Abstract
Research evidence guiding the identification of pragmatic and effective actions aimed at improving the selection, availability, affordability and rational prescribing of medicines for mental disorders is sparse and inconsistent. In order to boost the development of new research, in this commentary we suggest to organise and classify all the activities in this area under a common theoretical framework and nomenclature, adopting the term ‘public health psychopharmacology’. Public health psychopharmacology is proposed as a research discipline, based on contributions from the fields of regulatory science, health services research and implementation science. Implementing the term public health psychopharmacology may offer advantages, as the scientific community would be more focused on common goals and objectives, with, likely, an increasing body of research evidence of practical use.
Key words: Affordability, availability, psychotropic medicines, rational prescribing, selection of medicines
Appropriate use of medicines for mental disorders is a major challenge worldwide, being based on a complex interaction of different aspects, such as a functioning selection process, a dynamic health infrastructure assuring medicine availability and affordability, and an effective set of policies enhancing rational prescribing (Barbui et al. 2017a). Appropriate use of medicines has been conceptualised as the expectation that individuals receive medicines that are appropriate to their clinical needs, in doses that meet their individual requirements, for an adequate period of time, and at the lowest cost to them and their community (World Health Organization, 2015a).
In order to improve access and appropriate use of medicines for mental disorders, the Gulbenkian Mental Health Platform and the WHO Department of Mental Health and Substance Abuse recently worked on the identification of a set of practical key actions to be implemented by policy makers and clinicians in charge of governing mental health systems (Barbui et al. 2016). One aspect that the document clearly highlighted is the paucity and sparseness of research evidence to guide the identification of pragmatic and effective actions. As a consequence, most actions were recommended more on the basis of common sense and exemplary practice than on research findings.
Lack of an adequate evidence base needs to be properly addressed. One way to give more visibility to the existing research activities in this area, and to boost the development of new multidisciplinary projects and innovation initiatives, is to organise and classify all the research efforts aimed at increasing knowledge on aspects related to access, rational prescribing and appropriate use of medicines for mental disorders, under a common theoretical framework and nomenclature. Here we suggest the term ‘public health psychopharmacology’ (PHP).
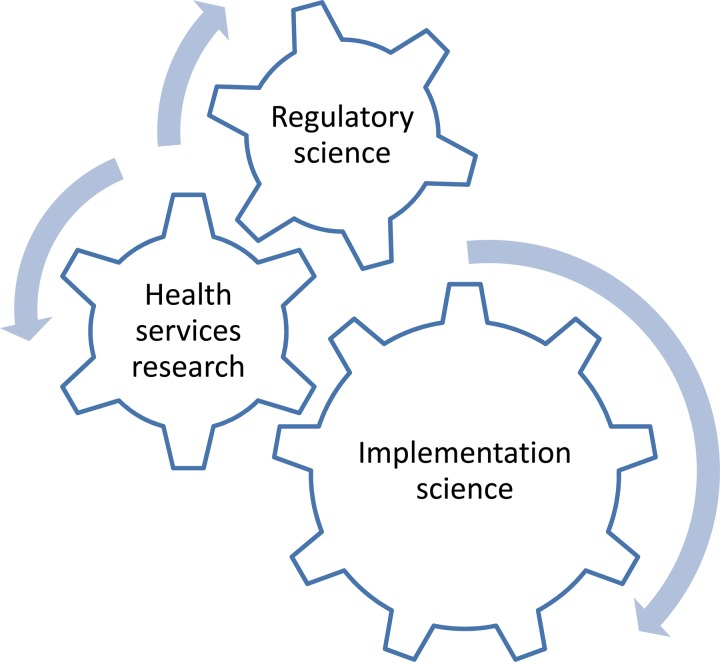
PHP should be considered a research discipline. Regulatory science, health services research and implementation science may represent research areas of interest (Fig. 1). For each of these research areas, challenges, aims and examples of research activities relevant to PHP may be identified (Table 1).
Fig. 1.
Research areas of interest for public health psychopharmacology
Table 1.
Description of the main aims and research activities of public health psychopharmacology
| Research area | Challenge | General aim | Examples of research activities |
|---|---|---|---|
| Regulatory science | Selection of psychotropic medicines | Improving the rules governing the approval process of psychotropic medicines | Analyses of the beneficial and harmful consequences of using placebo in conditions where active psychotropic medicines are available |
| Analyses of superiority v. non-inferiority v. equivalence designs | |||
| Analyses of the potential role of regulatory meta-analyses in the approval process for psychotropic medicines | |||
| Analyses of the consequences of using a systematic description of the evidence in support or against a new psychotropic medicine in public assessment reports | |||
| Health services research | Availability and affordability of psychotropic medicines | Improving timely obtainability of psychotropic medicines in the mental health system at affordable prices | Analyses of factors related to the development of sustainable and reliable supply systems |
| Development of studies on the beneficial and harmful consequences of different regulation systems for psychotropic medicines | |||
| Epidemiological studies on factors associated with counterfeit or substandard or degraded psychotropic medicines | |||
| Analyses of health system factors related to distribution strategies to reach those in need | |||
| Analyses of different financing systems to ensure economic sustainability for the health system | |||
| Epidemiological analyses of different policies for affordable prices for the end-user | |||
| Implementation science | Appropriate use of psychotropic medicines | Improving rational prescribing and appropriate use of psychotropic medicines | Production of systematic reviews of randomised and observational studies |
| Development of evidence-based guidelines and recommendations | |||
| Analyses of administrative information systems to monitor trends in psychotropic medicine prescribing and to establish associations between medicine exposure and outcome variables | |||
| Development of training and education activities, including audit and feedback initiatives | |||
| Development of epidemiological and pragmatic intervention studies on the beneficial and harmful consequences of psychotropic medicines |
A first challenge for PHP is to increase knowledge on how psychotropic medicines should be effectively selected. Research evidence is needed to help countries, regions, health districts and non-governmental organisations develop and implement effective rules governing the approval of psychotropic medicines. Examples of research areas that PHP should cover include:
-
•
analyses of the consequences of accepting the use of placebo in conditions where active psychotropic medicines are available (Barbui & Bighelli, 2013a; Ostuzzi et al. 2017), as well as ensuring appropriate doses of comparators, which was a concern with the second generation antipsychotics (Geddes et al. 2000);
-
•
the role of superiority v. non-inferiority v. equivalence designs (Garattini & Bertelè, 2007; Barbui & Bighelli, 2013b);
-
•
the potential added value of regulatory meta-analyses as an integral part of the drug approval process (Barbui et al. 2017b), ideally associated with a thorough qualitative appraisal of data (e.g., by employing the GRADE approach). The inclusion of systematic descriptions of the evidence in support or against a new psychotropic medicine in public assessment reports not only would ensure transparency, but could also orientate future research in effectively addressing evidence-to-practice gaps (Barbui et al. 2017b).
These regulatory themes are within the scope of PHP and should require careful research consideration (Garattini & Chalmers, 2009).
A second challenge for PHP is to increase knowledge on strategies to make psychotropic medicines available across mental health systems at affordable prices, especially in lower and middle income countries where an appreciable proportion of medical costs are out-of-pocket and illness can have catastrophic consequences on the family (World Health Organization, 2015b). Research evidence is needed to help countries, regions, health districts and non-governmental organisations develop and implement effective and sustainable policy actions (World Health Organization, 2003). Examples of research areas that PHP should cover include analyses of factors related to the development of sustainable and reliable medicine supply systems, studies on the impact of different regulation systems for psychotropic medicines recognising that boundaries between certain classes such as first and second generation anti-psychotic medicines are becoming blurred, studies investigating measures to limit the diffusion of counterfeit or substandard or degraded psychotropic medicines, and analyses of different financing systems to ensure economic sustainability at affordable prices for the end-user. This includes making quality generic medicines available at low prices (Woerkom et al. 2012), with typically no difference in outcomes between generics and originators to reduce co-payments as an issue. Furthermore, the area of health services research may be interested in evaluating particular needs of selected settings (for example prisons, emergency or humanitarian settings), where prescriptions may be particularly high in number, used off-label with poor clinical rationale, or not supported by adequate clinical resources (e.g., laboratory tests for monitoring adverse events) (Hassan et al. 2016). This would provide an evidence base for rationally addressing prescribing attitudes in settings where common guidelines do not easily apply. These health services themes are within the scope of PHP and should require careful research consideration.
Selected psychotropic medicines that are available at affordable prices should be rationally prescribed and appropriately used according to the context's features. Examples of irrational use of psychotropic medicines include prescribing or dispensing too many medicines per patient (polypharmacy), prescribing inappropriate dosages, poor adherence to correctly prescribed medications, as well as misuse, underuse or overuse (Jaracz et al. 2014). At a global level, more than 50% of all medicines are prescribed, dispensed or sold inappropriately, and half of all patients fail to take them correctly (Osterberg & Blaschke, 2005). Therefore, a third challenge for PHP is to produce knowledge that may support rational prescribing and appropriate use of medicines building on initiatives such as the Essential Medicines List (World Health Organization, 2015a). A number of research activities and study designs, with the background rationale of filling the gap between the production of evidence and its uptake in practice, may be employed (Table 1) including systematic reviews of randomised or observational evidence, evidence-based guidelines, pharmacoepidemiological studies based on administrative databases, observational or randomised studies with pragmatic designs. PHP should effectively implement these research tools to discover better and more effective strategies to support physicians in optimising the use of psychotropic medicines. We argue that more than the development of new and more effective psychotropic medicines, the greatest benefit in the next decade will derive from the generation of knowledge on how to provide better care based on current treatments.
Organising research activities on aspects related to access, rational prescribing and appropriate use of medicines for mental disorders under a common nomenclature may offer advantages. The scientific community would be more focused on common goals and objectives, with, likely, an increasing body of research evidence of practical use. International calls for funding may be interested in supporting research in the area of PHP, and scientific societies and journals might be more open to recognise the value of research in this area. Junior scientists and clinicians may appreciate that research activities in this area, being focused on implementation and quality of care, may effectively complement clinical practice.
Ultimately, the search for a better understanding of how psychotropic medicines should be effectively selected, made available to those in need at affordable prices, and prescribed rationally, is of importance to improving mental health care of individuals with these disorders in all countries. This is the ultimate goal of PHP.
Acknowledgement
None.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.
This Section of Epidemiology and Psychiatric Sciences appears in each issue of the Journal to stress the role of the epidemiological approach to promote advances in the field of clinical psychopharmacology, with a particular attention to controversial findings. The ultimate aims are to help develop a more critical attitude towards the results of research studies published in the international literature, to promote original research projects with higher methodological standards, and to implement the most relevant results of research in every-day clinical practice. These contributions are written in house by the journal's editorial team or commissioned by the Section Editor (no more than 1000 words, short unstructured abstract, 4 key-words, one Table or Figure and up to ten references).
Corrado Barbui, Section Editor
References
- Barbui C, Bighelli I (2013a). A new approach to psychiatric drug approval in Europe. PLoS Medicine 10, e1001530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbui C, Bighelli I (2013b). Regulatory science in Europe: the case of schizophrenia trials. Lancet 382, 1234–1235. [DOI] [PubMed] [Google Scholar]
- Barbui C, Dua T, Kolappa K, Saraceno B, Saxena S (2016). Access to psychotropic medicines in low-resource settings. Lancet Psychiatry 3, 913–915. [DOI] [PubMed] [Google Scholar]
- Barbui C, Dua T, Kolappa K, Saraceno B, Saxena S (2017a). Mapping actions to improve access to medicines for mental disorders in low and middle income countries. Epidemiology and Psychiatric Sciences (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbui C, Addis A, Amato L, Traversa G, Garattini S (2017b). Can systematic reviews contribute to regulatory decisions? European Journal of Clinical Pharmacology 73, 507–509. [DOI] [PubMed] [Google Scholar]
- Garattini S, Bertelè V (2007). Non-inferiority trials are unethical because they disregard patients’ interests. Lancet 370, 1875–1877. [DOI] [PubMed] [Google Scholar]
- Garattini S, Chalmers I (2009). Patients and the public deserve big changes in evaluation of drugs. BMJ 338, b1025. [DOI] [PubMed] [Google Scholar]
- Geddes J, Freemantle N, Harrison P, Bebbington P (2000). Atypical antipsychotics in the treatment of schizophrenia: systematic overview and meta-regression analysis. BMJ 321, 1371–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan L, Senior J, Webb RT, Frisher M, Tully MP, While D, Shaw JJ (2016). Prevalence and appropriateness of psychotropic medication prescribing in a nationally representative cross-sectional survey of male and female prisoners in England. BMC Psychiatry 16, 346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaracz J, Tetera-Rudnicka E, Kujath D, Raczynska A, Stoszek S, Czernas W, Wierzbinski P, Moniakowski A, Jaracz K, Rybakowski J (2014). The prevalence of antipsychotic polypharmacy in schizophrenic patients discharged from psychiatric units in Poland. Pharmacological Reports 66, 613–617. [DOI] [PubMed] [Google Scholar]
- Osterberg L, Blaschke T (2005). Adherence to medication. New England Journal of Medicine 353, 487–497. [DOI] [PubMed] [Google Scholar]
- Ostuzzi G, Papola D, Gastaldon C, Barbui C (2017). New EMA report on paliperidone 3-month injections: taking clinical and policy decisions without an adequate evidence base. Epidemiology and Psychiatric Sciences 26, 231–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woerkom M, Piepenbrink H, Godman B, Metz J, Campbell S, Bennie M (2012). Ongoing measures to enhance the efficiency of prescribing of proton pump inhibitors and statins in The Netherlands: influence and future implications. Journal of Comparative Effectiveness Research 1, 527–538. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2003). Effective Medicines Regulation: Ensuring Safety, Efficacy and Quality. Retrieved from http://apps.who.int/medicinedocs/pdf/s4921e/s4921e.Pdf
- World Health Organization (2015a). Essential Medicines and Basic Health Technologies for Noncommunicable Diseases: Towards a Set of Actions to Improve Equitable Access in Member States. Retrieved from http://www.who.int/nmh/ncd-tools/targets/Final_medicines_and_technologies_02_07_2015.pdf
- World Health Organization (2015b). WHO Guideline on Country Pharmaceutical Pricing Policies. Retrieved from http://apps.who.int/medicinedocs/documents/s21016en/s21016en.pdf