Abstract
Biosurfactants are structurally a diverse group of surface‐active molecules widely used for various purposes in industry. In this study, among 120 fungal isolates, M‐06 was selected as a superior biosurfactant producer, based on different standard methods, and was identified as Mucor circinelloides on the basis of its nucleotide sequence of the internal transcribed spacer (ITS) gene. M. circinelloides reduced the surface tension to 26 mN/m and its EI24 index was determined to be 66.6%. The produced biosurfactant exhibited a high degree of stability at a high temperature (121°C), salinity (40 g/L), and acidic pH (2–8). The fermentation broth's ability to recover oil from contaminated sand was 2 and 1.8 times higher than those of water and Tween 80, respectively. The ability of biosurfactant to emulsify crude oil in the sea and fresh water was 64.9 and 48% respectively. This strain could remove 87.6% of crude oil in the Minimal Salt Medium (MSM) crude oil as the sole carbon source. The results from a primary chemical characterization of crude biosurfactant suggest that it is of a glycolipid nature. The strain and its biosurfactant could be used as a potent candidate in bioremediation of oil‐contaminated water, soil, and for oil recovery processes.
Keywords: Bioremediation, Biosurfactant, Crude oil, Emulsification activity, Mucor circinelloides
Abbreviations
- E24
Emulsification Index (after 24 h)
- EI1
Emulsification Index (after 1 h)
- ITS
Internal Transcribed Spacer
- MEOR
Microbial Enhanced Oil Recovery
- TPH
Total Petroleum Hydrocarbon
1. Introduction
Surfactants are an important and diverse group of chemical compounds that have various applications in different fields such as cosmetics, food, biomedical, bio‐pesticide, household cleaning products, agriculture, and petrochemical industries. All of these applications are related to the amphiphilic nature of these compounds. Nowadays, most of the surfactants used are petrochemical‐based and could cause environmental problems because of their low biodegradability 1.
Biosurfactants are surface‐active agents of microbial origin. Many bacteria and some fungi have been reported to be biosurfactant producers. These compounds have some physiological roles in the production of microorganisms such as increasing the availability of hydrophobic substrates and performing antibiotic and anti‐adhesive activities 2. Biosurfactants offer several advantages over their synthetic counterparts such as biodegradability, low toxicity, ecological acceptance, probable activity at extreme temperatures, pH, and salinity, as well as their capacity to be modified by genetic engineering and the ability to be produced from renewable wastes and byproducts. Owing to these advantages of biosurfactants, they have the potential to replace chemical surfactants in various industries 3. So, it seems necessary to find a potent biosurfactant along with a more economical production process to justify the replacement of synthetic surfactants by biological molecules. The best known biosurfactant‐producing bacterial strains mainly belong to the genera Pseudomonas, Bacillus, Acinetobacter and Artherobacter. Fungi have a high yield of biosurfactants and can survive, grow, and reproduce in harsh environmental conditions compared to bacteria and it makes them more suitable than bacteria for bioremediation in extreme environments 4. Despite the high potential, biosurfactant production of fungi has been studied less compared to bacteria. Among fungi, Candida sp., Aspergillus ustus, Ustilago maydis and Fusarium sp. are the explored ones 5, 6.
Many biosurfactants and their production processes have been patented, but currently only a few of these compounds have been commercialized, of which the most important one is rhamnolipid 7. The greatest potential use of biosurfactants is in the oil industry. These compounds are considered key factors in the Microbial Enhanced Oil Recovery (MEOR) process, and bioremediation of oil contaminations due to their ability to emulsify oil in water and their high stability at extreme conditions 8. The application of biosurfactants in the remediation of organic compounds, such as hydrocarbons, aims at increasing their bioavailability or mobilizing and removing the contaminants by pseudosolubilization and emulsification in a washing treatment 1. It was generally observed that the degradation time and the adaptation time for microbes, in particular, was shortened by the use of biosurfactants. Several contaminated sites in the Middle East were remediated by the addition of biosurfactant to the contaminated environment in addition to other nutrients. These sites represented soil and sand contaminated by heavy hydrocarbons, primarily of industrial origin. Kosaric (2001) demonstrated that bioremediation accelerated when glycolipid biosurfactants were added (0.5 kg/ton of soil) to the nutrient applied to the soil 9.
In this study, we introduced a potent biosurfactant‐producing fungal strain and the remediation of oil contamination in water and soil was investigated. The surface tension activity of the biosurfactant was compared with common chemical surfactants including Tween80 and Triton X‐100 and the physiochemical characteristics of the biosurfactant were determined and compared with those of chemical surfactants.
2. Materials and methods
2.1. Sample collection and isolation of fungal strains
In total, 18 soil samples were collected from different oil‐contaminated areas of Iran. The isolation of fungi from the soil samples was carried out using the oil enrichment method as follows: Experiments were performed in 250 mL Erlenmeyer flasks containing 50 mL of the Minimal Salt Medium (MSM) with 1% w/v crude oil as sole carbon source 10. The flasks were inoculated with 0.5 g of each soil sample and cultivated at 28°C and 150 rpm for 21 days. After the incubation period, 0.1 mL of each culture was placed on MSM plates containing 1% (w/v) crude oil and 0.01 g/L chloramphenicol to inhibit bacterial growth. The inoculated plates were incubated at 28°C for 14 days; later, fungal colonies grown on the plates were purified and preserved at 4°C 10.
2.2. Screening of fungal strains with surface tension reduction activity
The composition of the screening medium was as follows: 3 g/L NaNO3, 0.25 g/L KH2PO4, 0.25 g/L MgSO4(7H2O), 1 g/L yeast extract and 5% waste frying oil as the main carbon source 11. The pH was adjusted to 7 with 1M NaOH and the media were sterilized at 121°C for 20 min. An equal amount of inoculum (5 × 105 spores) of each fungal isolate, grown in PDA for 5 days, was inoculated in 250 mL flasks containing 50 mL of the sterile medium. The flasks were incubated at 28°C and 150 rpm for 5 days. After the incubation period the fermentation broth was centrifuged at 4000 × g for 20 min, the supernatant was collected and passed through the Whatman filter paper NO.1. The remaining oil was separated from the cell‐free supernatant using centrifugation and decanter. The presence of biosurfactant in the cell‐free supernatant of each isolate was determined using the oil‐spreading and parafilm tests as the primary screening tests. The positive strains of oil‐spreading and the parafilm test isolates were also evaluated for their ability to reduce surface tension using the Du Nouy ring method and emulsifying activity.
2.3. Oil‐spreading and parafilm assays
For an oil‐spreading assay, 50 mL of distilled water was added to a petri dish of 15‐cm diameter. Twenty microliters of crude oil were added to the water surface. Subsequently, 10 μL of the fermentation broth was added to the oil surface. The diameter of the visually detectable clear zone on the oil surface was measured after 30 seconds. Each experiment was done with three different samples. Distilled water and an un‐inoculated medium were used as controls 12. For the parafilm test, the fungal cell‐free supernatant (25 μL) was added to the hydrophobic surface of parafilm. The shape of the drop on the surface was inspected after 1 min. The spreading of drops on the hydrophobic surface was considered an indicator of the presence of the biosurfactant. Distilled water and an un‐inoculated medium were used as controls 13.
2.4. Du Nouy ring method
The surface tension was measured using a Kruss K7 tensiometer (Atension 700 Germany). The sample temperature was adjusted to 25°C. A 20‐mL volume of each sample was poured into the sample container of the tensiometer and the surface tension was measured in triplicate for each sample in three different biological runs to increase the accuracy of the measurements. Distilled water and an un‐inoculated medium were used as controls 12.
2.4. Measurement of emulsification activity
The ability of the biosurfactant to emulsify diesel as a hydrophobic substrate was studied using the emulsification activity tests. An aliquot of 5 mL hydrocarbon was added to 5 mL of cell‐free culture supernatant in a glass test tube and vortexed at high speed (2300 rpm) for 2 min. The emulsion index was investigated after one (EI1) and 24 h (EI24). The EI1 (emulsification index after 1 h) and E24 (emulsification index after 24 h) indexes for each isolate were calculated by dividing the measured height of the emulsion layer by the total height of the mixture and multiplying by 100. In this experiment the EI24 index is also an indicator of emulsion stability after 24 h 14.
2.5. Fungal strain identification
The superior biosurfactant‐producing isolate was identified using the molecular ITS sequencing methodology. For this purpose, the selected isolate was incubated in 50 mL of the PDB (potato dextrose broth) for 48 h at 28°C and 150 rpm. The fungal mycelia were collected by centrifugation (4000 × g for 5 min) and then washed twice with normal saline of equal sterility. The 200 mg of mycelium was frozen with liquid nitrogen and grounded by a mortar and pestle. The DNA was isolated using phenol‐chloroform extraction and ethanol precipitation. The extracted DNA was amplified using ITS1 and ITS4 primers 15. The amplification was verified by continuous agarose gel electrophoresis (1%) stained with a green viewer and visualized under UV light. PCR products were then sequenced after the purification (Macrogene, South Korea), and sequence similarities were obtained using the blast tool from the National Center for Biotechnology Information (NCBI).
2.6. Time course of biosurfactant production and fungal growth
To evaluate the biosurfactant production time course for the superior strain, fungal spore (5 × 105) were inoculated in 250 mL flasks containing 50 mL of the biosurfactant production medium. The flasks were incubated at 28°C and 180 rpm. Biosurfactant production was measured at regular intervals (24 h) over 12 days using the oil‐spreading method. The composition of the biosurfactant production medium was as mentioned above 11. The weight of the produced dry biomass was also measured at regular intervals (24 h) over 12 days to evaluate the growth of the strain. The measurements were plotted against time to obtain the growth and biosurfactant production curves. All experiments were done in triplicate.
2.7. Evaluation of crude oil removal efficiency
The ability of the M‐06 isolate to degrade crude oil was evaluated using the total petroleum hydrocarbon assay (TPH) 16. For this purpose, fungal spores (5 × 106) were inoculated into the minimal salt medium (MSM) supplemented with 1% (w/v) crude oil as a sole carbon source. The un‐inoculated medium was considered as the control to confirm the amount of oil removed. The inoculated flasks and the control were incubated at 28°C and 180 rpm for 12 days. Every day, the fermentation broth was evaluated for the extraction of the total hydrocarbon content with toluene. Subsequently, to determine the amount of the remaining oil, the absorption of the sample was measured at 420 nm 16. All the experiments were done in triplicate.
2.8. Oil recovery assay
The applicability of the biosurfactant produced by the M‐06 isolate in oil recovery was evaluated using artificially contaminated sand containing 12.5% (w/w) of crude oil. Forty grams of sand were mixed with 5 g of the crude oil in 250 mL flasks by shaking, and allowed to age at room temperature for 24 h. Afterward, 40 mL of the fungal culture supernatant were added to the flask. The flasks were incubated at100 rpm and 40°C for 24 h. Then the laundering solution was separated from the sand, and the amount of oil remaining in the sand was later extracted by hexane. The hexane phases were passed through an anhydrous sodium sulfate to remove moisture. After evaporation of the solvent in a rotary evaporator, the amount of residual crude oil was weighed 17. This experiment also was done for high (12% v/v) and low (1.2% v/v) concentration solutions of Tween‐80 and Triton X‐100 as chemical surfactants. All the experiments were performed in three different batches. The control assay was performed using 40 mL of distilled water instead of the surfactant solution in the same condition.
2.9. Bioemulsifying activity
Bioemulsifying activity of the cell‐free supernatant was measured by the spectrophotometric method. Five milliliter of supernatant was taken in a glass tube, then 100 μL of crude oil was added, and the contents were vortexed vigorously for 2 min at full speed and then left undisturbed for 10 min. The bioemulsifying activity of the biosurfactant was measured at 550 nm spectrophotometrically in a glass cuvette against the blank of the un‐inoculated medium (5 mL) with 100 μL of crude oil vortexed, similar to the sample. The same experiments were done for 1.2 (v/v) Tween‐80 and Triton X‐100 solutions separately 18.
2.10. Biosurfactant extraction and partial purification
Using the afore mentioned biosurfactant‐production medium after 5 days of incubation, the fermentation broth obtained was centrifuged (14 000 × g for 20 min) and the cell‐free supernatant was acidified to pH 2 using 6 N HCl. The acidified cell free supernatant was kept overnight at 4°C for complete precipitation of the biosurfactant. The precipitated biosurfactant was then recovered with three extractions containing an equal volume of the organic solvent (ethylacetate‐methanol 5:1, v/v). The organic phase was then dried under vacuum in a rotary evaporator at 45°C. The dried, brownish extract thus obtained was used as the crude biosurfactant. The mass of freeze‐dried extract was measured to determine the yield of crude biosurfactant 12.
2.11. Primary structural characterization
For purification, 5 g of the crude biosurfactant extract was dissolved in chloroform (50 mL) and was separated by TLC using aluminum sheets of silica gel 60 F254 plates (Merck) with various solvent systems. The components were observed under UV light (wavelength 254 and 360 nm) and visualized by staining with ninhydrin, rhodamine B, alkali potassium permanganate, and iodine vapor in the presence of amino acids, lipids, organic compounds/sugar and sugar/lipids, respectively 19.
To investigate the role of peptides in the emulsification activity, the partially purified biosurfactant (1 mg/mL) was incubated with different concentrations of Proteinase K (viz. 0.5, 1, 2, 4 mg) at 37 °C. Samples were withdrawn at different time intervals (i.e. 10, 30, 60, 120 min) and assayed for biosurfactant activity Sekhon et al. 20. In addition, the partially purified biosurfactant was incubated with 100, 200, 500, 700 and 1000 μg of lipase and incubated at 37 °C. Samples were withdrawn at regular intervals (i.e. 30, 60, 90, 120 min) and assayed for biosurfactant activity 20.
2.12. Evaluating the ability of biosurfactant to emulsify crude oil in fresh and sea water
To determine the ability of the biosurfactant to emulsify crude oil in simulated sea water, 2 g of sea salt was added to 50 mL of fermentation broth in a 250 mL‐capacity conical flask, followed by the addition of 5 g crude oil, and incubated while being shaken (180 rpm) at room temperature for 1 h. Individual experiments were also carried out for 1.2 and 12% (v/v) solutions of Tween 80 and Triton X‐100 separately as chemical surfactants. The amount of crude oil removal was assessed by withdrawing the samples after 1 h, extracting the remaining oil on the surface of the liquid with n‐hexane and, then, weighing it after the evaporation of the solvent 21. The same experiment was done using freshwater instead of sea salt water.
2.13. Stability study of biosurfactant
The thermal stability of the biosurfactant was evaluated by heating the fermentation broth at 121°C for 30 min. To determine the stability of the biosurfactant at extreme pH, the pH of the cell‐free fermentation broth was adjusted to 2, 4, 6, 8, 10 and 12 with HCl (5 N) and NaOH (5 N) solutions. The salinity of the fermentation broth was adjusted to 10, 20, 30 and 40 g/L to investigate the effect of salinity on surface activity of the biosurfactant. Afterward the surface activity of these treated samples was measured and compared with the untreated samples (fermentation broth) 22.
For an emulsion stability study, the primary emulsion was obtained from the cell‐free fermentation broth and diesel by vortexing. The thermal stability of the emulsion was evaluated by heating the emulsion at 121°C for 30 min. To determine the effect of high‐speed centrifuging on emulsion stability, it was centrifuged at 12 000 × g for 20 min. The height of the emulsified layer was measured before and after applying heat and centrifuging. The control sample (the tube containing emulsion produced by diesel and the cell‐free culture broth) was not heated and centrifuged 23.
2.14. Emulsifying activity of biosurfactant using different hydrophobic substrates
In order to determine the ability of biosurfactant produced by M. circinelloides to emulsify different hydrophobic substrates, the EI24 index of the cell‐free fermentation broth was measured using kerosene, crude oil, olive oil, engine oil and diesel as the hydrophobic substrates 24. The EI24 test was done for each substrate as described previously.
2.15. Statistical analysis
The results obtained from different tests were analyzed using the SPSS Version 22. The statistical test used in this study was Tukey, One‐way ANOVA. A significance level of results was reported as p ≤ 0.05.
3. Results
3.1. Isolation and screening of biosurfactant‐producing fungal isolates
In total, 120 different fungal isolates were isolated from 18 crude oil‐contaminated soil samples. These fungal isolates were isolated from different soil samples. All of these isolates were screened for biosurfactant production using the oil‐spreading and parafilm tests. The potential of biosurfactant production for 8 selected isolates is represented in Table 1. The M‐6 isolate showed the best primary result and created a clear zone of 12.7 cm in diameter (Table 1). The biosurfactant production of the selected isolates was further investigated using the EI1, EI24 tests and the Du Nouy ring method. The results showed that all the 8 selected strains reduced the surface tension of the culture medium compared to the negative controls. The EI1 and EI24 tests determined the ability of the cell‐free fermentation broth of these isolates to emulsify diesel as a hydrophobic substrate (Table 1). Emulsification indexes (EI1and EI24) calculated for the selected isolates were significantly higher than those of controls. According to the results, M‐6 showed the highest surface tension reduction (26 mN/m) and BB‐02 showed the best emulsification ability (100%).
Table 1.
The results of the oil‐spreading, parafilm, EI24, EI1 tests and the surface tension measurement for 8 selected isolates
| Isolate | Parafilm test | The diameter of the clear zone (cm) | EI1% | EI24% | Surface tension reduction (mN/m) |
|---|---|---|---|---|---|
| SH‐03 | + | 9 | 77 | 53 | 26 |
| KH‐02 | + | 6.5 | 65 | 57 | 43 |
| KR‐05 | + | 11.2 | 49.3 | 14.5 | 38 |
| KR‐06 | + | 6 | 56 | 50 | 41 |
| KR‐01 | + | 6.2 | 46 | 20 | 43.5 |
| BB‐02 | + | 11 | 100 | 100 | 34 |
| KH‐08 | + | 11 | 85.7 | 78 | 34 |
| M‐06 | + | 12.7 | 100 | 66.6 | 26 |
| Control 1 (distilled water) | – | 0 | 0 | 0 | 70 |
| Control 2 | – | 0 | 10 | 0 | 47 |
| (Un‐inoculated medium) |
“+” Indicates distribution of sample drop on the parafilm surface compared to the controls.
Distilled water and un‐inoculated medium was used as negative control in all these tests.
3.2. Molecular identification of the best biosurfactant‐producing isolate
Based on the results of Table 1, the M‐06 isolate was chosen as the best isolate for production of surface‐active compounds in this study. Molecular identification of the M‐06 isolate by PCR of the ITS gene and alignment of the obtained sequence revealed 100% similarity to Mucor circinelloides and recorded in the University of Tehran Microorganisms Culture Collection (UTMC) and GenBank under accession number of UTMC 5032 and KR263057, respectively.
3.3. Kinetic of growth rate, biosurfactant production, and crude oil remediation
Biosurfactant production, crude oil degradation efficiency, and the growth rate of M. circinelloides were evaluated in a time course of 12 days. Biosurfactant production of the strain occurred during the logarithmic growth phase and the maximum production rate (12.7 cm oil‐spreading zone), maximum oil removal percentage (66.13%) and the maximum growth rate (1.45 g/L dry cell weight) were obtained after 3 days of incubation (Fig. 1). In addition, using the biosurfactant‐production medium, the highest yield of crude biosurfactant production (12.3 g/L) was obtained after 3 days incubation which is consistent with the results of the oil spreading method.
Figure 1.
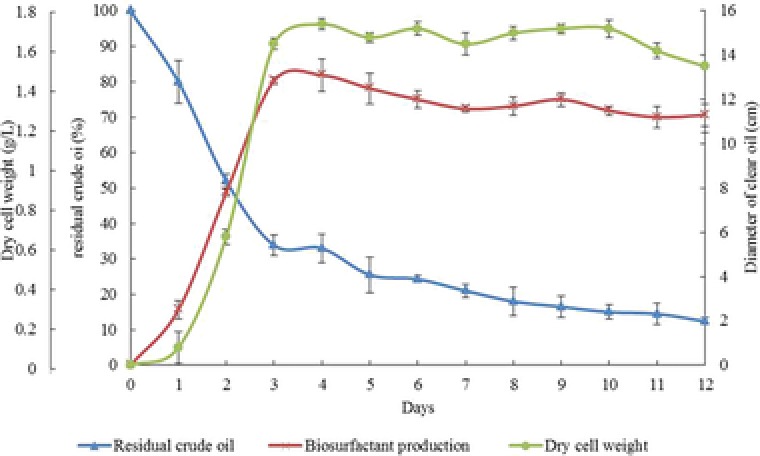
The kinetic of growth rate, biosurfactant production, and the crude oil remediation by M. circinelloides during 12 days.
M. circinelloides could utilize 87.6% of the petroleum compounds in the minimal salt medium in 12 days incubation (Fig. 1). The highest yield of biosurfactant production (12.3 g/L crude biosurfactant) was obtained after 3 days incubation.
3.4. Oil recovery assay
The ability of the biosurfactant produced by M. circinelloides to recover oil from artificially oil‐contaminated soil was evaluated using an oil recovery assay. The results obtained from the test demonstrated the fermentation broth of M. circinelloides was capable removing 84.4% of the crude oil adsorbed in the sand (Fig. 2). The ability of the fermentation broth to recover oil from the sand was two times as high as control (distillated water). We used chemical model surfactants for better comparison. The percentages of oil recovered by 1.2% (v/v) Tween‐80 and Triton X‐100 solutions were determined as 70 and 79.4% respectively. The oil recovery ability of 12% (v/v) Tween 80 and Triton X‐100 solutions was higher than that of the fermentation broth (Fig. 2).
Figure 2.
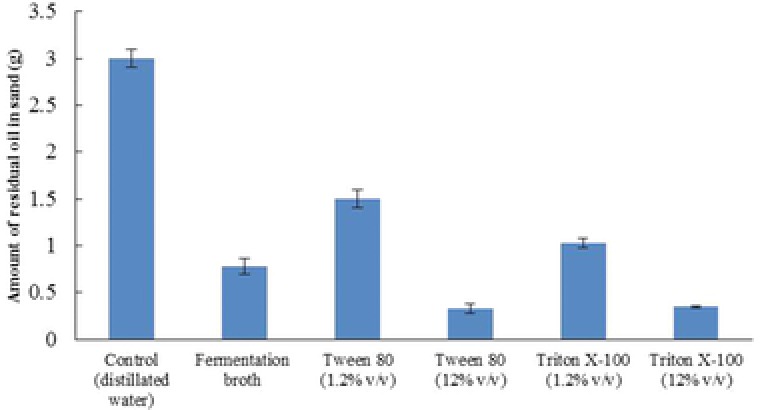
The ability of the biosurfactant produced by M. circinelloides to recover oil from artificially contaminated sand compared to chemical surfactants.
3.5. The ability of the biosurfactant to emulsify oil in fresh and sea water
The ability of the biosurfactant to remove crude oil from simulated sea water and freshwater was evaluated. The results showed that 64.9% of the crude oil was removed from 40 g/L sea salt solution within 60 min of exposure to the fermentation broth of M. circinelloides. The ability of the fermentation broth to remove crude oil from simulated sea water was 2 times less than the 12% (v/v) the Tween‐80 solution, and 2 and 3.1 times higher than the 1.2% (v/v) Tween‐80 solution and control (sea water) respectively (Fig. 3). The obtained results showed the amount of oil removed from sea water was higher compared to freshwater using the fermentation broth. That could be due to the effect of sea salt on biosurfactant activity, which will be dealt with in the stability studies. The evaluation of the emulsification activity showed the OD550 of the fermentation broth, 1.2 (v/v) Triton X‐100, and 1.2 (v/v) Tween 80 solutions were 1.35, 1.35, and 1.05 respectively compared to their controls.
Figure 3.
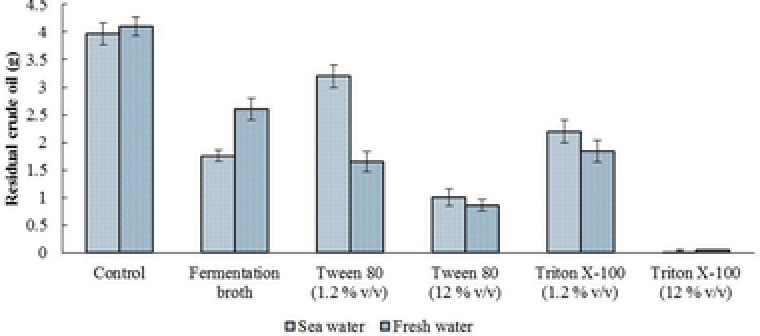
The ability of the produced biosurfactant by M. circinelloides to remove crude oil from fresh and sea water compared to the chemical surfactants. The sea and freshwater were used as controls.
3.6. Recovery of crude biosurfactant produced by M. circinelloides
After isolation and partial purification of the crude biosurfactant produced from the M. circinelloides, a brownish powder was obtained from the extraction. For detection of the partially purified biosurfactants by TLC study, the solvent system used was toluene/acetone (1/1). The TLC plate showed the presence of sugar and lipids when the components were stained with the developing agent, iodine vapor. The proteinase K test was negative, which indicated that the partially purified biosurfactant of the M. circinelloides did not contain peptide moiety. The incubation of biosurfactant with lipase resulted in an appreciable loss in biosurfactant activity (up to 100%). According to these results, the chemical nature of the produced biosurfactant could be a glycolipid type. The results of the emulsification and the surface activity of the two different levels of concentration of crude biosurfactant solutions compared to a chemical surfactant are shown in Fig. 4. The obtained results showed that the 15% (w/v) crude biosurfactant solution had higher surface and emulsification activities compared to 8% (w/v) of Tween 80 solution and the fermentation broth.
Figure 4.
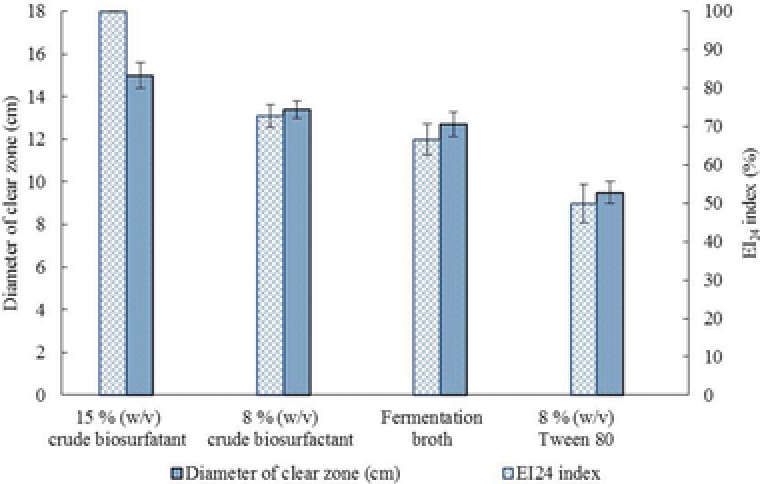
Evaluation of the surface activity and emulsification activity of 15 and 8% crude biosurfactant compared to 8% Tween‐80 and fermentation broth.
3.7. Stability study
The results obtained from the stability tests showed that increasing salinity of the fermentation broth of M. circinelloides up to 30 g/L enhances the surface activity of the biosurfactant (from a 12.5 to 13.5 cm oil‐spreading zone) and it was also stable and active at salinity levels up to 40 g/L NaCl (Fig. 5A). The thermal stability study revealed that the biosurfactant produced by M. circinelloides was a thermostable compound, whose activity was not affected by heating at 121°C for 30 min (Fig. 5B). This compound was also stable at the acidic and neutral pH (pH 2 to 8) but its activity was affected at the alkaline conditions (pH 8 to 12), and pH 12 led to 50% loss in its surface activity (Fig. 5C). The effect of high temperature and high‐speed centrifuging on the stability of the emulsion is shown in Fig. 5D. Heating the emulsion at 121°C for 30 min led to 70.1% destruction in the emulsified layer and the high‐speed centrifuging caused 43% destruction of the emulsified layer.
Figure 5.
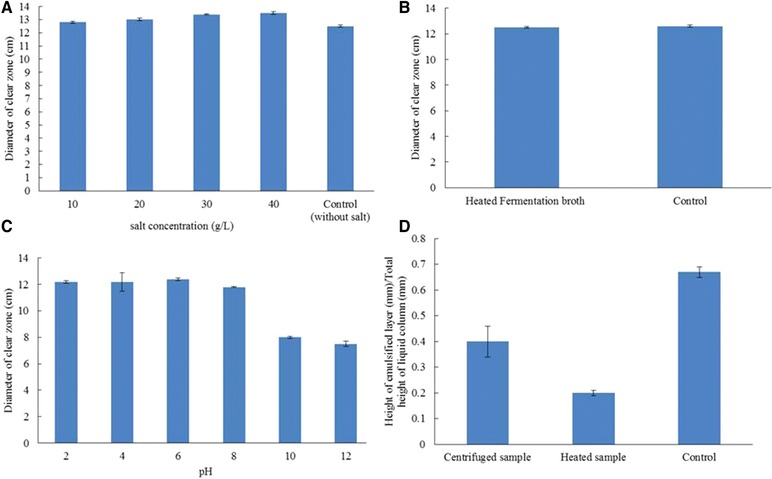
The effect of NaCl concentrations (A), high temperature (B), and pH (C) on stability of the biosurfactant produced by M. circinelloides, and the effect of high temperature and centrifugation on stability of emulsion produced by fermentation broth of the strain (D).
3.8. Evaluation of emulsification activity
The emulsification activity of the biosurfactant produced by M. circinelloides is shown in Fig. 6. According to the results, the fermentation broth of the strain could build stable emulsions with all of these hydrocarbons and the maximum (90%) and minimum (34%) EI24 indexes were obtained for the engine and olive oils, respectively.
Figure 6.
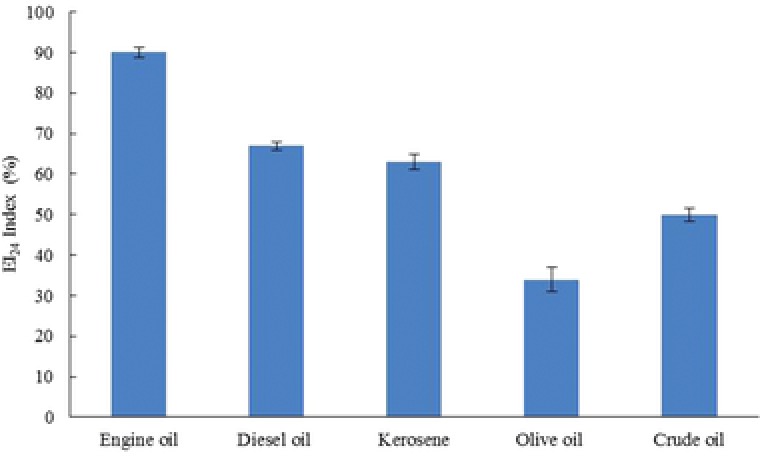
The emulsification activity of the biosurfactant produced by M. circinelloides on different hydrophobic substrates.
4. Discussion
Biosurfactants can fulfill various physiological roles and provide different advantages to their producing strains, and, therefore, biosurfactant‐producing microbes can be found in different environments 2. But it has been shown that the chance of success of the screening of biosurfactant‐producer strains in contaminated soils is more than in uncontaminated soils 25. Rahman et al. 26 obtained 130 oil‐degrading isolates using oil enrichment methods and two of these isolates were found to produce biosurfactants. In this study, 120 oil‐degrading isolates were isolated from oil‐contaminated soils and 8 of these isolates exhibited biosurfactant production activity.
The results obtained from the surface and the emulsification activity of the selected strain showed that M. circinelloides has a high ability to produce biosurfactant and these results are significant compared to the reported values. For example, Andrade Silva et al. 24 reported that Cunninghamella echinulata reduced the surface tension to 36 mN/m and created a clear zone with 4.5 cm diameter in the oil‐spreading test. Furthermore, the EI24 value reported for Bacillus cereus was 18.4% 27, while in this study, M. circinelloides reduced the surface tension to 26 mN/m and created a clear zone having a 12.7 cm diameter, and the EI24 calculated for this strain was 66.6% (Table 1). In addition, the observed emulsification index of the crude biosurfactant of M. circinelloides was 100% (Fig. 4), although this index for the pure biosurfactant produced by Fusarium sp. BS‐8 was 70% 12. Determination of the emulsification activity showed that the OD550 of the fermentation broth of M.circinelloides was higher than that of the Tween 80 solution (1.2% v/v). According to Sekhon et al. 20, the OD550 determined for the fermentation broth of Bacillus subtilis SK320 was 0.86, whereas this index was determined to be 1.35 for M. circinelloides. The fermentation broth of M. circinelloides also had the ability to emulsify the engine oil, crude oil, kerosene, and soybean oil in addition to emulsifying diesel (Fig. 6). The emulsification values obtained were compatible with those of the fungal and bacterial bioemulsifiers described in the literature 6, 12, 24. These results revealed the high ability of this biosurfactant to emulsify such hydrophobic compounds.
The convenience kinetic parameters of biosurfactant production can be grouped into the following types: (i) growth‐associated production, (ii) production under growth‐limiting conditions, (iii) production by resting or immobilized cells, and (iv) production with precursor supplementation 28. Our results show that the growth and the biosurfactant production of M. circinelloides occurred at the first 72 h of the incubation (Fig. 1) thus the kinetic of biosurfactant production by this strain follows the growth‐associated kinetic. The production of surfactin in the culture broth of Bacillus subtilis, rhamnolipids by Pseudomonas aeruginosa, emulsan in Acinetobacter calcoaceticus RAG‐1, exopolysaccharide in A. calcoaceticus BD4 and rhamnolipid AP‐6 in P. fluorescens 378 were all found to be growth associated 20, 29, 30.
One of the major problems in the bioremediation of the hydrophobic compounds is the low availability of these compounds. It has been reported that biosurfactants can act more effectively in the bioremediation of hydrophobic substrates by increasing the bioavailability of these compounds. For example, the positive effect of Alasan on bioremediation of different PAH compounds was reported 31. The results obtained by Manickam et al. 32 also confirm that biosurfactant‐supplementation is a feasible strategy for enhancing the biodegradation of halogenated hydrocarbons. It was observed that the biodegradation efficiency for all biosurfactant‐amended samples (rhamnolipids, sophorolipids, or trehalose lipids) was increased by 30–50% in 2 days compared to the degradation after 10 days in the absence of surfactant 32. Lai et al. 33 showed that rhamnolipid and surfactin showed superior performance on TPH removal, compared to Triton X‐100 and Tween 80. The obtained results from this study show that M. circinelloides could degrade 87.6% of the crude oil compounds in 12 days of incubation. The maximum oil removal rate was recorded in the first 3 days of incubation (Fig. 1). The evaluation of the biosurfactant production revealed that the production occurred in the first 3 days of incubation (Fig. 1) and 12.3 g/L was measured as a biosurfactant production yield, so the relationship between the biosurfactant production and oil removal was coincidental.
MEOR is a process that is applied for the extraction of unmined oil from oil reservoirs after extraction with conventional methods. Biosurfactants can be applied as a key factor in this process 34. With regard to the results of the oil recovery assay, the ability of the cell‐free fermentation broth from M. circinelloides to recover oil from artificially contaminated soil (84.4%) was twice higher than that of water as control (40%) and 20% higher than that of 1.2% (v/v) Tween‐80 solution with 70% oil recovery (Fig. 2). Similar results were obtained by Luna et al. 35 for the biosurfactant produced by Candida glabrata UCP 1002, which could recover 84% of the crude residual oil adsorbed in the sand, while distilled water removed about 56% of the oil. Experiments in a study on crude oil removal from sea and freshwater demonstrated the produced surface‐active agents were able to remove 64.9% of the crude oil within a very short period of time (Fig. 3). According to Gnanamani et al. 21, more than 90% of crude oil was removed after 60 min using biosurfactants of marine microbial origin. According to these results, the biosurfactant produced by M. circinelloides in fermentation broth of waste frying oil as a medium, directly can be suggested for use in the MEOR process, soil washing and bioremediation of oil slick from contaminated sea environments.
Application of biosurfactants in industries such as the oil and food depends on the stability of these compounds in harsh conditions such as high temperatures and salinity. The obtained results showed that heating at 121°C for 30 min had no effect on the surface activity of the biosurfactant produced by M. circinelloides, whereas temperatures up to 70°C leads to inactivation of most chemical surfactants 22. So this biosurfactant has the potential of being offered as a good candidate to be used in such fields. The results showed that this biosurfactant was stable and active in acidic and neutral conditions but its activity was affected at the alkaline pH. It has been reported that the biosurfactant produced by Nocardiopsis sp. B4 was stable and active at the alkaline and neutral pH but its surface activity decreased at the acidic pH. A salt concentration of 2–3% caused a loss of activity in chemical surfactants 22. According to the results, the biosurfactant produced by M. circinelloides was stable and active in high salinity (40 g/L NaCl). This degree of stability is remarkable compared to the reported values for biosurfactants 22, 23, 35.
Practical application
M. circinelloides was found to be a potent producer of biosurfactant through showing high surface reduction (26 mN/m) and emulsification activities (100%). The strain could degrade 87.6% of the crude oil in the medium in 12 days and the ability of the produced biosurfactant to recover crude oil from sand and remove oil from sea water was 84.4 and 64.9% respectively. These results were remarkable compared to those of the chemical surfactants obtained in this research. The stability study showed that the biosurfactant was stable at an extreme temperature (121°C), high salt concentration (40 g/L), and extreme acidic pH (2–8), which could be feasibly used during in situ bioremediation of oil contaminants from polluted water and soil. According to the results, M. circinelloides has a high ability to produce biosurfactant and could be a potent candidate for the bioremediation of oil‐contaminated fields, oil slicks, and in the MEOR process.
Conflict of interest
The authors have declared no conflict of interest.
Acknowledgments
This work has been partly supported by University of Tehran.
5 References
- 1. Banat, I. M. , Franzetti, A. , Gandolfi, I. , Bestetti, G. et al., Microbial biosurfactants production, applications and future potential. Appl. Microbiol. Biotechnol. 2010, 87, 427–444. [DOI] [PubMed] [Google Scholar]
- 2. Ron, E. Z. , Rosenberg, E. , Natural roles of biosurfactants. Environ. Microbiol. 2001, 3, 229–236. [DOI] [PubMed] [Google Scholar]
- 3. Sekhon, K. K. , Khanna, S. , Cameotra, S. S. , Biosurfactant production and potential correlation with esterase activity. J Pet Environ Biotechnol. 2012, 3, 1–10. [Google Scholar]
- 4. Gadd, G. M. , Fungi in bioremediation, Cambridge University Press, 2001. [Google Scholar]
- 5. Amaral, P. F. , Coelho, M. A. Z. , Marrucho, I. M. , Coutinho, J. A. , Biosurfactants from yeasts: characteristics, production and application, in Biosurfactants, In: Biosurfactants, Springer, New York: 2010, pp. 236–249. [DOI] [PubMed] [Google Scholar]
- 6. Bhardwaj, G. , Cameotra, S. S. , Chopra, H. K. , Biosurfactants from fungi: a review. J Pet Environ Biotechnol. 2013, 4, 1–6. [Google Scholar]
- 7. Müller, M. M. , Kügler, J. H. , Henkel, M. , Gerlitzki, M. et al., Rhamnolipids—next generation surfactants?. J. Biotechnol. 2012, 162, 366–380. [DOI] [PubMed] [Google Scholar]
- 8. Bustamante, M. , Durán, N. , Diez, M. , Biosurfactants are useful tools for the bioremediation of contaminated soil: a review. J Soil Sci Plant Nutr. 2012, 12, 667–687. [Google Scholar]
- 9. Kosaric, N. , Biosurfactants and their application for soil bioremediation. Food Technol Biotechnol. 2001, 39, 295–304. [Google Scholar]
- 10. Moghimi, H. , Heidary Tabar, R. , Hamedi, J. , Assessing the biodegradation of polycyclic aromatic hydrocarbons and laccase production by new fungus Trematophoma sp. UTMC 5003. World. J. Microbiol. Biotechnol. 2017, 33, 1–10. [DOI] [PubMed] [Google Scholar]
- 11. Zadeh, P. H. , Moghimi, H. , Hamedi, J. , Biosurfactant production by Mocur circinelloides on waste frying oil and possible uses in crude oil remediation. Water Sci. Technol. 2017, 76, 1706–1714. [DOI] [PubMed] [Google Scholar]
- 12. Qazi, M. A. , Kanwal, T. , Jadoon, M. , Ahmed, S. et al., Isolation and characterization of a biosurfactant‐producing Fusarium sp. BS‐8 from oil contaminated soil. Biotechnol. Prog. 2014, 30, 1065–1075. [DOI] [PubMed] [Google Scholar]
- 13. Hewald, S. , Josephs, K. , Bölker, M. , Genetic analysis of biosurfactant production in Ustilago maydis . Appl. Environ. Microbiol. 2005, 71, 3033–3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Youssef, N. H. , Duncan, K. E. , Nagle, D. P. , Savage, K. N. et al., Comparison of methods to detect biosurfactant production by diverse microorganisms. J. Microbiol. Methods. 2014, 56, 339–347. [DOI] [PubMed] [Google Scholar]
- 15. Kamyabi, A. , Nouri, H. , Moghimi, H. , Synergistic effect of Sarocladium sp. and Cryptococcus sp. co‐culture on crude oil biodegradation and biosurfactant production. Appl. Biochem. Biotechnol. 2017, 182, 324–334. [DOI] [PubMed] [Google Scholar]
- 16. Rahman, K. , Thahira‐Rahman, J. , Lakshmanaperumalsamy, P. , Banat, I. , Towards efficient crude oil degradation by a mixed bacterial consortium. Bioresour. Technol. 2002, 85, 257–261. [DOI] [PubMed] [Google Scholar]
- 17. Pereira, J. F. , Gudiña, E. J. , Costa, R. , Vitorino, R. et al., Optimization and characterization of biosurfactant production by Bacillus subtilis isolates towards microbial enhanced oil recovery applications. Fuel. 2013, 111, 259–268. [Google Scholar]
- 18. Cooper, D. G. , Goldenberg, B. G. , Surface‐active agents from two Bacillus species. Appl. Environ. Microbiol. 1987, 53, 224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Das, P. , Mukherjee, S. , Sen, R. , Antimicrobial potential of a lipopeptide biosurfactant derived from a marine Bacillus circulans . J. Appl. Microbiol. 2008, 104, 1675–1684. [DOI] [PubMed] [Google Scholar]
- 20. Sekhon, K. K. , Khanna, S. , Cameotra, S. S. , Enhanced biosurfactant production through cloning of three genes and role of esterase in biosurfactant release. Microb Cell Fact. 2011, 10, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gnanamani, A. , Kavitha, V. , Radhakrishnan, N. , Mandal, A. , Bioremediation of crude oil contamination using microbial surface‐active agents: isolation, production and characterization. J Bioremed Biodegrad. 2010, 1, 1–8. [Google Scholar]
- 22. Khopade, A. , Biao, R. , Liu, X. , Mahadik, K. et al., Production and stability studies of the biosurfactant isolated from marine Nocardiopsis sp. B4. Desalination. 2012, 285, 198–204. [Google Scholar]
- 23. Mackson, J. P. , Singh, S. P. , The effect of temperature and vibration on emulsion stability of mayonnaise in two different package types. Packag Technol Sci. 1991, 4, 81–90. [Google Scholar]
- 24. Andrade Silva, N. R. , Luna, M. A. , Santiago, A. L. , Franco, L. O. et al., Biosurfactant‐and‐Bioemulsifier Produced by a Promising Cunninghamella echinulata Isolated from Caatinga Soil in the Northeast of Brazil. Int. J. Mol. Sci. 2014, 15, 15377–15395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bodour, A. A. , Drees, K. P. , Maier, R. M. , Distribution of biosurfactant‐producing bacteria in undisturbed and contaminated arid southwestern soils. Appl. Environ. Microbiol. 2003, 69, 3280–3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rahman, K. , Rahman, T. , McClean, S. , Marchant, R. et al., Rhamnolipid biosurfactant production by strains of Pseudomonas aeruginosa using low‐cost raw materials. Biotechnol Prog. 2002, 18, 1277–1281. [DOI] [PubMed] [Google Scholar]
- 27. Bento, F. M. , de Oliveira Camargo F. A., Okeke B. C., Frankenberger W. T., Diversity of biosurfactant producing microorganisms isolated from soils contaminated with diesel oil. Microbiological research 2005, 160, 249–255. [DOI] [PubMed] [Google Scholar]
- 28. Karanth, N. G. K. , Deo, P. G. , Veenanadig, N. K. , Microbial production of biosurfactants and their importance. Curr. Sci. 1999, 77, 116–126. [Google Scholar]
- 29. Kaplan, N. , Rosenberg, E. , Exopolysaccharide distribution and bioemulsifier production in Acinetobacter calcoaceticus BD‐4 and BD413. Appl. Environ. Microbiol. 1982, 44, 1335–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peypoux, F. , Bonmatin, J. M. , Wallach, J. , Recent trends in the biochemistry of surfactin. App Microbiol Biotechnol. 1999, 51, 553–563. [DOI] [PubMed] [Google Scholar]
- 31. Ron, E. Z. , Rosenberg, E. , Biosurfactants and oil bioremediation. Curr. Opin. Biotechnol. 2002, 13, 249–252. [DOI] [PubMed] [Google Scholar]
- 32. Manickam, N. , Bajaj, A. , Saini, H. S. , Shanker, R. , Surfactant mediated enhanced biodegradation of hexachlorocyclohexane (HCH) isomers by Sphingomonas sp. NM05. Biodegradation. 2012, 23, 673–682. [DOI] [PubMed] [Google Scholar]
- 33. Lai, C. C. , Huang, Y. C. , Wei, Y. H. , Chang, J. S. , Biosurfactant‐enhanced removal of total petroleum hydrocarbons from contaminated soil. J. Hazard. Mater. 2009, 167, 609–614. [DOI] [PubMed] [Google Scholar]
- 34. Sen, R. , Biotechnology in petroleum recovery: the microbial EOR. Prog. Energy Combust. Sci. 2008, 34, 714–724. [Google Scholar]
- 35. Luna, J. M. D. , Sarubbo, L. , Campos‐Takaki, G. M. D. , A new biosurfactant produced by Candida glabrata UCP 1002: characteristics of stability and application in oil recovery. Braz Arch Biol Technol. 2009, 52, 785–793. [Google Scholar]


