Abstract
Elicitation is a possible aid to overcome various difficulties associated with the large‐scale production of most commercially important bioactive secondary metabolites from wild and cultivated plants, undifferentiated or differentiated cultures. Secondary metabolite accumulation in vitro or their efflux in culture medium has been elicited in the undifferentiated or differentiated tissue cultures of several plant species by the application of a low concentration of biotic and abiotic elicitors in the last three decades. Hairy root cultures are preferred for the application of elicitation due to their genetic and biosynthetic stability, high growth rate in growth regulator‐free media, and production consistence in response to elicitor treatment. Elicitors act as signal, recognized by elicitor‐specific receptors on the plant cell membrane and stimulate defense responses during elicitation resulting in increased synthesis and accumulation of secondary metabolites. Optimization of various parameters, such as elicitor type, concentration, duration of exposure, and treatment schedule is essential for the effectiveness of the elicitation strategies. Combined application of different elicitors, integration of precursor feeding, or replenishment of medium or in situ product recovery from the roots/liquid medium with the elicitor treatment have showed improved accumulation of secondary metabolites due to their synergistic effect. This is a comprehensive review about the progress in the elicitation approach to hairy root cultures from 2010 to 2019 and the information provided is valuable and will be of interest for scientists working in this area of plant biotechnology.
Keywords: elicitors, fungal extract, hairy root cultures, secondary metabolites, signal molecules
Abbreviations
- AMHRC
Astragalus membranaceus hairy root culture
- ASA
acetyl salicylic acid
- CAG
Ca‐alginate gel
- CHI
chitosan
- HRC
hairy root cultures
- JA
jasmonic acid
- LG
leoligin
- MJ
methyl jasmonate
- MLG
5‐methoxy‐leoligin
- NP
nanoparticles
- PAL
phenylalanine ammonia lyase
- RA
rosmarinic acid
- RC
rhinacanthin
- SA
salicylic acid
- SM
secondary metabolite
- SNP
sodium nitroprusside
- TAT
tyrosine aminotransferase
- TIA
terpenoid indole alkaloid
- YE
yeast extract
- YPS
yeast polysaccharide
1. INTRODUCTION
Higher plants may be considered as a biochemical factory to produce both primary metabolites (e.g. carbohydrates, lipids and amino acids) and high‐value usable secondary metabolites (SMs) such as alkaloids, terpenes, glycosides, flavonoids, polyketides, volatile oils, quinones, coumarins, tannins, glucosinolates, cyanogenic glycosides, resins, etc. 1. Unlike primary metabolites, SMs include a wide variety of low molecular weight compounds which do not have a direct role in the maintenance of fundamental life processes but are required for plant environmental interaction such as survival, adaptation, and competitiveness. SMs provide defense against biotic factors such as herbivores, fungi, bacteria, viruses, etc. as well as physical factors like UV radiation, high and low temperature, drought, etc. 2. They also act as attracting and/or stimulating agents during pollination, seed dispersal, oviposition, pharmacophagy, symbiotic association of nitrogen fixing bacteria and mycorrhiza 2. SMs are generally synthesized at a very low concentration from common precursors as the products of primary metabolism at specific physiological and developmental stage of the plant 3. Plant‐derived SMs have huge commercial importance in pharmaceutical industries as one fourth of all prescribed pharmaceuticals contain compounds that are directly or indirectly derived from plants 3.
Availability, overexploitation, and difficulties in cultivation of the source plant, low productivity, phytogeographical and seasonal variation in productivity, tissue/organ‐specific production, difficulties in purification, variability of impurities, and economic cost involved in the selection and implementation of appropriate screening bioassays are the limiting factors for industrial production of these phytochemicals from field‐grown plants. Furthermore, chemical synthesis is often not economically feasible because of their highly complex structures and stereospecificity 4, 5. Different basic biotechnological approaches such as cell culture, callus culture, organ culture, and micropropagation of whole plants have been developed for more than three decades as an attractive alternative for the production of economically important valuable SMs from field‐grown plant materials. However, to date, their application to large‐scale production of the target SMs has led to very limited commercial success due to low productivity of the culture. Various biotechnological strategies such as screening and selection of high yielding line, optimization of culture media composition and physical parameters, precursor feeding, elicitation, large scale cultivation in bioreactor system, hairy root culture (HRC), metabolic engineering, plant cell immobilization, biotransformation, etc. have been assayed to evaluate their effectiveness towards enhancement of SM production utilizing in vitro plant cell culture and/or organ culture of different plant species 6.
PRACTICAL APPLICATION
Elicitation with various biotic and abiotic elicitors has been widely applied to enhance the secondary metabolite production in hairy root cultures as well as in plant cell cultures of different plant species. In addition to enhancing the accumulation of specific product yield on per unit mass of roots, the application of elicitor in culture medium often stimulates the efflux of intracellular products that makes the product recovery or the purification of a desired compound easier. In situ product removal strategy is more easily applied in hairy root cultures than cell suspension cultures because the roots are self‐immobilized and retained within the culture vessel which allows the withdrawal of spent liquid medium and the addition of fresh medium. Thus, elicitation of hairy root culture along with integration of other biotic strategies can be used as a feasible alternative route for the commercial production of low volume pharmaceutically important secondary metabolites.
Elicitation is one of the most effective and widely employed biotechnological tools for the induction of novel SMs or enhanced biosynthesis as well as accumulation of SMs in in vitro plant tissue cultures 7, 8. Elicitors are the biotic or abiotic molecules which belong to several classes of compounds, do not share a common chemical structure, and can induce or enhance the biosynthesis of specific SM 3, 7, 8. Various parameters, such as elicitor type, concentrations, duration of exposure, treatment schedule, culture type, cell line, medium composition, presence or absence of growth regulation, and age or stage of the culture at the time of elicitor treatment are the major factors that can determine the effectiveness of the elicitation strategies on biomass and SM production 2, 9, 10.
Elicitors act as signal and elicitation starts with the signal perception by elicitor‐specific receptors present on the plant cell membrane followed by initiation of signal transduction cascade and ultimately change the expression level of various regulatory transcription factors/genes and rate‐limiting genes of the secondary metabolic pathways resulting in increased synthesis and accumulation of SMs 8, 11, 12, 13 (Figure 1).
Figure 1.
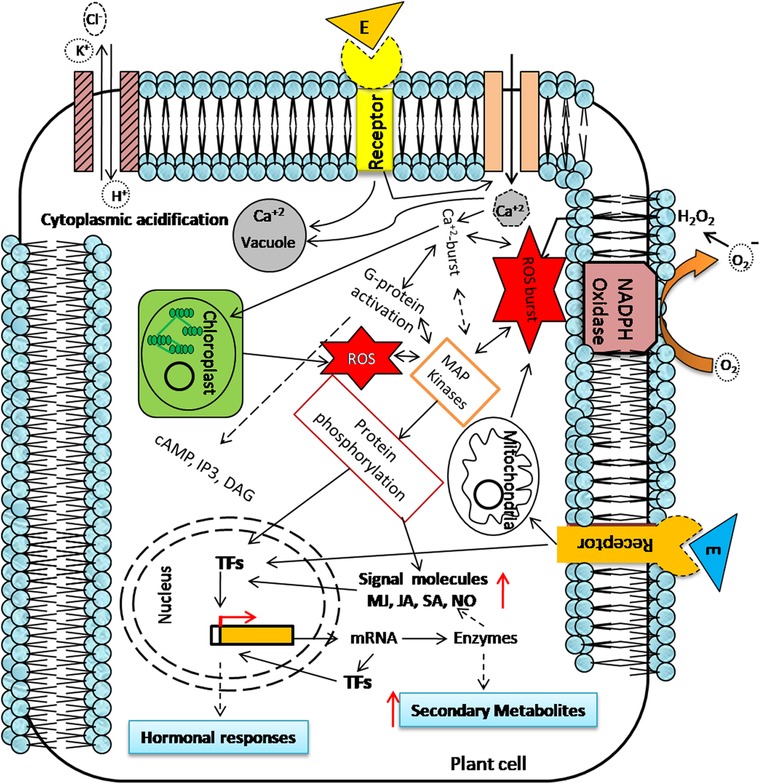
Schematic representation of mode of action of elicitor in plant cell
Several research studies showed that the enhanced production of the target SMs in HRCs as well as in cell suspension cultures was possible through the elicitation protocol that has opened a new avenue for the production of SMs by the pharmaceutical industry in near future 14, 15, 16, 17. Although treatment of undifferentiated cells with various elicitors enhanced production of SMs, it has several limitations such as low productivity compared to organ culture, genetic and biosynthetic instability in long term culture, irregular response to same elicitor, etc. 15, 17, 18. Additionally, in many species SMs are produced only in organ cultures, untransformed organ cultures are not favored due to slow growth and low biomass yield.
Transformed root cultures 6, 19 and shooty teratomas 19, 20 have been reported to accumulate high levels of SM characteristics of the parent plant. HRCs are preferred over undifferentiated cell suspension culture, callus culture, and untransformed root culture for application of elicitation due to their genetic and biosynthetic stability, high growth rate in growth regulator‐free media, equal or high biosynthetic capacity compared to the native mother culture, sizable biomass, and production consistence in response to elicitor treatment 6, 21, 22. Furthermore, HRCs often accumulate phytochemicals at a higher level than cell/callus cultures that contain undifferentiated cells 21, 22, 23, 24.
Till date, many research papers, reviews, books, and chapters have been published covering applications and future prospects of HRCs 14, 21, 22, 23, 24. This review provides an overview of various elicitation strategies applied on HRC of various plant species and their stimulating effects on enhancement of SMs from 2010–2019 and the information provided will be of interest for scientists working in this area of plant biotechnology.
2. ELICITORS AND THEIR EFFECTS ON HRCs
Elicitors are the chemically diverse group of biotic or abiotic signal which, when applied in low amounts to a living cell system, induce or enhance the biosynthesis of specific SM by induction of defense or stress‐induced responses 3, 7, 8, 25. Elicitors can be classified based on their ‘nature’ as abiotic elicitors or biotic elicitors or based on their ‘origin’ such as exogenous elicitors and endogenous elicitors 3 (Table 1). Biotic elicitors are either crude extracts or partially purified products derived from either pathogen (fungal, bacterial, yeast) or the plant itself. They are either of defined composition such as polysaccharides, glycoproteins, inactivated enzymes, purified chitosan (CHI), pectin, chitin, alginate, curdlan, xanthan, elicitin etc. or of complex composition such as yeast extract (YE) and fungal homogenate 11. Abiotic elicitors include various chemical and physical stressors such as light and UV‐radiation; salts of heavy metals (Ag2S2O3, AgNO3, CdCl2, CuCl2, CuSO4, VOSO4, NiSO4, selenium); temperature shift; osmotic stress induced by mannitol, sorbitol, sodium chloride, potassium chloride, cadmium chloride, PVP, etc.; intracellular signaling molecules such as jasmonic acid (JA), methyl jasmonate (MJ), salicylic acid (SA), acetyl salicylic acid (ASA), and systemin 8. Several researchers include intracellular signaling molecules and plant growth regulators in the group of biotic elicitors, whereas, others consider these molecules to be abiotic elicitors.
Table 1.
Basic classification of elicitors for the production of SMs
| Classification on based on the nature of elicitors |
| 1 Biotic elicitors: Either crude extracts or partially purified products derived from either pathogen (fungal, bacterial, yeast) or the plants itself. |
| 1.1 Defined composition: Composition was known. For example: purified polysaccharides, glycoproteins, glucans, chitin, chitosan, pectin, alginate, xanthan, elicitin, inactivated enzymes, etc. |
| 1.2 Non‐defined composition: Composition was unknown. For Example: crude extract of yeast, fungal homogenate, bacterial extract, etc. |
| 2 Abiotic elicitors |
| 2.1 Chemical stressors: |
| 2.1.1 Salts of heavy metals: Ag2S2O3, AgNO3, CdCl2, CuCl2, CuSO4, VOSO4, NiSO4, selenium, etc. |
| 2.1.2 Osmotic stressors: Mannitol, sorbitol, sodium chloride, potassium chloride, cadmium chloride, polyvinyl pyrrolidone, etc. |
| 2.1.3 Gaseous substances: NO, ethylene etc. |
| 2.2 Physical stressors: Light and UV‐radiation, temperature shift, salinity, drought, etc. |
| 2.3 Intracellular signaling molecules: Jasmonic acid, methyl jasmonate, salicylic acid, acetyl salicylic acid, systemin, etc. |
| Classification based on the origin of elicitors |
| 1 Exogenous elicitors: Originated outside the cell. For example: glucomannose, glucans, chitosan, monilicolin, polyamines, glycoproteins, polygalacturonase, endopolygalacturonic, acid lyase, cellulose, arachidonic acid, eicosapentanoic acid, etc. |
| 2 Endogenous elicitors: Jasmonic acid, methyl jasmonate, salicylic acid, acetyl salicylic acid, systemin, etc. |
Exogenous elicitors are chemicals originating from the microbial pathogen or the plant, such as peptides, polysaccharides, polyamines, fatty acids, and glycoproteins, whereas endogenous elicitors are chemicals within the cell of the host plant that play important role in the intracellular signal transduction system that includes pectic oligosaccharides released from plant cell wall and the intracellular signal compounds such as SA, MJ, JA, systemin, etc. 3. The schematic representation of the protocol for hairy root induction following infection with Agrobacterium rhizogenes and elicitation treatment of HRCs in liquid nutrient medium is shown in Figure 2.
Figure 2.
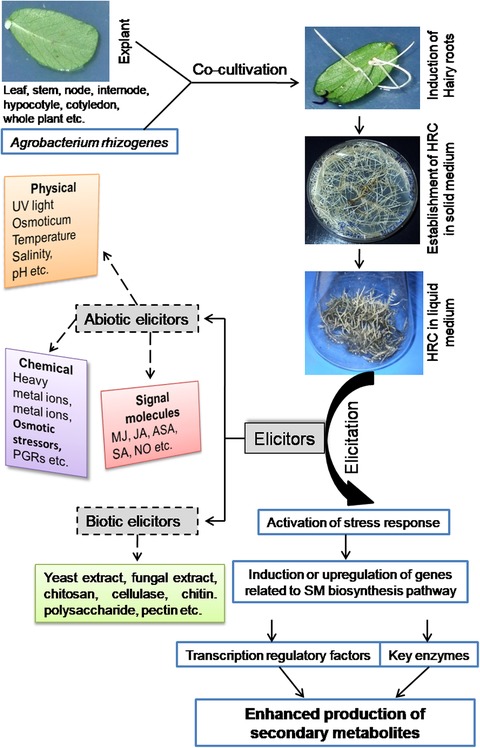
Schematic representation of hairy root induction from different explants and elicitation treatment of HRC in liquid nutrient medium
2.1. Effect of biotic elicitors on SM production
The growth and cryptotanshinone (a diterpene quinone) content in the HRCs of Salvia castanea Diels f. tomentosa were dramatically enhanced (2.84 mg g−1 DW) compared to untreated control when the medium was supplemented with an optimum concentration (200 mg L−1) of YE 26. The positive co‐relationship between accumulation of tanshinone (a diterpene) and expression of selected genes of the tanshinone biosynthetic pathway, such as 3‐hydroxy‐3‐methylglutaryl‐CoA reductase (HMGR), 1‐deoxy‐D‐xylulose‐5‐phosphate reductoisomerase (DXR), isopentenyl diphosphate isomerase (IPPI), and geranylgeranyl diphosphate synthase (GGPPS) in HRC during YE elicitation was also elucidated 26. Elicitation enhanced 13.9‐ and 16.7‐fold expression of isopentenyl diphosphate isomerase and geranylgeranyl diphosphate synthase genes at 12 and 24 h, respectively compared with that of the control 26.
The effect of abiotic and biotic elicitors (MJ, CHI, SA, Agrobacterium, and YE) at various concentrations on total isoflavonoid accumulation was studied in the HRCs of Pueraria candollei 27, 28. YE (0.5 mg mL−1) was the most effective elicitor which induced 4.5‐fold higher level of total isoflavonoid (60 ± 1 mg g−1 DW) on day 3 of elicitation, followed by CHI at 150 mg L−1 (34.5 ± 2 mg g−1 DW) and SA at 200 µM (20 ± 3.5 mg g−1 DW) at day 6 27. Similarly, HRCs of Scutellaria lateriflora, when treated with 50 µg mL−1 YE enhanced 1.4‐fold production of acteoside (a phenylethanoid glycoside) and 1.7‐fold flavone production after 7 and 14 days of elicitation 29. The elicitation treatment with exogenous YE was most effective in hairy roots of P. candollei var. mirifica for production of deoxymiroestrol (a phytoestrogen) and isoflavonoids, and the degree of SM production was found to be concentration dependent 30. On the other hand, hairy roots treated with 200 mg L−1 CHI yielded 1.68‐fold higher deoxymiroestrol (121 ± 5.75 µg g−1 DW) after 6 days whereas highest accumulation of each of isoflavonoids occurred after 3 days 30.
According to Zhao et al. 31, elicitation with yeast polysaccharide (YPS) effectively increased hairy root growth and flavonoids (rutin and quercetin) production of Fagopyrum tataricum in a concentration dependent manner by the stimulation of the phenylpropanoid pathway. Addition of YPS at 200 mg L−1 concentration resulted in 2.1‐fold enhancement of total rutin and quercentin content 31.
The extract of mycelium and the polysaccharide fraction derived from an endophytic root fungus Trichoderma atroviride D16 was used as biotic elicitor in hairy roots of Salvia miltiorrhiza. Polysaccharide fraction was found to be more active for promoting hairy root growth (increased ∼60% than the control) and stimulating biosynthesis of tanshinone (a diterpene) by influencing the expression of genes related to the SM biosynthetic pathway 32.
Mechanical wounding and treatment with chemical elicitors such as MJ, YE, and CHI promoted cardenolide (cardiac glycosides) production in HRCs of Calotropis gigantean 33. However, among the elicitors, CHI at 50 mg L−1 was more effective in enhancing cardenolide yield (1050 mg L−1) which was 2.7‐fold higher than the control 33. A recent study demonstrated that CHI at 150 mg L−1 could enhance total flavonoid productivity by 7.08‐fold (16.35 ± 0.88 mg g−1 DW) in 24‐day‐old HRCs of Isatis tinctoria after 36 h of elicitation 34. Accumulation of eight flavonoids, i.e. rutin, neohesperidin, buddleoside, liquiritigenin, quercetin, isorhamnetin, kaempferol, and isoliquiritigenin in CHI‐elicited hairy roots was elevated by 8.27‐, 4.19‐, 1.30‐, 4.88‐, 13.05‐, 7.63‐, 2.12‐, and 8.52‐fold, respectively, due to up‐regulation of chalcone synthase and flavonoid 3′‐hydroxylase genes of the flavonoid biosynthetic pathway 34. On the other hand, while CHI (100 mg L−1) showed a promotive effect on the growth of hairy roots of Agastache foeniculum, the supplementation of medium with SA (1 mM) adversely affected the growth of hairy roots compared with control roots 35.
Zhao et al. 36 demonstrated that addition of polysaccharide‐protein fractions (100 mg L−1) of a plant growth‐promoting rhizobacterium Bacillus cereus in culture medium few days before the stationary growth phase of S. miltiorrhiza hairy roots stimulated ∼seven‐fold accumulation of tanshinone (1.59 mg g−1) with an increase in biomass accumulation (13.6 g L−1) as compared to untreated control (0.19 mg g−1 and 11.3 g L−1, respectively) 36. This elicitation enhanced 10‐fold volumetric tanshinone yield in the hairy roots in comparison with the control HRCs (21.6 vs 2.11 mg L−1).
Different concentrations (1, 3, and 5%) of cell homogenates of root endophytic fungus Piriformospora indica (cell homogenate of Piriformospora indica [CHP]), used as biofertilizer, bioregulator, and bioprotector against stress conditions, was used as biotic elicitor in HRC of Withania somnifera for varying time periods 37. Treatment of W. somnifera HRCs for 48 h with 3% CHP enhanced biomass as well as withanolides viz., withanolide A, withaferin A, withanoside IV, and withanoside V production (1.15‐, 2.7‐, 2.5‐, 2.34‐, and 2.9‐fold, respectively) as compared to untreated hairy roots. Further, gene expression studies revealed that the application of 3% CHP for 48 h upregulated the expression of HMGR (3.2‐fold), farnesyl pyrophosphate synthase (FPPS) (3.55‐fold), squalene synthase (SS) (2.87‐fold), squalene epoxidase (SE) (3.25‐fold), cycloartenol synthase (CAS) (3.08‐fold), obtusifoliol‐14‐demethylase (4.42‐fold), sterol methyltransferase 1 (4.81‐fold), and sterol‐22‐desaturase (5.02‐fold) genes than in untreated hairy roots 37.
The influence of different concentrations of elicitors, viz. MJ, fungal elicitors‐ Alternaria alternate, Curvularia limata, Fusarium solani, and P. indica, farnesyl pyrophosphate (an inhibitor) and miconazole (a precursor) on artemisinin (a sesquiterpene lactone) accumulation in Artemisia annua HRCs was investigated by Ahlawat et al. 38. Treatment with P. indica was found to be the most effective elicitor that enhanced artemisinin production by 1.97 times 38. In another assay, 20‐day‐old hairy roots of A. annua showed stimulation in artemisinin content 0.7 mg g−1 DW to 1.3 mg g−1 DW when treated with oligosaccharide isolated from Fusarium oxysporum mycelium (at 0.3 mg total sugar mL−1) for 4 days, whereas treatment with 50 µM sodium nitroprusside (SNP), a NO donor had no effect on artemisinin synthesis 39.
Piriformospora indica cell wall was used as the biotic elicitor to evaluate the level of phenylpropanoid derivatives in hairy roots of Linum album 40. This fungal elicitor improved the content of lignin, lignans (lariciresinol, podophyllotoxin, and 6‐methoxy podophyllotoxin), phenolic acids (cinnamic acid, ferulic acid, and salicylic acid), flavonoids (myricetin, kaempferol, and diosmin) depending upon the exposure time by activating phenylalanine ammonia lyase (PAL), cinnamyl alcohol dehydrogenase (CAD), cinnamoyl‐CoA reductase (CCR), and pinoresinol‐lariciresinol reductase (PLR) genes of phenylpropanoid pathway 40. Furthermore, a shift from amino acid metabolism to phenylpropanoid pathway was noticed in response to treatment with P. indica cell wall in L. album hairy roots 40.
Application of biotic elicitors, Staphylococcus aureus and Bacillus cereus in HRCs of Datura metel increased root biomass accumulation, but reduced accumulation of the tropane alkaloid, atropine 41. Addition of filter‐sterilized fungal culture filtrate of Curvularia lunata (1% v/v) showed a most stimulatory effect on accumulation of azadirachtin (a complex tetranortriterpenoid limonoid) in HRCs of Azadirachta indica 42 with a yield of 7.1 mg g−1as compared to the untreated control (3.3 mg g−1).
The effect of various elicitors, namely, YE (1, 2, and 3 g L−1), SA (50, 100, 200, and 500 µM), and pectin (0.5, 1, 1.5, and 2%) on the glycoalkaloid solasodine production in HRCs of Solanum melongena was evaluated 43. The result demonstrated that among all the elicitors tested, maximum solasodine production (151.23 µg g−1 DW) was achieved by treatment with 1% pectin, which was 23‐fold higher than untreated HR control (6.5 µg g−1 DW) and 88‐fold over plants in the field (1.71 µg g−1 DW) 43. In hairy roots of Solanum khasianum, solasodine content was enhanced maximum four‐fold after 6 days of 100 mM NaCl treatment, whereas 1.6‐fold increased α‐solanine content increased 1.6‐fold after 24 h of treatment with 100 µg mL−1 cellulase 44. The investigators also studied the effect of biotic and abiotic elicitors on the production of important SMs in HRCs of Rauwolfia serpentine. Treatment with 100 mM NaCl and 100 mg L−1 mannan enhanced terpenoid alkaloids ajmalicine and ajmaline production up to 14.8‐fold and 2.9‐fold, respectively in HRCs of R. serpentina after 1 week of treatment 44.
The accumulation of wogonin, a dihydroxy‐ and monomethoxy‐flavone, in hairy roots of S. lateriflora was enhanced ∼three‐fold (30 mg g−1DW) by the application of Pectobacterium carotovorum lysate in the stationary phase of the HRC 29.
The effects of abiotic (SA, JA, and MJ) and biotic (CHI and YE) elicitors on the growth accumulation of xanthones, a commonly occurring group of SMs in Gentianaceae, were studied in two hairy root clones of Gentiana dinarica 45 and new xanthone compounds were detected in hairy roots treated with biotic elicitors. The obtained results showed clone specific effect to elicitor treatment and production of dominant xanthone norswertianin‐1‐O‐primeveroside was not significantly affected by either of SA, JA, and MJ, but was stimulated with CHI treatment (50 mg L− 1) which enhanced 24‐fold norswertianin content in comparison to control 45.
Recently a new approach of using fungal elicitor was developed by immobilizing the fungus in Ca‐alginate gel (CAG) and the study was carried out to evaluate the effect on production of astragalosides (AGs, a class of cycloartane‐type triterpene saponin). A co‐cultivation system of Astragalus membranaceus hairy root cultures (AMHRCs) with CAG facilitated immobilized endophytic fungus immobilized penicillium canescens (IPC) showed enhanced production of AG IV (1.585 ± 0.0106 mg g−1 DW) as compared to both control AMHRCs (0.187 ± 0.014 mg g−1 DW) and CAG‐treated AMHRCs (0.196 ± 0.009 mg g−1 DW) 46. However, the significant decrease in content of acetylated precursors (AG I, AG II, IAG II) of AG IV in IPC‐treated AMHRCs compared to untreated AMHRCs suggested that deacetylation of acetylated precursors may be responsible for the enhancement of AG IV content 46.
2.2. Abiotic elicitors
2.2.1. Effect of UV radiation on SM Production
UV radiation (UV‐A, UV‐B, and UV‐C) has been used as an abiotic elicitor in HRC of several species to enhance different groups of SMs 47, 48, 49, 50, 51, 52. Like other elicitors, dose, duration, plant species, etc. play a very vital role in achieving the goal.
UV‐A, UV‐B, and UV‐C radiation was used to enhance production of isoflavones and AGs in AMHRCs 48, 49. Application of optimal dose of UV‐A (32.4 kJ m−2), UV‐B (54 kJ m−2), and UV‐C (59.4 kJ m−2) radiation revealed 1.13‐, 1.30‐ and 1.21‐fold higher AG yield than in the non‐treated control (0 kJ m−2), respectively 49. Thus UV‐B elicitation is a feasible strategy to promote production of AG in AMHRCs that enhanced 1.30‐fold higher accumulation of total AGs (i.e. 3.43 mg g−1 DW) compared to the non‐treated control (2.64 mg g−1 DW) by the significant up‐regulation of HMGR gene expression among the tested eleven genes of the AG biosynthetic pathway 49.
Using UV radiation (UV‐A, UV‐B, and UV‐C) elicitation strategy, it was clearly demonstrated that the application of optimum dose of UV‐B (86.4 kJ m−2) radiation in 34‐day‐old AMHRCs was most effective in enhancing isoflavonoid yield (2.29‐fold, 533.54 µg g−1 DW) relative to control (232.93 µg g−1 DW) by up‐regulation of the transcriptional expressions of all investigated genes of the isoflavonoid biosynthetic pathway, specially PAL and C4H genes 48.
The elicitation effect of UV‐B radiation was also reported in HRC of Fagopyrum tataricum where the treatment resulted in a dramatic increase in flavonoid accumulation, for example, 4.82 mg g−1 DW for rutin and 0.04 mg g−1 DW for quercetin compared to 0.93 and 0.02 mg g−1 DW for rutin and quercetin, respectively in untreated control. Also, striking changes in gene expression of flavonoid biosynthetic pathway genes such as FtpAL, FtCHI, FtCHS, FtF3H, and FtFLS‐1 were obtained 50.
The biological effect of UV‐B on Anisodus luridus hairy roots was investigated based on gene expression (PMT, TRI, CYP80F1, and H6H), tropane alkaloids (scopolamine and hyoscyamine) biosynthesis and efflux in liquid medium 47. In UV‐B treated HRCs scopolamine accumulation increased, though there was significant enhancement in expression of all the four genes 47. UV‐B treatment did not affect efflux of TA in the liquid medium 47. Similar elicitation effect of UV‐B (40 µW cm−2) on the accumulation of a diterpene, tanshinone, was reported in S. miltiorrhiza HRCs 51.
2.2.2. Effect of heavy metal ions on SM production
Heavy metals such as nickel, selenium, and iron act as cofactor for many metallo‐enzymes, thus they are the essential trace elements required for plant growth and development 2. In plant tissue culture, heavy metals have tremendous potential to stimulate the production and accumulation of valuable SMs 52.
Ag+ is one of the most effective abiotic heavy metal elicitors for stimulating production of different SMs. Application of 15 µM Ag+ in the HRCs of S. castanea Diels f. tomentosa increased 1.8‐fold tanshinone IIA (a diterpenoid) content compared to untreated control by stimulation of gene expression of the tanshinone biosynthesis pathway 26. Similarly, application of Ag+ at 15 µM concentration dramatically enhanced lithospermic acid B, a dimer of rosmarinic acid (RA), from approx. 5.4% to 18.8% of DW in S. miltiorrhiza HRCs, and the rise in lithospermic acid B was found to be coincident with the decline of RA content at each time point after treatment 53. Hairy roots of Anisodus acutangulus showed up to 1.13 times improved accumulation of tropane alkaloids after 24 h treatment by increased expression of putrescine N‐methyltransferase I when elicited with Ag+ 54.
Elicitation with 2 mM of Ag+ increased 4.33‐fold lipoxigenase activity in treated hairy roots of Silybum marianum harvested 72 h after elicitation which stimulated signal transduction pathway resulting in two‐fold (0.56 mg g−1 DW) enhanced production of silymarin, a mixture flavonolignans, with slightly decreased DW in treated hairy roots compared to non‐treated hairy roots 55. The highest silymarin production (1.2 mg g−1 DW) that included silybin, isosilybin, taxifolin, silycristin, and silydianin at 0.069, 0.031, 0.688, 0.388, and 0.024 mg g−1 DW, respectively was obtained in HRCs treated with Ag+ for 96 h 55.
The effect of silver nitrate, YE and MJ was studied in Leontopodium nivale ssp. alpinum hairy root lines. Silver nitrate (15 µM) elicitation caused ∼five‐fold enhanced production of both leoligin (LG) and 5‐methoxy‐leoligin (MLG) with 30% reduction in biomass 56, while YE (2 g L−1) stimulated ∼five‐fold LG and four‐fold MLG production without any significant change in biomass accumulation, whereas eight‐fold higher LG and 3.8‐fold higher MLG accumulation were obtained by elicitation with 50 or 100 µM MJ 56.
Nanosilver was the most effective elicitor in HRCs of D. metel among the four elicitors, namely, S. aureus, B. cereus, AgNO3, and nanosilver that have been tested 41. Application of nanosilver enhanced both root biomass accumulation and yield of atropine (a tropane alkaloid), whereas other three elicitors promoted only root biomass accumulation 41. The highest hairy root biomass (237.23 mg DW) and atropine production (maximum 2.42‐fold in comparison to the control) was achieved by the application nanosilver 41.
The effectiveness of Ag‐SiO2 core‐shell nanosilver particles (AgNPs with an average size of 101.8 ± 8.9 nm) as a novel effective elicitor in plant biotechnology for stimulation of production of artemisinin (a sesquiterpene lactone) in HRCs of Artemisia annua was reported 57. Application of AgNPs at 900 mg L−1 for 3 days increased artemisinin content from 1.67 to 2.86 mg g−1 DW, induced oxidative stress, malonyldialdehyde accumulation, and enhanced activities of catalase in hairy roots 57, while 20‐day‐old elicited HRCs exhibited a 3.9‐fold increased artemisinin production (up to 13.3 mg L−1) in comparison to control 57.
Different concentrations of iron oxide nanoparticles (FeNPs) were used to study their effect on the accumulation of tropane alkaloids hyoscyamine and scopolamine in HRCs of Hyoscyamus reticulates 58. Elicitation with 900 and 450 mg L−1FeNPs for 24 and 48 h resulted in the accumulation of 5‐fold higher scopolamine in FeNP‐treated hairy roots than control, probably due to availability of sufficient Fe2+ required for the enzyme hyoscyamine‐6‐hydroxylase in catalyzing the conversion of hyoscyamine to scopolamine through hydroxylation 58.
Copper oxide nanoparticles (CuO NPs) act as novel elicitor. The level of ten glucosinolates (gluconasturtiin, glucobrassicin, 4‐methoxyglucobrassicin, neoglucobrassicin, 4‐hydroxyglucobrassicin, glucoallysin, glucobrassicanapin, sinigrin, progoitrin and gluconapin) significantly increased in CuO NPs elicited hairy roots of Brassica rapa spp. pekinensis compared to non‐elicited HRs due to higher expression of MYB34, MYB122, MYB28 and MYB29 that regulate genes encoding enzymes in glucosinolate biosynthesis pathway 59. Moreover, increased level of phenolic compounds (flavonols, hydroxybenzoic and hydroxycinnamic acids), total phenolic and flavonoid content were obtained in hairy roots treated with CuO NPs due to up regulation of phenolic biosynthetic genes such as PAL, CHI and FLS 59.
2.2.3. Effect of osmotic stress and other chemicals on SM production
Hairy roots of Valeriana officinalis, established using a mikimopine type strain of Agrobacterium rhizogenes ‘A13’ were used to investigate the effects of magnesium (Mg) and calcium (Ca) as abiotic elicitors for production of pharmacologically active sesquiterpene‐valerenic acid 60. When 28‐day old hairy roots were exposed to two‐ to six‐fold higher concentration of Mg and Ca than present in MS medium for 3 and 7 days, valerenic acid content increased to 1.83 ± 0.06 mg g−1 DW, which was 7.9 times higher as compared to control HRCs 60.
Two chemical elicitors, potassium chloride (KCl) and calcium chloride (CaCl2) were used to evaluate the effect of salt stress on accumulation of the tropane alkaloid, hyoscyamine in selected hairy root lines of Datura stramonium (LDS), D. innoxia (LDI), and D. tatula (LDT) 61. The concentrations of the elicitor and duration of exposure had a significant effect on hyoscyamine accumulation. Treatment with 1–2 g L−1 CaCl2 resulted in maximum hyoscyamine content 16.978 mg g−1 DW for LDT line, 10.828 mg g−1 DW for LDS line, and 12.697 mg g−1 DW for LDI line, i.e. an increase of 2.07‐, 2.08‐, and 1.85‐fold compared to the control, respectively. Similarly, the elicitation with KCl (2 g L−1) showed enhanced production of the hyoscyamine in the HRCs of the three species of Datura, i.e. 12.074 mg g−1 DW for LDS line (2.32‐fold), 16.978 mg g−1 DW for LDT (1.99‐fold), and 12.651 mg g−1 DW (1.85‐fold) 61.
Using hairy roots of Anisodus acutangulus, it was demonstrated that ethanol is the most effective among the tested chemicals as it improved the accumulation (1.51‐fold) of TAs after 24 h treatment by the up‐regulation of hyoscyamine‐6b‐hydroxylase 54.
Addition of five stress factors, namely, mannitol, sodium chloride, potassium chloride, cadmium chloride, and PVP (polyvinyl pyrrolidone) K‐30 in the statistically optimized medium for in vitro hairy root cultivation of Catharanthus roseus revealed that there was an increase of 182% (2.53 mg g−1 DW) and 227% (4.09 mg g−1 DW) ajmalicine (terpenoid indole alkaloid) accumulation by addition of PVP and KCl, respectively, than the untreated control 62. Furthermore, it was also reported that the maximum secretion of ajmalcine (5.4 mg L−1) in the culture medium was achieved by supplementation of medium with mannitol followed by PVP (2.192 mg L−1), NaCl (2.02 mg L−1), and cadmium chloride (1.74 mg L−1) compared to 1.32 mg L−1 ajmalcine incontrol HRCs 62.
Sodium acetate (10.2 mM) mediated elicitation in HRCs of Arachis hypogaea cv. Hull showed that the production of resveratrol, arachidin‐1, and arachidin‐3 (stilbenoids) was highly depended on the growth phase of the culture 63. Optimum elicitation was achieved by sodium acetate treatment during exponential growth of hairy roots on day 12 that resulted in ∼99‐fold enhanced accumulation of resveratrol. Interestingly, when 9‐day‐old HRC was elicited with sodium acetate, over 90% of the total resveratrol, arachidin‐1 and arachidin‐3 were secreted out and accumulated in the culture medium 63.
The effects of the three phytohormones namely, abscisic acid, gibberellin, and ethylene on production of phenolic acids (mainly caffeic acid, rosmarinic acid, and salvianolic acid B) in S. miltiorrhiza hairy roots were reported 64. The results showed that abscisic acid, gibberellin, and ethylene were all effective to induce production of phenolic acids and enhance the activities of PAL and tyrosine aminotransferase (TAT) in S. miltiorrhiza hairy roots 64.
2.2.4. Effect of signal molecules on SM production
JA, MJ, SA, and ASA have been recognized as another class of signal transducer in elicitation of plant defense responses 30, 42, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74. Application of MJ, JA, SA, ASA, and other signal molecules may or may not inhibit growth and thus effect biomass accumulation during the enhancement of SMs production or vice‐versa 8, 42.
Concentration‐dependent (2.0, 5.0, 10 and 15 µM for MJ and 10, 50, 100 and 150 µM for SA) decrease in biomass accumulation and increase in content of a group of naphthoquinone esters known as rhinacanthin (RC‐C, RC‐D and RC‐N) was observed in hairy roots of Rhinacanthus nasutus, harvested 7 days after elicitation with MJ and SA elicitors as compared to control in MS medium 65. Effectiveness of MJ to induce RC accumulation in hairy roots was higher than SA and the highest RC content (6.3 mg g−1 DW/1.7‐fold for RC‐C; 1.1 mg g−1 DW/2.5‐ fold for RC‐D; and 0.61 mg g−1 DW/3.5‐fold for RC‐N) was observed after treatment with 10 µM MJ 65.
Elicitation with MJ (50 µM) dramatically stimulated production of triptolide‐ a diterpenoid epoxide (maximum yield of 1448.43 µg per flask which was two‐fold higher than the untreated control) and wilforine, a sesquiterpene pyridine alkaloid (maximum yield of 3851.42 µg per flask which was 6.7‐fold higher than untreated control) with slight reduction in hairy root growth of Tripterygium wilfordii whereas 50 µM SA treatment had no apparent effect on hairy root growth with less stimulatory effects on the production of both the SMs 66. The majority of triptolide produced was secreted into the medium, whereas wilforine accumulated was mostly retained within the root tissue.
The effects of MJ and SA elicitation at different time points (6, 12, 24, and 48 h) on accumulation of morphinan alkaloids (thebaine, morphine, and codeine) and the relative expression level of six main regulatory genes of the morphinan pathway (COR, SalAT, SalR, T6ODM, CODM and Salsyn) was assessed in HRCs of Papaver orientale 69. The investigators found that the accumulation of morphinan alkaloids under elicitation through MJ and/or SA were directly related to the expression levels of key genes in the morphinan pathway at different time points. Application of MJ increased accumulation of 2.63‐fold thebaine (3.08 mg g−1), 6.18‐fold morphine (5.38 mg g−1) at 48 h, and 3.67‐fold codeine (2.57 mg g−1) at 24 h compared to control, which was directly correlated with the up‐regulation of Salsyn, SalR, SalAT, and CODM key genes to 27.79, 7.26, 6.85, and 3.42 times, respectively, in comparison with untreated control 69. However, SA treatment for 48 h increased accumulation of morphine (4.22‐fold/2.87 mg g−1) and thebaine (two‐fold/1.66 mg g−1) in comparison with control by the up‐regulation of Salsyn, T6ODM, and CODM genes 69.
The accumulation of hyoscyamine, a tropane alkaloid, was increased up to 1.42‐fold (11.58 ± 0.17 mg g−1 DW for LDS root lines), 2.09‐fold (17.94 ± 0.14 mg g−1 DW for LDT root lines), and 2.82‐fold (8.89 ± 0.29 mg g−1 DW for LDI root lines) in response to 0.1 mM ASA, whereas 0.1 mM SA significantly enhanced hyoscyamine content by 1.3‐fold (10.58 ± 0.43 mg g−1 DW for LDS root lines), 1.96‐fold (16.78 ± 0.21 mg g−1 DW for LDT root lines), and 2.1‐fold (6.61 ± 0.09 mg g−1 DW for LDI root lines) compared to untreated control roots 75. Such improved production of hyoscyamine in plant cells after elicitation is probably due to micro‐environmental changes in response to SA 75.
Elicitation of HRC of Catharanthus roseus with 250 µM MJ during mid‐exponential growth showed 150–370% increase of terpenoid indole alkaloid (TIA) as compared to untreated control 78. Moreover, the dose dependent stimulation of TIA production by MJ treatment correlated with 29–40‐fold, 8–15‐fold, and 2–7‐fold increased expression of the transcriptional activators (Orca), genes coding for key enzymes involved in TIA biosynthesis (G10h, Tdc, Str, and Sgd) and transcriptional repressors (Zct), respectively that indicated stimulation of TIA accumulation may be partly controlled through the relative levels of Orca and Zct family transcription factors 78.
Elicitation of Solanum trilobatum hairy roots (clone ST‐09) with 4 µM MJ for 2 weeks increased production of steroidal alkaloid solasodine (9.33 ± 0.04 mg g−1 DW) which was enhanced 1.9‐ and 6.5‐fold as compared to non‐treated HRCs and non‐transformed roots, respectively, by upregulation of hmgr (HMGCoA reductase) gene 67. The total phenolics (150.42 mg g−1 DE), total flavonoids (521.09 mg g−1 DE), and radical scavenging activity (83.3%) also significantly enhanced in 4 µM MJ treated roots 67.
To screen the best elicitor molecule for enhancing isoflavonoid production in AMHRCs, the effects of MJ, SA, and ASA was investigated 68. Optimal enhancement of isoflavonoid production was observed when 34‐day‐old AMHRCs was treated with 283 µM MJ for 33.75 h. Effective and feasible treatment of MJ promoted total isoflavonoid yield (2250.10 µg g−1 DW/9.71‐fold) compared to non‐treated control (231.64 µg g−1 DW) 68. Under the optimal elicitation conditions, MJ‐treated AMHRCs yielded 5.80‐ and 27.95‐fold higher contents of calycosin and formononetin respectively, compared to those in non‐treated control 68. Moreover, transcription of the eight isoflavonoid biosynthetic genes were significantly up‐regulated (43.02‐fold for CHI, 23.11‐fold for IFS, and 7.67‐fold for I3’H higher relative to control at 33.75 h and 7.74‐fold for PAL, 5.95‐fold for C4H, 16.84‐fold for 4CL, and 6.67‐fold for CHS as compared to control at 12 h) during the elicitation period suggesting some important preliminary molecular mechanism underlying isoflavonoid enhancement in response to MJ elicitation 68.
Similarly, the effect of MJ and SA on root biomass accumulation and production of iridoids (catalpol, harpagide, catalposide) and phenylethanoids (verbascoside and isoverbascoside) were evaluated in HRCs of Rehmannia glutinosa 70. Content of the iridoids and phenylethanoids was substantially enhanced in response to an optimal concentration (200 µM) and exposure time (72 h) of MJ in the 23‐day‐old HRCs of R. glutinosa 70. Accumulation of verbascoside and isoverbascoside increased up to 10‐fold (60.07 mg g−1 DW) and 6.4‐fold (1.77 mg g−1 DW), respectively than in the non‐treated control roots 70. Exposure to 150 µM MJ provided optimal harpagide content after 72 h (0.136 mg·DW−1; 7.5‐fold increase compared to the control) and catalpol content after 120 h (up to 2.145 mg·DW−1). SA alone was less effective in the enhancement of iridoids and phenylethanoids production than MJ, which might be due to differences in their mode of action 70.
HRCs of Prunella vulgaris established by Agrobacterium rhizogenes strain ATCC15834 produced 15–30 times higher RA, which is an ester of caffeic acid, than in the intact plant 71. Further enhancement of RA accumulation in HRC of P. vulgaris was achieved by optimizing the elicitation protocol. Maximum accumulation of RA, i.e. 1.66‐fold and 1.48‐fold was obtained in 8 days after ethephon (200 µg L−1) elicitation and in 2 days post SA (6.9 mg L−1) treatment, respectively 71. Up‐regulation of the expression of TAT and PAL correlated with the elicitation effect of ethephon, whereas elicitation caused by application of SA involved higher expression of TAT, 4‐hydroxyphenylpyruvate reductase, PAL, 4‐coumaric acid CoA‐ligase 1 (4CL1), and cytochrome P450‐dependent monooxygenase (CYP) genes 71.
Elicitation of 14 day‐old hairy roots of Rubia tinctorum cultured in optimized Gamborg B51/2 medium was performed with optimal concentration of MJ (100 µM) resulting in a massive accumulation of intracellular (up to 2.4‐fold increase) and extracellular anthraquinones (up to 8.1‐fold increase) with no significant difference in biomass production compared to control at 4 days post‐elicitation 73. HRC of S. miltiorrhiza when treated with 100 µM MJ increased total tanshinone content to 1.5 mg g−1 DW 51.
In HRC of Panax quinquefolium, the yield of ginsenosides (glycosylated triterpene) was maximum in shake flask (27.33 mg g−1 DW) and in bioreactor (51.0 mg g−1 DW) in modified Gamborg B5 medium elicited with 250 µM L−1 MJ after 7 days 74. This elevation could be correlated with higher activation of the squalene synthase (SSq) gene of ginsenoside biosynthetic pathway in response to MJ elicitation 74.
Addition of MJ or sodium acetate to half‐strength MS medium was reported to increase resveratrol (stilbenoid) content by ∼1.5‐ to two‐fold in the hairy root tissue of Vitis vinifera subsp. sylvesteris as well as to enhance release of resveratrol into the culture media compared to non‐treated hairy roots 72. Similar elicitation effect of MJ was observed in HRC of A. annua, where MJ treated HRCs showed enhanced artemisinin (a sesquiterpene lactone) content (10.33 mg l−1) than in control (4.63 mg L−1) HRCs. 76.
Putalun et al. 80 clearly demonstrated that the application of elicitors such as CHI, MJ, and YE enhanced the production of glycyrrhizin (saponin‐like compound) in hairy roots of Glycyrrhiza inflata. Among the tested elicitors, supplementation of MJ (100 µM) enhanced glycyrrhizin production up to 108.9 ± 1.15 µg g−1 DW on day 5 of elicitation 80. Sajjalaguddam and Paladugu 81 suggested that MJ is an effective elicitor for the enhancement of glycyrrhizin in hairy roots cultures of Abrus precatorius, as MJ (100 µM) treatment resulted 2.5 times higher glycyrrhizin production in hairy roots. Highest glycyrrhizin content, 80.01 and 72.01 µg g−1 DW was reported after 4 days in hairy roots established by A. rhizogenes strain MTCC 532 and strain MTCC 2364, respectively 81.
Accumulation of deoxymiroestrol (phytoestrogen) was 3.4‐fold higher (245 ± 12.0 µg g−1 DW), whereas the content of six isoflavonoids i.e. puerarin, daidzin, genistin, daidzein, genistein and kwakhurin increased 2.6‐, 2.5‐, 2.2‐, 1.7‐, 4.1‐ and 7.7‐fold, respectively in 21‐day old hairy roots of Pueraria candollei var. mirifica after 6 days of elicitation with 200 µM MJ 30.
The effects of different concentrations of MJ (100,150, 200 mM) and SA (125, 250 and 500 mM) on the production of dopamine in HRCs of Portulaca oleracea cultured for 4 weeks in 250 ml shake flasks containing ½MS liquid medium revealed that 100 mM MJ treatment was most effective in enhancing 4.3‐fold dopamine accumulation than the control, whereas no significant effect on dopamine accumulation was noticed following SA treatment 77.
Production of isoflavones in HRCs of Glycine max was studied by using sonication, vacuum infiltration, supplementation of medium with MJ and SA 82. It was observed that MJ and SA elicitation at optimum concentration (100 and 200 µM, respectively) and at optimum exposure period (72 and 96 h, respectively) enhanced total isoflavone production 10.67‐fold (53.16 mg g−1 DW) and 5.78‐fold (28.79 mg g−1 DW), respectively compared to control 82.
Similarly, in HRCs of Withania somnifera, the effect of MJ and SA at different concentrations for different period of exposure was studied and it was found that 150 µM SA treatment for 4 h resulted in optimum production of biomass (32.68 g FW and 5.54 g DW), and enhanced accumulation of withanolides such as withanolide A (132.44 mg g−1 DW/1.23‐fold), withanone (84.35 mg g−1 DW/58‐fold), and withaferin A (70.72 mg g−1 DW/42‐fold) whereas elicitation with 15 µM MJ showed 50‐, 38‐, and 34‐fold higher production of withanolide A (114.38 mg g−1 DW), withanone (69.89 mg g−1 DW), withaferin A (57.46 mg g−1 DW) in 40‐day‐old harvested hairy roots 83. Elicitation with SA was also reported in Azadirachta indica. Maximum 4.95 mg g−1 azadirachtin (a complex tetranortriterpenoid limonoid) yield in HRCs of A. indica was achieved by the elicitation with SA (15 mg L−1) compared to control (3.31 mg g−1), however growth was reduced 42.
HRC of Psoralea corylifolia when elicited with 1 and 10 µM JA led to increase of 2.8‐fold (5.09% DW) daidzin, an isoflavone, after second week, and 7.3‐fold (3.43% DW) after 10th week compared to untreated control 79. Addition of 10 µM ASA resulted in 1.7‐fold and 2.3‐fold increase in daidzin content after seventh week and eighth week, respectively when compared to untreated control 79.
Anisodus luridus HRCs treated with 1 mM ASA showed the highest capacity of TA biosynthesis, i.e. scopolamine and hyoscyamine content enhanced to 57.2 and 14.7 µg g−1 DW, respectively, due to dramatic increase in expression of PMT, TRI, and CYP80F1 genes 47. Treatment with 1 mM ASA also induced the efflux of scopolamine in the liquid medium (153.4 µg flask−1), about 6.2 folds higher as compared to control, whereas hyoscyamine was detected at trace level in liquid medium 47.
Both MJ and the phytotoxin coronatine (Cor) induced a significant accumulation of bioactive quinone diterpenes viz., aethiopinone and abietane in Salvia sclarea hairy roots by transcriptional activation of genes belonging to the plastidial MEP‐derived isoprenoid pathway but prolonged exposure to MJ inhibited hairy root growth 84. Treatment with Cor for 28 days produced 105.34 ± 2.30 mg L−1 aethiopinone, a significant increase of 24‐fold above the basal content in untreated hairy roots 84.
2.3. Effect of combined treatment with two elicitors on SM production
A few studies have shown that the application of two or more elicitors in different combinations in culture medium can enhance the SM production as compared to treatment with single elicitors in several species 38, 39, 51, 70, 85, 86.
Supplementation of medium with combination of MJ and cell homogenate of P. indica in the culture medium in combination showed 2.44 times enhancement in artemisinin content in HRCs of A. annua compared to control, which was positively correlated with regulatory genes of MVA, MEP, and artemisinin biosynthetic pathways, viz. hmgr, ads, cyp7, 1av1, aldh1, dxs, dxr, and dbr2 38.
Artemisia annua hairy roots when treated with NO donor SNP (at 50 µM) and an abiotic elicitor, i.e. oligosaccharide derived from Fusarium oxysporum mycelium (at 0.3 mg total sugar mL−1) together in combination showed synergized effect on artemisinin (a sesquiterpene lactone) production and artemisinin yield 39. Artemisinin production increased from 1.2 to 2.2 mg g−1 DW and maximum artemisinin yield of 28.5 mg l−1 was achieved, which was about two‐fold higher than that produced by the oligosaccharide treatment alone 39. Similar type of synergistic effect was also reported in HRCs of A. annua by Wang et al. 85. The combination of SNP (10 µM) and a fungus‐derived cerebroside C (30 µg mL−1) enhanced a 2.3‐fold artemisinin content (up to 22.4 mg L−1) in 20‐day‐old HRCs of A. annua 85.
Addition of MJ (50 µM) and SA (100 µM) in combination in 23‐day‐old HRC of Rehmannia glutinosa also promoted production of iridoids (catalpol, harpagide) and phenylethanoids (verbascoside and isoverbascoside) compared to the non‐treated HRCs, however the application of optimal concentration and exposure period of MJ alone was more effective 70.
The combination of 200 mg L−1 CHI and 80 µM MJ treatment in Plumbago indica hairy roots was more effective in increasing plumbagin (a naphthoquinone) yield than CHI alone in shake flask culture 86. Maximum plumbagin yield was 11.96 ± 0.76 mg g−l DW, which was 2.3‐fold higher than CHI induced plumbagin content. Moreover, similar synergistic effect was observed when 20‐days‐old HRCs in bioreactor‐culture were treated with same combination of elicitors, leading to significant improvement in plumbagin production, i.e. 13.16 ± 1.72 mg g−l DW with simultaneous leaching of plumbagin in culture media in the bioreactor 86.
The combined elicitation with UV‐B at 40 µW cm−2 for 40‐min and MJ (100 µM) for 9 days in 18‐day‐old HRCs of S. miltiorrhiza exhibited synergistic effects and induced a 4.9‐fold increase in tanshinone (a diterpene) production (28.21 mg L−1) over the control by stimulation of the expression levels of 3‐hydroxy‐3‐methylglutaryl‐CoA reductase (SmHMGR) and geranylgeranyl diphosphate synthase (SmGGPPS) genes in the tanshinone biosynthetic pathway 51.
Shi et al. 87 also showed that the combined use of a biotic elicitor (YE) along with hyperosmotic stress created with sorbitol can effectively enhance total tanshinone production in the HRC of S. miltiorrhiza. The combined use of sorbitol (50 g L−1) and YE (100 mg L−1) increased the tanshinone content 10‐fold (1481.6 vs 146.4 mg g−1 dry root) and the volumetric yield of tanshinone nine‐fold (16.3 vs 1.77 mg L−1) when compared with the control 87.
The combination of metabolic engineering and double elicitation was an effective strategy to increase the production of flavonoids in hairy roots of Glycyrrhiza uralensis 88. The transgenic hairy roots that over expressed chalcone isomerase gene when treated with a combination of polyethylene glycol (2%) and YE (0.1%) showed that the accumulation of total flavonoids (2.838 g/100 g DW) significantly increased in comparison to wild‐type hairy roots (0.842 g/100 g DW) and the untreated transgenic hairy roots (1.394 g/100 g DW) 88.
A combined treatment of sonication for 2 min and vacuum infiltration for 2 min in HRCs of Glycine max stimulated isoflavones production to 75.26 mg g−1 DW which was 15.11‐fold higher than control hairy roots at an optimal harvest time of 40 days 82.
HRCs of Scutellaria lateriflora when incubated under continuous light and treated with 15 mM methyl‐β‐cyclodextrin for 24 h produced significantly higher levels of the flavones, baicalein, and wogonin compared to cultures incubated under continuous darkness, suggesting that light may have a selected regulatory effect on the synthesis or accumulation of these phenolic compounds 89.
In HRCs of Tropaeolum majus, the combined application of 0.2 mM ASA and 50 µM MJ or 0.05% YE increased the glucotropaeolin production as compared to HRCs treated with these elicitors alone 90.
Co‐treatment with 100 µM MJ and 9 g L−1 methyl‐β‐cyclodextrin in HRCs of Arachis hypogaea induced high levels stilbenoids production, including resveratrol, piceatannol, arachidin‐1 (average yield 56 mg L−1), and arachidin‐3 (average yield 148 mg L−1) when compared to treatment with either MJ or methyl‐β‐cyclodextrin (CD) alone 91. Furthermore, MJ and CD had a synergistic effect on resveratrol synthase gene expression, which could explain the higher yield of resveratrol when compared to individual elicitor treatment 91.
2.4. Effect of integration of elicitation with other strategies on SM production
2.4.1. Combined effect of polyploidization and elicitation on SM production
The combination of polyploidy in hairy roots induced by colchicine and elicitation with ASA or SA can significantly improve accumulation of hyoscyamine due to their synergistic effects in the hairy roots of D. stramonium 92. SA elicitation improved hyoscyamine content 190% (7.697 mg g−1 DW) in diploid lines (2n = 24) and 126% (12.31 mg g−1 DW) in tetraploid lines (2n = 48) compared to non‐elicited control. Similarly, ASA elicitation improved hyoscyamine content 170% in diploid lines (6.33 mg g−1 DW) and 124% in tetraploid lines (11.309 mg g−1 DW) compared to non‐elicited control. Interestingly, the improvement in hyoscyamine content was maximally achieved in the tetraploid HR lines elicited by SA (279%) and ASA (256%), respectively; demonstrating the favorable combined effect of polyploidization and the elicitation on hyoscyamine content in HRCs of D. stramonium 92.
2.4.2. Elicitation and precursor feeding
The application of optimum concentration of MJ (300 µM) along with the precursors such as cholesterol (100 mM) and L‐arginine (1000 µM) supplemented modified liquid MS medium on 20‐days‐old HRCs (hairy root line‐ATCC31798 and line‐A4) of Solanum mammosum improved solasodine productivity five‐fold (4.5 mg g−1) with a high‐biomass accumulation (in average 190 mg DW) compared to control HRCs cultured without both the elicitor and precursor treatment 93.
2.4.3. Elicitation and nutrient feeding
Combination of YPS elicitation strategy with medium renewal process was more effective for flavonoids (rutin and quercetin) production in HRCs of Fagopyrum tatarium. The maximal yield of flavonoids was 47.13 mg L−1, i.e. ∼3.2‐fold higher in comparison with the control culture (14.88 mg l−1) 31. Moreover, this study revealed that the enhanced accumulation of these bioactive metabolites in HRCs was caused by the stimulation of the phenylpropanoid pathway by YPS treatment 31.
2.4.4. Elicitation and in situ product removal
Elicitation of hairy roots leads to increased productivity of SM and helps in designing metabolic traps to allow adsorption of the product that makes product recovery easier, preventing feedback inhibition of accumulated synthesized metabolites, mitigating toxicity caused by waste materials released from cells, and preventing their degradation in the culture media 8, 94, 95. Permeabilization and in situ product adsorption result in many folds increase in product yield 14. The introduction of an in situ product removal mechanism, such as a solid adsorbent (form solid–liquid systems) or an extraction solvent (liquid–liquid systems), to the culture medium can often effectively induce product release from plant cells and increase productivity 95. Nonionic polymeric ion‐exchange resins of Amberlite XAD series (Rohm and Haas, Philadelphia, PA) and macroporous polystyrene resin are commonly used as solid adsorbent. Solid–liquid system enables better outcome compared with liquid–liquid system.
Modeling of tanshinone synthesis and phase distribution under the combined effect of elicitation and in situ adsorption in HRCs of Salvia miltiorrhiza was studied by Yan et al. 95. The simulated results showed that the enhancement of tanshinone production was mainly due to the effect of the elicitor and that resin addition resulted in adsorbance of the tanshinones from the root and alteration of tanshinones distribution 95. The rate of transport of tanshinones from the root to the medium was an important factor that influenced tanshinones accumulation in the resin 95. This modeling can be used in similar plant tissue culture systems in future.
3. CONCLUDING REMARKS
Approximately 100 000 SMs have been recognized from 50 000 plant species and ∼4000 new SMs are being discovered every year from a variety of plant species. The supply of the high value, low amount bioactive SMs from the field grown plants for pharmaceutical industries often have several limitations. Although several tissue culture approaches have been developed in the last few decades to overcome the limitations, most of the times the production of desired SM in appreciable quantity and at competitive economic value for commercialization is not feasible due to the poor understanding of the basic secondary metabolic pathway and their regulation in plants.
Elicitation with various biotic and abiotic elicitors has been widely applied for enhancement of SM production in HRCs as well as in plant cell cultures of different species. Elicitors generally refer to the biotic or abiotic molecules with wide structural diversity, their application at very low concentration can induce or enhance the biosynthesis of specific SM in living system through the activation of defense responses. The differentiated cultures such as HRCs gained momentum over undifferentiated culture due to genetic stability, uniformity of the product formation, and high growth rate in the phytohormone free basal medium.
Among the many elicitors applied for stimulating SM production in HRCs, most common and effective elicitors are crude fungal extracts or partially purified polysaccharide fractions from fungal cells, UV‐radiation, JA and MJ, and heavy metal ions. Besides these, application of other chemical elicitors, hyperosmotic stress, hormones, temperature shift have been shown effective for some plant species/metabolites. Optimization of elicitor type, dose and duration is very much essential for their usefulness in SM production as their action may be plant species specific as well as biosynthetic pathway specific. Thus, an elicitor may not stimulate similar SM in all species as well as for different group of SM production in the same species.
In addition to enhancing the accumulation of specific product yield on per unit mass of roots, the application of elicitor in culture medium often stimulates the efflux of intracellular products that make product recovery or purification of desired compound quite easier. Elicitation also helps in better understanding of the regulation mechanisms of a particular biosynthesis pathway by evaluating the gene expression of the specific biosynthesis pathway which may be very effective in metabolic engineering in the near future.
Nowadays, integration of different strategies such as nutrient feeding along with traditional elicitation with single elicitors, in situ product removal from the roots and/or liquid medium, application of combinations of more than one elicitor simultaneously, have been examined and in most of the species these strategies improved volumetric yield of the SMs by synergistic effect of individual strategy. In situ product removal strategy is more easily applied in HRCs than cell suspension cultures because the roots are self‐immobilized and retained within the culture vessel allowing renewal of liquid medium. Thus, elicitation of HRC along with integration of other biotic strategies can be used as an alternative feasible route for the commercial production of low volume pharmaceutically important SMs. This review would be very useful for researchers working in biotechnological production systems such as those using different elicitors with HRCs of many plant species to study their effects in enhancing the production of SMs of interest.
CONFLICT OF INTEREST
The authors have declared no conflict of interest.
ACKNOWLEDGMENTS
SJ is thankful to the National Academy of Sciences (NASI, Allahabad, India) for the NASI Senior Scientist Fellowship award, and for providing the financial support to continue the research.
Halder M, Sarkar S, Jha S. Elicitation: A biotechnological tool for enhanced production of secondary metabolites in hairy root cultures. Eng Life Sci. 2019;19:880–895. 10.1002/elsc.201900058
REFERENCES
- 1. Neumann K. H., Kumar A., Imani J. (Eds.), Plant Cell and Tissue Culture‐ A Tool in Biotechnology: Basics and Application, Springer, Berlin, Heidelberg: 2009. [Google Scholar]
- 2. Kaur, K. , Pati, P. K. , Stress‐induced metabolite production utilizing plant hairy roots, in: Srivastava V., Mehrotra S., Mishra S. (Eds.), Hairy Roots‐ An Effective Tool of Plant Biotechnology, Springer, Singapore: 2018, pp. 123–145. [Google Scholar]
- 3. Namdeo, A. G. , Plant cell elicitation for production of secondary metabolites: a review. Pharmacogn. Rev. 2007, 1, 69–79. [Google Scholar]
- 4. Nandagopal, K. , Halder, M. , Dash, B. , Nayak, S. , Jha, S. , Biotechnological approaches for production of anti‐cancerous compounds resveratrol, podophyllotoxin and zerumbone. Curr. Med. Chem. 2018, 25, 4693–4717. [DOI] [PubMed] [Google Scholar]
- 5. Almagro, L. , Belchí‐Navarro, S. , Sabater‐Jara, A. B. , Vera‐Urbina, J. C. , Sellés‐Marchart, S. , Bru, R. , Pedreño, M. A. , Bioproduction of trans‐resveratrol from grapevine cell cultures, in: Ramawat K. G., Merillon J. M. (Eds.), Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes, Springer, Berlin: 2013, pp. 1683–1713. [Google Scholar]
- 6. Halder, M. , Roychowdhury, D. , Jha, S. , A critical review on biotechnological interventions for production and yield enhancement of secondary metabolites in hairy root cultures, in: Srivastava V., Mehrotra S., Mishra S. (Eds.), Hairy Roots, Springer Nature, Singapore: 2018, pp. 21–44 [Google Scholar]
- 7. Ramakrishna, A. , Ravishankar, G. A. , Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav. 2011, 6, 1720–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang, J. W. , Wu, J. Y. , Effective elicitors and process strategies for enhancement of secondary metabolite production in hairy root cultures, in: Doran P. M. (Ed.), Biotechnology of Hairy Root Systems. Advances in Biochemical Engineering/Biotechnology, Springer, Berlin, Heidelberg: 2013, pp. 55–89. [DOI] [PubMed] [Google Scholar]
- 9. Dhiman, N. , Patial, V. , Bhattacharya, A. , The current status and future applications of hairy root cultures, in: Kumar N. (Ed.), Biotechnological Approaches for Medicinal and Aromatic Plants, Springer, Singapore: 2018, pp. 87–155. [Google Scholar]
- 10. Naik, P. M. , Al–Khayri, J. M. , Abiotic and biotic elicitors–role in secondary metabolites production through in vitro culture of medicinal plant, in: Shanker A. K., Shanker C. (Eds.), Abiotic and Biotic Stress in Plants‐Recent Advances and Future Perspectives, InTech, Rijeka: 2016, pp. 247–277. [Google Scholar]
- 11. Vasconsuelo, A. , Boland, R. , Molecular aspects of the early stages of elicitation of secondary metabolites in plants. Plant Sci. 2007, 172, 861–875. [Google Scholar]
- 12. Mishra, A. K. , Sharma, K. , Misra, R. S. , Elicitor recognition, signal transduction and induced resistance in plants. J. Plant Interact. 2012, 7, 95–120. [Google Scholar]
- 13. Zhai, X. , Jia, M. , Chen, L. , Zheng, C. J. et al., The regulatory mechanism of fungal elicitor‐induced secondary metabolite biosynthesis in medical plants. Crit. Rev. Microbiol. 2017, 43, 238–261. [DOI] [PubMed] [Google Scholar]
- 14. Doran P. M. (Ed.), Biotechnology of Hairy Root Systems, Springer; 2013. [Google Scholar]
- 15. Ramirez‐Estrada, K. , Vidal‐Limon, H. , Hidalgo, D. , Moyano, E. et al., Elicitation, an effective strategy for the biotechnological production of bioactive high‐added value compounds in plant cell factories. Molecules 2016, 21, 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Srivastava, V. , Mehrotra, S. , Verma, P. K. , Biotechnological interventions for production of therapeutic secondary metabolites using hairy root cultures of medicinal plants, in: Dubey S. K., Pandey A., Sangwan R. S. (Eds.), Current Developments in Biotechnology and Bioengineering: Crop Modification, Nutrition, and Food Production, Elsevier, Netherland: 2017, pp. 259–282. [Google Scholar]
- 17. Singh, N. R. , Rath, S. K. , Behera, S. , Naik, S. K. , In vitro secondary metabolite production through fungal elicitation: an approach for sustainability, in: Prasad R., Kumar V., Kumar M., Wang S. (Eds.), Fungal Nanobionics: Principles and Applications, Springer, Singapore: 2018, pp. 215–242. [Google Scholar]
- 18. Cai, Z. , Kastell, A. , Knorr, D. , Smetanska, I. , Exudation: an expanding technique for continuous production and release of secondary metabolites from plant cell suspension and hairy root cultures. Plant Cell Rep. 2012, 31, 461–477. [DOI] [PubMed] [Google Scholar]
- 19. Jha, S. , Transgenic organ cultures and their use in plant secondary metabolism. Proc. Indian Natn. Sci. Acad. Part B 1995, 61, 63–72 [Google Scholar]
- 20. Spencer, A. , Hamill, J. D. , Rhodes, M. J. C. , In vitro biosynthesis of monoterpenes by Agrobacterium transformed shoot cultures of two Mentha species. Phytochemistry 1993, 32, 911–919. [Google Scholar]
- 21. Mehrotra, S. , Srivastava, V. , Rahman, L. U. , Kukreja, A. K. , Hairy root biotechnology—indicative timeline to understand missing links and future outlook. Protoplasma 2015, 252, 1189–1201. [DOI] [PubMed] [Google Scholar]
- 22. Srivastava V., Mehrotra S., Mishra S. (Eds.), Hairy Roots: An Effective Tool of Plant Biotechnology, Springer, Singapore: 2018. [Google Scholar]
- 23. Ono, N. N. , Tian, L. , The multiplicity of hairy root cultures: prolific possibilities. Plant Sci. 2011, 180, 439–446. [DOI] [PubMed] [Google Scholar]
- 24. Georgiev, M. I. , Agostini, E. , Ludwig‐Müller, J. , Xu, J. , Genetically transformed roots: from plant disease to biotechnological resource. Trends Biotechnol. 2012, 30, 528–537. [DOI] [PubMed] [Google Scholar]
- 25. Smetanska, I. , Production of secondary metabolites using plant cell cultures, in: Stahl U., Donalies U. E. B., Nevoigt E. (Eds.), Food Biotechnology, Springer, Berlin, Heidelberg: 2008, pp. 187–228. [DOI] [PubMed] [Google Scholar]
- 26. Li, B. , Wang, B. , Li, H. , Peng, L. et al., Establishment of Salvia castanea Diels f. tomentosa Stib. hairy root cultures and the promotion of tanshinone accumulation and gene expression with Ag+, methyl jasmonate, and yeast extract elicitation. Protoplasma 2016, 253, 87–100. [DOI] [PubMed] [Google Scholar]
- 27. Udomsuk, L. , Jarukamjorn, K. , Tanaka, H. , Putalun, W. , Improved isoflavonoid production in P. candollei hairy root cultures using elicitation. Biotechnol. Lett. 2011, 33, 369–374. [DOI] [PubMed] [Google Scholar]
- 28. Udomsuk, L. , Jarukamjorn, K. , Tanaka, H. , Putalun, W. , Isoflavonoid production in hairy roots culture of Pueraria candollei . Z. Naturforsch. 2009, 64, 687–691. [DOI] [PubMed] [Google Scholar]
- 29. Wilczańska‐Barska, A. , Królicka, A. , Głód, D. , Majdan, M. , Majdan, M. , Kawiak, A. , Krauze-Baranowska, M. , Enhanced accumulation of secondary metabolites in hairy root cultures of Scutellaria lateriflora following elicitation. Biotechnol. Lett. 2012, 34, 1757–1763. [DOI] [PubMed] [Google Scholar]
- 30. Udomsin, O. , Yusakul, G. , Kraithong, W. , Udomsuk, L. et al., Enhanced accumulation of high‐value deoxymiroestrol and isoflavonoids using hairy root as a sustainable source of Pueraria candollei var. mirifica . Plant Cell Tissue Organ Cult. 2019, 136, 141–151. [Google Scholar]
- 31. Zhao, J. L. , Zou, L. , Zhang, C. Q. , Li, Y. Y. et al., Efficient production of flavonoids in Fagopyrum tataricum hairy root cultures with yeast polysaccharide elicitation and medium renewal process. Pharmacogn. Mag. 2014, 10, 234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ming, Q. , Su, C. , Zheng, C. , Jia, M. et al., Elicitors from the endophytic fungus Trichoderma atroviride promote Salvia miltiorrhiza hairy root growth and tanshinone biosynthesis. J. Exp. Bot. 2013, 64, 5687–5694. [DOI] [PubMed] [Google Scholar]
- 33. Sun, J. , Xiao, J. , Wang, X. , Yuan, X. , Zhao, B. , Improved cardenolide production in Calotropis gigantean hairy roots using mechanical wounding and elicitation. Biotechnol. Lett. 2012, 34, 563–569. [DOI] [PubMed] [Google Scholar]
- 34. Jiao, J. , Gai, Q. Y. , Wang, X. , Qin, Q. P. et al., Chitosan elicitation of Isatis tinctoria L. hairy root cultures for enhancing flavonoid productivity and gene expression and related antioxidant activity. Ind. Crops Prod. 2018, 124, 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nourozi, E. , Hosseini, B. , Hassani, A. , A reliable and efficient protocol for induction of hairy roots in Agastache foeniculum . Biologia 2014, 69, 870–879. [Google Scholar]
- 36. Zhao, J. L. , Zhou, L. G. , Wu, J. Y. , Promotion of Salvia miltiorrhiza hairy root growth and tanshinone production by polysaccharide–protein fractions of plant growth‐promoting rhizobacterium Bacillus cereus . Process Biochem. 2010, 45, 1517–1522. [Google Scholar]
- 37. Saxena, P. , Ahlawat, S. , Ali, A. , Khan, S. , Abdin, M. Z. , Gene expression analysis of the withanolide biosynthetic pathway in hairy root cultures of Withania somnifera elicited with methyl jasmonate and the fungus Piriformospora indica . Symbiosis. 2017, 71, 143–154. [Google Scholar]
- 38. Ahlawat, S. , Saxena, P. , Alam, P. , Wajid, S. , Abdin, M. Z. , Modulation of artemisinin biosynthesis by elicitors, inhibitor, and precursor in hairy root cultures of Artemisia annua L. J. Plant Interact. 2014, 9, 811–824. [Google Scholar]
- 39. Zheng, L. P. , Guo, Y. T. , Wang, J. W. , Tan, R. X. , Nitric oxide potentiates oligosaccharide‐induced artemisinin production in Artemisia annua hairy roots. J. Integr. Plant Biol. 2008, 50, 49–55. [DOI] [PubMed] [Google Scholar]
- 40. Tashackori, H. , Sharifi, M. , Chashmi, N. A. , Behmanesh, M. , Safaie, N. , Piriformospora indica cell wall modulates gene expression and metabolite profile in Linum album hairy roots. Planta 2018, 248, 1289–1306. [DOI] [PubMed] [Google Scholar]
- 41. Shakeran, Z. , Keyhanfar, M. , Asghari, G. , Ghanadian, M. , Improvement of atropine production by different biotic and abiotic elicitors in hairy root cultures of Datura metel . Turk. J. Biol. 2015, 39, 111–118. [Google Scholar]
- 42. Srivastava, S. , Srivastava, A. K. , Effect of elicitors and precursors on azadirachtin production in hairy root culture of Azadirachta indica . Appl. Biochem. Biotechnol. 2014, 172, 2286–2297. [DOI] [PubMed] [Google Scholar]
- 43. Jain, A. , Singh, S. , Effect of growth regulators and elicitors for the enhanced production of solasodine in hairy root culture of Solanum melongena (L.). J. Indian Bot. Soc. 2015, 94, 23–39. [Google Scholar]
- 44. Srivastava, M. , Sharma, S. , Misra, P. , Elicitation based enhancement of secondary metabolites in Rauwolfia serpentina and Solanum khasianum hairy root cultures. Pharmacogn. Mag. 2016, 12, 315–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Krstić‐Milošević, D. , Janković, T. , Uzelac, B. , Vinterhalter, D. , Vinterhalter, B. , Effect of elicitors on xanthone accumulation and biomass production in hairy root cultures of Gentiana dinarica . Plant Cell Tissue Organ Cult. 2017, 130, 631–640. [Google Scholar]
- 46. Gai, Q. Y. , Jiao, J. , Luo, M. , Wang, W. , Yao, L.‐P. , Fu, Y.‐J. , Deacetylation biocatalysis and elicitation by immobilized Penicillium canescens in Astragalus membranaceus hairy root cultures: towards the enhanced and sustainable production of astragaloside IV. Plant Biotechnol. J. 2017, 15, 297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Qin, B. , Ma, L. , Wang, Y. , Chen, M. et al., Effects of acetylsalicylic acid and UV‐B on gene expression and tropane alkaloid biosynthesis in hairy root cultures of Anisodus luridus . Plant Cell Tissue Organ Cult. 2014, 117, 483–490. [Google Scholar]
- 48. Jiao, J. , Gai, Q. Y. , Wang, W. , Luo, M. et al., Ultraviolet radiation‐elicited enhancement of isoflavonoid accumulation, biosynthetic gene expression, and antioxidant activity in Astragalus membranaceus hairy root cultures. J. Agric. Food Chem. 2015, 63, 8216–8224. [DOI] [PubMed] [Google Scholar]
- 49. Gai, Q. Y. , Jiao, J. , Luo, M. , Wang, W. et al., UV elicitation for promoting astragaloside production in Astragalus membranaceus hairy root cultures with transcriptional expression of biosynthetic genes. Ind. Crops and Prod. 2016, 84, 350–357. [Google Scholar]
- 50. Huang, X. , Yao, J. , Zhao, Y. , Xie, D. et al., Efficient rutin and quercetin biosynthesis through flavonoids‐related gene expression in Fagopyrum tataricum Gaertn. hairy root cultures with UV‐B irradiation. Front. Plant Sci. 2016, 7, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang, C. H. , Zheng, L. P. , Tian, H. , Wang, J. W. , Synergistic effects of ultraviolet‐B and methyl jasmonate on tanshinone biosynthesis in Salvia miltiorrhiza hairy roots. J. Photochem. Photobiol. B. 2016, 159, 93–100. [DOI] [PubMed] [Google Scholar]
- 52. Lajayer, B. A. , Ghorbanpour, M. , Nikabadi, S. , Heavy metals in contaminated environment: destiny of secondary metabolite biosynthesis, oxidative status and phytoextraction in medicinal plants. Ecotoxicol. Environ. Saf. 2017, 145, 377–390. [DOI] [PubMed] [Google Scholar]
- 53. Xiao, Y. , Gao, S. , Di, P. , Chen J., Chen, W. , Zhang, L. , Lithospermic acid B is more responsive to silver ions (Ag+) than rosmarinic acid in Salvia miltiorrhiza hairy root cultures. Biosci. Rep. 2010, 30, 33–40. [DOI] [PubMed] [Google Scholar]
- 54. Kai, G. , Yang, S. , Zhang, Y. , Luo, X. et al., Effects of different elicitors on yield of tropane alkaloids in hairy roots of Anisodus acutangulus . Mol. Biol. Rep. 2012, 39, 1721–1729. [DOI] [PubMed] [Google Scholar]
- 55. Khalili, M. , Hasanloo, T. , Tabar, S. K. K. , Ag+ enhanced silymarin production in hairy root cultures of Silybum marianum (L.) Gaertn. Plant Omics 2010, 3, 109–114. [Google Scholar]
- 56. Wawrosch, C. , Schwaiger, S. , Stuppner, H. , Kopp, B. , Lignan formation in hairy root cultures of Edelweiss (Leontopodium nivale ssp. alpinum (Cass.) Greuter). Fitoterapia 2014, 97, 219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang, B. , Zheng, L. P. , Li, W. Y. , Wang, J. W. , Stimulation of artemisinin production in Artemisia annua hairy roots by Ag‐SiO2 core‐shell nanoparticles. Curr. Nanosci. 2013, 9, 363–370. [Google Scholar]
- 58. Moharrami, F. , Hosseini, B. , Sharafi, A. , Farjaminezhad, M. , Enhanced production of hyoscyamine and scopolamine from genetically transformed root culture of Hyoscyamus reticulatus L. elicited by iron oxide nanoparticles. In Vitro Cell. Dev. Biol. Plant 2017, 53, 104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chung, I. M. , Rekha, K. , Rajakumar, G. , Thiruvengadam, M. , Production of bioactive compounds and gene expression alterations in hairy root cultures of chinese cabbage elicited by copper oxide nanoparticles. Plant Cell Tissue Organ Cult. 2018, 134, 95–106. [Google Scholar]
- 60. Torkamani, M. R. D. , Abbaspour, N. , Jafari, M. , Samadi, A. , Elicitation of valerenic acid in the hairy root cultures of Valeriana officinalis L (Valerianaceae). Trop. J. Pharm. Res. 2014, 13, 943–949. [Google Scholar]
- 61. Harfi, B. , Khelifi‐Slaoui, M. , Bekhouche, M. , Benyammi, R. et al., Hyoscyamine production in hairy roots of three Datura species exposed to high‐salt medium. In Vitro Cell. Dev. Biol. Plant 2016, 52, 92–98. [Google Scholar]
- 62. Thakore, D. , Srivastava, A. K. , Sinha, A. , Enhanced production of antihypertensive drug ajmalicine in transformed hairy root culture of Catharanthus roseus by application of stress factors in statistically optimized medium, in: Khemani L., Srivastava M., Srivastava S. (Eds.), Chemistry of Phytopotentials: Health, Energy and Environmental Perspectives, Springer, Berlin, Heidelberg: 2012, pp. 39–42. [Google Scholar]
- 63. Condori, J. , Sivakumar, G. , Hubstenberger, J. , Dolan, M. C. , Sobolev, V. S. , Medina-Bolivar, F. , Induced biosynthesis of resveratrol and the prenylated stilbenoids arachidin‐1 and arachidin‐3 in hairy root cultures of peanut: effects of culture medium and growth stage. Plant Physiol. Biochem. 2010, 48, 310–318. [DOI] [PubMed] [Google Scholar]
- 64. Liang, Z. , Ma, Y. , Xu, T. , Cui, B. et al., Effects of abscisic acid, gibberellin, ethylene and their interactions on production of phenolic acids in Salvia miltiorrhiza Bunge hairy roots. PLoS One 2013, 8, e72806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cheruvathur, M. K. , Thomas, T. D. , Effect of plant growth regulators and elicitors on rhinacanthin accumulation in hairy root cultures of Rhinacanthus nasutus (L.) Kurz. Plant Cell Tissue Organ Cult. 2014, 118, 169–177. [Google Scholar]
- 66. Zhu, C. , Miao, G. , Guo, J. , Huo, Y. et al., Establishment of Tripterygium wilfordii Hook. f. hairy root culture and optimization of its culture conditions for the production of triptolide and wilforine. J. Microbiol. Biotechnol. 2014, 24, 823–834. [DOI] [PubMed] [Google Scholar]
- 67. Shilpha, J. , Satish, L. , Kavikkuil, M. , Largia, M. J. V. , Ramesh, M. , Methyl jasmonate elicits the solasodine production and anti‐oxidant activity in hairy root cultures of Solanum trilobatum L. Ind. Crops Prod. 2015, 71, 54–64. [Google Scholar]
- 68. Gai, Q.Y. , Jiao, J. , Luo, M. , Wang, W. et al., Tremendous enhancements of isoflavonoid biosynthesis, associated gene expression and antioxidant capacity in Astragalus membranaceus hairy root cultures elicited by methyl jasmonate. Process Biochem. 2016, 51, 642–649. [Google Scholar]
- 69. Hashemi, S. M. , Naghavi, M. R. , Production and gene expression of morphinan alkaloids in hairy root culture of Papaver orientale L. using abiotic elicitors. Plant Cell Tissue Organ Cult. 2016, 125, 31–41. [Google Scholar]
- 70. Piątczak, E. , Kuźma, Ł. , Wysokińska, H. , The influence of methyl jasmonate and salicylic acid on secondary metabolite production in Rehmannia glutinosa Libosch. hairy root culture. Acta Biol. Cracov. Ser. Bot. 2016, 58, 57–65. [Google Scholar]
- 71. Ru, M. , An, Y. , Wang, K. , Peng, L. et al., Prunella vulgaris L. hairy roots: culture, growth, and elicitation by ethephon and salicylic acid. Eng. Life Sci. 2016, 16, 494–502. [Google Scholar]
- 72. Hosseini, S. M. , Bahramnejad, B. , DouletiBaneh, H. , Emamifar, A. , Goodwin, P. H. , Hairy root culture optimization and resveratrol production from Vitis vinifera subsp. sylvesteris. World J. Microbiol. Biotechnol. 2017, 33, 67. [DOI] [PubMed] [Google Scholar]
- 73. Perassolo, M. , Cardillo, A. B. , Mugas, M. L. , Montoya, S. C. N. , Enhancement of anthraquinone production and release by combination of culture medium selection and methyl jasmonate elicitation in hairy root cultures of Rubia tinctorum . Ind. Crops Prod. 2017, 105, 124–132. [Google Scholar]
- 74. Kochan, E. , Balcerczak, E. , Lipert, A. , Szymańska, G. , Szymczyk, P. , Methyl jasmonate as a control factor of the synthase squalene gene promoter and ginsenoside production in American ginseng hairy root cultured in shake flasks and a nutrient sprinkle bioreactor. Ind. Crops Prod. 2018, 115, 182–193. [Google Scholar]
- 75. Harfi, B. , Khelifi, L. , Khelifi‐Slaoui, M. , Assaf‐Ducrocq, C. , Gontier, E. , Tropane alkaloids GC/MS analysis and low dose elicitors’ effects on hyoscyamine biosynthetic pathway in hairy roots of Algerian Datura species. Sci. Rep. 2018, 8, 17951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Patra, N. , Srivastava, A. K. , Mass scale artemisinin production in a stirred tank bioreactor using hairy roots of Artemisia annua . Int. J. Biosci. Biochem. Bioinforma. 2014, 4, 467–474. [Google Scholar]
- 77. Moghadam, Y. A. , Piri, K. , Bahramnejad, B. , Ghiasvand, T. , Dopamine production in hairy root cultures of Portulaca oleracea (Purslane) using Agrobacterium rhizogenes . J. Agr. Sci. Tech. 2014, 16, 409–420. [Google Scholar]
- 78. Goklany, S. , Rizvi, N. F. , Loring, R. H. , Cram, E. J. , Lee‐Parsons, C. W. T. , Jasmonate‐dependent alkaloid biosynthesis in Catharanthus roseus hairy root cultures is correlated with the relative expression of Orca and Zct transcription factors. Biotechnol. Prog. 2013, 29, 1367–1376. [DOI] [PubMed] [Google Scholar]
- 79. Zaheer, M. , Reddy, V. D. , Giri, C. C. , Enhanced daidzin production from jasmonic and acetyl salicylic acid elicited hairy root cultures of Psoralea corylifolia L. (Fabaceae). Nat. Prod. Res. 2016, 30, 1542–1547. [DOI] [PubMed] [Google Scholar]
- 80. Putalun, W. , Wongwicha, W. , Tanaka, H. , Shoyama, Y. , Enhanced glycyrrhizin production in Glycyrrhiza inflata hairy roots cultures using elicitation. Planta Med. 2011, 77, PB14. [DOI] [PubMed] [Google Scholar]
- 81. Sajjalaguddam, R. R. , Paladugu, A. , Influence of Agrobacterium rhizogenes strains and elicitation on hairy root induction and glycyrrhizin production from Abrus precatorius . J. Pharm. Sci. & Res. 2016, 8, 1353–1357. [Google Scholar]
- 82. Theboral, J. , Sivanandhan, G. , Subramanyam, K. , Arun, M. et al., Enhanced production of isoflavones by elicitation in hairy root cultures of soybean. Plant Cell Tissue Organ Cult. 2014, 117, 477–481. [Google Scholar]
- 83. Sivanandhan, G. , Kapil Dev, G. , Jeyaraj, M. , Rajesh, M. et al., Increased production of withanolide A, withanone, and withaferin A in hairy root cultures of Withania somnifera (L.) Dunal elicited with methyl jasmonate and salicylic acid. Plant Cell Tissue Organ Cult. 2013, 114, 121–129. [Google Scholar]
- 84. Vaccaro, M. C. , Mariaevelina, A. , Malafronte, N. , De Tommasi, N. , Leone, A. , Increasing the synthesis of bioactive abietane diterpenes in Salvia sclarea hairy roots by elicited transcriptional reprogramming. Plant Cell Rep. 2017, 36, 375–386. [DOI] [PubMed] [Google Scholar]
- 85. Wang, J. W. , Zheng, L. P. , Zhang, B. , Zou, T. , Stimulation of artemisinin synthesis by combined cerebroside and nitric oxide elicitation in Artemisia annua hairy roots. Appl. Microbiol. Biotechnol. 2009, 85, 285–292. [DOI] [PubMed] [Google Scholar]
- 86. Gangopadhyay, M. , Dewanjee, S. , Bhattacharya, S. , Enhanced plumbagin production in elicited Plumbago indica hairy root cultures. J. Biosci. Bioeng. 2011, 111, 706–710. [DOI] [PubMed] [Google Scholar]
- 87. Shi, M. , Kwok, K. W. , Wu, J. Y. , Enhancement of tanshinone production in Salvia miltiorrhiza Bunge (red or Chinese sage) hairy‐root culture by hyperosmotic stress and yeast elicitor. Biotechnol. Appl. Biochem. 2007, 46, 191–196. [DOI] [PubMed] [Google Scholar]
- 88. Zhang, H. C. , Liu, J. M. , Lu, H. Y. , Gao, S. L. , Enhanced flavonoid production in hairy root cultures of Glycyrrhiza uralensis Fisch by combining the over‐expression of chalcone isomerase gene with the elicitation treatment. Plant Cell Rep. 2009, 28, 1205–1213. [DOI] [PubMed] [Google Scholar]
- 89. Marsh, Z. , Yang, T. , Nopo‐Olazabal, L. , Wu, S. et al., Effect of light, methyl jasmonate and cyclodextrin on production of phenolic compounds in hairy root cultures of Scutellaria lateriflora . Phytochemistry 2014, 107, 50–60. [DOI] [PubMed] [Google Scholar]
- 90. Wielanek, M. , Urbanek, H. , Enhanced glucotropaeolin production in hairy root cultures of Tropaeolum majus L. by combining elicitation and precursor feeding. Plant Cell Tissue Organ Cult. 2006, 86, 177–186. [Google Scholar]
- 91. Yang, T. , Fang, L. , Nopo‐Olazabal, C. , Condori, J. et al., Enhanced production of resveratrol, piceatannol, arachidin‐1, and arachidin‐3 in hairy root cultures of peanut co‐treated with methyl jasmonate and cyclodextrin. J. Agric. Food Chem. 2015, 63, 3942–3950. [DOI] [PubMed] [Google Scholar]
- 92. Belabbassi, O. , Khelifi‐Slaoui, M. , Zaoui, D. , Benyammi, R. et al., Synergistic effects of polyploidization and elicitation on biomass and hyoscyamine content in hairy roots of Datura stramonium . Biotechnol. Agron. Soc. Environ. 2016, 20, 408–416. [Google Scholar]
- 93. Ooi, C. T. , Syahida, A. , Stanslas, J. , Maziah, M. , The influence of methyl jasmonate, cholesterol and L‐arginine on solasodine production in hairy root culture of Solanum mammosum . Eng. Life Sci. 2016, 16, 432–442. [Google Scholar]
- 94. Chandra, S. , Chandra, R. , Engineering secondary metabolite production in hairy roots. Phytochem. Rev. 2011, 10, 371–395. [Google Scholar]
- 95. Yan, Q. , Wu, J. Y. , Liu, R. , Modeling of tanshinone synthesis and phase distribution under the combined effect of elicitation and in situ adsorption in Salvia miltiorrhiza hairy root cultures. Biotechnol. Lett. 2011, 33, 813–819. [DOI] [PubMed] [Google Scholar]


