Abstract
Systemic corticosteroid use to manage uncontrolled asthma and its associated healthcare burden may account for important health-related adverse effects. We conducted a systematic literature review to investigate the real-world extent and burden of systemic corticosteroid use in asthma. We searched MEDLINE and Embase databases to identify English-language articles published in 2010–2017, using search terms for asthma with keywords for oral corticosteroids and systemic corticosteroids. Observational studies, prescription database analyses, economic analyses, and surveys on oral/systemic corticosteroid use in children (>5 yr old), adolescents (12–17 yr old), and adults with asthma were included. We identified and reviewed 387 full-text articles, and our review included data from 139 studies. The included studies were conducted in Europe, North America, and Asia. Overall, oral/systemic corticosteroids were commonly used for asthma management and were more frequently used in patients with severe asthma than in those with milder disease. Long-term oral/systemic corticosteroid use was, in general, less frequent than short-term use. Compared with no use, long-term and repeated short-term oral/systemic corticosteroid use were associated with an increased risk of acute and chronic adverse events, even when doses were comparatively low. Greater oral/systemic corticosteroid exposure was also associated with increased costs and healthcare resource use. This review provides a comprehensive overview of oral/systemic corticosteroid use and associated adverse events for patients with all degrees of asthma severity and exposure duration. We report that oral/systemic corticosteroid use is prevalent in asthma management, and the risks of acute and chronic complications increase with the cumulative oral corticosteroid dosage.
Keywords: asthma, oral corticosteroids, severe asthma, systematic literature review, systemic corticosteroids
Contents
Methods
Search Strategy and Selection Criteria
Data Analysis
Results
Manuscript Identification
Study Populations
Patterns of CS Use
Clinical Burden of SCS
Healthcare Resource Use and Economic Impact of SCS Use
Discussion
Systemic corticosteroids (SCS) became available in 1956, and their introduction provided effective treatment for the control of asthma symptoms and exacerbations (1). However, their widespread use quickly led to the recognition that long-term SCS use is associated with significant adverse events (AEs) (2). Inhaled CS (ICS), which have a reduced risk of AEs but are as effective as SCS for most patients, were introduced in 1972 as maintenance treatment for patients with asthma (1, 3). However, SCS, usually in the form of oral CS (OCS) and occasionally as injectable CS, remained the mainstay treatment for asthma exacerbations and severe disease over the next four decades (1, 4).
Today, ICS are the primary therapeutic intervention for persistent asthma along with other controller therapies, including predominantly long-acting β2-agonists (LABAs) and leukotriene receptor antagonists, as additional treatments to reduce ICS dosages, control asthma symptoms, and decrease exacerbation risk for patients with asthma (5). Add-on treatments, traditionally long-acting muscarinic antagonists or low-dosage OCS (before the introduction of targeted biologics), are recommended for patients with asthma that is not controlled by medium- to high-dosage ICS plus controller medications (5). In 2003, omalizumab (anti-IgE therapy), the first targeted biologic therapy for asthma, was approved by the U.S. Food and Drug Administration for add-on maintenance treatment (6). Subsequently, the anti–IL-5 treatments mepolizumab and reslizumab and the anti–IL-5 receptor α–directed cytolytic therapy benralizumab were approved for the treatment of severe, eosinophilic asthma (6, 7). More recently, the anti–IL-4 and anti–IL-13 therapy dupilumab was approved for the treatment of moderate-to-severe eosinophilic or OCS-dependent asthma (8). These targeted biologic treatments have demonstrated greater specificity for achieving disease control by reducing the risk of exacerbations and requirements for rescue medication and OCS use in their respective target patient populations, with limited AEs (7–17). Biologics are now also recommended in guidelines for the treatment of appropriate patients with severe asthma (5). For patients with severe asthma who are not eligible for the currently available biologic treatments, the 2019 Global Initiative for Asthma (GINA) guidelines recommend that several other strategies be considered before maintenance OCS/SCS (5).
Despite the availability of these new well-tolerated, effective, targeted biologic add-on treatments and the well-recognized AEs associated with SCS use, OCS are still treatment options in the current asthma treatment guidelines. The 2019 GINA guidelines recommend that if OCS are prescribed, they should be prescribed at lesser dosages. Prescribers should be fully aware of and monitor for AEs. OCS should be considered, along with targeted biologic treatments, for add-on treatment for patients with uncontrolled asthma despite the use of high-dosage ICS therapy (GINA step 5 treatment) (5). The GINA guidelines also recommend short-term OCS use for patients experiencing a severe exacerbation who do not respond to treatment (5). However, this guidance is relatively unspecific. Patients can repeatedly receive prescriptions for both short- and long-term OCS and ultimately become OCS dependent (18). The continued inclusion of OCS in guidelines, together with their worldwide easy accessibility, familiarity of use, and low acquisition costs compared with newer targeted treatments, contributes to the ongoing use of SCS for patients with severe asthma. In addition, it is likely that some patients fail to benefit significantly from the OCS-sparing effect of biologic treatments because of differences in their susceptibility to OCS-related adverse effects, or because of their unwillingness to initiate new treatment options and reduce OCS use.
Studies from France and the United Kingdom provide evidence that overall OCS use has increased over the last decade and continues to increase (19, 20). Respiratory disease is the most frequently recorded indication for OCS treatment, accounting for approximately 40% of prescriptions (20, 21). OCS use has declined in recent decades for other indications for which biologics have been available for more than 20 years. In rheumatology, for example, the introduction of biologics has significantly reduced OCS use, and this change has been cost-effective in the treatment of rheumatoid arthritis (22, 23).
Responses to SCS and the risk of AEs vary considerably among patients (24). Many patients with persistent SCS use demonstrate a relative resistance to treatment (24), but they continue to receive SCS prescriptions and increasing dosages, which further contributes to the prevalence of long-term use. In recent studies in which responses to SCS were comprehensively characterized, 25–35% of patients with asthma did not respond to OCS treatment by exhibiting evidence of a reduction in type 2 biomarkers, suggesting SCS resistance (25, 26). However, despite these findings and the extensive variation in prescription durations and dosages used in clinical practice, the optimal duration and dosage of SCS maintenance and short-term treatment have not been studied extensively (27). A recent systematic literature review evaluated the long-term use of OCS for patients with asthma and reported that the risk of developing OCS-related complications, including infections, diabetes, osteoporosis, and psychiatric disorders, was greater for patients with long-term OCS exposure compared with control groups, even for those receiving dosages below 5 mg/d (28). However, these findings were limited to adult patients with severe asthma and long-term OCS use and were based on just nine publications (seven large datasets).
In this systematic literature review, we investigated the extent and nature of real-world SCS use (oral and parenteral CS) for the treatment of asthma in children (>5 yr old), adolescents (12–17 yr old), and adults with asthma of any severity. We also examined available evidence regarding the clinical and economic impacts of sporadic, repeated short- and long-term SCS use in the general asthma population and by the degree of asthma severity. The findings of this review provide a comprehensive overview to improve our understanding of the risk of health-related adverse effects associated with SCS use for patients with asthma and the extent of SCS use for patients with all degrees of asthma severity.
Methods
Search Strategy and Selection Criteria
A literature search was conducted in MEDLINE and Embase databases via Ovid to identify English-language articles published between January 1, 2007, and December 4, 2017, using the following search terms: asthma AND ([corticosteroid OR corticosteroids OR glucocorticoid OR glucocorticoids OR prednisone OR prednisolone OR dexamethasone OR methylprednisolone OR hydrocortisone] AND [oral OR orally OR systemic OR parenteral OR intravenous OR intramuscular]) OR [SCS OR OCS]). Filters were applied to exclude reviews, letters, and information presented at scientific conferences. Full search details are provided in Table E1 in the online supplement. Searches were initially restricted to English-language articles published since 2007. However, because of the large number of relevant articles identified, and to ensure that the most up-to-date data relevant to current clinical practices were evaluated, the search was subsequently limited to articles published since January 1, 2010.
Abstracts and full-text articles were screened to determine their eligibility for inclusion according to prespecified patient-, intervention-, comparison-, outcome-, and study-design–related selection criteria (Table 1). To summarize, the systematic literature review included observational studies that reported information on frequency of SCS use and treatment patterns, as well as clinical complications and the economic burden associated with SCS use for children (>5 yr old), adolescents (12–17 yr old), and adults with asthma of any degree of severity. Studies that included <100 patients with asthma were excluded, as this sample size was considered too small to provide results likely to be representative of the overall asthma population.
Table 1.
Study Selection Criteria for the Systematic Literature Review on the Use of OCS and SCS for Treatment of Asthma
| Category | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | • Pediatric, adolescent, and adult patients (≥5 yr old) with asthma of any severity and all degrees of disease control • Mixed study populations or subpopulations in which ≥85% of patients met the above criteria (apart from age criteria, which had to be met by all patients) |
• Children <5 yr old with asthma • Patients who did not have asthma • Patients with ACOS • Patients with asthma during pregnancy • Mixed-study populations or subpopulations in which <85% of patients met the inclusion criteria • Studies containing <100 patients with asthma • Studies containing <500 patients with asthma, except for those reporting long-term OCS/SCS use or burden of OCS/SCS use |
| Intervention/comparators | • OCS, parenteral CS, or SCS | • No mention of OCS, parenteral CS, or SCS use • CS treatment in emergency department and/or in hospitalized patients with asthma only |
| Outcomes | • Frequency/patterns of OCS and SCS use (patient-level data) • Long-term OCS and SCS use, including Length of time (average duration) receiving long-term OCS Dosage and frequency of changing dosage |
• Outcome measures not listed in the inclusion criteria • Frequency/patterns of OCS and SCS use among physicians |
| • Clinical burden of OCS and SCS use: Asthma clinical features Exacerbation history Previous hospitalizations Degree of asthma control (based on ACQ or ACT) Phenotypes (eosinophilic, allergic, etc.) Comorbidities and complications for OCS and SCS users: Diabetes/metabolic Bone (e.g., osteoporosis) Cardiovascular disease Psychiatric (e.g., depression) Others as available |
||
| • Economic burden of OCS and SCS use: Healthcare resource use, including hospitalizations and doctor and emergency department visits Costs (including direct and indirect costs), both asthma-related and all costs Cost consequences of prolonged OCS and SCS use |
||
| Study design | • Observational studies, including prospective and retrospective cohort studies, and cross-sectional analyses • Prescription database analyses • Economic analyses • Patient, parent/guardian, and HCP surveys |
• Systematic and narrative reviews • Case reports and case series • Comments, letters, and editorials • Animal/in vitro studies • Meta-analyses/pooled analyses • Clinical trials • Asthma phenotyping analyses • Treatment guidelines • Patient education studies • Studies of healthcare management (e.g., physician prescribing preferences, physician/hospital management approaches, and care quality evaluation) |
| Time period | • January 1, 2007, to December 4, 2017 | • Studies published before January 1, 2007, or after December 4, 2017 |
| Other criteria | • Studies published in English • Limited to humans |
• Non–English language publications • Conference abstracts |
Definition of abbreviations: ACOS = asthma–chronic obstructive pulmonary disease overlap syndrome; ACQ = Asthma Control Questionnaire; ACT = Asthma Control Test; HCP = healthcare professional; OCS = oral corticosteroids; SCS = systemic corticosteroids.
Data Analysis
Data from the included articles were extracted into a Microsoft Excel spreadsheet. At extraction, studies were separated into the following categories of use: oral (OCS), parenteral, and systemic (both oral and parenteral; SCS). The evidence identified was summarized qualitatively, and studies were separated according to whether they reported long- or short-term use.
Results
Manuscript Identification
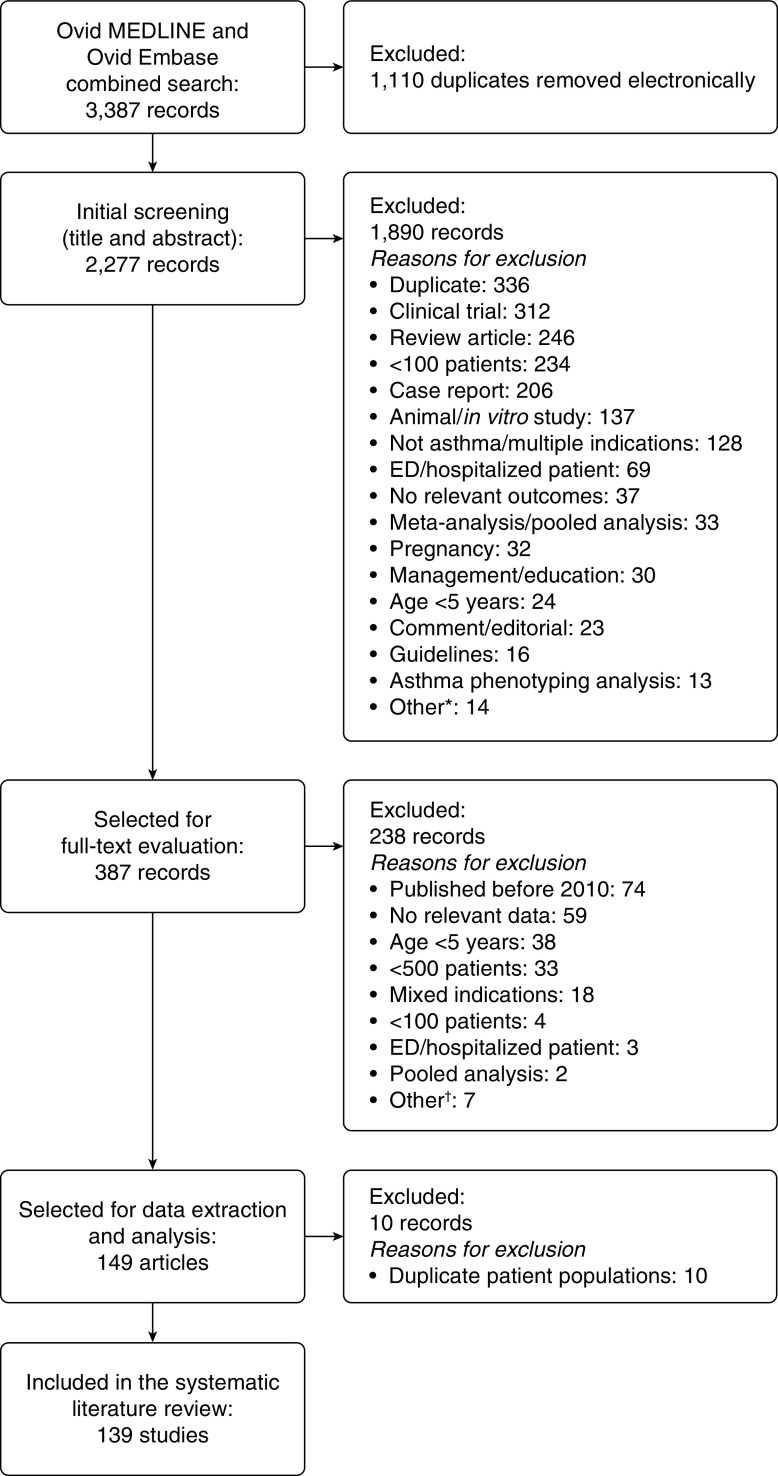
The search identified 2,277 unique references across the databases. Abstract screening identified 387 references that required further review of full-text articles. Of those, 149 publications met the criteria for inclusion (Figure 1). During data extraction, related publications reporting on the same patient populations or updates from the same studies were identified. To avoid study duplication, data from these publications were only included once in the analysis, resulting in 139 studies included in the final analyses. The most common reason for exclusion at the full-text level was no relevant data.
Figure 1.
Flow diagram of the article screening and evaluation process. *“Other” included asthma–chronic obstructive pulmonary disease overlap syndrome (n = 5), letter (n = 4), erratum (n = 2), not English language (n = 2), and retracted publication (n = 1). †“Other” included cost-effectiveness modeling, letter, not patients with asthma, not English language, asthma phenotyping analysis, controlled trial, and retracted publication (each n = 1). ED = emergency department.
Study Populations
The included studies were conducted in Europe, North America, and Asia (Table E2). However, 64% of the studies presented data from centers or databases in the United States (n = 55) or the United Kingdom (n = 34). UK studies accounted for 52% (n = 34/65) of all studies from Europe. Studies reporting data for adults (n = 48/139) or combined adult/adolescent populations (n = 44/139) accounted for approximately two-thirds of the studies (Table E2). Seventeen studies reported data for individuals ≥5 years of age. Age limits were undefined in 14 studies, but mean or median ages of ≥40 years were reported, suggesting that the studies consisted of largely adult populations.
Overall, 41% (n = 57) of the studies involved patients with any degree of disease severity; 23% (n = 32) included data for patients with GINA step 2 or greater treatment, and 19% (n = 26) involved only patients with severe asthma (GINA step 4 or 5). Studies of patients with severe asthma were smaller in size than those that covered a wider degree of disease severity, with 22 of 26 studies including fewer than 1,000 patients. The majority of studies reported data for OCS use (87%; n = 121), although some institutions, including centers participating in the Severe Asthma Research Program, exclusively recommended SCS use. However, because the results reported for SCS use were similar to those reported for OCS use, the rest of this review will focus on OCS use. The results for SCS use are reported in the relevant corresponding tables and online supplement.
Patterns of CS Use
Patterns of short-term CS use
Short-term use was defined by terms such as “acute,” “burst,” and “for asthma exacerbations,” or when OCS/SCS were used as reliever medications or had a defined short-term exposure. Overall, 58 studies reported short-term OCS/SCS use for patients with asthma (Table E3). Short-term OCS/SCS use in studies of patients with any degree of asthma severity ranged from 3.6% (underweight patients before OCS/SCS exposure in a U.S. study of children with asthma living in the vicinity of wildfires) (29) to 62.0% (U.S. observational study of primary care patients with asthma) (30). Short-term OCS/SCS use in studies of patients receiving GINA step 2 or greater treatment ranged from 2.1% (international study of SCS use for patients with asthma ≥12 yr old) (31) to 41.9% (fluticasone propionate/salmeterol fixed-dose combination inhaler use in a U.S. retrospective study) (32). Short-term OCS/SCS use in studies of patients with severe or difficult-to-treat asthma ranged from 23.2% (patients who experienced exacerbations within the prior 3 mo in a U.S. study of severe refractory asthma) (33) to 92.6% (UK study of OCS use for patients with severe asthma [age unspecified]) (34). The percentage of patients receiving short-term OCS/SCS therapy increased with increasing disease severity.
Patterns of short-term CS use by disease severity
Five retrospective cohort studies of adult patients with asthma of any degree of severity reported that approximately a quarter of patients required at least one short-term OCS course during a 1-year period (29, 35–38). Studies involving patients of all ages reported incidences of 16.2–30.9% for 1-year use of short-term OCS (39–41).
For patients with severe or difficult-to-treat asthma, short-term OCS use increased to 46.3–92.6% over a 1-year period (33, 34, 42–46). In one cross-sectional study of patients with uncontrolled asthma, 60% of whom had severe asthma, 24.4% overall were reported to have received greater than or equal to 3 short-term OCS courses in the previous year (42).
Short-term CS prescription use
The mean number of OCS short-term prescriptions ranged from 0.1 to 2.16 prescriptions per year in the nine studies that reported these data for patients with any degree of asthma severity or those using controller therapy (GINA step 2 or greater treatment) (32, 47–54). Studies involving patients with severe or uncontrolled asthma reported greater prescription rates compared with patients with less severe disease. A study that examined prescription rates in relation to treatment steps (according to the British Thoracic Society guidelines) reported that the mean number of OCS courses per year ranged from 1.2 to 2.1 at steps 1–4 and 5.3 at step 5 (maintenance OCS therapy) (47).
Patterns of long-term CS use
A total of 62 studies evaluated long-term OCS (54 studies) and SCS (eight studies) use for patients with asthma, defined as “daily” or “continuous” OCS/SCS use, or described OCS/SCS use as “chronic,” “maintenance,” or “controller medication,” or specified durations of long-term OCS/SCS exposure. For patients with any degree of asthma severity, long-term OCS/SCS use ranged from 1.2% (UK retrospective study of asthma therapy) (47) to 30.9% (patients with asthma and vitamin D insufficiency in a German study) (55) (Table 2 [extended version: Table E4]). For patients receiving GINA step 2 or greater treatment, long-term OCS/SCS use ranged from 0% (patients with nonsevere asthma in a U.S. study) to 100% (UK study of patients with severe asthma) (54). In general, long-term OCS/SCS use was less frequent than short-term use.
Table 2.
Frequency of Long-Term Use of OCS and SCS for Patients with Asthma, Categorized by Disease Severity
| Source | N | Long-Term OCS/SCS Definition | OCS/SCS Use at Follow-Up/Postindex, % (n/N) | OCS/SCS Use at Baseline/Preindex, % (n/N) |
|---|---|---|---|---|
| Any degree of asthma severity | ||||
| Allen-Ramey et al., 2013 (30) (U.S.) | 21,199 | An order quantity ≥30 with one or more refills | OCS | — |
| 8.9 (1,883/21,199) | ||||
| Arellano et al., 2011 (79) (U.S.) | 6–18 yr: 659,169 | Continuous OCS use >15 d | Addition of long-term OCS in first year in Tx-naive pts initiating Tx with: | — |
| 6–11 yr: 348,991 | SABA (n = 309,947): 22.8% | |||
| Tx-naive (6–18 yr): 595,619 | ICS (n = 16,783): 18.5% | |||
| ICS/LABA (n = 13,980): 15.1% | ||||
| Bottero et al., 2014 (125) (Italy) | 159 | Continuous or near continuous (≥50% of yr) oral prednisone use | OCS | — |
| 9.4 (15/159) | ||||
|
HLA-DRB4 positive: 16.7 (10/60) | ||||
|
HLA-DRB4 negative: 5.1 (5/99) | ||||
| Broder et al., 2010 (56) (U.S.) | 18,343 (uncontrolled asthma) | Total supply of ≥60 d in a 6-mo period |
— | OCS Preindex (EPR3 step 6): 0.6 (105/18,343) |
| Covvey et al., 2013 (47) (UK) | 12,319 | >14-d supply with no titration schedule (BTS/SIGN step 5) | 1.2 (149/12,319) | — |
| Dalal et al., 2016 (103) (U.S.) | 603,147; SCS users: 12,697 Nonusers: 590,450 |
SCS user: ≥6 mo of continuous long-term SCS use Nonuser: never exposed to SCS |
— | Long-term SCS use (overall pop): 2.1 (12,697/603,147) |
| Dodd and Mazurek, 2018* (59) (U.S.) | 14,915 | Controller therapy | OCS | — |
| % (95% CI) | ||||
| WRA: 5.5 (3.8–7.3) | ||||
| Possible WRA: 3.0 (1.8–4.2) | ||||
| Non-WRA: 2.5 (1.7–3.4) | ||||
| Fardet 2011 (57) (UK) | 4,518,753 (total) 167,886 (long-term OCS) |
Tx lasting ≥3 mo | OCS | — |
| Prevalence, % (95% CI): 1.3 (1.1–1.4)† | ||||
| Ferguson et al., 2014‡ (60) (U.S.) | 812 | Long-term OCS | OCS | — |
| 9 (70/812) | ||||
| Hasegawa et al., 2012‡§ (61) (Japan) | 1998: 3,347 | Controller medication | OCS | — |
| 1998: 18.8 | ||||
| 2000: 3,069 | 2000: 12.3 | |||
| 2002: 2,593 | 2002: 10.4 | |||
| 2004: 2,865 | 2004: 7.4 | |||
| 2006: 3,066 | 2006: 7.8 | |||
| 2008: 3,146 | 2008: 5.2 | |||
| P < 0.001 over study | ||||
| Korn 2013§|| (55) (Germany) | 280 | Daily OCS maintenance | OCS | — |
| 26.8 (75/280) | ||||
| Vitamin D concentrations | ||||
| 25(OH)D <30 ng/ml (insufficiency): | ||||
| 30.9 (58/188) | ||||
| 25(OH)D ≥30 ng/ml: | ||||
| 18.5 (17/92) | ||||
| P = 0.031 | ||||
| Lee et al., 2013 (98) (South Korea) | TB cases: 4,136 Matched control subjects: 20,538¶ | OCS user: cumulative dosage ≥1,680 mg of hydrocortisone equivalents during 1 yr before index date | — | OCS |
| Asthma pts | ||||
| TB cases: 11.2 (54/484) | ||||
| Control subjects: 4.8 (117/2,420) | ||||
| Lefebvre et al., 2017** (106) (U.S.) | SCS users: 3,628 Nonusers: 26,987 |
Daily doses ≥5 mg of prednisone equivalent with no gap of ≥14 d between 2 SCS claims | — | Overall population: 11.9 (3,628/30,615) |
| Luskin et al., 2016†† (49) (U.S.) | 3,604 (high OCS use) | High OCS use: pts who had a ≥30-d supply of OCS in each study year | OCS | — |
| 5.3 (3,604/67,860) | ||||
| Papaioannou et al., 2016 (62) (Greece) | 171 | Regular/continuous SCS | OCS | — |
| 20.5 (35/171) | ||||
| Price et al., 2015 (35) (UK) | 2,042‡‡ | BTS step 5 | — | OCS |
| 0.5 (10/2,042) | ||||
| Price et al., 2016 (58) (UK) | 130,547 | BTS step 5 | — | OCS |
| 0.8 (1,080/130,547) | ||||
| Reddy et al., 2011§§ (63) (U.S.) | (257‖‖) | Regular OCS | OCS | OCS |
| 5.8 (15/257) | 10.9 (28/257) | |||
| P = 0.055 vs. baseline | — | |||
| Sato et al., 2017 (64) (Japan) | 114 | Regular use of OCS | OCS | — |
| 4 (5/114) | ||||
| Shigemura et al., 2012‡ (65) (Japan) | 126 | Regular OCS | — | OCS |
| Baseline: 15.4 (18/117) | ||||
| Tattersall et al., 2015|| (66) (U.S.) | 667; Intermittent: 511 Persistent: 156 |
Controller medication | — | OCS |
| Overall: 4.8 (32/667) | ||||
| Persistent: 20.5 (32/156) | ||||
| Intermittent: NA | ||||
| Zeiger et al., 2017 (94) (U.S.) | 9,546 | Long-term OCS: Average daily dosage ≥2.5 mg in 2010 Short-term OCS: average daily dosage <2.5 mg or no OCS in 2010 |
Long-term OCS: 8.2 (782/9,546) | — |
| Long-term OCS: 782 Short-term OCS: 8,764 | ||||
| GINA step 2 or greater treatment | ||||
| Bengtson et al., 2017 (4) (U.S.) | Escalation: 5,044 Unchanged: 21,967 |
≥90 consecutive days of OCS coverage | Unchanged: 0.2 (52/21,967) Escalation: |
— |
| Before: 0.1 (6/5,044) After: 0.1 (7/5,044) | ||||
| Hawcutt et al., 2015¶¶ (126) (UK) | 525 | Regular maintenance | 10.8 (47/435) | — |
| Barry 2017†† (96) (UK) | 7,195; Severe: 808 Mild/moderate: 3,975 Nonasthma: 2,412 |
Severe asthma: regular OCS use*** | Severe: 100 (808/808) Mild/moderate: 25 (995/3,975) Nonasthma: 0 (0/2,412) |
— |
| Broder 2017 (92) (U.S.) | 3,355 | High OCS users: ≥1 OCS fill with ≥30 d of supply or ≥6 bursts of OCS | High OCS use: 15.4 (517/3,355) |
— |
| Chipps et al., 2017‡ (33) (U.S.) | 341††† | Long-term SCS | 11.2 (37/331) | — |
| Daugherty et al., 2017 (104) (UK) | 60,418; SCS nonuser: 24,994 SCS user: 35,444 |
SCS user: SCS use at baseline and observation periods‡‡‡ |
58.6 (35,444/60,418) | 25.6 (15,490/60,418) |
| SCS nonuser: no SCS use at baseline or observation periods‡‡‡ | (SCS use during baseline and observation periods) | — | ||
| Denlinger et al., 2017‡ (42) (U.S.) | 709 | Daily OCS | 11.0 (78/709) | — |
| Gibeon et al., 2015|| (43) (UK) | 346 | Maintenance OCS | 42.6 (123/289) | 41.2 (119/289) |
| Lefebvre et al., 2015** (105) (U.S.) | 3,628; SCS exposure (mg/d): Low (≤6): 368 Medium (>6–12): 1,630 High (>12): 1,630 |
Daily SCS dosage ≥5 mg of prednisone equivalent with no gap of ≥14 d between two SCS claims | At index date§§§ | — |
| Low SCS: 10 (368/3,628) Medium SCS: 45 (1,630/3,628) High SCS: 45 (1,630/3,628) | ||||
| Maio et al., 2017|| (46) (Italy) | 493 | Long-term OCS use | 16.0 (78/488) | — |
| Moore 2011|| (119) (U.S.) | 339; Nonsevere: 196 Severe: 102 Very severe: 41 |
OCS ≥20 mg/d for ≥50% of year | — | Baseline: % |
| Nonsevere: 1 (2/196) | ||||
| Severe: 21 (21/102) | ||||
| Very severe: 80 (33/41) | ||||
| P < 0.0001 | ||||
| O’Neill et al., 2015 (34) (UK) | 596; Severe: 516 Nonsevere: 80 |
Maintenance OCS | — | Overall: 34 (201/596) |
| Severe: 38 (196/516) | ||||
| Nonsevere: 5 (6/80) | ||||
| Phipatanakul et al., 2017|| (25) (U.S.) | 6–17 yr: 188; Nonsevere: 77 Severe: 111 |
≥3 mo with OCS use in past year | — | 6–17 yr |
| Nonsevere: 1.3 (1/77) | ||||
| Severe: 9.9 (11/111) P < 0.05 | ||||
| Adult (≥18 yr): 526; Nonsevere: 213 Severe: 313 |
Adult | |||
| Nonsevere: 0 (0/213) | ||||
| Severe: 22.4 (70/313) P < 0.01 | ||||
| Reddy et al., 2014§§ (67) (U.S.) | 228; Current (2003–2007): 65 Historic (1993–1997): 163 |
Daily OCS use | Current: 28 (11/41) Historic: 51 (28/55) P = 0.002 |
— |
| Rijssenbeek-Nouwens et al., 2012|| (127) (the Netherlands) | 137‖‖‖ | Daily OCS maintenance | Overall: 29.9 (41/137) | Overall: 51.1 (70/137) |
| HDM: 68 | HDM: 22 (15/68); P < 0.001 vs. baseline | HDM: 43 (29/68) | ||
| Non-HDM: 69 | Non-HDM: 38 (26/69); P < 0.001 vs. baseline | Non-HDM: 59 (41/69) | ||
| SEN: 92 | SEN: 29 (27/92); P < 0.001 vs. baseline | SEN: 49 (45/92) | ||
| Non-SEN: 45 | Non-SEN: 31 (14/45); P < 0.001 vs. baseline | Non-SEN: 56 (25/45) | ||
| Schleich et al., 2014‡ (128) (Belgium) | 350 | Daily maintenance SCS | 24 (84/350) | — |
| Shaw et al., 2015‡|| (75) (Europe) | 209; Severe nonsmoker: 311 Sever current/ex-smoker: 110 Mild/moderate: 88 |
Daily OCS | — | Severe all: 45.5 (181/398) |
| Severe nonsmoker: 45.8 (135/295) | ||||
| Severe ex-smoker: 44.7 (46/103) | ||||
| Mild/moderate: 0 (0/88) | ||||
| Sweeney et al., 2012 (76) (UK) | 349 | Maintenance OCS | 57 (199/349) | 42 (146/349) |
| Sweeney et al., 2016 (102) (UK) | 770¶¶¶ | Daily SCS | 57.1 (442/770) | — |
| Tay et al., 2017‡|| (129) (Singapore) | 423 | Maintenance OCS | — | 1.4 (6/423) |
| Severe: 4.1 (2/49) | ||||
| Nonsevere: 1.1 (4/374) | ||||
| Westerhof et al., 2016‡ (95) (the Netherlands) | 153; Current/ex-smoker: 83 Never-smoker: 70 |
Long-term OCS use >50% past yr | — | Current/ex-smoker: 28 (23/83) |
| Never-smoker: 29 (20/70) |
Definition of abbreviations: 25(OH)D = 25-hydroxyvitamin D; BTS = British Thoracic Society; CI = confidence interval; EPR = expert panel report; GINA = Global Initiative for Asthma; HCP = healthcare professional; HDM = house dust mite; ICS = inhaled corticosteroids; LABA = long-acting β2-agonists; NA = not applicable; OCS = oral corticosteroids; pts = patients; SABA = short-acting β2-agonists; SCS = systemic corticosteroids; SEN = sensitized; SIGN = Scottish Intercollegiate Guidelines Network; TB = tuberculosis; Tx = treatment; WRA = work-related asthma.
Studies used a retrospective cohort study design unless otherwise stated.
Patient survey.
Prevalence was determined by dividing the number of person-years with asthma receiving long-term OCS therapy by the total number of person-years with asthma.
Cross-sectional study.
Patient/HCP survey.
Prospective study.
Cases of TB identified after the treatment initiation date were matched with up to five control individuals without TB for age, sex, diagnosis of asthma or chronic obstructive pulmonary disease, and initiation date.
Longitudinal open cohort.
Retrospective matched cohort.
Patients who received tiotropium add-on therapy postindex (dry powder inhaler or soft mist inhaler).
Retrospective cross-sectional and historic cohort.
Patients with asthma who underwent bariatric surgery, consented to and had reached 1 yr of follow-up (n = 606), and returned for a follow-up survey (n = 257).
Prospective/retrospective cohort.
GINA Step 5 treatment and four or more OCS prescriptions per year for each of the two consecutive study years.
Data from TENOR (The Epidemiology and Natural History of Asthma. Outcomes and Treatment Regimens) II, a 10-year follow-up assessment of patients from TENOR I; age criteria provided for inclusion in TENOR I study.
Baseline period: 6 months before index date (date the patient was identified as having severe asthma [GINA Step 4/5]); observation period: follow-up after index date.
Index date was defined as the first day with a daily dosage of ≥5 mg of prednisone or equivalent after the first 6 months of long-term SCS use (baseline period).
High-altitude therapy for patients with severe asthma and without HDM sensitization (HDM and non-HDM groups) and patients with and without any allergic sensitization (sensitized and nonsensitized groups).
Patients with severe asthma at registry baseline assessment. Patients were divided into two groups: those who required daily SCS therapy to maintain asthma control and those who did not require maintenance SCS but required frequent rescue SCS courses.
Patterns of long-term CS use by disease severity
In general, <12% of patients with any degree of asthma severity received long-term OCS therapy (although values varied from 0.5% to 26.8% in different studies) (35, 55). This degree of variation could partly be related to how the studies defined long-term use, as well as differences in study inclusion criteria, geographic region, and availability of OCS-sparing treatments. Rates in 11 studies that applied precise definitions ranged from 0.5% to 9.4%, with five studies reporting rates of <3% (35, 47, 56–58). Studies that used more generalized terms, such as “regular OCS” and “controller medication,” reported rates of 4–20.5% (59–66).
Long-term OCS use was consistently reported as low across all age groups for patients with nonsevere disease (1.3% and 0.0% of children/adolescents and adults, respectively) (25). Among patients with severe or uncontrolled asthma, 20–60% were reported to have received long-term OCS therapy.
Long-term CS dosage
The dosage of long-term OCS was reported in 23 studies, most of which reported results for patients with severe disease. The mean daily OCS dosage (expressed as prednisolone-equivalent dosages) ranged from 4.0 to 21.4 mg (Table E5) (67, 68). In 12 of these studies, the mean daily dosage was 10–22 mg (34, 43, 55, 68–76).
Patterns of general CS use
Studies that did not specify short- or long-term use were classified as reporting general OCS use. These were studies that described any OCS/SCS use, or those in which OCS/SCS use was undefined and/or described with general terms, such as “OCS prescriptions,” “OCS use,” and “OCS claims.”
General CS use by disease severity
Forty-one studies reported general SCS/OCS use. Overall, OCS/SCS were reported to be commonly used for asthma management (Table 3 [extended version: Table E6]). Use was more frequent for patients with severe asthma than for those with milder disease. Reported OCS/SCS use varied considerably across a variety of patient populations, ranging from 2.8% (UK study of OCS use in patients 13–65 yr old with GINA step 2 or greater treatment) (77) to 93.5% (U.S. study of OCS use for patients of all ages with uncontrolled asthma) (78). Variations were likely related to differences in inclusion criteria, geography, and availability of OCS-sparing treatments. No clear geographic or age-related variations in general OCS use were observed in the identified studies (Tables 3 and E7). OCS use was typically reported for approximately 20–48% of patients in the general asthma population over a 1-year study period (Table 3) (79–83).
Table 3.
General OCS and SCS Use for Patients with Asthma, Categorized by Disease Severity
| Source | Sample Size | OCS or SCS Use at Follow-Up/Postindex, % (n/N) | OCS or SCS Use at Baseline/Preindex, % (n/N) |
|---|---|---|---|
| Any degree of asthma severity | |||
| Afshar et al., 2017 (130) (U.S.) | 14,012 (all individuals) | OCS | — |
| Asthma prevalence: 7.4% | 8.8 (95% CI, 6.2–11.3) | ||
| Arellano et al., 2011 (79) (U.S.) | 6–18 yr: 659,169 | OCS | — |
| 6–11 yr: 348,991 | 6–18 yr: 25.2 (165,783/659,169) | ||
| 6–11 yr: 27.6 (103,292/374,068) | |||
| Black et al., 2012 (80) (U.S.) | 74,057 | — | OCS |
| 31.2 | |||
| (23,115/74,057) | |||
| Björnsdóttir et al., 2014 (131) (Iceland) | 6,142 | — | OCS |
| 12.7 (783/6,142) | |||
| Butler et al., 2016 (132) (U.S.) | 123,868 | OCS | — |
| Jan 2005: 7.3% | |||
| Choi et al., 2017 (133) (South Korea) | 831,613 | OCS | — |
| 40.61 | |||
| Cooper et al., 2015 (134) (UK) | 2,624 | OCS | — |
| 12.0 (314/2,624) | |||
| Delate et al., 2017 (135) (U.S.) | 2,360 | SCS | SCS |
| 30.6 (723/2,360) | 35.8 (845/2,360) | ||
| P < 0.001 vs. preintervention | — | ||
| Farber et al., 2017 (81) (U.S.) | 5–8 yr: 20,645 | OCS | — |
| 9–12 yr: 14,716 | Rx (2,015) | ||
| 13–17 yr: 11,142 | 5–8 yr (N = 20,645) | ||
| 1: 31.9 (6,580) | |||
| 2: 6.6 (1,371) | |||
| ≥3: 3.5 (720) | |||
| 9–12 yr (N = 14,716) | |||
| 1: 28.2 (4,143) | |||
| 2: 5.3 (781) | |||
| ≥3: 2.2 (317) | |||
| 13–17 yr (N = 11,142) | |||
| 1: 30.1 (3,357) | |||
| 2: 4.3 (484) | |||
| ≥3: 2.4 (269) | |||
| Iribarren et al., 2012 (82) (U.S.) | 203,595 | — | OCS |
| 20% | |||
| Laforest et al., 2015 (136) (France) | UK: 38,637 | SCS | — |
| Non: 6,996 Low: 14,903 High: 16,738 |
Non/low/high ICS* | ||
| UK: 6.2%/22.5%/12.7% | |||
| France: 4,587 | P < 0.0001 | ||
| Non: 1,176 Low: 1,358 High: 2,053 |
France: 21.9%/36.1%/30.5% | ||
| P < 0.0001 | |||
| Lee et al., 2014 (137) (South Korea) | 736 | SCS | — |
| 71 (523/736) | |||
| Lin et al., 2016 (107) (Taiwan) | 24,109 | — | SCS |
| 3 mo: 8.0 (1,926/24,109) | |||
| 12 mo: 22.3 (5,378/24,109) | |||
| Luskin et al., 2016 (49) (U.S.) | 67,860 | Any OCS use | — |
| 66.0 (44,764/67,860) | |||
| Walters et al., 2011 (101) (UK) | 3,320 | — | OCS |
| Cases: 1,660 | Cases: 57.4% | ||
| Control subjects: 1,660† | Control subjects: 42.6%†P < 0.001 | ||
| Windt and Glaeske, 2010 (138) (Germany) | DMP: 317 | OCS | OCS |
| Not DMP (control): 317‡ | DMP: 26.5 (84/317) | DMP: 25.9 (82/317) | |
| Not DMP (all): 20,566 | Control: 24.3 (77/317)§ | Control: 20.5 (65/317)§P = 0.002 | |
| Not DMP: 25.9 (5,320/20,566) | |||
| Wong et al., 2010 (83) (U.S.) | 1,835 | OCS | OCS |
| 48.2 (884/1,835) | 48.1 (882/1,835) | ||
| GINA step 2 or greater treatment | |||
| Ali et al., 2015 (77) (UK) | 51,103 | — | OCS |
| ICS: 46,928 | Overall: 5.8 (2,976/51,103) | ||
| LABA: 714 | By postindex Tx | ||
| ICS/LABA: 3,461 | ICS: 5.7 (2,673/46,928) | ||
| LABA: 6.4 (46/714) | |||
| ICS/LABA: 7.4 (257/3,461) | |||
| Bengtson et al., 2017 (4) (U.S.) | Escalation: 5,044|| Unchanged: 21,967|| |
OCS | — |
| Unchanged|| | |||
| 31.9 (7,002/21,967) | |||
| Escalation|| | |||
| Pre: 29.8 (1,501/5,044) | |||
| Post: 30.7 (1,548/5,044) | |||
| Corrao et al., 2016 (84) (Italy) | 2,335 | OCS | — |
| 18.3 (428/2,335) | |||
| Hagiwara et al., 2010 (139) (U.S.) | FP: 469 | SCS | — |
| FSC: 3,881 | Matched¶: | ||
| FP: 32 (143/447) | |||
| FSC: 24 (106/447) P = 0.006 | |||
| Hagiwara et al., 2013 (140) (U.S.) | 18,283 | SCS | — |
| FSC: 14,044 | Matched**: | ||
| MF: 4,239 | FSC: 18.1 (688/3,799) | ||
| MF: 20.5 (780/3,799) P < 0.001 | |||
| Hagiwara et al., 2014 (141) (U.S.) | 7,779 | — | SCS claims and procedures |
| FP: 2,010 | FP: 69 (1,385/2,010) | ||
| FSC: 5,769 | FSC: 73 (4,232/5,769) P < 0.001 | ||
| Laforest et al., 2014 (85) (France) | 919 | — | OCS |
| Overall: 46.4 (394/849) | |||
| Laforest et al., 2014 (86) (France) | 2,162 | OCS | OCS |
| ICS: | 2008, % (n) | 2007, % (n) | |
| 1,757 | ICS (N = 1,757) | ICS (N = 1,757) | |
| ICS+LTRA: 1,826 | 0 units: 57.4 (1,009) | 0: 52.8 (928) | |
| 1 unit: 22.1 (388) | 1: 24.4 (428) | ||
| 2 units: 10.6 (187) | 2: 11.5 (202) | ||
| ≥3 units: 9.8 (173) | ≥3: 11.3 (199) | ||
| ICS+LTRA (N = 1,826) | ICS+LTRA | ||
| 0 units: 57.7 (1,053) | (N = 1,826) | ||
| 1 unit: 22.1 (403) | 0 units: 53.1 (970) | ||
| 2 units: 10.7 (195) | 1 unit: 24.3 (443) | ||
| ≥3 units: 9.6 (175) | 2 units: 11.4 (209) | ||
| ≥3 units: 11.2 (204) | |||
| Moderate-to-severe or severe asthma | |||
| Broder et al., 2011 (87) (U.S.) | 2003: 302 | OCS | — |
| 2004: 970 | 2007 cohort: 33.2 (459/1,382)†† |
||
| 2005: 1,301 | |||
| 2006: 1,361 | |||
| 2007: 1,382 | |||
| Bruno et al., 2014 (88) (France/Italy) | 102 | OCS | — |
| 64.7 (66/102) | |||
| DiSantostefano and Davis, 2011 (142) (UK) | 1,233 | SCS | SCS |
| 1–6 mo: 11% | 7–12 mo: 9% | ||
| 7–12 mo: 10% | 1–6 mo: 19% | ||
| Eisner et al., 2012 (89) (U.S.) | 2,878 | — | OCS |
| 51.2 (1,473/2,878) | |||
| Lafeuille et al., 2013 (90) (U.S.) | 3,044 | — | OCS |
| 49 (1,479/3,044) | |||
| Lafeuille et al., 2012 (78) (U.S.) | 644 | — | 93.5 (602/644) |
| (OCS) | |||
| Sposato et al., 2017 (143) (Italy) | 340 | OCS | — |
| OMB Tx duration | ≤12 mo: 13% | ||
| ≤12 mo: 39 | 12–≤24 mo: 9% | ||
| 12–≤24 mo: 94 | 24–≤60 mo: 6% | ||
| 24–≤60 mo: 171 | >60 mo: 3%‡‡ | ||
| >60 mo: 36 | ‡‡P = 0.044 vs. ≤12 mo | ||
| Sullivan et al., 2015 (91) (U.S.) | 25,297 | OCS | OCS |
| HDICS: 11,445 | HDICS: 34% | HDICS: 35% | |
| HICS: 6,926 | HICS: 65% | HICS: 53% | |
| OMB: 856 | OMB: 52% | OMB: 63% | |
| Sweeney et al., 2014 (144) (UK) | 2,670 pts with new ICS/LABA Rx and no prior ICS Rx | — | OCS |
| 5 (132/2,670) | |||
| Turner et al., 2017 (145) (UK) | 2,660 | OCS | — |
| FDC ICS/LABA: 6.5% | |||
| ICS+LABA: | |||
| 8.8% P = 0.084 |
Definition of abbreviations: CI = confidence interval; DMP = disease management program; FDC = fixed-dose combination; FP = fluticasone propionate; FSC = FP/SAL fixed-dose combination inhaler; GINA = Global Initiative for Asthma; HDICS = high-dosage ICS; HICS = high-intensity corticosteroids; ICS = inhaled corticosteroids; LABA = long-acting β2-agonists; LTRA = leukotriene receptor antagonists; MF = mometasone furoate; OCS = oral corticosteroids; OMB = omalizumab; Rx = prescription; SABA = short-acting β2-agonists; SCS = systemic corticosteroids; Tx = treatment.
Groups were defined according to the value of the ICS to total asthma medication ratio in 2008: R = 0% (non-ICS users), 0% < R < 50% (low ICS ratio group), and R ≥ 50% (high ICS ratio group). The ratio constituted the proportion of prescribed units of ICS out of the overall number of respiratory medication units prescribed during 2008.
Patients with asthma and a diagnosis of depression during the study period (cases) were matched to patients with asthma without depression (control subjects) according to the date of asthma diagnosis.
Occasional, intermittent, or continuous ICS/LABA use in baseline period.
Patients in the DMP group were propensity matched with non-DMP control subjects based on a range of variables, including demographics, asthma care/therapy, and comorbidities.
Escalation group: ICS or ICS-containing therapy dosage increase; a switch between ICS, LABA, or LTRA, or add-on of another controller within 12 months after the index date. Unchanged group: patients with ≥1 additional fill indicating continuation of index treatment regimen within 12 months after the index date.
Patients receiving FSC who stepped down to FSC at a smaller dosage of FP or switched to FP only at the same dosage. Patients in the FSC group were matched to those in the FP group through propensity score matching.
Each patient in the FSC group was matched to one patient in the MF group through propensity score techniques.
Based on OCS use in the top 10 medication patterns.
Pharmacist intervention to reduce SABA over-dispensing.
One study reported OCS use by 66% of patients over a 2-year period (U.S. study of adult patients) (49).
Similar incidences of general OCS use (range 18.3–47.2%) were observed in four of five studies involving patients receiving GINA step 2 or greater treatment across all age categories (Table 3) (4, 84–86). The exception was a UK-based retrospective cohort study (adolescent/adult patients) in which general OCS use was reported by 5.7%, 6.4%, and 7.4% of patients in the year before they initiated ICS, LABA, or ICS/LABA treatment, respectively (77).
General OCS use in studies involving patients with moderate or severe asthma was generally greater than that reported in studies involving a broader asthma population, although considerable interstudy variation existed (Table 3). OCS use ranged from 33.2% to 65% in five studies of patients with moderate-to-severe or severe asthma (6- to 12-mo assessment period) (87–91). A small cross-sectional study conducted in Italy and France reported general OCS use by 64.7% of patients with severe asthma in the previous year (88).
Clinical characteristics of patients prescribed CS
Overall, OCS users tended to be older than nonusers (Table E7) (30, 92–94). A U.S.-based prospective cohort study reported that for patients with severe asthma, long-term OCS use was more common among adults than among children/adolescents (22.4% and 9.9%, respectively, had at least 3 mo of OCS use in the previous year) (25). There was a greater percentage of female patients among OCS users in three U.S.-based studies reporting on use for patients with asthma overall, persistent asthma, and moderate-to-severe asthma (Table E7) (92–94). The percentage of patients receiving long-term OCS was greatest in the subgroups of patients who reported the greatest number of exacerbations (42, 46, 58, 62, 95). Disease severity and relative resistance to standard therapy (high-dosage ICS, LABA, and other controllers) are among the potential explanations for this finding. In addition, studies suggested that general and short-term OCS use was greater among obese patients than among nonobese patients. However, this may reflect the relatively poorer asthma control in this patient population (80, 88). A U.S.-based study reported that compared with nonusers or patients receiving short-term OCS therapy, adult patients who had uncontrolled asthma and were receiving long-term OCS were more likely to have received specialist asthma care (18.9% vs. 52.8%, respectively) and to have experienced more asthma exacerbations (rate: 0.3 vs. 1.6, respectively) (94). In all studies that reported on disease severity, for both long-term and short-term use, there was a strong correlation between increasing OCS use and increasing disease severity.
Clinical Burden of SCS
Comorbidities and complications associated with OCS/SCS use for patients with asthma were reported in 17 studies. Eleven of those studies reported OCS use (60, 72, 82, 93, 94, 96–101) and six reported SCS use (102–107).
Studies reported that the use of both short- and long-term OCS was consistently associated with a greater risk of acute and chronic CS-related complications compared with no OCS use. The risk of complications increased with increasing exposure (Table E8). A study that assessed the risk of any CS-related AEs reported a 1.3-fold increase in risk with long-term OCS use compared with no OCS use (108).
Acute complications
Identified studies reported acute OCS-associated complications that included infections and gastrointestinal events (Table E8). In a U.S.-based matched cohort study, approximately three times as many OCS users (≥30 d/yr) had pneumonia (28.4%) and opportunistic infections (1.5%) compared with non-OCS users (10.9% and 0.4%, respectively) (93). Four of five studies that reported gastrointestinal complications with OCS use found an increased risk associated with long-term use versus no use (odds ratio [OR], 1.035 [ulcers/bleeds] to 2.89 [gastroesophageal reflux]) (93, 94, 96, 97, 100).
Chronic complications
Identified studies reported chronic OCS-associated complications that included metabolic, bone-related, and cardiovascular events (Table E9). Most studies (U.S. and UK) reported increased risks of comorbid diabetes and obesity for patients with severe asthma and greater/long-term OCS/SCS use compared with patients with milder disease and less/no OCS use (93, 96, 100). However, one U.S.-based retrospective cohort study of adults with persistent asthma reported a similar prevalence of diabetes between patients with long- and short-term OCS use (94).
Bone- and muscle-related complications
Four studies reported an increased risk of bone- and muscle-related complications with long-term OCS use versus no use (Table E8) (93, 94, 96, 100). For example, one study reported that current OCS users with asthma (≥4 prescriptions/yr) had ORs of 1.44 and 1.21 for osteoporosis and bone fracture, respectively, compared with nonusers (100).
Cardiovascular complications
Identified studies consistently reported an association between long-term OCS use and increased risk of cardiovascular complications, hypertension, and hypercholesterolemia compared with no use or short-term use (Table E9) (60, 82, 93, 94, 96, 100). For example, in a UK-based matched cohort study of adults and adolescents, comorbid hypertension was more common with long-term OCS use among patients with severe asthma (34%) than with less OCS use among patients with mild-to-moderate asthma (29%) or without asthma (25%) (96). In a U.S.-based cross-sectional study of patients with asthma, long-term OCS use was significantly associated with systemic hypertension using univariate analysis. The association was no longer significant after adjustment for demographic and asthma-related variables and presence of obstructive sleep apnea (60). Importantly, in a U.S.-based retrospective cohort study, long-term OCS use was also associated with risk of coronary heart disease (hazard ratio, 2.59) and heart failure (hazard ratio, 3.48) (82).
Psychiatric complications
Identified studies reported mixed results for the association between OCS use and psychiatric complications (Table E9). A UK-based matched cohort study found that greater OCS use among patients with severe asthma was associated with a significant increase in the prevalence of comorbid psychiatric conditions compared with less OCS use (38% and 31%, respectively, vs. 25% for non–OCS use) (96). In a U.S.-based retrospective study, adults with persistent asthma reported an increased prevalence of anxiety (16.2% vs. 10.6%), but not depression (13.0% vs. 12.3%), with longer OCS use compared with short-term use (94). A database study of patients in the Netherlands with difficult-to-treat asthma reported an association between long-term OCS use and anxiety or depression (OR, 1.38) (97).
Ocular complications
Long-term OCS use was associated with an increased risk of cataracts, regardless of whether the risk was compared with short-term use or no exposure (Table E9) (93, 94, 96, 100). The OR for risk of cataracts in current versus nonusers of OCS was reported as 1.26 in one U.S.-based study (93). In a UK-based matched cohort study, cataracts were more common with long-term OCS use among patients with severe asthma (9%) than among those with less OCS use and mild-to-moderate asthma (5%) or without asthma (4%) (96). No studies reported an association between OCS use and increased risk of glaucoma.
Other complications
Included studies provided limited data regarding the effects of OCS therapy on the risk of other complications. Individual studies reported associations between long-term OCS use and asthma-related bronchiectasis (72), chronic kidney disease (96), and sleep disorders (96).
Healthcare Resource Use and Economic Impact of SCS Use
The impact of OCS/SCS use on healthcare resource use and costs for patients with asthma was reported in 12 studies (Tables E10 and E11). Across eight studies that evaluated the economic burden of OCS use for patients with asthma, long-term OCS and SCS use was consistently associated with increased healthcare costs compared with no or short-term use (34, 49, 92, 96, 103, 105, 106, 109). However, short-term use was also associated with increased costs and healthcare resource use compared with no OCS use.
Healthcare costs were reported to increase with greater OCS exposure. Data from a UK database estimated that the mean annual total costs of high, low, and no OCS use were £2,603, £978, and £560, respectively (96). Another UK-based database analysis reported that nonasthma-related medication costs were 58% greater for patients receiving long-term OCS than for nonusers (costs included prescriptions for proton pump inhibitors and bisphosphonates, treatments used to manage OCS-related AEs) (34). Nonmedication costs were 19% greater and total healthcare expenditures were 43% greater (34).
A U.S. claims-based analysis of adult patients with asthma receiving high-dosage OCS therapy reported that annual total healthcare costs were significantly greater for patients with OCS-related complications ($25,168) than for those without such complications ($21,882); however, asthma-related costs were comparable between the groups ($4,213 and $3,952, respectively) (49).
This association between healthcare costs and OCS exposure might reflect OCS use itself or disease severity. Patients with more severe asthma are likely to have had greater OCS exposure because of the need for treatment to control their symptoms. They are also likely to have incurred additional healthcare costs associated with increased clinic visits and hospitalizations for exacerbations. Currently, the literature does not appear to address indirect costs, such as lost productivity, associated with long-term OCS use. A link between treatment-related AEs and increased healthcare costs and resource use was identified in five articles that assessed the comorbidities and AEs associated with long-term OCS use (94, 96, 103, 105, 106). Furthermore, evaluation of data from the UK general practice database found that relative to patients with no OCS exposure, the additional costs for nonasthma-related medication use were notably greater for patients with greater OCS exposure (£772) than for those with less OCS exposure (£112) (96).
Discussion
This systematic literature review identified a large body of evidence from observational studies documenting real-world clinical practice regarding the use of OCS/SCS therapy for the general asthma population and especially for patients with more severe disease. Identified studies reported that short- and long-term OCS/SCS therapy is widely used to treat patients with asthma, particularly those with severe disease. Overall, OCS use was typically reported in approximately half of patients in the general asthma population over a 1-year period, and short-term use was reported in up to 36% of patients (35–38, 110). Guidelines recommend OCS for short-term treatment of serious exacerbations or as add-on maintenance therapy for patients with severe disease that is not controlled by high-dosage maintenance treatment (5). These recommendations restrict OCS use to an estimated 10% of patients with severe disease (111) and approximately 10% who experience an exacerbation in any given year (112). Thus, our findings indicate that in many instances, >10% of patients are receiving OCS treatment, suggesting that OCS may be overused for asthma management.
Long-term OCS use was consistently infrequent across all age groups for patients with nonsevere disease (<2%). However, up to 60% of patients with severe or uncontrolled asthma were reported to have received long-term OCS therapy. The extent of long-term OCS/SCS use has not been reported in other analyses. Furthermore, identified studies reported that OCS are used as long-term therapy at dosages up to 22 mg/d (67, 68), which is greater than the prednisone-equivalent dosage (≤7.5 mg/d) recommended in GINA guidelines as add-on therapy for patients with asthma that is not controlled by high-dosage therapy (GINA step 4 treatment) (5). These results suggest that patients are repeatedly treated with OCS at increasingly greater dosages, possibly despite their lack of response to OCS therapy or because of the nontargeted action of OCS. This may be because OCS are considered generally effective for all patients with asthma and are frequently prescribed in the absence of defined disease markers and objective response monitoring, such as lung function.
Notably, the identified studies predominantly reported on patients from Europe and North America, and therefore these findings may be biased toward approaches in these regions. It is possible that OCS are used more widely in low-income countries than in high-income ones because they are inexpensive. Indeed, until 2010, the World Health Organization recommended OCS as an essential medication to treat asthma (113). Moreover, payer criteria for biologics may impact OCS prescribing. For example, several reports from the United Kingdom (where four or more courses of OCS are required for eligibility for biologics) indicate that more than half of patients with severe asthma were prescribed four or more courses of OCS in 1 year. However, the percentages are lower for countries where there are no such requirements or fewer courses are required. In Australia, the payer requirement is two or more courses, and exemptions are available for OCS toxicity.
Studies have found that OCS are not equally effective for all patients and that most patients with severe asthma are steroid resistant to varying extents, by definition of their general lack of response to ICS (24, 25). Clinical characteristics of asthma, including eosinophilic airway inflammation, have been identified as being associated with response to OCS (1). Genetic factors have been identified that could also account for some of this patient variability in OCS response (24, 114–118). For example, genetic polymorphisms in the glucocorticoid-induced transcript gene GLCCI1 have been reported to account for 6.6% of the overall variability in clinical responses to ICS (117, 118). However, responses to both ICS and OCS are likely to be affected by several genetic variations, some of which have yet to be identified (24).
Identified studies reported that the use of both short- and long-term OCS/SCS is associated with an increased risk of acute and chronic complications, and this risk increases with greater exposure. The risk of any steroid-related AE was found to be up to 3.6-fold greater with long-term OCS use than with no use (103, 106, 108). Patients will become aware of any acute complications, if they occur, after treatment initiation. These complications can often have serious short-term consequences. However, patients may be less aware of the chronic complications associated with OCS/SCS use. In contrast, OCS/SCS-associated chronic conditions are often anticipated and monitored by physicians. Conversely, acute conditions, namely infections and gastrointestinal events, are frequently not considered by prescribers, although many OCS users experience acute complications, and relatively fewer studies have reported on these types of AEs. For example, pneumonia was the most frequently identified comorbidity associated with severe asthma in the Severe Asthma Research Program cohort of U.S. and UK patients with severe asthma (119). Even patients with low OCS exposure were reported to have an increased risk of infection (103). These findings are consistent with recent publications that were not included in our review (2, 120). One publication reported that AEs for patients with asthma receiving SCS began at cumulative exposures of 1.0 to <2.5 g (vs. >0 to <0.5 g reference), suggesting a relationship between cumulative SCS exposure and risk of AEs (2). Another publication reported that the 15-year cumulative incidence of type 2 diabetes was 9.5% for OCS/SCS users versus 5.6% for nonusers (120). However, the risk of type 2 diabetes began with a cumulative exposure of 0.5 to <1 g, which is equivalent to four lifetime OCS/SCS courses. This suggests that the incidence of comorbid type 2 diabetes is not necessarily exclusively influenced by the cumulative dosage of OCS (2). However, cumulative OCS/SCS exposure may not be an ideal measure because of possible variations among patients with regard to factors such as disease duration and severity. It is potentially an important metric for the medical community to understand and adopt in a clinical setting because it provides a means of assessing long-term exposure and associated adverse effects of OCS/SCS use. As with the response to OCS/SCS treatment, the risk of any patient experiencing an AE is likely to be influenced by underlying genetic factors, many of which have yet to be identified (24, 121).
The burden of comorbidities associated with both long-term and repeated short-term OCS/SCS use adds to the asthma burden, leading to increased risks of hospitalizations and emergency department visits, and corresponding increased healthcare resource use. We found reports of 43% greater overall healthcare expenditures for patients receiving long-term OCS therapy compared with nonusers, and 58% greater nonasthma-related costs (including costs of treatments used to manage OCS-related AEs) (108). Thus, although a prescriber’s decision to use OCS/SCS therapy instead of targeted treatments can be influenced by the initial unit price, incurred healthcare costs associated with OCS/SCS use may bring the validity of this choice into question. However, not all of the increased costs for patients receiving OCS are potentially directly attributable to OCS-related AEs: the direct costs arising from the management of severe asthma, such as treatment and hospitalization costs, are also likely to contribute to this increase. In an analysis of patients with asthma who were receiving intermittent or long-term SCS matched with patients who were not, 42% greater overall costs were reported for those who were receiving SCS (120). The associated average annual costs for adverse outcomes and asthma were £1,483 and £403, respectively, for patients receiving SCS, compared with £1,165 and £166, respectively, for nonusers. The individual contribution of OCS sparing to patients’ health-related quality of life is also difficult to elucidate. In future studies, the use of a global approach that includes generic and disease-related patient health-related quality of life questionnaires could help investigators assess the effects of OCS reduction and distinguish between improvements that result from reduced OCS use and those that are attributable to improved asthma control. It is also difficult to directly attribute the occurrence of some common comorbidities in the general population to OCS use. For example, gastroesophageal reflux disease is particularly common among patients with asthma. This condition is worsened by OCS use but can be improved by greater asthma control and increased patient activity (122).
Our review has several advantages over similar publications. This review included a search of both MEDLINE and Embase databases. We also used a strong methodology, with a study design that allowed the inclusion of a wide range of ages (children, adolescents, and adults), the full spectrum of disease, and the prevalence of both short- and long-term use. This resulted in the identification and reporting of results from 139 studies that included populations with varying degrees of asthma severity. Our findings are robust compared with recent reviews that identified fewer publications (<50 studies) and reported only on long-term OCS use among adult patients with severe asthma or AEs and their corresponding economic burden (28, 123, 124).
The limitations of our review include the fact that our search was conducted in December 2017, and congress abstracts were not included. Therefore, some more recent studies may have been omitted from our review. We also used the definitions of long-term and short-term use provided in the identified literature, but interpretations varied among the studies. Another potential limitation is our inclusion of 13 studies that reported results of patient surveys, as the data from such surveys are considered to be weaker than those obtained from cohort or database studies. Furthermore, some studies from which data were obtained were not designed to directly identify AEs associated with OCS/SCS use. Therefore, AEs may have been underreported in those studies. In addition, data on dose-dependent effects and the frequency of OCS/SCS use associated with AEs were not available for all studies and are not summarized here. The information provided on asthma severity varied among the identified studies, which prevented us from obtaining a more generalized synthesis of the data by GINA treatment step. Overall, a considerable amount of available data was obtained from retrospective reports from patients and physicians, and as such, was subject to recall bias. These data suggest that use remains a frequent component of treatment for patients with asthma, primarily related to the lack of control with other medications. They also suggest that OCS/SCS use is widespread and associated with significant adverse effects. Quantitation of these data would be helpful, but this was beyond the scope of this review. Future longitudinal studies using objective methods to collect data regarding the use of OCS and its associated clinical burden are needed.
Overall, this review demonstrates that OCS and SCS, including long-term OCS, continue to be commonly used and overused for the management of asthma across the disease spectrum and particularly for severe asthma. This use is associated with both acute and chronic complications. Importantly, patients receiving repeated short-term, high OCS dosages may incur a greater risk of AEs than those receiving long-term, low dosages, as the risk of AEs increases with the cumulative OCS dosage. The introduction of biologics has led to a reduction in OCS use in other disease areas, but our review shows that a similar change has not yet occurred for asthma (18). This is because biologics have only recently been approved for the treatment of severe asthma. Omalizumab was the only add-on biologic therapy available for the treatment of severe asthma during the time periods covered in many of the identified studies (18). The recent approval of further biologic treatments that reduce asthma symptoms and exacerbation risks and allow OCS tapering for OCS-dependent patients has the potential to reduce future OCS use for patients with asthma (10–14, 16, 17).
Supplementary Material
Acknowledgments
Acknowledgment
Writing and editing assistance, including preparation of a draft manuscript under the direction and guidance of the authors, incorporating author feedback, and manuscript submission, was provided by Debra Scates, Ph.D., of JK Associates, Inc., and Michael A. Nissen, E.L.S., of AstraZeneca. This support was funded by AstraZeneca.
Footnotes
Supported by AstraZeneca. The funder of the study collaborated in the study design, data collection, data analysis, data interpretation, and writing of the report.
Author Contributions: S.S., A.L.M., M.A., and T.N.T. conceived, designed, and executed the literature search. All authors had access to and analyzed and interpreted the data. All authors participated in the development and critical review of the manuscript. E.R.B. had full access to all of the data in the study and had final responsibility for the decision to submit for publication. All authors provided final approval for publication submission and are accountable for the accuracy and integrity of the work.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201904-0903SO on September 16, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Alangari AA. Corticosteroids in the treatment of acute asthma. Ann Thorac Med. 2014;9:187–192. doi: 10.4103/1817-1737.140120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Price DB, Trudo F, Voorham J, Xu X, Kerkhof M, Ling Zhi Jie J, et al. Adverse outcomes from initiation of systemic corticosteroids for asthma: long-term observational study. J Asthma Allergy. 2018;11:193–204. doi: 10.2147/JAA.S176026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.British Thoracic and Tuberculosis Association. Inhaled corticosteroids compared with oral prednisone in patients starting long-term corticosteroid therapy for asthma: a controlled trial by the British Thoracic and Tuberculosis Association. Lancet. 1975;2:469–473. [PubMed] [Google Scholar]
- 4.Bengtson LGS, Yu Y, Wang W, Cao F, Hulbert EM, Wolbeck R, et al. Inhaled corticosteroid-containing treatment escalation and outcomes for patients with asthma in a U.S. health care organization. J Manag Care Spec Pharm. 2017;23:1149–1159. doi: 10.18553/jmcp.2017.23.11.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Global Initiative for Asthma Management and Prevention (GINA) Difficult-to-treat and severe asthma in adolescent and adult patients, diagnosis and management. 2019 [accessed 2019 Dec 17]. Available from: www.ginasthma.org.
- 6.Israel E, Reddel HK. Severe and difficult-to-treat asthma in adults. N Engl J Med. 2017;377:965–976. doi: 10.1056/NEJMra1608969. [DOI] [PubMed] [Google Scholar]
- 7.Pelaia C, Vatrella A, Bruni A, Terracciano R, Pelaia G. Benralizumab in the treatment of severe asthma: design, development and potential place in therapy. Drug Des Devel Ther. 2018;12:619–628. doi: 10.2147/DDDT.S155307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drugs.com FDA approves Dupixent (dupilumab) for moderate-to-severe asthma 2018[accessed 2019 Jul 12]. Available from: https://www.drugs.com/newdrugs/fda-approves-dupixent-dupilumab-moderate-severe-asthma-4848.html
- 9.Humbert M, Busse W, Hanania NA. Controversies and opportunities in severe asthma. Curr Opin Pulm Med. 2018;24:83–93. doi: 10.1097/MCP.0000000000000438. [DOI] [PubMed] [Google Scholar]
- 10.Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, et al. MENSA Investigators. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198–1207. doi: 10.1056/NEJMoa1403290. [DOI] [PubMed] [Google Scholar]
- 11.Bleecker ER, FitzGerald JM, Chanez P, Papi A, Weinstein SF, Barker P, et al. SIROCCO study investigators. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388:2115–2127. doi: 10.1016/S0140-6736(16)31324-1. [DOI] [PubMed] [Google Scholar]
- 12.FitzGerald JM, Bleecker ER, Nair P, Korn S, Ohta K, Lommatzsch M, et al. CALIMA study investigators. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388:2128–2141. doi: 10.1016/S0140-6736(16)31322-8. [DOI] [PubMed] [Google Scholar]
- 13.Rabe KF, Nair P, Brusselle G, Maspero JF, Castro M, Sher L, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med. 2018;378:2475–2485. doi: 10.1056/NEJMoa1804093. [DOI] [PubMed] [Google Scholar]
- 14.Menzella F, Galeone C, Formisano D, Castagnetti C, Ruggiero P, Simonazzi A, et al. Real-life efficacy of omalizumab after 9 years of follow-up. Allergy Asthma Immunol Res. 2017;9:368–372. doi: 10.4168/aair.2017.9.4.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brusselle G, Germinaro M, Weiss S, Zangrilli J. Reslizumab in patients with inadequately controlled late-onset asthma and elevated blood eosinophils. Pulm Pharmacol Ther. 2017;43:39–45. doi: 10.1016/j.pupt.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Bel EH, Wenzel SE, Thompson PJ, Prazma CM, Keene ON, Yancey SW, et al. SIRIUS Investigators. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189–1197. doi: 10.1056/NEJMoa1403291. [DOI] [PubMed] [Google Scholar]
- 17.Nair P, Wenzel S, Rabe KF, Bourdin A, Lugogo NL, Kuna P, et al. ZONDA Trial Investigators. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376:2448–2458. doi: 10.1056/NEJMoa1703501. [DOI] [PubMed] [Google Scholar]
- 18.Menzies-Gow A, Canonica GW, Winders TA, Correia de Sousa J, Upham JW, Fink-Wagner AH. A charter to improve patient care in severe asthma. Adv Ther. 2018;35:1485–1496. doi: 10.1007/s12325-018-0777-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bénard-Laribière A, Pariente A, Pambrun E, Bégaud B, Fardet L, Noize P. Prevalence and prescription patterns of oral glucocorticoids in adults: a retrospective cross-sectional and cohort analysis in France. BMJ Open. 2017;7:e015905. doi: 10.1136/bmjopen-2017-015905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choo XN, Pavord ID. Morbidity associated with oral corticosteroids in patients with severe asthma. Thorax. 2016;71:302–304. doi: 10.1136/thoraxjnl-2015-208242. [DOI] [PubMed] [Google Scholar]
- 21.van Staa TP, Leufkens HG, Abenhaim L, Begaud B, Zhang B, Cooper C. Use of oral corticosteroids in the United Kingdom. QJM. 2000;93:105–111. doi: 10.1093/qjmed/93.2.105. [DOI] [PubMed] [Google Scholar]
- 22.Chen HC, Huang CD, Chang E, Kuo HP. Efficacy of omalizumab (Xolair) in patients with moderate to severe predominately chronic oral steroid dependent asthma in Taiwan: a retrospective, population-based database cohort study. BMC Pulm Med. 2016;16:3. doi: 10.1186/s12890-015-0156-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alten R, Nüßlein H, Galeazzi M, Lorenz HM, Nurmohamed MT, Bensen WG, et al. Decreased use of glucocorticoids in biological-experienced patients with rheumatoid arthritis who initiated intravenous abatacept: results from the 2-year ACTION study. RMD Open. 2016;2:e000228. doi: 10.1136/rmdopen-2015-000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyers DA, Bleecker ER, Holloway JW, Holgate ST. Asthma genetics and personalised medicine. Lancet Respir Med. 2014;2:405–415. doi: 10.1016/S2213-2600(14)70012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phipatanakul W, Mauger DT, Sorkness RL, Gaffin JM, Holguin F, Woodruff PG, et al. Severe Asthma Research Program. Effects of age and disease severity on systemic corticosteroid responses in asthma. Am J Respir Crit Care Med. 2017;195:1439–1448. doi: 10.1164/rccm.201607-1453OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peters MC, Kerr S, Dunican EM, Woodruff PG, Fajt ML, Levy BD, et al. National Heart, Lung and Blood Institute Severe Asthma Research Program 3. Refractory airway type 2 inflammation in a large subgroup of asthmatic patients treated with inhaled corticosteroids. J Allergy Clin Immunol. 2019;143:104–113, e14. doi: 10.1016/j.jaci.2017.12.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Normansell R, Kew KM, Mansour G. Different oral corticosteroid regimens for acute asthma. Cochrane Database Syst Rev. 2016;(5):CD011801. doi: 10.1002/14651858.CD011801.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Volmer T, Effenberger T, Trautner C, Buhl R. Consequences of long-term oral corticosteroid therapy and its side-effects in severe asthma in adults: a focused review of the impact data in the literature. Eur Respir J. 2018;52:1800703. doi: 10.1183/13993003.00703-2018. [DOI] [PubMed] [Google Scholar]
- 29.Tse K, Chen L, Tse M, Zuraw B, Christiansen S. Effect of catastrophic wildfires on asthmatic outcomes in obese children: breathing fire. Ann Allergy Asthma Immunol. 2015;114:308–311, e4. doi: 10.1016/j.anai.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allen-Ramey FC, Nelsen LM, Leader JB, Mercer D, Kirchner HL, Jones JB. Electronic health record-based assessment of oral corticosteroid use in a population of primary care patients with asthma: an observational study. Allergy Asthma Clin Immunol. 2013;9:27. doi: 10.1186/1710-1492-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papi A, Mansur AH, Pertseva T, Kaiser K, McIver T, Grothe B, et al. Long-term fluticasone propionate/formoterol fumarate combination therapy is associated with a low incidence of severe asthma exacerbations. J Aerosol Med Pulm Drug Deliv. 2016;29:346–361. doi: 10.1089/jamp.2015.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tunceli O, Williams SA, Kern DM, Elhefni H, Pethick N, Wessman C, et al. Comparative effectiveness of budesonide-formoterol combination and fluticasone-salmeterol combination for asthma management: a United States retrospective database analysis. J Allergy Clin Immunol Pract. 2014;2:719–726. doi: 10.1016/j.jaip.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 33.Chipps BE, Haselkorn T, Paknis B, Ortiz B, Bleecker ER, Kianifard F, et al. Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens Study Group. More than a decade follow-up in patients with severe or difficult-to-treat asthma: The Epidemiology and Natural History of Asthma. Outcomes and Treatment Regimens (TENOR) II. J Allergy Clin Immunol. 2018;141:1590–1597, e9. doi: 10.1016/j.jaci.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 34.O’Neill S, Sweeney J, Patterson CC, Menzies-Gow A, Niven R, Mansur AH, et al. British Thoracic Society Difficult Asthma Network. The cost of treating severe refractory asthma in the UK: an economic analysis from the British Thoracic Society Difficult Asthma Registry. Thorax. 2015;70:376–378. doi: 10.1136/thoraxjnl-2013-204114. [DOI] [PubMed] [Google Scholar]
- 35.Price D, Kaplan A, Jones R, Freeman D, Burden A, Gould S, et al. Long-acting muscarinic antagonist use in adults with asthma: real-life prescribing and outcomes of add-on therapy with tiotropium bromide. J Asthma Allergy. 2015;8:1–13. doi: 10.2147/JAA.S76639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schatz M, Zeiger RS, Yang S-J, Chen W, Sajjan S, Allen-Ramey F, et al. Prospective study on the relationship of obesity to asthma impairment and risk. J Allergy Clin Immunol Pract. 2015;3:560–565.e1. doi: 10.1016/j.jaip.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 37.Schatz M, Zeiger RS, Yang S-JT, Chen W, Crawford WW, Sajjan SG, et al. Relationship of asthma control to asthma exacerbations using surrogate markers within a managed care database. Am J Manag Care. 2010;16:327–333. [PubMed] [Google Scholar]
- 38.van Boven JFM, Hiddink EG, Stuurman-Bieze AGG, Schuiling-Veninga CCM, Postma MJ, Vegter S. The pharmacists’ potential to provide targets for interventions to optimize pharmacotherapy in patients with asthma. Int J Clin Pharm. 2013;35:1075–1082. doi: 10.1007/s11096-013-9829-1. [DOI] [PubMed] [Google Scholar]
- 39.Luskin AT, Antonova EN, Broder MS, Chang E, Raimundo K, Solari PG. Patient outcomes, health care resource use, and costs associated with high versus low HEDIS asthma medication ratio. J Manag Care Spec Pharm. 2017;23:1117–1124. doi: 10.18553/jmcp.2017.23.11.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silver HS, Blanchette CM, Kamble S, Petersen H, Letter MA, Meddis D, et al. Relationship between short-acting β2-adrenergic agonist use and healthcare costs. Am J Manag Care. 2011;17:19–27. [PubMed] [Google Scholar]
- 41.Stephenson JJ, Quimbo RA, Gutierrez B. Subacute lack of asthma control as a predictor of subsequent acute asthma exacerbation in a managed care population. Am J Manag Care. 2010;16:108–114. [PubMed] [Google Scholar]
- 42.Denlinger LC, Phillips BR, Ramratnam S, Ross K, Bhakta NR, Cardet JC, et al. National Heart, Lung, and Blood Institute’s Severe Asthma Research Program-3 Investigators. Inflammatory and comorbid features of patients with severe asthma and frequent exacerbations. Am J Respir Crit Care Med. 2017;195:302–313. doi: 10.1164/rccm.201602-0419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gibeon D, Heaney LG, Brightling CE, Niven R, Mansur AH, Chaudhuri R, et al. British Thoracic Society Difficult Asthma Network. Dedicated severe asthma services improve health-care use and quality of life. Chest. 2015;148:870–876. doi: 10.1378/chest.14-3056. [DOI] [PubMed] [Google Scholar]
- 44.Smith JR, Noble MJ, Musgrave S, Murdoch J, Price GM, Barton GR, et al. The At-Risk Registers in Severe Asthma (ARRISA) study: a cluster-randomised controlled trial examining effectiveness and costs in primary care. Thorax. 2012;67:1052–1060. doi: 10.1136/thoraxjnl-2012-202093. [DOI] [PubMed] [Google Scholar]
- 45.Deschildre A, Marguet C, Salleron J, Pin I, Rittié J-L, Derelle J, et al. Add-on omalizumab in children with severe allergic asthma: a 1-year real life survey. Eur Respir J. 2013;42:1224–1233. doi: 10.1183/09031936.00149812. [DOI] [PubMed] [Google Scholar]
- 46.Maio S, Baldacci S, Bresciani M, Simoni M, Latorre M, Murgia N, et al. AGAVE Group. RItA: the Italian severe/uncontrolled asthma registry. Allergy. 2018;73:683–695. doi: 10.1111/all.13342. [DOI] [PubMed] [Google Scholar]
- 47.Covvey JR, Johnston BF, Wood F, Boyter AC. Is the BTS/SIGN guideline confusing? A retrospective database analysis of asthma therapy. Prim Care Respir J. 2013;22:290–295. doi: 10.4104/pcrj.2013.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ismaila A, Corriveau D, Vaillancourt J, Parsons D, Stanford R, Su Z, et al. Impact of adherence to treatment with fluticasone propionate/salmeterol in asthma patients. Curr Med Res Opin. 2014;30:1417–1425. doi: 10.1185/03007995.2014.908827. [DOI] [PubMed] [Google Scholar]
- 49.Luskin AT, Antonova EN, Broder MS, Chang EY, Omachi TA, Ledford DK. Health care resource use and costs associated with possible side effects of high oral corticosteroid use in asthma: a claims-based analysis. Clinicoecon Outcomes Res. 2016;8:641–648. doi: 10.2147/CEOR.S115025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Makhinova T, Barner JC, Richards KM, Rascati KL. Asthma controller medication adherence, risk of exacerbation, and use of rescue agents among Texas Medicaid patients with persistent asthma. J Manag Care Spec Pharm. 2015;21:1124–1132. doi: 10.18553/jmcp.2015.21.12.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin RJ, Price D, Roche N, Israel E, van Aalderen WMC, Grigg J, et al. Cost-effectiveness of initiating extrafine- or standard size-particle inhaled corticosteroid for asthma in two health-care systems: a retrospective matched cohort study. NPJ Prim Care Respir Med. 2014;24:14081. doi: 10.1038/npjpcrm.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Price D, Small I, Haughney J, Ryan D, Gruffydd-Jones K, Lavorini F, et al. Clinical and cost effectiveness of switching asthma patients from fluticasone-salmeterol to extra-fine particle beclometasone-formoterol: a retrospective matched observational study of real-world patients. Prim Care Respir J. 2013;22:439–448. doi: 10.4104/pcrj.2013.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Price D, Thomas M, Haughney J, Lewis RA, Burden A, von Ziegenweidt J, et al. Real-life comparison of beclometasone dipropionate as an extrafine- or larger-particle formulation for asthma. Respir Med. 2013;107:987–1000. doi: 10.1016/j.rmed.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 54.Sadatsafavi M, Lynd L, Marra C, Bedouch P, Fitzgerald M. Comparative outcomes of leukotriene receptor antagonists and long-acting β-agonists as add-on therapy in asthmatic patients: a population-based study. J Allergy Clin Immunol. 2013;132:63–69. doi: 10.1016/j.jaci.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 55.Korn S, Hubner M, Jung M, Blettner M, Buhl R. Severe and uncontrolled adult asthma is associated with vitamin D insufficiency and deficiency. Respir Res. 2013;14:22. doi: 10.1186/1465-9921-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Broder MS, Chang EY, Sapra S. Care of asthma patients in relation to guidelines. Allergy Asthma Proc. 2010;31:452–460. doi: 10.2500/aap.2010.31.3369. [DOI] [PubMed] [Google Scholar]
- 57.Fardet L, Petersen I, Nazareth I. Prevalence of long-term oral glucocorticoid prescriptions in the UK over the past 20 years. Rheumatology (Oxford) 2011;50:1982–1990. doi: 10.1093/rheumatology/ker017. [DOI] [PubMed] [Google Scholar]
- 58.Price D, Wilson AM, Chisholm A, Rigazio A, Burden A, Thomas M, et al. Predicting frequent asthma exacerbations using blood eosinophil count and other patient data routinely available in clinical practice. J Asthma Allergy. 2016;9:1–12. doi: 10.2147/JAA.S97973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dodd KE, Mazurek JM. Asthma medication use among adults with current asthma by work-related asthma status, Asthma Call-back Survey, 29 states, 2012-2013. J Asthma. 2018;55:364–372. doi: 10.1080/02770903.2017.1339245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferguson S, Teodorescu MC, Gangnon RE, Peterson AG, Consens FB, Chervin RD, et al. Factors associated with systemic hypertension in asthma. Lung. 2014;192:675–683. doi: 10.1007/s00408-014-9600-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hasegawa T, Koya T, Sakagami T, Toyabe S, Kagamu H, Arakawa M, et al. Niigata Asthma Treatment Study Group. Asthma control and management changes in Japan: questionnaire survey. Intern Med. 2012;51:567–574. doi: 10.2169/internalmedicine.51.6586. [DOI] [PubMed] [Google Scholar]
- 62.Papaioannou AI, Kostikas K, Bakakos P, Papaporfyriou A, Konstantellou E, Hillas G, et al. Predictors of future exacerbation risk in patients with asthma. Postgrad Med. 2016;128:687–692. doi: 10.1080/00325481.2016.1220807. [DOI] [PubMed] [Google Scholar]
- 63.Reddy RC, Baptist AP, Fan Z, Carlin AM, Birkmeyer NJO. The effects of bariatric surgery on asthma severity. Obes Surg. 2011;21:200–206. doi: 10.1007/s11695-010-0155-6. [DOI] [PubMed] [Google Scholar]
- 64.Sato S, Saito J, Fukuhara A, Uematsu M, Suzuki Y, Togawa R, et al. The clinical role of fractional exhaled nitric oxide in asthma control. Ann Allergy Asthma Immunol. 2017;119:541–547. doi: 10.1016/j.anai.2017.09.059. [DOI] [PubMed] [Google Scholar]
- 65.Shigemura M, Konno S, Nasuhara Y, Shimizu C, Matsuno K, Nishimura M. Impact of asthmatic control status on serum cystatin C concentrations. Clin Chem Lab Med. 2012;50:1367–1371. [PubMed] [Google Scholar]
- 66.Tattersall MC, Guo M, Korcarz CE, Gepner AD, Kaufman JD, Liu KJ, et al. Asthma predicts cardiovascular disease events: the multi-ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:1520–1525. doi: 10.1161/ATVBAHA.115.305452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reddy MB, Doshi J, Covar R, Spahn JD. The changing face of severe childhood asthma: a comparison of two cohorts of children evaluated at National Jewish Health over the past 20 years. Allergy Asthma Proc. 2014;35:119–125. doi: 10.2500/aap.2014.35.3727. [DOI] [PubMed] [Google Scholar]
- 68.Barnes N, Menzies-Gow A, Mansur AH, Spencer D, Percival F, Radwan A, et al. Effectiveness of omalizumab in severe allergic asthma: a retrospective UK real-world study. J Asthma. 2013;50:529–536. doi: 10.3109/02770903.2013.790419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Braunstahl GJ, Chen CW, Maykut R, Georgiou P, Peachey G, Bruce J. The eXpeRience registry: the ‘real-world’ effectiveness of omalizumab in allergic asthma. Respir Med. 2013;107:1141–1151. doi: 10.1016/j.rmed.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 70.Gibson PG, Reddel H, McDonald VM, Marks G, Jenkins C, Gillman A, et al. Effectiveness and response predictors of omalizumab in a severe allergic asthma population with a high prevalence of comorbidities: the Australian Xolair Registry. Intern Med J. 2016;46:1054–1062. doi: 10.1111/imj.13166. [DOI] [PubMed] [Google Scholar]
- 71.James AJ, Reinius LE, Verhoek M, Gomes A, Kupczyk M, Hammar U, et al. BIOAIR (Longitudinal Assessment of Clinical Course and Biomarkers in Severe Chronic Airway Disease) Consortium. Increased YKL-40 and chitotriosidase in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2016;193:131–142. doi: 10.1164/rccm.201504-0760OC. [DOI] [PubMed] [Google Scholar]
- 72.Luján M, Gallardo X, Amengual MJ, Bosque M, Mirapeix RM, Domingo C. Prevalence of bronchiectasis in asthma according to oral steroid requirement: influence of immunoglobulin levels. BioMed Res Int. 2013;2013:109219. doi: 10.1155/2013/109219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Molimard M, Buhl R, Niven R, Le Gros V, Thielen A, Thirlwell J, et al. Omalizumab reduces oral corticosteroid use in patients with severe allergic asthma: real-life data. Respir Med. 2010;104:1381–1385. doi: 10.1016/j.rmed.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 74.Niven RM, Saralaya D, Chaudhuri R, Masoli M, Clifton I, Mansur AH, et al. Impact of omalizumab on treatment of severe allergic asthma in UK clinical practice: a UK multicentre observational study (the APEX II study) BMJ Open 20166e011857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shaw DE, Sousa AR, Fowler SJ, Fleming LJ, Roberts G, Corfield J, et al. U-BIOPRED Study Group. Clinical and inflammatory characteristics of the European U-BIOPRED adult severe asthma cohort. Eur Respir J. 2015;46:1308–1321. doi: 10.1183/13993003.00779-2015. [DOI] [PubMed] [Google Scholar]
- 76.Sweeney J, Brightling CE, Menzies-Gow A, Niven R, Patterson CC, Heaney LG British Thoracic Society Difficult Asthma Network. Clinical management and outcome of refractory asthma in the UK from the British Thoracic Society Difficult Asthma Registry. Thorax. 2012;67:754–756. doi: 10.1136/thoraxjnl-2012-201869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ali AK, Hartzema AG, Winterstein AG, Segal R, Lu X, Hendeles L. Application of multicategory exposure marginal structural models to investigate the association between long-acting beta-agonists and prescribing of oral corticosteroids for asthma exacerbations in the Clinical Practice Research Datalink. Value Health. 2015;18:260–270. doi: 10.1016/j.jval.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 78.Lafeuille MH, Dean J, Zhang J, Duh MS, Gorsh B, Lefebvre P. Impact of omalizumab on emergency-department visits, hospitalizations, and corticosteroid use among patients with uncontrolled asthma. Ann Allergy Asthma Immunol. 2012;109:59–64. doi: 10.1016/j.anai.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 79.Arellano FM, Arana A, Wentworth CE, Vidaurre CF, Chipps BE. Prescription patterns for asthma medications in children and adolescents with health care insurance in the United States. Pediatr Allergy Immunol. 2011;22:469–476. doi: 10.1111/j.1399-3038.2010.01121.x. [DOI] [PubMed] [Google Scholar]
- 80.Black MH, Smith N, Porter AH, Jacobsen SJ, Koebnick C. Higher prevalence of obesity among children with asthma. Obesity (Silver Spring) 2012;20:1041–1047. doi: 10.1038/oby.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Farber HJ, Silveira EA, Vicere DR, Kothari VD, Giardino AP. Oral corticosteroid prescribing for children with asthma in a Medicaid managed care program. Pediatrics. 2017;139:e20164146. doi: 10.1542/peds.2016-4146. [DOI] [PubMed] [Google Scholar]
- 82.Iribarren C, Tolstykh IV, Miller MK, Sobel E, Eisner MD. Adult asthma and risk of coronary heart disease, cerebrovascular disease, and heart failure: a prospective study of 2 matched cohorts. Am J Epidemiol. 2012;176:1014–1024. doi: 10.1093/aje/kws181. [DOI] [PubMed] [Google Scholar]
- 83.Wong MD, Manley RT, Stettin G, Chen W, Salmun LM. Intervention to reduce unnecessary dispensing of short-acting beta-agonists in patients with asthma. Ann Pharmacother. 2010;44:623–629. doi: 10.1345/aph.1M697. [DOI] [PubMed] [Google Scholar]
- 84.Corrao G, Arfè A, Nicotra F, Ghirardi A, Vaghi A, De Marco R, et al. CRD Real-World Evidence Scientific Board. Persistence with inhaled corticosteroids reduces the risk of exacerbation among adults with asthma: a real-world investigation. Respirology. 2016;21:1034–1040. doi: 10.1111/resp.12791. [DOI] [PubMed] [Google Scholar]
- 85.Laforest L, Licaj I, Devouassoux G, Chatté G, Belhassen M, Van Ganse E, et al. Relative exposure to controller therapy and asthma exacerbations: a validation study in community pharmacies. Pharmacoepidemiol Drug Saf. 2014;23:958–964. doi: 10.1002/pds.3668. [DOI] [PubMed] [Google Scholar]
- 86.Laforest L, Licaj I, Devouassoux G, Chatte G, Martin J, Van Ganse E. Asthma drug ratios and exacerbations: claims data from universal health coverage systems. Eur Respir J. 2014;43:1378–1386. doi: 10.1183/09031936.00100113. [DOI] [PubMed] [Google Scholar]
- 87.Broder MS, Zazzali JL, Chang E, Yegin A. Concomitant asthma medication use by patients receiving omalizumab 2003-2008. J Asthma. 2011;48:1058–1062. doi: 10.3109/02770903.2011.631241. [DOI] [PubMed] [Google Scholar]
- 88.Bruno A, Pace E, Cibella F, Chanez P.Body mass index and comorbidities in adult severe asthmatics Biomed Res Int 20142014607192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eisner MD, Yegin A, Trzaskoma B. Severity of asthma score predicts clinical outcomes in patients with moderate to severe persistent asthma. Chest. 2012;141:58–65. doi: 10.1378/chest.11-0020. [DOI] [PubMed] [Google Scholar]
- 90.Lafeuille MH, Gravel J, Zhang J, Gorsh B, Figliomeni M, Lefebvre P. Association between consistent omalizumab treatment and asthma control. J Allergy Clin Immunol Pract. 2013;1:51–57. doi: 10.1016/j.jaip.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 91.Sullivan PW, Campbell JD, Ghushchyan VH, Globe G. Outcomes before and after treatment escalation to Global Initiative for Asthma steps 4 and 5 in severe asthma. Ann Allergy Asthma Immunol. 2015;114:462–469. doi: 10.1016/j.anai.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 92.Broder MS, Raimundo K, Ngai KM, Chang E, Griffin NM, Heaney LG. Cost and health care utilization in patients with asthma and high oral corticosteroid use. Ann Allergy Asthma Immunol. 2017;118:638–639. doi: 10.1016/j.anai.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 93.Zazzali JL, Broder MS, Omachi TA, Chang E, Sun GH, Raimundo K. Risk of corticosteroid-related adverse events in asthma patients with high oral corticosteroid use. Allergy Asthma Proc. 2015;36:268–274. doi: 10.2500/aap.2015.36.3863. [DOI] [PubMed] [Google Scholar]
- 94.Zeiger RS, Schatz M, Li Q, Chen W, Khatry DB, Tran TN. Burden of chronic oral corticosteroid use by adults with persistent asthma. J Allergy Clin Immunol Pract. 2017;5:1050–1060, e9. doi: 10.1016/j.jaip.2016.12.023. [DOI] [PubMed] [Google Scholar]
- 95.Westerhof GA, de Groot JC, Amelink M, de Nijs SB, Ten Brinke A, Weersink EJ, et al. Predictors of frequent exacerbations in (ex)smoking and never smoking adults with severe asthma. Respir Med. 2016;118:122–127. doi: 10.1016/j.rmed.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 96.Barry LE, Sweeney J, O'Neill C, Price D, Heaney LG. The cost of systemic corticosteroid-induced morbidity in severe asthma: a health economic analysis. Respir Res. 2017;18:26. doi: 10.1186/s12931-017-0614-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hekking PPW, Amelink M, Wener RR, Bouvy ML, Bel EH. Comorbidities in difficult-to-control asthma. J Allergy Clin Immunol Pract. 2018;6:108–113. doi: 10.1016/j.jaip.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 98.Lee CH, Kim K, Hyun MK, Jang EJ, Lee NR, Yim JJ. Use of inhaled corticosteroids and the risk of tuberculosis. Thorax. 2013;68:1105–1113. doi: 10.1136/thoraxjnl-2012-203175. [DOI] [PubMed] [Google Scholar]
- 99.Majoor CJ, Kamphuisen PW, Zwinderman AH, Ten Brinke A, Amelink M, Rijssenbeek-Nouwens L, et al. Risk of deep vein thrombosis and pulmonary embolism in asthma. Eur Respir J. 2013;42:655–661. doi: 10.1183/09031936.00150312. [DOI] [PubMed] [Google Scholar]
- 100.Sullivan PW, Ghushchyan VH, Globe G, Schatz M. Oral corticosteroid exposure and adverse effects in asthmatic patients. J Allergy Clin Immunol. 2018;141:110–116.e7. doi: 10.1016/j.jaci.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 101.Walters P, Schofield P, Howard L, Ashworth M, Tylee A. The relationship between asthma and depression in primary care patients: a historical cohort and nested case control study. PLoS One. 2011;6:e20750. doi: 10.1371/journal.pone.0020750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sweeney J, Patterson CC, Menzies-Gow A, Niven RM, Mansur AH, Bucknall C, et al. British Thoracic Society Difficult Asthma Network. Comorbidity in severe asthma requiring systemic corticosteroid therapy: cross-sectional data from the Optimum Patient Care Research Database and the British Thoracic Difficult Asthma Registry. Thorax. 2016;71:339–346. doi: 10.1136/thoraxjnl-2015-207630. [DOI] [PubMed] [Google Scholar]
- 103.Dalal AA, Duh MS, Gozalo L, Robitaille M-N, Albers F, Yancey S, et al. Dose–response relationship between long-term systemic corticosteroid use and related complications in patients with severe asthma. J Manag Care Spec Pharm. 2016;22:833–847. doi: 10.18553/jmcp.2016.22.7.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Daugherty J, Lin X, Baxter R, Suruki R, Bradford E. The impact of long-term systemic glucocorticoid use in severe asthma: a UK retrospective cohort analysis. J Asthma. 2018;55:651–658. doi: 10.1080/02770903.2017.1353612. [DOI] [PubMed] [Google Scholar]
- 105.Lefebvre P, Duh MS, Lafeuille M-H, Gozalo L, Desai U, Robitaille M-N, et al. Acute and chronic systemic corticosteroid-related complications in patients with severe asthma. J Allergy Clin Immunol. 2015;136:1488–1495. doi: 10.1016/j.jaci.2015.07.046. [DOI] [PubMed] [Google Scholar]
- 106.Lefebvre P, Duh MS, Lafeuille M-H, Gozalo L, Desai U, Robitaille M-N, et al. Burden of systemic glucocorticoid-related complications in severe asthma. Curr Med Res Opin. 2017;33:57–65. doi: 10.1080/03007995.2016.1233101. [DOI] [PubMed] [Google Scholar]
- 107.Lin CS, Chang CC, Yeh CC, Chung CL, Chen TL, Liao CC. Postoperative adverse outcomes in patients with asthma: a nationwide population-based cohort study. Medicine (Baltimore) 2016;95:e2548. doi: 10.1097/MD.0000000000002548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sullivan PW, Globe G, Ghushchyan VH, Campbell JD, Bender B, Magid DJ. Exploring asthma control cutoffs and economic outcomes using the Asthma Control Questionnaire. Ann Allergy Asthma Immunol. 2016;117:251–257, e2. doi: 10.1016/j.anai.2016.07.020. [DOI] [PubMed] [Google Scholar]
- 109.Sullivan PW, Ghushchyan VH, Campbell JD, Globe G, Bender B, Magid DJ. Measuring the cost of poor asthma control and exacerbations. J Asthma. 2017;54:24–31. doi: 10.1080/02770903.2016.1194430. [DOI] [PubMed] [Google Scholar]
- 110.Tse SM, Li L, Butler MG, Fung V, Kharbanda EO, Larkin EK, et al. Statin exposure is associated with decreased asthma-related emergency department visits and oral corticosteroid use. Am J Respir Crit Care Med. 2013;188:1076–1082. doi: 10.1164/rccm.201306-1017OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–373. doi: 10.1183/09031936.00202013. [Published erratum appears in Eur Respir J 2014;43:1216 and Eur Respir J 2018;52:1352020.] [DOI] [PubMed] [Google Scholar]
- 112.Suruki RY, Daugherty JB, Boudiaf N, Albers FC. The frequency of asthma exacerbations and healthcare utilization in patients with asthma from the UK and USA. BMC Pulm Med. 2017;17:74. doi: 10.1186/s12890-017-0409-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bousquet J, Mantzouranis E, Cruz AA, Aït-Khaled N, Baena-Cagnani CE, Bleecker ER, et al. Uniform definition of asthma severity, control, and exacerbations: document presented for the World Health Organization Consultation on Severe Asthma. J Allergy Clin Immunol. 2010;126:926–938. doi: 10.1016/j.jaci.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 114.Qiu W, Guo F, Glass K, Yuan GC, Quackenbush J, Zhou X, et al. Differential connectivity of gene regulatory networks distinguishes corticosteroid response in asthma. J Allergy Clin Immunol. 2018;141:1250–1258. doi: 10.1016/j.jaci.2017.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Park HW, Dahlin A, Tse S, Duan QL, Schuemann B, Martinez FD, et al. Genetic predictors associated with improvement of asthma symptoms in response to inhaled corticosteroids. J Allergy Clin Immunol. 2014;133:664–669.e5. doi: 10.1016/j.jaci.2013.12.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Davis JS, Weiss ST, Tantisira KG. Asthma pharmacogenomics: 2015 update. Curr Allergy Asthma Rep. 2015;15:42. doi: 10.1007/s11882-015-0544-y. [DOI] [PubMed] [Google Scholar]
- 117.Dahlin A, Denny J, Roden DM, Brilliant MH, Ingram C, Kitchner TE, et al. CMTR1 is associated with increased asthma exacerbations in patients taking inhaled corticosteroids. Immun Inflamm Dis. 2015;3:350–359. doi: 10.1002/iid3.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tantisira KG, Lasky-Su J, Harada M, Murphy A, Litonjua AA, Himes BE, et al. Genomewide association between GLCCI1 and response to glucocorticoid therapy in asthma. N Engl J Med. 2011;365:1173–1183. doi: 10.1056/NEJMoa0911353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Moore WC, Evans MD, Bleecker ER, Busse WW, Calhoun WJ, Castro M, et al. National Heart, Lung, Blood Institute’s Severe Asthma Research Program. Safety of investigative bronchoscopy in the Severe Asthma Research Program. J Allergy Clin Immunol. 2011;128:328–336. doi: 10.1016/j.jaci.2011.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Voorham J, Xu X, Price D, Golam S, Davis J, Ling Zhi Jie J, et al. Healthcare resource utilization and costs associated with incremental systemic corticosteroid exposure in asthma. Allergy. 2019;74:273–283. doi: 10.1111/all.13556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hawcutt DB, Francis B, Carr DF, Jorgensen AL, Yin P, Wallin N, et al. Susceptibility to corticosteroid-induced adrenal suppression: a genome-wide association study. Lancet Respir Med. 2018;6:442–450. doi: 10.1016/S2213-2600(18)30058-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Havemann BD, Henderson CA, El-Serag HB. The association between gastro-oesophageal reflux disease and asthma: a systematic review. Gut. 2007;56:1654–1664. doi: 10.1136/gut.2007.122465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rice JB, White AG, Scarpati LM, Wan G, Nelson WW. Long-term systemic corticosteroid exposure: a systematic literature review. Clin Ther. 2017;39:2216–2229. doi: 10.1016/j.clinthera.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 124.Sarnes E, Crofford L, Watson M, Dennis G, Kan H, Bass D. Incidence and US costs of corticosteroid-associated adverse events: a systematic literature review. Clin Ther. 2011;33:1413–1432. doi: 10.1016/j.clinthera.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 125.Bottero P, Motta F, Bonini M, Vecchio F, Ierna F, Cuppari I, et al. Can HLA-DRB4 help to identify asthmatic patients at risk of Churg-Strauss Syndrome? ISRN Rheumatol 2014. 843804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hawcutt DB, Jorgensen AL, Wallin N, Thompson B, Peak M, Lacy D, et al. Adrenal responses to a low-dose short synacthen test in children with asthma. Clin Endocrinol (Oxf) 2015;82:648–656. doi: 10.1111/cen.12655. [Published erratum appears in Clin Endocrinol (Oxf) 2016;84:793.] [DOI] [PubMed] [Google Scholar]
- 127.Rijssenbeek-Nouwens LH, Fieten KB, Bron AO, Hashimoto S, Bel EH, Weersink EJ. High-altitude treatment in atopic and nonatopic patients with severe asthma. Eur Respir J. 2012;40:1374–1380. doi: 10.1183/09031936.00195211. [DOI] [PubMed] [Google Scholar]
- 128.Schleich F, Brusselle G, Louis R, Vandenplas O, Michils A, Pilette C, et al. Heterogeneity of phenotypes in severe asthmatics: the Belgian Severe Asthma Registry (BSAR) Respir Med. 2014;108:1723–1732. doi: 10.1016/j.rmed.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 129.Tay TR, Wong HS, Ihsan R, Toh HP, Choo X, Tee AK. Comparison of the proportion and healthcare utilisation of adult patients with uncontrolled severe asthma versus non-severe asthma seen in a Southeast Asian hospital-based respiratory specialist clinic. Ann Acad Med Singapore. 2017;46:217–228. [PubMed] [Google Scholar]
- 130.Afshar M, Wu D, Durazo-Arvizu R, Aguilar FG, Kalhan R, Davis SM, et al. Association of serum lipids and obstructive lung disease in Hispanic/Latino adults of diverse backgrounds. J Pulm Respir Med. 2017;7:419. doi: 10.4172/2161-105X.1000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Björnsdóttir US, Sigurðardóttir ST, Jonsson JS, Jonsson M, Telg G, Thuresson M, et al. Impact of changes to reimbursement of fixed combinations of inhaled corticosteroids and long-acting β2-agonists in obstructive lung diseases: a population-based, observational study. Int J Clin Pract. 2014;68:812–819. doi: 10.1111/ijcp.12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Butler MG, Zhou EH, Zhang F, Wu YT, Wu AC, Levenson MS, et al. Changing patterns of asthma medication use related to US Food and Drug Administration long-acting β2-agonist regulation from 2005-2011. J Allergy Clin Immunol. 2016;137:710–717. doi: 10.1016/j.jaci.2015.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Choi JY, Yoon HK, Lee JH, Yoo KH, Kim BY, Bae HW, et al. Current status of asthma care in South Korea: nationwide the health insurance review and assessment service database. J Thorac Dis. 2017;9:3208–3214. doi: 10.21037/jtd.2017.08.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cooper V, Metcalf L, Versnel J, Upton J, Walker S, Horne R. Patient-reported side effects, concerns and adherence to corticosteroid treatment for asthma, and comparison with physician estimates of side-effect prevalence: a UK-wide, cross-sectional study. NPJ Prim Care Respir Med. 2015;25:15026. doi: 10.1038/npjpcrm.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Delate T, Rader N, Jenkins DW, Lowe R. Multidisciplinary intervention to improve albuterol inhaler utilization among patients with asthma. J Asthma. 2017;54:105–110. doi: 10.3109/02770903.2015.1127934. [DOI] [PubMed] [Google Scholar]
- 136.Laforest L, Licaj I, Devouassoux G, Eriksson I, Caillet P, Chatte G, et al. Prescribed therapy for asthma: therapeutic ratios and outcomes. BMC Fam Pract. 2015;16:49. doi: 10.1186/s12875-015-0265-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee T, Kim J, Kim S, Kim K, Park Y, Kim Y, et al. COREA Study Group. Risk factors for asthma-related healthcare use: longitudinal analysis using the NHI claims database in a Korean asthma cohort. PLoS One. 2014;9:e112844. doi: 10.1371/journal.pone.0112844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Windt R, Glaeske G. Effects of a German asthma disease management program using sickness fund claims data. J Asthma. 2010;47:674–679. doi: 10.3109/02770900903556421. [DOI] [PubMed] [Google Scholar]
- 139.Hagiwara M, Delea TE, Stanford RH, Stempel DA. Stepping down to fluticasone propionate or a lower dose of fluticasone propionate/salmeterol combination in asthma patients recently initiating combination therapy. Allergy Asthma Proc. 2010;31:203–210. doi: 10.2500/aap.2010.31.3359. [DOI] [PubMed] [Google Scholar]
- 140.Hagiwara M, Delea TE, Stanford RH. Risk of asthma exacerbation, asthma-related health care utilization and costs, and adherence to controller therapy in patients with asthma receiving fluticasone propionate/salmeterol inhalation powder 100 μg/50 μg versus mometasone furoate inhalation powder. J Asthma. 2013;50:287–295. doi: 10.3109/02770903.2012.754028. [DOI] [PubMed] [Google Scholar]
- 141.Hagiwara M, Delea TE, Stanford RH. Health-care utilization and costs with fluticasone propionate and fluticasone propionate/salmeterol in asthma patients at risk for exacerbations. Allergy Asthma Proc. 2014;35:54–62. doi: 10.2500/aap.2014.35.3720. [DOI] [PubMed] [Google Scholar]
- 142.DiSantostefano RL, Davis KJ. Prescription patterns in asthma patients initiating salmeterol in UK general practice: a retrospective cohort study using the General Practice Research Database (GPRD) Drug Saf. 2011;34:511–520. doi: 10.2165/11587370-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 143.Sposato B, Scalese M, Latorre M, Novelli F, Scichilone N, Milanese M, et al. Xolair Italian Study Group. Can the response to omalizumab be influenced by treatment duration? A real-life study. Pulm Pharmacol Ther. 2017;44:38–45. doi: 10.1016/j.pupt.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 144.Sweeney J, Patterson CC, O’Neill S, O’Neill C, Plant G, Lynch V, et al. Inappropriate prescribing of combination inhalers in Northern Ireland: retrospective cross-sectional cohort study of prescribing practice in primary care. Prim Care Respir J. 2014;23:74–78. doi: 10.4104/pcrj.2014.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Turner S, Richardson K, Murray C, Thomas M, Hillyer EV, Burden A, et al. Respiratory Effectiveness Group. Long-acting β-agonist in combination or separate inhaler as step-up therapy for children with uncontrolled asthma receiving inhaled corticosteroids. J Allergy Clin Immunol Pract. 2017;5:99–106, e3. doi: 10.1016/j.jaip.2016.06.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.