Abstract
Plant biotechnology can be used to conserve the germplasm of natural forests, and to increase the productivity and sustainability of plantations. Both goals imply working with mature trees, which are often recalcitrant to micropropagation. Conventional in vitro culture uses closed containers and gelled medium with sugar supplementation. Bioreactor culture uses liquid medium and usually incorporates aeration. The increased absorption of nutrients via the liquid medium together with the renewal of the air inside the bioreactors may improve the physiological state of the explants. In this review, we will explore the feasibility of using bioreactors to overcome the recalcitrance of many trees to micropropagation and/or to decrease the cost of large‐scale propagation. We will focus on the recent use of bioreactors during the multiplication, rooting (plant conversion in the case of somatic embryos), and acclimation stages of the micropropagation of axillary shoots and somatic embryos of forest trees (including some shrubs of commercial interest), in both temporary and continuous immersion systems. We will discuss the advantages and the main obstacles limiting the widespread implementation of bioreactor systems in woody plant culture, considering published scientific reports and contributions from the business sector.
Keywords: axillary shoots, continuous immersion, rooting, somatic embryos, temporary immersion
Abbreviations
- CIS
continuous immersion system
- PAM
photoautotrophic micropropagation
- RITA
recipient for automated temporary immersion
- SS
semisolid medium
- TIS
temporary immersion system
1. INTRODUCTION
From an ecological point of view, forest species are essential for the preservation of ecosystems and the general equilibrium of the biosphere. From a human perspective, forest species also represent a source of raw materials in economically important sectors such as the buildings and paper industries. Trees provide food, resins, and medicinal products, and they can also be used in phytoremediation. Despite the difficulties associated with the economic quantification of this contribution, forest trees play a central role in maintaining landscapes and in establishing recreation areas.
Because of the wide range of possible uses for trees, many areas formerly occupied by natural forests have been transformed into plantations managed for productive purposes. In this context, scientific approaches should consider trees in natural forests and also the “domesticated” trees used in plantations 1. Increasing the productivity of plantations should decrease the pressure to allocate more land for this purpose, thus mitigating the associated risks, such as replacement of native species and potential long‐term loss of diversity at the landscape level 2, 3.
Natural forests are extraordinarily valuable as reservoirs of genetic diversity. In addition, underused “wild” woody plants will probably become more important in the fight against climate change or other environmental problems in the near future, and they may also be discovered to be important in new profitable applications. For these wild trees, germplasm conservation should be the main scientific focus 4, whereas for trees used in plantations the priority goals should be to balance the increased productivity with environmental sustainability.
Plant biotechnology (including in vitro culture) can be used to preserve valuable genotypes, to propagate superior material on a large scale, to develop physiological studies, and even to obtain genetically transformed trees. Protocols have been developed for the micropropagation of many different tree species 5, 6, 7, but recalcitrance to in vitro culture and some characteristics of forest trees hinder the applicability of these protocols to large‐scale propagation and to the preservation of elite trees.
The rapid improvement of trees through sexual breeding is restricted by the high heterozygosity and the long life cycles of forest trees 8. However, vegetative propagation enables the capture of additive and non‐additive genetic gain derived by selection 3, 9. Trees do not exhibit stable desirable traits until they have reached maturity. In order to maximize genetic gain, phenotypically characterized adult trees should be selected and used for micropropagation 10, 11, 12. However, vegetative propagation of woody plants becomes increasingly difficult as the trees age 13, 14. Despite major advances in forest biotechnology, clonal regeneration by somatic embryogenesis or organogenesis remains difficult for many tree species and is often limited to juvenile explants 10. In comparison with juvenile material, mature plants usually prove more recalcitrant to the establishment of aseptic and reactive cultures, multiplication (usually hindered by the several months required for culture stabilization), adventitious rooting (or plant conversion in the case of somatic embryos), and acclimation 15, 16, 17, 18. Moreover, genotypic differences within tree species in relation to the response to each stage of micropropagation suggest that current protocols are not efficient enough for commercial application, which requires homogenous performance for a wide spectrum of proven genotypes 19. Although it appears from the literature that micropropagation protocols have been successfully established for various forest tree species, this is probably only true at an experimental scale and not operationally, as further development of micropropagation remains hindered by serious limitations, as highlighted by Monteuuis 20.
In this review, we will explore the feasibility of using bioreactors to overcome these limitations. It has been claimed that the increased absorption of nutrients via the liquid medium, together with the renewal of the air inside the bioreactors may improve the physiological state of the explants and make them more competent to undergo rooting and acclimation 21, 22, 23, 24, 25, 26, 27. We will focus on the recent use of bioreactors in the micropropagation of axillary shoots or somatic embryos of forest trees (including some shrubs of commercial interest) during the following stages: i) multiplication, ii) rooting or plant conversion, and iii) acclimation. The advantages and the main obstacles limiting the widespread use of bioreactors in woody plant culture will be discussed, and published scientific reports and contributions from the business sector will be considered. Regarding the type of bioreactor, we will consider both temporary and continuous immersion systems (CIS). In order to stay within the length restrictions for this review paper, we will mainly focus on tree species that form an important part of natural forests or plantations, or that are currently being used for reforestation and afforestation activities. Regarding these trees, we will select those reports in which protocols are sufficiently well developed to be applied to plant production.
PRACTICAL APPLICATION
This review has been written as a contribution for the Special Issue Plant Cells and Algae in bioreactors. The aims of this work are: (1) To highlight the specific difficulties for the micropropagation of forest trees, (2) to review the current state of the application of bioreactors to these trees, and (3) to evaluate if using bioreactors is possible to overcome the recalcitrance of some trees for micropropagation.
2. BIOREACTORS SYSTEMS FOR THE PROPAGATION OF TREES
The term bioreactor describes large‐scale vessels used for plant biomass production. Bioreactors were first developed for culturing microorganisms, then for plant cell suspensions for secondary metabolite production, and later for plant propagation purposes. The aim of bioreactor application is to provide optimum growth conditions by regulating chemical or physical parameters, in order to achieve either both maximum yield and high quality of the explants, or to keep the production costs as low as possible by integration of automated facilities and simple low‐cost devices 28. Among the many categories in which bioreactors can be classified, here we will distinguish between continuous immersion and temporary immersion bioreactors.
The culture of a plant in a CIS means that the liquid medium is continuously in contact with at least one section of the explant. Stationary immersion of the whole explant usually causes hyperhydricity and malformations, since oxygen concentration in liquid media is often insufficient to meet the respiratory requirements of the submerged tissues 29, 30, 31. Oxygen depletion in plant cells induces oxidative stress, with production of reactive oxygen species, and therefore causes injury to the plant tissue 30. To avoid these problems, oxygen can be provided by agitation and/or aeration, or by maintaining part of the explant in contact with air 31.
Temporary immersion systems (TIS) represent another approach. TIS enable temporary contact between the plants and the liquid medium, thus avoiding continuous immersion and providing adequate oxygen transfer to the cultures 21, 22, 23. The thorough description and functioning of CIS and TIS, as well as the various particular designs are outside the scope of this review. Brief information about the bioreactors most frequently used for the propagation of trees is given below. For detailed information on these topics, readers are referred to several comprehensive reviews 21, 24, 25, 26, 27, 32, 33, 34, 35.
2.1. Bioreactors based on continuous immersion
Schemes showing the basic design and operation mode of some of the CIS bioreactors used for propagation of trees are shown in Figure 1. The first two bioreactors (Figure 1A and B) correspond to stirred tank and airlift, respectively, which are frequently used to culture somatic embryos. A stirred tank is a mechanically operated bioreactor that consists of an impeller or agitator along with different ports for aeration, medium addition or removal, in order to facilitate liquid circulation, mixing, and distribution of O2, and nutrients 26. The airlift is a pneumatic bioreactor equipped with a sparger for forming small bubbles of filtered air that rise through the column of liquid medium thereby aerating and mixing the culture. The key parameters for the efficient use of these bioreactors include control of shear, ease of gas and medium exchange, and maintenance of sterility. Although shear stress is caused in both mechanically and pneumatically operated bioreactors due to mechanical agitation and aeration respectively, its effects are less harmful in airlift vessels 26. Figure 1C and D represents some bioreactors used to culture axillary shoots, as a small vessel with natural ventilation (Figure 1C), a balloon bioreactor with net, in which the explants are partially submerged (Figure 1D), and a large vessel with forced ventilation and porous support material for inserting the explants 36 (Figure 1E). In the first case, air enters the vessel by simple diffusion through membrane filters. This approach can give acceptable results with small containers, but usually it is not suitable for large vessels due to the occurrence of hyperhydricity. The gaseous environment of large vessels (as those represented in Figure 1D and E) can be improved by forced ventilation, i.e. mechanically moving filtered air from the outside to the inside of a culture vessel and vice versa with the aid of an air pump 37. The use of continuous immersion with forced ventilation enables the size of bioreactors to be increased by adapting vessels intended for other uses (i.e. food containers) without the need to implement more complicated TISs, thereby lowering production costs.
Figure 1.
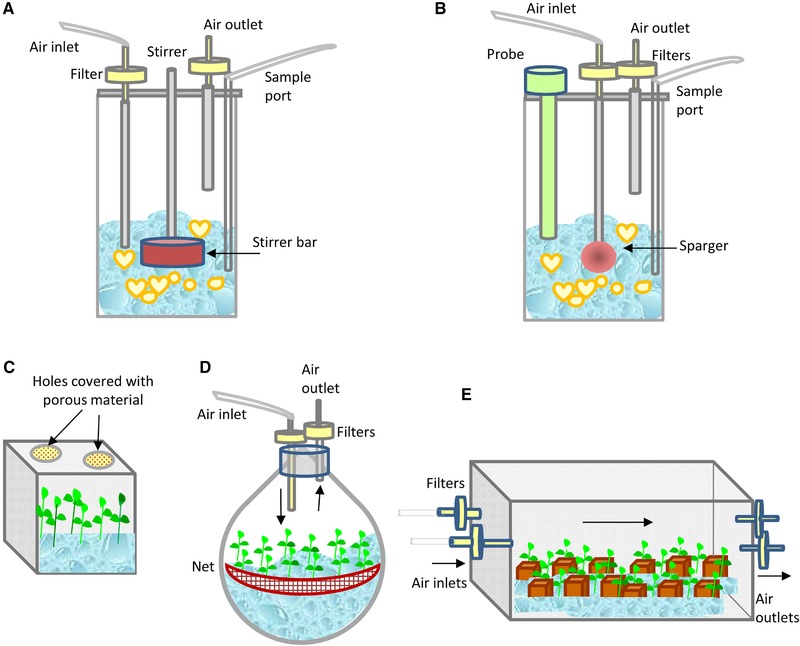
Schemes showing the basic design of some CIS bioreactors used for propagation of trees. (A) Stirred tank, (B) airlift bioreactor, (C) small flask with natural ventilation, (D) balloon with forced ventilation and a net to hold the explants, and (E) large vessel with forced ventilation and porous support material for inserting the explants
2.2. Bioreactors based in temporary immersion
TIS was first described by Steward in 1952 38, but its massive use began years later, after the studies of Alvard and Teisson 22, 23. The bioreactors most frequently used for micropropagation of trees are mainly those derived from the two‐flask system 39, commercial recipient for automated temporary immersion (RITA)® 23 and plantform™ 40 bioreactors, as well as others like the rocker system 41, 42. Figures 2, 3, 4, 5, 6 show some schemes of these apparatus. In all cases, the explants are placed separately from the liquid medium, either in a different compartment or zone of the same flask (RITA®, plantform™, and rocker bioreactors; Figures 2, 3, 4), or in an independent container connected by tubes (two‐flask system, Figures 5 and 6). The explants can be placed either directly on the bioreactor inner surface or by using different support materials (nets, glass beads, rockwool cubes, polyurethane foam, etc.). The medium reaches the explants by mechanical movement of the entire vessel (rocker bioreactors, Figure 4) or by the driven force of filtered air pumped at programmed intervals, as happens in RITA® (Figure 2), plantform™ (Figure 3), and two‐flask bioreactors (Figures 5 and 6). In the latter three cases, pumped air not only enables the contact of the explants with the medium but also causes the renewal of the gaseous atmosphere inside the vessels, thus promoting photoautotrophic behavior 43. Besides variations as regards type of material, size and shape, other features differentiate these designs and may influence their suitability for the micropropagation of specific species. For example, the rigid inner tubes of RITA® apparatus facilitate handling during medium exchange, which may be useful for plants that need several transfers during their culture cycle. Plantform™ vessels are not so easy to manage in these terms, but its arrangement of inlet/outlet holes allows to apply additional aerations independently of those directed to force the movement of the medium. As these additional aerations do not cause immersion of the explants, this system may be useful for plants that are especially prone to hyperhydricity, one of the most frequent hindrances associated to liquid medium 44, 45. Immersion time (duration and frequency) is the most decisive parameter for system efficiency, and once protocols have been optimized, plants cultured by TIS generally show increased vigor and better quality than those grown completely submerged in liquid medium or conventionally in semisolid medium (SS) 21.
Figure 2.
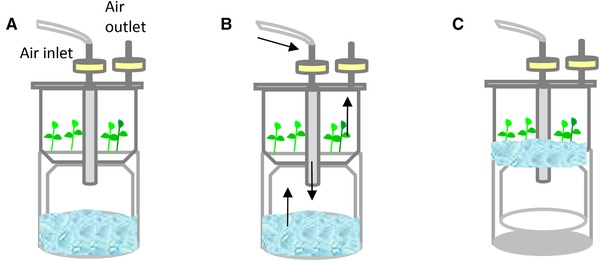
RITA® bioreactor. (A) The pump is off and the liquid medium is in the lower compartment, (B) the pump impulses air through the inlet filter, (C) the overpressure moves the medium up and cause immersion of the explants, as well as air expulsion through the outlet filter. When the pump is off, the medium goes down by gravity
Figure 3.
Plantform™ bioreactor. (A) The pump is off and the liquid medium is in the lower compartment, separate from the explants, (B) the pump impulses air through the central inlet filter, (C) the overpressure moves the medium up and cause immersion of the explants, as well as air expulsion through the outlet filter. When the pump is off, the medium goes down by gravity, and (D) additional aerations: the pump impulses air through any of the lateral inlet filters. The air circulates through the chamber containing the explants, but does not cause translocation of the medium
Figure 4.
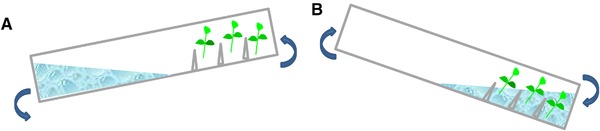
Rocker bioreactor. (A) Due to the angle of the container, the medium is a separate section from the explants and (B) the container moves and with the change of angle the explants are immersed in the medium
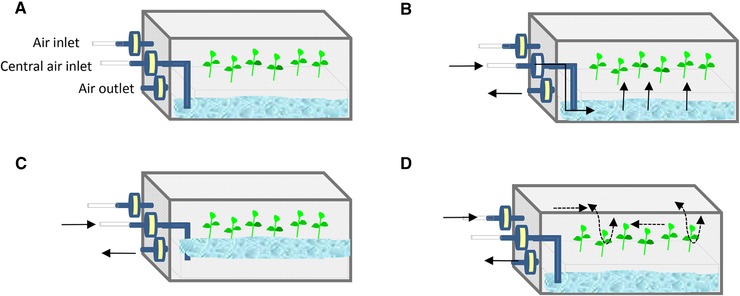
Figure 5.
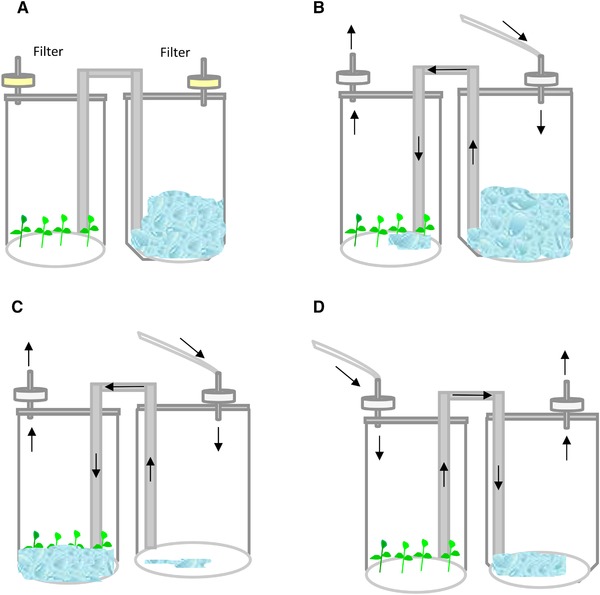
Two‐flask bioreactor. (A) The liquid medium is in a separate flask from the culture vessel that holds the explants, (B) the pump impulses air through the flask containing the medium, forcing its movement to the culture vessel, (C) the medium cause immersion of the explants, as well as air expulsion through the outlet filter, and (D) the pump impulses air through the culture vessel, forcing its movement to the empty flask
Figure 6.
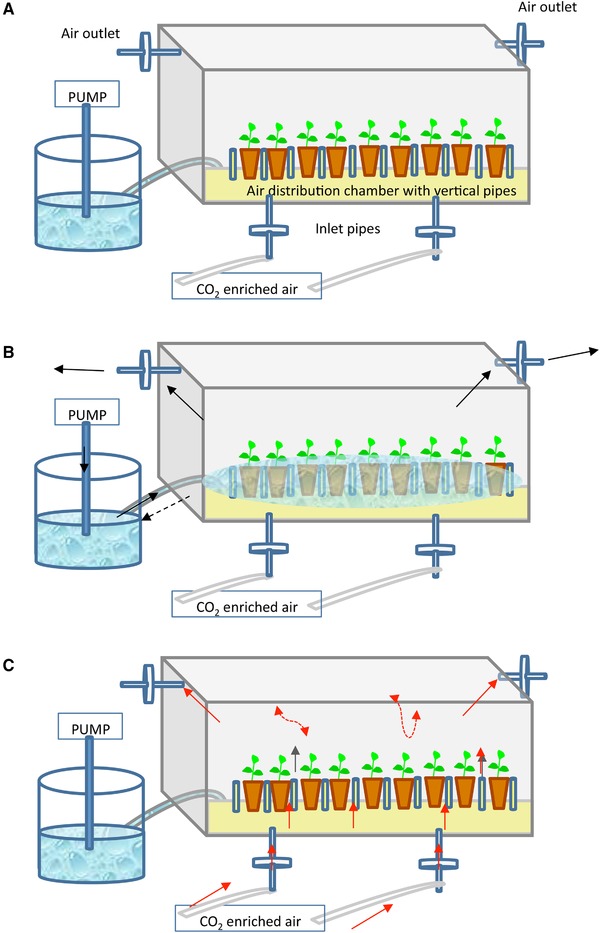
Two‐flask bioreactor with additional forced ventilation, TRI‐bioreactor. (A) The liquid medium is in a reservoir connected with the culture vessel, (B) the pump is switched on to impulse air through the flask containing the medium, forcing its movement into the culture vessel. Once the immersion is completed, the pump is switched off and the medium flows back in the reservoir under gravity, and (C) CO2‐enriched air is pumped through the inlet pipes and directed to the culture vessel headspace, without causing translocation of the medium
3. APPLICATION OF BIOREACTORS TO THE MICROPROPAGATION OF TREES
This section summarizes the state of the art on the application of CIS/TIS to the propagation of trees cultured both by axillary shoot propagation and by somatic embryogenesis.
3.1. Axillary shoots cultured by continuous immersion
Table 1 summarizes nine studies conducted with shoots immersed continuously in liquid medium. Only five genus are represented, as the use of this system is not extended in the case of trees. Shoots were proliferated by CIS in 78% of references but rooting and acclimation were reported only in 56% of cases. Within the examples cited in Table 1, eucalyptus is the best represented tree (one third of the references). Species of the genus Eucalyptus, native to Australia, are amongst the most widely trees grown in forest plantations, despite the widespread public concern originated by the ecological problems associated with its massive use 46, 47. Although eucalyptus are fast growing trees, many of the natural species and hybrids used commercially are recalcitrant to vegetative propagation (carried out either by cuttings or by conventional micropropagation). This recalcitrance, mainly due to poor adventitious rooting, causes in turn high plant losses during acclimation. CIS with natural or forced ventilation have been tested for solving these problems. Shoots of E. camaldulensis with their bases continuously exposed to liquid medium were cultured in glass flasks with forced ventilation 48 or in small flasks with natural ventilation 49. In both cases, the multiplication coefficients were higher than obtained in semi‐solid medium, although hyperhydricity was detected 48. This disorder also affected the propagation of apple shoots in a 5 L balloon with a net (Figure 1D), indicating that other parameters beside forced ventilation influence shoot quality 30. Other eucalyptus (Urophylla x Grandis) were cultured in different vessels as Miracle Pack and Vitron. These vessels were used with natural ventilation in a chamber with CO2‐enriched air, in order to achieve a photoautotrophic behavior 50, and rooted and acclimated plantlets were obtained.
Table 1.
Application of CIS to the propagation of trees by axillary shoots
| Performance or comparison with other systems | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Species | Common name | Plant material | No. of clones | Type of CIS bioreactor | Proliferation | Rooting | Acclimation | Observations | Reference |
| Castanea sativa x C. crenata | Chestnut | Adult trees | 8 | 10 L vessel | High | 60–70% (ex vitro rooting plus acclimation) | Forced ventilation, rockwool as support. No comparison with SS | 55 | |
| Castanea spp. | Chestnut | Adult trees | 15 | 10/16 L vessel | High | 70% (in vitro rooting plus acclimation) | Forced ventilation, CO2‐enriched air, rockwool, photoautotrophy. No comparison with SS | 53 | |
| Eucalyptus camaldulensis | Eucalyptus | Adult trees | 1 | 0.5 L flask | CIS > TIS > SS | 100% | 76% | Forced ventilation, hyperhydricity. | 48 |
| E. camaldulensis | Eucalyptus | n. s. | n. s. | 0.37 L flask | n. s. | CIS > SS | 90–100% | Natural ventilation, CO2‐enriched air, plastic or vermiculite support, photoautotrophy | 49 |
| E. urophylla x E. grandis | Eucalyptus | n. s. | n. s. | Miracle Pack and Vitron | High | High | 100% | Natural ventilation, CO2‐enriched air, photoautotrophy | 50 |
| Malus domestica | Apple | CCP | 1 | 5 L glass balloon with net | CIS > TIS | n. s. | n. s. | More hyperhydricity than in TIS. Physiological analysis. No comparison with SS | 30, 71 |
| Macadamia tetraphylla | Macadamia | Grafted seedlings | n. s. | Small flask | n. s. | 100% | n. s. | Natural ventilation, vermiculite, CO2‐enriched air, photoautotrophy. No comparison with SS | 52 |
| Samanea saman | Rain tree | Seedlings | n. s. | 0.24 L flask | High | High | n. s. | Natural ventilation, vermiculite, CO2‐enriched air, photoautotrophy. No comparison with SS | 51 |
CCP, characterized commercial plants; CIS, continuous immersion; n.s., not specified; SS, semisolid medium; TIS, temporary immersion
Photoautotrophic micropropagation (PAM) was also investigated in CIS in other species as rain tree 51, macadamia 52, and chestnut 53. In the case of rain tree and macadamia, small flasks with natural ventilation were used (Figure 1C), whereas chestnut was cultured in large flasks with forced ventilation (Figure 1E). Chestnut is a tree important for its fruits and timber. As European chestnut (C. sativa) is currently being threatened by ink disease (caused by Phytophthora cinnamomi and P. cambivora), resistant hybrids of European and Asian chestnut (Castanea sativa x C. crenata, C. sativa x C. mollissima) should be propagated vegetatively. Chestnut is difficult to root, mainly when the plant material is of mature origin 54, and the aim of the application of CIS was to obtain high number of shoots of good quality to undergo the rooting process 53. In a first study, we used 10 L bioreactors adapted from food containers, with rockwool cubes for support, and applied forced ventilation in photomixotrophic conditions (adding sugar to the medium) 55. Then, we proliferated and rooted chestnut by PAM 53. After a short dip with auxin, the shoots were inserted in rockwool cubes containing medium without sugar. The cubes were placed in 16 L vessels with forced ventilation with CO2‐enriched air 53. This way, more than 6000 vigorous shoots belonging to 15 genotypes were evaluated, and rooting and acclimation rates of more than 70% were obtained, significantly improving the performance of this difficult‐to‐root species regarding conventional micropropagation.
As a resume, hyperhydricity (reported in one third of the studies) was the main obstacle for successful application of CIS methodology. However, many examples (∽56%) of applying CIS to culture of axillary shoots of trees described high proliferation and good percentages of rooting and acclimation. It is worth noting that in most of these successful cases shoots were cultured in PAM 49, 50, 51, 52, 53, emphasizing the advantage of obtaining a “natural” physiological state for a good transition to ex vitro conditions 43, 56.
3.2. Axillary shoots cultured by temporary immersion
Table 2 includes 25 published articles dealing with the utilization of TIS for axillary shoot‐based tree production. Eleven families and 16 genus are represented, including species growing in natural forests and in plantations. Most of these references use adult trees or rejuvenated material obtained from characterized commercial plants, whereas six studies refer exclusively to seedlings. The most popular devices listed in this table are vessels in which the liquid medium reaches the explants driven by filtered air entering the system, including bioreactors derived from the two‐flask system (40%), followed by commercial RITA® (36%), and Plantform™ (16%) (Figures 2 and 3). Only two studies (8%) report the use of rocker bioreactors (Figure 4). Comparisons between TIS and SS performance are frequent, but less than 15% of reports analyzed more than one design of TIS 57, 58, 59. The use of TIS focused in the multiplication phase of micropropagation in 84% of references, and 88% of them reported the rooting of propagated plant material. Frequently (64%) the rooting phase occurred inside the bioreactors, whereas in other studies (24%) shoots produced by TIS were submitted either to in vitro rooting in SS or to ex vitro rooting. Acclimation success is mentioned in the 76% of the reports, as only six of them did not provide data on plant adaption to ex vitro conditions.
Table 2.
Application of TIS to the propagation of trees by axillary shoots
| Performance or comparison with other systems | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Species | Common name | Plant material | No. of clones | Type of TIS bioreactor | Proliferation | Rootinga , b | Acclimation | Observations | Reference |
| Betula pendula, B. pubescens | Birch | CCP | 2 | Two‐flasks | Species specific | TIS∼SSa | TIS∼SS | Slight hyperhydricity | 70 |
|
Chestnut | Adult trees | 10 | RITA Plantform (PF) | PF > RITA > SS | PF > RITA > SSb | PF > RITA > SS | Hyperhydricity (controlled using rockwool as support) | 58 |
| Cedrela odorata | Spanish red cedar | Seedlings + adult trees | High number | BioMINT® | TIS > SS | TIS > SSa | 98% | Juvenile > Mature material. No forced ventilation | 42 |
| Corylus spp. (Hybrids) | Hazelnut | CCP | 4 | Liquid Lab Rocker™ | TIS > SS | TIS < SSa | n. s. | No forced ventilation | 41 |
| Crescentia cujete | Calabash tree | Seedlings | n. s. | RITA | TIS > CIS > SS | TIS > CIS, SSa | TIS (75%) > CIS, SS | Tree with medicinal properties | 65 |
| Eucalyptus spp. and hybrids | Eucalyptus | CCP | 6 | RITA | TIS > SS | TIS > SSa | TIS > SS | Hyperhydricity (controlled by manipulation of immersion). Genotypical differences | 60 |
| E. camaldulensis | Eucalyptus | Seedlings | n. s. | Two‐flasks (20 L) + additional aeration | TIS > CM | TIS > CMa | TIS > CM | Photoautotrophy; Florialite as support in TIS and CM | 63 |
| E. camaldulensis | Eucalyptus | Seedlings | n. s. | Two‐flasks (4 L) + additional aeration | TIS > SS | TIS > SSa | TIS > SS | Photoautotrophy; Vermiculite and paper pulp as support in TIS and agar in SS | 61 |
| E. nitens | Eucalyptus | Seedlings | n. s. | Two‐flasks | n.s. | TIS > SSa | >70% | TIS only during rooting | 62 |
| Handroanthus heptaphyllus | Black lapacho | Seedlings | n. s. | Two‐flasks | TIS > SS | TIS > SSa | TIS > SS | Tree with medicinal properties | 68 |
| Ilex paraguariensis | Yerba mate | CCP | n. s. | Two‐flasks | TIS > CIS, SS | TIS > SSa | 80% | Tree with medicinal properties | 66 |
| Malus domestica | Apple M9 | CCP | 1 | Ebb & flood | TIS ∼ SS | >90%b | >90% | Hyperhydricity controlled with aeration. Rooting by hydroponic culture | 71 |
| M. domestica | Apple M26 | CCP | 1 | RITA | TIS > SS | >90%a | High | Hyperhydricity (controlled by manipulation of immersion) | 72 |
| M. domestica | Apple | CCP | 1 | PA‐TIS (two‐flasks) | n. s. | 60%a | n. s. | Photoautotrophy | 107 |
| Olea europaea | Olive | CCP | n. s. | LifeReactor©, in‐house design | TIS ∼ SS | n. s. | n. s. | Hyperhydricity, sometimes contamination | 59 |
| O. europaea | Olive | CCP | n. s. | RITA | TIS > SS | n. s. | n. s. | Improvement of leaf characteristics | 80 |
| O. europaea | Olive | CCP | 1 | Plantform | TIS > SS | n. s. | n. s. | Cost reduction due to less requirement of zeatin | 81 |
| Pistacea spp. | Pistachio | Seedlings, adult trees | 4 | RITA | TIS > SS | 50–70%a , b | 70–90% | Hyperhydricity (controlled by manipulation of immersion) | 75 |
| Populus deltoides x P. trichocarpa | Poplar | CCP | 3 | Two‐flasks | n. s. | TIS (97%) > SS a | TIS > SS | Photoautotrophy, mycorrhization | 67 |
| Prunus avium (and hybrid rootstocks) | Cherry | Adult trees | 4 | Two‐flasks | TIS > SS | TIS (100%) > SSb | TIS > SS | Hyperhydricity in some genotypes | 76 |
| P. cerasifera | Myrobolan | Young trees | 1 | RITA | TIS > SS | TIS > SSa | >80% | More [photosynthetic pigments] in RITA | 69 |
| Quercus robur | Oak | Seedlings | n. s. | Plantform | TIS ∼ SS | TIS∼SSb | n. s. | Hyperhydricity (controlled by manipulation of immersion) | 77 |
| Salix viminalis | Willow | Adult tree | 1 | RITA, Plantform (PF) | PF > RITA > SS | 100%a | 100% | Spontaneous rooting in all systems | 57 |
| Tectona grandis | Teak | Greenhouse tree | n. s. | Two‐flasks | TIS > SS | TIS (95 %) > SSa , b | 100% | Hyperhydricity (controlled by lowering cytokinin). Spontaneous rooting in TIS | 73 |
| T. grandis | Teak | Adult trees | n. s. | RITA | TIS > SS | TIS∼SS (∼90 %)b | TIS∼SS (∼90 %) | Hyperhydricity (controlled by lowering cytokinin, nº immersions, explant density) | 74 |
Rooting occurred in TIS.
Shoots proliferated by TIS were rooted in SS or ex vitro.
CCP, characterized commercial plant; CIS, continuous immersion; CM, conventional micropropagation with supports different from agar; n. s., not specified; TIS, temporary immersion; SS, semisolid medium.
As with continuous immersion, eucalyptus is the most commonly studied plant and possibly the one in which the most advantageous results were obtained. As mentioned above, these trees are recalcitrant to vegetative propagation, mainly due to difficulties during rooting and acclimation. These problems have been addressed by the use of different TIS with forced ventilation, such as RITA® 60 and various designs of the two‐flask system 61, 62, 63 (Figures 5 and 6). Bioreactors were used either for multiplication and rooting 60, 61, 63 or only for the phase of rooting 62. As reported by McAlister et al., great differences among clones regarding proliferation, rooting, and acclimation were detected 60. However, once hyperhydricity was controlled by optimizing immersion frequency, and duration 60, an overall increase in both proliferation and plant quality was obtained by the use of TIS 60, 61, 63.
The improvement of shoot quality observed by the use of RITA® 60 was attributed to the air supply inside the bioreactors, which can reduce the internal humidity and favor gas exchange (O2, CO2, and ethylene) between the plant and the surrounding environment. The promotion of normal metabolism of plant tissues (aerobic respiration and photosynthesis), which allows the acquisition of a photoautotrophic state and later facilitates the transition to ex vitro conditions, is one of the claimed benefits of using ventilated vessels 43, 56, 64. Indeed, eucalyptus shoots cultured in a two‐flask system subjected to PAM (without sugar supplementation and with CO2 enriched air; Figure 6), showed higher photosynthetic rates and epicuticular leaf‐wax contents (as well as better stomatal function) than shoots cultured in conventional SS 61. Moreover, gas exchange did not only favor the development of the aerial part of the plant, as rooting percentages and/or root quality were also improved by the use of TIS during the rooting phase 60, 62, 63. The supply of O2 in the rooting zone promoted the development of normal roots directly from the base of the stems, without callus interference 60. The formation of well‐developed roots enhances the nutrient/water uptake rate of plants during the acclimation process, which was easier in eucalypt plants cultured by TIS than in those cultured in SS 60, 62, 63.
Besides eucalyptus, other tree species showed better rooting in bioreactors than in SS, as reported for calabash tree 65, yerba mate 66, poplar 67, black lapacho 68, and myrobolan 69. In the two later cases, the use of liquid medium significantly reduced the apical necrosis detected when shoots grew in agar‐based medium, thereby increasing the number of shoots that could be rooted. Shoots of easy‐to‐root trees, as birch 70, willow 57, and two apple rootstocks 71, 72 showed high frequencies of rooting in TIS (90–100%). Although in these cases the rooting results were similar to those obtained in SS, shoots rooted by TIS were easier to manage and more cost‐effective. Successful acclimation was reported in all these examples.
In other plants, shoots obtained by TIS were rooted ex vitro, as reported for chestnut 58 and teak 73, 74, or in vitro by using SS, as reported for pistachio 75, cherry 76, and oak 77. Except for oak shoots, the use of TIS increased the number and quality of shoots destined to rooting, improving the overall process with regard to conventional micropropagation. Pistachio, a tree important for its fruits, was cultured in RITA® using juvenile and mature material of different species. As reported above for black lapacho 68 and myrobolan 69, pistachio shows apical necrosis when its shoots are proliferated in semi‐solid medium 78. TIS decreased the incidence of this disorder (thereby increasing the number of shoots suitable for rooting), once hyperhydricity was controlled by adjusting the duration and frequency of immersion 75.
In the case of chestnut, the aim of the application of TIS was to reduce micropropagation costs and too obtain high number of shoots of good quality for rooting 58. Since many chestnut genotypes are prone to developing hyperhydric symptoms even when cultured in semi‐solid medium 79, controlling this disorder was the major challenge faced in the application of TIS 58. In other species as eucalyptus, apple, teak, and pistachio 60, 72, 74, 75, hyperhydricity could be controlled by adjusting the duration and frequency of immersion. However, the only way to obtain a good proportion of normal chestnut shoots in bioreactors was to maintain the explants in an upright position during immersion, which was accomplished by using rockwool cubes as support material 58. This enabled successful propagation of ten ink‐resistant genotypes, which generally proliferated better in TIS than in semi‐solid medium. Plantform™ and RITA® bioreactors were compared during the proliferation phase 58. Longer and more vigorous shoots were obtained in Plantform™ vessels, probably because these bioreactors are larger than RITA®, have larger headspace, and additional aeration can be supplied without immersion of the medium (Figures 2 and 3). Chestnut shoots cultured in Plantform™ were subjected to ex vitro rooting and the rooting + acclimation success (ranging from 40 to 80% depending on the genotype) was higher than obtained with SS.
Plantform™ bioreactors were also tested with other emblematic trees, such as olive and oak, although these protocols have still to be fully developed. Olive (Olea europaea L.) is mainly cultivated in the Mediterranean basin and is used both for oil extraction and table consumption. This tree is recalcitrant to micropropagation due to low (and cultivar‐dependent) proliferation rates, as well as low rooting and acclimation rates. The first attempts to culture olive in several TIS devices were hindered by low proliferation, contamination and high hyperhydricity 59. The use of RITA® vessels enabled more shoots to be obtained than in semi‐solid cultures 80. Recently, healthy shoots of cv. Canino were produced in Plantform™ bioreactors using only half of the zeatin previously reported 81, thus potentially reducing production costs. Regarding pedunculated oak, a report of the proliferation of juvenile material of Quercus robur was recently published 77. Hyperhydricity was controlled by adjusting the immersion and aeration frequencies, and rooted shoots were obtained. Although the results were similar to those obtained in SS, the study findings demonstrated the feasibility of culturing axillary shoots of this tree in bioreactors.
TIS without ventilation (Figure 4) have also been applied to trees of economic importance for their fruits or wood, although with less frequency than the previous designs using forced ventilation. Hybrid hazelnut and Spanish red cedar were cultured in two different rocker system bioreactors named respectively Liquid Lab Rocker™ 41 and BioMINT® 42. By this approach, as outlined before, the liquid medium does not reach the explants forced by air entering the flask but by mechanical movement of the bioreactor (Figure 4). Hazelnut and cedar shoots proliferated more by TIS than by SS, showing higher content of photosynthetic pigments 41, as well as larger leaves and more vigorous shoots 41, 42. Although hazelnut shoots rooted better when adventitious rooting was induced in SS than by TIS without ventilation 41, the rocker system did enhance root formation and acclimation (up to 98%) in the case of Cedrela odorata 42.
As a resume, most of the examples (∽80 %) of applying TIS to culture of axillary shoots of trees described higher proliferation by this method than by conventional micropropagation. High percentages of rooting and/or high quality of the rooted shoots (which in turn facilitated acclimation success) were reported in a similar range of studies (∽80%), although clear comparisons with SS were not provided in all of them. As previously observed in CIS, the main hindrance to overcome was hyperhydricity (reported in ∽45% of the studies), which was controlled mainly by lowering cytokinin supply and by manipulation of immersion. It is worth noting that significant reduction of production costs were reported 60, 63, 71, 74, 81.
3.3. Somatic embryos cultured by temporary and continuous immersion
Recent reviews dealing with design and use of bioreactors for embryo culture are available 27, 33, 34, but those focusing in tree culture are relatively scarce 35. Table 3 includes 22 published articles dealing with the utilization of TIS and CIS for somatic embryo‐based tree production, most of them regarding angiosperm cultures (∽75%). Twelve genus are represented, and only in four of them somatic embryos were derived from mature or characterized commercial plants, whereas in the rest the explants were obtained from embryonic or unspecified material. The most popular devices listed in this table are commercial RITA® vessels (∽40%) and bioreactors derived from the two‐flask system (∽30%) (Figures 2 and 5). These apparatus are followed by airlift (Figure 1B) and stirred tank (Figure 1A) bioreactors (20 and 10%), together with other devices sometimes designed specifically for particular plants, as in the case of coffee 29, 82, 83, 84, 85, 86. Comparisons between bioreactors and SS are less frequent than in the case of axillary shoots, as are only shown in less than half of the references.
Table 3.
Application of bioreactors to the propagation of trees by somatic embryos
| Performance or comparison with other systemsa , b | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Species | Common name | Plant material | No. of clones | Type of bioreactor | Proliferation | Maturation | Germination/Plant conversion | Acclimation | Observations | Reference |
| Abies nordmanniana | Nordmann fir | Embryonic | 1 | Two‐ flasks (TIS) | TIS > SS | TIS > SS | n. s. | n. s. | TIS promoted maturation | 70 |
| Carica papaya | Papaya | Embryonic | n. s. | RITA (TIS) | SS | SS | TIS (95 %) > SS | n. s. | TIS used for germination of mature embryos | 89 |
| Castanea dentata (and hybrids) | American chestnut | Embryonic | n. s. | Airlift (CIS) | TIS > SF | TIS > SF | n. s. | n. s. | Used for obtaining targets for genetic transformation | 92 |
| Coffea arabica | Coffee | Greenhouse plants | 1 | RITA (TIS) | TIS | TIS | 75% ex vitro plant conversion plus acclimation | Physiological and chemical measurements | 29 | |
| C. arabica | Coffee | Greenhouse plants | High number | RITA, MATIS, Two‐flasks (TIS) | SF | High | 91% | High | Large‐scale propagation, histological and physiological measurements | 85, 86 |
| C. canephora ∼ C.robusta | Coffee | Greenhouse plants | 17 | Two‐flasks, box in bag (TIS) | SF | Two‐flasks (> 95 %) | 46% ex vitro plant conversion plus acclimation | Large‐scale propagation, variability between batches | 83 | |
| C. canephora ∼ C.robusta | Coffee | Greenhouse plants | n. s. | RITA, TRI‐bioreactor (TIS)) | n.s. | TRI‐bioreactor (84 %) > RITA > SS | TRI‐bioreactor (89 %) > SS > RITA | Photoautotrophy, physiological measurements | 82 | |
| C. canephora ∼ C.robusta | Coffee | Greenhouse plants | n. s. | Stirred tank (CIS), RITA, Two flask, box in bag (TIS) | Stirred tank | RITA, two flasks, box in bag | ∽100% | Large‐scale propagation, photoautotrophy | 84 | |
| Hevea brasiliensis | Rubber tree | Embryonic | n. s | ∽RITA (TIS) | TIS > SS | TIS > SS | TISa > SS | n. s. | TIS promoted synchronization of embryo development | 87 |
| Kalopanax septemlobus | Kalopanax | Grafted material | n. s. | TIS and CIS with net and forced ventilation | High | High | TIS > SS > CIS | 100% | The use of a net improved TIS | 88 |
| Picea abies | Norway spruce | Embryonic | 4 | Two‐ flasks (TIS) | High | High | High/Medium | n. s. | Genotypical differences | 101 |
| P. mariana, P. glauca‐engelmannii | Black and interior spruce | Embryonic | 2 | Air‐lift, Stirred tank (CIS) | High | TIS > SSb | n. s. | n. s. | Maturation was higher when embryos were previously cultured in airlift bioreactors | 102 |
| P. sitchensis | Sitka spruce | Embryonic | 2 | Stirred tank, Air‐lift, Bubble, Hanging stirrer bar (CIS) | High | Better in bubble bioreactors | n. s. | n. s. | Interaction bioreactor type/embryogenic line | 103 |
| Pinus kesiya | Khasi pine | Embryonic | n. s. | Bubble bioreactor (CIS) | TIS > SF | TIS > SFb | TIS ∽SF | n. s | 104 | |
| Psidium guajava | Guava | Embryonic | n. s. | RITA (TIS) | n. s. | TIS > SSa | n. s. | 90 | ||
| Quercus robur | Pedunculate oak | Mature trees | 2 | RITA (TIS) | TIS > SS | TIS < SSa | TIS > SSb | 95% | High genotypical differences | 108 |
| Q. robur | Pedunculate oak | Seedlings, Mature trees | 4 | RITA (TIS) | TIS > SS | TIS > SSb | TIS > SSa , b | TIS > SS | Selection phase of genetic transformation | 93, 94 |
| Q. suber | Cork oak | Embryonic | n. s. | RITA (TIS) | TIS∼SS | n. s. | n. s. | n. s. | 109 | |
| Santalum album | Sandalwood | n. s. | n. s. | Airlift (CIS) | High | n. s. | n. s. | n. s. | Metabolite production | 110 |
| Theobroma cacao | Cacao | Mature trees | 1 | Two‐flasks (TIS) | TIS > SS | TIS > SSa | Good | Biochemical analysis, direct sowing of germinated embryos | 91 | |
Process carried out in bioreactors
Process carried out with material previously cultured in bioreactors
CCP, characterized commercial plant; CIS, continuous immersion; n. s., not specified; TIS, temporary immersion; SF, Shaken flask; SS, semisolid medium
In angiosperm somatic embryos, the use of bioreactors focused in the multiplication phase of micropropagation in ∽80% of references, and 88% of them reported the plant conversion of propagated plant material. In some cases (68%) the last events of embryo development (maturation, germination, and plant conversion) occurred inside the bioreactors, whereas in other studies ( ∽20%) embryos produced in liquid medium were submitted either to in vitro maturation in SS or to ex vitro germination and plant conversion. Acclimation success is mentioned in ∽60% of the reports. Maybe coffee is the plant that has benefitted more from the development of temporary immersion bioreactors for large‐scale propagation at industrial level 86. Somatic embryos of selected clones derived from the two main commercial species, Coffea arabica and C. canephora were successfully cultured by using a combination of bioreactors of different shape and volume. Embryos of C. arabica and/or C. canephora (Robusta) were cultivated by CIS in shaken flasks and in a stirred tank 83, 84, 85 and by TIS using 1 L RITA® bioreactors, the 5 L MATIS®, two‐flask bioreactors, the TRI‐bioreactor (Figure 6) and the 10 L Box in Bag disposable bioreactor 29, 82, 83, 84, 85, 86. A high culture density positively affected embryo morphology by enhancing embryonic axis elongation, which allowed direct sowing of pre‐germinated embryos, greatly reducing the handling time. By the use of bioreactors handling decreased as plant production increased, allowing large‐scale propagation and successful industrial transfers to growers in Latin America, Africa, and Asia 86.
In other plants as rubber tree, kalopanax, papaya, guava, and cacao improvements in embryo germination and plant conversion were observed 87, 88, 89, 90, 91, although its commercial application did not reach the level of success of coffee.
Bioreactors were also used to improve genetic transformation protocols. The production of transgenic lines of several Fagaceae species, such as Castanea dentata 92 and Quercus robur 93, 94, was increased by culturing somatic embryos either in airlift bioreactors previously to transformation events 92, or by applying RITA® to select kanamycin resistant transformants after Agrobacterium inoculation 93, 94. In the case of oak, transgenic embryos were obtained faster and in higher frequencies than in SS. Since phenolics and other growth inhibitors diffuse faster in liquid medium 95, those exuded by non‐resistant dying cells were probably rapidly diluted to innocuous levels, thereby minimizing negative effects on growth of transgenic cells. Other advantage of using RITA® for oak transformation was that this bioreactor facilitated the plant conversion of transgenic lines originated from mature oak trees, both when the embryos were transferred to plates for maturation and germination treatments and when the embryos were maintained in the bioreactors 93.
In the case of conifers, however, the maturation, germination and plant conversion of embryos in liquid medium still remains as a challenge. Although there is a general agreement about the advantages of applying bioreactors to large‐scale gymnosperm production 96, 97, 98, currently the proliferation of cultures of these trees in liquid medium is mostly carried out using small flasks on rotary shakers 99, 100. Bioreactors have been used for the multiplication phase of somatic embryos of genus Abies, Picea, and Pinus 70, 101, 102, 103, 104, among other conifers. However, for maturation, germination and plant conversion, using either SS or different types of bioreactors are required 95, 96, 98, as current methods of proliferation in bioreactors can lead to problems such as failure to establish polarity or hyperhydricity 98. Also, light availability should be improved, as light is a critical factor especially during germination 98.
In general, the application of TIS to angiosperm embryo culture produced cases of clear success. Together with coffee, in which the use of various bioreactors allowed large‐scale propagation, Table 3 reports other examples in which TIS improved embryo quality. However, for the application of this technology to conifer production it is necessary to obtain synchronous cultures with well‐established polarity and competence to undergo plant conversion 98. This requires to solve some problems as the control the shear stress, which can damage the growing cells 27, 35, and to provide homogenous light to all the embryos 98.
4. ADVANTAGES, DISADVANTAGES, AND PROSPECTS OF THE USE OF BIOREACTORS
For several years, the use of bioreactors has become part of the daily routine in most plant tissue culture laboratories. These systems can improve proliferation, rooting, plant conversion and acclimation of a wide range of plants, including trees The industrial applications of bioreactors are widespread, and besides the references in which participation of companies is cited 53, 55, 58, 60, 76, 83, 84, 85, 86, many companies culturing woody plants use bioreactors at experimental or commercial levels. Figure 7 shows some examples of the propagation of woody plants in bioreactors on an industrial scale.
Figure 7.

Industrial applications of bioreactors. (A, B) Prunus rootstocks cultured in MATIS® bioreactors by Agromillora group. (C, D) Pistachio shoots cultured in Plantform™ by Vitrosur Lab SLU. (E, F) Chestnut shoots cultured by TRAGSA after being proliferated in Plantform™ (E) and during the acclimation phase (F)
However, it seems that the use of bioreactors to improve the propagation of forest trees has not yet reached its full potential. The decision as to whether to use bioreactors or not, and which type of bioreactor to use, is not always a matter of matching the characteristics of the bioreactor to the characteristics of the plant to be propagated, as economic concerns must also be taken into account. Although the use of bioreactors reduces the costs of consumables and personnel once the protocols are optimized 60, the initial outlay (for vessels, bombs, electrovalves, filters, etc.) is high. Commercial bioreactors are expensive and some parts have to be replaced after a few autoclaving cycles. Scientists working in research centres or in companies often have to make their own designs or improvise solutions using materials or devices designed for different purposes. The availability of cheap, customized bioreactors that could be acquired worldwide in a relatively short time would facilitate the implementation of liquid culture in many laboratories. Advances in 3‐D printing technology may allow the development of new and affordable designs on demand in the near future 105.
Bioreactors cannot be easily used with all types of plants. Problems such as hyperhydricity frequently arise during the development of new protocols in liquid medium for some species or genotypes, thus compromising the efficiency of the procedures 28, 58, 71, 106. Although physiological studies have been carried out in several species 29, 30, 61, 82, 85 there is necessary to get deeper knowledge of the physiological behavior of shoots and embryos in liquid medium.
In addition, although not always reported, the contamination risk has been highlighted in large‐scale somatic embryo and axillary shoot cultures 24, 28, 58, 59. Although contamination rates may be similar in bioreactors and in small vessels, bacteria and fungi proliferate faster in liquid medium. Greater losses occur due to contamination of larger vessels than with small flasks, which can discourage researchers from using bioreactors. Contamination depends not only on maintaining good laboratory practices during the work carried out in flow cabinets, but also on the environmental conditions and location of laboratory facilities (proximity to fields, ventilation, humidity, etc.). These conditions do not affect all bioreactor systems and all stages of experimental work to the same extent. Mireia Bordas (Agromillora Group) did not report any contamination problems when using MATIS® in experimental trials of culture of woody plants. Despite the rather excessive price of some components, the company plans to produce woody plants grown in bioreactors in the near future, especially for the pre‐acclimation phase (M. Bordas, pers. comm.). Beatriz Cuenca, a scientist working for TRAGSA nursery (Spain), has used Plantform™ bioreactors to multiply chestnut shoots 58 before rooting them photoautotrophically in a CIS 53 for more than 5 years. However, part of the multiplication phase has had to be performed in semi‐solid medium, after the Plantform™ bioreactors were severely affected by fungal contamination. This was probably influenced by the age of the facilities, which are in process of renewal, and their location, as they are surrounded by fields and forests (B. Cuenca, pers. comm.). Fortunately, the larger containers (without sugar) used for rooting were not as badly affected and were able to be used to improve chestnut acclimation. Susana Vilariño (Vitrosur Lab SLU) reported the use of “two‐flask system”, SETIS™ and Plantform™ to obtain eighty per cent of the annual production of eucalyptus and pistachio (S. Vilariño, pers. comm.). A common opinion among the three companies is that bioreactors can be used to complement semi‐solid culture, but not as a substitute. Aspects to consider before opting to use bioreactors include the excessive price of bioreactors and some components, such as spare parts and filters, and the need for careful training of operators in the correct installation and use of the devices.
5. CONCLUDING REMARKS
Bioreactors are useful tools for tree micropropagation and for the study of plant functioning. Use of these devices can help overcome the recalcitrance of some species and genotypes to proliferation, rooting, plant conversion, and acclimation. In addition, they can also be used to reduce the cost of large‐scale propagation. The number of tree species cultured in bioreactors is increasing steadily, and frequently the physiological state of plant propagules improves with these systems of culture, which also facilitate photoautotrophic propagation. However, two main types of challenges are still unresolved. At scientific level, it is necessary to unravel the physiological causes of hyperhydricity and to solve the difficulties to achieve maturation and plant conversion (especially in conifers). Besides, other issues such as the excessive cost and lack of availability of particular designs must be resolved to enable the general application of this promising technology.
CONFLICT OF INTEREST
The authors have declared no conflict of interest.
ACKNOWLEDGMENTS
The authors thank Susana Vilariño (Vitrosur Lab SLU), Mireia Bordas (Agromillora Group) and Beatriz Cuenca (TRAGSA) for their valuable comments on the industrial application of bioreactors for plant tissue culture.
Vidal N, Sánchez C. Use of bioreactor systems in the propagation of forest trees. Eng Life Sci. 2019;19:896–915. 10.1002/elsc.201900041
REFERENCES
- 1. Nehra, N. S. , Becwar, M. R. , Rottmann, W. H. , Pearson, L. et al., Forest biotechnology: innovative methods, emerging opportunities. In Vitro Cell Dev. Biol.‐Plant 2005, 41, 701–717. [Google Scholar]
- 2. Fenning, T. M. , Gershenzon, J. , Where will the wood come from? Plantation forests and the role of biotechnology. Trends Biotechnol. 2002, 20, 291–296. [DOI] [PubMed] [Google Scholar]
- 3. Wu, H. X. , Benefits and risks of using clones in forestry – a review. Scand. J. For. Res. 2019, 34, 352–359. [Google Scholar]
- 4. Ahuja M. R., Jain S. M. (Eds.), Biodiversity and Conservation of Woody Plants, (Sustainable Development and Biodiversity, Vol 17), Springer, Berlin, Germany: 2017. [Google Scholar]
- 5. Merkle, S. A. and Nairn, C. J. , Hardwood tree biotechnology. In Vitro Cell. Dev. Biol.‐Plant 2005, 41, 602–619. [Google Scholar]
- 6. Pijut, P. M. , Lawson, S. S. , Michler, C. H. , Biotechnological efforts for preserving and enhancing temperate hardwood tree biodiversity, health, and productivity. In Vitro Cell. Dev. Biol.‐Plant 2011, 47, 123–147. [Google Scholar]
- 7. Jain S. M., Haggman H. (Eds.), Protocols for Micropropagation of Woody Trees and Fruits, Springer, The Netherlands: 2007. [Google Scholar]
- 8. Bonga, J. M. , Vegetative propagation in relation to juvenility, maturity and rejuvenation, in: Bonga J. M., Durzan D. J. (Eds.), Tissue Culture in Forestry, Martinus Nijhoff/W. Junk Publishers, The Hague: 1982, pp. 387–412. [Google Scholar]
- 9. Libby, W.J. , Rauter, R. , Advantages of clonal forestry. For. Chron. 1984, 6, 145–149. [Google Scholar]
- 10. Bonga, J. M. , Klimaszewska, K. K. , von Aderkas, P. , Recalcitrance in clonal propagation, in particular of conifers. Plant Cell Tissue Organ Cult. 2010, 100, 241–254. [Google Scholar]
- 11. Monteuuis, O . Vegetatively propagating forest trees, in: Bonga J. M., Park Y.‐S., Trontin J.‐F. (Eds.), Proceedings of the 4th International Conference of the IUFRO Unit 2.09.02 on “Development and application of vegetative propagation technologies in plantation forestry to cope with a changing climate and environment”. September 19–23. La Plata, Argentina: 2016, pp. 37–57. [Google Scholar]
- 12. Shekhawat, N. S. , Rai, M. K. , Phulwaria, M. , Rathore, J. S. et al., Tree Biotechnology with special reference to species of fragile ecosystems and arid environments, in: Ramawat K., Mérillo J., Ahuja M. (Eds.), Tree Biotechnology, I. K. International Publishing House Pvt. Ltd., New Delhi: 2014, pp. 187–222. [Google Scholar]
- 13. Hackett, W.P. , Juvenility, maturation and rejuvenation in woody plants. Hortic. Rev. 1985, 7, 109–155. [Google Scholar]
- 14. Greenwood, M.S. , Rejuvenation of forest trees, in: Kossuth S. V., Ross S. D. (Eds.), Hormonal Control of Tree Growth, Martinus Nijhoff Publishers, Dordrecht: 1987, pp. 1–12. [Google Scholar]
- 15. Wendling, I. , Trueman, S. I. , Xavier, A. , Maturation and related aspects in clonal forestry‐Part I: concepts, regulation and consequences of phase change. New Forest 2014, 4, 449–471. [Google Scholar]
- 16. Díaz‐Sala, C. , Direct reprogramming of adult somatic cells toward adventitious root formation in forest tree species: the effect of the juvenile‐adult transition. Front. Plant Sci. 2014, 5, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bonga, J. M. , Conifer clonal propagation in tree improvement programs, in: Park Y. S., Bonga J. M., Moon H. K. (Eds.), Vegetative Propagation of Forest trees, National Institute of Forest Science, Seoul: 2016, pp. 3–31. [Google Scholar]
- 18. Guan, Y. , Li, S. , Fan, X. , Su, Z. , Application of somatic embryogenesis in woody plants. Front. Plant Sci. 2016, 7, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Durkovic, J. , Misalova, A. , Micropropagation of temperate noble hardwoods: an overview. Funct. Plant Sci. Biotech 2008, 2, 1–19. [Google Scholar]
- 20. Monteuuis, O. , Micropropagation and production of forest trees in: Park Y. S., Bonga J. M., Moon H. K. (Eds.), Vegetative Propagation of Forest Trees. National Institute of Forest Science, Seoul: 2016, pp. 32–55. [Google Scholar]
- 21. Etienne, H. , Berthouly, M. , Temporary immersion systems in plant micropropagation. Plant Cell. Tiss. Organ Cult. 2002, 69, 215–231. [Google Scholar]
- 22. Alvard, D. , Côte, F. , Teisson, C. , Comparison of methods of liquid medium culture for banana micropropagation. Effects of temporary immersion of explants. Plant Cell. Tiss. Org. Cult. 1993, 32: 55–60. [Google Scholar]
- 23. Teisson, C. , Alvard, D. , A new concept of plant in vitro cultivation liquid medium: temporary immersion, in: Terzi M., Cella R., Falavigna A. (Eds.), Current Issues in Plant Molecular and Cellular Biology. Kluwer Academic Publishers, Dordrecht: 1995, pp. 105–110. [Google Scholar]
- 24. Watt, M. P. , The status of temporary immersion system (TIS) technology for plant micropropagation. Afr. J. Biotechn. 2012, 11, 14025–14035. [Google Scholar]
- 25. Georgiev, V. , Schumann, A. , Pavlov, A. , Bley, T. , Temporary immersion systems in plant biotechnology. Eng. Life Sci. 2014, 14, 607–621. [Google Scholar]
- 26. Mamun, N. H. A. , Egertsdotter, U. , Aidun, C. K. , Bioreactor technology for clonal propagation of plants and metabolite production. Front. Biol. 2015, 10, 177–193. [Google Scholar]
- 27. Valdiani, A. , Hansen, O. K. , Nielsen, U. B. , Johannsen, V. K. et al., Bioreactor‐based advances in plant tissue and cell culture: Challenges and prospects. Crit. Rev. Biotechnol. 2019, 39, 20–34. [DOI] [PubMed] [Google Scholar]
- 28. Preil W., General introduction: a personal reflection on the use of liquid media for in vitro culture, in: Hvolslef‐Eide A. K., Preil W. (Eds.), Liquid Culture Systems for in vitro Plant Propagation. Springer, Netherlands: 2005, pp. 1–18. [Google Scholar]
- 29. Albarran, J. , Bertrand, B. , Lartaud, M. , Etienne, H. , Cycle characteristics in a temporary immersion bioreactor affect regeneration, morphology, water and mineral status of coffee (Coffea arabica L.) somatic embryos. Plant Cell Tiss. Org. Cult. 2005, 81, 27–36. [Google Scholar]
- 30. Chakrabarty, D. , Dewir, Y. H. , Hahn, E. J. , Datta, S. K. , Paek, K. Y. , The dynamics of nutrient utilization and growth of apple root stock ‘M9 EMLA’ in temporary versus continuous immersion bioreactors. Plant Growth Regul. 2007, 51, 11–19. [Google Scholar]
- 31. Preece, J. E. , Micropropagation in stationary liquid media. Prop. Orn. Plants 2010, 10, 183–187. [Google Scholar]
- 32. Carvalho, L. S. O. , Ozudogru, E. A , Lambardi, M. , Paiva, L. V. , Temporary immersion system for micropropagation of tree species: a bibliographic and systematic review. Not. Bot. Hortic. Agrobo. 2019, 47, 269–277. [Google Scholar]
- 33. Monja‐Mío, K. M. , Herrera‐Alamillo, M. A. , Robert, M. L. , Somatic embryogenesis in temporary immersion bioreactors, in: Loyola V. M., Ochoa‐Alejo N. (Eds.), Somatic Embryogenesis: Fundamental Aspects and Applications, Springer, Switzerland: 2016, pp. 435–454. [Google Scholar]
- 34. Fei, L. , Weathers, P. , Bioreactors for plant embryogenesis and beyond, in: Germana M. A., Lambardi M. (Eds.), In vitro Organogenesis in Higher Plants. Methods in Molecular Biology, vol 1359, Springer Science+Business Media, New York: 2016, pp. 245–259. [DOI] [PubMed] [Google Scholar]
- 35. Egertsdotter, U. , Ahmad, I. , Clapham, D. , Automation and scale up of somatic embryogenesis for commercial plant production, with emphasis on conifers. Front. Plant Sci. 2019, 10, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thorpe, T. , Stasolla, C. , Yeung, E. C. , De Klerk, G. J. et al., The components of plant tissue culture media II: organic additions, osmotic and pH effects, and support systems, in: George E. F., Hall M. A., De Klerk G. J. (Eds.), Plant Propagation by Tissue Culture, Volume 1. The background, 3rd Edition Springer; Dordrecht, 2008, pp. 115–173. [Google Scholar]
- 37. Zobayed, S. M. A. , Ventilation in micropropagation, in: Kozai T. (Ed.), Photoautotrophic (sugar‐free medium) Micropropagation as a New Propagation and Transplant Production System. Springer, The Netherlands: 2005, pp. 147–186. [Google Scholar]
- 38. Steward, F. C. , Caplin, S. , Millar, F. K. , Investigations on growth and metabolism of plant cells. I. New techniques for the investigation of metabolism, nutrition and growth in undifferentiated cells. Ann. Bot. 1952, 16, 57–77. [Google Scholar]
- 39. Escalona, M. , Lorenzo, J. C. , González, B. , Daquinta, M. et al., Pineapple (Ananas comosus L. Merr) micropropagation in temporary immersion systems. Plant Cell Rep. 1999, 18, 743–748. [Google Scholar]
- 40. Welander, M. , Persson, J. , Asp, H. , Zhu, L. H. , Evaluation of a new vessel system based on temporary immersion system for micropropagation. Sci. Hortic. 2014, 179, 227–232. [Google Scholar]
- 41. Latawa, Y. , Shukla, M. R. , Saxena, P. K. , An efficient temporary immersion system for micropropagation of hybrid hazelnut. Botany 2016, 94, 1–8. [Google Scholar]
- 42. Peña‐Ramírez, Y. , Juárez‐Gómez, J. , Gómez‐López, L. , Jerónimo‐Pérez, J. et al., Multiple adventitious shoot formation in Spanish Red Cedar (Cedrela odorata L.) cultured in vitro using juvenile and mature tissues: an improved micropropagation protocol for a highly valuable tropical tree species. In Vitro Cell. Dev. Biol. Plant 2010, 46, 149–160. [Google Scholar]
- 43. Xiao, Y. , Niu, G. , Kozai, T. , Development and application of photoautotrophic micropropagation plant system. Plant Cell. Tiss. Organ Cult. 2011, 105, 149–158. [Google Scholar]
- 44. Rojas‐Martínez, L. , Visser, R. G. F. , de Klerk, G.‐J. , The hyperhydricity syndrome: waterlogging of plant tissues as a major cause. Prop. Ornam Plants 2010, 10, 169–175. [Google Scholar]
- 45. Dewir, Y. H. , Indoliya, Y. , Chakrabarty, D. , Paek, K. P. , Biochemical and physiological aspects of hyperhydricity in liquid culture system, in: Paek K.‐Y. (Ed.), Production of Biomass and Bioactive Compounds Using Bioreactor Technology, Chapter 26, Springer Science+Business Media, Dordrecht: 2014, pp. 693–709. [Google Scholar]
- 46. Becerra, P. I. , Catford, J. A. , Inderjit, McLeod M. L. et al., Inhibitory effects of Eucalyptus globulus on understorey plant growth and species richness are greater in non‐native regions. Glob. Ecol. Biogeogr. 2018, 27, 68–76. [Google Scholar]
- 47. Goded, S. , Ekroos, J. , Domínguez, J. , Azcarate, J. G. et al., Effects of eucalyptus plantations on avian and herb species richness and composition in North‐West Spain. Glob. Ecol. Conserv. 2019, 19, e00690. [Google Scholar]
- 48. Mendonça, E. G. , Stein, V. C. , Carvalho, H. H. , de Santos, B. R. et al., The use of continuous, temporary immersion bioreactor system and semisolid culture medium for the production of Eucalyptus camaldulensis clones. Ciência Florestal 2016, 26, 1211–1224. [Google Scholar]
- 49. Kirdmanee, C. , Kitaya, Y. , Kozai, T. , Effects of CO2 enrichment and supporting material in vitro on photoautotrophic growth of Eucalyptus plantlets in vitro and ex vitro. In Vitro Cell Dev. Biol. Plant 1995, 31, 144–149. [Google Scholar]
- 50. Tanaka, M. , Giang, D. T. T. , Murakami, A. , Application of a novel disposable film culture system to photoautotrophic micropropagation of Eucalyptus uro‐grandis (Urophylia x grandis). In Vitro Cell Dev. Biol. Plant 2005, 41, 173–180. [Google Scholar]
- 51. Mosaleeyanon, K. , Cha‐um, S. , Kirdmanee, C. , Enhanced growth and photosynthesis of rain tree (Samanea saman Merr.) plantlets in vitro under a CO2‐enriched condition with decreased sucrose concentrations in the medium. Sci. Hortic. 2004, 103, 51–63. [Google Scholar]
- 52. Cha‐Um, S. , Chanseetis, C. , Chintakovid, W. , Pichakum, A. , Supaibulwatana, K. , Promoting root induction and growth of in vitro macadamia (Macadamia tetraphylla L. ‘Keaau’) plantlets using CO2‐enriched photoautotrophic conditions. Plant Cell Tiss Organ Cult 2011, 106, 435–444. [Google Scholar]
- 53. Vidal, N. , Aldrey, A. , Blanco, B. , Correa, B. et al., Proliferation and rooting of chestnut under photoautotrophic conditions, in: Bonga J. M., Park Y.‐S., Trontin J.‐F. (Eds.), Proceedings of the 4th International Conference of the IUFRO Unit 2.09.02 on “Development and application of vegetative propagation technologies in plantation forestry to cope with a changing climate and environment”. La Plata, Argentina: 2017, pp. 119–127. [Google Scholar]
- 54. Sánchez M. C., Vieitez A. M., In vitro morphogenetic competence of basal sprouts and crown branches of mature chestnut. Tree Physiol. 1991, 8, 59–70. [DOI] [PubMed] [Google Scholar]
- 55. Cuenca, B. , Sánchez, C. , Aldrey, A. , Bogo, B. et al., Micropropagation of axillary shoots of hybrid chestnut (Castanea sativa x C. crenata) in liquid medium in a continuous immersion system. Plant Cell Tiss. Organ Cult. 2017, 131, 307–320. [Google Scholar]
- 56. Nguyen, Q. T. , Xiao, Y. , Kozai, T. , Photoautotrophic micropropagation, in: Kozai T., Niu G., Takagaki M. (Eds.), Plant Factory ‐ An Indoor Vertical Farming System for Efficient uality Food Production, Edition: 1st, Chapter: 20, Elsevier, Academic Press, United Kingdom: 2016, pp. 271–283. [Google Scholar]
- 57. Regueira, M. , Rial, E. , Blanco, B. , Bogo, B. et al., Micropropagation of axillary shoots of Salix viminalis using a temporary immersion system. Trees 2018, 32, 61–71. [Google Scholar]
- 58. Vidal, N. , Blanco, B. , Cuenca, B. A temporary immersion system for micropropagation of axillary shoots of hybrid chestnut. Plant Cell Tiss. Org. Cult. 2015, 123, 229–243. [Google Scholar]
- 59. Grigoriadou, K. , Vasilakakis, M. , Tzoulis, T. , Eleftheriou, E. P. , Experimental use of a novel temporary immersion system for liquid culture of olive microshoots, in: Hvolslef‐Eide A. K., Preil W. (Eds.) Liquid Culture Systems for in vitro Plant Propagation. Springer, Netherlands: 2005, pp. 263–274. [Google Scholar]
- 60. McAlister, B. , Finnie, J. , Watt, M. P. , Blakeway, F. Use of temporary immersion system (RITA®) for production of commercial Eucalyptus clones in Mondi Forests (SA). Plant Cell Tiss. Organ Cult. 2005, 81, 347–358. [Google Scholar]
- 61. Zobayed, S. M. A. , Afreen, F. , Kozai, T. , Physiology of eucalyptus plantlets grown photoautotrophically in a scaled‐up vessel. In Vitro Cell. Dev. Biol‐Plant 2001, 37, 807–813. [Google Scholar]
- 62. Ayala, P. , Brugnoli, E. , Luna, C. , González, A. et al., Eucalyptus nitens plant regeneration from seedling explants through direct adventitious shoot bud formation. Trees: Structure and Function 2019. (in press). 10.1007/s00468-019-01888-5. [DOI] [Google Scholar]
- 63. Zobayed, S. M. A. , Afreen‐Zobayed, F. , Kubota, C. , Kozai, T. , Mass propagation of Eucalyptus camaldulensis in a scaled‐up vessel under in vitro photoautotrophic condition. Ann. Bot. 2000, 85, 587–592. [Google Scholar]
- 64. Kozai, T. , Photoautotrophic propagation‐ Environmental control for promoting photosynthesis. Prop. Ornam. Plants 2010, 10, 188–204. [Google Scholar]
- 65. Murch, S. J. , Chunzhao, L. , Romero, R. M. , Saxena, P. K. , In vitro culture and temporary immersion bioreactor production of Crescentia cujete . Plant Cell Tiss. Org. Cult. 2004, 78, 36–68. [Google Scholar]
- 66. Luna, C. V. , Gonzalez, A. M. , Mroginski, L. A. , Sansberro, P. A. , Anatomical and histological features of Ilex paraguariensis leaves under different in vitro shoot culture systems. Plant Cell Tiss. Organ. Cult. 2017, 129, 457–467. [Google Scholar]
- 67. Arencibia, A. D. , Gómez, A. , Poblete, M. , Vergara, C. , High‐performance micropropagation of dendroenergetic poplar hybrids in photomixotrophic Temporary Immersion Bioreactors (TIBs). Ind. Crop. Prod. 2017, 96, 102–109. [Google Scholar]
- 68. Duarte, E. , Sansberro, P. , Luna, C. , Micropropagation of Handroanthus heptaphyllus (Vell.) Mattos from seedling explants. Afr. J. Biotechn. 2016, 15, 1292–1298. [Google Scholar]
- 69. Nasri, A. , Baklouti, E. , Romdhane, A. B. , Maalej M. et al., Large‐scale propagation of Myrobolan (Prunus cerasifera) in RITA® bioreactors and ISSR‐based assessment of genetic conformity. Sci. Hortic. 2019, 245, 144–153. [Google Scholar]
- 70. Businge, E. , Trifonova, A. , Schneider, C. , Rodel, P. , Egertsdotter, U. , Evaluation of a new temporary immersion bioreactor system for micropropagation of cultivars of Eucalyptus, birch and fir. Forests 2017, 8, 196. [Google Scholar]
- 71. Chakrabarty, D. , Hahn, E. J. , Yoon, Y. S. , Paek, K. Y. , Micropropagation of apple root stock ‘M9 EMLA’ using bioreactor. J. Hortic. Sci. Biotechnol. 2003, 78, 605–609. [Google Scholar]
- 72. Zhu, L. H. , Li, X. Y. , Welander, M. , Optimisation of growing conditions for the apple rootstock M26 grown in RITA® containers using temporary immersion principle. Plant Cell Tiss. Organ Cult. 2005, 81, 313–318. [Google Scholar]
- 73. Quiala, E. , Cañal, M. J. , Meijón, M. , Rodriguez, R. et al., Morphological and physiological responses of proliferating shoots of teak to temporary immersion and BA treatments. Plant Cell Tiss. Organ Cult. 2012, 109, 223–234. [Google Scholar]
- 74. Aguilar, M. E. , Garita, K. , Kim, Y. W. , Kim, J.‐A. , Moon, H. K. , Simple protocol for the micropropagation of teak (Tectona grandis Linn.) in semi‐solid and liquid media in RITA© bioreactors and ex vitro rooting. Am. J. Plant Sci. 2019, 10, 1121–1141. [Google Scholar]
- 75. Akdemir, H. , Süzerer, V. , Onay, A. , Tilkat, E. et al., Micropropagation of the pistachio and its rootstocks by temporary immersion system. Plant Cell Tiss. Organ Cult. 2014, 117, 65–76. [Google Scholar]
- 76. Godoy, S. , Tapia, E. , Seit, P. , Andrade, D. et al., Temporary immersion systems for the mass propagation of sweet cherry cultivars and cherry rootstocks: development of a micropropagation procedure and effect of culture conditions on plant quality. In Vitro Cell. Dev. Biol.‐Plant. 2017. 53, 494–504. [Google Scholar]
- 77. Gatti, E. , Sgarbi, E. , Ozudogru, E. A. , Lambardi, M. , The effect of Plantform™ bioreactor on micropropagation of Quercus robur in comparison to a conventional in vitro culture system on gelled medium, and assessment of the microenvironment influence on leaf structure. Plant Biosyst. 2017, 151, 1129–1136. [Google Scholar]
- 78. Marín, J. A. , García, E. , Lorente, P. , Andreu, P. , Arbeloa, A. , A novel approach for propagation of recalcitrant pistachio cultivars that sidesteps rooting by ex vitro grafting of tissue cultured shoot tips. Plant Cell Tiss. Org. Cult. 2016, 124, 191–200. [Google Scholar]
- 79. Vieitez, A. M. , Ballester, A. , San Jose, M. C. , Vieitez, E. , Anatomical and chemical studies on vitrified shoots of chestnut regenerated in vitro. Physiol. Plant. 1985, 65, 177–184. [Google Scholar]
- 80. Lambardi, M. , Ozudogru, E. A. , Roncasaglia, R. , In vitro propagation of olive (Olea europaea L.) by nodal segmentation of elongated shoots, in: Lambardi M., Ozudogru E., Jain S. (Eds.) Protocols for Micropropagation of Selected Economically‐Important Horticultural Plants. Methods in Molecular Biology (Methods and Protocols), vol 994, Humana Press, Totowa: 2013, pp. 33–44. [DOI] [PubMed] [Google Scholar]
- 81. Benelli, C. , De Carlo, A. , In vitro multiplication and growth improvement of Olea europaea L. cv Canino with temporary immersion system (Plantform™). 3 Biotech 2018, 8, 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Afreen, F. , Zobayed, S. M. A. , Kozai, T. , Photoautotrophic culture of Coffea arabusta somatic embryos: development of a bioreactor for large‐scale plantlet conversion from cotyledonary embryos. Ann. Bot. 2002, 90, 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ducos, J. P. , Labbe, G. , Lambot, C. , Pétiard, V. , Pilot scale process for the production of pre‐germinated somatic embryos of selected robusta (Coffea canephora) clones. In Vitro Cell Dev. Biol. Plant 2007, 43, 652–659. [Google Scholar]
- 84. Ducos, J.‐P. , Lambot, C. , Pétiard, V. , Bioreactors for coffee mass propagation by somatic embryogenesis. Int. J. Plant Dev. Biol. 2007, 1, 1–12. [Google Scholar]
- 85. Etienne, H. , Bertrand, B. , Georget, F. , Lartaud, M. et al. Development of coffee somatic and zygotic embryos to plants differs in the morphological, histochemical and hydration aspects. Tree Physiol. 2013, 33, 640–653. [DOI] [PubMed] [Google Scholar]
- 86. Etienne, H. , Breton, D. , Breitler, J‐C. , Bertrand, B. et al., Coffee somatic embryogenesis: How did research, experience gained and innovations promote the commercial propagation of elite clones from the two cultivated species? Front. Plant Sci. 2018, 9, 1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Etienne, H. , Lartaud, M. , Michaux‐Ferrière, N. , Carron, M. P. et al., Improvement of somatic embryogenesis in Hevea brasiliensis (Müll. Arg.) using the temporary immersion technique. In Vitro Cell Dev Biol Plant 1997, 33, 81–87. [Google Scholar]
- 88. Kim, S. J. , Dewir, Y. H. , Moon, H. K. , Large‐scale plantlets conversion from cotyledonary somatic embryos of Kalopanax septemlobus tree using bioreactor cultures. J. Plant Biochem. Biotechnol. 2011, 20, 241–248. [Google Scholar]
- 89. Posada‐Pérez, L. , Montesinos, Y. P. , Guerra, D. G. , Daniels, D. , Gómez‐Kosky, R. , Complete germination of papaya (Carica papaya L. cv. `Maradol Roja´) somatic embryos using temporary immersion system type RITA® and phloroglucinol in semi‐solid culture medium. In Vitro Cell. Dev. Biol.‐Plant 2017, 53, 505–513. [Google Scholar]
- 90. Kosky, R. G. , Perozo, J. V. , Valero, N. A. , Peñalver, D. A. , Somatic embryo germination of Psidium guajava L. in the RITA‐temporary immersion system and on solid medium, in: Hvoslef‐Eide A. K., Preil W. (Eds.), Liquid Culture Systems for in vitro Plant Propagation. Springer, The Netherlands: 2005, pp. 225–229. [Google Scholar]
- 91. Niemenak, N. , Saare‐Surminski, K. , Rohsius, C. , Ndoumou, D. , Lieberei, R. , Regeneration of somatic embryos in Theobroma cacao L. in temporary immersion bioreactor and analyses of free amino acids in different tissues. Plant Cell Rep. 2008, 27, 667–676. [DOI] [PubMed] [Google Scholar]
- 92. Kong, L. , Holtz, C. T. , Nairn, C. J. , Houke, H. et al., Application of airlift bioreactors to accelerate genetic transformation in American chestnut. Plant Cell Tiss. Org. Cult. 2014, 117, 39–50. [Google Scholar]
- 93. Mallón, R. , Vieitez, A. M. , Vidal, N. , High‐efficiency Agrobacterium‐mediated transformation in Quercus robur: selection by use of a temporary immersion system and assessment by quantitative PCR. Plant Cell. Tiss. Organ Cult. 2013, 114, 171–185. [Google Scholar]
- 94. Mallón, R. , Valladares, S , Corredoira, E. , Vieitez, A. M. , Vidal, N. Overexpression of the chestnut CsTL1 gene coding for a thaumatin‐like protein in somatic embryos of Quercus robur . Plant Cell Tiss. Organ Cult. 2014, 116, 141–151. [Google Scholar]
- 95. Gupta, P. K. , Timmis, R. , Mass propagation of conifer trees in liquid cultures ‐ progress towards commercialization. Plant Cell Tiss. Organ Cult. 2005, 81, 339–346. [Google Scholar]
- 96. Trontin, J. F. , Teyssier, C. , Morel, A. , Harvengt, L. , Lelu‐Walter, M. A. , Prospects for new variety deployment through somatic embryo‐genesis in maritime pine in: Park Y. S., Bonga J. M., Moon H. K. (Eds.), Vegetative Propagation of Forest Trees, National Institute of Forest Science, Seoul: 2016, pp. 572–606. [Google Scholar]
- 97. Klimaszewska, K. , Hargreaves, C. , Lelu‐Walter, M. A. , Trontin, J. F. , Advances in conifer somatic embryogenesis since year 2000, in: Germana M. A., Lambardi M. (Eds.), In Vitro Organogenesis In Higher Plants. Methods in Molecular Biology vol 1359, Springer Science+Business Media, New York: 2016, pp. 131–166. [DOI] [PubMed] [Google Scholar]
- 98. Bonga, J. , Park, Y. S. , Ding, C. , What technical improvements are needed to achieve industrial application of conifer somatic embryogenesis? in: Bonga J. M., Park Y.‐S., Trontin J.‐F. (Eds.), Proceedings of the 5th International Conference of the IUFRO Unit 2.09.02 on “Clonal Trees in the Bioeconomy Age: Opportunities and Challenges.” September 10–15, 2018. Coimbra, Portugal: pp. 14–24. [Google Scholar]
- 99. Adams, G.W. , Kunze, H. A. , McCartney, A. , Millican, S. , Park, Y. S. , An industrial perspective on the use of advanced reforestation stock technologies, in: Park Y. S., Bonga J. M., Moon H. K. (Eds.), Vegetative Propagation of Forest Trees, National Institute of Forest Science, Seoul: 2016, pp. 323–334. [Google Scholar]
- 100. González‐Cabrero, N. , Ruiz‐Galea, M. , Alegre, J. , Toribio, M. , Celestino, C. , Growth, morphology and maturation ability of Pinus pinea embryogenic suspension cultures. Plant Cell Tissue Org. Cult. 2018, 135, 331–346. [Google Scholar]
- 101. Mamun, N. H. A. , Aidun, C. K. , Egertsdotter, U. , Improved and synchronized maturation of Norway spruce (Picea abies (L.) H.Karst.) somatic embryos in temporary immersion bioreactors. In vitro Cell. Dev. Biol. Plant 2018, 54, 612–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Tautorus, T. E. , Lulsdorf, M. M. , Kikcio, S. I. , Dunstan, D. I. , Nutrient utilisation during bioreactor culture, and regeneration of somatic embryo cultures of Picea mariana and Picea glauca‐engelmannii . In Vitro Cell Dev. Biol. Plant 1994, 30, 58–63. [Google Scholar]
- 103. Ingram, B. , Mavituna, F. , Effect of bioreactor configuration on the growth and maturation of Picea sitchensis somatic embryo cultures. Plant Cell Tiss. Org. Cult. 2000, 61, 87–96. [Google Scholar]
- 104. Choudhury, H. , Tandon, P. , Non‐destructive assessment of growth performance of embryogenic suspension culture of Pinus kesiya (Royle ex. Gord.) in shake‐flask and self‐designed bubble bioreactor and successful regeneration of plantlets from the culture systems. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2014, 84, 771–777. [Google Scholar]
- 105. Shukla, M. R. , Singh, A. S. , Piunno, K. , Saxena, P. K. , Jones, M. P. , Application of 3D printing to prototype and develop novel plant tissue culture systems. Plant Meth. 2017, 13, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. González, R. , Ríos, D. , Avilés, F. , Sánchez‐Olate, M. , In vitro multiplication of Eucalyptus globulus by a temporary immersion system. Bosque 2011, 32, 147–154. [Google Scholar]
- 107. Fuljahn, S. , Tantau, H.‐J. , Process engineering as a means of regulating the microclimate in a photoautotrophic in vitro culture. Acta Hort. 2009, 817, 143–150. [Google Scholar]
- 108. Mallón, R. , Covelo, P. , Vieitez, A. M. , Improving secondary embryogenesis in Quercus robur: application of temporary immersion for mass propagation. Trees 2012, 26, 731–741. [Google Scholar]
- 109. Perez, M. , Bueno, M. A. , Escalona, M. , Toorop, P. , Rodriguez, R. , Canal, M. J. , Temporary immersion systems (RITA) for the improvement of cork oak somatic embryogenic culture proliferation and somatic embryo production. Trees 2013, 27, 1277–1284. [Google Scholar]
- 110. Misra, B. B. , Dey, S. , Culture of East Indian sandalwood tree somatic embryos in air‐lift bioreactors for production of santalols, phenolics and arabinogalactan proteins. AoB Plants 2013, 5 plt025. [Google Scholar]


