Abstract
In this study, polyacrylic acid‐based nanofiber (NF) membrane was prepared via electrospinning method. Acetylcholinesterase (AChE) from Electrophorus electricus was covalently immobilized onto polyacrylic acid‐based NF membrane by demonstrating efficient enzyme immobilization, and immobilization capacity of polymer membranes was found to be 0.4 mg/g. The novel NF membrane was synthesized via thermally activated surface reconstruction, and activation with carbonyldiimidazole upon electrospinning. The morphology of the polyacrylic acid‐based membrane was investigated by scanning electron microscopy, Fourier Transform Infrared Spectroscopy, and thermogravimetric analysis. The effect of temperature and pH on enzyme activity was investigated and maxima activities for free and immobilized enzyme were observed at 30 and 35°C, and pH 7.4 and 8.0, respectively. The effect of 1 mM Mn2+, Ni2+, Cu2+, Zn2+, Mg2+, Ca2+ ions on the stability of the immobilized AChE was also investigated. According to the Michaelis–Menten plot, AChE possessed a lower affinity to acetylthiocholine iodide after immobilization, and the Michaelis–Menten constant of immobilized and free AChE were found to be 0.5008 and 0.4733 mM, respectively. The immobilized AChE demonstrated satisfactory reusability, and even after 10 consecutive activity assay runs, AChE maintained ca. 87% of its initial activity. Free enzyme lost its activity completely within 60 days, while the immobilized enzyme retained approximately 70% of the initial activity under the same storage time. The favorable reusability of immobilized AChE enables the support to be employable to develop the AChE‐based biosensors.
Keywords: Acetylcholinesterase, Covalent immobilization, Electrospinning, NF membrane, Polyacrylic acid
Abbreviations
- AChE
acetylcholinesterase
- AFM
atomic force microscopy
- ATChI
acetylthicholine iodide
- CDI
carbonyldiimidazole
- DTNB
5,5ʹ‐dithiobis‐(2‐nitrobenzoic acid)
- DW
deionized water
- NF
nanofiber
- PAA
poly(acrylic acid)
- TGA
thermogravimetric analysis
1. Introduction
Acetylcholinesterase (AChE; EC 3.1.1.7) is a hydrolase that converts neurotransmitter acetylcholine to choline and acetate and has substantial role for living organisms 1. Active ingredients found in organophosphorus pesticides are very effective acetylcholinesterase inhibitors and even trace amounts of these substances can be hazardous to human health 2. Based on the inhibition of the enzyme, AChE biosensors have been broadly utilized for the detection of these pesticides. Measurement of AChE inhibition has been used as a biosensing method for the monitoring of exposure to pesticides in occupational and environmental health 3. The initial step for the fabrication of AChE biosensors involves the immobilization of the AChE enzyme onto the various materials to monitor the inhibition. For this purpose, AChE has been immobilized onto various materials demonstrating significant mechanical strength, decent enzyme immobilization, such as, magnetic microbeads 4, polyamide sorbent 5, chitosan 6, mesoporous carbon/ferroferric oxide 2, acrylonitrile copolymer membranes 7, and so on. Immobilized enzymes are desirable over free enzymes owing to the simple separation from the reaction medium, which makes them reusable for the related applications. The most extensively utilized methods for the enzyme immobilization have been carried out through physical interaction, covalent bonds, or entrapment/encapsulation 8. Recently, electrospinning has emerged as a novel system for producing composite nanofibers (NFs) by attracting interest in nanotechnology. Electrospun materials have beneficial properties, such as high surface to volume ratio, decent mechanical quality, high porosity, biocompatibility, and supreme surface modification for the enzyme immobilization 9. Electrospinning is a suitable and versatile technique for producing NFs from a blend of chemical solution with NF distances across going from several micrometers down to various nanometers. Therefore, the NF membranes are preferable candidates for numerous areas such as, pharmaceutical industry, tissue engineering, cosmetics, clothing, electric devices, and enzyme immobilization 10, 11, 12. Poly(acrylic acid) (PAA) is an important industrial polymer with its low toxicity, which is generally used as a superabsorbent polymer, a scale inhibitor, and dispersing agent 13. In the literature, PAA based composites were synthesized via electrospinning for tissue engineering, drug delivery, fuel cell proton exchange membranes, environmental pollution treatment applications, cosmetic, pharmacy, or wound dressings 14, 15, as a water polluting dye adsorbent 16, and especially support material for the enzyme immobilization through covalent bonds using agents such as EDC/NHS 17, glutaraldehyde 18, and carbonyldiimidazole (CDI) 19.
In this study, polyacrylic acid/sorbitol NF membranes were prepared via electrospinning method. AChE enzyme was then covalently immobilized onto the CDI activated NF membranes. The morphology and chemical structure of produced NFs were characterized by using SEM and FTIR. Several features such as, the optima temperature and pH, kinetic parameters, reusability, and the storage stability of the immobilized enzyme were investigated. For the immobilization of AChE, a polyacrylic acid‐based NF membrane was evaluated to immobilize AChE. NF material was modified with sorbitol for the covalent immobilization of AChE.
2. Materials and methods
2.1. Materials
Acetylcholinesterase from Electrophorus electricus (Type VI‐S, lyophilized powder, 200–1000 units/mg protein), acetylthiocholine iodide (≥98%), Ellman's reagent (5,5'‐dithiobis‐(2‐nitrobenzoic acid) or DTNB), Tetrahydrofuran, D‐sorbitol, PAA, 1,1′‐CDI were purchased from Sigma Aldrich Chemical Co. (St. Louis, USA). Bradford reagent, ovalbumin were commercial product of BioRad (BioRad Laboratories, Hercules CA, USA). NaH2PO4·2 H2O, and Na2HPO4·12 H2O (Sigma‐Aldrich) were used for the preparation of PBS. Manganese (II) acetate 4H2O, Ni (II) chloride 6H2O, zinc nitrate 6H2O, calcium nitrate 4H2O were purchased from Sigma Aldrich, and copper (II) nitrate 3H2O, magnesium nitrate 6H2O were purchased from Merck. All other chemicals used were of analytical grade.
2.2. Characterization of polymeric support
Fourier Transform Infrared Spectroscopy (FTIR) measurements of the polymer, activated polymer, and immobilized AChE were obtained with Perkin–Elmer Spectrum 100 FTIR (Waltham, MA, USA) equipped with a universal attenuated total reflectance sampling accessory (ZnSe cell) with a diamond window. Spectral analyses were performed at a resolution of 2 cm−1 in the range of 400–4000 cm−1 at ambient temperature. SEM studies were carried out to study the surface morphology of polyacrylic acid‐based NF membrane at an accelerating voltage of 10 kV by using Philips XL30SEM FEG (Amsterdam, Netherlands). Atomic force microscopy (AFM) image of the membrane was performed on Universal Scanning Probe Microscope (Ambios Quesant) under room conditions. Thermogravimetric analysis (TGA) was conducted by using Setaram thermogravimetric analyzer (Setsys Evolution, France). The assay was performed at temperatures ranging from room temperature to 1000°C with a heating rate of 10°C/min under the inert atmosphere with the gas flow rate at 20 mL/min.
2.3. Preparation of electrospun PAA/sorbitol NFs
Electrospinning process was carried out by using Nano FMG NE100 lab scale electrospinning unit. Two grams of PAA and 2 g of D‐sorbitol were dissolved separately in 20 mL of deionized water (DW) and stirred gently at 500 rpm under ambient conditions for 2 h. Then the mixture was loaded to 10 mL syringe fitted with a stainless steel needle, and the flow rate was adjusted to 1 mL/h, and the distance between tip of syringe and collector was set as 15 cm with 27 V potential in the electrospinning process. This process was allowed to keep for 1 d. Nanofibers were carefully collected from the collector and thermally incubated at 140°C for 2 h in an oven.
2.4. Activation of PAA/sorbitol NFs
The hydroxyl groups of PAA/sorbitol NFs were activated with CDI. A 6.5 g of PAA/sorbitol NFs were transferred into a three‐neck round bottomed flask and purged with nitrogen atmosphere. Ten grams of CDI in dry THF (50 mL) was added into the same flask and stirred magnetically at 500 rpm for 24 h at 40°C under nitrogen atmosphere. Finally, THF was evaporated and the activated NF membrane was stored at 4°C until use. CDI was reacted with the hydroxyl groups of the PAA/sorbitol, and the resulting product contains an amine reactive imidazolyl‐carbamate group.
2.5. Immobilization of AChE on activated PAA/sorbitol NF membrane
For the immobilization of AChE, 200 μg of AChE (200–1000 units/mg AChE) was dissolved in 10 mL 10 mM pH 7.0 PBS. The enzyme solution and 0.5 g of amine active PAA/sorbitol NF were mixed on an orbital shaker at 100 rpm for 12 h at 4°C. After incubation, mixture was centrifuged (6000 g for 5 min) and the unbound enzyme solution was decanted into a beaker. After the centrifugation, the enzyme immobilized polymer membrane was removed from the solution and washed thrice with PBS (10 mM, pH 7.0) to remove the slightly bound AChE. The unbound enzyme was determined according to the Bradford Assay using a series of standard ovalbumin solutions 20.
2.6. AChE activity assay
Free and immobilized AChE activity assay was performed spectrophotometrically according to the Ellman method 21 by using acetylthicholine iodide (ATChI) as substrate and 5,5ʹ‐dithiobis‐(2‐nitrobenzoic acid) (DTNB) as chromogen. Hundred microliters of 2 mM Acetylthiocholine iodide solution in DW, 2 mM of 100 μL DTNB solution in 10 mM pH 8.0 PBS, and 1.8 mL of 10 mM pH 8.0 PBS were taken into an Eppendorf tube. Then, 50 μL (0.308 μg) of enzyme solution was added to the same tube, and the mixture was incubated for 10 min at 37°C at 100 rpm in a stirring water bath. At the end of the incubation, absorbance of the yellow solution was measured at 412 nm against. The activity determination of the immobilized enzyme was carried out as described above except that corresponding amount of immobilized enzyme was taken instead of free enzyme. One unit of enzymatic activity was defined as the amount of enzyme that catalyzes 1 μmol of ATChI to thiocholine and acetate per minute.
2.7. Effect of pH and temperature on immobilized and free AChE
Activity assay was carried out in the pH range of 6.5–8.4 for the investigation of pH effect. To study the temperature effect, activity assay was also performed at the temperature range of 20–40°C while acetylthiocholine iodide concentration was kept constant at 10 × Km for every assay to maintain the maximum velocity during the enzymatic reaction. By considering the maxima activities as 100, the relative activity versus pH and temperature plots were drawn.
2.8. Storage stability and reusability studies
To further investigate the immobilization method, the storage stability of immobilized and free AChE was studied by determining the residual activity of immobilized and free AChE by keeping in 10 mM PBS (pH 7.5 for free and pH 8.0 for immobilized AChE) at 4°C. Samples were stored for 60 d and then removed from the PBS at 10‐day intervals for the enzyme activity test. In addition, the reusability of immobilized AChE was investigated by measuring the activity of immobilized AChE consecutively. The reusability test was performed at room temperature with 10 repeated cycles in 10 mM PBS buffer (pH 8.0), by taking the immobilized AChE from the solution with 10‐min intervals and by washing with 10 mM PBS (pH 7.4) five times for consecutive measurement.
2.9. The effect of metal ions on the stability of the immobilized AChE, and determination of kinetic parameters
The effect of Mn2+, Ni2+, Cu2+, Zn2+, Mg2+, Ca2+ ions on the stability of the immobilized AChE was investigated by preparing 1 mM solutions of related metals, and carrying out the activity assay. Michaelis–Menten kinetic behavior of free and immobilized AChE was investigated by carrying out the activity assay to determine the initial reaction rates of immobilized and free AChE by using the acetylthiocholine iodide solutions at the range of 0.01–1.5 mM concentrations under optima pH and temperature. The Michaelis–Menten kinetic parameters (Km) and maxima rates (Vmax) of free and immobilized enzymes were determined according to the Michaelis–Menten plot.
3. Results and discussion
3.1. Covalent immobilization of AChE
The study was illustrated in Fig. 1. In the process of NF synthesis, PAA was cross‐linked with sorbitol, and upon the electrospinning, the obtained cross‐linked membrane was thermally incubated. Here, sorbitol was chosen as crosslinking agent owing to its appropriate molecular size compared to other polyalcohols. Sorbitol can thermally bind to the carboxylic acid functional groups through ester bonds 19. Thus, three dimensional nanofiber membrane was synthesized. The surface activation was carried out via CDI binding to sorbitol moieties through carbamate bonds. Finally, AChE was covalently immobilized onto the amine active PAA/sorbitol NF through covalent ester bonds. Thus, a novel material for the enzyme immobilization was achieved, and 96% of the enzyme was successfully immobilized onto NF. The morphologies of NF membrane and enzyme immobilized membrane prepared by electrospinning were demonstrated in Fig. 2A and B. The NF membrane exhibits a smooth network of interlaid fibers that yields mechanical strength with a narrow fiber diameter distribution of 175–225 nm. The presence of fully interconnected micron‐sized pores in the structure provides an ideal host matrix for the enzyme immobilization owing to the high surface area, and decent permeability of analyte molecules into the inner cavities. In addition, the parallel arranged nanofiber units endow electrospun nanofibers with rigidity 22. As can be seen from the AFM image in Fig. 3, the surface of electrospun nanofiber over an area 20 μm × 20 μm was further revealed, and the lighter regions were related to the highest points, and the darker regions were related to pores and valleys. The general appearance of the NF membrane was similar to that obtained from the SEM analysis. Surface activation and enzyme immobilization were confirmed by FTIR results. As can be seen from Fig. 4A(a), the peaks located at 2956, 1721, 1470, 1403 cm−1 originate from the pure PAA that can be assigned to the stretching and bending vibrations of CH2, stretching vibrations of C=O and C–O of COOH groups, respectively, and the peaks at 1545 cm−1, 1160 cm−1 come from the C–O moieties of the PAA 23. In addition, a broad shoulder band in the 3150 cm−1 region is attributed to the –OH of COOH 24. Upon the CDI activation, the peaks appeared at 1767 cm−1, 1448 cm−1, and 1366 cm−1 were attributed to the imidazole moiety 25 of the activated membrane as shown in Fig. 4A(b). In order to confirm the AChE immobilization, FTIR spectrum of the immobilized enzyme was also investigated. As can be seen from the Fig. 4A(c), the characteristic absorption bands observed at 1635 cm−1 is associated to the C=O stretching of the amide I peak; and the absorption band at 1547 cm−1 of the amide II band is assigned to N–H bending and C–N stretching 26. The peak at 1260 cm−1 corresponds to the amide III region. Also, the peaks corresponding to CDI moieties disappeared, which confirmed the covalent bond formation. The broad band at 3280 cm−1 originates from N‐H stretching vibrations. The peak at ca. 3280 cm−1 is attributed to be N–H bending vibration. In all spectra, the peak at 1400 cm−1 confirms the carbonyl groups in the covalent bonds, and carboxylic acid side‐chains of protein 27. The covalent immobilization through carbamate bonds was confirmed with the peaks at 1535 cm−1 associated with the N–H deformation and C–N stretching, at 1257 cm−1 attributed to the C–N and C–O stretching vibration of carbamate group 28.
Figure 1.
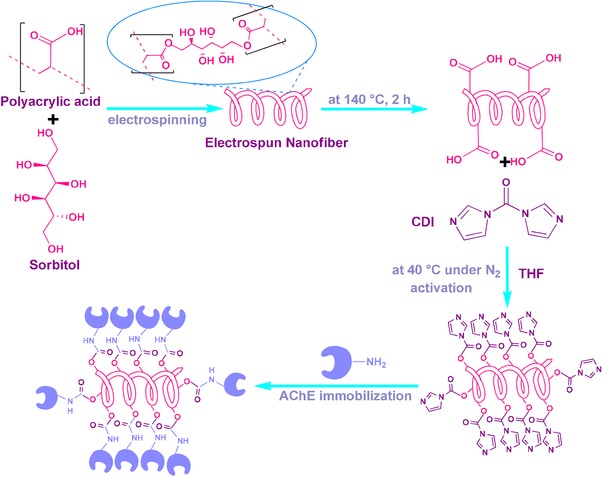
Schematic illustration of immobilization of AChE on electrospun NF membrane.
Figure 2.
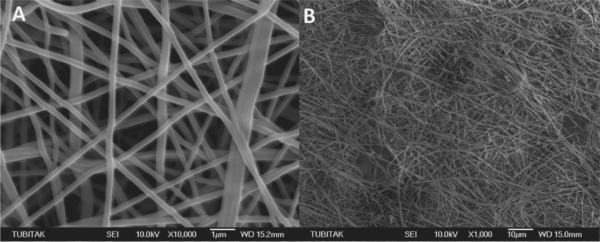
(A) SEM images of electrospun PAA/sorbitol NFs before, and (B) after AChE immobilization.
Figure 3.
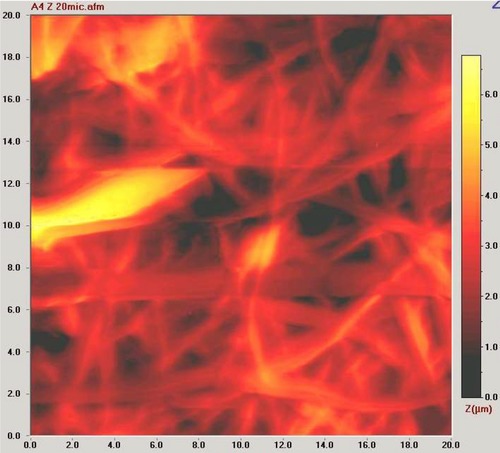
AFM image of the electrospun PAA/sorbitol NFs.
Figure 4.
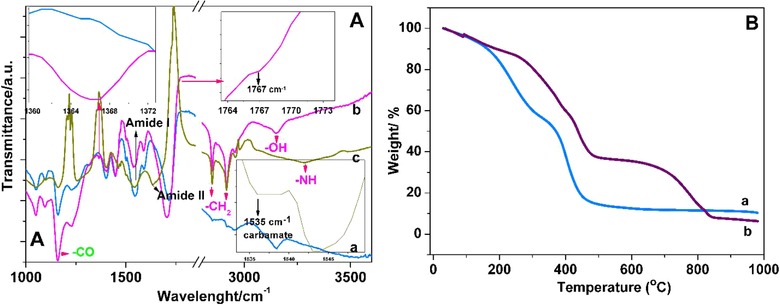
(A) Fourier transform infrared spectra (FTIR) of electrospun PAA/sorbitol NFs (a), activated electrospun PAA/sorbitol NFs (b), and AChE immobilized NFs (c). (B) TGA curves of electrospun PAA/sorbitol NFs (a), and AChE immobilized NFs (b) over the temperature range of 25–1000°C (B).
To further investigate the enzyme immobilization efficiency, the TGA was carried out. As shown in Fig. 4B(a), the mass loss of ca.15% observed at the temperature range from 27 to 200°C was attributed to the evaporation of the physically absorbed water molecules. In addition, the substantial mass loss of 70% in the range of 200–450°C was associated to the decomposition of PAA‐based NF. Upon the AChE immobilization (Fig. 4B(b)), the mass loss increased to some extent, which confirms the enzyme presence.
3.2. Effect of pH and temperature on immobilized and free AChE
The pH of medium is of great importance for the immobilized enzymes. Upon the immobilization process, optimum pH is generally shifted and this change can be beneficial for the specific applications. The influence of pH on free and immobilized AChE was displayed in Fig. 5A. According to the figure, enhanced enzyme activity was observed in the range of pH 7.75–8.5 upon the AChE immobilization. The activity of free and immobilized AChE decreased substantially from the optima pH with the pH decrement. This finding originates from the acid labile nature of the AChE, along with the repellence of the same positively charged substrate acetylthiocholine and the immobilized AChE below the isoelectric point (pH 6) 29. Optima pH values of free and immobilized enzyme were found to be 7.5 and 8.0, respectively. The observed optimum pH value for the immobilized AChE was shifted 0.5 pH unit toward the alkaline side when compared with free AChE. This phenomenon was explained by the partitioning of hydrogen ions in our previous study 30. According to this phenomenon, the negatively charged supports lead to an increment in the optimum pH, and the positively charged supports diminish the optimum pH upon the immobilization. Also, covalent immobilization can impact on the AChE conformation, by altering the optimum pH for AChE, and various enzyme catalyzed reactions release different charged products, which alter the solution pH. All in all, the immobilized AChE was more active in the alkaline pH range than the free AChE, as shown in Fig. 5A. Similar pH values were found to be at pH 8 for AChE immobilized on mesoporous silicon surface 31, on SiO2 hybrid membranes 32, on styrene–maleic anhydride copolymer 33, and acrylonitrile copolymer membranes 7.
Figure 5.
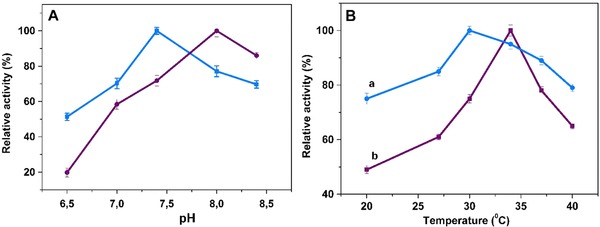
(A) Effect of pH, and (B) temperature on the activity of free (a), and immobilized AChE (b) (error bars represent ± SD, n = 3).
The influence of temperature on the activity of the free and immobilized AChE was investigated by varying the temperature from 20 to 40°C and the enzyme activity behaviors were demonstrated in Fig. 5B. It was observed that the free and immobilized AChE activity increased with increasing temperature up to the optima values, indicating that moderately high temperature was beneficial for both free and immobilized AChE. The results in Fig. 5B showed that optimum temperature for free AChE was approximately 30°C, while it shifted to 35°C for immobilized AChE. According to the literature, the optimum temperature of the enzyme increases owing to a mass of covalent cross‐linking between the enzymes and supporting materials upon the immobilization. The enzyme activities decreased substantially due to the enzyme denaturation above the optima temperatures 34.
3.3. The effect of metal ions on the stability of the immobilized AChE, and kinetic parameters of free and immobilized AChE
The effect of 1 mM Mn2+, Ni2+, Cu2+, Zn2+, Mg2+, Ca2+ solutions on the stability of the immobilized AChE was demonstrated in Fig. 6A. Alkaline earth metals used in the study slightly diminished the AChE activity. While, used transition earth metals, esp. Ni2+, and Mn2+ led to a substantial decrement in the activity, owing to the inhibition effect of the heavy metal ions on the enzyme activity. Also, Cu2+ can inhibit the AChE enzyme noncompetitively, and lead to the decrement in the AChE activity 35. The enzyme affinity to its respective substrate is determined with the Michaelis constant (K m). For this, the K m and maximum reaction rate (Vmax) were assessed from the Michaelis–Menten plot for free and immobilized AChE, at the optima temperature and pH values, with the certain concentration range of ATChI solutions. As shown in Fig. 6B, for free AChE, K m and Vmax were calculated as 0.4733 mM and 0.5 mM/min, respectively, while for immobilized AChE, K m and Vmax values were determined as 0.5008 mM and 0.509 mM/min, respectively. The immobilized AChE possesses a higher K m value compared to free AChE. The observed increment in K m of immobilized AChE reveals the diminished affinity to ATChI. This finding can be attributed to the impairment in the active site of AChE, along with the enzyme flexibility upon covalent immobilization. The Vmax value of immobilized enzyme was nearly comparable to that of free enzyme. This result can be attributed to the small impairment of the enzyme conformation upon immobilization. Similar changes in the kinetic parameters of AChE after immobilization have been reported, and the K m values of immobilized AChE were found to be 0.1241 mM on electrospun poly(acrylic acid)/multiwalled carbon nanotube NF 18, 27.24 mM on chitosan‐SiO2 36, 0.02630 mM on polyacrylamide‐functionalized multiwalled carbon nanotube NF 37, 0.0272 mM on Polymethyl Methacrylate NF 38. Therefore, the immobilization procedure enables the utilization of immobilized enzyme for the immobilization applications. Also, the K m value of free AChE was found to be between the values for the immobilized AChE on nanofibrous PVA/BSA membranes (0.3 Mm) 39 and modified acrylonitrile copolymer membranes (0.9 Mm) 7.
Figure 6.
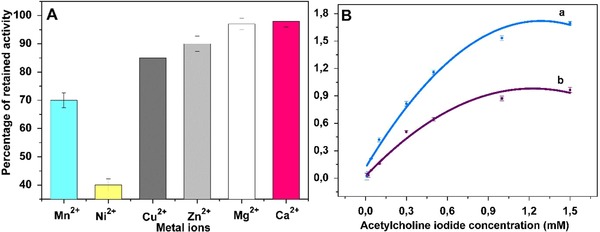
(A) The effect of 1 mM Mn2+, Ni2+, Cu2+, Zn2+, Mg2+, Ca2+ ions on the stability of the immobilized AChE (error bars represent ± SD, n = 3). (B) Michelis–Menten plot of free (a) and immobilized AChE (b).
3.4. Storage stability and reusability
Storage stability is one of the significant indexes to evaluate the properties of the enzyme. Generally, enzymes are not stable in solution, owing to the susceptibility and their activities decrease substantially during storage compared to the immobilized enzymes. Therefore, the reusability and storage stability studies are of great importance to evaluate the immobilized enzyme for the various applications. The storage stability graph was given in Fig. 7A. Free enzyme lost its activity completely within 60 d, while the immobilized enzyme retained approximately 70% of the initial activity under the same storage time. This result was comparable to the retained activity of immobilized AChE on acrylonitrile copolymer membranes (75%) 7. Thus, the enzyme gained more stable character compared to the free counterpart upon the immobilization by providing a longer shelf life. The satisfactory reusability of immobilized enzyme is of great importance in the immobilization‐based applications. As can be seen from Fig. 7B, the residual activity of the immobilized AChE was decreased with the increasing number of washes. However, at the end of 10th cycle, the residual activity of the immobilized AChE corresponded to 87% of its initial activity by indicating the excellent operational stability, which is desirable for the enzyme‐related applications. The observed reusability was comparable or better compared to the AChE immobilized on acrylonitrile copolymer membranes 7, on styrene electrospun mats 33, on PVA/BSA NF 39.
Figure 7.
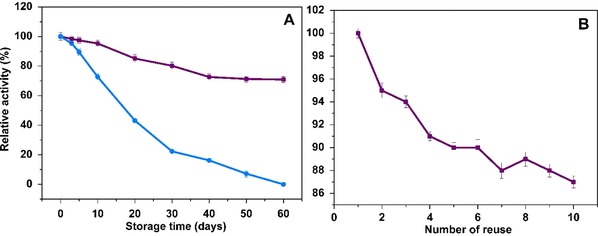
(A) The storage stability of free (a) and immobilized AChE (b) and (B) operational stability of immobilized AChE for subsequent 10 cycles (error bars represent ± SD, n = 3).
4. Conclusions
The highly pure PAA‐based NF membrane was successfully synthesized by electrospinning method. The NF membrane activation with CDI and immobilization of AChE to NF via covalent bonds during immobilization process was confirmed by FTIR, TGA. After immobilization, enzyme activity was improved to some extent at the pH range of 8.0–8.5 and at the temperature value of 35°C. Upon the addition of 1 mM metal salt solutions to the activity assay solution, Mn2+and Ni2+ ions decreased the activity substantially owing to the enzyme inhibition, and Cu2+, Zn2+, Mg2+, Ca2+ solutions slightly diminished the immobilized AChE activity. The enzyme's affinity to acetylthiocholine iodide decreased upon immobilization owing to the conformational changes after the covalent immobilization. The immobilized AChE was found to be more stable than the free AChE according to the stability experiments. The operational and storage stabilities of AChE were also significantly improved after immobilization. The superior immobilization strategy carried out in this study offers great advantages for various applications, not only for AChE‐related systems but also for other enzymes and biomolecules‐related applications.
Practical application
A support polyacrylic acid‐based electrospun membrane was synthesized for the enzyme immobilization applications. In this study, AChE was used as a model, and efficient enzyme binding was achieved.
The authors have declared no conflict of interest.
Acknowledgments
We thank to Özkan Danış and Başak Yüce Dursun for their valuable contributions.
5 References
- 1. Khaldi, K. , Sam, S. , Gouget‐Laemmel, A. C. , Henry De Villeneuve, C. et al., Active acetylcholinesterase immobilization on a functionalized silicon surface. Langmuir 2015, 31, 8421–8428. [DOI] [PubMed] [Google Scholar]
- 2. Zhang, Q. , Xu, Q. , Guo, Y. , Sun, X. et al., Acetylcholinesterase biosensor based on the mesoporous carbon/ferroferric oxide modified electrode for detecting organophosphorus pesticides. RSC Adv. 2016, 6, 24698–24703. [Google Scholar]
- 3. Dorraki, N. , Safa, N. N. , Jahanfar, M. , Ghomi, H. et al., Surface modification of chitosan/PEO nanofibers by air dielectric barrier discharge plasma for acetylcholinesterase immobilization. Appl. Surf. Sci. 2015, 349, 940–947. [Google Scholar]
- 4. Shriver‐Lake, L. C. , Charles, P. T. , Adams, A. A. , Fontana, J. et al., A simple approach to a vastly improved acetylcholinesterase activity and stability at elevated temperatures using magnetic microbeads and poly(N‐(3‐aminopropyl methacrylamide)) hydrogel supports. J. Mol. Catal. B Enzym. 2016, 134, 61–69. [Google Scholar]
- 5. Godjevargova, T. , Dimov, A. , Ivanova, D. , Immobilization of glucose oxidase and acetylcholinesterase onto modified polyamide sorbent. J. Appl. Polym. Sci. 1998, 68, 323–329. [Google Scholar]
- 6. Warner, J. , Andreescu, S. , An acetylcholinesterase (AChE) biosensor with enhanced solvent resistance based on chitosan for the detection of pesticides. Talanta 2016, 146, 279–284. [DOI] [PubMed] [Google Scholar]
- 7. Gabrovska, K. , Nedelcheva, T. , Godjevargova, T. , Stoilova, O. et al., Immobilization of acetylcholinesterase on new modified acrylonitrile copolymer membranes. J. Mol. Catal. B Enzym. 2008, 55, 169–176. [Google Scholar]
- 8. Bornscheuer, U. T. , Immobilizing enzymes: how to create more suitable biocatalysts. Angew Chem.– Int. Ed. 2003, 42, 3336–3337. [DOI] [PubMed] [Google Scholar]
- 9. Ding, B. , Wang, M. , Wang, X. , Yu, J. et al., Electrospun nanomaterials for ultrasensitive sensors. Mater. Today 2010, 13, 16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aragon, J. , Navascues, N. , Mendoza, G. , Irusta, S. , Laser‐treated electrospun fibers loaded with nano‐hydroxyapatite for bone tissue engineering. Int. J. Pharm. 2017, 525, 112–122. [DOI] [PubMed] [Google Scholar]
- 11. Santos, D. , Correia, C. O. , Silva, D. M. , Gomes, P. S. et al., Incorporation of glass‐reinforced hydroxyapatite microparticles into poly(lactic acid) electrospun fibre mats for biomedical applications. Mater. Sci. Eng. C 2017, 75, 1184–1190. [DOI] [PubMed] [Google Scholar]
- 12. Kumuthini, R. , Ramachandran, R. , Therese, H. A. , Wang, F. , Electrochemical properties of electrospun MoS2 @C nanofiber as electrode material for high‐performance supercapacitor application. J. Alloy Compd. J. 2017, 705, 624–630. [Google Scholar]
- 13. Zhou, Q. , Luo, W. , Zhang, X. , Ingenious route for ultraviolet‐induced graft polymerization achieved on inorganic particle: fabricating magnetic poly(acrylic acid) densely grafted nanocomposites for Cu2+ removal. Appl. Surf. Sci. 2017, 413, 181–190. [Google Scholar]
- 14. Shami, Z. , Sharifi‐Sanjani, N. , Khanyghma, B. , Farjpour, S. et al., Ordered exfoliated silicate platelets architecture: hydrogen bonded poly(acrylic acid)–poly(ethylene oxide)/Na–montmorillonite complex nanofibrous membranes prepared by electrospinning technique. RSC Adv. 2014, 4, 40892–40897. [Google Scholar]
- 15. Lubasova, D. , Niu, H. , Zhao, X. , Lin, T. , Hydrogel properties of electrospun polyvinylpyrrolidone and polyvinylpyrrolidone/poly(acrylic acid) blend nanofibers. RSC Adv. 2015, 5, 54481–54487. [Google Scholar]
- 16. Yan, J. , Huang, Y. , Miao, Y. E. , Tjiu, W. W. et al., Polydopamine‐coated electrospun poly(vinyl alcohol)/poly(acrylic acid) membranes as efficient dye adsorbent with good recyclability. J. Hazard. Mater. 2015, 283, 730–739. [DOI] [PubMed] [Google Scholar]
- 17. Li, C. , Zhou, L. , Wang, C. , Liu, X. et al., Electrospinning of a PMA‐co‐PAA/FP biopolymer nanofiber: enhanced capability for immobilized horseradish peroxidase and its consequence for p‐ nitrophenol disposal. RSC Adv. 2015, 5, 41994–41998. [Google Scholar]
- 18. Ebadi, S. V. , Fakhrali, A. , Ranaei‐Siadat, S. O. , Gharehaghaji, A. A. et al., Immobilization of acetylcholinesterase on electrospun poly(acrylic acid)/multi‐walled carbon nanotube nanofibrous membranes. RSC Adv. 2015, 5, 42572–42579. [Google Scholar]
- 19. Bastürk, E. , Demir, S. , Danis, O. , Kahraman, M. V. , Covalent immobilization of α‐amylase onto thermally crosslinked electrospun PVA/PAA nanofibrous hybrid membranes. J. Appl. Polym. Sci. 2013, 127, 349–355. [Google Scholar]
- 20. Bradford, M. M. , A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Anal. Biochem. 1976, 72, 248–254. [DOI] [PubMed] [Google Scholar]
- 21. Ellman, G. L. , Courtney, K. D. , Andres, V. , Featherstone, R. M. , A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [DOI] [PubMed] [Google Scholar]
- 22. Hwang, E. T. , Tatavarty, R. , Lee, H. , Kim, J. et al., Shape reformable polymeric nanofibers entrapped with QDs as a scaffold for enzyme stabilization. J. Mater. Chem. 2011, 21, 5215–5218. [Google Scholar]
- 23. Ge, J. , Hu, Y. , Biasini, M. , Dong, C. et al., One‐step synthesis of highly water‐soluble magnetite colloidal nanocrystals. Chem. A Eur. J. 2007, 13, 7153–7161. [DOI] [PubMed] [Google Scholar]
- 24. Wichaita, W. , Samart, C. , Yoosuk, B. , Kongparakul, S. , Cellulose graft poly(acrylic acid) and polyacrylamide: grafting efficiency and heavy metal adsorption performance. Macromol. Symp. 2015, 354, 84–90. [Google Scholar]
- 25. Boufi, S. , Vilar, M. R. , Parra, V. , Ferraria, A. M. et al., Grafting of porphyrins on cellulose nanometric films. Langmuir 2008, 24, 7309–7315. [DOI] [PubMed] [Google Scholar]
- 26. Rodríguez‐deLuna, S. E. , Moreno‐Cortez, I. E. , Garza‐Navarro, M. A. , Lucio‐Porto, R. et al., Thermal stability of the immobilization process of horseradish peroxidase in electrospun polymeric nanofibers. J. Appl. Polym. Sci. 2017, 134, 44811–44821. [Google Scholar]
- 27. Hsiao, M.‐H. , Lin, K.‐H. , Liu, D.‐M. , Improved pH‐responsive amphiphilic carboxymethyl‐hexanoyl chitosan–poly(acrylic acid) macromolecules for biomedical applications. Soft Matter 2013, 9, 2458. [Google Scholar]
- 28. Oliveira, J. R. , Martins, M. C. L. , Mafra, L. , Gomes, P. , Synthesis of an O‐alkynyl‐chitosan and its chemoselective conjugation with a PEG‐like amino‐azide through click chemistry. Carbohydr. Polym. 2012, 87, 240–249. [DOI] [PubMed] [Google Scholar]
- 29. Zhu, M. C. , Xin, Y. B. , Sun, M. J. , Fang, Y. Z. , Purification and properties of acetylcholinesterase from human brain. Sci. China B 1993, 36, 1207–1215. [PubMed] [Google Scholar]
- 30. Altun, S. , Çakiroğlu, B. , Özacar, M. , Özacar, M. , A facile and effective immobilization of glucose oxidase on tannic acid modified CoFe2O4 magnetic nanoparticles. Colloids Surf. B Biointerfaces 2015, 136, 963–970. [DOI] [PubMed] [Google Scholar]
- 31. Saleem, M. , Rafiq, M. , Seo, S.‐Y. , Lee, K. H. , Acetylcholinesterase immobilization and characterization, and comparison of the activity of the porous silicon‐immobilized enzyme with its free counterpart. Biosci. Rep. 2016, 36, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yotova, L. , Medhat, N. , Coimmobilization of acetylcholinesterase and choline oxidase on new nanohybrid membranes obtained by sol gel technology. Biotechnol. Biotechnol. Equip. 2012, 26, 3039–3043. [Google Scholar]
- 33. Stoilova, O. , Ignatova, M. , Manolova, N. , Godjevargova, T. et al., Functionalized electrospun mats from styrene‐maleic anhydride copolymers for immobilization of acetylcholinesterase. Eur. Polym. J. 2010, 46, 1966–1974. [Google Scholar]
- 34. Atacan, K. , Çakiroğlu, B. , Özacar, M. , Improvement of the stability and activity of immobilized trypsin on modified Fe3O4 magnetic nanoparticles for hydrolysis of bovine serum albumin and its application in the bovine milk. Food Chem. 2016, 212, 460–468. [DOI] [PubMed] [Google Scholar]
- 35. Pohanka, M. , Copper, aluminum, iron and calcium inhibit human acetylcholinesterase in vitro. Environ. Toxicol. Pharmacol. 2014, 37, 455–459. [DOI] [PubMed] [Google Scholar]
- 36. Ye, Y. H. , Li, C. , Yang, J. , Ma, L. et al., Construction of an immobilised acetylcholinesterase column and its application in screening insecticidal constituents from Magnolia officinalis . Pest Manag. Sci. 2015, 71, 607–615. [DOI] [PubMed] [Google Scholar]
- 37. Amini, N. , Mazinani, S. , Ranaei‐Siadat, S. O. , Kalaee, M. R. et al., Acetylcholinesterase immobilization on polyacrylamide/functionalized multi‐walled carbon nanotube nanocomposite nanofibrous membrane. Appl. Biochem. Biotechnol. 2013, 170, 91–104. [DOI] [PubMed] [Google Scholar]
- 38. Amini, N. , Mazinani, S. , Ranaei‐Siadat, S. O. , Kalaee, M. et al., Manufacturing polymethyl methacrylate nanofibers as a support for enzyme immobilization. Fibers Polym. 2012, 8, 994–998. [Google Scholar]
- 39. Moradzadegan, A. , Ranaei‐Siadat, S. O. , Ebrahim‐Habibi, A. , Barshan‐Tashnizi, M. et al., Immobilization of acetylcholinesterase in nanofibrous PVA/BSA membranes by electrospinning. Eng. Life Sci. 2010, 10, 57–64. [Google Scholar]


