Abstract
Aims.
For nearly a century, the incidence of cancer in people with schizophrenia was lower than in the general population. In the recent decade, the relationship between cancer and schizophrenia has become obscured. Thus, we investigated the cancer risk among young and middle-aged patients with schizophrenia.
Methods.
Records of newly admitted patients with schizophrenia (n = 32 731) from January 2000 through December 2008 were retrieved from the Psychiatric Inpatient Medical Claims database in Taiwan, and the first psychiatric admission of each patient during the same period was defined as the baseline. We obtained 514 incident cancer cases that were monitored until December 2010. Standardised incidence ratios (SIRs) were calculated to compare the risk of cancer between those with schizophrenia and the general population. Stratified analyses of cancer incidences were performed by gender, site of cancers and duration since baseline (first psychiatric admission).
Results.
The incidence of cancer for all sites was slightly higher than that of the general population for the period (SIR = 1.15 [95% CI 1.06–1.26], p = 0.001). Men had a significantly higher incidence of colorectal cancer (SIR = 1.48 [95% CI 1.06–2.06], p = 0.019). Women had a higher incidence of breast cancer (SIR = 1.47 [95% CI 1.22–1.78], p < 0.001). Intriguingly, the risk for colorectal cancer was more pronounced 5 years after the first psychiatric admission rather than earlier (SIR = 1.94 [1.36–2.75], p < 0.001) and so was the risk for breast cancer (SIR = 1.85 [1.38–2.48], p < 0.001). The cancer incidence was higher in patients with schizophrenia contradicting the belief that schizophrenia was protective of cancers.
Conclusions.
Our analyses suggest that men and women with schizophrenia were more vulnerable to certain types of cancers, which indicates the need for gender-specific cancer screening programs. The fact that risk of colorectal cancer was more pronounced 5 years after the first psychiatric admission could imply the impact of unhealthy lifestyles or the possibility of delayed diagnoses.
Key words: Breast cancer, cancer, colorectal cancer, schizophrenia, standardised incidence ratio (SIR)
Introduction
For nearly a century, the medical community has had the notion that people with schizophrenia have a lower risk of cancer than the general population (Goldacre et al. 2005), which was supported by many biological hypotheses (Basu & Dasgupta, 2000; Yovel et al. 2000). However, recent epidemiologic research on the protective effects of schizophrenia for protection against cancer has produced mixed results (Chou et al. 2016). Although several studies from Sweden, Israel and Australia (Barak et al. 2005; Grinshpoon et al. 2005; Gal et al. 2012; Ji et al. 2013; Kisely et al. 2016) have shown lower cancer incidences in patients with schizophrenia, studies from Taiwan, Finland and the USA have revealed increased incidences of cancers in patients with schizophrenia (Lichtermann et al. 2001; McGinty et al. 2012; Lin et al. 2013). A large-scale study in the UK (Goldacre et al. 2005) demonstrated similar incidences of cancers in patients with schizophrenia and the general population, except for skin cancers. A meta-analysis (Catts et al. 2008) showed similar cancer incidences in patients with schizophrenia but lower incidences in their relatives compared with general population. This finding implies an inherently protective effect for schizophrenia on cancer, considering the higher exposure to cancer risk factors in patients with schizophrenia.
These inconsistencies may be explained in several ways. First, both genetic and environmental factors (such as lifestyle factors) contribute to cancer development in patients with schizophrenia. However, these lifestyle factors were not considered in most of the previous studies. The impact of lifestyle factors is best demonstrated in the following example. Mortensen (Mortensen, 1989) concluded that the incidence of lung cancer was higher for individuals with schizophrenia in a Danish cohort, but he later revealed a lower lung cancer incidence after accounting for smoking habits (Mortensen, 1994). Previous studies have revealed a link between schizophrenia and unhealthy lifestyles such as smoking, alcohol abuse and obesity (IARC, 2004, 2010; Hodgson et al. 2010; Hung et al. 2014). The impact of unhealthy lifestyles on the development of cancers should be investigated in patients with schizophrenia.
Second, different types or sites of cancers have different aetiologies (Hodgson et al. 2010; Chou et al. 2016). Therefore, it might be misleading to look at the overall cancer incidence in patients with schizophrenia. For instance, an Israeli study (Grinshpoon et al. 2005) showed lower cancer incidences, except for breast cancers, in patients with schizophrenia. Another UK study (Goldacre et al. 2005) reported low rates of skin cancers in schizophrenic patients than in the general population but no difference in overall cancer incidence. Failure to examine the site-specific cancer incidences in patients with schizophrenia may explain the inconsistency of the findings in the previous literature.
Third, age and gender play significant roles in the development of cancer in the population with schizophrenia. A previous study revealed that age at diagnosis of schizophrenia modified the cancer incidences and schizophrenia (Lin et al. 2013). Furthermore, a Swedish study showed different cancer incidences between men and women with schizophrenia, with higher risks of liver, lung, breast and endometrium cancers in women than the general population (Ji et al. 2013). These findings indicate the significance of incorporating the possible effects modification of age and sex in such studies.
To fill in the gap in the literature, we investigated the following questions using a longitudinal health insurance database, National Health Insurance Research Database. First, we sought to determine how site-specific cancer incidences differ by age and sex in individuals with schizophrenia compared with that of the general population. Second, we aimed to learn how unhealthy lifestyles affected the incidences of cancers at different sites. Thus, we categorised site-specific cancers based on their association with unhealthy lifestyles. Third, since early-onset cancers tend to have a genetic origin, and late-onset cancers are more lifestyle-related (Lin et al. 2013), the disease duration-stratified analysis helped us characterise the impacts of various lifestyles.
Methods
Data sources
The data source was described in detail elsewhere (Hung et al. 2014) and is briefly summarised here. Taiwan introduced a single-payer National Health Insurance program on March 1, 1995, and 98% of the 23 million of the Taiwanese population are enrolled. In 1996, the National Health Research Institute in Taiwan established the National Health Insurance Research Database. The database constitutes medical claim files representative of the entire population in Taiwan. All investigators signed an agreement guaranteeing patient confidentiality before using the database. The identities of subjects were fully encrypted to preserve patient anonymity during the statistical analysis. A waiver was granted for informed consent due to the minimal risk to the privacy of the individual subjects. This study was approved by the Institutional Review Board of the Committee on Human Subjects of Taipei City Hospital, Taipei, Taiwan.
We used the Psychiatric Inpatient Medical Claims database, a subset of the National Health Insurance Research Database, comprising a cohort of patients hospitalised for any psychiatric disorder between 1996 and 2008 (n = 187 117). The database included patients with at least one psychiatric inpatient record and one discharge diagnosis of mental illness coded by the International Classification of Diseases, Ninth Revision (ICD-9-CM codes: 290–319). The database includes patients’ demographic characteristics, diagnoses, medical expenditures and prescription claims data. Each prescription record contains the type of medication, dosage, time of prescription and duration of drug supply. The Bureau of National Health Insurance Information scrambled data that could be used to identify the beneficiaries and medical care providers.
Identification of the study cohort
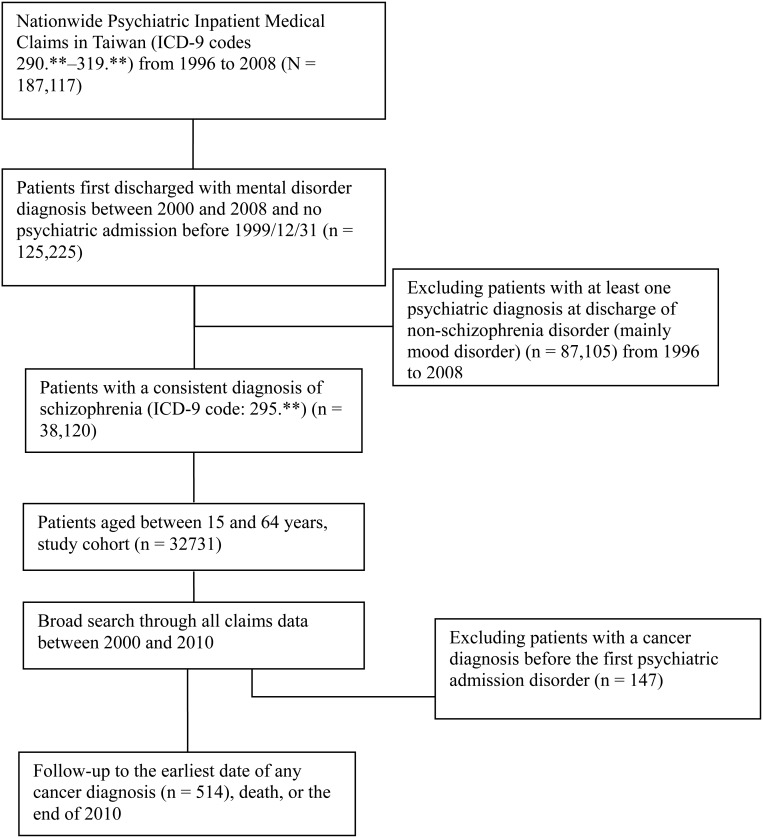
We conducted a prospective cohort study to examine cancer incidence in patients with schizophrenia. For enrolling the study cohort with probable incident schizophrenia (ICD-9-CM code: 295.**), the subjects analysed were identified through the Psychiatric Inpatient Medical Claims database. Figure 1 shows the detailed study algorithm. We excluded patients with any psychiatric admission between 1996 and 1999 and enrolled the patients with at least one psychiatric admission between 2000 and 2008 (n = 125 225). We then excluded patients with at least one psychiatric diagnosis at discharge of non-schizophrenia disorder (mainly mood disorder) (n = 87 105) between 1996 and 2008. The primary inclusion criterion for the study cohort was that a patient's diagnosis at each discharge must have fulfilled the principal diagnosis of schizophrenia (ICD-9-CM code: 295.**) when they had more than one psychiatric admission. We defined the first psychiatric admission between 2000 and 2008 as the baseline. The age of the patients at the first psychiatric admission was restricted from 15 to 64 years. Subsequently, 32 731 patients with schizophrenia were designated as the study cohort.
Fig. 1.
Study flow diagram.
Identification of cancer events
In the database, cancer was defined as a ‘catastrophic illness,’ and thus, the status of ‘catastrophic illnesses’ enabled the co-payment for the treatment of the disease to be waived. Cytological or other pathological reports or evidence supportive of malignancy was required to apply for a catastrophic illness certificate for cancer. Cancer events in the present study were identified by linking the study cohort with the catastrophic illness database (ICD-9-CM codes: 140–208).
By retrieving all of the cohort medical records from 1996 through 2010, all patients (n = 32 731) had been monitored from the baseline to cancer diagnosis and death, or until the end of 2010, whichever came first. Of them, 147 subjects had had a cancer diagnosis before the first psychiatric admission and were excluded from the study. Eventually, 514 cases with incident cancers were included. We then identified the cancers sites (ICD-9-CM codes), including the buccal cavity (140–149), oesophagus (150), larynx (161), liver (155), lung (162), nasal cavity and paranasal sinuses (160), pancreas (157), stomach (151), kidney (189), urinary bladder (188), myeloid leukemia (205), colon and rectum (153–154), skin cancer (172–173), brain tumor (191) and thyroid cancer (193). We also identified female-specific cancers, including breast (174), cervical cancers (180).
Estimation of standardised incidence ratios (SIRs)
We used SIRs to determine whether the occurrence of cancer in patients with schizophrenia was higher or lower than in the general population (Hung et al. 2014). This methodology has been used previously (Hung et al. 2014). SIRs were computed as the ratio of the observed number to the expected number of cancer events in the study cohort. The expected number of cancer events was calculated by multiplying the number of subjects at risk by the national cancer incidence and the length of follow-up time (years). The length of follow-up for each subject was estimated from the baseline to cancer diagnosis, death, or until December 31, 2010.
We stratified the entire cohort into various age subgroups in 5-year intervals (from 15 to 64 years) and summed the expected number of cancer events in each age stratum as the expected total number of cancer events. Then, the age-stratified adjusted SIR was computed.
The national incidences of all and site-specific cancers were retrieved from the cancer statistics website of the Health Promotion Administration, Ministry of Health and Welfare (https://cris.bhp.doh.gov.tw/). The data are recorded and updated annually (https://cris.bhp.doh.gov.tw/), with the most recent data available at the time of this study for the year 2010. Thus, we used the average incidences from 1999 to 2010 to estimate the expected number of cancer events.
The SAS System for Windows (Version 9.2) was used to manage and analyze the data. We employed SAS software to calculate the observed number and expected number of events. Confidence intervals for the SIRs were estimated using the Boice–Monson Method (Rothman & Greeland, 1998), which was also used for the estimation of confidence intervals for the SMRs (standardised mortality ratios). The calculator is available on the website (http://web1.sph.emory.edu/cdckms/exact-midP-SMR.html; by Dr. Sullivan KM).
Results
Incidence of cancer in patients with schizophrenia
The incidences of cancer in the schizophrenia cohort were 2.15 per 1000 person-years. The incidence of all-site cancers was slightly higher than that in the general population (SIR = 1.15 [95% CI 1.06–1.26], p = 0.001). Some age subgroups had a higher incidence of all-site cancers than the general population did, including the 25–29, 50–54 and 60–64-year age groups. Thus, we standardised the age for estimating the incidence of cancer (Table 1).
Table 1.
Baseline characteristics and incident cancer risk in patients with schizophrenia (cohort: 2000–2008) monitored from 2000 through 2010 (n = 32 731)
| N | Follow-up duration, year Mean (SD) | Number of cancers observed, n | Total person-years | Crude incidencea | Expected incidence, n | SIRb (95% CI) | p | |
|---|---|---|---|---|---|---|---|---|
| Agec, year | ||||||||
| 15–19 | 1741 | 7.48 (2.24) | 2 | 13024 | 15.36 | 2.07 | 0.97 (0.24–3.86) | 0.961 |
| 20–24 | 4195 | 7.58 (2.15) | 13 | 31785 | 40.88 | 9.26 | 1.40 (0.82–2.42) | 0.219 |
| 25–29 | 4731 | 7.37 (2.24) | 25 | 34851 | 71.70 | 16.59 | 1.51* (1.02–2.23) | 0.039 |
| 30–34 | 4915 | 7.42 (2.27) | 34 | 36481 | 93.23 | 34.04 | 1.00 (0.71–1.40) | 0.995 |
| 35–39 | 4849 | 7.45 (2.25) | 60 | 36147 | 166.09 | 64.20 | 0.93 (0.73–1.20) | 0.600 |
| 40–44 | 4190 | 7.17 (2.31) | 85 | 30062 | 282.93 | 79.24 | 1.07 (0.87–1.33) | 0.518 |
| 45–49 | 3352 | 7.08 (2.31) | 101 | 23724 | 425.58 | 80.66 | 1.25* (1.03–1.52) | 0.024 |
| 50–54 | 2432 | 6.83 (2.27) | 94 | 16617 | 565.91 | 68.26 | 1.38** (1.13–1.69) | 0.002 |
| 55–59 | 1414 | 6.70 (2.34) | 36 | 9476 | 380.00 | 46.44 | 0.78 (0.56–1.07) | 0.126 |
| 60–64 | 912 | 6.95 (2.45) | 64 | 6339 | 1009.72 | 45.71 | 1.40** (1.10–1.79) | 0.007 |
| Total | 32731 | 7.29 (2.28) | 514 | 238509 | 215.42 | 446.47 | 1.15** (1.06–1.26) | 0.001 |
Incidence: incident number/100 000 person-years.
Standardised incidence ratio (SIR), the observed number of cases/expected number of cases; the expected numbers of cancers were computed by multiplying the number in the study cohort (standardised by gender, stratified by age) by the national cancer incidence (2000–2010).
Indicates age at earliest psychiatric admission between 2000 and 2008.
*p < 0.05; **p < 0.01.
Site-specific cancers in schizophrenia by gender
Based on the estimated age- and gender-SIRs, the incidences of the site-specific cancers did not differ significantly from those of the general population, except for breast cancer (only in women) (SIR = 1.47 [1.22–1.78], p < 0.001) and bladder cancer (SIR = 2.07 [1.18–3.65], p = 0.010) (Table 2). The stratified analyses revealed that women with schizophrenia had higher incidences of bladder and breast cancers while men had a significantly higher incidence of colorectal cancer (SIR = 1.48 [1.06–2.06], p = 0.019) than in the general population. As for gender-specific cancers, the incidence of breast cancer was greater in women with schizophrenia than that in the general population (SIR = 1.47 [1.22–1.78], p < 0.001); however, no significant difference was found in cervical cancer.
Table 2.
Standardized incidence ratios (SIRs) for site-specific cancer in patients with schizophrenia stratified by gender in Taiwan, 2000–2010 (n = 32 731)
| Male (n = 17971) | Female (n = 14760) | Total (n = 32731) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Site of cancer (modified ICD-9-CM) | Observed n | SIRa (95% CI) | p | Observed n | SIRa (95% CI) | p | Observed n | SIRb (95% CI) | p |
| Total (140–208) | 230 | 1.05 (0.92–1.19) | 0.503 | 284 | 1.25 (1.12–1.41) | <0.001 | 514 | 1.15** (1.06–1.26) | 0.001 |
| Buccal cavity (140–149) | 54 | 0.88 (0.67–1.15) | 0.348 | 8 | 1.01 (0.50–2.01) | 0.983 | 62 | 0.89 (0.70–1.15) | 0.381 |
| Oesophagus (150) | 15 | 1.55 (0.93–2.57) | 0.087 | 1 | 1.53 (0.22–10.92) | 0.664 | 16 | 1.55 (0.95–2.53) | 0.078 |
| Colorectal cancer (153–154) | 35 | 1.48* (1.06–2.06) | 0.019 | 20 | 0.93 (0.60–1.44) | 0.732 | 55 | 1.22 (0.93–1.58) | 0.146 |
| Liver(155) | 44 | 1.04 (0.77–1.39) | 0.808 | 14 | 1.26 (0.75–2.13) | 0.388 | 58 | 1.08 (0.84–1.40) | 0.542 |
| Larynx (161) | 4 | 1.56 (0.58–4.15) | 0.372 | 0 | 0.00 | – | 4 | 1.09 (0.41–2.90) | 0.863 |
| Lung (162) | 17 | 1.06 (0.66–1.70) | 0.811 | 8 | 0.59 (0.29–1.18) | 0.129 | 25 | 0.84 (0.57–1.25) | 0.395 |
| Nasal cavity and paranasal sinuses (160) | 1 | 1.01 (0.14–7.17) | 0.992 | 1 | 2.11 (0.30–15.10) | 0.440 | 2 | 1.37 (0.34–5.48) | 0.655 |
| Pancreas (157) | 5 | 1.74 (0.73–4.18) | 0.209 | 4 | 2.00 (0.75–5.33) | 0.157 | 9 | 1.85 (0.96–3.55) | 0.061 |
| Stomach (151) | 7 | 0.85 (0.41–1.79) | 0.675 | 11 | 1.61 (0.89–2.90) | 0.113 | 18 | 1.20 (0.75–1.90) | 0.447 |
| Kidney (189) | 3 | 1.04 (0.34–3.23) | 0.944 | 4 | 2.56 (0.96–6.83) | 0.051 | 7 | 1.58 (0.75–3.31) | 0.224 |
| Bladder (188) | 7 | 1.71 (0.82–3.59) | 0.150 | 5 | 2.94* (1.22–7.07) | 0.011 | 12 | 2.07** (1.18–3.65) | 0.010 |
| Skin cancer (172–173) | 5 | 0.95 (0.39–2.28) | 0.906 | 2 | 0.46 (0.12–1.85) | 0.264 | 7 | 0.73 (0.35–1.53) | 0.403 |
| Brain tumour (191) | 4 | 1.35 (0.51–3.60) | 0.546 | 4 | 2.03 (0.76–5.38) | 0.151 | 8 | 1.62 (0.81–3.24) | 0.167 |
| Thyroid cancer (193) | 2 | 0.53 (0.13–2.13) | 0.366 | 11 | 0.96 (0.53–1.74) | 0.903 | 13 | 0.86 (0.50–1.48) | 0.577 |
| Other and unspecified sites | 21 | 0.63* (0.41–0.96) | 0.032 | 58 | 1.43** (1.11–1.86) | 0.006 | 79 | 1.07 (0.86–1.33) | 0.544 |
| Myeloid leukaemia (205) | 6 | – | – | 4 | – | – | 10 | – | – |
| Breast (174), women only | – | – | – | 105 | 1.47** (1.22–1.78) | <0.001 | – | – | – |
| Cervical cancer (180), women only | – | – | – | 24 | 0.85 (0.57–1.26) | 0.415 | – | – | – |
SIR: observed number of cases/expected number of cases; the expected numbers of cancer were computed by multiplying the number in the study cohort by the national cancer incidence rate (age standardisation used the strata in Table 1).
Age- and gender-SIRs.
*p < 0.05; **p < 0.01.
Duration-stratified colorectal and breast cancers
As for the site-specific cancers that had statistically significantly elevated risks in Table 2 with incident numbers >50 (i.e., colorectal and breast cancers), we conducted further analysis based on duration-stratified risks. Table 3 shows estimated risks of colorectal and breast cancers across various lengths of follow-up, respectively.
Table 3.
Standardized incidence ratios for colorectal and breast cancers after the first psychiatric admission (baseline) among inpatients with schizophrenia in Taiwan, 2000–2010 (n = 32 731)
| All subjects | 1 Year after baseline | 2–5 Years after baseline | >5 Years after baseline | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis | Observed | Observed | SIRa (95% CI) | p | Observed | SIRa (95% CI) | p | Observed | SIRa (95% CI) | p |
| Total (140–208) | ||||||||||
| Colorectal cancerb | 55 | 5 | 0.77 (0.32–1.86) | 0.566 | 19 | 0.77 (0.49–1.21) | 0.263 | 31 | 1.94** (1.36–2.75) | <0.001 |
| Breast cancer (women only)b | – | – | – | – | – | – | – | – | – | – |
| Males | ||||||||||
| Colorectal cancer | 35 | 3 | 0.89 (0.29–2.76) | 0.840 | 15 | 1.17 (0.70–1.94) | 0.545 | 17 | 1.98** (1.23–3.18) | 0.004 |
| Breast cancer | – | – | – | – | – | – | – | – | – | – |
| Females | ||||||||||
| Colorectal cancer | 20 | 2 | 0.65 (0.16–2.58) | 0.532 | 4 | 0.34* (0.13–0.91) | 0.024 | 14 | 1.89* (1.12–3.19) | 0.016 |
| Breast cancerb | 105 | 16 | 1.58 (0.97–2.57) | 0.066 | 44 | 1.15 (0.85–1.54) | 0.365 | 45 | 1.85** (1.38–2.48) | <0.001 |
SIR: observed number of cases/expected number of cases.
The site-specific cancers had statistically significant elevated risks in Table 2 with incident numbers >50.
*p < 0.05; **p < 0.01.
Intriguingly, colorectal cancer did not increase in the early period (1–5 years after baseline) but increased significantly in the period more than 5 years after baseline (SIR = 1.94 [1.36–2.75], p < 0.001). Analogous results were noted for all subjects and both genders. Similarly, the incidence of breast cancer in women was increased significantly in the period more than 5 years after the first psychiatric admission (SIR = 1.85 [1.38–2.48], p < 0.001).
Discussion
The present study investigated an Asian national cohort with schizophrenia to examine the risk of cancer in the past decade. The age-SIR was used as an index to measure the incidence of cancer in patients with schizophrenia. We would expect the cancer rates in schizophrenia to be higher than in the general population, especially those cancers associated with unhealthy lifestyles, such as smoking, alcohol use and metabolic syndrome. Our findings showed that the SIRs in several site-specific cancers (e.g., colorectal, breast and bladder cancers) went in the expected (i.e., increasing) direction and the SIRs in other cancers did not; thus, they only partly mirrored our overall preliminary expectations.
In this study, four main findings were observed. First, we found a slightly higher incidence of all-site cancer than in the general population (SIR = 1.15). Second, we identified the age groups at high risk of cancer as 25–29, 45–49, 50–54 and 60–64 years. Third, women with schizophrenia had higher incidences of breast and bladder cancers than in the general population while men with schizophrenia were more likely to have colorectal cancer compared with their counterparts in the general population. Fourth, when investigating the interval from the first psychiatric admission (baseline) to the diagnosis of incident cancer, we found that the incidence of colorectal cancer significantly increased more than 5 years after the baseline psychiatric admission in both sexes.
Incidences of all-site cancers
Previous studies have shown inconsistent results regarding the incidence of cancer in patients with schizophrenia (Lichtermann et al. 2001; Barak et al. 2005; Goldacre et al. 2005; Grinshpoon et al. 2005; Gal et al. 2012; McGinty et al. 2012; Lin et al. 2013; Kisely et al. 2016). Unhealthy lifestyle choices such as smoking and alcohol consumption may contribute to increased cancer risks in patients with schizophrenia. However, several investigators have proposed that the genes causing schizophrenia have possible protective effects against cancer development. A Finnish study revealed an increased risk of cancers in patients with schizophrenia but significantly lower risks in their relatives, indicating the protective effects of schizophrenia-related genes (Lichtermann et al. 2001). The genetic association of cancer-related and schizophrenia-related genes may offer an explanation. P53, a tumour suppressor gene central to the regulation of cell apoptosis is postulated as a candidate susceptibility gene for schizophrenia (Catts & Catts, 2000). However, we found that patients with schizophrenia had a higher cancer incidence than in the general population, suggestive of pronounced effects of unhealthy lifestyles in recent decades.
Age as an effect modifier
The age at which patients with schizophrenia had the highest cancer incidences were 25–29, 45–49, 50–54 and 60–64 years. Older patients with schizophrenia (≥45 years) had a higher incidence of cancer than in the general population. One possible reason for this finding is the effects of unhealthy lifestyles (Lin et al. 2013) or medications (Yamazawa et al. 2003). In addition to smoking and alcohol use (Drake et al. 1990; Sagud et al. 2009), schizophrenia is also associated with a higher risk of metabolic syndrome (Monteleone et al. 2009), which shows a dose-response relationship with colorectal cancer (Ahmed et al. 2006). Nevertheless, we found that relatively young (25–29 years of age) patients with schizophrenia were at high risk of a cancer diagnosis. One possible hypothesis for this result could be the medical surveillance bias (Haut & Pronovost, 2011), in which higher diagnostic rates of other psychiatric or physical comorbidities occur due to the patient's initial entry into the medical system for schizophrenia. As the age of onset of schizophrenia is usually in the second decade of life (Johnstone et al. 1989), it is possible that these patients with schizophrenia were referred to medical doctors for their physical complaints, and thus, cancer incidence increased. Further investigation is required to determine the effect of age on the development of cancer in patients with schizophrenia.
Gender effect on site-specific cancer incidence
Women with schizophrenia had higher incidences of breast and bladder cancers than in the general population. Men with schizophrenia were more likely to be diagnosed with colorectal cancer. As for the viewpoint of lifestyles, according to the World Health Organization (WHO) International Agency for Research on Cancer Monographs and recent literature (IARC, 2004, 2010; Baan et al. 2007; Esposito et al. 2012), breast cancer was identified in relation to alcohol (Baan et al. 2007; IARC, 2010) and also metabolic syndrome (Esposito et al. 2012). Bladder cancer was associated with tobacco use (IARC, 2004). Colorectal cancer was related to alcohol use (Baan et al. 2007; IARC, 2010) and metabolic syndrome (Esposito et al. 2012).
The association between schizophrenia and breast cancers in women is well established. A slight elevation in the risk of breast cancer among women with schizophrenia (rate ratio = 1.19) was found two decades ago (Mortensen, 1989). An 11-year cohort study of 3470 patients with schizophrenia revealed a higher standardised mortality rate for breast cancer in women than in the general population (standardised mortality rate = 2.2; 95% CI 1.6–3.3) (Tran et al. 2009). A meta-analysis showed inconsistent results due to methodological issues, but a trend of increased breast cancer risk was observed (Bushe et al. 2009). The pathogenesis of this association could be hyperprolactinemia due to antipsychotic use (Tworoger et al. 2007); however, a recent studies (Esposito et al. 2012) showed metabolic syndrome or unhealthy lifestyle (e.g., alcohol use) was highly associated with this finding.
Additionally, we found that women with schizophrenia had a higher incidence of bladder cancer. This result could also be attributed to lifestyle habits since women with schizophrenia are three times more likely to smoke cigarettes than women in the general population are (de Leon & Diaz, 2005).
Men with schizophrenia were more likely to be diagnosed with colorectal cancer. Some studies reported a lower rate of colorectal cancer (Goldacre et al. 2005; Abel et al. 2006) while others demonstrated an increased rate of colorectal cancer in men with schizophrenia (Hippisley-Cox et al. 2007; Lin et al. 2013). The association between colorectal cancer and schizophrenia was more pronounced in men than in women in our study. This result could be attributable to metabolic factors or alcohol use (Esposito et al. 2012, 2010). One review article (Ochoa et al. 2012) indicated that the prevalence of alcohol consumption was much greater in men with schizophrenia than in women with schizophrenia. Additionally, obesity is a known risk factor for colorectal cancer, and the relationship between obesity and colorectal cancer is more prominent in men than in women (Dai et al. 2007).
Notably, both genders revealed increased risk for colorectal cancer in the late period (5 years after baseline), which could be due to unhealthy lifestyles (such as obesity) or medications. For instance, the increased risk of colorectal cancer is particularly marked in patients taking antipsychotic drugs (Hippisley-Cox et al. 2007) and antipsychotics could also lead to obesity (Sicras-Mainar et al. 2008). Whether antipsychotics use increased the risk of colorectal cancer through obesity requires further investigation.
Potential effects of unhealthy lifestyles on cancer incidence
One significant finding of the study was that the critical period for the development of colorectal and breast cancers in patients with schizophrenia was 5 years after the baseline. Both of these site-specific cancers were associated with unhealthy lifestyles (IARC, 2004, 2010; Baan et al. 2007; Esposito et al. 2012). There could be two possible explanations for this result. One is that the influences from genetic factors were outweighed by environmental factors in the development of the cancers in our cohort (Lin et al. 2013). Evidence shows that patients with schizophrenia have less healthy lifestyles than people do in the general population, with increased prevalences of obesity, smoking, diabetes, hypertension and dyslipidemia, which could contribute to cancer development (Newcomer & Sernyak, 2007; De Hert et al. 2009), such as colorectal and breast cancers. The other one is that patients with schizophrenia experienced delayed cancer diagnoses, which is supported by their excess mortality rates from cancers (Tran et al. 2009; Wildgust et al. 2010) in spite of improvements in medical service delivery (Capasso et al. 2008; Nielsen et al. 2013). The delayed diagnoses could be attributed to lack of recognition of the patients’ physical problems by health professionals and the patients themselves (Muir-Cochrane, 2006).
Limitations
In spite of the large sample size and generalisability, there are several limitations to the study. First, an inherent limitation of the National Health Insurance Research Database is that data on sociodemographic variables, lifestyle variables, psychotropic drug use and cancer biomarkers were not available. We also could not obtain information on the changes of lifestyles after the first psychiatric admissions; thus, the genetic and environmental causes could not be dissociated in this study.
Second, the age at first psychiatric admission was used as the baseline instead of age at onset. However, the mean (SD) time lag between the actual age at onset and first psychiatric admission in the study subjects was 1.92 (2.57) years, which is not long. Since we used the age at first psychiatric admission in the age-stratified analysis, the cancer incidences did not necessarily match the age-specific cancer incidences due to the different length of follow-up for each subject.
Third, although we categorised cancers based on unhealthy lifestyles (Hung et al. 2014) to examine the impact of each habit, there was some overlapping of these unhealthy lifestyle-related cancers. For instance, both smoking and metabolic syndrome were related to colorectal cancer. Therefore, discerning specific effects of each lifestyle was not possible.
Fourth, this study extracted the patients with schizophrenia from the administrative database based on a service diagnosis instead of a research diagnosis. Nonetheless, since we selected the patients with schizophrenia based on the ICD 9-CM system (code 295.** schizophrenic disorders), patients with schizoaffective disorder could be included (code 295.7*).
Fifth, the incidences of some site-specific cancers were quite small, and thus, their generalisability is limited. Last, the study population was restricted to ages 15–64, and the follow-up duration was <10 years; therefore, the generalisability is also limited.
Conclusions
The results of this study have clinical and public health implications. The findings show a modest 15% increase in cancer risk in patients with schizophrenia, which challenges the belief that schizophrenia is protective of cancer. Our analyses suggest the impact of an unhealthy lifestyle could have a pronounced influence on the incidence of cancers in patients with schizophrenia, highlighting the significance of cancer screening and lifestyle modifications in this patient population. The results of gender differences in cancer incidences at different sites highlight the urgent need for sex-specific cancer screening programs.
Acknowledgements
The authors thank Yen-Chung Chen, M.S. and Jia-Rong Jhong, B.S., both with the Department of General Psychiatry, Taipei City Psychiatric Center, Taipei City Hospital, for data management and help with the statistical analyses.
Financial Support
This research was supported by grants from the National Science Council, Taiwan (MOST 105-2314-B-532-006-MY3; NSC 102-2628-B-532-001-MY3) and Taipei City Hospital (10401-62-059; 10501-62-039).
Conflict of Interest
The authors have declared that there are no conflicts of interest related to the subject of this study.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Author Contributions
Dr Lian-Yu Chen, Dr Hung, and Dr Kuo conceived and designed the study. Dr Yang acquired the data and performed the statistical analysis. Dr Ying-Yeh Chen, Dr Pan, and Dr Chiao-Chicy Chen provided administrative and material support. Dr Lian-Yu Chen and Dr Kuo drafted the manuscript. Dr Ying-Yeh Chen made critical revisions to the manuscript for important intellectual content. Dr Ying-Yeh Chen and Dr Kuo supervised the study.
Availability of data and materials
Please find the website (http://nhird.nhri.org.tw/en/) to request the information of database this study used, such as a point of contact, data protection, and content of data files. The database constitutes medical claim files representative of the entire population in Taiwan.
References
- Abel KM, Allin MP, Jirtle RL (2006). Schizophrenia, cancer and imprinting: early nutritional influences. British Journal of Psychiatry 188, 394. [DOI] [PubMed] [Google Scholar]
- Ahmed RL, Schmitz KH, Anderson KE, Rosamond WD, Folsom AR (2006). The metabolic syndrome and risk of incident colorectal cancer. Cancer 107, 28–36. [DOI] [PubMed] [Google Scholar]
- Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, Altieri A, Cogliano V (2007). Carcinogenicity of alcoholic beverages. Lancet Oncology 8, 292–293. [DOI] [PubMed] [Google Scholar]
- Barak Y, Achiron A, Mandel M, Mirecki I, Aizenberg D (2005). Reduced cancer incidence among patients with schizophrenia. Cancer 104, 2817–2821. [DOI] [PubMed] [Google Scholar]
- Basu S, Dasgupta PS (2000). Role of dopamine in malignant tumor growth. Endocrine 12, 237–241. [DOI] [PubMed] [Google Scholar]
- Bushe CJ, Bradley AJ, Wildgust HJ, Hodgson RE (2009). Schizophrenia and breast cancer incidence: a systematic review of clinical studies. Schizophrenia Research 114, 6–16. [DOI] [PubMed] [Google Scholar]
- Capasso RM, Lineberry TW, Bostwick JM, Decker PA, St Sauver J (2008). Mortality in schizophrenia and schizoaffective disorder: an Olmsted County, Minnesota cohort: 1950–2005. Schizophrenia Research 98, 287–294. [DOI] [PubMed] [Google Scholar]
- Catts VS, Catts SV (2000). Apoptosis and schizophrenia: is the tumour suppressor gene, p53, a candidate susceptibility gene? Schizophrenia Research 41, 405–415. [DOI] [PubMed] [Google Scholar]
- Catts VS, Catts SV, O'Toole BI, Frost AD (2008). Cancer incidence in patients with schizophrenia and their first-degree relatives – a meta-analysis. Acta Psychiatrica Scandinavica 117, 323–336. [DOI] [PubMed] [Google Scholar]
- Chou FH, Tsai KY, Wu HC, Shen SP (2016). Cancer in patients with schizophrenia: what is the next step? Psychiatry and Clinical Neurosciences doi: 10.1111/pcn.12420. [DOI] [PubMed] [Google Scholar]
- Dai Z, Xu YC, Niu L (2007). Obesity and colorectal cancer risk: a meta-analysis of cohort studies. World Journal of Gastroenterology 13, 4199–4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hert M, Dekker JM, Wood D, Kahl KG, Holt RI, Moller HJ (2009). Cardiovascular disease and diabetes in people with severe mental illness position statement from the European Psychiatric Association (EPA), supported by the European Association for the Study of Diabetes (EASD) and the European Society of Cardiology (ESC). European Psychiatry 24, 412–424. [DOI] [PubMed] [Google Scholar]
- de Leon J, Diaz FJ (2005). A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophrenia Research 76, 135–157. [DOI] [PubMed] [Google Scholar]
- Drake RE, Osher FC, Noordsy DL, Hurlbut SC, Teague GB, Beaudett MS (1990). Diagnosis of alcohol use disorders in schizophrenia. Schizophrenia Bulletin 16, 57–67. [DOI] [PubMed] [Google Scholar]
- Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D (2012). Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care 35, 2402–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal G, Goral A, Murad H, Gross R, Pugachova I, Barchana M, Kohn R, Levav I (2012). Cancer in parents of persons with schizophrenia: is there a genetic protection? Schizophrenia Research 139, 189–193. [DOI] [PubMed] [Google Scholar]
- Goldacre MJ, Kurina LM, Wotton CJ, Yeates D, Seagroat V (2005). Schizophrenia and cancer: an epidemiological study. British Journal of Psychiatry 187, 334–338. [DOI] [PubMed] [Google Scholar]
- Grinshpoon A, Barchana M, Ponizovsky A, Lipshitz I, Nahon D, Tal O, Weizman A, Levav I (2005). Cancer in schizophrenia: is the risk higher or lower? Schizophrenia Research 73, 333–341. [DOI] [PubMed] [Google Scholar]
- Haut ER, Pronovost PJ (2011). Surveillance bias in outcomes reporting. JAMA 305, 2462–2463. [DOI] [PubMed] [Google Scholar]
- Hippisley-Cox J, Vinogradova Y, Coupland C, Parker C (2007). Risk of malignancy in patients with schizophrenia or bipolar disorder: nested case-control study. Archives of General Psychiatry 64, 1368–1376. [DOI] [PubMed] [Google Scholar]
- Hodgson R, Wildgust HJ, Bushe CJ (2010). Cancer and schizophrenia: is there a paradox? Journal of Psychopharmacology 24, 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung YN, Yang SY, Huang MC, Lung FW, Lin SK, Chen KY, Kuo CJ, Chen YY (2014). Cancer incidence in people with affective disorder: nationwide cohort study in Taiwan, 1997–2010. British Journal of Psychiatry 205, 183–188. [DOI] [PubMed] [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans (2004). Tobacco smoke and involuntary smoking. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans 83, 1–1438. [PMC free article] [PubMed] [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans (2010). Alcohol consumption and ethyl carbamate. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans 96, 3–1383. [PMC free article] [PubMed] [Google Scholar]
- Ji J, Sundquist K, Ning Y, Kendler KS, Sundquist J, Chen X (2013). Incidence of cancer in patients with schizophrenia and their first-degree relatives: a population-based study in Sweden. Schizophrenia Bulletin 39, 527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone EC, Owens DG, Bydder GM, Colter N, Crow TJ, Frith CD (1989). The spectrum of structural brain changes in schizophrenia: age of onset as a predictor of cognitive and clinical impairments and their cerebral correlates. Psychological Medicine 19, 91–103. [DOI] [PubMed] [Google Scholar]
- Kisely S, Forsyth S, Lawrence D (2016). Why do psychiatric patients have higher cancer mortality rates when cancer incidence is the same or lower? Australian and New Zealand Journal of Psychiatry 50, 254–263. [DOI] [PubMed] [Google Scholar]
- Lichtermann D, Ekelund J, Pukkala E, Tanskanen A, Lonnqvist J (2001). Incidence of cancer among persons with schizophrenia and their relatives. Archives of General Psychiatry 58, 573–578. [DOI] [PubMed] [Google Scholar]
- Lin GM, Chen YJ, Kuo DJ, Jaiteh LE, Wu YC, Lo TS, Li YH (2013). Cancer incidence in patients with schizophrenia or bipolar disorder: a nationwide population-based study in Taiwan, 1997–2009. Schizophrenia Bulletin 39, 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinty EE, Zhang Y, Guallar E, Ford DE, Steinwachs D, Dixon LB, Keating NL, Daumit GL (2012). Cancer incidence in a sample of Maryland residents with serious mental illness. Psychiatric Services 63, 714–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone P, Martiadis V, Maj M (2009). Management of schizophrenia with obesity, metabolic, and endocrinological disorders. Psychiatric Clinics of North America 32, 775–794. [DOI] [PubMed] [Google Scholar]
- Mortensen PB (1989). The incidence of cancer in schizophrenic patients. Journal of Epidemiology and Community Health 43, 43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen PB (1994). The occurrence of cancer in first admitted schizophrenic patients. Schizophrenia Research 12, 185–194. [DOI] [PubMed] [Google Scholar]
- Muir-Cochrane E (2006). Medical co-morbidity risk factors and barriers to care for people with schizophrenia. Journal of Psychiatric and Mental Health Nursing 13, 447–452. [DOI] [PubMed] [Google Scholar]
- Newcomer JW, Sernyak MJ (2007). Identifying metabolic risks with antipsychotics and monitoring and management strategies. Journal of Clinical Psychiatry 68, e17. [DOI] [PubMed] [Google Scholar]
- Nielsen RE, Uggerby AS, Jensen SO, McGrath JJ (2013). Increasing mortality gap for patients diagnosed with schizophrenia over the last three decades – a Danish nationwide study from 1980 to 2010. Schizophrenia Research 146, 22–27. [DOI] [PubMed] [Google Scholar]
- Ochoa S, Usall J, Cobo J, Labad X, Kulkarni J (2012). Gender differences in schizophrenia and first-episode psychosis: a comprehensive literature review. Schizophrenia Research and Treatment 2012, 916198. doi: 10.1155/2012/916198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ, Greeland S (1998). Modern Epidemiology, 2nd edn Lippincott Williams & Wilkins: Philadelphia, PA. [Google Scholar]
- Sagud M, Mihaljevic-Peles A, Muck-Seler D, Pivac N, Vuksan-Cusa B, Brataljenovic T, Jakovljevic M (2009). Smoking and schizophrenia. Psychiatria Danubina 21, 371–375. [PubMed] [Google Scholar]
- Sicras-Mainar A, Navarro-Artieda R, Rejas-Gutierrez J, Blanca-Tamayo M (2008). Relationship between obesity and antipsychotic drug use in the adult population: a longitudinal, retrospective claim database study in Primary Care settings. Neuropsychiatric Disease and Treatment 4, 219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran E, Rouillon F, Loze JY, Casadebaig F, Philippe A, Vitry F, Limosin F (2009). Cancer mortality in patients with schizophrenia: an 11-year prospective cohort study. Cancer 115, 3555–3562. [DOI] [PubMed] [Google Scholar]
- Tworoger SS, Eliassen AH, Sluss P, Hankinson SE (2007). A prospective study of plasma prolactin concentrations and risk of premenopausal and postmenopausal breast cancer. Journal of Clinical Oncology 25, 1482–1488. [DOI] [PubMed] [Google Scholar]
- Wildgust HJ, Hodgson R, Beary M (2010). The paradox of premature mortality in schizophrenia: new research questions. Journal of Psychopharmacology 24, 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazawa K, Matsui H, Seki K, Sekiya S (2003). A case-control study of endometrial cancer after antipsychotics exposure in premenopausal women. Oncology 64, 116–123. [DOI] [PubMed] [Google Scholar]
- Yovel G, Sirota P, Mazeh D, Shakhar G, Rosenne E, Ben-Eliyahu S (2000). Higher natural killer cell activity in schizophrenic patients: the impact of serum factors, medication, and smoking. Brain, Behavior, and Immunity 14, 153–169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Please find the website (http://nhird.nhri.org.tw/en/) to request the information of database this study used, such as a point of contact, data protection, and content of data files. The database constitutes medical claim files representative of the entire population in Taiwan.