Abstract
Background
Symptomatic vasospasm (sVSP) is a common complication during the course of aneurysmal subarachnoid hemorrhage (aSAH). We aimed to evaluate the efficacy and accuracy of transcranial Doppler ultrasound (TCD), performed within the first 3 days of aSAH to predict the development of sVSP.
Methods
We performed a retrospective analysis of our institutional prospectively collected database of patients with aSAH. Patients with aSAH and World Federation of Neurosurgical Societies (WFNS) grades I–III were included in the analysis. A receiver operating characteristic (ROC) curve was generated to determine cut-off values for mean flow velocities (MFVs) in the middle cerebral artery (MCA) and anterior cerebral artery (ACA) bilaterally to predict sVSP.
Results
Fifty-one patients were included in the study. Mean age was 49.8 ± 10.2 years, and 84.3% (43 patients) were women. The accuracy of measured MFVs to predict sVSP was 0.79 [95% confidence interval (CI), 0.69–0.89] and 0.77 (95% CI, 0.64–0.91) for the MCA and the ACA, respectively. In the MCA, an MFV ≥ 74 cm/s was significantly associated with a six-fold increased risk of sVSP, achieving sensitivity greater than 70%. In the ACA, an MFV ≥ 64 cm/s was significantly associated with a nine-fold increased risk of sVSP.
Conclusion
Early TCD evaluation of MFVs in the MCA and ACA is a useful tool to predict the development of sVSP in patients with acute aSAH.
Keywords: cerebral vasospasm, transcranial Doppler, ultrasound, subarachnoid hemorrhage, intracranial aneurysm
INTRODUCTION
During the early clinical course of aneurysmal subarachnoid hemorrhage (aSAH), symptomatic vasospasm (sVSP) is one of the most frequent and dreaded complications, worsening functional and neurological outcomes [1, 2]. Standard tests used to determine the source of bleeding and diagnose sVSP include neuroimaging studies that administer contrast either intravenously (computed tomography angiography [CTA]) or intraarterially (digital subtraction angiography [DSA]). Recently, it has been proposed that cerebral blood flow measurements using computed tomography (CT) perfusion techniques may detect the degree of cerebral ischemia in a very early stage [3]. Although well-tolerated, these studies cannot be readily performed on the bedside and expose the patient to additional radiation, thus significantly restricting their use in daily cerebral hemodynamics monitoring [4, 5]. Moreover, they involve patient transportation to the CT scanner and utilization of resources such as nurses, technologists, and ancillary personnel.
Transcranial Doppler ultrasound (TCD) is a valuable tool in the detection of increased mean flow velocities (MFVs) and diagnosis of sVSP. Intracranial blood flow assessments can be safely performed with bedside TCDs during early stages of aSAH [6]. While the utility of TCD in the diagnosis and monitoring of sVSP is well-known, its validity to predict sVSP has not yet been fully assessed [6–9]. In this study, we aimed to evaluate the efficacy and accuracy of early TCD, performed within the first 3 days of aSAH, to predict the development of sVSP.
METHODS
Population
A retrospective longitudinal analysis was conducted in our institutional prospectively collected database of patients with aSAH admitted to the stroke unit of the Hermanos Ameijeiras Clinical-Surgical Hospital in Havana-Cuba, between January 2009 and December 2012. Institutional Review Board approval was obtained from our institution.
We included patients with the following characteristics: spontaneous aSAH with favorable clinical status (grades I–III based on the WFNS scale) [10], and with TCD studies performed during the first 3 days of the aSAH. We excluded patients with poor clinical status (WFNS grades IV–V) since they tend to have extensive hemorrhages with increased intracranial pressure (ICP) that may modify MFVs. Subjects with aneurysms in the posterior circulation were also excluded since only vessels in the anterior circulation were assessed systematically.
Patients without a suitable cranial window to perform TCD studies were excluded. Also, since increased ICP could potentially modify MFVs measurements on TCD studies, patients with sustained increased ICPs despite maximal medical and surgical therapy were excluded from the study. Moreover, patients with >50% stenosis of any carotid artery confirmed by Duplex Doppler ultrasound were excluded, as proximal arterial stenosis affects MFVs in the middle cerebral artery (MCA) and anterior cerebral artery (ACA). Finally, patients who developed hydrocephalus or experienced rebleeding, infection, metabolic or hydro-electrolytic disturbances during hospitalization were excluded from the study, as these events may affect the neurological examination.
Management of aneurysmal subarachnoid hemorrhage
aSAH was diagnosed in patients referring the “worst headache of their lives” and/or loss of consciousness at presentation, and evidence of hemorrhage on CT or lumbar puncture. The presence of an intracranial aneurysm was determined by helical multislice CTA and/or DSA. Patients were managed by a multidisciplinary team of radiologists, vascular neurosurgeons, and neurocritical care neurologists. Every patient admitted to the stroke unit underwent the same standardized care that sought prompt diagnosis and securement of the aneurysm by microsurgical clipping and within the first 72 hours of aSAH. Medical therapy and surgical interventions were performed according to guidelines from the American Heart Association.[11].
TCD protocol
The TCD study was performed with an Embo-Dop machine (DWL Elektronische Systeme GmbH, Singen, Germany) by application of a 2-MHz probe over the temporal window, according to the method previously described by Aaslid et al. [12]. MFVs were measured daily from admission. For the purpose of this study, we analyzed the highest MFV recorded in the first 3 days of aSAH. MFVs were measured in the proximal segments of the MCA (M1) and anterior cerebral ACA (A1) of each patient bilaterally. All sonographies at our institution were performed by the same vascular neurologist with training and experience in the technique.
Outcome
sVSP was diagnosed in patients who, between days 4th and 14th of aSAH, developed temporary or permanent clinical signs and symptoms of any global or focal neurological deficit: confusion, decreased level of consciousness, motor or sensory deficits, aphasia, and/or dysarthria. Following recognition of these signs, TCD sonography was performed to determine if the MFVs were within the range of sVSP: ≥120 cm/s in the MCA and ≥90 cm/s in the ACA [13–15]. Further studies such as CTA and/or DSA were performed to confirm the presence of radiological vasospasm.
Statistical analysis
Data are summarized as mean ± standard deviation (SD) and range for continuous variables, and as frequency for categorical variables. Development of sVSP during hospitalization was treated as a binary categorical variable. A univariate statistical analysis was performed using χ2 and Fisher exact tests in categorical variables and Student t-test for comparison of means in continuous variables, as appropriate. P values < 0.05 were considered statistically significant. Odds ratios (ORs) were estimated for the magnitude of effect with 95% confidence intervals (CIs).
To determine the most accurate cut-off points for MFVs, a receiver operating characteristic (ROC) curve was constructed considering the highest values measured in the first 72 hours of aSAH evolution. Optimal cut-off points for MFVs in the MCA and ACA were established in two ways: (1) by calculating the Youden’s index, a function of sensitivity and specificity; and (2) by performing percentile analyses (50th and 75th percentiles) to which positive predictive values (PPVs) and negative predictive values (NPVs) were calculated. Finally, a binary logistic regression was performed to determine the independent predictive capacity of established cut-off points. IBM SPSS 20.0 software for Windows was used for statistical analyses.
RESULTS
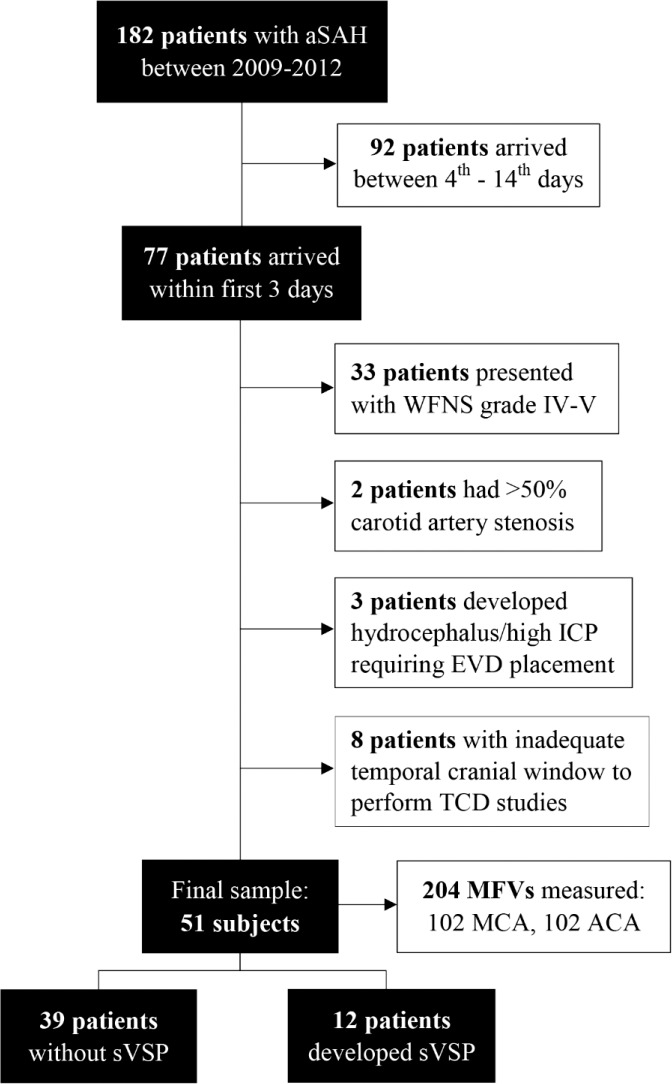
During the study period, 182 patients were diagnosed with spontaneous aSAH. Of them, 77 (42.3%) patients presented within the first 3 days after aSAH onset. Following our strict criteria, 26 subjects were further excluded: 13 presented with WFNS grades IV–V, two had >50% carotid artery stenosis, three required external ventricular device placement for management of hydrocephalus/sustained increased ICP, and eight patients had inadequate temporal cranial window to perform TCD studies. A total of 51 patients were included in the final sample (Figure 1).
Figure 1. Flowchart of the cohort. ACA, anterior cerebral artery; aSAH, aneurysmal subarachnoid hemorrhage; EVD, external ventricular drain; ICP, intracranial pressure; MCA, middle cerebral artery; MFV, mean flow velocity; sVSP, symptomatic vasospasm; TCD, transcranial Doppler; WFNS, World Federation of Neurological Surgeons Scale.
Baseline characteristics are presented in Table 1. Mean age was 49.8 ± 10.2 years (range 35–75), and 84.3% were women. Forty (78.4%) patients presented with significant hemorrhage (Fisher 3–4). Aneurysms were located in the ACA, MCA, or internal carotid artery. There was no evidence of radiological vasospasm on CTAs performed upon admission.
Table 1. Baseline characteristics of the sample.
| sVSP n = 12 (24 MCA, 24 ACA) |
No sVSP n = 39 (78 MCA, 78 ACA) |
P | |
|---|---|---|---|
| Age, mean ± SD | 46.4 ± 5.8 | 50.9 ± 11.0 | 0.193 |
| Women (%) | 11 (91.6) | 32 (82.0) | 0.230 |
| Hypertension (%) | 9 (75.0) | 19 (48.7) | 0.020* |
| Smoking (%) | 5 (41.6) | 20 (51.2) | 0.280 |
| Alcohol (%) | 2 (16.6) | 8 (20.5) | 0.670 |
| SBP, mean ± SD | 156.5 ± 30.5 | 149.2 ± 55.6 | 0.300 |
| Glucose, mean ± SD | 6.02 ± 2.4 | 5.7 ± 1.3 | 0.536 |
| Hematocrit, mean ± SD | 39.0 ± 3.0 | 38.3 ± 4.0 | 0.533 |
| Fisher grade 3–4 (%) | 8 (66.6) | 32 (82.0) | 0.262 |
ACA, anterior cerebral artery; MCA, middle cerebral artery; n, number of patients; P, p-value; SD, standard deviation; SBP, systolic blood pressure; sVSP, symptomatic vasospasm.
Indicates p-value < 0.05.
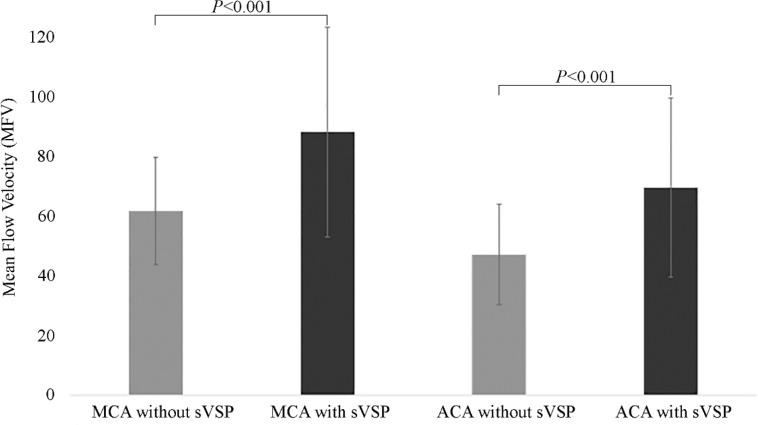
Twelve patients (23.5%) were diagnosed with sVSP between days 7th and 10th of aSAH. History of hypertension was significantly associated with development of sVSP (P = 0.02) (Table 1). Average systolic blood pressure (SBP) upon admission was slightly higher in patients who developed sVSP during hospitalization (156.5 ± 30.5 mmHg) versus those who did not (149.2 ± 55.6 mmHg, P = 0.30). MFVs measured within the first 72 hours in patients who developed sVSP (MCA = 88.1 ± 35.2 cm/s, ACA = 69.5 ± 30.0 cm/s) were significantly higher than MFVs recorded in patients without sVSP (MCA = 61.7 ± 17.9 cm/s, ACA = 47.1 ± 16.8 cm/s), with P ≤ 0.001 for both vessels (Figure 2).
Figure 2. Comparison of mean flow velocities (MFVs) recorded within the first 3 days of admission according to diagnosis of symptomatic vasospasm (sVSP). Measurements were obtained from the middle cerebral artery (MCA) and the anterior cerebral artery (ACA). MCA without sVSP = 61.7 ± 17.9 cm/s; MCA with sVSP = 88.1 ± 35.2 cm/s; ACA without sVSP = 47.1 ± 16.8 cm/s; ACA with sVSP = 69.5 ± 30.0 cm/s.
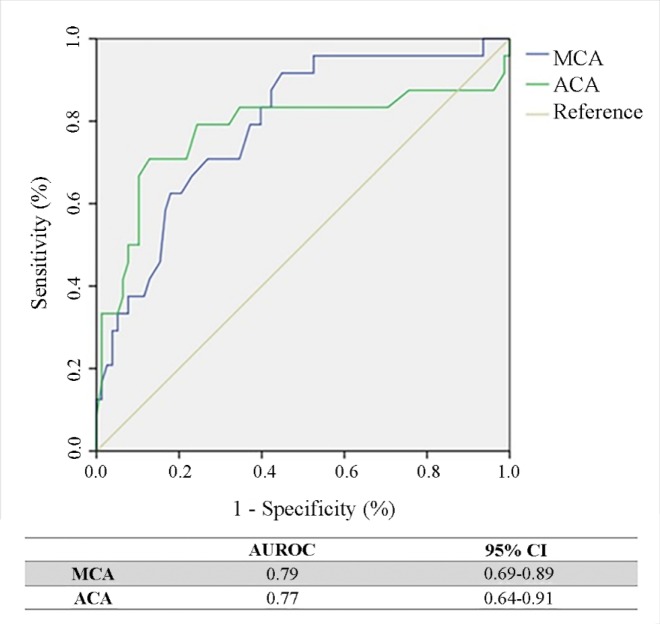
Table 2 shows the MFV cut-off points established for the prediction of sVSP with respective PPVs and NPVs. In the MCA, the Youden’s Index method calculated the most accurate cut-off point at MFV = 74 cm/s, which provided sensitivity, specificity, PPV, and NPV above 70%. The 75th percentile method calculated a cut-off point of MFV = 80 cm/s, resulting in improved specificity (83%), but decreased sensitivity (58%). In the ACA, placing the cut-off point at MFV = 64 cm/s achieved a sensitivity, specificity, and PPV greater than 70%, with an NPV close to 70%. The overall performance of the cut-off points determined by the ROC curve was 0.79 (95% CI, 0.69–0.89) and 0.77 (95% CI, 0.64–0.91) for the MCA and ACA, respectively (Figure 3). Finally, a binary logistic regression demonstrated that an MFV ≥ 74 cm/s in the MCA is significantly associated with a six-fold increased risk of sVSP (OR 6.59, 95% CI 2.40–18.14, P ≤ 0.01), whereas an MFV ≥ 64 cm/s in the ACA is significantly associated with an almost 10-fold increased risk of sVSP (OR 9.93, 95% 2.34–42.17, P ≤ 0.01) (Table 3).
Table 2. Predictive values of MFVs measured by TCD within the first 3 days for development of sVSP.
| Artery | Method (percentile) | MFV cut-off (cm/s) | Sensibility | Specificity | PPV | NPV | P |
|---|---|---|---|---|---|---|---|
| MCA | Youden’s Index | 74 | 0.71 | 0.73 | 0.85 | 0.71 | ≤0.01 |
| 50th | 64 | 0.83 | 0.60 | 0.60 | 0.83 | ≤0.01 | |
| 75th | 80 | 0.58 | 0.83 | 0.86 | 0.46 | ≤0.01 | |
| ACA | Youden’s Index | 64 | 0.71 | 0.87 | 0.89 | 0.67 | ≤0.01 |
| 50th | 49 | 0.83 | 0.63 | 0.63 | 0.83 | ≤0.01 | |
| 75th | 64 | 0.71 | 0.87 | 0.89 | 0.67 | ≤0.01 |
ACA, anterior cerebral artery; MCA, middle cerebral artery; MFV, mean flow velocity; NPV, negative predictive value; P, p-value; PPV, positive predictive value; sVSP, symptomatic vasospasm; TCD, transcranial Doppler sonography.
Figure 3. Receiver operating characteristic (ROC) curves for the determination of the cut-off points for the mean flow velocity in the middle cerebral artery (MCA) and the anterior cerebral artery (ACA). AUROC, area under ROC curve; CI, confidence interval.
Table 3. Binary logistic regression values for MFV cut-off points.
| Artery | Method (Percentile) |
MFV cut-off (cm/s) | P | OR | 95% CI |
|---|---|---|---|---|---|
| MCA | Youden’s Index | ≥74 | ≤0.01 | 6.59 | 2.40–18.14 |
| 50th | ≥64 | ≤0.01 | 7.58 | 2.36–24.31 | |
| 75th | ≥80 | ≤0.01 | 4.65 | 1.69–12.80 | |
| ACA | Youden’s Index | ≥64 | ≤0.01 | 9.93 | 2.34–42.17 |
| 50th | ≥49 | ≤0.01 | 8.45 | 2.63–27.17 | |
| 75th | ≥64 | ≤0.01 | 9.93 | 2.34–42.17 |
ACA, anterior cerebral artery; CI, confidence interval; MCA, middle cerebral artery; MFV, mean flow velocity; P, p-value; OR, odds ratio
DISCUSSION
sVSP is defined as focal or diffuse narrowing of large and medium caliber arteries. Often, it is as a consequence of aSAH. The identification of sVSP is complex and can be easily misdiagnosed when other cofounders such as hydrocephalus or electrolyte abnormalities are present.[1, 16] In more than 50% of cases, sVSP presents with focal neurological deficits between days 4th and 14th of aSAH, which can resolve spontaneously or progress to delayed cerebral infarction.[1, 14] Despite current advances in neuroimaging techniques and improved therapeutic management, sVSP continues to be the main cause of morbidity and mortality in patients with aSAH. Even after maximal medical therapy, around 15% of patients suffer an ischemic event or die as a consequence of sVSP.[17–19].
Several factors have been associated with the development sVSP after aSAH, including a higher Fisher scale [2, 20]. Our results did not show a significant association between Fisher scale grade and sVSP, which might be explained by the exclusion of patients with WFNS grades IV–V.
TCD has been shown to be useful in the diagnosis and monitoring of arterial vasospasm. Recently, Connolly et al. [21] have proposed a new arteriovenous index between the MCA and the basal vein of Rosenthal using MFVs and peak systolic velocities to diagnose cerebral VSP with a specificity as high as 93%–94%, PPV 86%–88%, and accuracy 84%–88%.
Although numerous studies have supported the use of TCD to predict delayed cerebral ischemia following aSAH [22–24], few studies have evaluated the predictive value of early TCD in the development of sVSP. In 2013, Toi et al. [25] studied 45 patients and reported an MFV cut-off point in the MCA of 72.5 cm/s to predict sVSP with a sensitivity of 71.4% and specificity of nearly 70%. Similar results were reproduced by Muñoz-Sanchez et al. [26], with a specificity of 96.6% and sensitivity of 85.7%.
In our study, an MFV cut-off point of 74 cm/s in the MCA and 64 cm/s in the ACA predicted the development of sVSP with a sensitivity and specificity greater than 70%. These results emphasize the importance of performing TCDs in patients with aSAH early upon admission to monitor MFVs and possibly predict the development of sVSP.
To the authors’ knowledge, this study is the first to determine predictive values for MFVs measured in two arteries of the anterior circulation, the MCA and the ACA, simultaneously. Moreover, we calculated MFV cut-off points using two different rigorous statistical methods, Youden’s Index and 75th percentile, achieving high specificity performance at 70% for both vessels. These principal differences compared to previous studies [25, 26] allowed us to thoroughly evaluate the effectiveness of TCD in predicting sVSP. We plan on performing a larger study of TCDs within the first 72 hours of aSAH to predict and even preemptively treat patients at risk of developing sVSP.
Limitations
One of the main limitations of this study is its small sample size. In addition, the following factors were not considered in the analysis: Lindegaard index (MCA/internal carotid artery), location of the hemorrhage on CT scans, and MFVs of the arteries in the posterior circulation. Despite these limitations, our results demonstrated that early TCD studies reliably predict the development of sVSP in aSAH, thus potentially expanding its use in clinical practice. Numerous studies have evaluated the limitations of TCD in the diagnosis and follow-up of VSP, arguing that it is only able to measure relative changes in cerebral blood flow (i.e., blood velocity, not blood volume) with a highly operator-dependent variability [6, 12]. However, when performed by an experienced vascular neurologist in appropriately selected patients with good temporal cranial windows, TCD provides real-time and cost-effective information about cerebral hemodynamics. Moreover, CT, MRI, or DSA are not readily available in developing countries.
CONCLUSION
Early TCD assessment may be a useful tool to predict the development of sVSP in selected patients with aSAH. MFVs higher than 74 cm/s in the MCA and 64 cm/s in the ACA may trigger preemptive treatments and closer neurological monitoring.
Table 4. Studies evaluating TCD in early stages of aSAH to predict sVSP.
| Carrera et al. [24] | Muñoz-Sanchez et al. [26] | Toi et al. [25] | Our study | |
|---|---|---|---|---|
| Sample size | 199 patients | 122 patients | 45 patients | 51 patients |
| MFV cut-off definition | Author criteria: MFV ≥ 90 cm/s | Calculated ROC curve | Calculated ROC curve | Calculated ROC curve, Youden index, and percentiles |
| Vessels included | MCA | MCA | MCA | MCA and ACA |
| Timing of TCD from aSAH onset | First 48 hours | First 72 hours | First 72 hours | First 72 hours |
| Findings | Early MFV elevations correlate with acute angiographic vasospasm and are associated with a significantly increased risk of DCI | An increase of 21 cm/s/24 hours in MCA-MFV during first 3 days was associated with development of sVSP: sensibility = 0.86, specificity = 0.97, PPV = 0.86, NPV = 0.97 | MCA-MFV ≥ 72cm/s on day 3 may predict future occurrence of sVSP: sensitivity = 0.82, specificity = 0.68 | MCA-MFV ≥ 74 cm/s, sensibility = 0.71, specificity = 0.73; ACA-MFV ≥ 64 cm/s, sensibility = 0.71, specificity = 0.87; MCA-MFV 75th percentile = 80 cm/s, sensibility = 0.58, specificity = 0.83; ACA-MFV 75th percentile = 64 cm/s, sensitivity = 0.71, specificity = 0.87 |
aSAH, aneurysmal subarachnoid hemorrhage; DCI, delayed cerebral ischemia, MCA, middle cerebral artery; MFV, mean flow velocity; ROC, receiver operating characteristic; sVSP, symptomatic vasospasm; TCD, transcranial Doppler.
Acknowledgments:
We thank the Fulbright U.S. Program for the fellowship opportunity provided to K. Sam to contribute to this study. We also thank the neuroradiology technicians for performing patient examinations and the Neurology medical/nursing team for their contributions to the patients care.
References
- Janjua N, Mayer SA. Cerebral vasospasm after subarachnoid hemorrhage. Curr Opin Crit Care. 2003;9(2):113–119. doi: 10.1097/00075198-200304000-00006. [DOI] [PubMed] [Google Scholar]
- Fisher CM, et al. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6(1):1–9. doi: 10.1227/00006123-198001000-00001. [DOI] [PubMed] [Google Scholar]
- Fotakopoulos G, et al. The value of computed tomography perfusion & transcranial Doppler in early diagnosis of cerebral vasospasm in aneurysmal & traumatic subarachnoid hemorrhage. . Future Sci OA. 2018;4(6):FSO313. doi: 10.4155/fsoa-2018-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann TJ, et al. Complications of diagnostic cerebral angiography: evaluation of 19,826 consecutive patients. Radiology. 2007;243(3):812–819. doi: 10.1148/radiol.2433060536. [DOI] [PubMed] [Google Scholar]
- Choudhri O, et al. Increased risk for complications following diagnostic cerebral angiography in older patients: trends from the Nationwide Inpatient Sample (1999-2009) J Clin Neurosci. 2016;32:109–114. doi: 10.1016/j.jocn.2016.04.007. [DOI] [PubMed] [Google Scholar]
- Rigamonti A, et al. Transcranial Doppler monitoring in subarachnoid hemorrhage: a critical tool in critical care. Can J Anaesth. 2008;55(2):112–123. doi: 10.1007/BF03016323. [DOI] [PubMed] [Google Scholar]
- Ekelund A, et al. Is transcranial Doppler sonography useful in detecting late cerebral ischaemia after aneurysmal subarachnoid haemorrhage? Br J Neurosurg. 1996;10(1):19–25. doi: 10.1080/bjn.10.1.19. [DOI] [PubMed] [Google Scholar]
- Lysakowski C, et al. Transcranial Doppler versus angiography in patients with vasospasm due to a ruptured cerebral aneurysm: a systematic review. Stroke. 2001;32(10):2292–2298. doi: 10.1161/hs1001.097108. [DOI] [PubMed] [Google Scholar]
- Marshall SA, et al. The role of transcranial Doppler ultrasonography in the diagnosis and management of vasospasm after aneurysmal subarachnoid hemorrhage. Neurosurg Clin N Am. 2010;21(2):291–303. doi: 10.1016/j.nec.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Rosen DS, Macdonald RL. Grading of subarachnoid hemorrhage: modification of the world World Federation of Neurosurgical Societies scale on the basis of data for a large series of patients. Neurosurgery. 2004;54(3):566–575. discussion 575–576. [PubMed] [Google Scholar]
- Bederson JB, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40(3):994–1025. doi: 10.1161/STROKEAHA.108.191395. [DOI] [PubMed] [Google Scholar]
- Aaslid R, et al. A transcranial Doppler method in the evaluation of cerebrovascular spasm. Neuroradiology. 1986;28(1):11–16. doi: 10.1007/BF00341759. [DOI] [PubMed] [Google Scholar]
- Scherle-Matamoros C, Pérez-Nellar J. Eficacia del Doppler transcraneal para la detección del vasoespasmo en las arterias cerebrales anteriores. Rev Neurol. 2010;50(5):273–278. [PubMed] [Google Scholar]
- Scherle-Matamoros C, et al. Vasoespasmo sintomático. Caracterización clínica. Neurocirugía. 2011;22:116–122. [PubMed] [Google Scholar]
- Scherle-Matamoros CE, et al. Clinical usefulness of transcranial Doppler ultrasound imaging in the diagnosis of cerebral vasospasm in subarachnoid haemorrhage. A validation study. Rev Neurol. 2008;47(6):295–298. [PubMed] [Google Scholar]
- Ritzenthaler T, et al. “Vasospasm mimic” after aneurysmal subarachnoid hemorrhage. World Neurosurg”. 2019;124:295–297. doi: 10.1016/j.wneu.2019.01.034. [DOI] [PubMed] [Google Scholar]
- Adams HP, Jr, et al. Predicting cerebral ischemia after aneurysmal subarachnoid hemorrhage: influences of clinical condition, CT results, and antifibrinolytic therapy. A report of the Cooperative Aneurysm Study. Neurology. 1987;37(10):1586–1591. doi: 10.1212/wnl.37.10.1586. [DOI] [PubMed] [Google Scholar]
- Haley EC, Jr, et al. The International Cooperative Study on the Timing of Aneurysm Surgery. The North American experience. Stroke. 1992;23(2):205–214. doi: 10.1161/01.str.23.2.205. [DOI] [PubMed] [Google Scholar]
- Kassell NF, et al. The international cooperative study on the timing of aneurysm surgery. part 1: overall management results. J Neurosurg. 1990;73(1):18–36. doi: 10.3171/jns.1990.73.1.0018. [DOI] [PubMed] [Google Scholar]
- Nomura Y, et al. Retrospective analysis of predictors of cerebral vasospasm after ruptured cerebral aneurysm surgery: influence of the location of subarachnoid blood. J Anesth. 2010;24(1):1–6. doi: 10.1007/s00540-009-0836-2. [DOI] [PubMed] [Google Scholar]
- Connolly F, et al. Assessment of intracranial venous blood flow after subarachnoid hemorrhage: a new approach to diagnose vasospasm with transcranial color-coded duplex sonography. J Neurosurg. 2018;129(5):1136–1142. doi: 10.3171/2017.5.JNS17232. [DOI] [PubMed] [Google Scholar]
- Grosset DG, et al. Use of transcranial Doppler sonography to predict development of a delayed ischemic deficit after subarachnoid hemorrhage. J Neurosurg. 1993;78(2):183–187. doi: 10.3171/jns.1993.78.2.0183. [DOI] [PubMed] [Google Scholar]
- Calviere L, et al. Prediction of delayed cerebral ischemia after subarachnoid hemorrhage using cerebral blood flow velocities and cerebral autoregulation assessment. Neurocrit Care. 2015;23(2):253–258. doi: 10.1007/s12028-015-0125-x. [DOI] [PubMed] [Google Scholar]
- Carrera E, et al. Transcranial Doppler for predicting delayed cerebral ischemia after subarachnoid hemorrhage. Neurosurgery. 2009;65(2):316–323. doi: 10.1227/01.NEU.0000349209.69973.88. discussion 323–324. [DOI] [PubMed] [Google Scholar]
- Toi H, et al. Prediction of cerebral vasospasm using early stage transcranial Doppler. Neurol Med Chir (Tokyo) 2013;53(6):396–402. doi: 10.2176/nmc.53.396. [DOI] [PubMed] [Google Scholar]
- Muñoz-Sanchez MA, et al. Emergency transcranial doppler ultrasound: predictive value for the development of symptomatic vasospasm in spontaneous subarachnoid hemorrhage in patients in good neurological condition. Med Intensiva. 2012;36(9):611–618. doi: 10.1016/j.medin.2012.01.013. [DOI] [PubMed] [Google Scholar]