Abstract
Current global environmental issues raise unavoidable challenges for our use of natural resources. Supplying the human population with clean water is becoming a global problem. Numerous organic and inorganic impurities in municipal, industrial, and agricultural waters, ranging from microplastics to high nutrient loads and heavy metals, endanger our nutrition and health. The development of efficient wastewater treatment technologies and circular economic approaches is thus becoming increasingly important. The biomass production of microalgae using industrial wastewater offers the possibility of recycling industrial residues to create new sources of raw materials for energy and material use. This review discusses algae‐based wastewater treatment technologies with a special focus on industrial wastewater sources, the potential of non‐conventional extremophilic (thermophilic, acidophilic, and psychrophilic) microalgae, and industrial algae‐wastewater treatment concepts that have already been put into practice.
Keywords: bioeconomy, bioreactors, extremophiles, microalgae, wastewater treatment
Abbreviations
- ATS
Algal Turf Scrubber
- PBR
photobioreactor
- RAB
revolving algal biofilm
- WWT
wastewater treatment
1. INTRODUCTION
Water is one of the most important natural resources on our planet. However, in addition to an inadequate clean water supply in many developing countries, water quality in industrialized nations has reached a worrying state 1, 2. The pollution of municipal, agricultural, and industrial wastewater with a huge number of organic and inorganic contaminants, such as microplastics 3, xenobiotics 1, heavy metals 4, and high concentrations of nitrates 5, phosphates 6, and carbon (C) compounds 2, puts a strain on the food chain and thus the basis of human life. Wastewater treatment (WWT) is a global issue that cannot be managed by a single technology because of the extremely variable scales, types of contaminants, and regional conditions involved (Figure 1).
Figure 1.
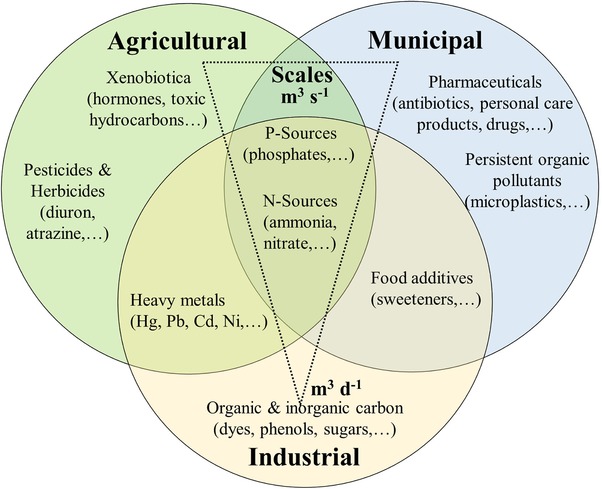
Wastewater sources and their typical impurities
Conventional WWT plants focus on the removal of suspended solids (mostly mechanically) and the reduction of biological oxygen demand by activated sludge 7. This biodegradation involves the breakdown of organic molecules and inorganic constituents (nitrogen [N] and phosphorous [P] compounds), which is of great importance to prevent the eutrophication of downstream waters such as rivers and lakes. The degradation capacity of these conventional technologies is limited, especially with regard to heavy metals, extremely high nutrient loads, and xenobiotics, leading to an increasing accumulation of these substances in groundwater 1, 2, 3, 4, 5, 6.
Because of the metabolic flexibility of microalgae, i.e. their ability to perform photoautotrophic, mixotrophic, or heterotrophic metabolism 8, 9, they represent promising biological systems for treating a variety of sources of wastewater. In particular, in the context of a circular and bio‐based economy and the development of biorefinery concepts 10, microalgae biomass produced from wastewater streams offers a great potential for sustainable bioproducts (dependent on national legislation on reusing microalgae biomass/bioproducts), such as proteins 11, fatty acids 12, pigments 13, biofertilizers/biochar 14, 15, and animal feed 16. Algae‐based WWT technologies have in fact been researched since the 1950s, mainly because of their very efficient fixation of inorganic N and P.
The usage of microalgae in WWT plants has two main aims: (1) the direct uptake or transformation of water contaminants, and/or (2) improving the purification performance of bacterial systems (microalgae‐bacteria aggregates) by providing additional oxygen from photosynthesis (symbiotic cocultures), thus reducing the total energy costs of direct (gassing performance) or indirect (stirring performance) oxygen supply 17. Until now, research on algae‐based WWT has focused mainly on the conventional microalgae and cyanobacteria such as Chlorella ssp. 18, Arthrospira ssp. 19, Scenedesmus ssp. 20, and Nannochloropsis ssp. 21, 22 because of their potential to accumulate high levels of lipids and starch. This review provides an overview of these biological systems, with a particular focus on the potential application of extremophilic microalgae (thermophilic, psychrophilic, and acidophilic), the technological systems used for WWT (suspension vs. immobilized systems), and algae based WWT approaches that have already been put into practice.
PRACTICAL APPLICATION
This minireview presents the biological and technological approaches concerning microalgae‐based wastewater treatment technologies. The biological and technical systems must be adapted to the respective wastewater conditions, since the scale and composition of the wastewater sources can vary greatly. The minireview shows different solution strategies, especially for the treatment of industrial wastewater. The special focus is on the distinction between immobilized and suspended biological systems, the potential of extremophilic microalgae and the presentation of plant concepts that have already been implemented on a technical scale.
2. CONVENTIONAL MICROALGAE USED FOR WWT
Photosynthetic microorganisms comprise a wide spectrum of photosynthetically active green, red, and brown algae as well as cyanobacteria. Because of their ability to fix carbon dioxide (CO2) using light as the sole source of energy, they are promising cell factories to produce bio‐based energy carriers and products. Along with photoautotrophy, several microalgal species are capable of performing chemoheterotrophic or mixotrophic metabolism, which is of interest for the treatment of industrial wastewater containing a high organic load. Among the most studied conventional microalgal strains is Chlorella vulgaris, which has been examined recently for its biomass production from food waste compost 23, sludge extracts 24, corn steep liquor, cheese whey and vinasse 25, textile waste effluent 26, tofu wastewater 27, and industrial dairy effluent 28. Zhai et al. 19 have demonstrated the high N (81.51%) and P (80.52%) removal efficiency of the widely used cyanobacterium Arthrospira platensis using a synthetic wastewater. Hena et al. have evaluated the ability of A. platensis to accumulate lipids while undergoing mixotrophic growth on dairy farm wastewater 29, obtaining a total biomass concentration of 4.98 g L−1 and lipid content of 30.23 wt% and thus demonstrating the potential for the production of biofuels. A. platensis was also been applied to the treatment of piggery wastewater 30, http://confectionary effluent 31, composite media made of mineral medium, beet vinasse 32, and distillery wastewater 33.
3. EXTREMOPHILE MICROALGAE — SPECIALISTS FOR HARSH PROCESSING CONDITIONS
Typically, WWT of municipal and agricultural wastewater by microalgae is performed in outdoor conditions at physiological temperatures and pH. However, the parameters of industrial wastewater can fluctuate widely, ranging from highly acidic (2.0 < pH < 8.0) to extreme temperatures (>40°C for process industries, e.g. fermentation residues of bioenergy industry, <10°C in the food processing industry) and high organic loads (>100 g L−1 in fruit processing), which is not compatible with the physiology of most conventional microalgae species. Microalgal specialists that are adapted to thermophilic, psychrophilic, or acidophilic environmental conditions are therefore necessary to realize the degradation of water impurities on the spot of origin (see Table 1). Some of those extremophiles not only tolerate such conditions but require them for their metabolic activity 34.
Table 1.
Overview on wastewater treatment approaches using extremophilic microalgae
| Species | Strain | Cultivation system | Growth conditions | Removal rates | Product | Source |
|---|---|---|---|---|---|---|
| Galderia sulphuraria | CCMEE 5587.1 | 700 L field scale open system | Mixotrophic on raw primary effluent diluted with media and CO2 enriched headspace |
After 3 days: BOD5 36 to 13 mg L−1 N 23 to 2.6 mg L−1 P 4.5 to 0.6 mg L−1 |
Biomass OD750 1.9 | 40 |
| 074G | 3 L bioreactor, 2.5 L culture volume | Heterotrophic on complex media with glucose |
After 100 h: NH3 0.31 to 0.15 g L−1 |
c‐Phycocyanin 250–400 mg L−1 | 36 | |
| CCMEE 5587.1 | Glass tubes, 6 mL culture volume | Heterotrophic in media with primary effluent |
After 7 days: NH3 4.85 mg L−1 d−1 PO4 1.21 mg L−1 d−1 |
Biomass 2.5 g L−1 | 41 | |
| CCMEE 5587.1 | Closed outdoor reactor, 300 L culture volume | Mixotrophic in media with primary effluent and 1‐2% CO2 sparged |
‐ |
Biomass 2.5 g L−1 | 41 | |
| 074G | 500 mL shake flasks, 150 mL culture volume | Heterotrophic bakery and restaurant waste hydrolysates with supplemented N‐sources | ‐ | ‐ | 42 | |
| Chlamydomonas acidophila | River water isolates | 1 L batch reactor | Mixotrophic on several carbon sources | ‐ |
Lutein: 9–10 mg g−1 Zeaxanthin: 7–8 mg g−1 |
43 |
| Chlorella sorokiniana | UTEX 2805 | 1 L batch reactor, 400 mL culture volume | Phototrophic cultivation, cells immobilized in alginate beads, aeriated |
After 4 days: NH3 from 10 to 0 mg L−1 |
‐ | 44 |
| Open pond isolates | Shake flasks, no volume information | Phototrophic growing on post‐chlorinated wastewater supplemented with various N‐sources | ‐ | Max. 0.220 g L−1 d−1 with urea supplementation | 45 | |
| UTEX 1230 | 1 L batch reactor | Phototrophic growth on anaerobic digester centrate and final effluent from municipal WWTP supported with diesel engine flue gas |
CO 20–30% CO2 30–45% NOx 95–100% |
Biomass 250 mg L−1 d−1 | 46 | |
| UTEX 2714 | Hanging bags, 80 L culture volume | Phototrophic growth in 10% anaerobic digester effluent fed with cattle waste, aeriated |
PO4‐P 57.70% TP 64.10% NH3‐N 72.17% TN 87.35% |
Biomass 13–17 mg L−1 d−1 | 47 | |
| Isolated wildtyp | 2 L shake flasks | Phototrophic growth on filtered raw sewage |
COD 69.38% N 86.93% P 68.24% coliforms 99.78% faecal coliforms 100% |
Biomass with 22.36% lipids | 48 |
COD, chemical oxygen demand; N, nitrogen; P, phosphorus.
Galdieria sulphuraria, also denoted as Cyanidium caldarium, is one of the most interesting microalgae with extremophilic growth properties. Gross and Schnarrenberger reported in 1995 that strains of this Rhodophyta (red algae) species are able to grow mixotrophically and heterotrophically on 27 different sugars and sugar alcohols 35, 36. Galdieria sulphuraria is able to grow not only in neutral environments but also in highly acidic environments, down to pH 1.8 37, and G. sulphuraria is able to acidify its environment by an active proton efflux, thus reducing the costs of pH control and the risk of contamination 38, 39.
Besides its acidophilic nature, G. sulphuraria shows thermophilic growth behaviour up to 56°C 41. The economic value of G. sulphuraria is enhanced by high levels of the phycobiliprotein phycocyanin, which is increasingly being accepted as a natural colorant/nutraceutical in the food industry 49, 50, cosmetics industry 51, and as a fluorescence marker in molecular biology 52. This metabolic versatility, coupled with the ability to produce value‐added phycocyanin, makes G. sulphuraria a very promising candidate for treating high chemical oxygen demand‐loaded, acidic or high‐temperature wastewater 53. Sloth et al. 42 have shown the potential of growing G. sulphuraria 074G heterotrophically on hydrolysates of food waste from restaurants and bakeries. In a first field study, Lammers and co‐workers 40 have reported that G. sulphuraria was able to grow well in primary‐settled wastewater while significantly reducing levels of organic carbon (46–72%), ammonium (NH4‐N) (63–89%), and phosphate (PO4) (71‐95%). Further promising acidophilic microalgal strains can be found within the Chlorophyta (green algae). Chlamydomonas acidophila has been isolated from an acidic river in a mining area, with pH values ranging between 1.7 and 3.1 43. It has been shown that C. acidophila can grow mixotrophically without CO2 by using different carbon sources, especially glucose, glycerol, and starch, at pH 2.5 43, and its capacity to remove NH4 54. The added value of C. acidophila biomass from waste sources is its ability to accumulate high concentrations of antioxidants such as the carotenoid lutein 55.
Chlorella protothecoides var. acidicola has been isolated from acidic (pH 2.5–2.6) mine water and has shown good heterotrophic growth on glycolic acid 56, which is part of the wastewater load of fruit and vegetable processing industries. Chlorella sorokiniana, a well‐studied thermophilic green microalgae, has revealed high photoautotrophic growth rates up to 43°C 57. Kim et al. 58 have shown efficient P and N removal rates in heterotrophically grown C. sorokiniana cultures, which is an essential precondition for many WWT processes. In a following study Kim et al. have described superior removal behaviour for heterotrophic C. sorokiniana cultures, compared with photo‐ and mixotrophic cultures 59. Cells of C. sorokiniana can accumulate high levels of valuable bioproducts, e.g. lutein 60, fatty acids 61, 62, and proteins 63, making the sustainably produced biomass a good source for animal feed or biofuel production. The co‐immobilization with the microalgae growth‐promoting bacterium Azospirillum brasilense significantly enhanced the P‐removal efficiency of C. sorokiniana 64, 65.
Another challenge is the energy‐efficient treatment of low‐temperature wastewater. Psychrophilic species such as Koliella antarctica have temperature optima below 10°C 66, making them an interesting potential biological system for treating wastewater from fresh fruit processing industries. Koliella antarctica has also been shown to produce high levels of EPA, DHA, astaxanthin, and lutein 67.
4. (PHOTO‐)BIOREACTOR SYSTEMS FOR ALGAE‐BASED WWT
Activated bacterial sludge processes in stirred ponds are the most widely used WWT technologies, especially for municipal and industrial wastewater. However, as introduced, activated sludge is limited regarding the sufficient N and P removal or elimination of heavy metals without using chemical precipitation 7. The usage of microalgae in WWT is associated with additional technological requirements regarding photobioreactor (PBR) systems. This is mainly because of the photoautotrophic processes, for which a sufficient supply of light energy and CO2 is needed. In general, microalgae PBRs are categorized as open and closed systems, which have already been described in several reviews 68, 69. However, for WWT, classification into suspended and immobilized methods provides a more useful comparison of existing technological approaches.
4.1. Suspended WWT systems
Pond systems are common in bacterial WWT 70. They are also the most widely applied type of large‐scale reactor for microalgae cultivation, because of their simple construction and low investment costs 71. However, because of a higher light path of >30 cm, resulting in a limited light supply, fluctuating outdoor temperatures, and poor mixing capacity, the biomass yield of pond systems is lower compared to tubular systems or more specific PBRs such as flat‐panel PBRs 72, 73 (Figure 2). High‐rate algal ponds try to bypass some of these problems by enhancing the mixing efficiency via paddlewheel stirrers and gas introduction 70. The insufficient supply of CO2 limits algal biomass production because of the unfavourable C:N:P ratio in wastewater 74, but it has been shown that specific aeration and the addition of CO2 can enhance biomass productivity and removal rates of undesired water constituents. The addition of N or P is sometimes used to ensure molar ratios of nutrients for optimal algal growth 75, 76, and co‐cultivation with bacteria can be favourable in relation to heterotrophic oxidation of organic compounds in wastewater by microorganisms that benefit from increased oxygen levels, induced by photoautotrophic algal growth 77, 78, 79. The removal efficiency of total N and P by microalgae from wastewater has been determined to be between 10 and 97% and is highly dependent on culture mode, tank size, type of wastewater, and the microalgae strain 72, 80, 81, 82, 83, indicating that there is no single technology/species combination that is able to fulfil every WWT goal.
Figure 2.
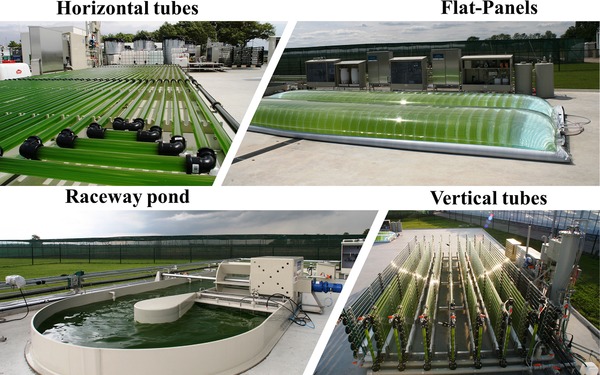
Types of photobioreactors (PBR) systems located at the AlgaePARC at Wageningen University and Research. With kind permission of Marcel Janssen 88
Alternative PBR technologies, such as tubular or flat‐panel PBR systems, are designed to improve light distribution by minimizing the thickness of the surface layer and therefore providing a more efficient light penetration even in highly concentrated suspensions 84. In combination with a controlled environment and effective aeration, for example with bubble columns or other gas‐liquid contractors such as flat‐panel airlift PBRs, the growth rates and productivities are usually higher compared with pond‐based systems 85, 86. However, the investment and maintenance costs of advanced PBRs significantly exceed open pond systems 71, and therefore such systems do not currently prevail in large‐scale WWT but are applied in the production of high‐value metabolites or food products, or the generation of sterile inoculua for further cultivation in raceway ponds 73, 87.
4.2. Immobilized WWT systems
Because of the typically low biomass concentrations at photoautotrophic growth conditions, harvesting and downstream processing are still the most costly steps in microalgae cultivation 84, 85. The small cellular size and high water content of most microalgae further exacerbate the problem of keeping processing costs acceptable. Therefore, microalgae immobilization offers a promising approach to obtaining both processing goals: metabolic conversion of wastewater components and easy and cost‐efficient harvesting of the produced biomass 91.
The technological implementation can be realized in different ways. For pond systems, the Algal Turf Scrubber (ATS) process uses an immobilized community of bacteria, algae, and cyanobacteria in the form of periphyton for the removal of N and P from agricultural and municipal wastewater, inspired by natural wetland ecosystems. It is based on a raceway with a slight slope, covered with a liner as a substrate for periphyton attachment 92. The water is streamed through the growing biomass while the pollutants are degraded or filtrated. To maintain higher growth rates and removal efficiencies, mechanical harvesting is applied periodically 93.
Immobilized systems can be divided into two groups. Fixed‐bed systems rely on a stationary matrix for biomass immobilization, using different types of construction, usually based on porous matrices, fibers, or surfaces. High surface‐to‐volume ratios are crucial for effective growth conditions 74. Hydrophobicity and micro‐ or macro‐structured surfaces promoting stable biofilm formation and material selection seem to be important for biofilm adhesion strength, and therefore in terms of growth potential and removal rates 75, 93, 94. Sukčová et al. were able to demonstrate removal rates of up to 92% by using naturally occurring algae and cyanobacteria on a horizontal flat‐panel PBR made from a concrete slab 95. The revolving algal biofilm reactor, presented in the study of Gross et al. provides an example of another application, with growth rates higher than in suspended culture for Chlorella and an easy harvesting technology 96.
Fluidized bed systems immobilize biomass on a floating substratum that increases the surface‐to‐volume‐ratios even more and enhances light distribution because of an improved mixing capability, adjustable by the movability of the immobilized cells. Examples of common applications include the use of alginate, chitosan, or carrageen beads to fix the biomass. The cells penetrate the porous matrix of the bead and also grow inside it. Fluidized bed systems integrate well with other reactor concepts, such as bubble columns, stirred tanks or ponds, and allow benefit from synergizing characteristics. Growth rates and removal rates can be similar to suspension systems, and sometimes higher. However, in general a direct comparison of removal rates for nutrients or heavy metals is difficult because of the strong dependence on cultivation system, organisms used, immobilization matrix, and pollutant composition. Chevalier et al., Lau et al., and Travesio et al. have reported N removal rates of 100, 95, and 82%, respectively 97, 98, 99; all reported that the rate of phosphate removal was not as high as N, because of the lower demand for cell growth and N:P ratio in cells. However, the experiments of Fierro et al. showed an opposite trend in relation to nitrate and phosphate 100, and Lau et al. demonstrated slightly higher growth constants for carrageen‐immobilized cells of C. vulgaris and the same nutrient‐removal rates as in suspended cultures 101. As described in previous studies, the chlorophyll content of immobilized microalgae was higher than in suspension, because of the self‐shading effect inside the bead matrix 101, 102, 103.
The long‐term stability of bead‐immobilized algal cultures still has to be improved to maintain the high removal rates achieved so far 104, thus knowledge of biofilm formation is needed. As in suspended cultures, a mixture of microalgae and bacterial growth can be beneficial for removal rates, particularly of organic compounds 105, 106. Su et al. successfully co‐cultivated algae and bacteria from activated sludge and reported removal efficiencies for N and P of more than 90% 79. Plant growth‐promoting bacteria such as Azospirillum spp. were also tested to support microalgae cells in attached biofilm cultivations. In immobilized cultures, an increase in growth capacity, higher pigment content or N:P, and changing physiological parameters have been detected, showing a distinct relationship between these bacteria and microalgae populations of different Chlorella species 107, 108, 109. It has been shown that a bacterially overgrown surface can promote biofilm formation or exopolysaccharide production 110, 111. Covarrubias et al. were able to demonstrate a protective function of immobilization in alginate beads. A surface‐attached biofilm of bacteria protected algal cells inside the gel matrix against indigenous natural wastewater micro‐fauna 112.
5. MICROALGAE‐BASED WWT APPROACHES THAT HAVE BEEN PUT INTO PRACTICE
During the last decade, several companies, mainly from the US, UK, and Australia, have started working on algae biomass production using wastewater sources. Despite the limited information on the microalgal species used by these companies, a brief overview of the technological approaches is given here.
The algae WWT plant of Algal Enterprises (Australia) can be applied to the whole spectrum of wastewater sources: municipal, industrial, and agricultural. Photosynthetically active radiation is used as the main energy source by local algae species in a closed PBR system. The algal biomass produced is co‐digested anaerobically to obtain a methane‐rich biogas that is further converted to electricity 113.
The RNEW® technology of Microbio Engineering (US) uses mechanically mixed, CO2‐gassed open raceway ponds to treat N‐ and P‐rich municipal wastewater to produce feedstock biomass for biofuel production 114, 115, 116. Solimeno et al. predicted the proportion of algal and bacterial biomass within the open raceway ponds to 58–68% and 23–30% of total suspended solids, respectively 117. In a following study the algae microbiome of the high‐rate algal ponds was found to be dominated by species of Micractinium ssp., Scenedesmus ssp., Chlorella ssp., and pennate diatoms 118. Oswald Green Technologies has developed the Advanced Integrated Wastewater Pond System (AIWPS®), also known as Energy Ponds™, which works with a symbiotic bacterial algal consortium to capture both organic and inorganic pollutants of municipal, agricultural, and industrial wastewater 119, 120. In a first pretreatment step, the wastewater solids are removed by anaerobic ponds or gravity settlers, followed by the assimilation of organic and inorganic matter by the microalgae in high‐rate algae ponds. The captured algal biomass from the Energy Ponds™ is processed as fertilizer, animal feed and raw material for plastics and biofuel.
Another approach is offered by AlgaeSystems. This US company has developed a low‐cost offshore floating PBR system that is applied in environmental light and CO2 conditions to take up nutrients downstream from their original source 121. The offshore PBR was demonstrated to treat 50 000 gal day−1 of raw municipal wastewater with removal efficiencies of 75% (total N), 93% (total P), and 93% (BOD). After one year of operation, the originally inoculated pure culture of the genus Scenedesmus dimorphus shifted towards a stable operating polyculture of Chlorella ssp., Scenedesmus ssp., and Cryptomonas ssp. 122. The raw algal biomass is processed onshore by hydrothermal liquefaction to yield renewable fuels and fertilizers 122.
Besides the approaches using suspended cultures for WWT, there is a trend in using microalgal biofilms, immobilized microalgae, or microalgae–bacteria co‐cultures, such as those of HydroMentia, OneWater, and Gross‐Wen Technologies (Figure 3). The Algal Turf Scrubber® (ATS) of HydroMentia consists of a flow‐way that is pulsed in waves with the treated wastewater 123, 124. Periphytic algae, which are harvested periodically from the surface of the flow‐way, fix excess nutrients and CO2 from the wastewater. Kangas and Mulbry found a non‐linear relationship between daily operation time and ATS productivity 125. The N and P removal rates for an agricultural drainage ditch were accounted to 125 mg N m−2 d−1 and 25 mg m−2 d−1 125 at the highest flow characteristics and continuous ATS operation. Later, the ATS system was further validated in a couple of studies dealing (waste‐)water originated from an oyster aquaculture facility 126, 127, a Chinese drinking water reservoir 128, and rivers 129. The authors found a high variability in the ATS community structure (∼182 species at 28 m2 growing area) and seasonal biomass productivities (peak production during July/August). The ATS produced algae biomass serves as soil‐enhancing compost and livestock feed but is also intended as a resource for biofuel production 124. The technological approaches of OneWater and Gross‐Wen Technologies are based on immobilized cells in rotating parts of the WWT plant. OneWater has developed the AlgaeWheel® system, an advanced algal‐fixed film technology. The biofilm ecosystem attached to the AlgaeWheels® comprises a diverse group of algae and bacteria, and the synergetic effect of both types of microorganism enhances the treatment efficiency of the overall system 130. The microalgae use sunlight to fix CO2, which is released by the bacteria. The polysaccharides, which are produced by photosynthesis, act as both bacterial nutrient source and solid settlement. In turn, the bacteria are able to use the photosynthetically produced oxygen, resulting in a stable self‐regulating and ecological WWT system 130.
Figure 3.
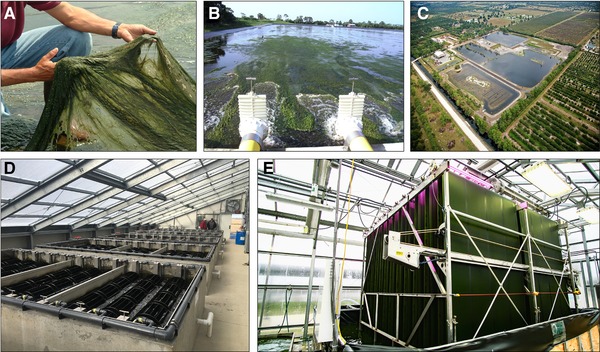
(A‐C) Images of the Algal Turf Scrubber® of HydroMentia, kindly provided by Mark Zivojnovich; (D) AlgaeWheel® system of OneWater Inc. kindly provided by Daniel Johnson and Steve Kingsland; (E) Revolving algal biofilm (RAB) system of Gross‐Wen Technologies kindly provided by Martin Gross
The revolving algal biofilm (RAB) system of Gross‐Wen Technologies is made of an algae biofilm attached to vertically oriented rotating conveyor belts. While performing photoautotrophic growth at the gaseous phase, the attached microalgae fix N and P from the nutrient‐rich liquid 96. The algal biomass of the RAB system can be easily scrapped from the surface of the RAB system avoiding expensive harvesting operations 96. Gross and Wen presented the results of a year‐round operation of the RAB WWT pilot plant at a greenhouse facility at Iowa/USA 131. The authors found a 302% increased biomass productivity compared to control raceway ponds yielding a biomass productivity of 18.9 ash free g m−2 d−1, which was further increased to 46.8 g m−2 d−1 by using a trough‐based RAB configuration 132. Zhou and co‐workers validated the RAB system processing sulphate‐loaded mining wastewater at low pH conditions obtaining a sulphate removal efficiency of 46% with a rate of 0.56 g L−1 d−1 133. A further RAB validation study at pilot scale was performed at supernatant from sludge sedimentation yielding removal rates of 80% (total P) and 87% (total N), respectively. Actually, the biomass produced is sold as fertilizer or feedstock for bioplastics 134.
6. CONCLUDING REMARKS
Clean water has become a limiting resource in many regions of the world. The most efficient approach to reduce the pollution of water resources with nitrates, phosphates, and high organic loads is to remove these components at the point of origin, i.e. at the processing sites. However, conventional biological WWT systems are often unable to fulfil this cleaning task because the pH values, high organics, or temperatures are often non‐compatible to microbiological physiology. Extremophilic microalgae offer a potential means, so‐far largely unexplored, to solve this problem. Microalgae in general, conventional and extremophile can play an important role in a circular bio‐economy by providing high‐quality products, such as proteins, lipids, and colorants, within the biomass produced by the WWT cleaning process. Some selected examples of algae‐based WWT technologies have been reviewed here, with a special focus on concepts that have been validated at technical scale.
CONFLICT OF INTEREST
The authors have declared no conflict of interest
ACKNOWLEDGMENTS
This work was supported by the Federal Ministry of Education and Research (BMBF grant ID Algae4Cycle: 031B0699). The authors further greatly acknowledge Daniel Johnson and Steve Kingsland (OneWater Inc.), Mark Zivojnovich (HydroMentia), and Martin Gross (Gross‐Wen Technologies) for providing pictures of their Algae WWT plants.
Wollmann F, Dietze S, Ackermann J‐U, et al. Microalgae wastewater treatment: Biological and technological approaches. Eng Life Sci. 2019;19:860–871. 10.1002/elsc.201900071
REFERENCES
- 1. Muñoz, I. , Gómez‐Ramos, M. J. , Agüera, A. , Fernández‐Alba, A. R. et al., Chemical evaluation of contaminants in wastewater effluents and the environmental risk of reusing effluents in agriculture. Trends Anal. Chem. 2009, 28, 676–694. [Google Scholar]
- 2. Sousa, J. C. G. , Ribeiro, A. R. , Barbosa, M. O. , Pereira, M. F. R. et al., A review on environmental monitoring of water organic pollutants identified by EU guidelines. J. Hazard. Mater. 2018, 344, 146–162. [DOI] [PubMed] [Google Scholar]
- 3. Eerkes‐Medrano, D. , Leslie, H.A. and Quinn, B. , Microplastics in drinking water: a review and assessment. Curr. Opin. Env. Sci. & Health 2019, 7, 69–75. [Google Scholar]
- 4. Chowdhury, S. , Mazumder, M. A. J. , Al‐Attas, O. and Husain, T. , Heavy metals in drinking water: occurrences, implications, and future needs in developing countries. Sci. Total Environ. 2016, 569–570, 476–488. [DOI] [PubMed] [Google Scholar]
- 5. Menció, A. , Mas‐Pla, J. , Otero, N. , Regàs, O. et al., Nitrate pollution of groundwater; all right…, but nothing else?. Sci. Total Environ. 2016, 539, 241–251. [DOI] [PubMed] [Google Scholar]
- 6. Farmer, A. M. , Phosphate pollution: a global overview of the problem, in: Schaum, C. (Ed.), Phosphorus: Polluter and Resource of the Future, IWA Publishing, London: 2018. [Google Scholar]
- 7. Wang, Q. , Wei, W. , Gong, Y. , Yu, Q. et al., Technologies for reducing sludge production in wastewater treatment plants: state of the art. Sci. Total Environ. 2017, 587–588, 510–521. [DOI] [PubMed] [Google Scholar]
- 8. Hu, J. , Nagarajan, D. , Zhang, Q. , Chang, J.‐S. et al., Heterotrophic cultivation of microalgae for pigment production: a review. Biotechnol. Adv. 2018, 36, 54–67. [DOI] [PubMed] [Google Scholar]
- 9. Subashchandrabose, S. R. , Ramakrishnan, B. , Megharaj, M. , Venkateswarlu, K. et al., Mixotrophic cyanobacteria and microalgae as distinctive biological agents for organic pollutant degradation. Environ. Int. 2013, 51, 59–72. [DOI] [PubMed] [Google Scholar]
- 10. Venkata Mohan, S. , Nikhil, G. N. , Chiranjeevi, P. , Nagendranatha Reddy et al., Waste biorefinery models towards sustainable circular bioeconomy: critical review and future perspectives. Bioresour. Technol. 2016, 215, 2–12. [DOI] [PubMed] [Google Scholar]
- 11. Soto‐Sierra, L. , Stoykova, P. and Nikolov, Z. L. , Extraction and fractionation of microalgae‐based protein products. Algal Res. 2018, 36, 175–192. [Google Scholar]
- 12. Ramesh Kumar, B. , Deviram, G. , Mathimani, T. , Duc, P. A. et al., Microalgae as rich source of polyunsaturated fatty acids. Biocatal. Agric. Biotechnol. 2019, 17, 583–588. [Google Scholar]
- 13. D'Alessandro, E. B. and Antoniosi Filho, N. R. , Concepts and studies on lipid and pigments of microalgae: a review. Renew. Sust. Energ. Rev. 2016, 58, 832–841. [Google Scholar]
- 14. Santos, F. M. and Pires, J. C. M. , Nutrient recovery from wastewaters by microalgae and its potential application as bio‐char. Bioresour. Technol. 2018, 267, 725–731. [DOI] [PubMed] [Google Scholar]
- 15. Yu, K. L. , Show, P. L. , Ong, H. C. , Ling, T. C. et al., Microalgae from wastewater treatment to biochar – feedstock preparation and conversion technologies. Energ. Convers. Manage. 2017, 150, 1–13. [Google Scholar]
- 16. Madeira, M. S. , Cardoso, C. , Lopes, P. A. , Coelho, D. et al., Microalgae as feed ingredients for livestock production and meat quality: a review. Livest. Sci. 2017, 205, 111–121. [Google Scholar]
- 17. Quijano, G. , Arcila, J. S. and Buitrón, G. , Microalgal‐bacterial aggregates: applications and perspectives for wastewater treatment. Biotechnol. Adv. 2017, 35,00 772–781. [DOI] [PubMed] [Google Scholar]
- 18. Chiu, S.‐Y. , Kao, C.‐Y. , Chen, T.‐Y. , Chang, Y.‐B. et al., Cultivation of microalgal Chlorella for biomass and lipid production using wastewater as nutrient resource. Bioresour. Technol. 2015, 184, 179–189. [DOI] [PubMed] [Google Scholar]
- 19. Zhai, J. , Li, X. , Li, W. , Rahaman, M. H. et al., Optimization of biomass production and nutrients removal by Spirulina platensis from municipal wastewater. Ecol. Eng. 2017, 108, 83–92. [Google Scholar]
- 20. Ansari, F. A. , Ravindran, B. , Gupta, S. K. , Nasr, M. et al., Techno‐economic estimation of wastewater phycoremediation and environmental benefits using Scenedesmus obliquus microalgae. J. Environ. Manage. 2019, 240, 293–302. [DOI] [PubMed] [Google Scholar]
- 21. Şirin, S. and Sillanpää, M. , Cultivating and harvesting of marine alga Nannochloropsis oculata in local municipal wastewater for biodiesel. Bioresour. Technol. 2015, 191, 79–87. [DOI] [PubMed] [Google Scholar]
- 22. Mitra, M. , Shah, F. , Bharadwaj, S. V. V. , Patidar, S. K. et al., Cultivation of Nannochloropsis oceanica biomass rich in eicosapentaenoic acid utilizing wastewater as nutrient resource. Bioresour. Technol. 2016, 218, 1178–1186. [DOI] [PubMed] [Google Scholar]
- 23. Chew, K. W. , Chia, S. R. , Show, P. L. , Ling, T. C. et al., Food waste compost as an organic nutrient source for the cultivation of Chlorella vulgaris . Bioresour. Technol. 2018, 267, 356–362. [DOI] [PubMed] [Google Scholar]
- 24. Chen, X. , Zhou, T. , Wang, X. , Xu, P. et al., Cultivation of Chlorella vulgaris in sludge extract from resorcinol‐rich wastewater: the removal and inhibitory effect of sludge toxicity. J. Chem. Technol. Biot. 2019, 94, 1240–1248. [Google Scholar]
- 25. Melo, R. G. d. , Andrade, A. F. d. , Bezerra, R. P. , Correia, D. S. et al., Chlorella vulgaris mixotrophic growth enhanced biomass productivity and reduced toxicity from agro‐industrial by‐products. Chemosphere 2018, 204, 344–350. [DOI] [PubMed] [Google Scholar]
- 26. El‐Kassas, H. Y. and Mohamed, L. A. , Bioremediation of the textile waste effluent by Chlorella vulgaris . Egypt. J. Aquat. Res. 2014, 40, 301–308. [Google Scholar]
- 27. Dianursanti, Rizkytata, B. T. and Gumelar, M. T. , Abdullah, T. H. , industrial tofu wastewater as a cultivation medium of microalgae Chlorella vulgaris . Energy. Proced 2014, 47, 56–61. [Google Scholar]
- 28. Abreu, A. P. , Fernandes, B. , Vicente, A. A. , Teixeira, J. et al., Mixotrophic cultivation of Chlorella vulgaris using industrial dairy waste as organic carbon source. Bioresour. Technol. 2012, 118, 61–66. [DOI] [PubMed] [Google Scholar]
- 29. Hena, S. , Znad, H. , Heong, K. T. and Judd, S. , Dairy farm wastewater treatment and lipid accumulation by Arthrospira platensis . Water Res. 2018, 128, 267–277. [DOI] [PubMed] [Google Scholar]
- 30. Depraetere, O. , Foubert, I. and Muylaert, K. , Decolorisation of piggery wastewater to stimulate the production of Arthrospira platensis . Bioresour. Technol. 2013, 148, 366–372. [DOI] [PubMed] [Google Scholar]
- 31. El‐Kassas, H. Y. , Heneash, A. M. M. and Hussein, N. R. , Cultivation of Arthrospira (Spirulina) platensis using confectionary wastes for aquaculture feeding. J. Gen. Eng. Biotechnol. 2015, 13, 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Coca, M. , Barrocal, V. M. , Lucas, S. , González‐Benito, G. et al., Protein production in Spirulina platensis biomass using beet vinasse‐supplemented culture media. Food Bioprod. Process. 2015, 94, 306–312. [Google Scholar]
- 33. Krishnamoorthy, S. , Manickam, P. and Muthukaruppan, V. , Evaluation of distillery wastewater treatability in a customized photobioreactor using blue‐green microalgae – laboratory and outdoor study. J. Environ. Manage. 2019, 234, 412–423. [DOI] [PubMed] [Google Scholar]
- 34. Varshney, P. , Mikulic, P. , Vonshak, A. , Beardall, J. et al., Extremophilic micro‐algae and their potential contribution in biotechnology. Bioresour. Technol. 2015, 184, 363–372. [DOI] [PubMed] [Google Scholar]
- 35. Gross, W. and Schnarrenberger, C. , Heterotrophic growth of two strains of the acido‐thermophilic red alga Galdieria sulphuraria . Plant Cell Physiol. 1995, 36, 633–638. [Google Scholar]
- 36. Schmidt, R. A. , Wiebe, M. G. and Eriksen, N. T. , Heterotrophic high cell‐density fed‐batch cultures of the phycocyanin‐producing red alga Galdieria sulphuraria . Biotechnol. Bioeng. 2005, 90, 77–84. [DOI] [PubMed] [Google Scholar]
- 37. Merola, A. , Castaldo, R. , Luca, P. D. , Gambardella, R. et al., Revision of Cyanidium caldarium. Three species of acidophilic algae. Giornale botanico italiano. 1981, 115, 189–195. [Google Scholar]
- 38. Enami, I. and Kura‐Hotta, M. , Effect of Intracellular ATP Levels on the light‐induced H+ efflux from intact cells of Cyanidium caldarium1. Plant Cell Physiol. 1984, 25, 1107–1113. [Google Scholar]
- 39. Delanka‐Pedige, H. M. K. , Munasinghe‐Arachchige, S. P. , Cornelius, J. , Henkanatte‐Gedera, S. M. et al., Pathogen reduction in an algal‐based wastewater treatment system employing. Galdieria sulphuraria. Algal Res. 2019, 39, 101423. [Google Scholar]
- 40. Henkanatte‐Gedera, S. M. , Selvaratnam, T. , Karbakhshravari, M. , Myint, M. et al., Removal of dissolved organic carbon and nutrients from urban wastewaters by Galdieria sulphuraria: laboratory to field scale demonstration. Algal Res. 2017, 24, 450–456. [Google Scholar]
- 41. Selvaratnam, T. , Pegallapati, A. K. , Montelya, F. , Rodriguez, G. et al., Evaluation of a thermo‐tolerant acidophilic alga, Galdieria sulphuraria, for nutrient removal from urban wastewaters. Bioresour. Technol. 2014, 156, 395–399. [DOI] [PubMed] [Google Scholar]
- 42. Sloth, J. K. , Jensen, H. C. , Pleissner, D. and Eriksen, N. T. Growth and phycocyanin synthesis in the heterotrophic microalga Galdieria sulphuraria on substrates made of food waste from restaurants and bakeries. Bioresource Technol. 2017, 238, 296–305. [DOI] [PubMed] [Google Scholar]
- 43. Cuaresma, M. , Casal, C. , Forján, E. and Vílchez, C. , Productivity and selective accumulation of carotenoids of the novel extremophile microalga Chlamydomonas acidophila grown with different carbon sources in batch systems. J. Ind. Microbiol. Biot. 2011, 38, 167–177. [DOI] [PubMed] [Google Scholar]
- 44. de‐Bashan, L. E. , Trejo, A. , Huss, V. A. R. , Hernandez, J.‐P. et al., Chlorella sorokiniana UTEX 2805, a heat and intense, sunlight‐tolerant microalga with potential for removing ammonium from wastewater. Bioresour. Technol. 2008, 99, 4980–4989. [DOI] [PubMed] [Google Scholar]
- 45. Ramanna, L. , Guldhe, A. , Rawat, I. and Bux, F. , The optimization of biomass and lipid yields of Chlorella sorokiniana when using wastewater supplemented with different nitrogen sources. Bioresour. Technol. 2014, 168, 127–135. [DOI] [PubMed] [Google Scholar]
- 46. Lizzul, A. M. , Hellier, P. , Purton, S. , Baganz, F. et al., Combined remediation and lipid production using Chlorella sorokiniana grown on wastewater and exhaust gases. Bioresour. Technol. 2014, 151, 12–18. [DOI] [PubMed] [Google Scholar]
- 47. Kobayashi, N. , Noel, E. A. , Barnes, A. , Watson, A. et al., Characterization of three Chlorella sorokiniana strains in anaerobic digested effluent from cattle manure. Bioresour. Technol. 2013, 150, 377–386. [DOI] [PubMed] [Google Scholar]
- 48. Gupta, S. K. , Ansari, F. A. , Shriwastav, A. , Sahoo, N. K. et al., Dual role of Chlorella sorokiniana and Scenedesmus obliquus for comprehensive wastewater treatment and biomass production for bio‐fuels. J. Clean. Prod. 2016, 115, 255–264. [Google Scholar]
- 49. Fernández‐Rojas, B. , Hernández‐Juárez, J. and Pedraza‐Chaverri, J. Nutraceutical properties of phycocyanin. J. Func. Food. 2014, 11, 375–392. [Google Scholar]
- 50. Greque, d. M. M. , Denise, d. F. P. , Botelho, M. J. , Hartwig, D. J. et al., Phycocyanin from microalgae: properties, extraction and purification, with some recent applications. Ind. Biotechnol. 2018, 14, 30–37. [Google Scholar]
- 51. Couteau, C. and Coiffard, L. , Chapter 15 ‐ microalgal application in cosmetics, in: Levine I. A. and Fleurence J. (Eds.) Microalgae in Health and Disease Prevention, Academic Press, 2018, pp. 317–323. [Google Scholar]
- 52. Pagels, F. , Guedes, A. C. , Amaro, H. M. , Kijjoa, A. et al., Phycobiliproteins from cyanobacteria: chemistry and biotechnological applications. Biotechnol. Adv. 2019, 37, 422–443. [DOI] [PubMed] [Google Scholar]
- 53. Wan, M. , Wang, Z. , Zhang, Z. , Wang, et al., A novel paradigm for the high‐efficient production of phycocyanin from Galdieria sulphuraria . Bioresour. Technol. 2016, 218, 272–278. [DOI] [PubMed] [Google Scholar]
- 54. Escudero, A. , Blanco, F. , Lacalle, A. and Pinto, M. Ammonium removal from anaerobically treated effluent by Chlamydomonas acidophila . Bioresour. Technol. 2014, 153, 62–68. [DOI] [PubMed] [Google Scholar]
- 55. Garbayo, I. , Cuaresma, M. , Vílchez, C. and Vega, J. M. , Effect of abiotic stress on the production of lutein and β‐carotene by Chlamydomonas acidophila . Process Biochem. 2008, 43, 1158–1161. [Google Scholar]
- 56. Nancucheo, I. and Barrie Johnson, D. , Acidophilic algae isolated from mine‐impacted environments and their roles in sustaining heterotrophic acidophiles. Front. Microbiol. 2012, 3, 325‐325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Varshney, P. , Beardall, J. , Bhattacharya, S. and Wangikar, P. P. Isolation and biochemical characterisation of two thermophilic green algal species‐ Asterarcys quadricellulare and Chlorella sorokiniana, which are tolerant to high levels of carbon dioxide and nitric oxide. Algal Res. 2018, 30, 28–37. [Google Scholar]
- 58. Kim, S. , Lee, Y. and Hwang, S.‐J. , Removal of nitrogen and phosphorus by Chlorella sorokiniana cultured heterotrophically in ammonia and nitrate. Int Biodeter Biodeg. 2013, 85, 511–516. [Google Scholar]
- 59. Kim, S. , Park, J.‐e. , Cho, Y.‐B. and Hwang, S.‐J. , Growth rate, organic carbon and nutrient removal rates of Chlorella sorokiniana in autotrophic, heterotrophic and mixotrophic conditions. Bioresour. Technol. 2013, 144, 8–13. [DOI] [PubMed] [Google Scholar]
- 60. Chen, C.‐Y. , Lu, I.C. , Nagarajan, D. , Chang, C.‐H. et al., A highly efficient two‐stage cultivation strategy for lutein production using heterotrophic culture of Chlorella sorokiniana MB‐1‐M12. Bioresour. Technol. 2018, 253, 141–147. [DOI] [PubMed] [Google Scholar]
- 61. Chen, F. and Johns, M. R. , Effect of C/N ratio and aeration on the fatty acid composition of heterotrophic Chlorella sorokiniana . J. Appl. Phycol. 1991, 3, 203–209. [Google Scholar]
- 62. León‐Vaz, A. , León, R. , Díaz‐Santos, E. , Vigara, J. , et al., Using agro‐industrial wastes for mixotrophic growth and lipids production by the green microalga Chlorella sorokiniana . New Biotechnol. 2019, 51, 31–38. [DOI] [PubMed] [Google Scholar]
- 63. Blackburn, S. I. and Volkman, J. K. , Microalgae: a renewable source of bioproducts, in: Dunford N. T. (Eds.) Food and Industrial Bioproducts and Bioprocessing, John Wiley & Sons, Ltd, 2012, pp. 221–241. [Google Scholar]
- 64. Hernandez, J.‐P. , de‐Bashan, L. E. and Bashan, Y. , Starvation enhances phosphorus removal from wastewater by the microalga Chlorella spp. co‐immobilized with Azospirillum brasilense . Enzyme Microb. Tech. 2006, 38, 190–198. [Google Scholar]
- 65. Hernández, D. , Riaño, B. , Coca, M. and García‐González, M. C. , Treatment of agro‐industrial wastewater using microalgae–bacteria consortium combined with anaerobic digestion of the produced biomass. Bioresour. Technol. 2013, 135, 598–603. [DOI] [PubMed] [Google Scholar]
- 66. Andreoli, C. , Lokhorst, G. M. , Mani, A. M. , Scarabel, L. , et al., Koliella antarctic sp. nov. (Klebsormidiales) a new marine green microalga from the Ross Sea (Antarctica). Algological Studies/Archiv Für Hydrobiologie. Suppl. Vol. 1998, 90, 1–8. [Google Scholar]
- 67. Fogliano, V. , Andreoli, C. , Martello, A. , Caiazzo, M. et al., Functional ingredients produced by culture of Koliella antarctica . Aquaculture 2010, 299, 115–120. [Google Scholar]
- 68. Christenson, L. and Sims, R. , Production and harvesting of microalgae for wastewater treatment, biofuels, and bioproducts. Biotechnol. Adv. 2011, 29, 686–702. [DOI] [PubMed] [Google Scholar]
- 69. Cai, T. , Park, S. Y. and Li, Y. , Nutrient recovery from wastewater streams by microalgae: status and prospects. Renew. Sust. Energ. Rev. 2013, 19, 360–369. [Google Scholar]
- 70. Young, P. , Taylor, M. and Fallowfield, H. J. , Mini‐review: High rate algal ponds, flexible systems for sustainable wastewater treatment. World J. Microb. Biot. 2017, 33, 117. [DOI] [PubMed] [Google Scholar]
- 71. Barry, A. , Wolfe, A. , English, C. , Ruddick, C. et al., National Algal Biofuels Technology Review , 2016.
- 72. Park, J. B. K. , Craggs, R. J. and Shilton, A. N. , Wastewater treatment high rate algal ponds for biofuel production. Bioresour. Technol. 2011, 102, 35–42. [DOI] [PubMed] [Google Scholar]
- 73. Pulz, O. , Photobioreactors: production systems for phototrophic microorganisms. App. Microbiol. Biot. 2001, 57, 287–293. [DOI] [PubMed] [Google Scholar]
- 74. Kesaano, M. and Sims, R. C. , Algal biofilm based technology for wastewater treatment. Algal Res. 2014, 5, 231–240. [Google Scholar]
- 75. Christenson, L. B. and Sims, R. C. , Rotating algal biofilm reactor and spool harvester for wastewater treatment with biofuels by‐products. Biotechnol. Bioeng. 2012, 109, 1674–1684. [DOI] [PubMed] [Google Scholar]
- 76. Boelee, N. C. , Temmink, H. , Janssen, M. , Buisman, C. J. N. et al., Scenario analysis of nutrient removal from municipal wastewater by microalgal biofilms. Water 2012, 4, 460–473. [DOI] [PubMed] [Google Scholar]
- 77. Craggs, R. , Sutherland, D. and Campbell, H. , Hectare‐scale demonstration of high rate algal ponds for enhanced wastewater treatment and biofuel production. J. Appl. Phycol. 2012, 24, 329–337. [Google Scholar]
- 78. El Hamouri, B. , Rethinking natural, extensive systems for tertiary treatment purposes: the high‐rate algae pond as an example. Desal. Water Treat. 2012, 4, 128–134. [Google Scholar]
- 79. Su, Y. , Mennerich, A. and Urban, B. , Synergistic cooperation between wastewater‐born algae and activated sludge for wastewater treatment: influence of algae and sludge inoculation ratios. Bioresour. Technol. 2012, 105, 67–73. [DOI] [PubMed] [Google Scholar]
- 80. Chen, P. , Zhou, Q. , Paing, J. , Le, H. et al., Nutrient removal by the integrated use of high rate algal ponds and macrophyte systems in China. Water Sci. Technol. 2003, 48, 251–257. [PubMed] [Google Scholar]
- 81. Shelef, G. , Azov, Y. and Moraine, R. , Nutrients removal and recovery in a two‐stage high‐rate algal wastewater treatment system. Water Sci. Technol. 1982, 14, 87–100. [Google Scholar]
- 82. El Hafiane, F. and El Hamouri, B. , Anaerobic reactor/high rate pond combined technology for sewage treatment in the Mediterranean area. Water Sci. Technol. 2005, 51, 125–132. [PubMed] [Google Scholar]
- 83. Craggs, R. J. , Davies‐Colley, R. J. , Tanner, C. C. and Sukias, J. P. , Advanced pond system: performance with high rate ponds of different depths and areas. Water Sci. Technol. 2003, 48, 259–267. [PubMed] [Google Scholar]
- 84. Posten, C. , Aquatische biomasse: verfahrenstechnische grundlagen, in: Kaltschmitt M., Hartmann H., Hofbauer H. (Eds.), Energie aus Biomasse: Grundlagen, Techniken und Verfahren, Springer Berlin Heidelberg, Berlin, Heidelberg: 2016, pp. 254–272. [Google Scholar]
- 85. Arbib, Z. , Ruiz, J. , Álvarez‐Díaz, P. , Garrido‐Pérez, C. et al., Long term outdoor operation of a tubular airlift pilot photobioreactor and a high rate algal pond as tertiary treatment of urban wastewater. Ecol. Eng. 2013, 52, 143–153. [Google Scholar]
- 86. Mata, T. M. , Martins, A. A. , Caetano, N. S. , Microalgae for biodiesel production and other applications: a review. Renew. Sust. Energ. Rev. 2010, 14, 217–232. [Google Scholar]
- 87. Rodolfi, L. , Chini Zittelli, G. , Bassi, N. , Padovani, G. et al., Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low‐cost photobioreactor. Biotechnol. Bioeng. 2009, 102, 100–112. [DOI] [PubMed] [Google Scholar]
- 88. de Vree, J. H. , Bosma, R. , Janssen, M. , Barbosa, M. J. et al., Comparison of four outdoor pilot‐scale photobioreactors. Biotechnol. Biofuels 2015, 8, 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Greenwell, H. C. , Laurens, L. M. L. , Shields, R. J. , Lovitt, R. W. et al., Placing microalgae on the biofuels priority list: a review of the technological challenges. J. R. Soc. Interface. 2010, 7, 703–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sander, K. and Murthy, G. S. , Life cycle analysis of algae biodiesel. Int. J. Life Cycle Assess. 2010, 15, 704–714. [Google Scholar]
- 91. Lam, M. K. and Lee, K. T. , Immobilization as a feasible method to simplify the separation of microalgae from water for biodiesel production. Chem. Eng. J. 2012, 191, 263–268. [Google Scholar]
- 92. Craggs, R. J. , Adey, W. H. , Jenson, K. R. , St. John, M. S. et al., Phosphorus removal from wastewater using an algal turf scrubber. Water Sci. Technol. 1996, 33. [Google Scholar]
- 93. Sekar, R. , Venugopalan, V. P. , Satpathy, K. K. , Nair, K. V. K. et al., Laboratory studies on adhesion of microalgae to hard substrates. Hydrobiologia. 2004, 512, 109–116. [Google Scholar]
- 94. Ozkan A., Berberoglu H. (Eds.), Adhesion of Chlorella vulgaris on hydrophilic and hydrophobic surfaces. ASME 2011 International Mechanical Engineering Congress and Exposition, Denver, Colorado, USA, November 11–17, 2011, ASME, New York, NY: 2012. [Google Scholar]
- 95. Sukačová, K. , Trtílek, M. and Rataj, T. , Phosphorus removal using a microalgal biofilm in a new biofilm photobioreactor for tertiary wastewater treatment. Water Res. 2015, 71, 55–63. [DOI] [PubMed] [Google Scholar]
- 96. Gross, M. , Henry, W. , Michael, C. and Wen, Z. , Development of a rotating algal biofilm growth system for attached microalgae growth with in situ biomass harvest. Bioresource Technol. 2013, 150, 195–201. [DOI] [PubMed] [Google Scholar]
- 97. Chevalier, P. , Noüe, La and de, J. , Efficiency of immobilized hyperconcentrated algae for ammonium and orthophosphate removal from wastewaters. Biotechnol. Lett. 1985, 7, 395–400. [Google Scholar]
- 98. Lau, P. S. , Tam, N. F. Y. and Wong, Y. S. , Wastewater nutrients (N and P) removal by carrageenan and alginate immobilized Chlorella Vulgaris . Environ. Technol. 1997, 18, 945–951. [Google Scholar]
- 99. Travieso, L. , Benitez, F. , Weiland, P. , Sánchez, E. , et al., Experiments on immobilization of microalgae for nutrient removal in wastewater treatments. Bioresour. Technol. 1996, 55, 181–186. [Google Scholar]
- 100. Fierro, S. , Sánchez‐Saavedra, M. d. P. and Copalcúa, C. , Nitrate and phosphate removal by chitosan immobilized Scenedesmus . Bioresour. Technol. 2008, 99, 1274–1279. [DOI] [PubMed] [Google Scholar]
- 101. Lau, P. S. , Tam, N. F. Y. and Wong, Y. S. , Effect of carrageenan immobilization on the physiological activities of Chlorella vulgaris . Bioresour. Technol. 1998, 63, 115–121. [Google Scholar]
- 102. Robinson, P. K. , Dainty, A. L. , Goulding, K. H. , Simpkins, I. et al., Physiology of alginate‐immobilized Chlorella . Enzyme Microb. Tech. 1985, 7, 212–216. [Google Scholar]
- 103. Bailliez, C. , Largeau, C. , Berkaloff, C. and Casadevall, E. , Immobilization of Botryococcus braunii in alginate: Influence on chlorophyll content, photosynthetic activity and degeneration during batch cultures. Appl. Microbiol. Biotechnol. 1986, 23. [Google Scholar]
- 104. Ruiz‐Marin, A. , Mendoza‐Espinosa, L. G. and Stephenson, T. , Growth and nutrient removal in free and immobilized green algae in batch and semi‐continuous cultures treating real wastewater. Bioresour. Technol. 2010, 101, 58–64. [DOI] [PubMed] [Google Scholar]
- 105. Muñoz, R. and Guieysse, B. , Algal‐bacterial processes for the treatment of hazardous contaminants: a review. Water Res. 2006, 40, 2799–2815. [DOI] [PubMed] [Google Scholar]
- 106. Muñoz, R. , Köllner, C. , Guieysse, B. and Mattiasson, B. , Salicylate biodegradation by various algal‐bacterial consortia under photosynthetic oxygenation. Biotechnol. Lett. 2003, 25, 1905–1911. [DOI] [PubMed] [Google Scholar]
- 107. de‐Bashan, L. E. , Moreno, M. , Hernandez, J.‐P. and Bashan, Y. , Removal of ammonium and phosphorus ions from synthetic wastewater by the microalgae Chlorella vulgaris coimmobilized in alginate beads with the microalgae growth‐promoting bacterium Azospirillum brasilense . Water Res. 2002, 36, 2941–2948. [DOI] [PubMed] [Google Scholar]
- 108. de‐Bashan, L. E. , Bashan, Y. , Moreno, M. , Lebsky, V. K. et al., Increased pigment and lipid content, lipid variety, and cell and population size of the microalgae Chlorella spp. when co‐immobilized in alginate beads with the microalgae‐growth‐promoting bacterium Azospirillum brasilense. Can. J . Microbiol. 2002, 48, 514–521. [DOI] [PubMed] [Google Scholar]
- 109. de‐Bashan, L. E. and Bashan, Y. , Joint immobilization of plant growth‐promoting bacteria and green microalgae in alginate beads as an experimental model for studying plant‐bacterium interactions. Appl. Environ. Microbiol. 2008, 74, 6797–6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Hodoki, Y. , Bacteria biofilm encourages algal immigration onto substrata in lotic systems. Hydrobiologia 2005, 539, 27–34. [Google Scholar]
- 111. Holmes, P. E. , Bacterial enhancement of vinyl fouling by algae. Appl. Environ. Microbiol. 1986, 52, 1391–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Covarrubias, S. A. , de‐Bashan, L. E. , Moreno, M. and Bashan, Y. , Alginate beads provide a beneficial physical barrier against native microorganisms in wastewater treated with immobilized bacteria and microalgae. Appl. Environ. Microbiol. 2012, 93, 2669–2680. [DOI] [PubMed] [Google Scholar]
- 113. Montingelli, M. E. , Tedesco, S. and Olabi, A. G. , Biogas production from algal biomass: a review. Renew Sust Energ Rev. 2015, 43, 961–972. [Google Scholar]
- 114. Craggs, R. J. , Heubeck, S. , Lundquist, T. J. and Benemann, J. R. , Algal biofuels from wastewater treatment high rate algal ponds. Water Sci Technol. 2011, 63, 660–665. [DOI] [PubMed] [Google Scholar]
- 115. Bailey Green, F. , Bernstone, L. S. , Lundquist, T. J. and Oswald, W. J. , Advanced integrated wastewater pond systems for nitrogen removal. Water Sci Technol. 1996, 33, 207–217. [Google Scholar]
- 116. Craggs, R. J. , Lundquist, T. J. and Benemann, J. R. , Wastewater treatment and algal biofuel production, in: Borowitzka M. A., Moheimani N. R. (Eds.), Algae for Biofuels and Energy, Springer Netherlands, Dordrecht: 2013, pp. 153–163. [Google Scholar]
- 117. Solimeno, A. , Parker, L. , Lundquist, T. and García, J. , Integral microalgae‐bacteria model (BIO_ALGAE): Application to wastewater high rate algal ponds. Sci Total Environ. 2017, 601–602, 646–657. [DOI] [PubMed] [Google Scholar]
- 118. Bohutskyi, P. , Spierling, R. E. , Phan, D. , Kopachevsky, A. M. et al., Bioenergy from wastewater resources: nutrient removal, productivity and settleability of indigenous algal‐bacteria polyculture, and effect of biomass composition variability on methane production kinetics and anaerobic digestion energy balance. Algal Res. 2018, 36, 217–228. [Google Scholar]
- 119. Green, F. B. , Lundquist, T. J. and Oswald, W. J. , Energetics of advanced integrated wastewater pond systems. Water Sci Technol. 1995, 31, 9–20. [PubMed] [Google Scholar]
- 120. Nurdogan, Y. and Oswald, W. J. , Enhanced nutrient removal in high‐rate ponds. Water Sci Technol. 1995, 31, 33–43. [Google Scholar]
- 121. Green, F. B. , Lundquist, T. J. , Quinn, N. W. T. , Zarate, M. A. et al., Selenium and nitrate removal from agricultural drainage using the AIWPS® technology. Water Sci Technol. 2003, 48, 299–305. [PubMed] [Google Scholar]
- 122. Novoveská, L. , Zapata, A. K. M. , Zabolotney, J. B. , Atwood, M. C. et al., Optimizing microalgae cultivation and wastewater treatment in large‐scale offshore photobioreactors. Algal Res. 2016, 18, 86–94. [Google Scholar]
- 123. Mulbry, W. , Kangas, P. and Kondrad, S. , Toward scrubbing the bay: nutrient removal using small algal turf scrubbers on Chesapeake Bay tributaries. Ecol Eng. 2010, 36, 536–541. [Google Scholar]
- 124. Adey, W. H. , Kangas, P. C. and Mulbry, W. , Algal turf scrubbing: cleaning surface waters with solar energy while producing a biofuel. Bioscience 2011, 61, 434–441. [Google Scholar]
- 125. Kangas, P. and Mulbry, W. , Nutrient removal from agricultural drainage water using algal turf scrubbers and solar power. Bioresour. Technol. 2014, 152, 484–489. [DOI] [PubMed] [Google Scholar]
- 126. Ray, N. E. , Li, J. , Kangas, P. C. and Terlizzi, D. E. , Water quality upstream and downstream of a commercial oyster aquaculture facility in Chesapeake Bay, USA. Aquacult Eng. 2015, 68, 35–42. [Google Scholar]
- 127. Ray, N. E. , Terlizzi, D. E. and Kangas, P. C. , Nitrogen and phosphorus removal by the Algal Turf Scrubber at an oyster aquaculture facility. Ecol Eng.2015, 78, 27–32. [Google Scholar]
- 128. Chen, N. , Li, J. , Wu, Y. , Kangas, P. C. et al., Nutrient removal at a drinking water reservoir in China with an algal floway. Ecol Eng.2015, 84, 506–514. [Google Scholar]
- 129. Kangas, P. , Mulbry, W. , Klavon, P. , Laughinghouse, H. D. et al., High diversity within the periphyton community of an algal turf scrubber on the Susquehanna river. Ecol Eng. 2017, 108, 564–572. [Google Scholar]
- 130. Johnson, D. B. , Schideman, L. C. , Canam, T. and Hudson, R. J. M. , Pilot‐scale demonstration of efficient ammonia removal from a high‐strength municipal wastewater treatment sidestream by algal‐bacterial biofilms affixed to rotating contactors. Algal Res. 2018, 34, 143–153. [Google Scholar]
- 131. Gross, M. and Wen, Z. , Yearlong evaluation of performance and durability of a pilot‐scale Revolving Algal Biofilm (RAB) cultivation system. Bioresour. Technol. 2014, 171, 50–58. [DOI] [PubMed] [Google Scholar]
- 132. Gross, M. , Mascarenhas, V. and Wen, Z. , Evaluating algal growth performance and water use efficiency of pilot‐scale revolving algal biofilm (RAB) culture systems. Biotechnol Bioeng. 2015, 112, 2040–2050. [DOI] [PubMed] [Google Scholar]
- 133. Zhou, H. , Sheng, Y. , Zhao, X. , Gross, M. et al., Treatment of acidic sulfate‐containing wastewater using revolving algae biofilm reactors: sulfur removal performance and microbial community characterization. Bioresour. Technol. 2018, 264, 24–34. [DOI] [PubMed] [Google Scholar]
- 134. Zhao, X. , Kumar, K. , Gross, M. A. , Kunetz, T. E. et al., Evaluation of revolving algae biofilm reactors for nutrients and metals removal from sludge thickening supernatant in a municipal wastewater treatment facility. Water Res. 2018, 143, 467–478. [DOI] [PubMed] [Google Scholar]


