Abstract
Biomachining has become a promising alternative to micromachining metal pieces, as it is considered more environmentally friendly than their physical and chemical machining counterparts. In this research work, two strategies that contribute to the development of this innovative technology and could promote its industrial implementation were investigated: preservation of biomachining microorganisms (Acidithiobacillus ferrooxidans) for their further use, and making valuable use of the liquid residue obtained following the biomachining process. Regarding the preservation method, freeze‐drying, freezing, and drying were tested to preserve biomachining bacteria, and the effect of different cryoprotectants, storage times, and temperatures was studied. Freezing at –80°C in Eppendorf cryovials using betaine as a cryoprotective agent reported the highest bacteria survival rate (40% of cell recovery) among the studied processes. The treatment of the liquid residue in two successive stages led to the precipitation of most of the total dissolved iron and divalent copper (99.9%). The by‐products obtained (iron and copper hydroxide) could be reused in several industrial applications, thereby enhancing the environmentally friendly nature of the biomachining process.
Keywords: Acidithiobacillus ferrooxidans, Biomachining, Liquid residue treatment, Preservation of microorganisms
Abbreviation
- BLR
biomachining liquid residue
1. Introduction
Recent advances in biotechnology have led to the development of biomachining as a promising alternative to the physical and chemical micromachining methods used in manufacturing microelectromechanical devices. The technology refers to the use of microorganisms to selectively form microstructures on a workpiece through the removal or dissolution of metal 1, 2, and regarding material removal, it is considered more environmentally friendly than other techniques, due to lower energy consumption, lower cost, and the nonuse of hazardous components 1, 3, 4, 5.
Acidithiobacillus ferrooxidans has been widely used in the bioleaching and indirect biomachining of copper 6, 7, 8, 9, 10. This bacterium utilizes the energy generated by oxidation of ferrous ions to ferric ions to fix carbon dioxide in the air (Eq. 1). In a second step (Eqs. 2‐3), the biogenic ferric ion brings about the dissolution of copper metal 4, 7, 11.
| (1) |
| (2) |
| (3) |
Full‐scale biomachining has not yet been implemented, despite the numerous advantages of this method as complement or alternative to traditional machining processes. Therefore, having a deeper knowledge of the process and optimizing operational aspects such as the preservation of microorganisms and the valorization of the biomachining liquid residue, could promote the industrial implementation of this green technology.
A reliable preservation method that enables long‐term storage of A. ferrooxidans while maintaining cell viability would ensure the ready availability of the bacteria and prevent mutations and contamination of cultures 12, 13, 14, 15. Successful techniques for preserving A. ferrooxidans have already been studied 16, 17, 18, 19. A. ferrooxidans is highly susceptible to cryoinjury 17 and the addition of a suitable protective agent is therefore critical for cell survival or for improving cell viability 17, 19, 20, 21. In the particular case of A. ferrooxidans, protective agents such as GP (glycerate 3‐phosphate molecule), glycine betaine, skimmed milk, sucrose, sucrose + mannitol, and glycerol have already been tested in the different preservation methods 16, 17, 19. Preservation in the absence of cryoprotective agents is also possible with the aid of cryoballs, which are now widely used 22.
Regarding the treatment of the liquid residue generated in the biomachining process, no research has been carried out with the aim of making use of this by‐product.
In this research work, strategies for promoting the industrial application of this innovative micromachining technology have been investigated. Three methods of preserving A. ferrooxidans cells were tested (freeze‐drying, freezing, and drying), and the effect of different cryoprotectants and storage times, among other key parameters, were evaluated. In addition, a procedure for treating the liquid residue generated in the process was also described, with the aim of obtaining a less contaminating residue and of recovering the dissolved metals as high purity precipitates.
2. Materials and methods
2.1. Preservation of A. ferrooxidans
2.1.1. Culture medium
A. ferrooxidans (DSM‐14882) bacterial cells were cultured in a specific broth (9K) the chemical composition and initial pH value (adjusted with H2SO4) of which are summarized in Table 1.
Table 1.
Culture media: Chemical composition and initial pH
| Medium | Chemical composition (g/L) | Reference |
|---|---|---|
| Liquid broth | ||
| 9K | 30 FeSO4·7H2O | 39 |
| 3 (NH4)2SO4 | ||
| 0.5 Mg(SO4)·7H2O | ||
| 0.5 K2HPO4: | ||
| 0.1 KCl | ||
| 0.01Ca(NO3)2·4H2O | ||
| pH = 1.8 | ||
| Solid medium | ||
| A | 3.0 (NH4)2SO4 | 24 |
| 0.1KCl | ||
| 0.5 K2HPO4 | ||
| 0.5 MgSO4·7H2O | ||
| 0.01Ca(NO3)2 | ||
| 16.5 FeSO4·7H2O | ||
| 0.7% (w/v) Agarose | ||
| pH 2.5 | ||
| B | Solution A: | |
| 20% (w/v) FeSO4 (36.57 g/L FeSO4·7H2O) | 25 | |
| pH 2.0 | ||
| Solution B: | ||
| 1.8 (NH4)2SO4 | ||
| 0.7 MgSO4·7H2O | ||
| 0.35 TSB | ||
| pH 2.5 | ||
| Solution C: | ||
| Agarose 2.8% (w/v) | ||
| No pH adjustment | ||
| A:B:C solutions mixed in 1:14:5 ratio | ||
| C | Solution A: | 26 |
| 0.5 (NH4)2SO4 | ||
| 0.5 Mg(SO4)·7H2O | ||
| 0.5 K2HPO4·7H2O | ||
| 5.0 mL H2SO4 (15 N) | ||
| pH 1.3 | ||
| Solution B: | ||
| 167 g FeSO4·7H2O | ||
| 50 mL H2SO4 (15 N) | ||
| pH 1.3 | ||
| A:B solutions mixed in 4:1 ratio and agarose added to 0.7% (w/v) | ||
In addition, three different culture media (A, B, and C) were tested separately in order to assess their suitability for determining cell viability. The chemical composition and the initial pH (adjusted with H2SO4) of the three solid media tested are shown in Table 1.
2.1.2. Cell preservation
Freeze‐drying, freezing, and drying were tested as methods of preserving A. ferrooxidans.
2.1.2.1. Freeze‐drying
Two different experiments (FD1 and FD2) were carried out to determine the effects of the duration of primary drying (12 or 35 h, respectively) and of three cryoprotective agents (sucrose, glycerol, and trehalose) on the viability of freeze‐dried A. ferrooxidans cells. The conditions for each experiment are defined in Table 2.
Table 2.
Freeze‐drying experiments
| FD1 | FD2 | FD3 | |
|---|---|---|---|
| Culture time (days) | 14 | 18 | 4 |
| Initial cell concentration (CFU/mL) | 1.8·109 | 5.2·108 | 2.1·109 |
| Primary drying temperature (°C) | 5 | 5 | 5 |
| Primary drying duration (h) | 12 | 35 | 35 |
| Secondary drying temperature (oC) | 30 | 30 | 30 |
| Secondary drying duration (h) | 3 | 3 | 3 |
| Cryprotectant (% w/v in distilled water) | Glycerol (5%) Sucrose (18%) Trehalose (15%) | Glycerol (5%) Sucrose (18%) Trehalose (15%) | Glycerol (5%) Sucrose (18%) Trehalose (15%) Betaine (6%) |
| Storage time | None | None | None |
| 4 weeks at 4ºC |
In a third experiment (FD3), the influence of using younger cells from the beginning of the process was studied and a fourth cryoprotectant was also tested (betaine) (Table 2). In this third experiment, duplicate samples were freeze‐dried in order to study how storage at 4ºC for one month (4 weeks) affected cell viability.
In the three experiments, the first step in sample preparation was the removal (by deposition) of the solid ferrous residues that may be present in solution after cell cultivation. Thus, the cell cultures (200–400 mL) were incubated at room temperature for 10 min without stirring and then the steps indicated in Table 3 were performed.
Table 3.
Freeze‐drying and freezing process steps
| Freeze‐drying | |||||
|---|---|---|---|---|---|
| Process steps | 1 | 2 | 3 | 4 | |
| Description | Cell harvesting by centrifugation | Resuspension of the cell pellet | Mixing of the aliquots in a single sample and cell harvesting by centrifugation | Supernatant removal and resuspension of the cell pellet | |
| Conditions | 10 000 rpm, 20 min, 4ºC (Eppendorf 5810‐R, Eppendorf AG, Hamburg/Germany) | 15 mL of 9K medium (Table 1) | 10 000 rpm, 15 min, 4ºC (Eppendorf 5810‐R, Eppendorf AG, Hamburg/Germany) | 15 mL of distilled water | |
| Volume per aliquot (mL) | 65 | 65 | 325 | 325 | |
| Number of aliquots | 5 | 5 | 1 | 1 | |
| Process steps | 5 | 6 | 7 | 8 | |
| Description | Cell harvesting by centrifugation | Supernatant removal and resuspension of the cell pellet | Incubation | Freeze‐drying | |
| Conditions | 10 000 rpm, 20 min, 4ºC (Eppendorf 5415‐R, Eppendorf AG, Hamburg/Germany) | 0.5 mL of cryoprotectant (Table 2) | 30ºC, 1 h | Freezing (–40ºC) Primary drying (5ºC, 0.250 mbar) Secondary drying (30ºC, 0.250 mbar) | |
| Volume per aliquot (mL) | 1.5 | 0.5 | 0.5 | 0.5 | |
| Number of aliquots | 18 | 18 | 18 | 18 | |
| Freezing | |||||
|---|---|---|---|---|---|
| Initial process | Process steps | I | II | III | IV |
| Description | Cell harvesting by centrifugation | Resuspension of the cell pellet | Mixing of the aliquots in a single sample and cell harvesting by centrifugation | Supernatant removal and resuspension of the cell pellet | |
| Conditions | 10 000 rpm, 15 min, 4ºC (Eppendorf 5810‐R, Eppendorf AG, Hamburg/Germany) | 15 mL distilled water | 10 000 rpm, 15 min, 4ºC (Eppendorf 5810‐R, Eppendorf AG, Hamburg/Germany) | 15 mL distilled water | |
| Volume per aliquot (mL) | 65 | 65 | 325 | 325 | |
| Number of aliquots | 5 | 5 | 1 | 1 | |
| Eppendorf cryovials | Process steps | V | VI | VII | VIII |
| Description | Cell harvesting by centrifugation | Supernatant removal and resuspension of the cell pellet | Refrigeration and incubation | Freezing | |
| Conditions | 10 000 rpm, 15 min, 4ºC (Eppendorf 5415‐R, Eppendorf AG, Hamburg/Germany) | 1 mL of cryoprotectant | Refrigeration: 4ºC, 30–40 min Incubation: –20ºC, 1 h | –80ºC, 28 days | |
| Volume per aliquot (mL) | 1 | 1 | 1 | 1 | |
| Number of aliquots | 13 | 3 (glycerol) 3 (betaine) 3 (glycerol‐betaine) 4 (distilled water) | 3 (glycerol) 3 (betaine) 3 (glycerol‐betaine) 4 (distilled water) | 3 (glycerol) 3 (betaine) 3 (glycerol‐betaine) 4 (distilled water) | |
In all three experiments, an additional sample was resuspended in distilled water as a control.
The viability of A. ferrooxidans was tested before and immediately after freeze‐drying the samples obtained in all experiments (FD1, FD2, FD3, and FD3 after 4 weeks) (see “Cell viability” section).
2.1.2.2. Freezing
The effect of two freezing procedures on the viability of A. ferrooxidans was tested. The first method consisted of cryofreezing the samples in Eppendorf cryovials using different cryoprotectants, while the second method involved the use of commercial Cryoinstant vials (Scharlab, Barcelona/Spain). The experiments were carried out using several storage temperatures and times as these aspects are key to obtaining a high survival rate. In both cases, the process started with the procedure summarized in Table 3 (steps I–IV).
2.1.2.2.1. Freezing in Eppendorf cryovials
The freezing procedure was based on the protocols reported by Wu et al. 12, Wu et al. 17, and Cleland et al. 19 and is summarized in Table 3 (steps V–VIII). The composition of each cryoprotectant (step VI) was: autoclaved glycerol solution (30% in distilled water, w/v); filter sterilized betaine solution (6% in distilled water, w/v) and a sterile‐mixed solution containing glycerol and betaine (30% glycerol and 6% betaine in distilled water, w/v). The aliquots resuspended in distilled water were considered as controls.
The initial cell concentration of the samples prior to freezing was determined by spread plating one of the control samples onto the surface of a Petri plate containing medium B. The initial cell concentration (approximately 1.4·109 CFU m/L) was assumed to be the same for all samples before freezing.
Cell viability was calculated on days 14 and 28, to evaluate the effect of preservation time on the viability of A. ferrooxidans as described in the “Cell viability” section. For this purpose, the Eppendorf vials were thawed in a water bath (at 37ºC), and the cryoprotectant was removed by centrifugation (10 000 rpm, 10 min, 4ºC). The cell pellet was washed twice with 1 mL of fresh 9 K broth (by centrifuging at 10 000 rpm, 10 min, 4ºC) before being resuspended and diluted tenfold in distilled water.
2.1.2.2.2. Freezing in commercial cryovials
Two samples, each of 1 mL, subjected to steps I–IV as summarized in Table 3, were frozen in commercially available Cryoinstant vials (Scharlab, Barcelona/Spain) containing porous beads in culture medium and glycerol as a cryoprotectant. One of the cryovials was stored at –20ºC for 28 days. The second vial was frozen at –20ºC for 1 h before being stored at –80ºC for 28 days. On days 14 and 28, the commercial cryovials were thawed at room temperature and two beads were extracted from each. Cell viability was then determined by the methodology indicated in the “Cell viability” section by spreading the beads on solid medium B until the development of bacterial colonies.
2.1.2.3. Drying
Prior to drying, a suspension of A. ferrooxidans in culture media 9 K (cell viability = 8.7·107 CFU m/L) was concentrated by filtering in a 0.22 μm nitrocellulose vacuum filter. The concentrated cells retained on the filter (cell viability = 3.5·109 CFU m/L) were dried at 30ºC for 24 h in an empty sterile Petri plate. The filter containing the dry biomass was stored at 4°C and, after 14 days, the bacteria were resuspended in 10 mL of distilled water. Finally, dilution of the resuspended bacteria was spread on solid medium B and incubated at 30ºC for 12 days.
2.1.2.4. Cell viability
Cell viability was determined, after resuspending the samples in 1 mL of distilled water, by calculating the number of colony forming units per milliliter (CFU m/L). Viability was determined before (CFU0) and after (CFUt) submitting the cells to the corresponding method of preservation. Both CFU0 and CFUt were calculated by making serial dilutions of the liquid samples and plating these onto the surface of Petri plates containing medium B (Table 1). The plates were incubated at 30ºC for 12–14 days. The viability rate (V%) was expressed by the cell survival rate: V (%) = 100·CFTt/CFU0 23.
2.2. Treatment of the biomachining liquid residue (BLR)
2.2.1. Preparation of synthetic BLR
A synthetic BLR was prepared. The composition simulated the typical liquid residue generated in the biomachining process, i.e. an acidic liquid (pH = 2.3) containing approximately 3.0 g Fe2+ L−1, 3.0 g Fe3+ L−1, 6.7 g Cu2+ L−1, and 1.0 g NH4+ L−1. Thus, 3.8 g of FeSO4·7H2O, 6.5 g of (NH4)Fe(SO4)2·12H2O and 6.6 g of CuSO4·5H2O were dissolved in 250 mL of deionized water. The initial pH was adjusted to 2.27 by adding H2SO4 (25% w/v). The initial value of the Redox potential was 436.9 mV.
2.2.2. BLR treatment procedure
A 100 mL sample of the BLR was subjected to two successive stages, in duplicate. The objective of this sequential treatment was to remove the ferrous iron and the copper (II) ion in Stages I and II, respectively, by recovering the metals as pure metal hydroxides.
In Stage I, iron (II) was oxidized to iron (III) by adding hydrogen peroxide (30% w/v) to a continuously stirred 100 mL BLR sample until a change in the redox potential trend was observed. The pH was then increased from 2.27 to 4.0 by adding NaOH (97 mg/mL). Finally, the precipitated Fe(OH)3 was separated by simple filtration (paper filter, FilterLab‐1240).
In Stage II, the pH of the solution obtained (by filtration) in the previous stage was increased to 8.0 by adding NaOH (97 mg/mL), and the resulting precipitate was filtered (paper filter, FilterLab‐1240). Finally, a cylindrical piece of iron was submerged in the filtrate for 2 h to remove the remaining dissolved Cu2+ by reduction to Cu0 in the presence of iron. The sample solution was stirred and, after 1 h and at the end of the experiment, the pH was measured and the concentrations of Cu2+ and total Fe (FeT) in solution were determined. At the end of the process, the solution was characterized by determining the dissolved salt content as the weight of dry residue.
The same procedure was carried out by submerging a second piece of iron in an aqueous solution containing 20 g/L of Na2SO4 (main compound in the final filtrate). This was considered a blank solution for purposes of comparison.
2.3. Analytical methods
The redox potential was measured with a Thermo‐Orion 920A+ potentiometer and the pH, with a Crison GLP 21 pH‐meter.
The Cu2+ content was determined by atomic absorption (Perkin Elmer, Aanalyst 100, LabX, Midland/Canada). Total Fe (FeT) was measured by colorimetry. For this purpose, 5 mL of an ammonium acetate/acetic acid buffer solution (pH = 5.5) was added to a 50 mL flask containing 5 mL of sample. The sample was stirred and 2 mL of hydroxylamine hydrochloride solution (10% w/v) was added. The sample was stirred again and, after 5 min, 2 mL of a 2,2’‐bipyridine solution (0.5% w/v) was added. After 5 min, the absorbance of the 50 mL diluted solution was measured at 520 nm by spectrophotometry (Vis/UV Thermo‐Helios, Thermo Electron Corporation, Spain).
The dissolved salt content was determined by weighing the dry residue of the solution after evaporating the water content of an aliquot at 105ºC to constant weight.
3. Results
3.1. Preservation of A. ferrooxidans
3.1.1. Culture medium
Colonies of A. ferrooxidans grew on medium A 24 and medium B 25 within 6–7 days of incubation at 28ºC (Fig. 1A and B, respectively). However, no microbial growth was observed on medium C 26, even after incubation for 11 days (Fig. 1C).
Figure 1.
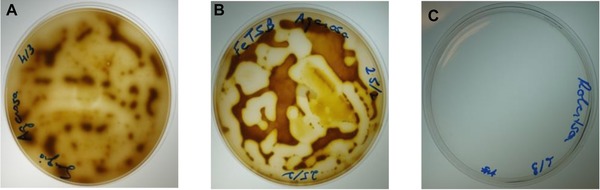
Growth of A. ferrooxidans on the three media tested: (A) Medium A, (B) Medium B, and (C) Medium C.
3.1.2. Cell preservation
3.1.2.1. Freeze‐drying
In FD1 (primary drying = 12 h), only the negative control was successfully freeze‐dried, while the samples resuspended in glycerol, sucrose, and trehalose remained damp at the end of the process. However, when the length of the primary drying stage was increased to 35 h in experiments FD2 and FD3, freeze‐drying of the control and the sample resuspended in the trehalose solution was successful.
Cell counts in the samples preserved in FD1 using cryoprotectants ranged between 2.8·102 CFU/mL and 8.1·103 CFU/mL (Table 4). The survival rate increased when the duration of the primary drying was prolonged to 35 h in experiment FD2. Nevertheless, by comparison to FD2, cell viability in experiment FD3 was reduced although in this case the primary drying was also established at 35 h. It should be noted that no A. ferrooxidans colonies were detected in the sample in which glycerol was used as a cryoprotectant.
Table 4.
Survival rate and concentration of A. ferrooxidans cells before and after freeze‐drying in experiments FD1 (primary drying = 12 h), FD2 (primary drying = 35 h), and FD3 (primary drying = 35 h) using different cryoprotective agents
| Cryoprotectant (% w/v in distilled water) | FD1 | FD2 | FD3 | FD3–4 weeks | |
|---|---|---|---|---|---|
| Control | Final cell concentration (CFU/mL) | 1.1·105 | 9.0·106 | 1.5·105 | No bacteria |
| Survival rate (%) | 0.0057 | 1.7 | 0.0073 | — | |
| Glycerol (5%) | Final cell concentration (CFU/mL) | 2.8·102 | 8.8·106 | No bacteria | No bacteria |
| Survival rate (%) | 0.000015 | 1.7 | — | — | |
| Sucrose (18%) | Final cell concentration (CFU/mL) | 3.4·103 | 4.5·106 | 2.8·105 | 7.0·101 |
| Survival rate (%) | 0.00018 | 0.90 | 0.013 | 0.025 | |
| Trehalose (15%) | Final cell concentration (CFU/mL) | 8.1·103 | 5.2·106 | 2.6·105 | 2.3·103 |
| Survival rate (%) | 0.00044 | 1.0 | 0.013 | 0.87 | |
| Betaine (6%) | Final cell concentration (CFU/mL) | — | — | 4.6·104 | 2.0·101 |
| Survival rate (%) | — | — | 0.0022 | 0.043 |
Cell viability in the aliquots preserved for 4 weeks using sucrose, trehalose and betaine decreased respectively to 7.0·101 CFU/mL, 2.3·103 CFU/mL and 2.0·101 CFU/mL. No colonies were detected in the control sample or in the sample supplemented with glycerol after storage for 4 weeks.
3.1.2.2. Freezing
Regarding the samples stored in Eppendorf tubes, cell concentration was highest in the one with 6% betaine (5.1·108 CFU/mL) after 28 days (Fig. 2). In the rest of the samples, the cell concentration varied from 4.4·105 CFU/mL to 2.1·108 CFU/mL.
Figure 2.
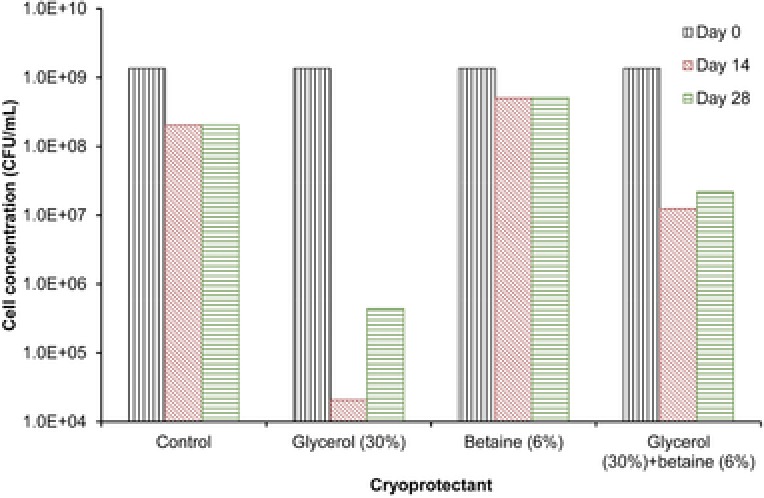
Concentration of A. ferrooxidans cells after freezing at –80°C in Eppendorff vials for 14 and 28 days and using different cryoprotectants.
In the samples stored in commercial cryovials, the cell viability decreased with time in both the sample frozen at –20ºC and the one frozen at –80ºC (Fig. 3). Cell growth was not observed after 28 days in the samples stored at –20ºC (Fig. 3C), while the bacterium was recovered from those samples stored at –80ºC for the same period of time (Fig. 3F).
Figure 3.
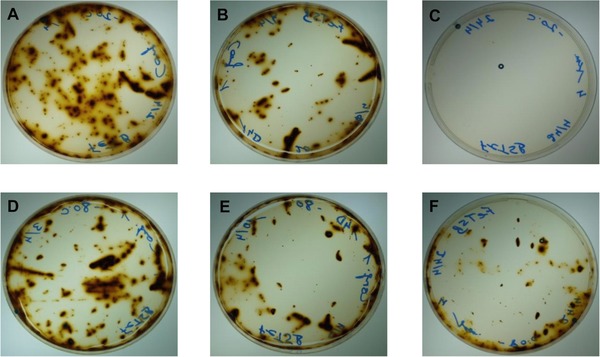
Cell viability in the samples stored at –20ºC (A–C) and –80ºC (D–F) in commercial cryovials: (A) T = –20ºC, after 7 days; (B) T = –20ºC, after 14 days; (C) T = –20ºC, after 28 days; (D) T = –80ºC, after 7 days; (E) T = –80ºC, after 14 days and (F) T = –80ºC, after 28 days.
3.1.2.3. Drying
In this study, no growth was observed after drying the filter at 30ºC for 24 h. No microbial growth was observed after incubation of samples for 12 days at 30ºC, even when samples obtained by washing the filter were seeded directly on medium B.
3.2. Treatment of synthetic BLR
The Fe2+ and Cu2+ content in the synthetic BLR was reduced in two consecutive stages: Stage I (Fe2+ removal) and Stage II (Cu2+ removal), as described below.
In Stage I, the initial redox potential (436.9 mV) of the synthetic BLR solution increased as a consequence of the continuous addition of H2O2. Once 0.45 mL of hydrogen peroxide had been added, this trend changed, and in the addition of another drop of H2O2, the Redox potential decreased from 616.8 to 590.3 mV, indicating complete oxidation of Fe2+ to Fe3+.
In the next step (Stage II), 9.3 mL of NaOH was added to the continuously stirred BLR solution, until the pH of the solution increased to 4.0. During this step, the total Fe3+ precipitated as Fe(OH)3 that was then recovered by filtering the solution.
Approximately 95 mL of the initial 100 mL of BLR solution was recovered after the removal of Fe(OH)3 by filtration.
The color changes in the BLR solution during Stage I are shown in Fig. 4A–C.
Figure 4.
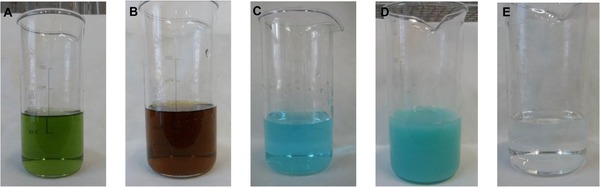
(A) Initial BLR, (B) BLR after Fe2+ oxidation (Stage I.i), (C) BLR after Fe(OH)3 precipitation and filtration (Stage I.iii), (D) BLR solution after precipitation of Cu2+ and (E) BLR solution after removal of Cu(OH)2 by filtration.
In Stage II, the pH was increased to 8.27 by adding 5.3 mL of NaOH. Regarding the solution volume, 80 mL of liquid was recovered after removal of Cu(OH)2 by filtration. Figs. 4D‐4E show the color of the BLR solution before and after removal of Cu(OH)2 by filtration. The submersion of an iron piece in the solution did not have the expected effect on Cu2+ removal (data not shown) and, therefore, this method was not considered effective for the desired purpose. The dry residue in the final solution, which was measured experimentally, was 25.7 g/L.
4. Discussion
4.1. Preservation of A. ferrooxidans
Several methods could be used for the estimation of cell viability. Several novel counting methods, including fluorescence analysis, staining technique, or protein measurement have been developed to date. These methods may require expensive consumables, complex equipment, and also incur high costs per test 27. Compared to the aforementioned techniques, the conventional bacteria counting method based on plate colony counting is simple and is therefore usually used in laboratories. Nevertheless, it is characterized by being labor intensive, time consuming, and dependent on the operator's ability 21, 28, which could make the method both error‐prone and less efficient.
In this case, for A. ferrooxidans plate colony counting, three solid media were tested in order to test bacterial growth. Of these, bacterial growth was only observed in medium A and medium B (Table 1). The latter of these two was chosen to determine the cell viability for the aforementioned bacteria counts due to it being less complex than medium A.
Focusing on the possibility of the industrial application of the biomachining process as a green alternative to conventional physical‐chemical manufacturing procedures, one of the most challenging aspects would be to define a preservation process for the reliable maintenance of production strains. Even if serial subculturing techniques are well established and widely used, their low stability and high labor cost, render them less adequate for long‐term storage 29.
Methods of storing microorganisms generally include passage culture, sterile sand tube preservation, freeze‐drying, and freezing 30. When one of the latter methods is mentioned in relation to the preservation of microorganisms, it is nearly always in regard to long‐term storage 20, 28. Wherever possible, long‐term storage must be considered as the most appropriate option, as this involves halting the growth of the cells and maintaining them in a viable state 22.
Freeze‐drying, also known as lyophilisation, was tested in three experiments (FD1, FD2, and FD3), as it is currently one of the most economical and preferred methods of preserving food products, biological materials, and is also used in drug delivery systems worldwide 31, 32, 33, 34. In this procedure, samples are frozen and the water content is then reduced in two consecutive steps: sublimation (primary drying) and desorption (secondary drying) 22. It is essential to determine the duration of culture prior to harvesting cells and also to select an appropriate cryoprotective agent 33, 34. The cryoprotectants used in this study were: glycerol (5% w/v, 0.007 €/mL), sucrose (18% w/v, 0.012 €/mL), trehalose (15% w/v, 16.26 €/mL), and betaine (6% w/v, 0.012 €/mL).
The cell survival rate may vary greatly depending on the equipment and the conditions of the process 30, 33. As expected, the length of the primary drying stage influenced the effectiveness of the freeze‐drying process, obtaining best results when this period was set at 35 h of duration. Morais et al. 34 indicated that the primary drying step is usually long, having a significant impact on the overall cost of the process. Thus, defining an optimal duration for this step is essential in order to ensure both successful freeze‐drying and an acceptable cost.
After FD1 (primary drying = 12 h), the cell viability decreased strongly, and the survival rate was below 0.1% in all the samples (Table 4). The best results were obtained in the control sample, although cell viability decreased by at least four orders of magnitude (from 1.8·109 CFU/mL to 1.1·105 CFU/mL). With an increase in the duration of the primary drying in FD2, 0.90–1.7% of the cells survived after freeze‐drying; survival rates were highest in the control and the sample supplemented with glycerol. Finally, in FD3 the survival rate was higher in the samples supplemented with sucrose and trehalose (around 0.013% in both cases).
Even if the survival rate of the FD1 and FD2 experiments were low, it has been suggested that a survival rate of 0.10% of the original cell population after freeze‐drying may be sufficient 35. However, much higher poststorage survival rates (70–80%) have been reported as being necessary 22, 36.
Regarding FD3, the different survival obtained in comparison to FD2 may be related to the early stage at which the samples were harvested prior to freeze‐drying (18 days for FD2 compared with 4 days for FD3). Indeed, the operational conditions were the same as in FD2 (primary drying stage, 35 h). This result could be in accordance with the ones obtained for microalgae freezing preservation by Morschett et al. 21 who reported that, contrary to what is commonly accepted, the preservation of stationary phase cells proved superior to freezing cells at their growing phase.
Furthermore, the absence of bacterial growth in the sample with glycerol as cryoprotectant may be due to the complicated retrieval procedure involved, which makes glycerol unsuitable as a cryoprotectant for many of the bacteria used in bioleaching 17.
The influence of storage time on cell survival was determined in the samples from experiment FD3, although cell survival immediately after freeze‐drying was extremely low. Storing the samples in FD3 for 4 weeks at 4ºC after freeze‐drying had a negative impact on all samples, as the survival rate decreased significantly (Table 4). The samples stored in the presence of sucrose, trehalose, and betaine accounted for the 0.025, 0.87, and 0.043%, respectively, of viable cells remaining immediately after freeze‐drying. Dimitrellou et al. 37 also noted the negative effect that storing samples for 15 days (at 4ºC) after being freeze‐dried had on cell viability in L. casei cells.
With this all, it could be concluded that freeze‐drying adversely affected the viability of the strain studied regardless of the primary drying length and cryoprotectant used. The viability rate did not exceed 2% in any of the cases presented herein.
Together with freeze‐drying, freezing is considered to be the most feasible and effective method for long‐term storage of pure strains and mixed cultures 30. Cryopreservation of bioleaching bacteria and microalgae was first reported about 40 years ago 17, 21. However, several protocols and cryoprotectants have since been developed. In this study, glycerol (30% w/v, 0.041 €/mL), betaine (6% w/v, 0.012 €/mL), and a glycerol (30% w/v)‐betaine (6% w/v) mixture (0.026 €/mL) were used as cryoprotective agents.
This storage method is based on freezing cells that have been suspended in a liquid medium, which may or may not contain a cryoprotective agent (although inclusion is advisable). Storage of samples at temperatures below 0ºC maintains the intracellular and extracellular water in a solid state 22. The low temperature reduces cell metabolism and cell growth is prevented due to the absence of liquid water.
Storage time (14 or 28 days) did not have a significant impact on cell survival in the samples stored at –80ºC in Eppendorf tubes and treated with the same cryoprotectant, except in those to which glycerol was added (Fig. 2). However, the cryoprotectant influenced the viability of cells after freezing. Cell viability was highest in samples stored in 6% betaine, with a 37% survival rate after 28 days. The latter parameter was somewhat lower in the sample preserved in the absence of the protective agents (control, 15.2%) and much lower in the samples stored with the glycerol and glycerol + betaine solution (0.032 and 1.6%, respectively).
In the cases in which samples were stored in commercial cryovials, cell viability was influenced by both storage time and freezing temperature. The decrease was more significant in the samples stored at –20ºC (Fig. 3A–C) than in those stored at –80ºC (Fig. 3D–F). In general, the intracellular recrystallization of water decreased with storage temperature, thus enabling the cells to remain viable for longer 22.
Hence, the freezing procedure in Eppendorf vials showed high dependency on the type of cryoprotectant used, with betaine obtaining the highest recovery percentage. From an industrial point of view, it is interesting to point out that betaine was the less expensive cryoprotectant among the agents used for this preservation technique in this study. By contrast, freezing time had no influence on cell viability (up to 28 days). Conversely, the viability of the samples frozen in commercial cryovials decreased significantly during the freezing time. The temperature also proved to be an important factor, with better results in the samples stored at –80ºC.
Finally, desiccation negatively affected cell viability, being inadequate for the preservation of A. ferrooxidans. Thus, drying has proven to be unsuitable for the preservation of A. ferrooxidans as it annulled all possibilities of microbial growth after processing.
4.2. Treatment of synthetic BLR
The indirect mechanism of the biomachining process is a cyclic combination of chemical and microbiological processes (Eqs. 1–3). This indirect pathway uses an intermediate redox couple (such as Fe2+/Fe3+ ions) to dissolve the metal from the workpiece surface, and no direct contact is established between the bacteria and the metal. Thus, the biomachining liquid residue will contain a certain concentration of both Fe2+ and Fe3+. In addition, when the biomachining is carried out on a copper workpiece, Cu2+ is released to the liquid residue and dissolved Cu2+ concentration increases with time. Both iron and copper are considered to be toxic heavy metals at high concentrations, so the release of these metal ions in high quantities could be dangerous for the environment 38. Therefore, their total or partial removal from the liquid residue is of great importance in order to avoid any pollution caused by the effluent generated in the biomachining process. Additionally, if iron and copper are removed from the liquid residue as pure precipitates, two useful by‐products can be obtained: Fe(OH)3 and Cu (OH)2.
The sequential treatment for the synthetic BLR consisted in the oxidation of Fe2+ to Fe3+, its precipitation as Fe(OH)3, and the precipitation of Cu2+ as Cu(OH)2 (Eqs. 4–6).
| (4) |
| (5) |
| (6) |
Regarding reactive consumption, in Stage I 0.5 mg of H2O2 and 1.5 mg of NaOH was consumed per mg of oxidized Fe2+ and per mg of precipitated Fe3+, respectively. In Stage II, 0.77 mg of NaOH was consumed per mg of Cu2+ dissolved in the BLR. Therefore, based on the price of these chemicals in the market (Sigma‐Aldrich), the treatment of a residue containing 3 gFe2+/L, 3gFe3+/L, and 6.7 gCu2+/L would have a total cost of 2.5 € per liter of treated dissolution.
The pH played an important role in both precipitation reactions. An increase in the pH above 4.0 during Fe(OH)3 precipitation Eq. (5) could lead to the coprecipitation of Cu2+ and the consequent reduction of the quality of the precipitate. Regarding Cu(OH)2 precipitation reaction Eq. (6), ammonium can be partly transformed to NH3 when the pH increases above 8.0. The NH3 and the Cu2+ can form a soluble complex that would solubilize the precipitated Cu(OH)2, thus reducing the amount of product obtained. Therefore, the pH was carefully controlled in both steps.
The FeT and Cu2+ contents were both greatly reduced in the process. Precipitation of both Fe(OH)3 and Cu(OH)2 led to 99.9% of the initial FeT and Cu2+ contents being insolubilized, as the concentrations of FeT and Cu2+ were reduced from 6.0 g/L in the initial BLR to respectively 3.6 and 3.4 mg/L in the final solution. The concentration of FeT was presumably related to the Fe2+ that had not been completely oxidized in the first step; optimization of the Fe2+ to Fe3+ oxidation step could therefore reduce the concentration of FeT in the final solution.
This result of the dry residue was consistent with the theoretical Na2SO4 concentration in the solution (25.1 g/L), calculated by taking into account the stoichiometric Na+ concentration (from the NaOH consumed in both Fe3+ and Cu2+ precipitation steps) and assuming that all the Na+ was in the form Na2SO4. Thus, it can be concluded that the dry residue essentially consists of Na2SO4 (17.0 g/L and 8.1 g/L SO4 2‐ and Na+ in solution, respectively).
The method used in this study generated very pure Fe(OH)3 and Cu(OH)2 that are valuable chemicals that could be sold in order to yield a return that would reduce the overall cost of the treatment. For example, Fe(OH)3 can be used as a cement additive, and Cu(OH)2 can be redissolved and recovered as highly pure copper by electrolysis.
As a general conclusion, regarding the preservation study of A. ferrooxidans, it could be concluded that freezing at –80ºC using Eppendorf cryovials is the procedure that provides the best results regarding the feasibility for the microorganisms studied in these assays. The procedure developed for treating the liquid residue generated in the biomachining process enabled the metal content of the effluent to be reduced, which then only contained significant amounts of sulfates and sodium as well as the ammonia nitrogen (from the 9K broth). Moreover, metal recovery as high purity precipitates that could be used in industrial applications was proven to be possible. These two aspects are of great interest for the implementation of future biomachining industrial‐scale plants.
Practical application
The use of microorganisms to remove material from metal workpieces, known as biomachining, provides an effective and sustainable alternative to conventional chemical and physical manufacturing processes. Despite this technology being researched thoroughly nowadays because of its potential benefits, its industrial application is still pending. The research here presented could promote the industrial implementation of this technology as it presents solutions to two important aspects of the process. First, the possibility of preserving the bacteria used in the process offers the opportunity to have the biomachining tool always ready for use. Second, treating the liquid residue obtained in the process is a way of boosting its “environmentally friendly” nature, reducing wastewater treatment costs and obtaining potentially useful by‐products.
The authors have declared no conflicts of interest.
Acknowledgments
Authors are grateful to Basque Government (BFI 2011–119) and the University of the Basque Country UPV/EHU (UFI 11/29 and Convocatoria de ayudas para la Contratación de Doctores Recientes hasta su Integración en Programas de Formación Postdoctoral del Vicerrectorado de Investigación de la UPV/EHU) for providing financial support. GUSERBIOT, S.L. is acknowledged for collaboration and technical support.
5 References
- 1. Ting, Y. P. , Senthil, Kumar A. , Rahman, M. , Chia, B. K. , Innovative use of Thiobacillus ferrooxidans for the biological machining of metals. Acta Biotechnol. 2000, 20, 87–96. [Google Scholar]
- 2. Hocheng, H. , Jadhav, U. , Process of biological machining, in: Nee A. Y. C. (Ed.), Handbook of Manufacturing Engineering and Ttehcnology, Springer‐Verlag, London: 2015, pp. 1687–1713. [Google Scholar]
- 3. Johnson, D. , Warner, R. , Shih, A. J. , Surface roughness and material removal rate in machining using microorganisms. J. Manuf. Sci. Eng. 2007, 129, 223–227. [Google Scholar]
- 4. Hocheng, H. , Chang, J. H. , Jadhav, U. U. , Micromachining of various metals by using Acidithiobacillus ferrooxidans 13820 culture supernatant experiments. J. Cleaner Prod. 2012, 20, 180–185. [Google Scholar]
- 5. Istiyanto, J. , Saragih, A. S. , Jo‐Ko, T. , Metal based micro‐feature fabrication using biomachining process. Microelectron. Eng. 2012, 98, 561–565 [Google Scholar]
- 6. Yang, Y. , Wang, X. , Liu, Y. , Wang, S. et al., Techniques for micromachining using Thiobacillus ferrooxidans based on different culture medium. Appl. Mech. Mater. 2009, 16–19, 1053–1057. [Google Scholar]
- 7. Jadhav, U. , Hocheng, H. , Weng, W. H. , Innovative use of biologically produced ferric sulfate for machining of copper metal and study of specific metal removal rate and surface roughness. J. Mater. Process. Technol. 2013, 213, 1509–1515. [Google Scholar]
- 8. Díaz‐Tena, E. , Rodríguez‐Ezquerro, A. , López de Lacalle, L. N. , Gurtubay, L. et al., A sustainable process for material removal on pure copper by use of extremophile bacteria. J. Cleaner Prod. 2014, 84, 752–760. [Google Scholar]
- 9. Akcil, A. , Erust, C. , Ozdemiroglu, S. , Fonti, V. et al., A review of approaches and techniques used in aquatic contaminated sediments: metal removal and stabilization by chemical and biotechnological processes. J. Cleaner Prod. 2015, 86, 24–36. [Google Scholar]
- 10. Johnson, D. B. , du Plessis, C. A. , Biomining in reverse gear: using bacteria to extract metals from oxidised ores. Miner. Eng. 2015, 75, 2–5. [Google Scholar]
- 11. Istiyanto, J. , Jo‐Ko, T. , Yoon, II‐C., A study on copper micromachining using microorganisms. Int. J. Precis. Eng. Manuf. 2010, 11, 659–664. [Google Scholar]
- 12. Wu, X. L. , Xin, X. H. , Jiang, Y. , Liang, R. X. et al., Liquid‐nitrogen cryopreservation of three kinds of autotrophic bioleaching bacteria. Trans. Nonferrous Met. Soc. China 2008, 18, 1386–1391. [Google Scholar]
- 13. Kurtzman, C. P. , Labeda, D. P. , Type culture collections and their databases, in: Schaechter M. (Ed.), Encyclopedia of Microbiology, Elsevier, San Diego: 2009, pp. 306–312. [Google Scholar]
- 14. Hegler, F. , Kappler, A. , Cryopreservation of anoxygenic phototrophic Fe(II)‐oxidizing bacteria. Cryobiology 2010, 61, 158–160. [DOI] [PubMed] [Google Scholar]
- 15. Overmann, J. , Principles of enrichment, isolation, cultivation, and preservation of prokaryotes, in: Roseberg E., DeLong E. F., Stackebrandt E., Lory S., Thompson F. (Eds.), The Prokaryotes‐Prokariotic Biol and Symbiotic Associations, Springer‐Verlag, Berlin Heidelberg: 2013, pp. 150–207. [Google Scholar]
- 16. Gupta, S. G. , Agate, A. D. , Preservation of Thiobacillus ferrooxidans and Thiobacillus thiooxidans with activity check. Antonie van Leeuwenhoek 1986, 52, 121–127. [DOI] [PubMed] [Google Scholar]
- 17. Wu, X. L. , Hu, Q. , Hou, D. M. , Xin, X. H. et al., Preservation of new cryoprotectant used for Acidithiobacillus ferrooxidans in liquid nitrogen. Trans. Nonferrous Met. Soc. China 2013, 23, 818–823. [Google Scholar]
- 18. Barron, J. L. , Lueking, D. R. , Growth and maintenance of Thiobacillus ferrooxidans cells. Appl. Environ. Microbiol. 1990, 56, 2801–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cleland, D. , Krader, P. , McCree, C. , Tang, J. et al., Glycine betaine as a cryoprotectant for prokaryotes. J. Microbiol. Methods 2004, 58, 31–38. [DOI] [PubMed] [Google Scholar]
- 20. Chotiko, A. , Sathivel, S. , Effects of enzymatically‐extracted purple rice bran fiber as a protectant of L. plantarum NRRL B‐44796 during freezing, freeze drying and storage. LWT‐Food Sci. Technol. 2014, 59, 59–64. [Google Scholar]
- 21. Morschett, H. , Reich, S. , Wiechert, W. , Oldiges, M. , Simplified cryopreservation of the microalga Chlorella vulgaris integrating a novel concept for cell viability estimation. Eng. Life. Sci. 2016, 16, 36–44. [Google Scholar]
- 22. García, M. D. , López‐Coronado, J. M. , López‐Ocaña, L. , Uruburu Fernández, F. , Preservation of microbial strains in the wine industry, in: Carrascosa A. V., Muñoz R., González R. (Ed.), Molecular Wine Microbiology, Elsevier; 2011, pp. 303–318. [Google Scholar]
- 23. Zeng, W. M. , Zhou, H. B. , Wan, M. X. , Chao, W. L. et al., Preservation of Acidithiobacillus caldus: a moderately thermophilic bacterium and the effect on subsequent bioleaching of chalcopyrite. Hidrometallurgy 2009, 96, 333–336. [Google Scholar]
- 24. Sugio, T. , Domatsu, C. , Tano, T. , Imai, K. , Role of ferrous ions in synthetic cobaltous sulfide leaching of Thiobacillus ferrooxidans . App. Environ. Microbiol. 1984, 48, 461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Johnson, D. B. , McVivar, J. H. M. , Rolfe, S. , A new solid medium for the isolation and enumeration of Thiobacillus ferrooxidans and acidophilic heterotrophic bacteria. J. Microbiol. Methods 1987, 7, 9–18. [Google Scholar]
- 26. Robertson, L. A. , Gijs‐Kuenen, J. , The genus Thiobacillus . Prokariotes 2006, 5, 812–827. [Google Scholar]
- 27. Zhihua, L. , Xuetao, H. , Jiyong, S. , Xiaobo, Z. et al., Bacteria counting method based on polyaniline/bacteria thin film. Biosens. Biolectron. 2016, 81, 75–79. [DOI] [PubMed] [Google Scholar]
- 28. Hallas, G. , Monis, P. , Evaluation of heterotrophic plate and chromogenic agar colony counting in water quality laboratories. MethodsX 2015, 2, 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fourrier, M. C. S. , Arnold, M. F. F. , Collet, B. , Munro, E. S. , The effect of sub‐culturing on the basal level of type I interferon (IFN) gene expression in the Salmon Head Kidney (SHK‐1) cell line. Fish Shellfish Immunol. 2009, 27, 535–538. [DOI] [PubMed] [Google Scholar]
- 30. Zeng, W. M. , Zhou, H. B. , Liu, X. D. , Qiu, G. Z. , Preservation of moderately thermophilic culture by freeze drying and frozen preservation way and effect on subsequent bioleaching of chalporite. Trans. Nonferrous Met. Soc. China 2010, 20, 882–887. [Google Scholar]
- 31. Morgan, C. A. , Herman, N. , White, P. A. , Vesey, G. , Preservation of micro‐organisms by drying: a review. J. Microbiol. Methods 2006, 66, 183–193. [DOI] [PubMed] [Google Scholar]
- 32. Safronova, V. I. , Novikova, N. I. , Comparison of two methods for root nodule bacteria preservation. Lyophilization and liquid nitrogen freezing. J. Microbiol. Methods 1996, 24, 231–237. [Google Scholar]
- 33. Morgan, C. , Vesey, G. , Freeze‐drying of microorganisms, in: Schaechter M. (Ed.), Encyclopedia of Microbiology, Elsevier, San Diego: 2009, pp. 162–173. [Google Scholar]
- 34. Morais, A. R. , Alencar, E. N. , Junior, F. H. X. , de Oliveira, C. M. et al., Freeze‐drying of emulsified sistems: a review. Int. J. Pharm. 2016, 503, 102–114. [DOI] [PubMed] [Google Scholar]
- 35. Bozoglu, T. F. , Ozilgen, M. , Bakir, U. , Survival kinetics of lactic acid starter cultures during and after freeze drying. Enzyme Microbiol. Technol. 1987, 9, 531–537. [Google Scholar]
- 36. Morales‐García, Y. E. , Duque, E. , Rodríguez‐Andrade, O. , de la Torre, J. et al., Bacterias preservadas, una fuente importante de recursos biotecnológicos. BioTecnologia 2010, 14, 11–29. [Google Scholar]
- 37. Dimitrellou, D. , Kandylis, P. , Kourkoutas, Y. , Effect of cooling rate, freeze‐drying, and storage on survival of free and immobilized Lactobacillus case ATCC 393. LWT Food Sci. Technol. 2016, 69, 468–473. [Google Scholar]
- 38. Sotero‐Santos, R. B. , Rocha, O. , Povinelli, J. , Toxicity of ferric chloride sludge to aquatic organisms. Chemosphere 2007, 68, 628–636. [DOI] [PubMed] [Google Scholar]
- 39. Silverman, M. P. , Lundgren, D. G. , Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans. I An improved medium and harvesting procedure for securing high cell yields. J. Bacteriol. 1959, 77, 642–647. [DOI] [PMC free article] [PubMed] [Google Scholar]


