation of this case report is very unusual; therefore, it is very difficult to identify steps to prevent similar cases in the future. If diagnosed and managed in a timely fashion, choriocarcinoma is considered to be the most curable gynecologic cancer, even in the setting of metastasis. Nongestational trophoblastic neoplasia in the postmenopausal period is extremely rare [6]. In our review of the literature, we found very few studies of gestational trophoblastic disease in postmenopausal women. There are a few reports of long latent periods between the last pregnancy and the occurrence of choriocarcinoma, but the mechanism for this remains uncertain [7–9]. Clinical signs and symptoms of postmenopausal choriocarcinoma are not specific. In previous case reports, the most common presenting symptom has been postmenopausal vaginal bleeding, for which these patients underwent thorough workup for endometrial cancers, including transvaginal ultrasounds and endometrial biopsies [5,10].
Choriocarcinoma can be diagnosed by histologic examination of the tumor showing malignant cytotrophoblasts, intermediate trophoblasts, and syncytiotrophoblasts [11]. The tissue is typically arranged in a biphasic pattern, with absence of chorionic villi (although the presence of villi does not exclude the diagnosis) [1]. Elevated serum β-hCG and human placental lactogen (h-PL) are found in choriocarcinoma [1].
Our patient presented with neurologic symptoms secondary to brain metastases with pathologic diagnosis of choriocarcinoma. Elevated serum β-hCG confirmed the diagnosis. Given her recent postmenopausal bleeding, a mass in the uterus, and endometrial sampling being positive for choriocarcinoma, this was thought to be a primary uterine tumor with metastasis to the brain. The cause of the long latency period between last pregnancy and tumor growth with subsequent development of symptoms is not well understood.
Very rarely, choriocarcinoma can develop from germ cells or from dedifferentiation of endometrial carcinoma into choriocarcinoma [12]. A postmenopausal woman presenting with vaginal bleeding from a mass and with β-hCG elevation should be immunohistochemically analyzed to rule out the possibilities of a germ cell origin of the tumor or dedifferentiation of an epithelial tumor. To definitively differentiate a gestational choriocarcinoma from a non-gestational type, a DNA analysis of the tumor confirming paternal DNA would need to be performed [12]. Unfortunately, we were unable to perform this testing.
Despite its invasive properties, metastatic choriocarcinoma is a very chemo-sensitive disease with first-line therapy of combination chemotherapy using the EMA/CO regimen (etopo-side, methotrexate, and dactinomycin, followed by cyclophosphamide and vincristine). Serial β-hCG levels should be used to measure appropriate response to chemotherapy. In many cases, chemotherapy can be curative without need for surgical excision.
Conclusions
The present case is an unusual and exceedingly rare occur-rence of postmenopausal choriocarcinoma. Our patient presented with neurologic symptoms secondary to a brain mass. Pathology diagnosed choriocarcinoma; therefore, further testing was done, confirming a primary uterine cancer.
Although few cases of choriocarcinoma in a postmenopausal woman have been described, we have been unable to locate another case in the literature in which the patient presented with neurologic symptoms or a brain mass.
The etiology of postmenopausal choriocarcinoma is poorly understood. It is possible that our patient has had disease since her elective abortion more than 50 years ago. However, this is unlikely given that the patient has been asymptomatic and was able to carry 2 normal pregnancies to term after her abortion.
In conclusion, choriocarcinoma should be included in the differential diagnosis for all women with evidence of metastasis, regardless of the site and patient age. Additionally, all women, again regardless of age, should have a urine pregnancy test as part of a metastatic workup, given that our patient’s diagnosis was delayed until we received the pathology report regarding the brain mass. A brain mass with a positive urine pregnancy test result would have immediately instigated further investigation into a diagnosis of metastatic choriocarcinoma, and initiation of aggressive chemotherapy may have preceded and possibly even prevented the need for craniotomy and tumor resection.
Footnotes
Conflict of interest
None.
References:
- 1.Marcu M, Chefani A, Sajin M, et al. Postmenopausal choriocarcinoma : A case report. Roma J Morphol Embryol. 2005;46(2):145–48. [PubMed] [Google Scholar]
- 2.Vincent M, Court-Fortune I, Karpathiou G, et al. An extremely rare case of postpartum gestational choriocarcinoma with long-term survival. Tumori. 2018;103(1):S16–18. doi: 10.5301/tj.5000660. [DOI] [PubMed] [Google Scholar]
- 3.Dilek S, Pata O, Tok E, et al. Extraovarian nongestational choriocarcinoma in a postmenopausal woman. Int J Gynecol Cancer. 2004;14:1033–35. doi: 10.1111/j.1048-891X.2004.014548.x. [DOI] [PubMed] [Google Scholar]
- 4.Newlands ES, Bagshawe KD, Begent RH, et al. Results with EMA/CO regimen in high-risk gestational trophoblastic tumours, 1979 to 1989. Br J Obstet Gynaecol. 1991;98(6):550–57. doi: 10.1111/j.1471-0528.1991.tb10369.x. [DOI] [PubMed] [Google Scholar]
- 5.Yousefi G, Saied S, Davachi B, et al. Postmenopausal choriocarcinoma: Case report and literature review. Int J Cancer Manag. 2017;20:e4400. [Google Scholar]
- 6.Mukherjee U, Thakur V, Katiyar D, et al. Uterine choriocarcinoma in a post-menopausal woman. Med Oncol. 2006;23(2):301–3. doi: 10.1385/MO:23:2:301. [DOI] [PubMed] [Google Scholar]
- 7.Lathrop JC, Wachtel TS, Meissner GF. Uterine choriocarcinoma fourteen years following bilateral tubal ligation. Obstet Gynecol. 1978;51:477–82. doi: 10.1097/00006250-197804000-00019. [DOI] [PubMed] [Google Scholar]
- 8.Coulson LE, Kong CS, Zaloudek C. Epithelioid trophoblastic tumor of the uterus in a postmenopausal woman: A case report and review of the literature. Am J Surg Pathol. 2000;24(11):1558–62. doi: 10.1097/00000478-200011000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Guo N, Yin R, Li Q, et al. Postmenopausal choriocarcinoma: A rare case report and review of the literature. Menopause. 2018;25(2):239–41. doi: 10.1097/GME.0000000000000968. [DOI] [PubMed] [Google Scholar]
- 10.Evsen M, Erdal S, Ender S, et al. A rare cause of postmenopausal bleeding: choriocarcinoma. J Clin Gyneco Obstet. 2012:79–81. [Google Scholar]
- 11.Chittenden B, Adamed E, Maheshwari A, et al. Choriocarcinoma in a post-menopausal woman. Obstet Gynecol. 2009;114(2 Pt 2):462–65. doi: 10.1097/AOG.0b013e3181aa97e7. [DOI] [PubMed] [Google Scholar]
- 12.Desai NR, Gupta S, Said R, et al. Choriocarcinoma in a 73-year-old woman: A case report and review of the literature. J Med Case Rep. 2010;25:379. doi: 10.1186/1752-1947-4-379. [DOI] [PMC free article] [PubMed] [Google Scholar]