Abstract
In order to achieve continuous industrial production of astaxanthin in Xanthophyllomyces dendrorhous, a moderate temperature (25–37°C) fermentation process was needed. In this study, a two‐step process with a 20°C pre‐culture for 18 h and a 30°C culture for 30 h was performed to achieve the astaxanthin yields of 116.42 μg g−1 dry cell weight, which was lower than that in the normal process (20°C, 96 h). However, cell yield (YX/S) and product yield (YP/S) showed no significant differences between the two processes, suggesting that moderate temperature did not affect the productivity of astaxanthin. The transcriptional levels of genes involved in astaxanthin synthesis were compared in different culture times and a negative correlation between temperature and expression of carotenogenic genes was found. This work provided a potential method for continuous production of astaxanthin using X. dendrorhous at moderate temperature throughout the year.
Keywords: Astaxanthin, Kinetics, Moderate temperature, Transcriptional level, Xanthophyllomyces dendrorhous
Abbreviation
- DCW
dry cell weight
1. Introduction
Astaxanthin as a natural carotenoid has been widely used in food additive as a pigment or antioxidant and in nutraceutical for its high antioxidant activity 1, 2, 3. Xanthophyllomyces dendrorhous is the main microorganism to produce astaxanthin, in which astaxanthin accounts for 70% of carotenoids 4, 5. For the commercial importance of astaxanthin, more researches are focused on the increased astaxanthin yield using mutation breeding, genetic engineering or optimized fermentation process 6, 7.
To date, a major factor restricting the industrialization of astaxanthin is the fermentation temperature, because astaxanthin is produced by X. dendrorhous at 18–22°C, which means more costs are spent on the cooling process 8, 9. A huge reaction heat was generated during a large‐scale fermentation, indicating a normal water cooling system could not meet the cooling demand. Therefore, a moderate temperature strain or fermentation process was urgently required. However, X. dendrorhous could not survive above 25°C, causing only a few mutant strains to grow at 25°C 7.
As a strategy for the increase of fermentation temperature of X. dendrorhous, a different fermentation process may achieve moderate temperature culture. Therefore, in the present investigation, we started from 20°C and increased the temperature step by step, thus increasing the culture temperature and accumulating astaxanthin. A two‐step process (20°C, 18 h followed by 30°C, 30 h) was performed to achieve the astaxanthin yields of 116.42 μg g−1, and the transcriptional levels of genes involved in astaxanthin synthesis were compared in different culture times in order to find potential courses leading to heat resistance in astaxanthin accumulation.
2. Materials and methods
2.1. Strains and culture conditions
X. dendrorhous CBS 6938 was purchased from Westerdijk Fungal Biodiversity Institute. The strain was maintained on potato dextrose agar slants at 4°C. One colony was selected and inoculated into a shake flask with 50 mL of potato dextrose medium and cultured at 20°C for 48 h. Then 80 mL of the seed liquid was inoculated into a 5 L bioreactor for batch fermentation. The standard culture conditions were: temperature, 20°C; agitation, 200 rpm; natural pH; air‐flow, 1 vv−1m−1. The overall production period of fermentation culture was 10 days with samples taken at 6 or 12 h intervals, during which dry cell weight (DCW), astaxanthin production, and glucose consumption were monitored. All experiments had three repetitions.
2.2. Determination of the pre‐culture time
A two‐step process in 250 mL shake flasks at 180 rpm with a pre‐culture at 20°C and a culture at 30°C was performed. The total culture time was 48 h. The astaxanthin yield was determined after the two‐step process with the pre‐culture time of 12, 15, 18, 21, and 24 h.
2.3. Astaxanthin extraction and detection
2 mL of the samples were centrifuged at 5000 g for 5 min to collect cells. Pellets were mixed with 300 μL of 3 mol L−1 HCl and incubated for 1 h. The mixture was maintained sequentially in boiling water and ice for 3 min each. Pellets were centrifuged (5000 g, 5 min) and washed with distilled water twice. Broken cells were extracted with 900 μL acetone and centrifuged at 5000 g for 5 min. The supernatant was filtered with a 0.22 μm membrane. This process was repeated until the pellets showed no red color.
HPLC analysis of astaxanthin was performed on a Waters‐e2695 system equipped with Waters 2998 photodiode array detector. The extracts were separated on a Hypersil ODS2 column (C18, 5 μm, 250 × 4.6 mm, Dalian Elite Analytical Instruments Co. Ltd., China): temperature 25°C, flow rate 1.4 mL min−1, and wavelength 478 nm. The mobile phase consisted of methanol 90%, and acetonitrile 10%. Astaxanthin standards were from Sigma.
2.4. Transcriptional levels of genes involved in astaxanthin synthesis in different culture times
RT‐PCR was used to evaluate gene expression in astaxanthin synthesis in different culture times. Total RNA (2 μg) from each sample was reverse‐transcribed into cDNA in the presence of random primers using the Revert Aid First Strand cDNA Synthesis Kit (Thermo Scientific). The synthesized cDNA was used as the template for RT‐PCR. Actin in X. dendrorhous CBS 6938 was used as an internal reference to normalize the amount of total RNA present in each reaction. RT‐PCR experiments were performed using TransStart Top Green qPCR Super Mix (Beijing TransGen Biotech Co., Ltd.). The transcriptional levels of the genes were calculated from the threshold cycle according to the 2−ΔΔCT method.
3. Results
3.1. Determination of the process
X. dendrorhous cannot grow above 25°C, so changing the culture process is a viable way to increase the culture temperature. A pre‐culture at 20°C may increase the activity of X. dendrorhous and achieve the followed moderate temperature production of astaxanthin. Different pre‐culture times were set to evaluate its impact on the production of astaxanthin (Fig. 1). The results showed that X. dendrorhous could grow and produce astaxanthin at 30°C after 15 h of pre‐culture, however the 12 h of pre‐culture had no effect. After 18 h of pre‐culture, the astaxanthin yield reached to 78.16 μg g−1 which was significantly higher (p < 0.05) than that after 15 h of pre‐culture. No significant difference between 18 h of pre‐culture and more time was observed. The two‐step process was a pre‐culture at 20°C for 18 h followed by a culture at 30°C for 30 h.
Figure 1.
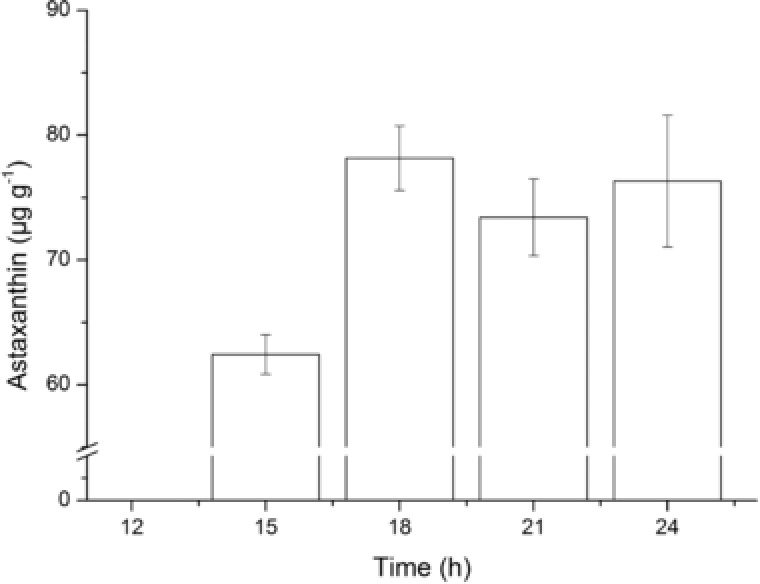
Determination of the pre‐culture time. The astaxanthin yield was determined after the two‐step process with the pre‐culture time of 12, 15, 18, 21, and 24 h.
3.2. Kinetics of astaxanthin production
Fermentation kinetics showed the dynamics of interactions between various environmental factors and microbial metabolic activities over time. It could help to understand the physiological characteristics of microbes, the appropriate conditions for cell growth and product formation, and the relationship among various fermentation parameters. The cell growth, glucose consumption, and astaxanthin production in the normal and two‐step process are shown in Fig. 2. As shown by these data, X. dendrorhous grew faster under normal conditions with OD600 reaching to 40 (Fig. 2A), while OD600 only reached to 20 under the two‐step process (Fig. 2B). In the normal process, cell mass, astaxanthin production and glucose consumption did not change significantly after 96 h. In the two‐step process, OD600 significantly reduced after 48 h of the culture which indicated the decline in population for the high temperature. Accordingly, the glucose consumption and astaxanthin yield were reduced. As a result, a 48 h culture was suitable for the two‐step process with the astaxanthin yield at 116.42 μg g−1 DCW. In the normal process, the astaxanthin yield reached to 118.70 μg g−1 DCW at 48 h and 140.28 μg g−1 DCW at 96 h. 30°C is lethal temperature for X. dendrorhous, resulting in poor growth and less astaxanthin production in the two‐step process after 48 h culture. However, the two‐step process saved more costs and the production was not limited by the season.
Figure 2.
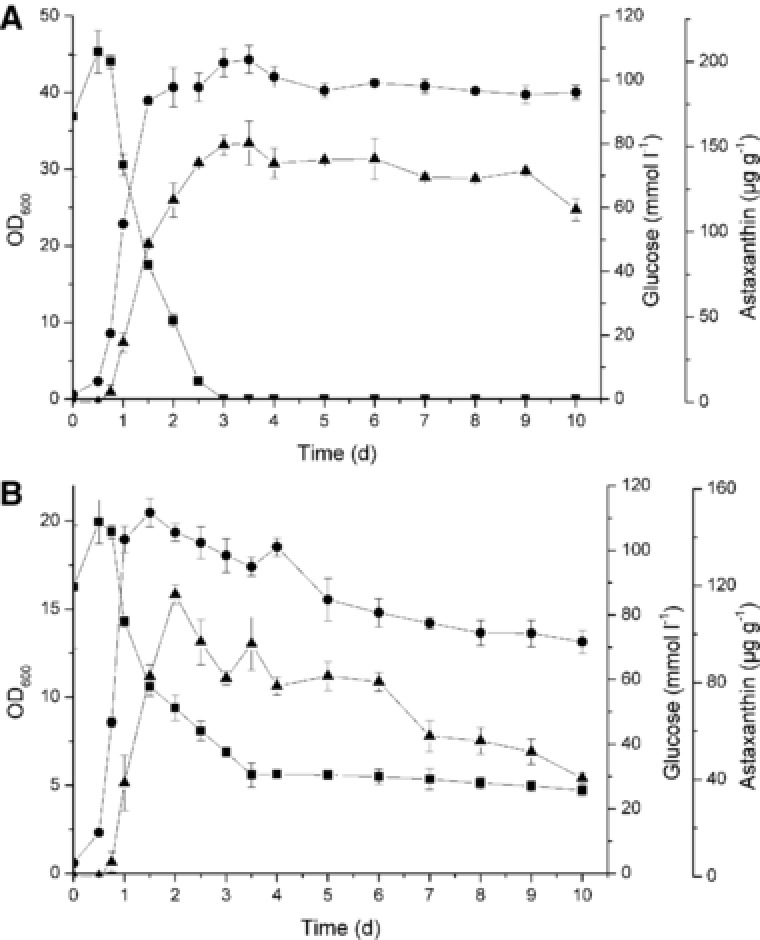
Kinetics of astaxanthin production in X. dendrorhous. Cell growth, glucose consumption, and astaxanthin production in normal process (A) and the two‐step process (B). The circle, square and triangle symbols represent the cell growth, glucose consumption and astaxanthin production, respectively.
The kinetic parameters of the two processes are shown in Table 1. The DCWmax and μmax in the two‐step process (4.12 g L−1 and 0.181 h−1) were lower than that in the normal process (6.23 g L−1 and 0.195 h−1) indicating the negative effect of the high temperature on the growth performance. However, cell yield (YX/S) and product yield (YP/S) showed no significant differences between the two processes, suggesting that high temperature did not affect the production capacity of astaxanthin. The two‐step process improved the culture temperature and reduced the culture time, thus saving at least 4200 kJ energy costs each cubic meter of the fermentation broth for reducing the temperature by 1°C.
Table 1.
Kinetic parameters in batch fermentation by X. dendrorhous
| Parameters | Fermentation process | |
|---|---|---|
| 20°C, 96 h | 20°C, 18 h and 30°C, 30 h | |
| Fermentation time (h) | 96 | 48 |
| Glucose consumption (g L−1) | 20.86 ± 1.34 | 11.63 ± 1.90 |
| DCWmax (g L−1) | 6.23 ± 0.13 | 4.12 ± 0.14 |
| Astaxanthin (μg g−1) | 140.28 ± 8.80 | 116.42 ± 3.93 |
| μmax (h−1) | 0.195 ± 0.002 | 0.181 ± 0.004 |
| YX/S (g g−1) | 0.30 ± 0.02 | 0.36 ± 0.04 |
| YP/S (mmol mol−1) | 0.013 ± 0.001 | 0.013 ± 0.001 |
3.3. Transcriptional levels of genes involved in astaxanthin synthesis in different culture times
In order to understand the effect of the changed temperature on gene expression, RT‐PCR was performed to compare the transcriptional levels of genes involved in astaxanthin synthesis in different culture times of the two processes. The results are shown in Fig. 3 and the astaxanthin synthesis pathway could be found in Alcaíno's work 10. As shown in Fig. 3, the transcriptional levels of crtE are the lowest among carotenogenic genes, suggesting that crtE might be an important factor limiting the production of astaxanthin, especially in the two‐step process. The transcriptional level of crtE in the two‐step process was significantly lower than that in the normal process after 48 h of culture, which indicated the negative effect of high temperature on the transcription level of crtE. crtE catalyzes the formation of geranylgeranyl diphosphate, which is generally considered the first step of the carotenoid pathway, because crtE is part of the carotenogenic gene cluster in all microorganisms investigated 7.
Figure 3.
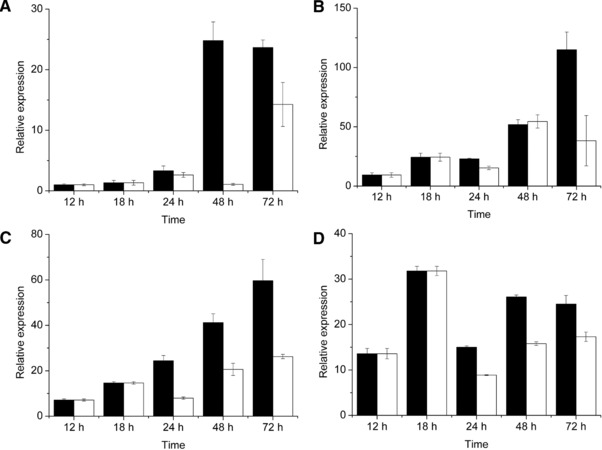
Relative expression of carotenogenic genes in normal process (black) and the two‐step process (white). The relative expression of the crtE gene was designated as 1 in the normal process at 12 h (A). The numbers of relative expression of other genes were folds of the crtE gene in the normal process at 12 h. (A) crtE; (B) crtI; (C) crtS; (D) crtY.
crtI is a phytoene desaturase gene. The transcriptional level of crtI showed no difference between the two processes during the first 48 h. However, in the two‐step process, the transcription of crtI was 33% of that in the normal process at 72 h of culture. The other two carotenogenic genes (crtS and crtYB) showed lower transcriptional levels in the two‐step process than in the normal process. The higher expression of crtS decreased the intermediate levels resulting in a higher percentage of astaxanthin per total carotenoids and a higher absolute amount in the cells 11.
4. Discussion
Over decades, many researches have been focused on the increase of astaxanthin production by chemical mutagenesis or genetic engineering 12, 13, 14. Although significant progress has been made, the commercial astaxanthin production is still limited by the low fermentation temperature (20°C) resulting in only half of the time per year for production. Astaxanthin as a secondary metabolite belonged to the conserved mevalonate pathway whose precursor was acetyl‐CoA 15. As shown in Fig. 2A, the production of astaxanthin was partly associated with cell growth and glucose consumption. Astaxanthin generated from the beginning of the exponential phase and reached to the maximal value when the glucose consumed up. It seemed different from a theory that in cultures supplemented with glucose, carotenogenesis was induced only after the culture medium ran out of glucose 16. The reason might be starch or other organic carbon source existing in the potato dextrose medium weakened the catabolic repression of glucose.
In the two‐step process, both OD600 and astaxanthin production were reduced after 48 h. Glucose was almost no longer consumed after 84 h. All these conditions indicated that the high temperature caused cell growth inhibition and even death. Although the pre‐culture enhanced the cell activity and enabled the growth of X. dendrorhous at 30°C, the high temperature still had a negative effect on X. dendrorhous. So the culture time of the two‐step process was determined to be 48 hours. Compared to the normal process, astaxanthin production was reduced in the two‐step process, however the YX/S and YP/S did not change. Moreover, by increasing the culture temperature, the two‐step process saved at least $ 50 per cubic meter of the fermentation broth in the energy and refrigeration equipment costs. Through continuous production throughout the year, total annual production had also increased.
The investigation of transcriptional levels of genes involved in astaxanthin synthesis in different culture times of the two processes provided the possible mechanism of the effect of temperature on the production of astaxanthin. The findings suggest a negative correlation between high temperature and expression of carotenogenic genes. The carotenogenic pathway was active throughout the entire culture in both the processes, indicating that the pre‐culture enhanced the adaptability of X. dendrorhous to high temperature. High temperature showed a great influence on the expression of crtS and crtI which could be the reason for the low astaxanthin production in the two‐step process, because the enzyme CrtS had become a bottleneck of the carotenoid pathway 11.
5. Concluding remarks
A two‐step process was performed to increase the fermentation temperature in astaxanthin production. X. dendrorhous could be alive at 30°C after a 18 h of pre‐culture at 20°C. The astaxanthin yield reached to 116.42 μg g−1 DCW without affecting the production capacity. Transcriptional levels of carotenogenic genes were negatively correlated with temperature. The two‐step process enabled the growth of X. dendrorhous and production of astaxanthin at 30°C; however, the optimization for astaxanthin production at 30°C is needed in the future work.
Practical application
Astaxanthin is a natural carotenoid widely used in health care products for its antioxidant property. The production of astaxanthin has been seasonally restricted for the low fermentation temperature of Xanthophyllomyces dendrorhous. This work reported a two‐step process which could increase the fermentation temperature to moderate temperature. The transcriptional levels of genes involved in astaxanthin synthesis were compared in different culture times in order to find potential courses leading to heat resistance in astaxanthin accumulation. To our knowledge, it is the first report on astaxanthin production at 30°C using X. dendrorhous.
The authors have declared no conflict of interest.
Acknowledgments
This work was supported by National Natural Science Foundation of China (Grant No. 31701587), Department of Education of Liaoning Province (2016J053) and Natural Science Foundation of Liaoning Province (20170520198).
6 References
- 1. Martinez‐Delgado, A. A. , Khandual, S. , Villanueva‐Rodriguez, S. J. , Chemical stability of astaxanthin integrated into a food matrix: Effects of food processing and methods for preservation. Food. Chem. 2017, 225, 23–30. [DOI] [PubMed] [Google Scholar]
- 2. Sakai, S. , Nishida, A. , Nishino, K. , Ohno, M. , et al., Astaxanthin, a xanthophyll carotenoid, suppresses the development of experimental colitis by inhibiting the activation of NF‐kappa B and AP‐1. Gastroenterology. 2017, 152, S573–S573. [Google Scholar]
- 3. Pan, J. L. , Wang, H. M. , Chen, C. Y. , Chang, J. S. , Extraction of astaxanthin from Haematococcus pluvialis by supercritical carbon dioxide fluid with ethanol modifier. Eng. Life Sci. 2012, 12, 638–647. [Google Scholar]
- 4. Rodriguez‐Saiz, M. , de la Fuente, J. L. , Barredo, J. L. , Xanthophyllomyces dendrorhous for the industrial production of astaxanthin. Appl. Microbiol. Biotechnol. 2010, 88, 645–658. [DOI] [PubMed] [Google Scholar]
- 5. Wang, W. J. , Yu, L. J. , Zhou, P. P. , Effects of different fungal elicitors on growth, total carotenoids and astaxanthin formation by Xanthophyllomyces dendrorhous . Bioresour. Technol. 2006, 97, 26–31. [DOI] [PubMed] [Google Scholar]
- 6. Yamamoto, K. , Hara, K. Y. , Morita, T. , Nishimura, A. , et al., Enhancement of astaxanthin production in Xanthophyllomyces dendrorhous by efficient method for the complete deletion of genes. Microb. Cell Fact. 2016, 15, 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miao, L. L. , Chi, S. A. , Tang, Y. C. , Su, Z. Y. , et al., Astaxanthin biosynthesis is enhanced by high carotenogenic gene expression and decrease of fatty acids and ergosterol in a Phaffia rhodozyma mutant strain. FEMS Yeast Res. 2011, 11, 192–201. [DOI] [PubMed] [Google Scholar]
- 8. Amado, I. R. , Vazquez, J. A. , Mussel processing wastewater: A low‐cost substrate for the production of astaxanthin by Xanthophyllomyces dendrorhous. Microb. Cell Fact. 2015, 14, 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sanpietro, L. M. D. , Kula, M. R. , Studies of astaxanthin biosynthesis in Xanthophyllomyces dendrorhous (Phaffia rhodozyma). Effect of inhibitors and low temperature. Yeast 1998, 14, 1007–1016. [DOI] [PubMed] [Google Scholar]
- 10. Alcaíno, J. , Bravo, N. , Córdova, P. , Marcoleta, A. E. , et al., The involvement of Mig1 from Xanthophyllomyces dendrorhous in catabolic repression: An active mechanism contributing to the regulation of carotenoid production. PLoS One. 2016, 11, e0162838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gassel, S. , Breitenbach, J. , Sandmann, G. , Genetic engineering of the complete carotenoid pathway towards enhanced astaxanthin formation in Xanthophyllomyces dendrorhous starting from a high‐yield mutant. Appl. Microbiol. Biotechnol. 2014, 98, 345–50. [DOI] [PubMed] [Google Scholar]
- 12. Dursun, D. , Dalgic, A. C. , Optimization of astaxanthin pigment bioprocessing by four different yeast species using wheat wastes. Biocatal. Agric. Biotechnol. 2016, 7, 1–6. [Google Scholar]
- 13. Castelblanco‐Matiz, L. M. , Barbachano‐Torres, A. , Ponce‐Noyola, T. , Ramos‐Valdivia, A. C. , et al., Carotenoid production and gene expression in an astaxanthin‐overproducing Xanthophyllomyces dendrorhous mutant strain. Arch. Microbiol. 2015, 197, 1129–1139. [DOI] [PubMed] [Google Scholar]
- 14. Kim, J. h. , Chang, H. I. , High‐level production of astaxanthin by Xanthophyllomyces dendrorhous mutant JH1, using chemical and light induction. J. Microbiol. Biotechnol. 2006, 16, 381–385. [DOI] [PubMed] [Google Scholar]
- 15. Schmidt, I. , Schewe, H. , Gassel, S. , Jin, C. , et al., Biotechnological production of astaxanthin with Phaffia rhodozyma/Xanthophyllomyces dendrorhous . Appl. Microbiol. Biotechnol. 2011, 89, 555–571. [DOI] [PubMed] [Google Scholar]
- 16. Cordova, P. , Alcaino, J. , Bravo, N. , Barahona, S. , et al., Regulation of carotenogenesis in the red yeast Xanthophyllomyces dendrorhous: The role of the transcriptional co‐repressor complex Cyc8‐Tup1 involved in catabolic repression. Microb. Cell Fact. 2016, 15, 193. [DOI] [PMC free article] [PubMed] [Google Scholar]


