Abstract
Microbial community in soil is a complex and dynamic system. Using traditional culture experiments it is difficult to model the stochastic distribution of single organisms of microbial communities in the soil pore's structure. Droplet‐based micro‐segmented flow technique allows the transfer of the principle of stochastic confinement of stochastically reduced communities from soil micro pores into nanoliter droplets. Microfluidics was applied for the investigation and comparison of soil samples from ancient mining areas by highly resolved concentration‐dependent screenings. As results, the generation, incubation, and in situ optical characterization of nanoliter droplets of suspensions of unknown soil microbial communities allowed the identification of different response characteristics toward heavy metal exposition. The investigations proved the high potential of microfluidics for investigations of soil microbial communities. It may be in the future helpful to detect bacteria and consortia with special biosorption characteristics, which could be useful for the development of biological accumulation and detoxification strategies.
Keywords: Dose‐response screening, Microfluidics, Micro pores, Nanoliter droplets, Soil microbial communities
Abbreviations
- AM
actinomycete
- PVP
polyvinylpyrrolidone
- PP9
perfluoromethyldecalin
1. Introduction
Many soils, particularly in agriculture, are exposed to strong external stresses by changing local ecological conditions. Open surface, complete removal of plant communities, monocultures, and high, but changing concentrations of fertilizers, herbicides, and pesticides, cause extreme shifts of conditions on comparatively small time scales 1, 2. In addition, the micro morphology of soil is highly complex and it is difficult to simulate this whole complexity under well‐controlled experimental conditions. In this situation, the diversity of soil micro communities is currently characterized by culture‐independent methods such as DNA sequencing or metagenomics. These analyses provide information on genotypes, but do not allow to investigating the physiological behavior of involved organisms. The physiological and ecological function of the single constituents or interacting groups of them can hardly be analyzed. And, it is particularly difficult to model the stochastic distribution of single organisms of microbial communities in the soil pore's structure into a higher number of separated small compartments by traditional culture experiments.
The recent development in microfluidics opens the possibility of an ultimate miniaturization and parallelization of culture experiments under varied compositions of culture media 3, 4, 5, 6. The utilization of micro droplet generators, computer‐controlled nearly pulsation‐free micro pumps allow a precise dosing and automated step‐wise or continuously variation of droplet compositions. Micro flow‐through photometric and fluorimetric sensors 7, miniaturized Raman probes 8, 9, and micro imaging systems were used for the noncontact characterization of microbial growth inside micro fluid segments. Micro flow‐through photometry provides general information on the increase of the mean cell density. In contrast, micro flow‐through fluorimetry supplies autofluorescence activities, which may even better relate to the formation of secondary metabolites. The micro‐segmented flow technique 10 was applied, in particular, for the determination of highly resolved dose/response functions of bacterial growth on various toxic substances. This technique is well suited for the evaluation of combinatorial effects of two or three toxic substances on microbial populations 11. The microfluidic technique was applied for the characterization of the responses of soil microorganisms on toxic substances 12. Recently, the microfluidic technique enabled for the evaluation of the response behavior of unknown communities from soil samples 13.
The principle of stochastic confinement was introduced for the separation of single cells and can be both used for the separation of complex composed communities and populations of single species 14, 15, 16. The number of organisms in each droplet in case of a population with dispersed single cells leads to a binomial distribution 17. The probability to find one, two, three, or no cells in one droplet can be controlled by the cell density and the droplet volume.
In this work, the soil samples from two ancient copper mining areas were implemented in nanoliter droplets in order to identify different types of growth responses. The application of microfluidic technique allows to realizing the principle of “stochastically reduced communities”: In an experimental run with several hundreds of small separated droplets, the suspended microorganisms from the unknown soil community were in this case stochastically distributed into the single droplets. The limited droplet size means a low total number of cells in a single droplet. Therefore, each individual droplet contains a certain accidental mixture of a reduced number of cell types. This random distribution can be regarded in analogy to limited numbers of individual microorganisms in small pores of soil.
2. Materials and methods
2.1. Chemicals
For the experiment, the following chemicals were utilized: CuSO4·6H2O, NiSO4·6H2O, and Co(NO3)2·6H2O (Merck, Darmstadt, Germany). Cycloheximide was obtained from BioChemica (Düsseldorf, Germany). The actinomycete (AM) minimal medium is that described by Amoroso et al. and composed of 0.5 g/L asparagine, 0.5 g/L K2HPO4, 0.2 g/L MgSO4, 0.01 g/L FeSO4 and 10 g/L D‐glucose 18. AM medium has to be used in order to minimize complexation of the heavy metal ions by different components in case of other more complex composed nutrient medium. For the preparation of colloidal metal nanoparticles, the following chemicals were used: Palladium(II) nitrate dihydrate (Carl Roth GmbH, Karlsruhe, Germany), polyvinylpyrrolidone (PVP) and 2‐hydroxy‐4′‐(2‐hydroxyethoxy)‐2‐methylpropiophenone from Sigma‐Aldrich (Germany); perfluoromethyldecalin (PP9) (F2 Chemicals Ltd., UK).
2.2. Synthesis of palladium nanoparticles
A photochemical segmented flow strategy was chosen for the synthesis of colloidal noble metal nanoparticles 19. Palladium nanoparticles were prepared from a 1 mM palladium nitrate solution. This solution was mixed with the polyvinylpyrrolidone/photoinitiator solution (PVP/PI) in a 4‐port manifold and segmented with PP9. The PVP/PI solution was previously prepared from a 2 wt% PVP solution and 3 mM 2‐hydroxy‐4′‐(2‐hydroxyethoxy)‐2‐methylpropiophenone solution. The nucleation starts with the irradiation (UV – mercury high pressure source, mean emission peak at 366 nm) of the segments in the photo initiation element with 2 mm length of the focus and an irradiation time of 135 ms. The size of obtained particles was about 2.5 nm.
2.3. Soil sample preparation
Soil samples from two ancient copper mining areas in Thuringia and Saxony‐Anhalt have been used in the experiments (Table 1). The motivation behind the application of these soil samples was the idea, that soil from such places should contain a higher number of metal‐tolerant bacterial species. This assumption is due to the fact, that in the time of the active mining and even later from time to time metal ions have been released in dependence on humidity and erosion conditions. This temporal release led to a stochastic activation of the “ecological memory” of the bacterial pool in soil, among them organisms specialized on enhanced metal concentrations.
Table 1.
Soil sample description
| Soil sample location | ||
|---|---|---|
| Sample | Location | GPS coordinates (Gauss‐Krueger) |
| N1 | Neuenhof, historic copper mine | 3585,405/5651,421 |
| N6 | Neuenhof, historic copper mine | 3584,622/5649,727 |
| E63 | Hettstedt, preindustrial copper mine | 4467,258/5722,002 |
| E65 | Hettstedt, preindustrial copper mine | 4467,320/5722,189 |
Two samples (N1 and N6) were taken from a copper mining area in a recent forest (Neuenhof, near Eisenach, Thuringia) with the main mining activity in the 18th century. The two other samples (E63 and E65) are originating from a copper mining area of the beginning of the 19th century near Hettstedt (Sachsen‐Anhalt).
After the collection the soil samples were air‐dried under sterile conditions in the laboratory. 1 g soil was mixed with 15 ml distilled water and vortexed thoroughly. It was expected that dispensable bacteria and bacterial spores could be dispergated in the aqueous phase. This is important for the following incubation in the aqueous phase of nanoliter droplets despite the fact that a considerable part of particle‐adherent bacteria gets lost. The solution was centrifuged at 237 × g for 20 min and the supernatant liquid was filtered through a sterile filter paper. To prevent the growth of soil‐derived fungi in segments, cycloheximide with a final concentration of 75 μg/mL was added. This solution containing bacterial spores and vegetative bacteria was used for inoculation of the micro droplets or inoculation in flask culture.
2.4. Experimental concept and arrangement
The water and microorganisms containing pores in soils can have very different sizes. Soil pores can span a large size spectrum from the dimensions of single small cells (femtoliter range) over pore sizes in the micrometre range (picoliter and nanoliter scale) up to large microliter pores and milliliter holes. The distribution of the pores structure is dependent mainly on the structure and distribution of mineral and organic particles. Their separation from each other, the exchange of nutrients, oxygen, dissolved organic compounds, and bacteria between them vary to a large extent. The principle of small local spaces is realized by all these pores, in which a certain restricted group of microorganisms can develop. Many of them are fairly closed and allow the evolution of populations of slowly growing microorganisms.
The principle of subdividing a culture volume into a larger set of small culture volumes, which are stochastically occupied by a single or a few microorganisms can be evolved in the lab either by an emulsion technology or by the technique of microfluidics (Fig. 1). The latter technique has the advantage that droplets of equal size are generated, the chemical composition of the single droplets can be tuned, and the initial order of droplets can be preserved over incubation times of days, weeks, or even months. The micro fluidic technique is well suited for a stochastic confinement of single organisms as well as for a stochastic confinement of reduced microbial communities by a random distribution into fluid segments during the segment generation. The number of commonly growing organisms in one segment ranges between a few or some dozen, even in the case that hundreds of species might growth under the experimental incubation conditions and the total number of dormant bacterial genotypes is still much higher. The micro segmented flow technique is a convenient method for the realization of concentration programs by controlling the flow rate ratios of several inlet liquid flows by computer‐controlled syringe pumps during the droplet generation in a microfluidic manifold. The details on the fluidic devices, the micro optical devices and the applied methods for realizing concentration gradients were described earlier 20.
Figure 1.
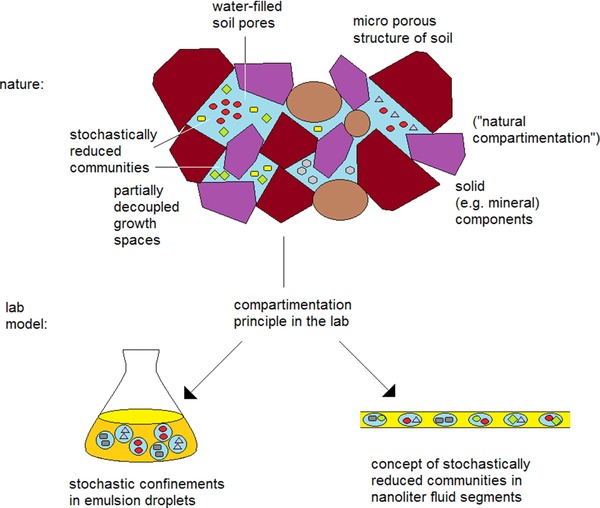
Space structure, community separation, and the principle of stochastic confinement (schematic)
The experimental procedures of this report are illustrated in Fig. 2, where the soil sample was dispersed in water and large soil particles were separated by sedimentation. The filtered suspension was then brought into a syringe pump and mixed with a nutrient solution and stressor additives during the generation of the microfluidic segments of 0.5 μL volume each. Segment sequences with 200–250 segments and a variation of the concentration of heavy metal ions in equal steps between a prechosen minimum and maximum concentration were generated. The quality of generated segment series–segment size and distance of all generated fluid segments–and simultaneous measurement of extinction and fluorescence of the segment content were monitored by micro flow‐through dual‐photo‐fluorimetry. As light source a LED with a peak wavelength of 470 nm (Ledxon, Germany) is both for extinction measurement and fluorescence measurement. The latter was combined with a shortpass filter at 470 nm and longpass filter with 515 nm (Laser components, Germany). This arrangement ensures the exact assignment of optical density and fluorescence data of each individual liquid segment. After the formation and measurements, the segment sequence containing soil slurry and concentration gradients of heavy metals were stored in PTFE tubes (0.5 mm id, 1.0 mm od) with a length of four meters for 2 up to 5 weeks at 28°C.
Figure 2.
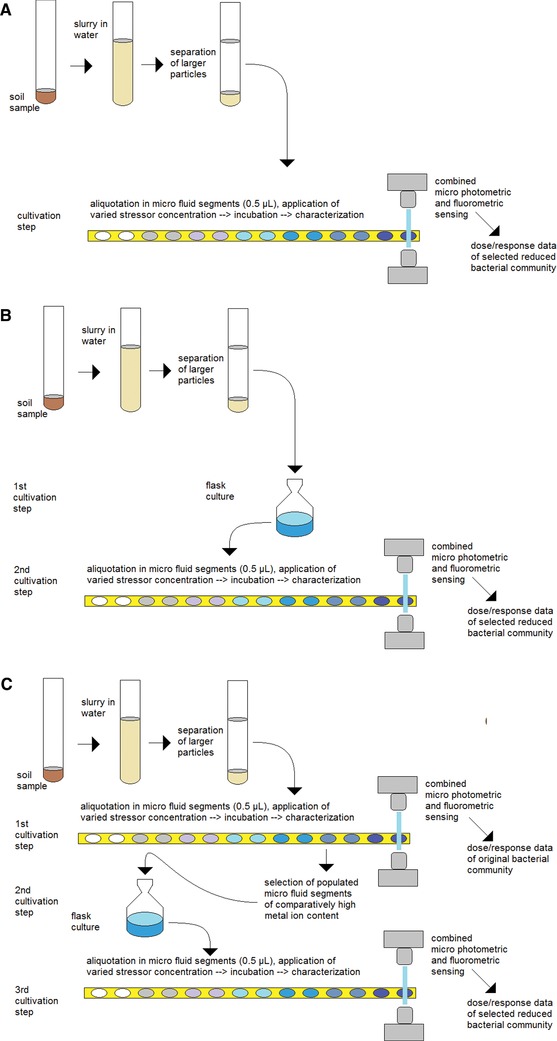
Experimental procedure for stochastic confinement, concentration‐dependent culture in micro fluid segments for dose/response characterization of reduced communities: (A) without preselection, (B) with one selection step, (C) with two selection steps.
To investigate the effects, all segments were moved after a certain incubation time from one tube coil through the micro flow‐through photometer and fluorimeter to another tube coil. The concentration‐dependent transmission and fluorescence supply the information on the growth of bacteria in the single segments. In experiments in which a strong growth of bacteria at higher metal ion concentrations was observed, well‐populated micro fluid segments from the range of higher stressor concentration were transferred into a new culture flask under the same metal ion stress. After a cultivation time of 4 days the obtained suspension of grown bacteria was brought into a syringe pump again, and another sequence of micro fluid segments with a step‐wise varied metal ion concentration was generated (Fig. 2C). As a result, dose response functions of preselected input communities could be obtained after incubation.
3. Results
3.1. Stochastic confinement and stochastic response
In this experiment (Fig. 3) two culture steps were carried out: 1st culture step: A slurry of soil sample N6 and N1 was transferred into flask filled with AM minimal medium cultivated under 5 mM nickel stress for N6 and 1.4 mM cobalt stress for N1 for 25 days. Second culture step: the growing cell culture was distributed into micro fluid segments containing AM solutions with an increasing content of the colloidal solution of palladium nanoparticles for sample N6 (Fig. 3A) and CuSO4 for sample N1 (Fig. 3B).
Figure 3.
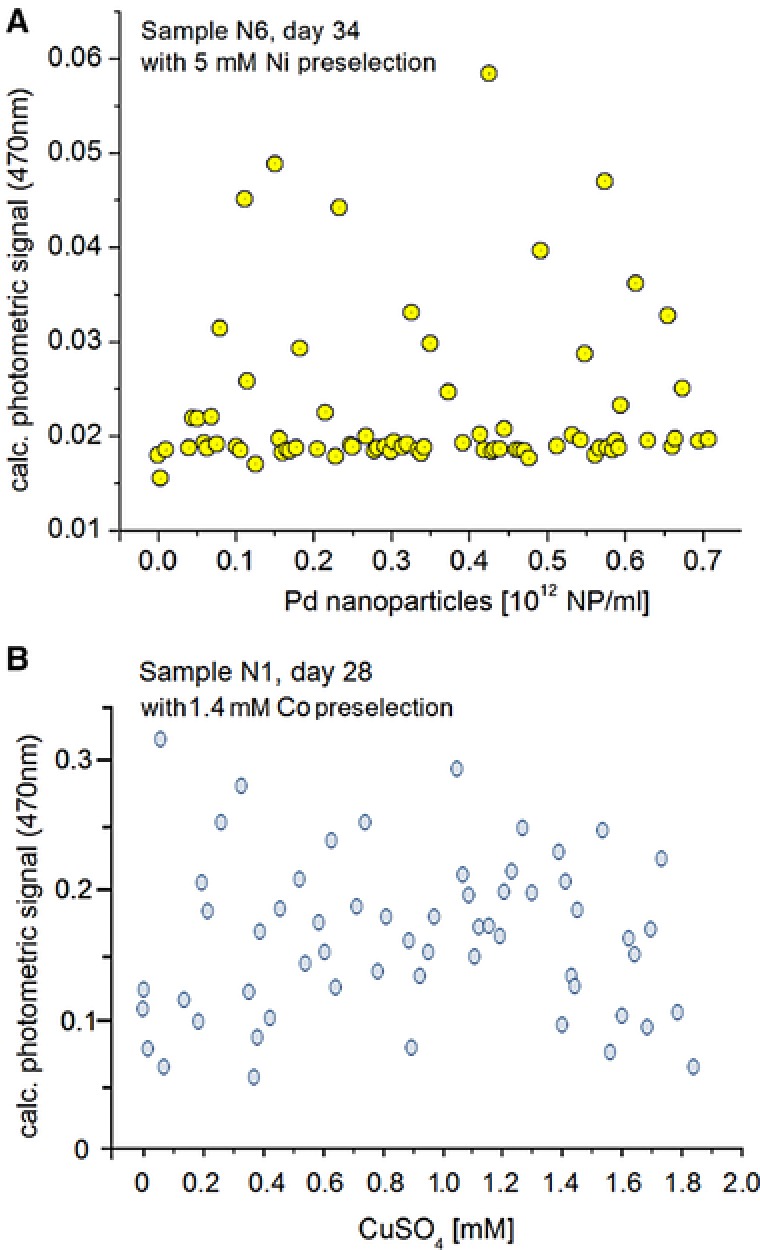
Stochastic response of growth of microorganisms of soil samples: (A) sample N6 (preselected with 5 mM Ni(II) in flask incubation) incubated in micro fluid segments containing different concentrations of Pd nanoparticles in the concentration range between (0‐0.7)·1012 NP/mL; measurement of the photometric signal (470 nm) after 34 days of incubation; (B) sample N1 (preselected with 1.4 mM Co(II) in flask incubation) incubated in micro fluid segments containing different concentrations of Cu(II) (0‐1.9 mM); measurement of the photometric signal (470 nm) after 28 days of incubation.
As a results, sample N6 after an incubation period of 34 days inside micro droplets the growth‐related micro photometric signal obviously indicated two different types of response over the whole range of nanoparticle concentrations between about (0‐0.7)·1012 NP/ml (Fig. 3A). The majority of segment has shown none or a very low increase of photometric signal. Certain segments showed a significant increase regarding the photometric signal. This can be interpreted as an increased optical density of the bacterial population.
In case of the incubation of a slurry of sample N1 in AM medium under copper stress (0‐1.9 mM Cu concentration) nearly no significant growth inhibition after an incubation period of 28 days was found (Fig. 3B).
3.2. Comparison of response of two soil samples
In case of soil samples E63 and N1, a significant sensitivity against nickel ions was observed. Above a concentration of about 1.2 mM nickel the growth was suppressed for the microbiological communities of both samples (Fig. 4A). Below this critical value, a subdivision of segments in two response groups was found in the nickel concentration range between about 0.3 and 1.2 mM. In this range, the majority of segments were also marked by a strong suppression of bacterial growth, except that some segments populated and showed a moderate increase in the optical density. This subdivision into a higher number of no growing and a smaller number of moderate growing populations was found for sample E63, as well as for sample N1. Nearly no segments with complete growth suppression have been found for nickel concentrations below 0.3 mM.
Figure 4.
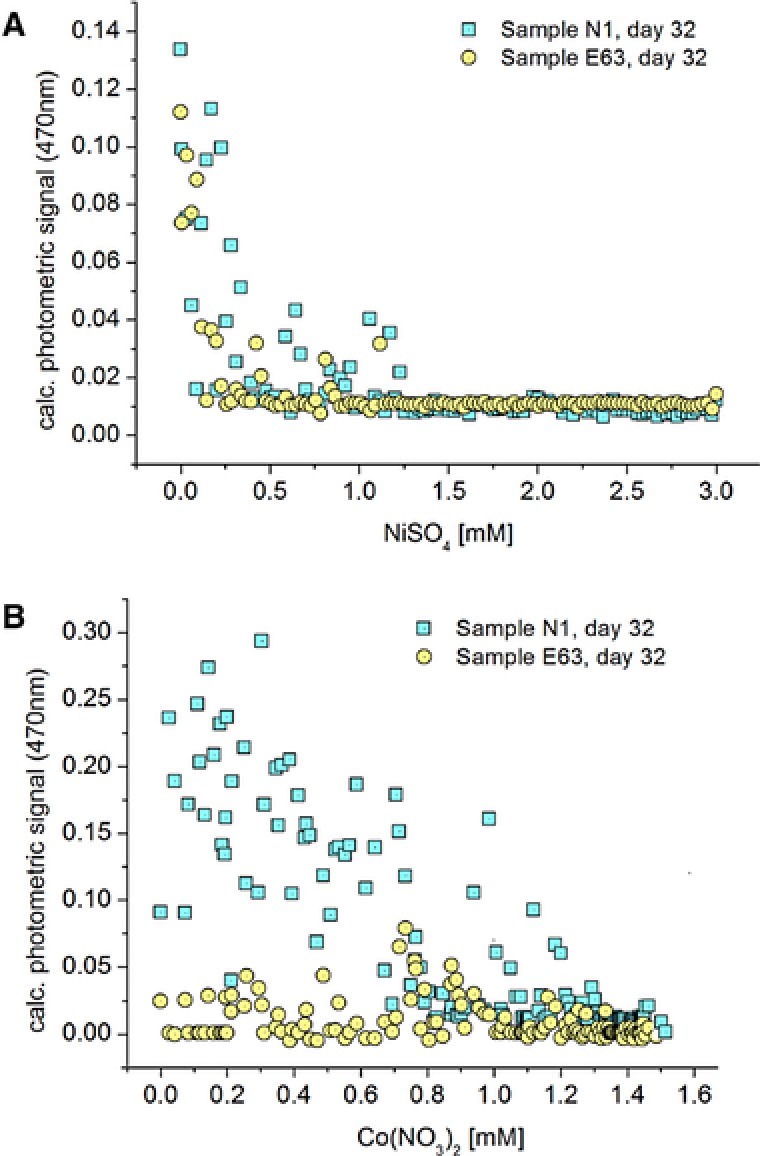
Comparison of the dose/response function of two different soil samples (N1, E63) cultivated inside nanoliter segments of 32 days. (A) soil samples against NiSO4; (B) soil samples against Co(II), characterized by micro photometric sensing.
Whereas the soil samples E63 and N1 showed a similar dose‐response relationship against Ni, in case of these soil samples against cobalt showed quite different response behavior (Fig. 4B). Sample N1 showed a much higher growth than sample E63. In particular, in the concentration range between 0 and 0.7 mM Co, the growth in segments of soil sample E63 was strongly suppressed, whereas all segments of sample N1 showed a high optical density.
3.3. Concentration‐dependent response classes
Despite all scattering in the stochastic response, the majority of samples reflect the expected general decrease of photometric and fluorimetric signals with increasing metal ion concentrations. Beside this general behavior, in a few cases, a significant increase from lower to higher optical signals was observed with increasing concentrations of heavy metal ions. The changing characteristics of the optical signals are frequently marked by a sharp transition within a small concentration interval. Two of these significant sharp transitions separating three clearly distinguishable concentration ranges have been found by sample E65 in the presence of cobalt (Fig. 5A). In this case, the highest autofluorescence intensity can be found in the range between 1.6 and 2.3 mM Co.
Figure 5.
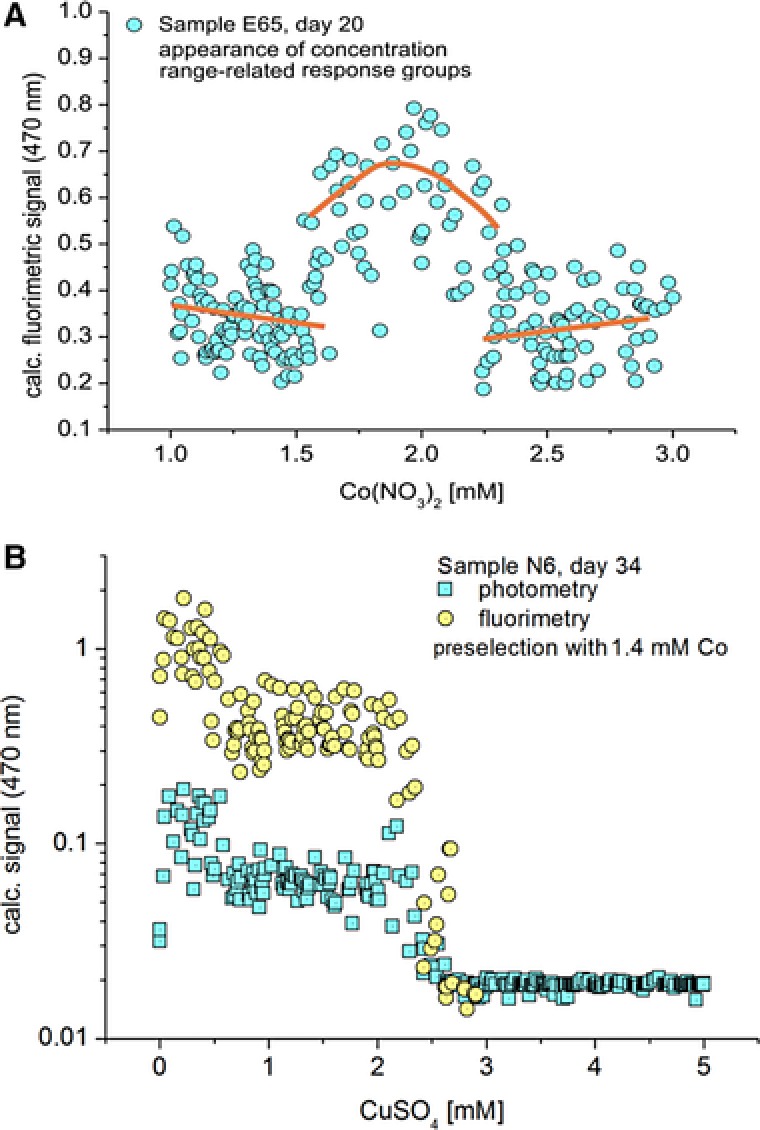
(A) Formation of three characteristic concentration‐dependent response groups in the case of the exposition of microbial communities from soil sample E65 in AM medium containing Co(II) in the concentration range between 1 and 3 mM (The lines are drawn in order to illustrate the significant changes in the character of responses in the three different concentration ranges); (B) dose/response function in dependence on Cu(II) of a reduced community obtained by micro segmented flow technique after two preselection steps by first culture step in micro fluid segments with enhanced Co(II) concentration and a second culture step in a flask (soil sample N6).
The structure of dose/response functions becomes clearer after a preselection. The whole procedure followed the scheme of Fig. 2C. After a first culture in micro fluid segments with varied cobalt concentration and a following culture of selected segments in a flask, the obtained bacterial cultures have been introduced into a segment sequence with step‐wise varied copper concentration, again. As a result, two concentration ranges with step‐wise reduced bacterial growth have been observed in this case (Fig. 5B). A first transition between the highest and the intermediate growth activity was found at about 0.6 mM Cu and a second transition between intermediate growth and growth suppression was found at about 2.5 mM Cu concentration. This transition points were well reflected by the fluorimetric signal as well as by the optical density.
3.4. Soil microbial response in case of partial compensation of nickel toxicity by histidine
Here, the effect of nickel and histidine on the concentration‐dependent dose/response functions of a bacterial community from a soil sample (N1) was investigated. The bacterial community of sample N1 showed a strong bacterial growth up to a Ni concentration of about 0.3 mM. In this lowest concentration range, all segments showed a bacterial growth after the incubation time of 32 days (Fig. 6). However, this activity was strongly reduced above 0.3 mM nickel. Above this critical value, the majority of segments showed no bacterial growth and only a few segments with moderate bacterial growth have been found. No significant bacterial growth was observed at nickel concentrations above 1.25 mM. This situation changed drastically after the addition of 7.5 mM histidine. No segments without bacterial growth existed up to 1.7 mM Ni. In this range the extinction values were threefold higher than in the absence of the complexing amino acid. The limit for complete growth suppression had shifted up to about 2.5 mM nickel concentration.
Figure 6.
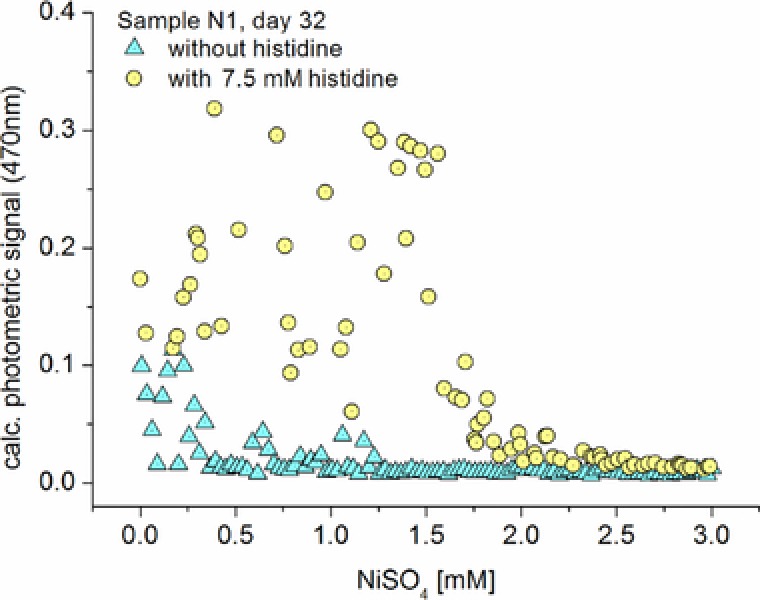
Effect of complexation of nickel ions by histidine on the response of a bacterial community of a soil sample (N1) on the nickel content in AM medium in the concentration range up to 3 mM.
4. Discussion
In many cases, typical consequences of stochastic confinements can be observed in applications of droplet‐based microfluidics for the investigation of soil samples. A stochastic distribution of various organisms is reflected by different types of growth intensity. A typical example is shown in Fig. 3A. Nevertheless, some of segments were marked by a significant increase of the photometric signal, which hints to an increase of the bacterial populations inside these segments. It seems that the majority of segments did not contain nickel‐tolerant bacteria while some segments did and therefore showed the enhanced signals. Obviously, all segments having their final optical density around or below a value of 0.02 are nickel‐sensitive (about 69%), whereas the segments with optical density above 0.02 (about 31%) are populated by nickel‐tolerant species. It is assumed, that the ratio of droplets with significant and of suppressed growth is due to the statistical distribution of growing nickel‐tolerant bacteria. The appearance of populated droplets at higher nickel concentrations and the approximatively constant ratio of populated and growth‐suppressed droplets reflect the high nickel tolerance of the growing organisms.
The fact of nearly no inhibition up to a content of 1.9 mM copper as found for the sample N1 indicates for the presence of copper‐tolerating bacteria in nearly all segments (Fig. 3B). The observation of heavy metal‐tolerant organisms in the investigated samples correlates well with the fact that the exposition of soil with heavy metal ions enhances the tolerance of bacterial community 21. It is known that bacterial communities are modified under heavy metal stress 22. This modification means a shift in the species composition that seems to be typical for any stress application 23, but the diversity can remain high 24. Even temporal and short‐term changes influence the composition of bacterial communities, whereby cumulative effects could be observed 25. The data of concentration‐dependent response of bacteria from soil samples reflect the stability and the potential of functional resilience of communities after perturbation. This corresponds to the fact that bacterial diversity promotes the ecological robustness of communities in case of perturbations 26.
In sample N1 and E63 up to 0.3 mM nickel, high photometric signals in some segments indicated high densities of bacteria after incubation inside micro droplets (Fig. 4). The signals underline the presence of different growing organisms in this lowest concentration range. It is remarkable, that the main features of the dose/response functions of both soil samples–originating from different geographical regions—were identical. This observation changed regarding the sensitivity of the bacterial communities of the same both soil samples against cobalt. In Fig. 4A it can be seen, that the samples from the two different places show a similar response on nickel exposition. But their response varies significantly in case of the exposition to cobalt (Fig. 4B).
The assumption was made, that the sharp transition in the character of the response of the ensemble of micro fluid segments was due to a change in the dominance of one or several species within a certain concentration interval. Such behavior was observed in case of sample E65 in dependence on the cobalt exposition (Fig. 5A). Probably, the transition borderlines mark critical toxic concentrations for a species or a special community. A clear dose response‐relationship of sample N6 against Cu indicates a strong reduction of the number of growing species (Fig. 5B). The photometric and fluorimetric sensor signals are scattering in a comparatively small range, only. This refers a high homogeneity of bacteria growth within single segments. In the lower concentration range the scattering of the fluorescence signal is more enhanced than the signal itself. This can be interpreted by the existence of several organisms, which grow within this concentration range.
Obviously, the different responses in Fig. 5 illustrate the differences character of the communities of soil samples. The dose/response functions in Fig. 5B seems to be exclusively determined by the response of a single species or, at least, it is dominated by uniform populations because of the observed a sharp transition between high and suppressed growth in a small concentration interval (around 2.3 mM Cu). Additionally growing organisms might only be existent below 0.5 mM Cu. In contrast, the community in Fig. 5A must be more complex composed. The species, which is dominating the fluorescence intensity in the range between 1.6 mM and 2.6 mM Co, is suppressed below 1.6 mM as well as above 2.6 mM cobalt concentration.
It is well known that histidine forms stable coordinative compounds with nickel ions 27. Thus, the concentration of freely available nickel ions is strongly reduced. However, nickel can be supplied by a release of itself from the reversible formed complexes. These effects are obviously reflected by the dose‐dependent culture experiments in the presence of nickel and histidine with the sample N1 (Fig. 6). Obviously, moderate nickel sensitive organisms, which cannot grow in the absence of histidine, were able to grow at enhanced nickel concentration in the presence of histidine. The observed effect of histidine on the nickel toxicity corresponds well with the observed nickel accumulation and storage 28, for example in histidine‐rich proteins in Heliobacter pylori 29 and Pseudomonas putida 30, but also in eukaryotic cells like yeast 31.
The investigations proved that microfluidic technique allows the transfer of the principle of stochastic confinement of reduced communities from soil micropores into nanoliter droplets. In addition, different stress conditions can be automatically realized by computer‐controlled flow rate programs during aliquotation of microorganism suspensions and generation of micro droplets. Optical micro sensing supplies empirical information on the concentration‐dependent growth behavior and on fluorescence activity of the single fluid segments in large sets of droplets.
Based on the microfluidic technique, different characteristic response behaviors were recognized. A strategy was shown, (1) to determine stochastic responses in case of domination of populated segments as well as in case of growth‐suppressed segments, and (2) to compare soil samples concerning their sensitivity and critical toxicity thresholds against different heavy metal ions. Thus, similarities in the dose/response functions of two soil samples against nickel ions have been observed, whereas the same pair of samples showed strong differences in their dose/response functions for the exposition by cobalt ions. In some cases, concentration ranges with a typical response pattern and sharp concentration‐modulated transitions between the concentration‐dependent response classes were identified. Finally, microfluidic technique for analysing the concentration dependence of antagonistic effects of different components in the culture media, which was investigated by highly concentration‐resolved effect of nickel on the bacterial community of a soil sample in the presence of histidine, was successfully applied.
In contrast, to metagenomics studies, the used strategy was based on cultivation and supplies, therefore, information on the effect of heavy metal ions on the part of microbial genepool that is able to grow under the given conditions. Additionally, in the future it might be helpful to detect bacteria and consortia with special biosorption characteristics 32, 33, which could become useful for the development of biological accumulation and detoxification strategies 34.
In summary, it can be concluded that micro fluidics, and in particular the technique of micro segmented flow, offers promising new strategies for the screening of soil microorganisms and for searching for new types, for the characterization of communities and for the improvement of the economic utilization.
Practical application
Microfluidics was successful applied for evaluation of stress response of soil microbial communities. Micro droplets are a model for occasional fractionating of microbial communities in soil. Different soil samples show different response patterns on chemical stress.
The authors have declared no conflicts of interest.
Acknowledgments
The financial support of the BMBF (Beo Jülich, project Bactocat, Kz. 031A161A) is gratefully acknowledged.
5 References
- 1. Kennedy, A. C. , Smith, K. L. , Soil microbial diversity and the sustainability of agricultural soils. Plant Soil. 1995, 170, 75–86. [Google Scholar]
- 2. van Bruggen, A. H. C. , Semenov, A. M. , In search of biological indicators for soil health and disease suppression. Appl. Soil Ecol. 2000, 15, 13–24. [Google Scholar]
- 3. Guo, M. T. , Rotem, A. , Heyman, J. A. , Weitz, D. A. , Droplet microfluidics for high‐throughput biological assays. Lab. Chip. 2012, 12, 2146–2155. [DOI] [PubMed] [Google Scholar]
- 4. Kaminski, T. S. , Scheler, O. , Garstecki, P. , Droplet microfluidics for microbiology: techniques, applications and challenges. Lab. Chip. 2016, 16, 2168–2187. [DOI] [PubMed] [Google Scholar]
- 5. Jiang, L. , Boitard, L. , Broyer, P. , Chareire, A. C. et al., Digital antimicrobial susceptibility testing using the MilliDrop technology. Eur. J. Clin. Microbiol. 2016, 35, 415–422. [DOI] [PubMed] [Google Scholar]
- 6. Lok, C. , Mining the microbial dark matter. Nature 2015, 522, 270–273. [DOI] [PubMed] [Google Scholar]
- 7. Kursten, D. , Kothe, E. , Wetzel, K. , Bergmann, K. et al., Micro‐segmented flow and multisensor‐technology for microbial activity profiling. Environ. Sci‐Proc. Imp. 2014, 16, 2362–2370. [DOI] [PubMed] [Google Scholar]
- 8. Marz, A. , Henkel, T. , Cialla, D. , Schmitt, M. et al., Droplet formation via flow‐through microdevices in Raman and surface enhanced Raman spectroscopy‐concepts and applications. Lab. Chip. 2011, 11, 3584–3592. [DOI] [PubMed] [Google Scholar]
- 9. Walter, A. , Marz, A. , Schumacher, W. , Rosch, P. et al., Towards a fast, high specific and reliable discrimination of bacteria on strain level by means of SERS in a microfluidic device. Lab. Chip. 2011, 11, 1013–1021. [DOI] [PubMed] [Google Scholar]
- 10. Kohler, J. M. , Henkel, T. , Grodrian, A. , Kirner, T. et al., Digital reaction technology by micro segmented flow—components, concepts and applications. Chem. Eng. J. 2004, 101, 201–216. [Google Scholar]
- 11. Cao, J. L. , Kohler, J. M. , Droplet‐based microfluidics for microtoxicological studies. Eng. Life Sci. 2015, 15, 306–317. [Google Scholar]
- 12. Cao, J. L. , Kursten, D. , Krause, K. , Kothe, E. et al., Application of micro‐segmented flow for two‐dimensional characterization of the combinatorial effect of zinc and copper ions on metal‐tolerant Streptomyces strains. Appl. Microbiol. Biot. 2013, 97, 8923–8930. [DOI] [PubMed] [Google Scholar]
- 13. Kursten, D. , Moller, F. , Gross, G. A. , Lenk, C. et al., Identification of response classes from heavy metal‐tolerant soil microbial communities by highly resolved concentration‐dependent screenings in a microfluidic system. Methods Ecol. Evol. 2015, 6, 600–609. [Google Scholar]
- 14. Boedicker, J. Q. , Li, L. , Kline, T. R. , Ismagilov, R. F. , Detecting bacteria and determining their susceptibility to antibiotics by stochastic confinement in nanoliter droplets using plug‐based microfluidics. Lab. Chip. 2008, 8, 1265–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martin, K. , Henkel, T. , Baier, V. , Grodrian, A. et al., Generation of larger numbers of separated microbial populations by cultivation in segmented‐flow microdevices. Lab. Chip. 2003, 3, 202–207. [DOI] [PubMed] [Google Scholar]
- 16. Liu, W. S. , Kim, H. J. , Lucchetta, E. M. , Du, W. B. et al., Isolation, incubation, and parallel functional testing and identification by FISH of rare microbial single‐copy cells from multi‐species mixtures using the combination of chemistrode and stochastic confinement. Lab. Chip. 2009, 9, 2153–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clausell‐Tormos, J. , Lieber, D. , Baret, J. C. , El‐Harrak, A. et al., Droplet‐based microfluidic platforms for the encapsulation and screening of mammalian cells and multicellular organisms. Chem. Biol. 2008, 15, 427–437. [DOI] [PubMed] [Google Scholar]
- 18. Amoroso, M. J. , Schubert, D. , Mitscherlich, P. , Schumann, P. et al., Evidence for high affinity nickel transporter genes in heavy metal resistant Streptomyces spec. J. Basic Microb. 2000, 40, 295–301. [DOI] [PubMed] [Google Scholar]
- 19. Hafermann, L. , Köhler, J. M. , Photochemical micro‐continuous flow synthesis of noble metal nanoparticles of the platinum group. Chem. Eng. Technol. 2015, 38, 1138–1143. [Google Scholar]
- 20. Cao, J. L. , Kursten, D. , Schneider, S. , Knauer, A. et al., Uncovering toxicological complexity by multi‐dimensional screenings in microsegmented flow: modulation of antibiotic interference by nanoparticles. Lab. Chip. 2012, 12, 474–484. [DOI] [PubMed] [Google Scholar]
- 21. Li, J. , Zheng, Y. M. , Liu, Y. R. , Ma, Y. B. et al., Initial copper stress strengthens the resistance of soil microorganisms to a subsequent copper stress. Microb. Ecol. 2014, 67, 931–941. [DOI] [PubMed] [Google Scholar]
- 22. Sitte, J. , Loffler, S. , Burkhardt, E. M. , Goldfarb, K. C. et al., Metals other than uranium affected microbial community composition in a historical uranium‐mining site. Environ. Sci. Pollut. R. 2015, 22, 19326–19341. [DOI] [PubMed] [Google Scholar]
- 23. Griffiths, B. S. , Kuan, H. L. , Ritz, K. , Glover, L. A. et al., The relationship between microbial community structure and functional stability, tested experimentally in an upland pasture soil. Microb. Ecol. 2004, 47, 104–113. [DOI] [PubMed] [Google Scholar]
- 24. Li, J. , Hu, H. W. , Ma, Y. B. , Wang, J. T. et al., Long‐term nickel exposure altered the bacterial community composition but not diversity in two contrasting agricultural soils. Environ. Sci. Pollut. R. 2015, 22, 10496–10505. [DOI] [PubMed] [Google Scholar]
- 25. Ranjard, L. , Lignier, L. , Chaussod, R. , Cumulative effects of short‐term polymetal contamination on soil bacterial community structure. Appl. Environ. Microb. 2006, 72, 1684–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Girvan, M. S. , Campbell, C. D. , Killham, K. , Prosser, J. I. et al., Bacterial diversity promotes community stability and functional resilience after perturbation. Environ. Microbiol. 2005, 7, 301–313. [DOI] [PubMed] [Google Scholar]
- 27. Lascelles, K. , Morgan, L. G. , Nicholls, D. , Beyersmann, D. , Nickel compounds, Ullmann's Encyclopedia of Industrial Chemistry. Wiley‐VCH, Weinheim, 2000. [Google Scholar]
- 28. Dosanjh, N. S. , Michel, S. L. J. , Microbial nickel metalloregulation: NikRs for nickel ions. Curr. Opin. Chem. Biol. 2006, 10, 123–130. [DOI] [PubMed] [Google Scholar]
- 29. Ge, R. G. , Watt, R. M. , Sun, X. S. , Tanner, J. A. et al., Expression and characterization of a histidine‐rich protein, Hpn: potential for Ni2+ storage in Helicohacter pylori . Biochem. J. 2006, 393, 285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ray, P. , Girard, V. , Gault, M. , Job, C. et al., Pseudomonas putida KT2440 response to nickel or cobalt induced stress by quantitative proteomics. Metallomics 2013, 5, 68–79. [DOI] [PubMed] [Google Scholar]
- 31. Pearce, D. A. , Sherman, F. , Toxicity of copper, cobalt, and nickel salts is dependent on histidine metabolism in the yeast Saccharomyces cerevisiae (vol 181, pg 4774, 1999). J. Bacteriol. 1999, 181, 6856–6856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haferburg, G. , Merten, D. , Buchel, G. , Kothe, E. , Biosorption of metal and salt tolerant microbial isolates from a former uranium mining area. Their impact on changes in rare earth element patterns in acid mine drainage. J. Basic Microb. 2007, 47, 474–484. [DOI] [PubMed] [Google Scholar]
- 33. Hassen, A. , Saidi, N. , Cherif, M. , Boudabous, A. , Effects of heavy metals on Pseudomonas aeruginosa and Bacillus thuringiensis . Bioresource Technol. 1998, 65, 73–82. [Google Scholar]
- 34. Bruins, M. R. , Kapil, S. , Oehme, F. W. , Microbial resistance to metals in the environment. Ecotox Environ. Safe. 2000, 45, 198–207. [DOI] [PubMed] [Google Scholar]


