Abstract
Objective:
Characterize differences in adult cochlear implant outcomes and programming parameters for a straight (CI422/522) and a precurved (CI532) electrode array.
Setting:
Cochlear implant (CI) program at a tertiary otologic center.
Patients:
Fifty-eight adults were included in the study; 29 were implanted with CI422 or CI522 and 29 were implanted with CI532. Each CI532 recipient was matched to a CI422/522 recipient in terms of age and preoperative hearing thresholds for comparison purposes.
Main Outcome Measures:
Consonant-Nucleus-Consonant (CNC) words, AzBio sentences, residual audiometric thresholds, and Speech Spatial Qualities (SSQ) questionnaire collected 6 months postoperatively were used to characterize outcomes. Pulse duration, maxima, impedances, and overall charge measurements were used to characterize programming parameters.
Results:
Postoperative unaided low frequency pure-tone average (LFPTA) was significantly better for the CI532 group. CNC scores were significantly better for the CI532 group. Impedances and pulse duration were significantly lower for the CI532 group, but there was no difference in overall charge between the groups.
Conclusion:
The CI532 group showed either similar or statistically superior results on all measures when compared with the CI422/522 suggesting that the CI532 electrode may be an advantageous substitute for the CI522.
Keywords: Audiology, Cochlear implant, Electrode array, Electrode type, Outcomes
In adults with moderate to profound sensorineural hearing loss, cochlear implants (CIs) improve communi-cation abilities following implantation; nevertheless, CI recipients continue to show high variability in outcomes (1–3). Many patient-specific factors are known to influ-ence audiologic outcomes such as duration of deafness, age at implantation, preoperative hearing aid use, and preoperative hearing thresholds, however, these factors account for less than 30% of the variability in patient outcomes (3–8). In addition to patient-specific factors, factors associated with implant technology, surgical technique, and placement of the device are also known to impact outcomes. These factors include electrode design, intrascalar location, surgical approach, and atraumatic insertion technique (2,3,8–15).
The two main types of electrode arrays, precurved and straight, have been designed for two very different use cases. Ideal placement of precurved arrays results in closer proximity to the modiolus and spiral ganglion cells. Closer proximity is thought to improve stimulation specificity (16) and reduce charge required for upper stimulation levels (17–20). However, several previous studies have shown that precurved electrodes are prone to scalar translocation (Wanna et al. (21) reported 42%) and intracochlear trauma, which is not conducive to structure, and subsequently, hearing preservation (15,21–24). Con-versely, straight arrays are inserted into the cochlea and track along the lateral wall during insertion, which reduces the frequency of scalar translocation and improves odds of hearing preservation (15,23,24). However, the resting place of the straight electrode is further from the spiral ganglion cells, potentially necessitating higher charge for upper stimulation levels and lower channel specificity (17–19,25–29). Additionally, straight arrays may in theory be more prone to variability in impedances due to a greater amount of fluid between the electrode and the neural tissue and because bone has a higher resistivity than tissue. Overall, precurved devices are thought to provide higher stimulation specificity (16) using lower stimulation levels (18–20), which results in better speech recognition scores in the electric only condition (3,19,30,31). However, there are studies which diverge with these findings (32,33), which are largely focused on poorer hearing preservation and trauma inflicted by previous generation precurved electrodes.
Atraumatic electrode insertion is of particular interest due to an increasing number of patients with significant residual hearing pursuing CI (34). When low frequency hearing is preserved, the addition of an acoustic compo-nent can be used present the low frequency portion of a signal acoustically. When combined with the electrical stimulation from the implant (Electric Acoustic Stimulation), higher levels of speech understanding in noise (12,35) and improvement in music appreciation (36) are observed. Because straight electrodes are associated with higher rates of acoustic hearing preservation (15,23,24), patients with significant preoperative hearing are typically implanted with straight arrays. This general practice has resulted in a lack of hearing preservation outcome data with precurved electrodes. As Fabie et al. (1) point out in their recent electrode array evaluation study, there is a need for “future studies comparing hearing performance between array designs to consider underlying preoperative auditory discrepancies among [electrode array] groups,” which is a key motivator for the current study.
Cochlear Ltd. currently offers two precurved electrodes, the Nucleus CI512 electrode and the Nucleus CI532 electrode, and one primary conventional length straight electrode, the Nucleus slim straight array used in the CI422 and CI522. The CI512 and CI532 differ in their insertion technique; the CI512 uses an advanced off-stylet technique, while the CI532 uses a sheath–based delivery system. Cochlear’s initial clinical trial found 100% of CI532 electrodes maintained scala tympani placement (37). Postoperative CT scans from patients implanted with CI532 at our center yielded similar results, which indicate much lower translocation rates compared with previous studies of precurved devices (42% [21]). This information suggests that the CI532 is less traumatic to the inner ear structures and thus could offer the surgeon better opportunity to preserve acoustic hearing than traditional precurved arrays, including the CI512.
In addition to electrode location, some previous studies have shown differences in programming parameters between the CI512 and CI422/522 electrodes (17,18,27–29), while others have not (28,30). For exam-ple, the default programming parameters are different for the two devices; the CI422/522 device defaults to a pulse duration of 37 μs per phase and the CI532 and 512 devices default to a pulse duration of 25 μs per phase. Due to the inherent limitations of the present system, patients that require wider pulse durations are unable to take advantage of the highest maxima settings, which have been preliminarily shown to provide an average 12-percentage point improvement for sentence recognition in noise (16). A better understanding of how electrode type affects these programming parameters and subsequent patient outcomes is necessary when choosing the best device for an individual.
The current study aims to compare a newer generation precurved electrode, CI532, with a straight electrode, CI422/522. A single manufacturer was chosen to limit the impact of signal processing as a confounding variable. CI532 recipients were matched to CI422/522 recipients with respect to age and preoperative hearing thresholds. Outcomes measured included acoustic hearing preservation, speech understanding, and programming parameters. We hypothesized that in comparison to the CI422/522, the CI532 electrode array would provide 1) compa-rable hearing preservation due to similar rates of scalar translocation, 2) better speech understanding scores due to lower electrode to modiolus distance, and 3) preferable programming parameters due to lower electrode to modiolus distance.
MATERIALS AND METHODS
Participants
Data were collected with Vanderbilt’s IRB approval in a prospective manner for all adults undergoing implantation with the Cochlear Nucleus CI532 CI electrode (n = 29, 17 men). Eleven CI532 recipients were excluded from this study for the following reasons: non-English speaking (1/11), less than 6 months of postoperative audiologic follow-up (7/11), devel-opmental disability (1/11), stroke (1/11), and/or revision surgery (1/11). Included participants were matched with adults who underwent CI with a Cochlear Nucleus CI422/522 CI electrode (n = 29,12 CI422, 19 men) by retrospectively reviewing our aggregate clinical database developed and maintained in conjunction with a National Institute of Health (NIH) funded study (R01 DC13117). CI532 recipients were individually matched with CI422/522 counterparts in age and preoperative hearing thresholds to create similar groups for comparing the two electrodes.
Etiology of the participants’ hearing losses was variable. Patient report was used to determine etiology when physician diagnosis was not present. The majority of the participant’s etiology was due to a progressive hearing loss of unknown cause (43/58), followed by sudden sensorineural hearing loss (6/58), Meniere’s disease (5/58), Labyrinthitis (2/58), Otosclerosis (1/58), and hereditary cause (1/58). All patients underwent cochlear implantation through a standard mastoid-ectomy/facial recess approach and electrode insertion via round window (RW), extended round window (ERW), or cochleos-tomy (C). For the CI422/522 group the insertion approach was as follows: RW = 23, ERW = 3, and C = 3. For the CI532 group the insertion approach was as follows: RW = 18, ERW = 5, C = 6.
Audiologic Procedures
Each participant underwent postoperative evaluation and programming according to Vanderbilt University Medical Center’s standard CI protocol as outlined below. Outcome data including residual acoustic hearing thresholds, speech recognition scores, and questionnaire data were prospectively collected. Additionally, programming software parameters such as pulse duration, impedances, maxima, and datalogging were recorded and analyzed retrospectively. Data were collected and managed using the Research Electronic Data Capture (REDCap) secure data management tool (38).
Audiometric Thresholds
Pre and postoperative acoustic hearing thresholds were obtained in a double-walled sound treated booth. For the purposes of characterizing hearing preservation, the low frequency pure-tone average (LFPTA, average of 125, 250, and 500 Hz) was recorded for each participant preoperatively and 6 months postoperatively.
Speech Recognition
Speech recognition results reported herein follow the revised Minimum Speech Test Battery (MSTB) for adult CI recipients (39). Speech recognition testing was completed in a double-walled sound treated booth with a presentation level of 60 dBA through a single loud speaker positioned at 0 degree azimuth approximately 1 m from the listener. To ensure appropriate calibration, rooms were equipped with Larson Davis LxT sound level meters positioned at the level of the patient’s head. For all materials, the participant was instructed to repeat as much as possible and encouraged to guess when necessary. Participants completed CNC word recognition (50 word list) (40) and AzBio sentence recognition (20 sentence list) (41). Sentences were presented in quiet and +5 dB signal-to-noise ratio (SNR). Scores were recorded as the percentage of words correctly repeated. Our rationale for selecting +5 dB SNR for assessing candidacy criteria and postoperative performance is based on the following: 1) patients’ most frequent complaint is speech understanding in noise (42), 2) +5 dB is the most common SNR present in real-world circumstances (43,44), and 3) our own clinical data and data reported by Mudery et al. (45) show that listeners meeting candidacy criteria at +5 dB SNR demonstrate significant improvement in quiet and noise following implantation (34).
Speech Spatial Qualities (SSQ)
The SSQ questionnaire assesses subjective hearing abilities across three listening domains: speech understanding, spatial hearing, and overall quality of speech using a visual analog scale ranging from 1 (poor) to 10 (perfect) (46). The overall score, which is an average of these three subscales, was reported. Note that SSQ data were only available for 28 out of the 58 participants.
Programming Software Parameters
Pulse duration, maxima, and upper stimulation levels (clinical levels/c-levels) were recorded for all participants from the program they were using 6 months post-activation. The following formula was provided by Cochlear Ltd. and used to convert clinical levels to charge per phase [nanocoulombs (nC)]:(100(clinicallevel ÷ 255) × 17.5) × (pulsewith ÷ 1000). Additionally, impedance data were collected from the clinical software at the 1, 3, and 6 months post-activation time points on all active electrodes in the common ground mode to measure variability across the electrode and over time.
Statistical Analysis
Independent-samples t tests were used to compare paramet-ric data between the two groups. A mixed-effects general linear model was used to compare impedances between the two groups over time. All statistical analyses were performed with SPSS (IBM, Armonk, NY).
RESULTS
Participant Matching
Age, preoperative LFPTA, preoperative CNC scores, and daily processor usage are reported in Table 1. The average age for the CI422/522 and CI532 groups were 66.90 and 67.00 years, respectively. The average preoperative LFPTA was 84.54- and 83.62-dB HL for the CI422/522 and CI532 groups, respectively. The average preoperative CNC word score was 5.51 and 7.31% for the Cl422/522 and CI532 groups, respectively. Lastly, the average daily usage reported in hours was 12.75 and 12.27 for the CM22/522 and CI532 groups, respectively.
TABLE 1.
Mean data are shown for each electrode
| Straight 422/522 (n = 29) | Precurved 532 (n = 29) | Significance | |
|---|---|---|---|
| Age at implant (yr) | 66.90 (34–84) | 67.00 (32–87) | - |
| Male/Female | 19/10 | 17/12 | - |
| Preoperative LFPTA (dB HL) | 84.54 (53.33–106.67) | 83.62 (48.33–106.67) | - |
| Preoperative CNC (%) | 5.51 (0–25) | 7.31 (0–40) | - |
| Datalogging (h/d) | 12.75 (3.90–17.00) | 12.27 (6.20–15.30) | - |
| Overall charge (nC) | 13.88 (6.73–35.73) | 12.29 (5.47–22.55) | 0.225 |
| Pulse duration (μs) | 36.00 (25–88) | 28.79 (22–50) | 0.005 |
| Maxima | 9.59 (5–16) | 11.79 (5–16) | 0.003 |
| Average impedance (kΩ) | 9.46 (5.41–19.91) | 7.39 (7.04–20.01) | 0.001 |
| Postoperative LFPTA (dB HL) | 102.13 (68.33–106.67) | 94.71 (66.67 – 106.67) | 0.028 |
| CNC (%) | 43.34 (4–80) | 56.97 (8–80) | 0.016 |
| AzBio (%) | 54.44 (5–92) | 67.17 (0–100) | 0.065 |
| AzBio +5 (%) | 15.93 (0–68) | 21.48 (0–57) | 0.404 |
| SSQ | 4.87 (0.82–8.17) | 4.76 (0.58–8.30) | 0.889 |
Ranges are shown in parentheses. The significance of the differences between groups determined using t tests is shown in the significance column, p < 0.05 is shown in bold. CNC indicates Consonant-Nucleus-Consonant; LFPTA, low frequency pure-tone average; SSQ, speech spatial qualities.
Audiologic Outcomes
Outcome measures were obtained at approximately 6 months after activation (422/522 average = 6.70 mos, 532 average = 6.90 mos). All outcome measures are summarized in Table 1 and illustrated in Figures 1 and 2. Postoperative acoustic hearing LFPTA was available for all 58 participants. Preoperatively, there was no significant difference between the groups, t (56) = 0.176, p = 0.861; however, postoperatively, the CI532 group had a significantly lower (i.e., better) LFPTA (M = 94.71 dB HL, standard error [SE] = 2.75) than the CI422/522 group (M = 102.13dB HL, SE = 1.78), t (47.96) = 2.266, p = 0.028 (Fig. 1). Twenty five of 58 participants had a LFPTA of less than 80 dB HL preoperatively, and nine patients (CI532 = 7) maintained a LFPTA of less than 80 dB HL at 6 months following surgery. CNC word scores were available for 58 participants with an overall mean score of 50.16%. The 532 group’s mean CNC score (56.97%), was found to be significantly higher than the mean score for the 422/522 group (43.34%), t (56) = −2.478, p = 0.016. AzBio sentence scores were available for 58 participants with an overall mean score of 60.81%. The 532 group’s mean AzBio score (67.17%) was found to be higher than the mean score for the 422/522 group (54.44%); however, this 12.7-percentage point difference did not reach statistical significance t (56) = −1.884, p = 0.065. AzBio +5dB SNR sentence scores were available for 37 (CI422/522 = 14) participants with an overall mean score of 19.38%. The 532 group’s mean AzBio +5 score (21.48%) was found to be higher than the mean score for the 422/522 group (15.93%); however, this 5.6-percentage point difference was not found to be statistically significant, t (35) = −0.844, p = 0.404 (Fig. 2). It should be noted that the AzBio +5 dB SNR scores were not available for all participants (CI422/522 = 14, CI 532 = 23), which could have affected the comparison for this measure. Part of this discrepancy can be explained by our clinical protocol, which states that if the patient scores less than 30% on AzBio sentences in quiet, AzBio sentences in noise are deferred. No significant differences were observed for the available SSQ data (n = 29), t (27) = 0.141, p = 0.889.
FIG. 1.
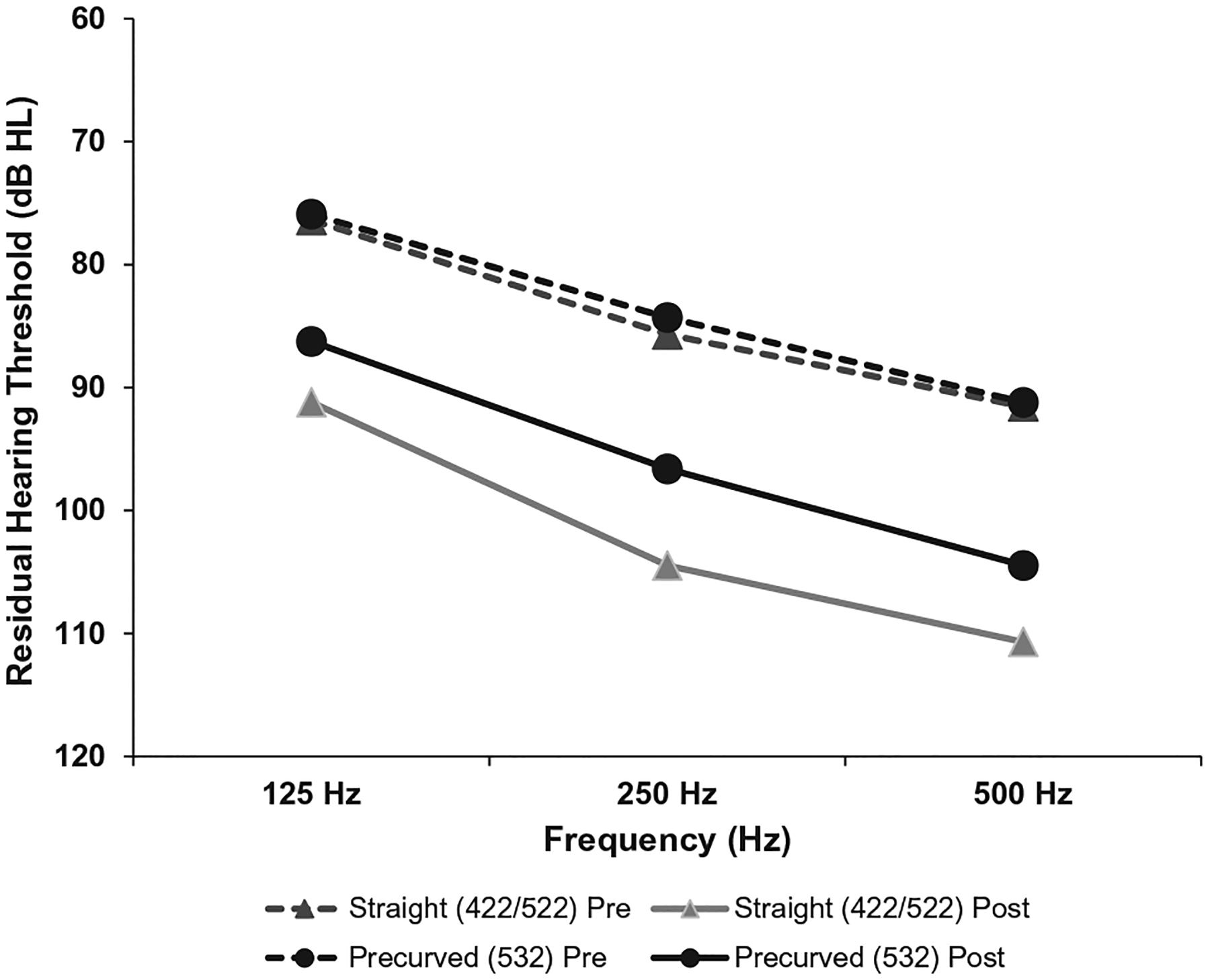
Preoperative (dashed line) and postoperative (solid line) acoustic hearing are shown for 125, 250, and 500 Hz.
FIG. 2.
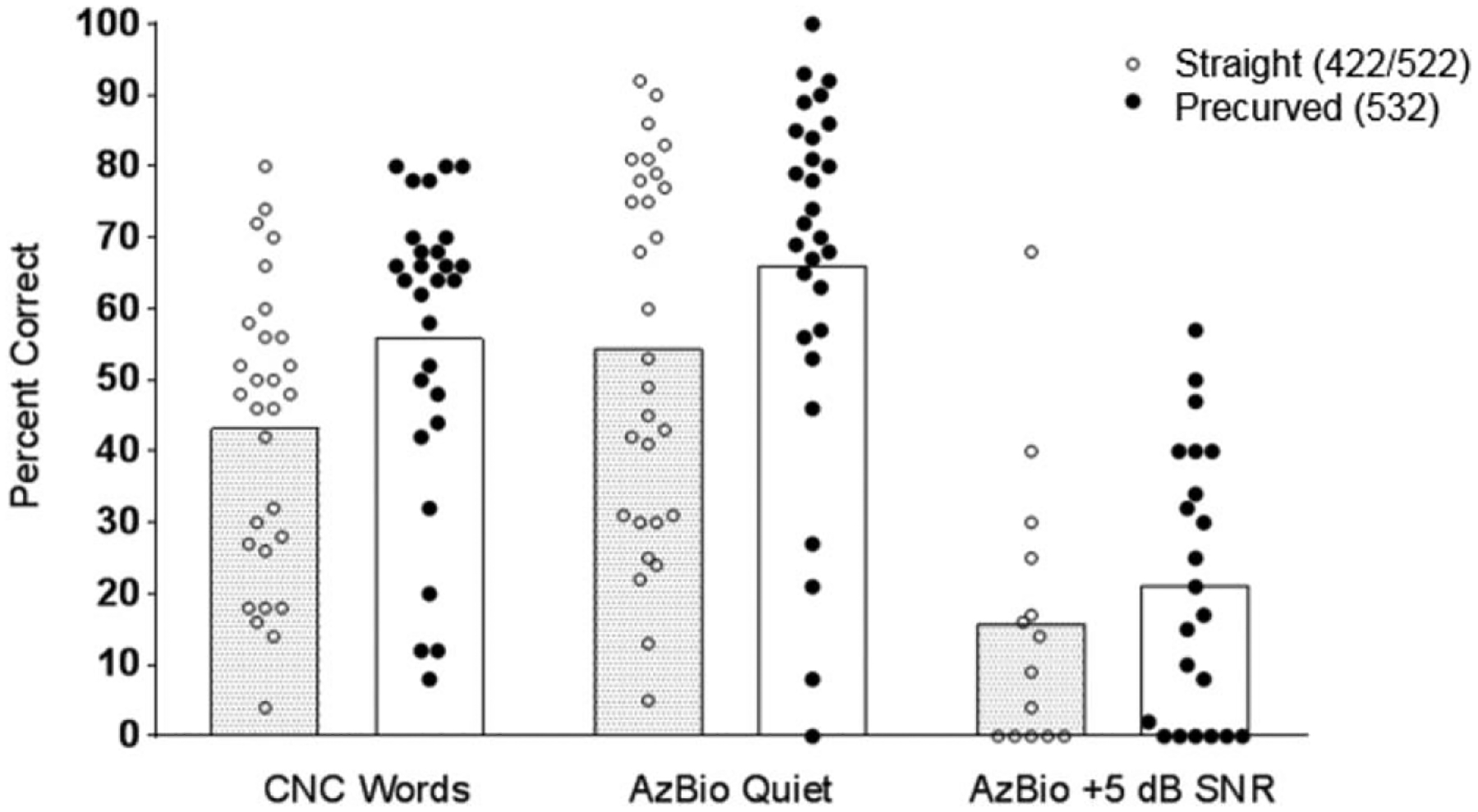
Individual speech recognition outcomes collected at approximately 6 months after implantation are shown as percent correct. Bars represent mean data. Difference in Consonant-Nucleus-Consonant (CNC) was found to be statistically significant (p=0.016).
Programming Software Parameters
Impedance data were available for 53 participants at 6 months post-activation. The average impedance at 6 months post-activation was 9.46 kΩ for the 422/522 group and 7.39 kΩ for the 532 group. Impedances were found to be significantly lower for the 532 group (F1,3138 = 400.7, p < 0.0005). A mixed-effects general linear model was conducted to analyze change in impedance over time for each electrode group (Fig. 3). The model revealed a significant effect of measurement time point (F1,1992 = 25.9, p < 0.0005) for both groups. Follow-up analyses revealed that for the CI422/522 group, impedances were significantly lower at 3 months than at 1 and 6 months, and for the CI532 group, impedances were significantly lower at 3 and 6 months than at 1 month.
FIG. 3.
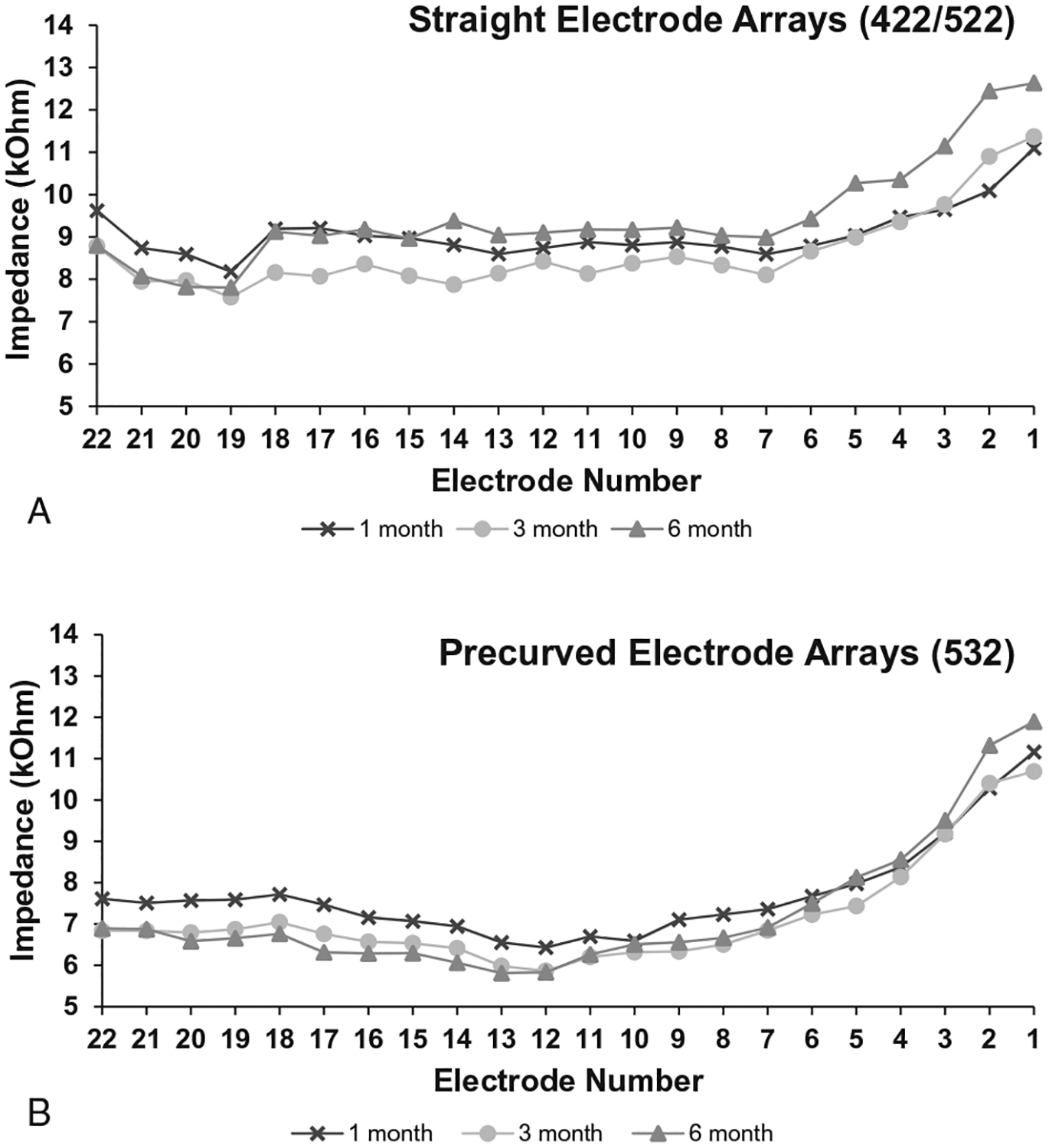
Mean impedances measured from each electrode contact are shown for 1 month, 3 months, and 6 months post-activation. A, shows the 422/522 data, and (B) shows the 532 data.
Charge data were available for 57 participants at 6 months post-activation. On average, charge was higher for the CI422/522 (M = 13.88nC, SE = 1.17) than CI532 (M = 12.29nC, SE = 0.79); however, the difference of 6nC was not significant, t (55) = 1.140, p = 0.225. When comparing charge for each of the primary electrodes (1, 6, 11, 16, and 22), there were also no significant differences (Fig. 4).
FIG. 4.
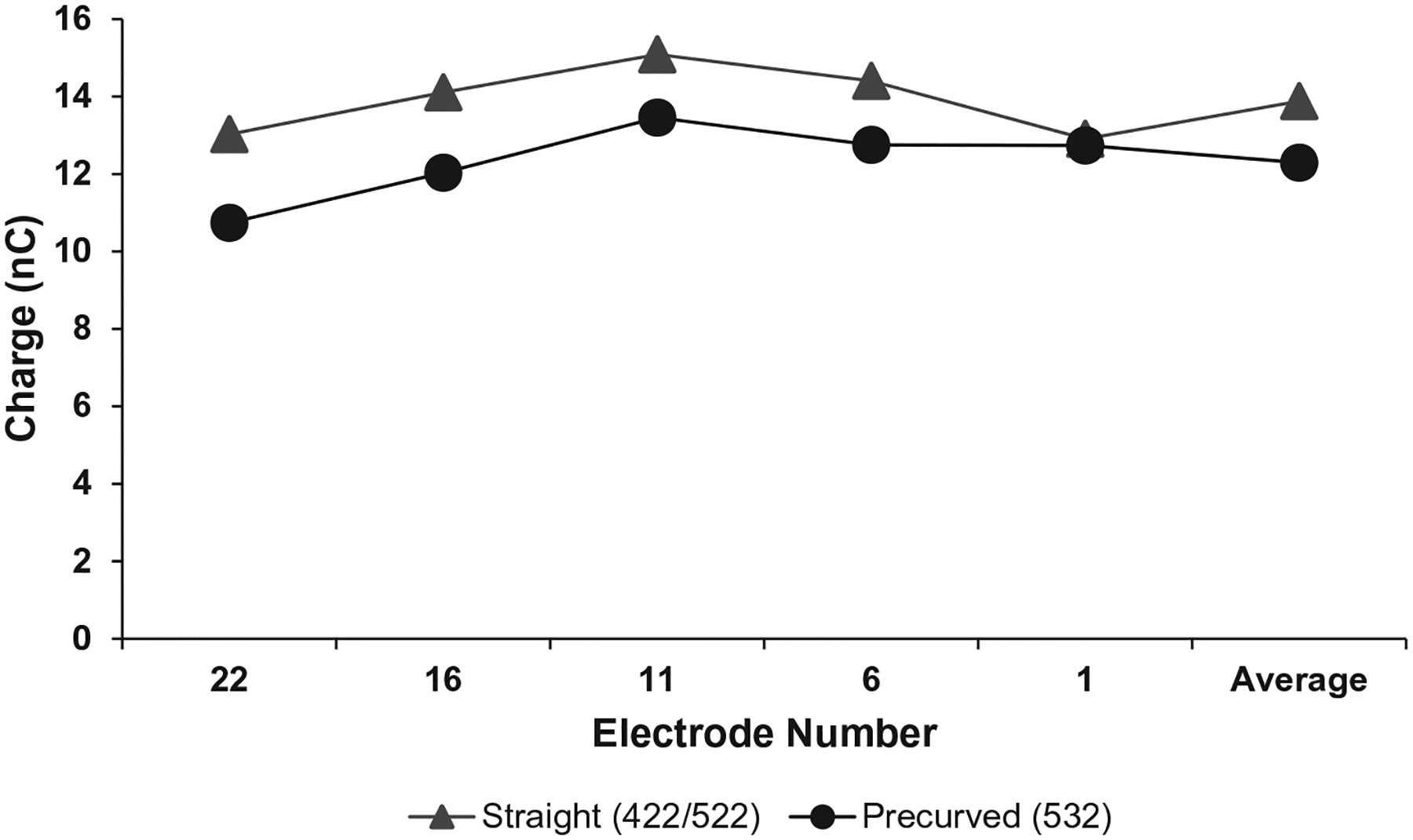
Mean charge levels are shown for five primary electrode contacts, as well as the average across the array for each electrode array type.
Pulse duration data were available for all 58 participants. The average pulse duration was 32.39 μs overall, 36.00 μs for the 422/522 group, and 28.79 μs for the 532 group. The difference in pulse duration was found to be significant, t (56) = 2.570, p = 0.013. Maxima data were also available for all 58 participants. The average maxima was 10.69 overall, 9.59 for the 422/522 group, and 79 for the 532 group. The difference in maxima was significant, t (56) = −3.185, p = 0.002.
DISCUSSION
Several previous studies have compared audiologic outcomes and programming parameters for Cochlear’s earlier precurved and straight devices (15,29,31,32); however, previous precurved electrodes were especially prone to scalar translocation resulting in poor hearing preservation due to intracochlear trauma (15,21–24). O’Connell et al. (15) reported that earlier precurved electrodes are 22.4 times more likely to translocate than straight electrodes, which despite many reports of higher speech recognition outcomes for precurved electrodes (3,19,30,31), remains a disadvantage of previous designs. In light of Cochlear’s release of the CI532 precurved electrode and report of low rates of translocation (37), the aim of the current study was to evaluate the newer generation CI532 in terms of audiologic outcomes and programming parameters and describe how it compares to the CI422/522, Cochlear’s straight electrode typically recommended for hearing preservation.
The most notable finding of the current study is that the CI532 group had significantly better postoperative acoustic LFPTA than the CI422/522 group even when age and preoperative LFPTA were matched across groups. Previous studies comparing LFPTA for straight versus precurved electrodes have shown much poorer hearing preservation for precurved arrays (15,21–24). This difference in results could be explained by lower rates of translocation for the CI532, which suggests less trauma on the cochlear structures. Of note here is that only 25 of 58 participants had a LFPTA of less than 80 dB HL before surgery, and nine patients (CI532 = 7) maintained a LFPTA of less than 80 dB HL following implantation. Future work should investigate hearing preservation with CI522 and CI532 for individuals with much greater preoperative acoustic hearing.
Similar to Park et al. (30), the current results demon-strated significantly higher CNC word scores for the CI532 group. These findings support the notion that precurved electrodes provide more focused stimulation required to encode individual words in the absence of context (20,26), but when context clues are provided, such as with the AzBio sentences, the listener can employ “top-down” processing resulting in more similar performance between the two groups on sentence recognition measures.
Contrary to Telmesani and Said (27) who found no difference between CI422 and CI24RE (previous generation precurved array), the current precurved group’s impedances were significantly lower than the straight array group. Impedance measurements are important for estimating the voltage compliance limits for the CI system. Should the upper stimulation levels exceed compliance on a channel, the channel will cease to deliver additional current beyond said limit. Thus, higher impedances increase the likelihood of exceeding compliance. Additionally, the straight array recipients showed greater variation in impedances over time. Changes in impedances occurring between CI mapping appointments can result in exceeding compliance and/or changes to performance and sound quality for the recipient. The CI532 group’s impedances showed an expected decrease over time, while the CI422/522 group showed unpredictable variation in impedance values. As shown in Figure 3A, impedances at 6 months post-activation are significantly higher than 3 months post-activation for the CI422/522 group. Impedance levels are often affected by changes to the resistive properties of the surrounding fluid and tissue. It is reasonable to conclude that straight electrodes showed higher impedances than the precurved group due to greater distance between the electrode and neural tissue (30). Similarly, previous studies may have found no difference in impedances (27) due to higher rates of translocation with precurved electrodes resulting in greater separation between the electrode and neural tissue than the current precurved group.
The programming software for these 58 patients indi-cated that significantly longer pulse durations were used for the CI422/522 group. Although we found significantly higher impedances and pulse durations for the 422/522 group, the difference in overall charge was not significant between groups. This finding related to overall charge is incongruent with our clinical experience and the experience of other clinics, but it is supported by Park et al. (30). A possible explanation for this finding is related to the software defaults. It is possible that in this subset of patients, the clinical default pulse duration (37 μs) is longer than necessary to achieve comfortable loudness and that these patients may be adequately served by the default pulse duration of the CI532 (25 μs per phase). This is an important consideration in light of recent findings from our lab, which indicate that patients can improve speech in noise scores by approximately 12-percentage points on average by using 16 maxima (16). Note that 16 maxima can only be achieved while using a 25-μs pulse duration. Study in this area is currently ongoing in our lab.
CONCLUSION
The current study design matched participants in terms of age and preoperative hearing thresholds, variables known to affect residual hearing and speech recognition outcomes, to more precisely assess the effect of electrode choice (CI532 versus CI522). The CI532 electrode showed equal or better outcomes on all measures of audiometric outcomes including hearing preservation and speech understanding. Additionally, CI532 impedances and pulse duration were significantly lower allowing for the imple-mentation of greater maxima, which has recently been shown to positively impact speech understanding in complicated listening situations. Overall, in comparison to a matched cohort, the CI532 group showed favorable or similar results on all measures when compared with the CI422/522 suggesting that the CI532 electrode may be an advantageous substitute for the CI522.
Acknowledgments:
The authors would like to express sincere gratitude to Dr. Mary Dietrich for her insight on statistical analyses and to the cochlear implant surgeons and audiologists involved in caring for the participants enrolled in our study.
Financial Material & Support: NIH R01 DC13117 – PI: René Gifford.
René Gifford: Advisory board for Advanced Bionics, Cochlear, and Frequency Therapeutics, Robert Labadie: Consultant for Advanced Bionics, Johnson & Johnson, and Ototronix, Alejandro Rivas: Consultant for Med-El, Advanced Bionics, Cochlear, Grace Medical, Stryker, and Cook Medical.
REFERENCES
- 1.Fabie JE, Keller RG, Hatch JL, et al. Evaluation of outcome variability associated with lateral wall, mid-scalar, and perimodiolar electrode arrays when controlling for preoperative patient characteristics. Otol Neurotol 2018;39:1122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finley CC, Holden T, Holden LK, et al. Role ofelectrode placement as a contributor to variability in cochlear implant outcomes. Otol Neurotol 2008;29:920–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holden LK, Finley CC, Firszt JB, et al. Factors affecting open-set word recognition in adults with cochlear implants. Ear Hear 2013;34:342–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tong YC, Busby PA, Clark GM. Perceptual studies on cochlear implant patients with early onset of profound hearing impairment prior to normal development of auditory, speech, and language skills. J Acoust Soc Am 1988;84:951–62. [DOI] [PubMed] [Google Scholar]
- 5.Rubinstein JT, Parkinson WS, Tyler RS, Gantz BJ. Residual speech recognition and cochlear implant performance: effects of implantation criteria. Am J Otol 1999;20:445–52. [PubMed] [Google Scholar]
- 6.Lazard DS, Vincent C, Dé F, et al. Pre-, per-and postoperative factors affecting performance of postlinguistically deaf adults using cochlear implants: a new conceptual model over time. PLoS One 2012;7:e48739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blamey P, Artieres F, BaŞkent D, et al. Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants: an update with 2251 patients. Audiol Neurotol 2012; 18:36–47. [DOI] [PubMed] [Google Scholar]
- 8.Holden LK, Firszt JB, Reeder RM, Uchanski RM, Dwyer NY, Holden TA. Factors affecting outcomes in cochlear implant recipients implanted with a perimodiolar electrode array located in scala tympani. Otol Neurotol 2016;37:1662–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wanna GB, Noble JH, McRackan TR, et al. Assessment of electrode placement and audiological outcomes in bilateral cochlear implantation. Otol Neurotol 2011;32:428–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aschendorff A, Kromeier J, Klenzner T, Laszig R. Quality control after insertion of the nucleus contour and contour advance electrode in adults. Ear Hear 2007;28 (suppl):75S–9S. [DOI] [PubMed] [Google Scholar]
- 11.Carlson ML, Driscoll CLW, Gifford RH, et al. Implications of minimizing trauma during conventional cochlear implantation. Otol Neurotol 2011;32:962–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gifford RH, Dorman MF, Skarzynski H, et al. Cochlear implantation with hearing preservation yields significant benefit for speech recognition in complex listening environments. Ear Hear 2013; 34:413–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skinner MW, Holden TA, Whiting BR, et al. In vivo estimates of the position of advanced bionics electrode arrays in the human cochlea. Ann Otol Rhinol Laryngol 2007;116:2–24. [PubMed] [Google Scholar]
- 14.O’Connell BP, Hunter JB, Haynes DS, et al. Insertion depth impacts speech perception and hearing preservation for lateral wall electrodes. Laryngoscope 2017;127:2352–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Connell BP, Hunter JB, Gifford RH, et al. Electrode location and audiologic performance after cochlear implantation: a comparative study between Nucleus CI422 and CI512 Electrode Arrays. Otol Neurotol 2016;37:1032–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berg KA, Noble J, Dawant B, Dwyer R, Labadie R, Gifford R. Speech recognition as a function of the number of channels in perimodiolar electrode recipients. JAcoustSoc Am 2019;145:1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis TJ, Zhang D, Gifford RH, Dawant BM, Labadie RF, Noble JH. Relationship between electrode-To-modiolus distance and current levels for adults with cochlear implants. Otol Neurotol 2016;37:31–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saunders E, Cohen L, Aschendorff A, et al. Threshold, comfortable level and impedance changes as a function of electrode-modiolar distance. Ear Hear 2002;23 (1 suppl):28S–40S. [DOI] [PubMed] [Google Scholar]
- 19.Gordin A, Papsin B, James A, Gordon K. Evolution of cochlear implant arrays result in changes in behavioral and physiological responses in children. Otol Neurotol 2009;30:908–15. [DOI] [PubMed] [Google Scholar]
- 20.Cohen LT. Practical model description of peripheral neural excitation in cochlear implant recipients: 2. Spread of the effective stimulation field (ESF), from ECAP and FEA. Hear Res 2009; 247:100–11. [DOI] [PubMed] [Google Scholar]
- 21.Wanna GB, Noble JH, Carlson ML, et al. Impact ofelectrode design and surgical approach on scalar location and cochlear implant outcomes. Laryngoscope 2014;124:S1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sweeney AD, Hunter JB, Carlson ML, et al. Durability of hearing preservation after cochlear implantation with conventional-length electrodes and scala tympani insertion. Otolaryngol Head Neck Surg 2016;154:907–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wanna GB, Noble JH, Gifford RH, et al. Impact of intrascalar electrode location, electrode type, and angular insertion depth on residual hearing in cochlear implant patients: preliminary results. Otol Neurotol 2015;36:1343–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyer E, Karkas A, Attye A, Lefournier V, Escude B, Schmerber S. Scalar localization by cone–beamcomputed tomography of cochlear implant carriers: a comparative study between straight and periomodiolar precurved electrode arrays. Otol Neurotol 2015;36:422–9. [DOI] [PubMed] [Google Scholar]
- 25.Van Wermeskerken GKA, Van Olphen AF, Graamans K. Imaging of electrode position in relation to electrode functioning after cochlear implantation. Eur Arch Otorhinolaryngol 2009; 266:1527–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shepherd RK, Hatsushika S, Clark GM. Electrical stimulation of the auditory nerve: the effect of electrode position on neural excitation. Hear Res 1993;66:108–20. [DOI] [PubMed] [Google Scholar]
- 27.Telmesani LM, Said NM. Effect of cochlear implant electrode array design on auditory nerve and behavioral response in children. Int J Pediatr Otorhinolaryngol 2015;79:660–5. [DOI] [PubMed] [Google Scholar]
- 28.Tykocinski M, Cohen LT, Pyman BC, et al. Comparison of electrode position in the human cochlea using various perimodiolar electrode arrays. Am J Otol 2000;21:205–11. [DOI] [PubMed] [Google Scholar]
- 29.Cohen LT, Saunders E, Knight MR, Cowan RSC. Psychophysical measures in patients fitted with Contour™ and straight Nucleus electrode arrays. Hear Res 2006;212:160–75. [DOI] [PubMed] [Google Scholar]
- 30.Park LR, Teagle HFB, Brown KD, Gagnon EB, Woodard JS, Buchman CA. Audiological outcomes and map characteristics in children with perimodiolar and slim straight array cochlear implants in opposite ears. Otol Neurotol 2017;38:e320–6. [DOI] [PubMed] [Google Scholar]
- 31.Bacciu A, Pasanisi E, Vincenti V, et al. Comparison of speech perception performance between the Nucleus 24 and Nucleus 24 Contour cochlear implant systems. Acta Otolaryngol 2004; 124:1155–8. [DOI] [PubMed] [Google Scholar]
- 32.Doshi J, Johnson P, Mawman D, et al. Straight versus modiolar hugging electrodes: does one perform better than the other? Otol Neurotol 2015;36:223–7. [DOI] [PubMed] [Google Scholar]
- 33.Fitzgerald MB, Shapiro WH, McDonald PD, et al. The effect of perimodiolar placement on speech perception and frequency discrimination by cochlear implant users. Acta Otolaryngol 2007; 127:378–83. [DOI] [PubMed] [Google Scholar]
- 34.Holder JT, Reynolds SM, Sunderhaus LW, Gifford RH. Current profile of adults presenting for preoperative cochlear implant evaluation. Trends Hear 2018;22:233121651875528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gifford RH, Davis TJ, Sunderhaus LW, et al. Combinedelectric and acoustic stimulation with hearing preservation: effect of cochlear implant low-frequency cutoff on speech understanding and perceived listening difficulty. Ear Hear 2017;38:539–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gfeller K, Olszewski C, Turner C, Gantz B, Oleson J. Music perception with cochlear implants and residual hearing. Audiol Neurootol 2006;11 (suppl):12–5. [DOI] [PubMed] [Google Scholar]
- 37.Aschendorff A, Briggs R, Brademann G, et al. Clinical investigation of the Nucleus Slim Modiolar electrode. Audiol Neurootol 2017;22:169–79. [DOI] [PubMed] [Google Scholar]
- 38.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MSTB. Minimum Speech Test Battery For Adult Cochlear Implant Users; 2011. Available at: http://auditorypotential.com/MSTBfiles/MSTBManual2011-06-20.pdf. Accessed January 5, 2018.
- 40.Peterson GE, Lehiste I. Revised CNC lists for auditory tests. J Speech Hear Disord 1962;27:62. [DOI] [PubMed] [Google Scholar]
- 41.Spahr AJ, Dorman MF, Litvak LM, et al. Development and validation of the AzBio sentence lists. Ear Hear 2012;33:112–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kochkin S MarkeTrak VIII: consumer satisfaction with hearing aids is slowly increasing. Hear J 2010;63:19–27. [Google Scholar]
- 43.Pearsons K, Bennett R, Fidell S. Speech Levels in Verious Noise Environments (Report No. EPA-600/1-77-025). Washington, DC: US, 1977. [Google Scholar]
- 44.Smeds K, Wolters F, Rung M. Estimation ofsignal-to-noise ratios in realistic sound scenarios. J Am Acad Audiol 2015;26:183–96. [DOI] [PubMed] [Google Scholar]
- 45.Mudery JA, Francis R, McCrary H, Jacob A. Older individuals meeting medicare cochlear implant candidacy criteria in noise but not in quiet: are these patients improved by surgery? Otol Neurotol 2017;38:187–91. [DOI] [PubMed] [Google Scholar]
- 46.Gatehouse S, Noble W. The Speech, spatial and qualities ofhearing scale (SSQ). Int J Audiol 2004;43:85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]


