To the Editor:
17β-estradiol (E2), the most abundant female sex steroid, has been implicated in the development and progression of pulmonary arterial hypertension (PAH) (1, 2). Although some studies have found that E2 drives PAH progression, others have found a protective effect (3–6). The majority of these studies used right ventricular (RV) end systolic or mean pulmonary artery (PA) pressure as the primary metric of pulmonary vascular function and RV afterload. However, these endpoints are affected by RV function and do not fully capture RV afterload (7, 8). To accurately determine the impact of E2 in PAH, a comprehensive assessment of pulmonary vascular function, including multipoint pressure–flow relationships and impedance to flow at physiological frequencies, is necessary (8, 9). To date, no studies have quantified mechanical pulmonary vascular function in PAH using such an approach. A better understanding of the effects of E2 on mechanical pulmonary vascular function would help explain why prior studies yielded seemingly discrepant results and may help solve the “estrogen puzzle” of PAH. We investigated the impact of endogenous and exogenous E2 on pulmonary vascular mechanics in a rat model of PAH.
Healthy female Sprague-Dawley rats with cyclical endogenous E2 production (labeled Intact), ovariectomized rats replete with exogenous E2 in a continuous manner (75 μg ⋅ kg−1 ⋅ day−1 via subcutaneous pellets [labeled OVX+E2]), or ovariectomized rats treated with vehicle (OVX+Veh) as previously described (3) were exposed to Sugen and hypoxia (SuHx) to generate experimental PH. Rats exposed to room air served as controls. Given the female predominance of PAH, and to study the effects of endogenous E2, we used only female rats. Seven weeks after SuHx exposure, RV systolic pressure (RVSP) was measured via closed-chest right-heart catheterization (3). This protocol was designed to generate a chronic, progressive, and severe model of PAH and has been well characterized. E2 repletion was initiated before SuHx exposure as a model of PAH prevention. Pulmonary vascular mechanics were subsequently assessed ex vivo via isolated lung perfusion with both pulsatile and steady flow at baseline and after treatment with the rho kinase inhibitor Y27632 (Y27, 10−5 M). Y27 was used to eliminate persistent vasoconstriction. The main PA and left atria were cannulated (10), and perfusion was carried out via a protocol adapted from one we previously developed for mice (11). Small variations in the steady flow rate were due to the flow pump’s sensitivity to downstream impedance, which varied with individual animals but did not affect the distensibility calculations because the flow ranges achieved were equivalent.
To analyze PA remodeling, Verhoeff-Van Giesson immunohistochemical staining was performed on paraffin-embedded sections from lungs fixed with agarose-formalin (to a pressure of 23 cm H2O) (12). Lungs fixed in formalin did not undergo ex vivo perfusion testing and were not treated with Y27. We determined the PA wall area by calculating the ratio between the area defined by the internal lumen border and the external elastic layer (as identified by Verhoeff-Van Giesson staining), and then expressing this area as a percentage of the entire vessel area (determined by external elastic layer) in arteries <200 μm in diameter (identified by proximity to terminal bronchioles or alveolar ducts) (12). We used nine animals per experimental group. One animal in the Intact SuHx group died before the terminal time point with symptoms of RV failure. Additional losses in data occurred owing to technical factors in the in vivo and ex vivo procedures. All data are presented as mean ± SD. Two-way ANOVA was used to compare differences between groups, and repeated-measures ANOVA was used to evaluate the effect of Y27 on pulmonary vascular mechanics. Although the sample sizes for individual groups were small for some endpoints, the combined sample sizes were sufficient for ANOVA to evaluate model assumptions. Residual plots and normal probability plots were examined, and there was no evidence of model assumption violations.
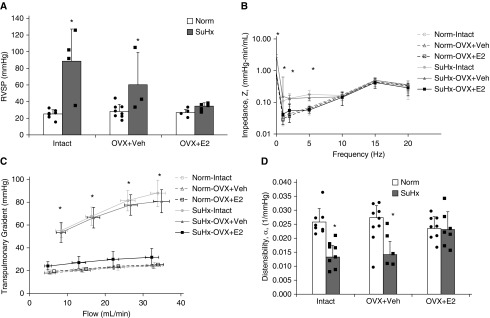
RVSP measured in vivo increased after SuHx exposure in the Intact and OVX+Veh groups. In contrast, in vivo pressures were similar to those obtained under normoxia (Norm) after SuHx in the OVX+E2 group (Figure 1A). We next performed an ex vivo assessment of the opposition to pulsatile flow across a range of oscillatory frequencies, from 0 Hz (steady flow) well into the physiological range of 15–20 Hz (Figure 1B). We noted a significant increase in impedance at 0 Hz (Z0; measuring distal PA narrowing) and up to 10 Hz (reflecting intermediate PA narrowing and stiffening) after SuHx exposure in the Intact and OVX+Veh groups (Figure 1B). Interestingly, this was not seen in the SuHx-OVX+E2 group (Figure 1B). Increased impedance after SuHx exposure was not seen at higher frequencies in any group. Consistent with this, there was no difference in characteristic impedance (reflecting proximal PA stiffness, calculated as the average impedance from 5 Hz to the highest frequency imposed [20 Hz]) after SuHx exposure.
Figure 1.
Exogenous estrogen treatment is protective for pulmonary arterial mechanics after Sugen/hypoxia (SuHx) exposure. (A) Right ventricular systolic pressure (RVSP) was measured via closed-chest right-heart catheterization in intact females (Intact) and ovariectomized females with continuous 17β-estradiol (E2) repletion (OVX+E2) or placebo (OVX+Veh) before either normoxia (Norm) or SuHx exposure; n = 3–8 per group. (B) The pulmonary vascular impedance magnitude (Z) was measured (logarithmic scale on the y-axis) for varying pulsatile flow frequencies in Intact, OVX+E2, and OVX+Veh isolated rat lungs after either Norm or SuHx exposure; n = 4–9 per group. (C) The transpulmonary gradient was measured for varying flow rates in Intact, OVX+E2, and OVX+Veh isolated rat lungs after either Norm or SuHx exposure; n = 4–9 per group. (D) Distal pulmonary artery distensibility in Intact, OVX+E2, and OVX+Veh isolated rat lungs after Norm or SuHx exposure; n = 4–9 per group. *P < 0.05 versus Norm and SuHx-OVX+E2.
A steady-flow evaluation of pulmonary vascular mechanics ex vivo in isolated perfused lungs showed an increased transpulmonary gradient (TPG; determined as the main PA pressure minus the left atria pressure) after SuHx in Intact and OVX+Veh rats, whereas E2 repletion prevented this increase (Figure 1C). Consistent with these findings, distal PA distensibility determined from multipoint pressure–flow curves (13) decreased nearly 50% after SuHx exposure in both the Intact and OVX+Veh groups, whereas the OVX+E2 group exhibited preserved distensibility (Figure 1D).
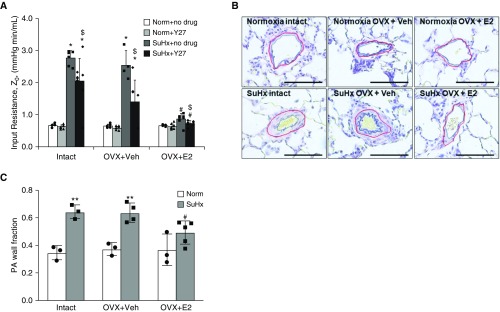
To determine the contribution of vasoconstriction to the increased resistance, increased impedance, and decreased distensibility noted in the SuHx-Intact and OVX+Veh groups, we repeated the evaluation of pulmonary vascular mechanics after treatment with Y27. As expected, both groups demonstrated a decrease in Z0 (Figure 2A), decrease in TPG (data not shown), and increase in distensibility (data not shown) in response to Y27. These findings demonstrate that vasoconstriction is a significant component of increased RV afterload after SuHx in the absence of continuous, exogenous E2 repletion.
Figure 2.
Exogenous estrogen prevents vasoconstriction and pulmonary artery (PA) remodeling after Sugen/hypoxia (SuHx) exposure. (A) Pulsatile perfusion of isolated rat lungs in intact females (Intact) and ovariectomized females with continuous 17β-estradiol (E2) repletion (OVX+E2) or placebo (OVX+Veh) before either normoxia (Norm) or SuHx exposure demonstrates increased impedance magnitude (Z) at 0 Hz (input resistance, Z0) after SuHx exposure in the Intact and Veh groups, but not in the E2 groups. Treatment with the rho kinase inhibitor Y27 induced a stronger response in the SuHx-OVX+Veh group than in the Intact or OVX+E2 group; n = 4–9 per group, *P < 0.05 versus Norm, #P < 0.05 versus SuHx-Intact and SuHx-OVX+Veh, and $P < 0.05 versus no Y27. (B and C) PA remodeling was assessed by Verhoeff-Van Giesson staining and subsequent determination of the PA wall fraction in Intact, OVX+E2, and OVX+Veh rat lungs after either Norm or SuHx exposure. The PA wall fraction was determined by dividing the PA wall area (area between the blue line and the red line) by the total vessel area (total area outlined by the red line). The red line denotes external elastic lamina, and the blue line denotes the internal lumen border. Note the decreased PA wall fraction in SuHx-OVX+E2 versus SuHx-Intact or SuHx-OVX+Veh rat lungs. Scale bars, 100 μm. (B) Representative images. (C) Quantification of the PA wall fraction; n = 3–5 rats per group; 20 vessels per rat were analyzed. **P < 0.01 versus Norm and #P < 0.05 versus SuHx-Intact and SuHx-OVX+Veh.
To identify a structural correlate of the improvements in RVSP, Z0, TPG, and distensibility noted with E2 repletion in SuHx rats, we analyzed PA remodeling (Figures 2B and 2C). We found that E2 repletion was associated with a 60% reduction in the PA wall area as compared with the Intact and OVX+Veh groups.
This study is the first to comprehensively demonstrate that continuous, exogenous E2 treatment confers protection to pulmonary vascular mechanics in rats with angioproliferative PAH. In particular, this is the first investigation of pulsatile pulmonary vascular mechanics in SuHx-PH rats. We demonstrated distal PA narrowing with relative preservation of proximal PA mechanics. Although exogenous E2 has previously been shown to reduce RVSP (14), we expand our prior observations beyond RVSP and now demonstrate the novel finding that E2 also attenuates PH-induced alterations in impedance and distensibility. This is important because RVSP can be confounded by other factors, and because impedance and distensibility are critical determinants of RV adaptation in PAH. Impedance measurements and the use of an ex vivo lung perfusion preparation allowed us to dissect, for the first time, how endogenous and exogenous E2 affects various pulmonary vascular compartments. Importantly, continuous exogenous E2 was superior to both physiologically cyclical endogenous estrogen (intact females) and little to no estrogen (ovariectomized females) in limiting PH development. Although several recent studies from our group and others demonstrated a protective effect of E2 on RV function (1, 3, 4), we now identify a novel and direct protective effect of E2 on distal PA structure and function. In particular, exogenous, continuous E2 repletion attenuated the distal PA remodeling, increase in Z0, increase in TPG, and decrease in distensibility induced by SuHx in intact and OVX female rats. A prior study performed in female mice found that E2 attenuated proximal, but not distal, PA remodeling (15), which highlights potentially important differences between the two species. The previously demonstrated beneficial effects of E2 on RV function and the currently demonstrated protective effects of E2 on distal PA structure and function suggest that exogenous E2 repletion could be a potent tool to combat PAH-induced changes in several compartments of the cardiopulmonary system. In future studies, we will focus on the use of rescue protocols and male rats. Our findings highlight a key role for E2 in attenuating PA remodeling and dysfunction in PAH and identify the distal PA as the target of E2’s vasculoprotective effects in SuHx-PH rats. This provides a rationale and basis for further studies to understand the complex mechanisms by which E2 regulates pulmonary vascular mechanics.
Supplementary Material
Footnotes
Supported in part by a Thoracic Surgery Foundation Nina Starr Braunwald Research Fellowship (J.L.P.); NIH grants R01-HL086939 (N.C.C.), R56-HL134736 (T.L. and N.C.C.), and 1R01HL144727 (T.L.); American Heart Association grant 17POST33670365 (A.L.F); and VA Merit grant 1I01BX002042 (T.L.).
Originally Published in Press as DOI: 10.1164/rccm.201906-1217LE on October 29, 2019
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Lahm T, Kawut SM. Inhibiting oestrogen signalling in pulmonary arterial hypertension: sex, drugs and research. Eur Respir J. 2017;50:1700983. doi: 10.1183/13993003.00983-2017. [DOI] [PubMed] [Google Scholar]
- 2. Foderaro A, Ventetuolo CE. Pulmonary arterial hypertension and the sex hormone paradox. Curr Hypertens Rep. 2016;18:84. doi: 10.1007/s11906-016-0689-7. [DOI] [PubMed] [Google Scholar]
- 3. Frump AL, Goss KN, Vayl A, Albrecht M, Fisher A, Tursunova R, et al. Estradiol improves right ventricular function in rats with severe angioproliferative pulmonary hypertension: effects of endogenous and exogenous sex hormones. Am J Physiol Lung Cell Mol Physiol. 2015;308:L873–L890. doi: 10.1152/ajplung.00006.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu A, Schreier D, Tian L, Eickhoff JC, Wang Z, Hacker TA, et al. Direct and indirect protection of right ventricular function by estrogen in an experimental model of pulmonary arterial hypertension. Am J Physiol Heart Circ Physiol. 2014;307:H273–H283. doi: 10.1152/ajpheart.00758.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mair KM, Wright AF, Duggan N, Rowlands DJ, Hussey MJ, Roberts S, et al. Sex-dependent influence of endogenous estrogen in pulmonary hypertension. Am J Respir Crit Care Med. 2014;190:456–467. doi: 10.1164/rccm.201403-0483OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen X, Talati M, Fessel JP, Hemnes AR, Gladson S, French J, et al. Estrogen metabolite 16α-hydroxyestrone exacerbates bone morphogenetic protein receptor type II-associated pulmonary arterial hypertension through microRNA-29-mediated modulation of cellular metabolism. Circulation. 2016;133:82–97. doi: 10.1161/CIRCULATIONAHA.115.016133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bellofiore A, Chesler NC. Methods for measuring right ventricular function and hemodynamic coupling with the pulmonary vasculature. Ann Biomed Eng. 2013;41:1384–1398. doi: 10.1007/s10439-013-0752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chesler NC, Argiento P, Vanderpool R, D’Alto M, Naeije R. How to measure peripheral pulmonary vascular mechanics. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:173–176. doi: 10.1109/IEMBS.2009.5333299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Naeije R. Pulmonary vascular resistance: a meaningless variable? Intensive Care Med. 2003;29:526–529. doi: 10.1007/s00134-003-1693-3. [DOI] [PubMed] [Google Scholar]
- 10. Uhlig S, Wollin L. An improved setup for the isolated perfused rat lung. J Pharmacol Toxicol Methods. 1994;31:85–94. doi: 10.1016/1056-8719(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 11.Vanderpool RR, Chesler NC.Characterization of the isolated, ventilated, and instrumented mouse lung perfused with pulsatile flow J Vis Exp 2011. pii:2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goss KN, Cucci AR, Fisher AJ, Albrecht M, Frump A, Tursunova R, et al. Neonatal hyperoxic lung injury favorably alters adult right ventricular remodeling response to chronic hypoxia exposure. Am J Physiol Lung Cell Mol Physiol. 2015;308:L797–L806. doi: 10.1152/ajplung.00276.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Linehan JH, Haworth ST, Nelin LD, Krenz GS, Dawson CA. A simple distensible vessel model for interpreting pulmonary vascular pressure-flow curves. J Appl Physiol (1985) 1992;73:987–994. doi: 10.1152/jappl.1992.73.3.987. [DOI] [PubMed] [Google Scholar]
- 14. Hunter KS, Lee PF, Lanning CJ, Ivy DD, Kirby KS, Claussen LR, et al. Pulmonary vascular input impedance is a combined measure of pulmonary vascular resistance and stiffness and predicts clinical outcomes better than pulmonary vascular resistance alone in pediatric patients with pulmonary hypertension. Am Heart J. 2008;155:166–174. doi: 10.1016/j.ahj.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu A, Hacker T, Eickhoff JC, Chesler NC. Estrogen preserves pulsatile pulmonary arterial hemodynamics in pulmonary arterial hypertension. Ann Biomed Eng. 2017;45:632–643. doi: 10.1007/s10439-016-1716-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.