Abstract
Oleochemical activities (e.g. biodiesel production, fat saponification) generate annually very high amounts of concentrated glycerol‐containing waters (called crude glycerol) as the principal residues of these processes. Crude glycerol is an industrial residue the valorization of which attracts remarkable and constantly increasing interest. In the current investigation, biodiesel‐derived glycerol was employed as substrate for yeast and fungal strains cultivated under nitrogen‐limited conditions in shake flasks. Glucose was employed as reference substrate. Several yeasts (Candida diddensiae, Candida tropicalis, Pichia ciferrii, Williopsis saturnus, Candida boidinii, and Candida oleophila) rapidly assimilated glucose and converted it into ethanol, despite aerobic conditions imposed, and were Crabtree‐positive. None of these yeasts produced ethanol during growth on glycerol or accumulated significant quantities of lipid during growth on glucose or glycerol. Only Rhodosporidium toruloides produced notable lipid quantities from glucose and to lesser extent from glycerol. Yarrowia lipolytica LFMB 20 produced citrate ≈58 g/L growing on high‐glucose media, while on high‐glycerol media ≈42 g/L citrate and ≈18 g/L mannitol. During growth on glucose/glycerol blends, glycerol was assimilated first and thereafter glucose was consumed. Fungi produced higher lipid quantities compared with yeasts. High lipid quantities were produced by Mortierella ramanniana, Mucor sp., and mainly Mortierella isabellina, with glycerol being more adequate for M. ramanniana and glucose for Mucor sp. and M. isabellina. M. isabellina ATHUM 2935 produced lipids of 8.5 g/L, 83.3% w/w in dry cell weight (DCW) and conversion yield per unit of glucose consumed ≈0.25 g/g. The respective values on glycerol were 5.4 g/L, 66.6% w/w in DCW and ≈0.22 g/g. Lipids of all microorganisms were analyzed with regards to their fatty acid composition, and M. isabellina presented the closest similitude with rapeseed oil. Crude lipids produced by this fungus and extracted with chloroform/methanol blend, were composed mostly of triacylglycerols, thus indicating that these solvents are adequate for triacylglycerol extraction.
Keywords: Biodiesel‐derived glycerol, Citric acid, Ethanol, Mannitol, Single cell oil
Abbreviations
- Cit
citric acid
- DCW
dry cell weight
- Ery
erythritol
- EtOH
ethanol
- FA
fatty acid
- Glc
glucose
- Glol
glycerol
- L
lipid
- Lac
lactic acid
- Ml
mannitol
- Oxl
oxalic acid
- Pyr
pyruvate
- X
biomass (dry cell weight)
- YA/Glol or YA/Glc
conversion yield of the product A per unit of glycerol (Glol) or glucose (Glc) consumed
- YL/X
lipid in dry cell weight (%, w/w)
1. Introduction
Biodiesel production originated from trans‐esterification of vegetable oils, microbial lipids and animal fats constitutes a constantly growing industrial application. In 2021, the worldwide biodiesel production from edible vegetable oils is expected to be up to 30 × 106 T. 1. With the production of 10 kg of biodiesel, 1 kg of glycerol is generated; therefore, the expected biodiesel production will result in the generation of 3 × 106 T. of glycerol as side by‐product 1. On the other hand, very high quantities of glycerol‐containing water can also be generated through bioethanol and/or alcoholic beverage production units 2 or through oleochemical production facilities 3, thus, valorization of glycerol through microbial and/or chemical conversions presents enormous interest 4, 5. Concerning the former ones, glycerol has been used in many reports as a substrate for the production of single cell oils – SCOs 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, citric acid 25, 26, 27, 28, 29, 30, polyols 10, 31, 32, and other compounds. Nevertheless, the research for new microbial strains able to convert efficiently industrial glycerol into microbial metabolites of interest is still of high significance.
Aim of the present study was to investigate the capability of a relatively broad spectrum of eukaryotic microbial strains (yeasts and fungi), toward their potentiality to assimilate biodiesel‐derived glycerol and convert it into secondary metabolites, useful in the Industrial and Food Biotechnology. The yields recorded on glycerol containing media were compared to those obtained on glucose, which is considered as reference substrate, being preferably assimilated by most yeasts and fungi. Biochemical and technological considerations concerning the microbial behavior were assessed and critically discussed.
2. Materials and methods
2.1. Microorganisms, media and culture conditions
The yeast strains used in the current study were as follows: Candida diddensiae NRRL Y‐7589, C. tropicalis NRRL Y‐12968, Pichia ciferrii NRRL Y‐1031, Williopsis saturnus NRRL Y‐17396, C. boidinii ATCC 32195, C. oleophila NRRL Y‐1613, Rhodosporidium toruloides DSM 4444, C. guilliermondii NRRL Y‐2075, Yarrowia lipolytica LFMB 20, Y. lipolytica ACA‐YC 5030, Y. lipolytica ACA‐YC 5029, Lipomyces starkeyi LGAM 235, and Pichia membranifaciens LFMB 1. The fungal strains employed were as follows: Aspergillus sp. ATHUM 3482, Cunninghamella echinulata LFMB 5, Mortierella ramanniana MUCL 9235, Mucor sp. LGAM 366, Rhizopus sp. LFMB 6, and M. isabellina ATHUM 2935. Strains with the characteristic code NRRL Y were provided by the NRRL culture collection (Peoria, USA), the strain DSM was provided by the DSMZ culture collection (Leibniz, Germany), strains with the code LGAM were provided by the Laboratory of General and Agricultural Microbiology (Agricultural University of Athens – Greece), strains with the code LFMB were isolates from the Laboratory of Food Microbiology and Biotechnology (Agricultural University of Athens – Greece), strains with the code ATHUM were purchased by the culture collection of the Department of Biology of the National and Kapodistrian University of Athens (Greece), while strains with the code ACA‐YC were isolates from the Laboratory of Dairy Science and Technology (Agricultural University of Athens – Greece). Finally the strain with the code MUCL was purchased by the Laboratory of Mycology (Catholic University of Louvain, Belgium). All strains were maintained on potato dextrose agar at T = 4 ± 1°C. For all trials performed, the salt composition of the basal medium used was the following one (in g/L): KH2PO4, 7.0; Na2HPO4, 2.5; MgSO4·7H2O, 1.5; FeCl3·6H2O, 0.15; ZnSO4·7H2O, 0.02; MnSO4·H2O, 0.06; CaCl2·2H2O, 0.15. The nitrogen sources employed were (ΝΗ4)2SO4 (containing c. 21%, w/w, of nitrogen) and yeast extract (containing c. 7%, w/w, of nitrogen and c. 8%, w/w, of carbon), both at concentration of 0.5 g/L. The carbon source used in all trials was industrial (biodiesel‐derived) glycerol (Agroinvest SA – Achladi, Fthiotis prefecture, Greece) with a purity of c. 81% w/w. The impurities were composed of: 11–12% w/w water, 5–6% w/w potassium salts, 1% w/w free‐fatty acids, and less than 0.2% w/w methanol. Commercial glucose provided by the “Hellenic Industry of Sugar SA” (Thessaloniki, Greece) [purity 95%; impurities composed of maltose (2%, w/w), malto‐dextrines (0.5%, w/w), water (1.5%, w/w), and salts (1.0%, w/w)] was used as control experiment. Both substrates, glucose and glycerol, were employed at initial concentration (Glc0 and Glol0) of c. 30 g/L. The initial molar ratio in these cultures was high (≈100 moles/moles) since it was desirable mainly to direct the cellular metabolism of the yeast and fungal strains used toward the synthesis of microbial lipids 3, 33. Moreover, given that it was desirable to study in more details the strain Y. lipolytica LFMB 20, in a number of trials performed with this microorganism glucose and industrial glycerol were employed at higher initial concentrations. In these cases, (ΝΗ4)2SO4 and yeast extract were employed at initial concentration of 1.0 g/L. It is evident that when industrial glycerol was used as substrate, the appropriate calculations were performed in order to achieve the requested, in each experiment, Glol0 concentration.
All experiments were carried out in 250‐mL Erlenmeyer flasks, containing 50 ± 1 mL of growth medium, sterilized at T = 121°C/20 min and inoculated with either 1 mL of spore suspension (in the case of fungi), or 1 mL of 24‐h exponential pre‐culture in the case of yeasts. The yeast pre‐culture was carried out in yeast‐peptone‐dextrose medium with 10 g/L of each. Irrespective of the trials performed with yeast or fungal strains, the initial dry cell weight (DCW) concentration (X0) was c. 0.15 g/L. All cultures were done in an orbital shaker (New Brunswick Sc, USA) at T = 28 ± 1°C and 185 ± 5 rpm and periodically removed from the incubator for further analyses. In all of the trials performed, medium pH value was maintained within 5.0–6.0 by adding into the flasks when needed, an appropriate volume of KOH (5Μ) (e.g. 500–600 μL) under aseptic conditions.
2.2. Analytical methods
The kinetics of DCW production, carbon substrate (glucose and/or glycerol) assimilation, ammonium ions decrease, organic acids (e.g. citric acid, oxalic acid, etc.) and alcohols (ethanol, mannitol, erythritol) secretion and intra‐cellular lipid production was assessed. In the case of Y. lipolytica LFMB 20, besides intra‐cellular total lipids, also total intra‐cellular sugars were quantified. Erlenmeyer flasks were removed from the incubator and initially the dissolved oxygen (DO) concentration was determined with the aid of a selective electrode (OXI 96, B‐SET, Germany) 34. In all cases, the DO concentration was >5% v/v for all growth steps of the cultures. Thereafter, pH in the culture medium was measured (Jenway 3020 pH‐meter, UK), and, as previously stated, it was corrected (if needed) in order to be maintained within the range of 5.0–6.0. Moreover, and before the quantitative determination of the substrate (glycerol or glucose) and the metabolic compounds, after the measurement of DO concentration and the tentative correction of pH, the volume of the collected cultures was corrected to the value of 50 ± 1 mL. Due to the water evaporation, the volume of medium in the flasks after their harvesting was 46 ± 2 mL). It is evident that the more the culture duration was elevated, the more water evaporation of the medium occurred. Nevertheless, the volume of the medium was never lower than 44 mL, it was always corrected (to 50 ± 1 mL) and the whole content of the flask was used in order to quantify the DCW concentration of the cultures. Cells, pellets, or mycelia were collected, either by filtration through a 0.09 mm stainless steel sieve (for the case of fungi, except the fungus M. isabellina) or centrifugation (12 000 × g/15 min) (for yeasts and M. isabellina) in a Hettich Universal 320‐R (Germany) centrifuge and washed twice with distilled water. Thereafter, wet pellets, mycelia, or cells were placed at c. 95°C/24 h and, thus, DCW (X, g/L) was determined. Glycerol (Glol, g/L), glucose (Glc, g/L) and metabolic products were determined with the aid of HPLC analysis 10. In the case of Y. lipolytica LFMB 20, total intra‐cellular sugars were quantified 35. Moreover, total cellular lipids (L, in g/L) were extracted from the DCW with a mixture of chloroform/methanol (C/M) 2/1 (v/v) (“Folch” extract) 36 and were determined gravimetrically. In addition, in some cases cellular lipids were fractionated into their lipid fractions over a column of silicic acid while lipids were converted to their fatty acid methyl‐esters and were analyzed with the aid of GC 37. Ammonium ions determination was done with the aid of an ammonium selective electrode (Hach 95‐12, Germany).
In some cases (e.g. lipids of M. isabellina), the crude lipid extract (“Folch” extract) was washed with one fourth its volume of 0.88% w/v KCl solution 38, was dried over anhydrous Na2SO4, and TLC analysis was performed in both the crude lipid (“Folch” extract) and the washed lipid (“Folch” washed extract). The separation was performed on glass precoated silica‐gel G plates (Merck, Germany) (20 cm × 20 cm; thickness = 0.25 mm), with development of 17 cm and origin 2 cm from the bottom of the plate. The plates were prewashed before use, by running them with C/M 2/1 (v/v) and were activated by heating for 1 h in an oven at T = 110°C. The separation of crude lipid and “Folch” washed extract was carried out with petroleum ether/diethyl ether/glacial acetic acid (80:20:1, v/v/v), in an all‐glass chromatography chamber at room temperature. The plate application of samples and lipid standards was carried out with a semi‐automatic injector Linomat IV, Camag (Switzerland). Visualization was achieved by spraying with 3% cupric acetate in 8% phosphoric acid solution and heating at T = 180°C for 30 min. Lipid standards were used for the identification of the main bands on TLC plates.
3. Results and discussion
3.1. Growth of yeast strains on industrial glycerol; comparisons with glucose
In the first part of this work, trials were performed on industrial glycerol and commercial glucose used as control employed at initial concentration (Glol0 or Glc0) of c. 30 g/L and initial C/N molar ratio adjusted at c. 100 moles/moles. As indicated, the fermentations were performed under nitrogen limitation in order to investigate the potential of the microorganisms tested to accumulate lipids and/or produce secondary metabolites. The obtained results for the screening on glucose‐based media are illustrated in Table 1A, while the respective ones on industrial glycerol are shown in Table 1B.
Table 1.
Growth of yeast strains on glucose (Glc0≈30 g/L, nitrogen added into the medium in the form of ammonium sulfate at 0.5 g/L and yeast extract at 0.5 g/L) (A) and biodiesel‐derived glycerol (Glol0≈30 g/L, nitrogen added into the medium in the form of ammonium sulfate at 0.5 g/L and yeast extract at 0.5 g/L) (B).
| A | ||||||
|---|---|---|---|---|---|---|
| Yeast strains | Time | Glccons (g/L) | X (g/L) | L (g/L) | YL/X (%, w/w) | |
| Candida diddensiae NRRL Y‐7589 | a, b*1 | 112 | 29.5 | 7.9 | 0.48 | 6.0 |
| c**1 | 44 | 26.5 | 5.9 | 0.13 | 2.2 | |
| Candida tropicalis NRRL Y‐12968 | a, b*2 | 141 | 33.7 | 7.3 | 0.44 | 6.1 |
| c**2 | 42 | 33.2 | 5.8 | 0.26 | 4.4 | |
| Pichia ciferrii NRRL Y‐1031 | a, b*3 | 72 | 31.2 | 7.8 | 0.55 | 7.0 |
| c**3 | 44 | 31.2 | 4.5 | 0.30 | 6.6 | |
| Williopsis saturnus NRRL Y‐17396 | a, b*4 | 68 | 33.4 | 8.7 | 0.52 | 5.7 |
| c**4 | 44 | 33.4 | 7.0 | 0.05 | 0.7 | |
| Candida boidinii ATCC 32195 | a, b*5 | 100 | 31.1 | 7.6 | 0.36 | 4.7 |
| b**5 | 31 | 29.2 | 4.9 | 0.10 | 2.0 | |
| Candida oleophila NRRL Y‐1613 | a, b*6 | 166 | 32.9 | 5.2 | 0.52 | 10.0 |
| c**6 | 20 | 32.2 | 3.4 | 0.14 | 4.1 | |
| Rhodosporidium toruloides DSMZ 4444 | a, b | 120 | 33.4 | 9.7 | 3.84 | 39.6 |
| Candida guilliermondii NRRL Y‐2075 | a, b | 112 | 31.0 | 9.3 | 0.57 | 6.1 |
| Yarrowia lipolytica ACA‐YC 5030 | a*7 | 144 | 35.5 | 6.2 | 0.30 | 4.8 |
| b | 48 | 12.5 | 5.9 | 0.59 | 10.0 | |
| Yarrowia lipolytica ACA‐YC 5029 | a*8 | 118 | 25.7 | 4.9 | 0.20 | 4.0 |
| b | 48 | 5.9 | 3.9 | 0.37 | 9.5 | |
| Yarrowia lipolytica LFMB 20 | a*9 | 120 | 29.5 | 6.9 | 0.35 | 5.0 |
| b | 48 | 8.2 | 4.1 | 0.33 | 8.1 | |
| Lipomyces starkeyi LGAM 235 | a, b | 120 | 28.1 | 10.7 | 1.28 | 11.9 |
| Pichia membranifaciens LFMB 1 | a, b | 180 | 28.9 | 11.2 | 1.02 | 9.1 |
| B | ||||||
|---|---|---|---|---|---|---|
| Yeast strains | Time | Glccons (g/L) | X (g/L) | L (g/L) | YL/X (%, w/w) | |
| Candida diddensiae NRRL Y‐7589 | a | 164 | 33.3 | 9.1 | 0.03 | 0.3 |
| b | 21 | 7.0 | 3.9 | 0.45 | 11.3 | |
| Candida tropicalis NRRL Y‐12968 | a | 96 | 4.9 | 3.1 | 0.16 | 5.1 |
| b | 67 | 2.4 | 2.1 | 0.14 | 6.5 | |
| Pichia ciferrii NRRL Y‐1031 | a, b | 238 | 30.7 | 9.5 | 1.32 | 13.9 |
| Williopsis saturnus NRRL Y‐17396 | a | 96 | 30.3 | 10.0 | 0.20 | 2.0 |
| b | 16 | 6.5 | 3.3 | 0.29 | 8.8 | |
| Candida boidinii ATCC 32195 | a, b | 111 | 16.4 | 6.0 | 0.08 | 1.2 |
| Candida oleophila NRRL Y‐1613 | a | 118 | 29.8 | 13.3 | 0.09 | 0.6 |
| b | 24 | 6.1 | 3.9 | 0.18 | 4.5 | |
| Rhodosporidium toruloides DSMZ 4444 | a, b | 160 | 26.9 | 8.0 | 1.91 | 23.7 |
| Candida guilliermondii NRRL Y‐2075 | a, b | 272 | 33.3 | 11.2 | 0.92 | 8.2 |
| Yarrowia lipolytica ACA‐YC 5030 | a# | 110 | 30.2 | 7.3 | 0.37 | 5.1 |
| b | 22 | 6.7 | 5.7 | 1.91 | 33.5 | |
| Yarrowia lipolytica ACA‐YC 5029 | a## | 95 | 29.1 | 5.5 | 0.21 | 3.8 |
| b | 22 | 5.9 | 3.2 | 0.29 | 9.0 | |
| Yarrowia lipolytica LFMB 20 | a### | 120 | 31.0 | 8.7 | 0.44 | 5.1 |
| b | 48 | 9.0 | 5.0 | 0.49 | 9.8 | |
| Lipomyces starkeyi LGAM 235 | a | 188 | 28.1 | 8.8 | 0.62 | 7.0 |
| b | 95 | 19.2 | 5.2 | 0.46 | 8.9 | |
| Pichia membranifaciens LFMB 1 | a | 190 | 31.5 | 10.7 | 0.16 | 1.5 |
| b | 24 | 1.6 | 1.5 | 0.23 | 15.6 | |
A: **1EtOH = 2.9 g/L, *1EtOH = 0.5 g/L; **2EtOH = 8.9 g/L, Cit = 1.9 g/L, *2EtOH = 0.2 g/L; **3EtOH = 5.3 g/L, *3EtOH = 0.5 g/L; **4EtOH = 3.4 g/L, *4EtOH = 0.2 g/L; **5EtOH = 5.1 g/L, *5EtOH = 1.2 g/L; **6EtOH = 10.0 g/L, *6EtOH = 2.1 g/L; *7Cit = 12.9 g/L; *8Cit = 11.0 g/L, Ace = 1.9 g/L; *9Cit = 13.9 g/L.
X: biomass in g/L; Glc: glucose in g/L; L: lipid in g/L; EtOH: ethanol in g/L; Cit: citric acid in g/L; Ace: acetic acid in g/L.
B: #Cit = 2.5 g/L, Ml = 9.0 g/L; ##Cit = 8.5 g/L, Ml = 6.9 g/L; ###Cit = 5.5 g/L, Ml = 4.0 g/L.
X: biomass in g/L; Glol: glycerol in g/L; L: lipid in g/L; Cit: citric acid in g/L; Ml: mannitol in g/L.
Representations: (a) When the maximum concentration of biomass (X, g/L) is achieved; (b) When the maximum quantity of lipids in dry cell weight (YL/X, % w/w) is achieved; (c) when the maximum ethanol (EtOH, g/L) is achieved (ethanol production occurred only from glucose). Each point is the mean value of two independent measurements (SE < 10%).
According to the results obtained during growth on glucose, most of the yeast strains produced low lipid quantities, i.e. 0.05–0.59 g/L, while only R. toruloides DSMZ 4444 presented lipids in DCW (YL/X) values >20% w/w. The strains Williopsis saturnus, Pichia ciferrii, Candida tropicalis, C. boidinii, C. oleophila, and C. diddensiae assimilated the carbon source in a short period of 20–44 h and converted glucose to ethanol in maximum quantities 2.9–10.0 g/L. C. oleophila demonstrated the highest ethanol production with a maximum quantity of ethanol achieved of 10 g/L and a concomitant conversion yield of ethanol produced per glucose consumed = 0.31 g/g. We can also consider the strain C. tropicalis that produced a maximum ethanol quantity of c. 9 g/L with a concomitant yield of 0.27 g/g. All other ethanol‐producing yeast strains produced lower quantities of ethanol (conversion yield lower than 0.15 g/g). All these yeasts are positive to the so‐called “Crabtree” effect, since they can produce ethanol under aerobic conditions if glucose concentration is higher than a critical value 2, 39, 40, 41, 42, 43.
The conversion yield of ethanol produced per glucose consumed in the present study was lower than the maximum theoretical one ( = 0.51 g/g; see: Sarris and Papanikolaou 2). Other Crabtree‐positive yeast strains demonstrated significant differentiations in relation with the currently presented results as far as ethanol production was concerned; for instance, Saccharomyces cerevisiae cultivated in shake flasks under aerobic conditions demonstrated an ethanol yield on glucose assimilated ranging between 0.34 and 0.49 g/g, depended on the initial concentration of sugar, the initial C/N ratio and the addition of natural substances into the medium (like phenol‐containing wastes) 41, 42. Higher conversion of glucose into ethanol than in the current study has been reported by other Crabtree‐positive yeasts like Sac. weihenstephan (0.50 g/g), Sac. paradoxus (0.43 g/g), Sac. uvarum (0.43 g/g), and Kazachstania exiguous (0.43 g/g) 40. Other Crabtree‐positive yeast strains cultivated under aerated conditions, like Nakaseomyces glabrata, Nak. castellii, Vanderwaltozyma polysporus, Lachancea kluyveri, Zygosaccharomyces rouxii and Zsa. bisporus, presented conversion yields similar with the current investigation (0.04–0.36 g/g) 40.
For the ethanol‐producing yeasts during growth on glucose and considering the conversion yield of DCW produced per unit of sugar consumed without taking into account ethanol oxidation (in most but not all of the cases of the microorganisms tested here, the maximum concentration of ethanol achieved coincided with the complete disappearance of glucose from the medium) this yield ranged between 0.22 g/g (case of C. diddensiae) and 0.11 g/g (case of C. oleophila). Concerning this yield (always without considering ethanol oxidation), for the classical Sac. cerevisiae, discrepancies in its value were presented that were attributed on the initial sugar concentration, the addition of natural compounds into the medium (e.g. phenol‐containing wastewaters), the culture configuration (e.g. batch bioreactors versus shake‐flasks), the initial C/N molar ratio and the strain itself, with values recorded between 0.08 and 0.18 g/g 40, 41, 42, 43. Other Crabtree‐positive yeasts like Eremothecium sinecaudum, Zygotorulaspora mrakii, and Kluyveromyces wickerhamii presented much higher conversion yields on DCW produced per glucose consumed (e.g. ranging between 0.40 and 0.50 g/g), while in other cases this yield was comparable (e.g. 0.19 g/g for Kaz. exiguous; 40) with the current investigation. On the other hand, as seen in Table 1A, for all of the Crabtree‐positive strains studied, ethanol assimilation was followed by a (significant in some cases) increment of the biomass produced. This fact is in agreement with the results reported by Sac. cerevisiae cultivated in shake‐flask 42 or aerated batch bioreactors 40. Moreover, in the current investigation, increasing accumulation of total lipids was also observed during alcohol oxidation phase; in particular, in the case of the P. ciferrii and C. diddensiae strains the lipids quantity (in g/L) was doubled or tripled (see Table 1A). W. saturnus strain exhibited the most impressive lipid accumulation rate among the Crabtree‐positive yeasts strains tested; the second phase of the culture in which glucose had been already assimilated, and ethanol re‐consumption occurred, was accompanied by a ten‐fold increase in the amount of the cellular total lipids produced (0.05 g/L at the time of the complete glucose assimilation, 0.52 g/L at the time of ethanol exhaustion). Characteristic results concerning biomass production, substrate assimilation, DO concentration, and cellular lipid evolution (in % of lipids per DCW) during growth on glucose for C. oleophila NRRL Y‐1613 are presented in Fig. 1A and B).
Figure 1.
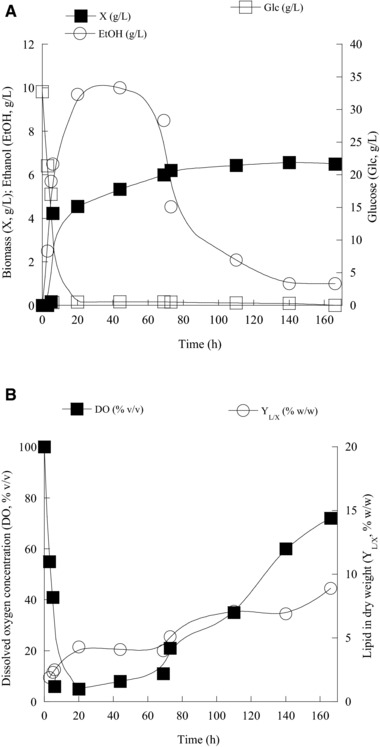
(A) Kinetics of biomass (X, g/L), ethanol (EtOH, g/L), glucose (Glc, g/L) and (B) intra‐cellular lipids produced per unit of dry weight (YL/X, % w/w) and dissolved oxygen (DO, % v/v) of Candida oleophila NRRL Y‐1613 during growth on glucose, at initial glucose concentration (Glc0) of c. 30 g/L, under nitrogen‐limited conditions. Each point is the mean value of two independent measurements (SE<10%).
The Crabtree‐negative yeast strains followed different metabolic profiles during their cultivation on glucose (see Table 1A); in all cases, glucose assimilation was significantly more decreased as compared with the Crabtree‐positive yeast strains, while despite the nitrogen limitation imposed, in C. guillermondii, L. starkeyi, and P. membranifaciens the metabolism was shifted mainly toward the synthesis of DCW that did not contain significant quantities of lipids (i.e.: X > 9.3 g/L, YL/Xmax≈12.5% w/w). Potentially, intra‐cellular storage compounds other than lipids (e.g. polysaccharides) were produced by the above‐mentioned yeasts strains, in accordance with recent literature reports 24, 35. In the case of Yarrowia lipolytica strains cultivated on glucose, equally a different biochemical behavior was observed, since growth was accompanied by significant secretion of citric acid into the growth medium, specifically after nitrogen depletion. What was interesting and coincided with previously published literature refers to the fact that these yeasts in many instances, during their cultivation on glucose or similarly metabolized compounds (e.g. glycerol), presented higher YL/X values at the initial stages of the cultures, decreasing afterward 13, 28, 44, 45, 46. In general, the production of citric acid and/or microbial lipid during growth of Y. lipolytica strains on glucose or similarly metabolized compounds (e.g. glycerol or other sugars) seems strain dependent, since in some cases predominantly SCO is produced (YL/X > 35% w/w; 7, 18, 47, 48) whereas in other studies citric acid (and potentially sugar‐alcohols) is secreted into the growth medium with relatively low quantities of lipid (e. g. YL/X < 22% w/w) being synthesized 13, 27, 44, 49, 50. Summarizing the results of Table 1A, only R. toruloides DSMZ 4444 presented noticeable lipid accumulation on glucose‐based media (Lmax≈3.9 g/L, YL/X≈40% w/w), indicating, thus, oleaginous character, in agreement with previously published reports concerning this strain 23, 51.
Summarizing the obtained results of the yeast strains growing on biodiesel‐derived industrial glycerol (Table 1B) and comparing these with the previous trials performed on glucose (Table 1A), the observations that one can made are as follows: no one of the Crabtree‐positive on glucose strains was capable of producing ethanol during growth on industrial glycerol (thus, these strains were “Crabtree‐negative” on glycerol). This result seems to be in contradiction with (the few) results already found in the literature; i.e. the microorganism Pachysolen tannophilus CBS4044 when cultivated on industrial glycerol produced non‐negligible quantities of ethanol, with a maximum ethanol concentration of 28.1 g/L with a concomitant volumetric productivity of c. 0.06 g L–1 h–1 achieved 52, 53, while strains of this species are characterized as potential ethanol producers during their cultivation on glucose‐ or xylose‐based media 2. Moreover, Rivaldi et al 54 revealed the capability of at least some yeast strains (e.g. belonging to the genus Hansenula) to perform alcoholic fermentation on industrial glycerol. On the other hand, in the current study, during growth on glycerol some of the Crabtree‐positive on glucose strains, produced significant quantities of DCW (e.g. W. saturnus, C. oleophila), that in most of the cases did not contain significant quantities of SCO (YL/X < 15% w/w). For the microorganism C. oleophila cultivated on glycerol or similarly metabolized compounds (e.g. glucose), discrepancies in the literature concerning its potentialities have been performed; for instance, while in the current submission it has been demonstrated that this microorganism could perform ethanol fermentation from glucose under aerobic conditions while it was unable to do so from glycerol under nitrogen‐limited conditions, other reports have indicated its capability to perform production of citric acid from glucose under nitrogen‐limited and aerated batch and continuous cultures with no indication at all of production of ethanol 55, 56. More recently and in agreement with the current investigation, the strain C. oleophila ATCC 20177 was revealed capable to produce significant quantities of biomass containing restricted quantities of lipids during growth on biodiesel‐derived glycerol under nitrogen‐limited conditions 50. In the latter case though, slightly higher maximum lipid quantities had been produced in comparison with the current submission (i.e. the strain ATCC 20177 has showed a YL/Xmax value of 15.3% w/w, while in the current investigation for the strain NRRL Y‐1613 the YL/Xmax value was only 4.5% w/w).
For most of the yeast strains tested, due to their virtual inability to produce appreciable quantities of cellular lipids, these microorganisms seemed to produce other storage compounds (like i.e. polysaccharides) during growth on nitrogen‐limited glycerol‐based media (in agreement with Tchakouteu et al. 24). Only the microorganisms R. toruloides DSMZ 4444 and Y. lipolytica ACA‐YC 5030 produced lipid in non‐negligible quantities (i.e. YL/X > 20% w/w). Specifically, the microorganism Y. lipolytica ACA‐YC 5030 synthesized lipids in a DCW value presenting the non‐negligible quantity of 33.5% w/w. However, as in the previous trials of Y. lipolytica strains performed on glucose, lipid in DCW values were elevated at the first growth steps in the presence of nitrogen, decreasing significantly afterward (similar trend with lower YL/Xmax values was observed also for the strain Y. lipolytica ACA‐YC 5029 – see Table 1B). This fact could suggest that in at least some strains of this species, lipid accumulation could be performed with the presence of some quantities of nitrogen found into the culture medium, before lipid breakdown in favor of the synthesis of extracellular metabolites 13, 24, 28, 45. Finally, concerning the yeast strains of Y. lipolytica that were studied in the current investigation, besides citric acid that was the main metabolic compound produced during growth on glucose, the above‐mentioned strains produced polyols (mostly mannitol) during growth on glycerol. In one of the trials (Y. lipolytica ACA‐YC 5030), this compound was the principal metabolite produced. The kinetics of biomass and lipid production and substrate assimilation in one of the yeasts cultivated on biodiesel‐derived glycerol in shake flasks (case of the yeast C. guilliermondii NRRL Y‐2075) is illustrated in Fig. 2.
Figure 2.
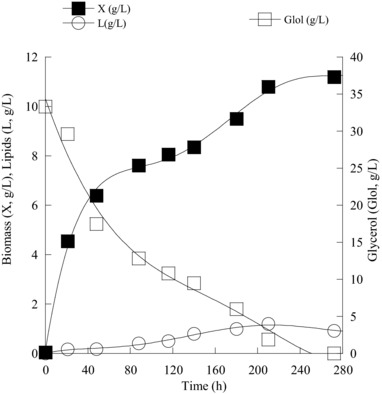
Kinetics of biomass (X, g/L), lipid (L, g/L), and glycerol (Glol, g/L) of Candida guilliermondii NRRL Y‐2075 during growth on industrial glycerol, at initial glycerol concentration (Glol0) of c. 30 g/L, under nitrogen‐limited conditions. Each point is the mean value of two independent measurements (SE<10%).
From all the above‐mentioned results, it may be deduced that although theoretically glucose and glycerol are metabolized through almost the same metabolic pathways (glycerol in the eukaryotes is catabolized through the C3 pathway of the EMP glycolysis; 2, 46, 57), finally the metabolism of glucose can result in very important variations compared with that of glycerol when all fermentation parameters are identical, except carbon substrate, that is employed at the same initial concentration of these compounds.
3.2. Growth of fungal strains on industrial glycerol; comparisons with glucose
In the next step, the potential of fungal strains growing on industrial glycerol under nitrogen‐limited conditions was evaluated while control experiments on glucose employed at the same initial concentration (Glol0 or Glc0 adjusted at a concentration of c. 30 g/L) were performed. The results on glucose are shown in Table 2A, the respective ones on glycerol in Table 2B.
Table 2.
Growth of fungal strains on glucose (Glc0≈30 g/L, nitrogen added into the medium in the form of ammonium sulfate at 0.5 g/L and yeast extract at 0.5 g/L) (A) and biodiesel‐derived glycerol (Glol0≈30 g/L, nitrogen added into the medium in the form of ammonium sulfate at 0.5 g/L and yeast extract at 0.5 g/L) (B).
| A | ||||||
|---|---|---|---|---|---|---|
| Fungal strains | Time | Glccons (g/L) | X (g/L) | L (g/L) | YL/X (%, w/w) | |
| Aspergillus sp. ATHUM 3482 | a* | 92 | 27.7 | 5.7 | 0.18 | 3.1 |
| b | 50 | 7.0 | 3.5 | 0.47 | 13.4 | |
| Cunninghamella echinulata LFMB 5 | a, b | 336 | 32.0 | 11.5 | 3.83 | 33.3 |
| Mortierella ramanniana MUCL 9235 | a, b** | 264 | 33.6 | 4.4 | 1.10 | 25.0 |
| Mucor sp. LGAM 366 | a, b | 310 | 31.5 | 8.0 | 3.46 | 43.3 |
| Rhizopus sp. LFMB 6 | a, b*** | 77 | 33.1 | 3.8 | 0.57 | 15.1 |
| Mortierella isabellina ATHUM 2935 | a | 92 | 34.2 | 10.2 | 8.50 | 83.3 |
| b | 155 | 34.9 | 13.7 | 4.05 | 29.6 | |
| B | ||||||
|---|---|---|---|---|---|---|
| Fungal strains | Time | Glccons (g/L) | X (g/L) | L (g/L) | YL/X (%, w/w) | |
| Aspergillus sp. ATHUM 3482 | a | 122 | 18.6 | 3.9 | 0.67 | 17.2 |
| b# | 192 | 21.0 | 3.5 | 1.07 | 30.3 | |
| Cunninghamella echinulata LFMB 5 | a, b | 262 | 12.4 | 2.9 | 1.61 | 55.6 |
| Mortierella ramanniana MUCL 9235 | a | 255 | 24.0 | 9.3 | 3.91 | 42.0 |
| b | 142 | 14.9 | 7.6 | 3.30 | 43.4 | |
| Mucor sp. LGAM 366 | a | 357 | 25.5 | 8.0 | 2.08 | 26.0 |
| b | 310 | 20.4 | 6.8 | 2.93 | 43.1 | |
| Rhizopus sp. LFMB 6 | a## | 140 | 24.1 | 4.6 | 0.84 | 18.2 |
| b | 71 | 23.6 | 3.7 | 0.86 | 23.3 | |
| Mortierella isabellina ATHUM 2935 | a | 340 | 27.5 | 9.7 | 2.99 | 30.8 |
| b | 222 | 24.5 | 8.1 | 5.40 | 66.7 | |
A: *Oxl = 13.8 g/L, Cit = 1.5 g/L; **Pyr = 8.8 g/L, Cit = 1.5 g/L; ***Lac = 10.1 g/L.
X: biomass in g/L; Glc: glucose in g/L; L: Lipid in g/L; Oxl: Oxalic acid in g/L; Pyr: pyruvate in g/L; Cit: Citric acid in g/L; Lac: Lactic acid in g/L.
B: #Oxl = 7.5 g/L; ##Ace = 2.5 g/L.
X: biomass in g/L; Glol: Glycerol in g/L; L: Lipid in g/L; Oxl: Oxalic acid in g/L; Ace: Acetic acid in g/L).
Representations: (a) When the maximum concentration of biomass (X, g/L) is achieved; (b) When the maximum quantity of lipids in dry cell weight (YL/X, % w/w) is achieved. Each point is the mean value of two independent measurements (SE<10%).
From the obtained results of the fungi cultivated on glucose and the comparison with the equivalent trials of the yeasts, it can be seen that fungi produced higher lipid quantities than the yeasts; during growth on glucose by the tested fungi, the lower YL/Xmax quantity produced was that of 13.4% w/w, obtained by the fungus Aspergillus sp. ATHUM 3482. (In the trials performed on yeasts cultivated on glucose, with the exception of the microorganism R. toruloides DSMZ 4444, all other yeast strains screened presented YL/Xmax values < 12%, w/w). In the case of Aspergillus sp. ATHUM 3482, the metabolism of the studied strain was shifted toward the production of oxalic acid (Oxl = 13.8 g/L, yield of oxalic acid produced per glucose consumed; YOxl/Glc≈0.50 g/g). Interestingly, in the above‐mentioned case, the microorganism presented some accumulation of storage lipids at the relatively early growth steps (YL/X = 13.4% w/w) decreasing afterward. In fact, this trend has already been reported for several Aspergillus niger strains cultivated on biodiesel‐derived waste glycerol; lipid and oxalic acid production in these experiments, as in the current investigation, was sequential since at the first growth steps, nitrogen limitation induced lipid accumulation (lipogenic phase) while oxalic acid biosynthesis was not very high. Thereafter, as in trials performed on glucose, lipid turnover (degradation) occurred simultaneously with significant oxalic acid secretion into the growth medium, while, as in the current investigation, decrease of the YL/Xmax value occurred although non‐negligible amounts of substrate (glucose) remained unconsumed into the medium 11. On the other hand, in the current investigation Aspergillus sp. ATHUM 3482 did not show the same behavior during growth on glycerol, in which simultaneously accumulated lipids (YL/Xmax = 30.3% w/w) and produced oxalic acid (up to 7.5 g/L).
The maximum production of oxalic acid achieved in the current investigation (Oxl = 13.8 g/L, YOxl/Glc≈0.50 g/g on glucose; Oxl = 7.5 g/L, YOxl/Glol≈0.36 g/g on glycerol), although it is non‐negligible, compares unfavorably with the maximum values achieved in the literature; for instance, the mutant strain A. niger XP produced oxalate to quantities ranging between 34 and 49 g/L when cultivated on media containing 30 g/L of post‐refining fatty acids used as the sole substrate with conversion yield ranging between 1.3 and 1.8 g of oxalic acid produced per gram of fatty acids consumed (trials performed in batch bioreactors) 58. Even higher quantities of oxalic acid (e.g. c. 65 g/L) have been achieved by the same mutant strain when it was batch cultured on crude rapeseed oil, with conversion yields higher than 1.0 g/g 59. Musiał and Rymowicz 60 have reported a maximum oxalic acid concentration of 42.0 g/L by growing the same strain a biodiesel manufacture by‐product that contained besides industrial glycerol also significant quantities of free‐fatty acids. Moreover, during growth on industrial glycerol, A. niger NRRL 364 presented a maximum oxalic acid concentration of c. 21 g/L (conversion yield 0.55 g per g of glycerol assimilated) 11. On sucrose, A. niger CMB 120.49 was reported to produce 38.4 g/L of oxalic acid in fed‐batch bioreactor experiments, with lower conversion yield compared with the current submission (≈0.23 g/g) 61, while the genetically engineered A. niger NW 131 (deriving from the wild strain N 400 and containing the gox‐C17 mutation being a glucose‐oxidizing negative mutant) growing glucose produced 34.1 g/L of oxalic acid with a conversion yield on glucose consumed of 0.51 g/g 62.
In the current investigation and during trials on glucose, slightly higher maximum lipid quantities compared with Aspergillus sp. ATHUM 3482 (YL/Xmax = 15.1% w/w) were obtained by Rhizopus sp. LFMB 6, the metabolism of which also shifted toward the production of organic acids (mainly lactate at a maximum concentration of 10.1 g/L). Biotechnological production of lactic acid is achieved via pyruvate with lactate dehydrogenase in lactic acid bacteria, including but not limited to Carnobacterium, Enterococcus, Lactobacillus, Lactococcus, Leuconostoc, and Weissella genera 1. Fungal strains like Mucor, Monilia, and especially Rhizopus are also used for the production of lactic acid, but in general in higher titers than the current investigation 1, 63. For instance, in batch bioreactor experiments, R. oryzae ATCC 9363 was reported to produce quantities of lactic acid >100 g/L with conversion yield of 0.87 g per g of glucose consumed 64. Bai et al. 65 reported lactic acid production of 76 g/L by the strain R. oryzae R1021 cultured in batch bioreactor experiments during growth on glucose (conversion yield 0.78 g/g) while the respective values for R. oryzae NRRL 395 growing on glucose were 93.8 g/L and 0.78 g/g 66. Interestingly, during the current investigation Rhizopus sp. LFMB 6 when flask‐cultivated on industrial glycerol employed as substrate, did not produce at all lactic acid while it produced small quantities of acetic acid ( = 2.5 g/L). Moreover, growth on glycerol was followed by somehow higher biomass and total lipid production compared with the one glucose (see Tables 2A and 2B).
The four remaining Zygomycetes namely Mortierella ramanniana MUCL 9235, Cunninghamella echinulata LFMB 5, Mucor sp. LGAM 366, and M. isabellina ATHUM 2935 during their cultivation on glucose presented the typical features of the oleaginous microorganisms; they accumulated lipids in DCW basis of at least 25% w/w, after considerable glucose exhaustion from the growth medium. Nevertheless, for the case of the microorganism M. ramanniana MUCL 9235, non‐negligible quantities of extra‐cellular organic acids were identified and quantified into the medium (mostly pyruvate at a concentration of c. 9 g/L). In general, the production of pyruvic acid is not a common feature for several microorganisms, and has been carried out mostly by mutant or metabolically engineered strains (e.g. Torulopsis glabrata, Sac. cerevisiae, Y. lipolytica, Escherichia coli, etc.), with concentrations higher than the current investigation (i.e. pyruvate ranging between 36.9 and 90 g/L; 63, 67. Interestingly, the same strain M. ramanniana MUCL 9235 presented a completely different metabolic activity during its cultivation of industrial glycerol; unlike the results achieved on glucose, in the trial carried out on glycerol significantly higher DCW and total lipid quantities were achieved, while no production at all of extra‐cellular organic acids was reported (see Table 2B). This microorganism produced the appreciable quantity of SCO of c. 4 g/L, with concomitant lipid in DCW quantities ranging between 42 and 44% w/w. The conversion yield of total lipid produced per unit of glycerol consumed ranged between 0.16 and 0.22 g/g. The same microorganism cultivated on a similar type of industrial glycerol but in media presenting higher initial availability of nitrogen showed lower lipid production compared with the current submission (L = 2.71 g/L, YL/Xmax = 37.1% w/w; 50). The same strain during in its culture on pure glycerol in shake‐flask experiments produced lipid quantities of c. 3.7 g/L (respective lipid in DCW value of 53.1% w/w). Interestingly, the growth on bioreactor shifted toward the synthesis of lipid‐free material (L = 3.2 g/L, YL/Xmax = 32.7%, w/w) 15.
The remaining Zygomycetes, namely C. echinulata LFMB 5, Mucor sp. LGAM 366, and M. isabellina ATHUM 2935 produced significant quantities of lipid during their cultivation on glucose, in shake‐flask trials (in all cases YL/Xmax > 33.3% w/w; Lmax > 3.46 g/L). Industrial glycerol was not so adequate substrate like glucose, by taking into consideration both total biomass and microbial lipid produced in absolute values (g/L) and glycerol assimilation compared with that of glucose (see Tables 2A and 2B). The most affected by the change of the substrate strain was that of C. echinulata LFMB 5 that produced 11.5 g/L of total biomass containing lipids of c. 33% w/w, while almost complete exhaustion of glucose from the growth medium occurred 336 h after inoculation. The same strain produced much lower total biomass quantities (e.g. c. 3 g/L) and assimilated significantly lower glycerol quantities (12.4 g/L) after 262 h of inoculation. Interestingly, in the above‐mentioned experiment, lipid in DCW of 55.6% w/w was produced. However, further incubation did not ameliorate the consumption of glycerol by this strain. Apparently, glycerol is not an adequate substrate for C. echinulata growth and lipid production since similar mediocre results concerning growth and lipid production on glycerol had also been reported for the strain C. echinulata ATHUM 4411, which in contrast, demonstrated excellent results on glucose or other sugars 68, 69.
Lower quantities of substrate assimilated, biomass produced and lipid produced were recorded during growth on glycerol compared with that on glucose for both Mucor sp. LGAM 366 and M. isabellina ATHUM 2935 (Tables 2A and 2B). Apparently and in accordance with the results achieved for C. echinulata LFMB 5, in most oleaginous fungi, poor regulation of the enzymes involved in the primary metabolic steps of glycerol assimilation (e.g. glycerol kinase, 3‐P‐glycerol dehydrogenase) seems to be the reason for which lower biomass and lipid production and decreased lipid accumulation are observed during growth on glycerol compared with the one on glucose 57, 68, 70. Remarkable exception in this rule was the microorganism Thamnidium elegans CCF‐1465, that produced significant and almost equivalent quantities of biomass and total lipids during its cultivation in flasks when glucose or biodiesel‐derived glycerol had been used as individual substrates 50, 71. As far as M. isabellina ATHUM 2935 was concerned, its cultivation on glucose demonstrated, indeed, excellent results; the Lmax concentration achieved was 8.5 g/L with concomitant YL/Xmax value of 83.3% w/w. This lipid in DCW value achieved is one of the highest ones ever reported in the international literature, while the simultaneous conversion yield of lipid produced per unit of glucose consumed (≈0.25 g/g) was, equally among the highest ones ever reported. Microscopic observation of the strain using a light microscope was performed when the maximum amount of lipid had been accumulated and revealed that the mycelia were, indeed, completely and excessively saturated with storage lipids that were found in distinct lipid droplets of different sizes (Supporting Information Fig. S1). On the other hand, culture of M. isabellina ATHUM 2935 on industrial glycerol resulted in lower substrate assimilation rate compared with that on glucose, while DCW and lipid production were lower compared with growth on glucose (see Tables 2A and 2B). Though, appreciable quantities of storage lipids were produced even on glycerol employed as substrate; the Lmax quantity achieved was 5.4 g/L with a concomitant YL/Xmax value of 66.6% w/w, while the conversion yield of lipid produced per unit of glycerol consumed (≈0.22 g/g) was very interesting.
In the case of lipid accumulation performed through de novo biochemical synthesis (i.e. through utilization of glucose, glycerol, or similarly catabolized carbon sources as substrates), for the formation of 1 mole of triacylglycerol c. 30 moles of CH3COSCoA are required 72. The stoichiometry of lipid accumulation indicates that in the (completely ideal) case in which all of the CH3COSCoA produced through the intermediate cellular metabolism is channeled toward storage lipid biosynthesis, the maximum theoretical yield of lipid produced per unit of glucose consumed would be around 0.32 g/g 72. With reference to glycerol, the maximum theoretical yield of lipid produced from this substrate is around 0.30 g/g 46, 57, 73. However, as previously indicated, this is a completely ideal case in which all CH3COSCoA would have been channeled toward the synthesis of SCO, which does not happen in real fermentation conditions. As a general rule, under ideal conditions for SCO production (e.g. highly aerated chemostat cultures) lipid yield on glucose consumed cannot be higher than 0.22 g/g 72, 74, whereas in other reports this threshold is considered to be in the level of 0.20 g/g 57. Even for genetically engineered strains cultivated in highly aerated bioreactors, the conversion yield of lipid produced per glucose consumed is in the above‐mentioned level; for instance, in a strain of Y. lipolytica, delta‐9 stearoyl‐CoA desaturase (SCD) was identified as the rate‐limiting step and target for the metabolic engineering in order to enhance SCO production. Simultaneous over‐expression of SCD, acetyl‐CoA carboxylase (ACC1), and diacylglyceride acyl‐transferase (DGA1) resulted in the construction of an engineered strain exhibiting highly desirable phenotypes of fast cell growth and lipid over‐production, with conversion yield of lipid produced per unit of glucose consumed of 0.234 g/g 75 (even in the above‐mentioned case the achieved conversion yield is lower than that of M. isabellina obtained in the current investigation). As far as glycerol as substrate is concerned, its conversion threshold into SCO production under real fermentation conditions is around 0.10 ± 0.02 g/g, which, according to the previously presented analysis, is somewhat lower than that obtained on glucose, for many oleaginous strains used 6, 7, 12, 13, 68, 76. However, the respective YL/Glol value is slightly higher (e. g. ≈0.15 g/g) for the case of algae like Schizochytrium limacinum growing heterotrophically on glycerol 8, 9, or of the yeast R. toruloides NRRL Y‐27012 24, while only in a very scarce number of reports SCO conversion yields on glycerol assimilated higher than 0.20 g/g have been reported 50.
The same strain that was used in the current investigation (M. isabellina ATHUM 2935) has been successfully employed for the production of SCO from glucose and other sugars and specifically when glucose had been employed as substrate excellent conversion yields of c. 0.19–0.22 g/g (close or even higher to the maximum potentially achievable ones) have been obtained in both shake‐flask and batch bioreactor experiments 69, 77 demonstrating that, in contrast to several reports found in the literature that strongly claimed the opposite 72, 74, shake flasks are an adequate mode of cultivating oleaginous Zygomycetes. The results obtained in the current shake‐flask experiments (YL/Xmax = 83.3% w/w; conversion yield of SCO produced per unit of glucose consumed ≈0.25 g/g, that is one of the highest ever reported) provide evidence, once more, of the above‐mentioned conclusion.
The kinetics of DCW (g/L), lipid (g/L), glucose (g/L), lipid in DCW (%, w/w), and ammonium ions (mg/L) evolution by M. isabellina ATHUM 2935 during its shake‐flask experiment on glucose is presented in Fig. 3A and B. Significant accumulation of storage lipid was observed immediately after virtual depletion of assimilable nitrogen (ammonium ions) from the medium (see Fig. 3B), whereas, previously accumulated lipids were subjected to degradation (turnover) at the late growth step, after drop of glucose concentration below a critical value 70. Cellular lipid biodegradation occurs regardless of the culture “pre‐history” (i.e. the carbon source that had been previously employed in order SCO to be produced; 11, 13, 33, 46, 69, 70), while, in any case, the cellular triacylglycerols should be subjected to enzymatic hydrolysis through the use of intra‐cellular lipases, the released cellular fatty acids should be catabolized via the process of β‐oxidation and the produced acetyl‐CoA should be further converted via the anaplerotic by‐pass of glyoxylic acid into storage polysaccharides (in Fig. 3A it is clear that lipid biodegradation was accompanied by significant rise in the production of total, and hence, lipid‐free biomass). Moreover, the kinetics of DCW (g/L), lipid (g/L), glycerol (g/L), lipid in DCW (%, w/w), and ammonium ions (mg/L) evolution by Mucor sp. LGAM 366 during its culture on biodiesel‐derived glycerol is presented in Fig. 4A and B. Despite the disappearance from the growth medium of the assimilable nitrogen (ammonium ions were almost exhausted from the medium at c. 100‐h after inoculation – see Fig. 4B), at the relevant fermentation period small quantities of biomass and storage lipids had been produced, while equally insignificant amounts of glycerol had been assimilated by the microorganism (see Fig. 4A). Thereafter, at 130–150‐h after inoculation, onset of glycerol assimilation was observed resulting in non‐negligible glycerol consumption and DCW and lipid production (310 h after inoculation, DCW≈7 g/L containing total lipids of c. 43%) was observed, followed by lipid turnover and new lipid‐free material creation being realized after glycerol concentration drop at low levels (see Fig. 4A).
Figure 3.
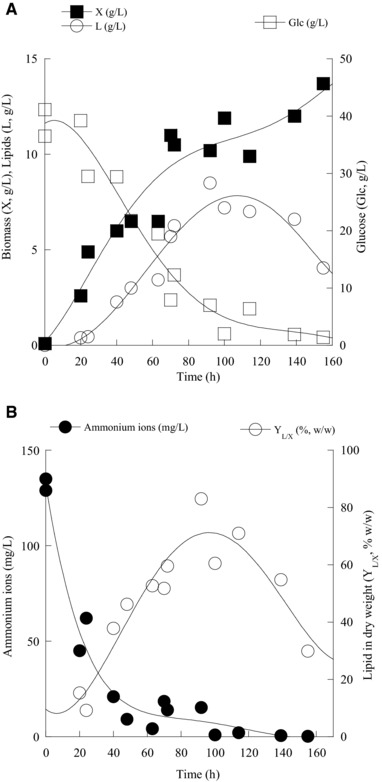
(A) Kinetics of biomass (X, g/L), lipid (L, g/L), glucose (Glc, g/L) and (B) intra‐cellular lipids produced per unit of dry weight (YL/X, % w/w) and ammonium ions (mg/L) of Mortierella isabellina ATHUM 2935 during growth on glucose, at initial glucose concentration (Glc0) of c. 30 g/L, under nitrogen‐limited conditions. Each point is the mean value of two independent measurements (SE<10%).
Figure 4.
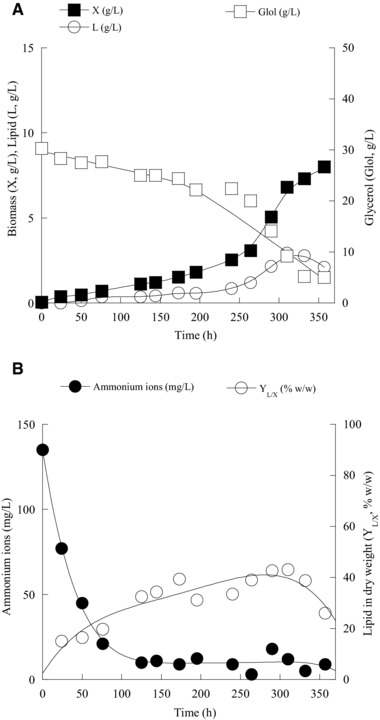
(A) Kinetics of biomass (X, g/L), lipid (L, g/L), glycerol (Glol, g/L) and (B) intra‐cellular lipids produced per unit of dry weight (YL/X, % w/w) and ammonium ions (mg/L) of Mucor sp. LGAM 366 during growth on industrial glycerol, at initial glycerol concentration (Glol0) of c. 30 g/L, under nitrogen‐limited conditions. Each point is the mean value of two independent measurements (SE < 10%).
3.3. Trials of Yarrowia lipolytica LFMB 20 on high glucose and glycerol media
The microorganism Y. lipolytica LFMB 20 presented non‐negligible production of citric acid during growth on glucose, while it also produced quantities of citric acid and mannitol growing on glycerol. Thus, it was decided to further study this strain. Trials were performed at higher Glc0 and Glol0 concentrations (≈100 g/L) in which nitrogen, in the form of (ΝΗ4)2SO4 and yeast extract was employed at higher initial concentration ( = 1.0 g/L) in order to somehow enhance the production of biomass and, hence, the assimilation of substrate by the strain. The trial performed on glucose is seen in Fig. 5A. The microorganism consumed all available assimilable nitrogen within 70 ± 10 h after inoculation (data not presented – at that fermentation time, the DCW concentration was at 10.4 ± 0.7 g/L) and afterward citric acid was secreted. The highest citrate concentration achieved was 58.1 g/L with concomitant yield of total citrate produced per glucose consumed ≈0.55 g/g.
Figure 5.
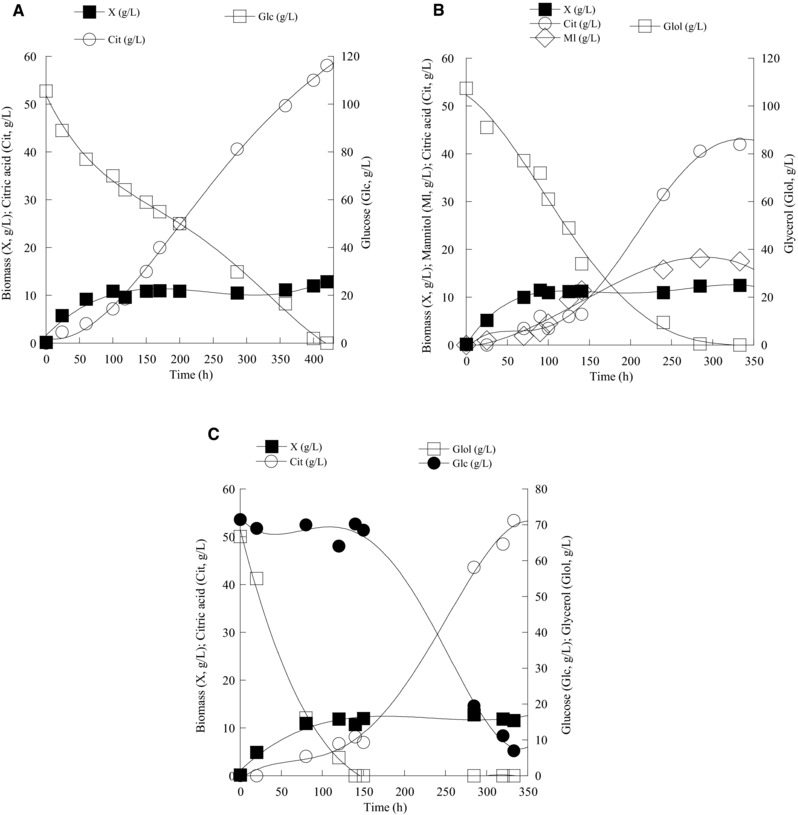
(A) Kinetics of biomass (X, g/L), substrate (glucose‐Glc, glycerol‐Glol or glucose/glycerol blend, g/L), citric acid (Cit, g/L), and mannitol (Ml, g/L) of Yarrowia lipolytica LFMB 20 during growth on glucose, at initial glucose concentration (Glc0) of c. 100 g/L, (B) industrial glycerol, at initial glycerol concentration (Glol0) of c. 100 g/L and (C) blend of industrial glycerol and glucose, at initial concentration of each carbon source of c. 70 g/L, under nitrogen‐limited conditions. Each point is the mean value of two independent measurements (SE < 10%).
Shake‐flask culture of Y. lipolytica LFMB 20 on glycerol revealed a different kinetic profile compared with that on glucose (Fig. 5B and Supporting Information Fig. S2), fortifying the previously indicated conclusion, meaning that that although glucose and glycerol theoretically are metabolized through almost the same metabolic pathways, finally the metabolism of glucose can result in important variations compared with that of glycerol. Concerning the microorganism Y. lipolytica LFMB 20, the cultivation on biodiesel‐derived glycerol resulted in slightly higher DCW production (at the stationary phase of the trial on glycerol, the Xmax concentration achieved was at 12.5 g/L) compared with the trial on glucose. During growth on glycerol, assimilable nitrogen disappeared 70 ± 10‐h after inoculation, and thereafter secondary metabolites were accumulated into the medium. At the end of the culture, citric acid, at lower quantities compared with glucose fermentation (Citmax = 42 g/L; YCit/Glol = 0.39 g/g), was the principal metabolite. In contrast to the trial performed on glucose, mannitol at non‐negligible concentrations (Mlmax = 18.2 g/L; YMl/Glol = 0.17 g/g) was secreted into the medium, while its production profile coincided with the one of citric acid and was performed at the stationary growth phase, after virtual arrival of biomass at its plateau value, due to nitrogen exhaustion from the culture medium (see Fig. 5B). What was also interesting during the culture of Y. lipolytica LFMB 20 on high Glol0 concentration media referred to the fact that at the initial stages after stationary growth phase had arrived (as indicated within 70 ± 10 h after inoculation), citric acid was not the principal metabolite produced; 140 h after inoculation, the secondary metabolites that had been detected into the medium were citrate (at 6.5 g/L), mannitol (at 11.4 g/L), and erythritol (at 13.3 g/L). At that fermentation point, the remaining into the medium glycerol concentration was c. 34 g/L and the concomitant conversion yields concerning the polyols produced were YMl/Glol = 0.16 g/g and YEry/Glol ≈ 0.19 g/g. Thereafter, and for reasons that cannot be identified, onset of erythritol re‐consumption was observed together with the simultaneous and continuous assimilation of glycerol, while the intra‐cellular carbon flow toward the synthesis of mannitol was maintained constant (the value of the yield YCit/Glol at the end of the culture was ≈0.17 g/g, a value that was almost equal with that recorded 140 h after inoculation) and citric acid in elevated quantities was finally synthesized (see evolution of the polyols mannitol, glycerol and erythritol during growth of Y. lipolytica LFMB 20 in Supporting Information Fig. S2).
In a relatively scarce number of reports, secretion of polyols into the culture medium, together with or independently from the production of citric acid, has been reported when glycerol was utilized as the sole carbon source in nitrogen‐limited submerged experiments 78, 79, 80. When mutant Y. lipolytica strains (e.g. Wratislavia AWG7 and Wratislavia K1 strains) were used in bioreactor experiments, erythritol was the principal polyol produced, in some cases in significant concentrations (up to 47 g/L), while mannitol was secreted in smaller quantities (up to 9 g/L) 78. Nevertheless, citric acid was always the most important metabolite produced (and in some cases in very high quantities, higher than these of the current investigation, e.g. in quantities ranging between 87 and 139 g/L) 78, 79, 80. On the other hand, production of erythritol, in some cases in indeed high final concentrations (e.g. quantities >45 or even 80 g/L) has been reported when industrial or pure glycerol has been employed as fermentation substrate by wild or mutant Y. lipolytica strains 31, 32, but in any case according to our knowledge, it is the first time in the literature that Y. lipolytica displays the biochemical behavior previously indicated (increase of erythritol concentration followed by its almost compete degradation whilst simultaneously significant quantities of glycerol remained into the medium, in favor of citric acid biosynthesis).
The culture of Y. lipolytica LFMB 20 was also investigated on media composed of blends of industrial glycerol and glucose (Glc0 and Glol0 concentrations adjusted to c. 70 g/L of each substrate) in order to investigate the dynamics of this microorganism on this type of dual substrates employed (see Fig. 5C). Glycerol was rapidly and preferentially assimilated by the microorganism, while at the same time glucose remained almost intact into the growth medium, in accordance with previously presented results for other Y. lipolytica strains like LGAM S(7)1 and IBT 446 49, 81, suggesting that this feature (preferential assimilation of glycerol versus glucose in glycerol/glucose co‐fermentations) is a common behavior for all Y. lipolytica strains. In similar types of investigations though and for other yeast strains studied (e.g. in R. toruloides), a completely inverted trend was observed, since glucose was much more rapidly assimilated and after its virtual exhaustion from the medium, onset of glycerol consumption was given 23. In the current investigation and in the glycerol/glucose co‐fermentation experiment, citric acid was the main metabolite produced (see Fig. 5C) with a maximum concentration achieved of 53.4 g/L (simultaneous conversion yield per unit of glucose + glycerol assimilated ≈0.41 g/g), while also 7.8 g/L of mannitol were synthesized. Comparisons of the results achieved in the current investigation related with the production of citric acid with the ones achieved in the earlier or more recent literature are indicated in Table 3.
Table 3.
Synopsis of results concerning citric acid production by yeasts growing on various fermentation configurations. Comparisons with the current investigation.
| Strain | Substrate | Culture mode | Cit (g/L) | YCit/S a (g/g) | References |
|---|---|---|---|---|---|
| Yarrowia lipolytica Wratislavia 1.31 | Industrial glycerol | Batch culture, bioreactor | 124.5 | 0.62 | Rymowicz et al. 25 |
| Yarrowia lipolytica A‐101‐1.22 | Industrial glycerol | Batch culture, bioreactor | 112.0 | 0.60 | Rymowicz et al. 26 |
| Yarrowia lipolytica ACA‐DC 50109 | Industrial glycerol | Batch culture, flasks | 62.5 | 0.56 | Papanikolaou et al. 27 |
| Yarrowia lipolytica JMY 1203 | Industrial glycerol | Batch culture, flasks | 57.7 | 0.92 | Papanikolaou et al. 28 |
| Yarrowia lipolytica Wratislavia AWG7 | Industrial glycerol | Repeated batch culture, bioreactor | 154.0 | 0.78 | Rywińska and Rymowicz 29 |
| Yarrowia lipolytica N15 | Pure glycerol | Batch culture, flasks | 19.1 | 0.55 | Kamzolova et al. 30 |
| Yarrowia lipolytica N15 | Pure glycerol | Fed‐batch culture, bioreactor | 98.0 | 0.70 | Kamzolova et al. 30 |
| Yarrowia lipolytica ACA‐DC 50109 | Industrial glycerol | Batch culture, flasks | 35.1 | 0.44 | Papanikolaou et al. 49 |
| Candida oleophila ATCC 20177 | Glucose | Batch culture, flasks, bioreactor | 50.1–79.1 | 0.55 | Anastassiadis et al. 55 |
| Saccharomycopsis lipolytica NRRL Y7576 | Glucose | Batch culture, bioreactor | 51.5 | 0.71 | Klasson et al. 82 |
| Yarrowia lipolytica ATCC 20346 | Glucose | Fed‐batch culture, bioreactor | 50.0–69.0 | 0.52 | Moresi 83 |
| Candida lipolytica Y 1095 | Glucose | Continuous culture recycling, bioreactor | 40.0–50.0 | 0.72 | Rane and Sims 84 |
| Yarrowia lipolytica N1 | Ethanol | Fed‐batch culture, bioreactor | 120.0 | 0.85 | Kamzolova et al. 85 |
| Yarrowia lipolytica 187/1 | Rapeseed oil | Fed‐batch culture, bioreactor | 135.1 | 1.55 | Kamzolova et al. 86 |
| Yarrowia lipolyrica NG40/UV7 | Pure glycerol | Fed‐batch culture, bioreactor | 115.0 | 0.64 | Morgunov et al. 87 |
| Yarrowia lipolyrica NG40/UV7 | Industrial glycerol | Fed‐batch culture, bioreactor | 112.0 | 0.90 | Morgunov et al. 87 |
| Yarrowia lipolytica LFMB 20 | Glucose | Batch culture, flasks | 58.1 | 0.55 | This study |
| Yarrowia lipolytica LFMB 20 | Industrial glycerol | Batch culture, flasks | 42.0 | 0.39 | This study |
| Yarrowia lipolytica LFMB 20 | Industrial glycerol/glucose blend | Batch culture, flasks | 53.4 | 0.41 | This study |
YCit/S is the conversion yield, in g of citrate produced per g of carbon substrate consumed.
In all of the trials performed with Y. lipolytica LFMB 20 growing at increased Glol0 or Glc0 concentration media, quantification of intra‐cellular lipids and sugars was performed for all growth stages, and similar results have been observed; at the relatively early growth stages of the culture and in the presence of nitrogen into the medium, the highest quantities of cellular lipids per unit of DCW (YL/X = 9–12% w/w) were reported, constantly decreasing afterward with the final values at the end of culture being of c. 2–4% w/w. The inverted trend was observed for the total intra‐cellular polysaccharide's evolution, the concentration of which at the initial growth stages was 22–28% w/w, and their final concentration at the end of the culture was 40–45% w/w. As it was previously indicated, in many Y. lipolytica strains growing in batch shake‐flask or bioreactor experiments with glucose or glycerol employed as the sole carbon source, somehow elevated quantities of total lipid in DCW values have been reported at the early growth stages, decreasing afterward, and simultaneously with citric acid secretion into the medium 13, 28, 44, 45. In contrast, according to our knowledge there is no available information concerning the evolution of total intra‐cellular polysaccharides as function of the fermentation time by Y. lipolytica strains, while in at least one case, inverted trend compared with the current investigation (i.e. increased quantity of total intra‐cellular sugars per unit of DCW at the first stages of the fermentation decreasing afterwards) has been observed for the oleaginous strain Cryptococcus curvatus NRRL Y‐1511 35.
3.4. Cellular lipid analysis
Analysis in the fatty acid (FA) composition of the cellular lipids produced by the several yeast and fungal strains cultivated on glucose or glycerol was performed and results of many of the microorganisms tested are presented in Table 4A. In this table, there is also presentation of an average FA composition of the rapeseed oil (an oil that is broadly used in order to be converted into biodiesel and is considered as a “model” oil for this purpose 51, 70, in order to be compared with that of the microorganisms studied in the present investigation. The results obtained in Table 4A can be summarized as follows: some differentiations but, as a general rule, not significant can be observed between the FA composition of the lipids produced when microorganisms are growing on glucose in comparison with the results obtained during growth on glycerol. Also, some differentiations (equally in most cases not very important and not following a systematic trend for the microorganisms tested) in the FA composition are seen as function of the fermentation time of the culture. The above‐mentioned observations are in agreement with most of the reports found in the literature 33, 46, 70, 72, 74 in which it is indicated that the carbon source as well the fermentation time can have an impact upon the total FA composition of the cellular lipids produced by the microorganisms tested. In general, literature suggests that fatty (and not hydrophilic, as in the current investigation) materials employed as substrates can have the most important impact upon the microbial FA composition 33, 46, 70.
Table 4.
Fatty acid composition of the cellular lipids produced by microorganisms cultivated on industrial glycerol and glucose (comparisons of fatty acid composition with the one of rapeseed oil, for the presentation of fungi, C18:3 is the γ‐ isomer; C18:3 ω‐6, while for the yeasts it is the α‐ isomer; C18:3 ω‐3) (A) and distribution of lipid fractions for several screened strains (growth of Mortierella isabellina ATHUM 2935 on glucose, Rhodosporidium toruloides DSMZ 4444 on glucose, Mortierella ramanniana MUCL 9235 on glycerol and Yarrowia lipolytica ACA‐YC 5029 on glycerol, under nitrogen‐limited conditions), in which analysis was performed when the maximum quantity of lipids in DCW was recorded for the respective cultures (N: Neutral lipids; G+S: Glycolipids plus sphingolipids; P: Phospholipids) (B).
| A | |||||
|---|---|---|---|---|---|
| Cellular fatty acids | C16:0 | C18:0 | C18:1 | C18:2 | C18:3 |
| Cunninghammella echinulata on glycerol | |||||
| 94 h | 23.5 | 5.9 | 46.2 | 16.7 | 5.6 |
| 144 h | 22.0 | 5.4 | 41.0 | 22.7 | 6.3 |
| 262 h | 21.7 | 6.0 | 42.5 | 20.8 | 7.5 |
| Cunninghammella echinulata on glucose | |||||
| 57 h | 17.2 | 6.3 | 49.4 | 15.8 | 10.4 |
| 147 h | 16.8 | 4.5 | 51.5 | 16.0 | 10.0 |
| 336 h | 16.6 | 3.8 | 51.9 | 16.0 | 10.6 |
| Mortierella ramanniana on glycerol | |||||
| 24 h | 26.9 | 6.5 | 37.2 | 20.5 | 8.9 |
| 93 h | 22.1 | 6.1 | 47.2 | 17.5 | 5.9 |
| 142 h | 21.4 | 5.4 | 48.9 | 17.8 | 5.4 |
| 255 h | 21.6 | 4.1 | 48.8 | 19.3 | 5.1 |
| Mortierella ramanniana on glucose | |||||
| 24 h | 28.7 | 7.0 | 37.2 | 15.8 | 11.3 |
| 92 h | 21.4 | 5.0 | 43.4 | 18.6 | 10.5 |
| 165 h | 23.8 | 4.1 | 38.7 | 21.4 | 10.4 |
| 264 h | 22.1 | 3.9 | 41.9 | 22.2 | 10.0 |
| Mucor sp. on glycerol | |||||
| 76 h | 29.2 | 12.6 | 28.0 | 16.7 | 16.5 |
| 240 h | 22.8 | 3.8 | 27.8 | 28.1 | 14.4 |
| 310 h | 25.0 | 4.0 | 26.4 | 27.0 | 10.0 |
| Mucor sp. on glucose | |||||
| 24 h | 21.9 | 7.2 | 31.5 | 20.5 | 17.0 |
| 125 h | 26.1 | 5.6 | 38.8 | 22.0 | 5.5 |
| 310 h | 24.1 | 5.0 | 39.2 | 22.8 | 7.0 |
| Mortierella isabellina on glycerol | |||||
| 25 h | 22.1 | 5.9 | 48.1 | 18.0 | 7.1 |
| 222 h | 22.0 | 3.9 | 51.2 | 18.8 | 4.0 |
| Mortierella isabellina on glucose | |||||
| 23 h | 25.5 | 4.4 | 50.0 | 9.9 | 3.0 |
| 92 h | 20.0 | 2.9 | 53.1 | 12.8 | 3.1 |
| 155 h | 19.1 | 1.9 | 55.1 | 15.1 | 4.1 |
| Candida diddensiae on glycerol | |||||
| 21 h | 23.1 | 3.9 | 26.6 | 33.8 | 6.3. |
| 116 h | 26.8 | 9.9 | 33.8 | 25.1 | Tr. |
| Candida diddensiae on glucose | |||||
| 16 h | 23.7 | 7.0 | 38.5 | 19.8 | 6.0 |
| 112 h | 22.7 | 8.4 | 40.1 | 17.8 | 7.5 |
| Pichia ciferri on glycerol | |||||
| 21 h | 21.7 | 6.0 | 43.3 | 12.1 | 2.0 |
| 140 h | 20.8 | 5.1 | 35.2 | 10.8 | 1.0 |
| Pichia ciferri on glucose | |||||
| 16 h | 25.9 | 10.7 | 42.7 | 13.2 | Tr. |
| 112 h | 21.0 | 5.3 | 31.7 | 10.7 | Tr. |
| Candida guillermondii on glycerol | |||||
| 16 h | 21.4 | 22.9 | 40.2 | 11.3 | Tr. |
| 112 h | 22.4 | 4.5 | 59.0 | 5.0 | Tr. |
| Candida guillermondii on glucose | |||||
| 16 h | 19.8 | 15.1 | 47.3 | 13.3 | Tr. |
| 112 h | 21.0 | 5.7 | 57.5 | 7.7 | Tr. |
| Rhodosporidium toruloides on glycerol | |||||
| 46 h | 21.8 | 10.9 | 42.3 | 19.2 | 1.3 |
| 160 h | 30.3 | 14.3 | 42.0 | 10.7 | 1.9 |
| Rhodosporidium toruloides on glucose | |||||
| 24 h | 26.9 | 10.1 | 40.0 | 17.0 | 4.5 |
| 120 h | 27.3 | 10.8 | 48.8 | 9.9 | 3.1 |
| Rapeseed Oil | 2–7 | 1–3 | 50–66 | 18–28 | 6–14 |
| B | |||||
|---|---|---|---|---|---|
| Lipid fraction | %, w/w | ||||
| Mortierella isabellina ATHUM 2935 | N | 90.1 | |||
| G+S | 9.1 | ||||
| P | 0.8 | ||||
| Rhodosporidium toruloides DSMZ 4444 | N | 83.5 | |||
| G+S | 9.5 | ||||
| P | 7.0 | ||||
| Mortierella ramanniana MUCL 9235 | N | 90.0 | |||
| G+S | 8.1 | ||||
| P | 1.9 | ||||
| Yarrowia lipolytica ACA‐YC 5029 | N | 76.5 | |||
| G+S | 14.8 | ||||
| P | 8.7 | ||||
A: Tr.: Fatty acid concentration <0.5% w/w.
Another result that can be deduced from the Table 4A indicates that, in agreement with the literature 33, 46, 70, 72, fungal lipids are more unsaturated than yeast lipids. The Zygomycetes studied produced the γ‐ isomer of linolenic acid (C18:3 ω‐6) while the yeasts the α‐ one (C18:3 ω‐3). γ‐Linolenic acid is an important FA presenting various therapeutic activities 33, 88. The lipids of some plants that contain γ‐linolenic acid are currently commercialized (it is the case of the oil deriving from the plant Oenothera biennis called also evening primrose oil, that contains this FA in concentrations 8–10% w/w) and their cost is estimated to 45–50 $ per kg 27, 72. Therefore, there is increasing interest in the production of microbial lipids containing this FA. In the current investigation, the highest quantities of γ‐linolenic acid produced were by C. echinulata LFMB 5 growing on glucose (≈405 mg per L of medium), M. ramanniana MUCL 9235 growing on biodiesel‐derived glycerol (≈200 mg/L), M. isabellina ATHUM 2935 growing on biodiesel‐derived glycerol (= 216 mg/L), and M. isabellina ATHUM 2935 growing on glucose (≈350 mg/L). The highest quantities of γ‐linolenic acid that have been produced by wild‐type Zygomycetes are recorded by C. echinulata ATHUM 4411, C. echinulata CCRC 31840, and T. elegans CCF‐1465 (quantities ranging between 900 and 1200 mg/L of medium) 68, 89, 90, 91.
Some of the microorganisms that presented noticeable lipid production on glucose or biodiesel‐derived glycerol, were further studied in relation with the fractionation of their lipids into neutral lipids (NL), glycolipids plus sphingolipids (G+S) and phospholipids (P) (Table 4B). In all cases and in full agreement with the literature 28, 37, 44, 50, 57, 69, 90 NL fraction was by far the most abundant one of the microbial produced lipids. It is interesting to indicate that even for the microorganism Y. lipolytica ACA‐YC 5029 that did not accumulate significant quantities of lipids (at the time in which analysis of total lipid into its classes was performed, lipid in DCW quantity was of 9% w/w – see Table 1B), the NL fraction was richest one from all fractions quantified.
Given that M. isabellina ATHUM 2935 presented very interesting results concerning SCO production in both media composed of glucose or glycerol (see Tables 2A and 2B), the total lipids of this microorganism contained a very high quantity of NL while also their total FA composition presented remarkable similarities with the “model” rapeseed oil (as stated the most adequate oil employed for the creation of biodiesel 70), it was decided to study these lipids in more details. TLC analysis revealed that the fraction of triacylglycerols was (by far) the most abundant one of the (crude) microbial lipids produced (extraction performed with C/M 2/1 – “Folch” extract – see Fig. 6). Visual inspection of the chromatoplate (Fig. 6), demonstrates that the typical washing procedure 36 performed on the “Folch” extract of the crude microbial lipid had no effect on the concentration of the neutral lipids. The results of visual inspection were further supported from the integrated optical densities of the spots obtained after processing the image with Image software (a public domain, Java‐based image processing program developed at the National Institute of Health, USA) (results not shown). Therefore, “wash” of the diluted into Folch mixture “crude” microbial lipid with saturated solution of KCl, revealed negligible differences compared with “crude” microbial lipid, demonstrating that the Folch extract is completely adequate in order to collect the microbial triacylglycerols, amenable to be converted in next step into biodiesel. Moreover, M. isabellina lipids contained steryl‐esters and sterols (in fact, fungal lipids cannot contain cholesterol; most probably this spot corresponds to ergosterol and/or potentially diacylglycerols that were not sufficiently separated) (Fig. 6). (In all cases, lipid analyses were performed at the stationary growth phase of M. isabellina cultivated on glucose when the maximum quantity of lipids in DCW was recorded). From all the above‐mentioned analysis it can be deduced that although in microbial lipids several compounds can be found, triacylglycerols are, by far, the most abundant compound.
Figure 6.
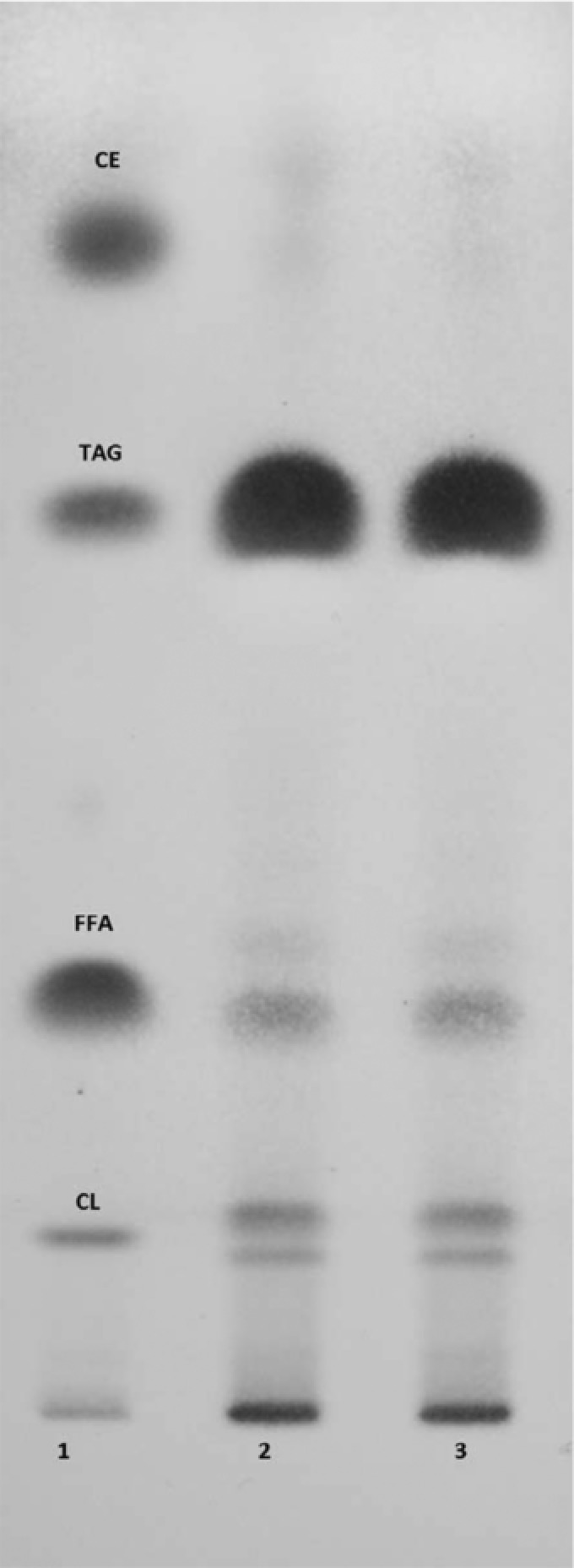
TLC analysis of the “crude” “Folch” extract before (lane 3) and after washing with saturated KCl solution (lane 2) of the cellular lipid by Mortierella isabellina ATHUM 2935, cultivated on glucose. Lane 1: mix of neutral lipid standards (cholesterol‐CL, oleic acid‐FFA, glyceryl trioleate‐TAG, cholesteryl linoleate, CE). Analysis was performed when lipid in DCW presented its highest value.
4. Concluding remarks
Several Crabtree‐positive yeasts produced ethanol from glucose but not from glycerol, a substrate that was used mainly for the production of biomass. Despite nitrogen limitation imposed, in most of the yeast species tested, no virtual lipid accumulation was recorded.
Although glucose and glycerol are theoretically subjected to similar catabolism within the microbial cells, differentiations (in some cases very important) were observed when glucose or glycerol was employed as substrate by yeast and fungal strains. Therefore in several cases completely different final spectra of synthesized metabolites were recorder during growth on glucose compared with that on glycerol.
The fungi tested presented higher production of lipid than the yeasts.
With the exception of Mortierella ramanniana MUCL 9235, glucose was a more adequate substrate for lipid accumulation than glycerol for the employed fungal species tested. On the other hand, M. ramanniana MUCL 9235 produced appreciable quantities of lipid during growth on biodiesel‐derived glycerol (c. 4 g/L), a relatively correct quantity of the medically important γ‐linolenic acid (≈200 mg/L) was produced, while the conversion yield of total lipid produced per unit of glycerol was very high (between 0.16–0.22 g/g). Concerning the potential of SCO production by this microorganism during growth on waste glycerol, more attention in larger‐scale production should be paid in the future.
M. isabellina ATHUM 2935 presented a very interesting lipid production on both glucose and glycerol, with conversion yields per unit of substrate (glucose or glycerol) consumed, almost the highest ones ever reported (ranging between 0.22 and 0.25 g/g). Moreover, a relatively correct quantity of the medically important γ‐linolenic acid (up to 350 mg/L) was produced. The lipids produced by this fungus consisted mostly of triacylglycerols. Concerning the potential of SCO production by this microorganism cultivated either on waste glycerol or on waste streams composed of glucose, more attention in larger‐scale production should be paid in the future.
Even in the case that insignificant lipid accumulation occurred, microbial lipids were composed mainly of neutral fractions.
Yarrowia lipolytica LFMB 20 is a potential producer of citric acid and mannitol from biodiesel‐derived glycerol. Erythritol was also produced into the medium but it was re‐consumed in favor of citric acid production. On the other hand, growth on glucose was accompanied by the production of citric acid with no polyols having been produced. The highest mannitol concentration achieved during growth on biodiesel‐derived glycerol (18.2 g/L) is among the quite correct ones recorded in the international literature. Likewise, the production of citric acid from both glucose and waste glycerol by this strain was interesting, thus concerning the potential of citric acid production by this microorganism cultivated either on waste glycerol or on waste streams composed of glucose, more attention in larger‐scale production should be paid in the future.
Practical application
The potential increase of biodiesel production in the near future leads to the necessity of discovering various integrated (bio)‐processes for valorization of crude glycerol, the main residue deriving from this process. Here, this residue was employed by several yeast and fungal species, while glucose was employed as “reference” substrate. Despite the significant similitude of the metabolism between glucose and glycerol, differences on the final spectrum of the metabolites synthesized were observed between growths on those two substrates. Several yeasts produced ethanol from glucose under aerobic conditions but not from glycerol. Yarrowia lipolytica LFMB 20 produced remarkable quantities of citric acid and mannitol during growth on glycerol. Mortierella isabellina ATHUM 2935 produced lipid amenable to be converted in high‐quality biodiesel growing on both glucose and glycerol. The current study provides alternative ways of valorization of industrial glycerol, by using it as substrate by yeasts or fungi, which produced a plethora of metabolic compounds on this residue.
The authors have declared no conflict of interest.
Supporting information
Figure S1. Microscopic observation of Mortierella isabellina ATHUM 2935 during growth on glucose, in the fermentation point in which the highest lipid production was observed. Magnification ×100
Figure S2. Kinetics of glycerol (Glol, g L–1), mannitol (Ml, g L–1) and erythritol (Ery, g L–1) of Yarrowia lipolytica LFMB 20 during growth on industrial glycerol, at initial glycerol concentration (Glol0) of c. 100 g L–1, under nitrogen‐limited conditions. Each point is the mean value of two independent measurements (SE<10%).
Acknowledgments
The current investigation was financially supported by the project entitled “New bioprocess for microbial oil from crude glycerol and cellulosic sugars” (Acronym: BIO4OIL, project code 2359) financed by the General Secretariat for Research and Technology, Ministry of National Education and Religious Affairs, Greece (project action: “Bilateral S&T cooperation between Greece and Germany 2013–2015”).
5 References
- 1. Koutinas, A. A. , Vlysidis, A. , Pleissner, D. , Kopsahelis, N. et al., Valorization of industrial waste and by‐product streams via fermentation for the production of chemicals and biopolymers. Chem. Soc. Rev. 2014, 43, 2587–2627. [DOI] [PubMed] [Google Scholar]
- 2. Sarris, D. , Papanikolaou, S. , Biotechnological production of ethanol: Biochemistry, processes and technologies. Eng. Life Sci. 2016, in press, DOI: 10.1002/elsc.201400199. [DOI] [Google Scholar]
- 3. Papanikolaou, S. , Aggelis, G. , Biotechnological valorization of biodiesel derived glycerol waste through production of single cell oil and citric acid by Yarrowia lipolytica . Lipid Technol. 2009, 21, 83–87. [Google Scholar]
- 4. Bozell, J. J. , Petersen, G. R. , Technology development for the production of biobased products from biorefinery carbohydrates‐the US Department of Energy's “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar]
- 5. Clomburg, J. M. , Gonzalez, R. , Anaerobic fermentation of glycerol: A platform for renewable fuels and chemicals. Trend. Biotechnol. 2013, 31, 20–28. [DOI] [PubMed] [Google Scholar]
- 6. Meesters, P. A. E. P. , Huijiberts, G. N. M. , Eggink, G. , High‐cell‐density cultivation of the lipid accumulating yeast Cryptococcus curvatus using glycerol as a carbon source. Appl. Microbiol. Biotechnol. 1996, 45, 575–579. [Google Scholar]
- 7. Papanikolaou, S. , Aggelis, G. , Lipid production by Yarrowia lipolytica growing on industrial glycerol in a single‐stage continuous culture. Bioresour. Technol. 2002, 82, 43–49. [DOI] [PubMed] [Google Scholar]
- 8. Chi, Z. , Pyle, D. J. , Wen, Z. , Frear, C. et al., A laboratory study of producing docosahexaenoic acid from biodiesel‐waste glycerol by microalgal fermentation. Proc. Biochem. 2007, 42, 1537–1545. [Google Scholar]
- 9. Pyle, D. J. , Garcia, R. A. , Wen, Z. , Producing docosahexaenoic acid (DHA)‐rich algae from biodiesel‐derived crude glycerol: effects of impurities on DHA production and algal biomass composition. J. Agric. Food Chem. 2008, 56, 3933–3939. [DOI] [PubMed] [Google Scholar]
- 10. André, A. , Chatzifragkou, A. , Diamantopoulou, P. , Sarris, D. et al., Biotechnological conversions of bio‐diesel derived crude glycerol by Yarrowia lipolytica strains. Eng. Life Sci. 2009, 9, 468–478. [Google Scholar]
- 11. André, A. , Diamantopoulou, P. , Philippoussis, A. , Sarris, D. et al., Biotechnological conversions of bio‐diesel derived waste glycerol into added‐value compounds by higher fungi: Production of biomass, single cell oil and oxalic acid. Ind. Crops Prod. 2010, 31, 407–416. [Google Scholar]
- 12. Liang, Y. N. , Cui, Y. , Trushenski, J. , Blackburn, J. W. , Converting crude glycerol derived from yellow grease to lipids through yeast fermentation. Bioresour. Technol. 2010, 101, 7581–7586. [DOI] [PubMed] [Google Scholar]
- 13. Makri, A. , Fakas, S. , Aggelis, G. , Metabolic activities of biotechnological interest in Yarrowia lipolytica grown on glycerol in repeated batch cultures. Bioresour. Technol. 2010, 101, 2351–2358. [DOI] [PubMed] [Google Scholar]
- 14. Ethier, S. , Woisard, K. , Vaughan, D. , Wen, Z. , Continuous culture of the microalgae Schizochytrium limacinum on biodiesel‐derived crude glycerol for producing docosahexaenoic acid. Bioresour. Technol. 2011, 102, 88–93. [DOI] [PubMed] [Google Scholar]
- 15. Bellou, S. , Moustogianni, A. , Makri, A. , Aggelis, G. , Lipids containing polyunsaturated fatty acids synthesized by zygomycetes grown on glycerol. Appl. Biochem. Biotechnol. 2012, 166, 146–158. [DOI] [PubMed] [Google Scholar]
- 16. Dedyukhina, E. G. , Chistyakova, T. I. , Kamzolova, S. V. , Vinter, M. V. et al., Arachidonic acid synthesis by glycerol‐grown Mortierella alpina . Eur. J. Lipid Sci. Technol. 2012, 114, 833–841. [Google Scholar]
- 17. Dedyukhina, E. G. , Chistyakova, T. I. , Mironov, A. A. , Kamzolova, S. V. et al., Arachidonic acid synthesis from biodiesel‐derived waste by Mortierella alpina . Eur. J. Lipid Sci. Technol. 2014, 116, 429–437. [Google Scholar]
- 18. Fontanille, P. , Kumar, V. , Christophe, G. , Nouaille, R. et al., Bioconversion of volatile fatty acids into lipids by the oleaginous yeast Yarrowia lipolytica . Bioresour. Technol. 2012, 114, 443–449. [DOI] [PubMed] [Google Scholar]
- 19. Xu, J. , Zhao, X. , Wang, W. , Du, W. et al., Microbial conversion of biodiesel byproduct glycerol to triacylglycerols by oleaginous yeast Rhodosporidium toruloides and the individual effect of some impurities on lipid production. Biochem. Eng. J. 2012, 65, 30–36. [Google Scholar]
- 20. Chang, G. , Luo, Z. , Gu, S. , Wu, Q. et al., Fatty acid shifts and metabolic activity changes of Schizochyrium sp. S31 cultured on glycerol. Bioresour. Technol. 2013, 142, 255–260. [DOI] [PubMed] [Google Scholar]
- 21. Cui, Y. , Blackburn, J. W. , Liang, Y. , Fermentation optimization for the production of lipid by Cryptococcus curvatus: Use of response surface methodology. Biomass Bioenerg. 2012, 47, 410–417. [Google Scholar]
- 22. Yang, X. , Jin, G. , Gong, Z. , Shen, H. et al., Recycling biodiesel‐derived glycerol by the oleaginous yeast Rhodosporidium toruloides Y4 through the two‐stage lipid production process. Biochem. Eng. J. 2014, 91, 86–91. [Google Scholar]
- 23. Bommareddy, R. R. , Sabra, W. , Maheshwari, G. , Zeng, A. P. , Metabolic network analysis and experimental study of lipid production in Rhodosporidium toruloides grown on single and mixed substrates. Microb. Cell Fact. 2015, 14, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tchakouteu, S. S. , Kalantzi, O. , Gardeli, C. , Koutinas, A. A. et al., Lipid production by yeasts growing on biodiesel‐derived crude glycerol: Strain selection and impact of substrate concentration on the fermentation efficiency. J. Appl. Microbiol. 2015, 118, 911–927. [DOI] [PubMed] [Google Scholar]
- 25. Rymowicz, W. , Rywińska, A. , Źarowska, B. , Juszczyk, P. , Citric acid production from raw glycerol by acetate mutants of Yarrowia lipolytica . Chem. Pap. 2006, 60, 391–394. [Google Scholar]
- 26. Rymowicz, W. , Fatykhova, A. R. , Kamzolova, S. V. , Rywinska, A. et al., Citric acid production from glycerol‐containing waste of biodiesel industry by Yarrowia lipolytica in batch, repeated batch, and cell recycle regimes. Appl. Microbiol. Biotechnol. 2010, 87, 971–979. [DOI] [PubMed] [Google Scholar]
- 27. Papanikolaou, S. , Fakas, S. , Fick, M. , Chevalot, I. et al., Biotechnological valorisation of raw glycerol discharged after bio‐diesel (fatty acid methyl‐esters) manufacturing process: Production of 1,3‐propanediol, citric acid and single cell oil. Biomass Bioenerg. 2008, 32, 60–71. [Google Scholar]
- 28. Papanikolaou, S. , Beopoulos, A. , Koletti, A. , Thevenieau, F. et al., Importance of the methyl‐citrate cycle on glycerol metabolism in the yeast Yarrowia lipolytica . J. Biotechnol. 2013, 168, 303–314. [PubMed] [Google Scholar]
- 29. Rywińska, A. , Rymowicz, W. , High‐yield production of citric acid by Yarrowia lipolytica on glycerol in repeated‐batch bioreactors. J. Ind. Microbiol.Biotechnol. 2010, 37, 431–435. [DOI] [PubMed] [Google Scholar]
- 30. Kamzolova, S. V. , Fatykhova, A. R. , Dedyukhina, E. G. , Anastassiadis, S. G. et al., Citric acid production by yeast grown on glycerol‐containing waste from biodiesel industry. Food Technol. Biotechnol. 2011, 49, 65–74. [Google Scholar]
- 31. Tomaszewska, L. , Rywińska, A. , Gładkowski, W. , Production of erythritol and mannitol by Yarrowia lipolytica yeast in media containing glycerol. J. Ind. Microbiol. Biotechnol. 2012, 39, 1333–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rywińska, A. , Tomaszewska, L. , Rymowicz, W. , Erythritol biosynthesis by Yarrowia lipolytica yeast under various culture conditions. Afr. J. Microbiol. Res. 2013, 7, 3511–3516. [Google Scholar]
- 33. Bellou, S. , Triantaphyllidou, I. E. , Aggeli, D. , Elazzazy, A. M. et al., Microbial oils as food additives: recent approaches for improving microbial oil production and its polyunsaturated fatty acid content. Curr. Opin. Biotechnol. 2016, 37, 24–35. [DOI] [PubMed] [Google Scholar]
- 34. Papanikolaou, S. , Sarantou, S. , Komaitis, M. , Aggelis, G. , Repression of reserve lipid turnover in Cunninghamella echinulata and Mortierella isabellina cultivated in multiple‐limited media. J. Appl. Microbiol. 2004, 97, 867–875. [DOI] [PubMed] [Google Scholar]
- 35. Tchakouteu, S. S. , Chatzifragkou, A. , Kalantzi, O. , Koutinas, A. A. et al., Oleaginous yeast Cryptococcus curvatus exhibits interplay between biosynthesis of intracellular sugars and lipids. Eur. J. Lipid Sci. Technol. 2015, 117, 657–672. [Google Scholar]
- 36. Folch, J , Lees, M ., Slane‐Stanley, J. , A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [PubMed] [Google Scholar]
- 37. Fakas, S. , Papanikolaou, S. , Galiotou‐Panayotou, M. , Komaitis, M. et al., Lipids of Cunninghamella echinulata with emphasis to γ‐linolenic acid distribution among lipid classes. Appl. Microbiol. Biotechnol. 2006, 73, 676–683. [DOI] [PubMed] [Google Scholar]
- 38. Christie, W. W. , Han, X. , Lipid Analysis ‐ Isolation, Separation, Identification and Lipidomic Analysis, 4th ed., Oily Press, Bridgwater, U.K. 2010, p. 446. [Google Scholar]
- 39. Piškur, J. , Rozpędowska, E. , Polakova, S. , Merico, A. et al., How did Saccharomyces evolve to become a good brewer? Trends Genet. 2006, 22, 183–186. [DOI] [PubMed] [Google Scholar]
- 40. Hagman, A. , Torbjörn, S. , Compagno, C. , Piskur, J. , Yeast ‘‘Make‐Accumulate‐Consume’’ life strategy evolved as a multi‐step process that predates the whole genome duplication. PLoS One 2013, 8, e68734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sarris, D. , Kotseridis, Y. , Linga, M. , Galiotou‐Panayotou, M. et al., Enhanced ethanol production, volatile compound biosynthesis and fungicide removal during growth of a newly isolated Saccharomyces cerevisiae strain on enriched pasteurized grape musts. Eng. Life Sci. 2009, 9, 29–37. [Google Scholar]
- 42. Sarris, D. , Giannakis, M. , Philippoussis, A. , Komaitis, M. et al., Conversions of olive mill wastewater‐based media by Saccharomyces cerevisiae through sterile and non‐sterile bioprocesses. J. Chem. Technol. Biotechnol. 2013, 88, 958–969. [Google Scholar]
- 43. Sarris, D. , Matsakas, L. , Aggelis, G. , Koutinas, A. A. et al., Aerated vs non‐aerated conversions of molasses and olive mill wastewaters blends into bioethanol by Saccharomyces cerevisiae under non‐aseptic conditions. Ind. Crops Prod. 2014, 56, 83–93. [Google Scholar]
- 44. Papanikolaou, S. , Chatzifragkou, A. , Fakas, S. , Galiotou‐Panayotou, M. et al., Biosynthesis of lipids and organic acids by Yarrowia lipolytica strains cultivated on glucose. Eur. J. Lipid Sci. Technol. 2009, 111, 1221–1232. [Google Scholar]
- 45. Sarris, D. , Galiotou‐Panayotou, M. , Koutinas, A. A. , Komaitis, M. et al., Citric acid, biomass and cellular lipid production by Yarrowia lipolytica strains cultivated on olive mill wastewater‐based media. J. Chem. Technol. Biotechnol. 2011, 86, 1439–1448. [Google Scholar]
- 46. Papanikolaou, S. , Aggelis, G. , Lipids of oleaginous yeasts. Part I: Biochemistry of single cell oil production. Eur. J. Lipid Sci. Technol. 2011, 113, 1031–1051. [Google Scholar]
- 47. Tsigie, Y. A. , Wang, C.‐Y. , Truong, C.‐T. , Ju, Y.‐H. , Lipid production from Yarrowia lipolytica Po1g grown in sugarcane bagasse hydrolysate. Bioresour. Technol. 2011, 102, 9216–9222. [DOI] [PubMed] [Google Scholar]
- 48. Tsigie, Y. A. , Wang, C. ‐Y. , Kasim, N. S. , Diem, Q. D. et al., Oil production from Yarrowia lipolytica Po1g using rice bran hydrolysate. J. Biomed. Biotechnol. 2012, 2012, 378384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Papanikolaou, S. , Muniglia, L. , Chevalot, I. , Aggelis, G. et al., Yarrowia lipolytica as a potential producer of citric acid from raw glycerol. J. Appl. Microbiol. 2002, 92, 737–744. [DOI] [PubMed] [Google Scholar]
- 50. Chatzifragkou, A. , Makri, A. , Belka, A. , Bellou, S. et al., Biotechnological conversions of biodiesel derived waste glycerol by yeast and fungal species. Energy 2011, 36, 1097–1108. [Google Scholar]
- 51. Leiva‐Candia, D. E. , Tsakona, S. , Kopsahelis, N. , García, I. L. et al., Biorefining of by‐product streams from sunflower‐based biodiesel production plants for integrated synthesis of microbial oil and value‐added co‐products. Bioresour. Technol. 2015, 190, 57–65. [DOI] [PubMed] [Google Scholar]
- 52. Liu, X. , Jensen, P. R. , Workman, M. , Bioconversion of crude glycerol feedstocks into ethanol by Pachysolen tannophilus . Bioresour. Technol. 2012, 104, 579–586. [DOI] [PubMed] [Google Scholar]
- 53. Liu, X. , Mortensen, U. H. , Workman, M. , Expression and functional studies of genes involved in transport and metabolism of glycerol in Pachysolen tannophilus . Microb. Cell Fact. 2013, 12, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rivaldi, J. D. , Sarrouh, B. F. , Branco, R. F. , de Mancilha, I. M. et al., Biotechnological utilization of biodiesel‐derived glycerol for the production of ribonucleotides and microbial biomass. Appl. Biochem. Biotechnol. 2012, 167, 2054–2067. [DOI] [PubMed] [Google Scholar]
- 55. Anastassiadis, S. , Aivasidis, A. , Wandrey, C. , Citric acid production by Candida strains under intracellular nitrogen limitation. Appl. Microbiol. Biotechnol. 2002, 60, 81–87. [DOI] [PubMed] [Google Scholar]
- 56. Anastassiadis, S. , Rehm, H. J. , Continuous citric acid secretion by a high specific pH dependent active transport system in yeast Candida oleophila ATCC 20177. Electronic J. Biotechnol. 2005, 8, 146–161. [Google Scholar]
- 57. Fakas, S. , Papanikolaou, S. , Galiotou‐Panayotou, M. , Komaitis, M. et al., Biochemistry and biotechnology of single cell oil, in: Pandey A., Larroche C., Soccol C. R., Dussard C. G. (Eds.), New Horizons in Biotechnology, AsiaTech Publishers Inc, New Delhi, India: 2009, pp. 53–75. [Google Scholar]
- 58. Musiał, I. , Rymowicz, W. , Witkowska, D. , Effect of Span 20 concentration on oxalic acid production from post‐refining fatty acids by Aspergillus niger XP. Chem. Pap. 2006, 60, 388–390. [Google Scholar]
- 59. Rymowicz, W. , Lenart, D. , Oxalic acid production from lipids by a mutant of Aspergillus niger at different pH. Biotechnol. Lett. 2003, 25, 955–958. [DOI] [PubMed] [Google Scholar]
- 60. Musiał, I. , Rymowicz, W. , Biodiesel by‐products used as substrates for oxalic acid production by Aspergillus niger, in: Aggelis G. (Ed.), Microbial Conversions of Raw Glycerol, Nova Science Publishers Inc, New York: 2009, pp. 31–40. [Google Scholar]
- 61. Strasser, H. , Burgstaller, W. , Schinner, F. , High yield acid production of oxalic acid for metal leaching processes by Aspergillus niger . FEMS Microbiol. Lett. 1994, 119, 365–370. [DOI] [PubMed] [Google Scholar]
- 62. Ruijter, G. J. G. , van de Vondervoort, P. J. I. , Visser, J. , Oxalic acid production by Aspergillus niger: An oxalate‐non‐producing mutant produces citric acid at pH 5 and in the presence of manganese. Microbiol. (UK) 1999, 145, 2569–2576. [DOI] [PubMed] [Google Scholar]
- 63. Becker, J. , Lange, A. , Fabarius, J. , Wittmann, C. , Top value platform chemicals: Bio‐based production of organic acids. Curr. Opin. Biotechnol. 2015, 36, 168–175. [DOI] [PubMed] [Google Scholar]
- 64. Yu, M. C. , Wang, B. C. , Wang, C. Y. , Duan, K. J. et al., Enhanced production of L(+)‐lactic acid by floc‐form culture of Rhizopus oryzae . J. Chin. Inst. Chem. Eng. 2007, 38, 223–228. [Google Scholar]
- 65. Bai, D. M. , Jia, M. Z. , Zhao, X. M. , Ban, R. et al., L(+)‐Lactic acid production by pellet‐form Rhizopus oryzae R1021 in a stirred tank fermentor. Chem. Eng. Sci. 2003, 58, 785–791. [Google Scholar]
- 66. Soccol, C. R. , Marin, B. , Lebeault, J. M. , Raimbault, M. , Potential of solid state fermentation for production of L(+)‐lactic acid by Rhizopus oryzae . Appl. Microbiol. Biotechnol. 1994, 41, 286–290. [Google Scholar]
- 67. Li, Y. , Chen, J. , Lun, S. Y. , Biotechnological production of pyruvic acid. Appl. Microbiol. Biotechnol. 2001, 57, 451–459. [DOI] [PubMed] [Google Scholar]
- 68. Fakas, S. , Papanikolaou, S. , Batsos, A. , Galiotou Panayotou, M. et al., Evaluating renewable carbon sources as substrates for single cell oil production by Cunninghamella echinulata and Mortierella isabellina . Biomass Bioenerg. 2009, 33, 573–580. [Google Scholar]
- 69. Chatzifragkou, A. , Fakas, S. , Galiotou‐Panayotou, M. , Komaitis, M. et al., Commercial sugars as substrates for lipid accumulation in Cunninghamella echinulata and Mortierella isabellina . Eur. J. Lipid Sci. Technol. 2010, 112, 1048–1057. [Google Scholar]
- 70. Papanikolaou, S. , Aggelis, G. , Lipids of oleaginous yeasts. Part II: Technology and potential applications. Eur. J. Lipid Sci. Technol. 2011, 113, 1052–1073. [Google Scholar]
- 71. Papanikolaou, S. , Diamantopoulou, P. , Chatzifragkou, A. , Philippoussis, A. et al., Suitability of low‐cost sugars as substrates for lipid production by the fungus Thamnidium elegans . Energy Fuel. 2010, 24, 4078–4086. [Google Scholar]
- 72. Ratledge, C. , Wynn, J. , The biochemistry and molecular biology of lipid accumulation in oleaginous microorganisms. Adv. Appl. Microbiol. 2002, 51, 1–51. [DOI] [PubMed] [Google Scholar]
- 73. Ratledge, C. , Biochemistry, stoichiometry, substrates and economics, in: Moreton R. S. (Ed.), Single Cell Oil, Longman Scientific &Technical, Harlow, UK: 1988, pp. 33–70. [Google Scholar]
- 74. Ratledge, C. , Cohen, Z. , Microbial and algal lipids: Do they have a future for biodiesel or as commodity oils? Lipid Technol. 2008, 20, 155–160. [Google Scholar]
- 75. Qiao, K. , Imam Abidi, S. H. , Liu, H. , Zhang, H. et al., Engineering lipid overproduction in the oleaginous yeast Yarrowia lipolytica . Metab. Eng. 2015, 29, 56–65. [DOI] [PubMed] [Google Scholar]
- 76. Fakas, S. , Bellou, S. , Makri, A. , Aggelis, G. , Single cell oil and gamma‐linolenic acid production by Thamnidium elegans grown on raw glycerol, in: Aggelis G. (Ed.), Microbial Conversions of Raw Glycerol, Nova Science Publishers, Inc, New York: 2009, pp. 85–99. [Google Scholar]
- 77. Papanikolaou, S. , Komaitis, M. , Aggelis, G. , Single cell oil (SCO) production by Mortierella isabellina grown on high‐sugar content media. Bioresour. Technol. 2004, 95, 287–291. [DOI] [PubMed] [Google Scholar]
- 78. Rywińska, A. , Rymowicz, W. , Źarowska, B. , Wojtatowicz, M. , Biosynthesis of citric acid from glycerol by acetate mutants of Yarrowia lipolytica in fed‐batch fermentation. Food Technol. Biotechnol. 2009, 47, 1–6. [Google Scholar]
- 79. Rywińska, A. , Rymowicz, W. , Citric acid production from raw glycerol by Yarrowia lipolytica Wratislavia 1.31, in: Aggelis G. (Ed.), Microbial Conversions of Raw Glycerol, Nova Science Publishers Inc., New York: 2009, pp. 19–30. [Google Scholar]
- 80. Rywińska, A , Rymowicz, W ., Marcinkiewicz, M. , Valorization of raw glycerol for citric acid production by Yarrowia lipolytica yeast. Electron. J. Biotechnol. 2010, 13, 4. [Google Scholar]
- 81. Workman, M. , Holt, P. , Thykaer, J. , Comparing cellular performance of Yarrowia lipolytica during growth on glucose and glycerol in submerged cultivations. AMB Express 2013, 3, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Klasson, K. T. , Clausen, E. C. , Gaddy, J. L. , Continuous fermentation for the production of citric acid from glucose. Appl. Biochem. Biotechnol. 1989, 20–21, 491–509. [Google Scholar]
- 83. Moresi, C. , Effect of glucose concentration on citric acid production by Yarrowia lipolytica – kinetics of the trophophase, citrate lag phase and idiophase. J. Chem. Technol. Biotechnol. 1994, 60, 387–395. [Google Scholar]
- 84. Rane, K. D. , Sims, K. A , Citric acid production by Candida lipolytica Y 1095 in cell recycle and fed‐batch fermentors, Biotechnol. Bioeng. 1995, 46, 325–332. [DOI] [PubMed] [Google Scholar]
- 85. Kamzolova, S. V. , Shishkanova, N. , Morgunov, I. , Finogenova, T. , Oxygen requirements for growth and citric acid production of Yarrowia lipolytica , F.E.M.S. Yeast Res. 2003, 3, 217–222. [DOI] [PubMed] [Google Scholar]
- 86. Kamzolova, S. V. , Morgunov, I. , Aurich, A. , Perevoznikova, S. et al., Lipase secretion and citric acid production in Yarrowia lipolytica yeast grown on animal and vegetable fat. Food Technol. Biotechnol. 2005, 43, 113–122. [Google Scholar]
- 87. Morgunov, I. G. , Kamzolova, S. V. , Lunina, J. N. , The citric acid production from raw glycerol by Yarrowia lipolytica yeast and its regulation. Appl. Microbiol. Biotechnol. 2013, 97, 7387–7397. [DOI] [PubMed] [Google Scholar]
- 88. Alakhras, R. , Bellou, S. , Fotaki, G. , Stephanou, G. et al., Fatty acid lithium salts from Cunninghamella echinulata have cytotoxic and genotoxic effects on HL‐60 human leukemia cells. Eng. Life Sci. 2015, 15, 243–253. [Google Scholar]
- 89. Chen, H. C. , Liu, T. M. , Inoculum effects on the production of γ‐linolenic acid by the shake culture of Cunninghamella echinulata CCRC 31840. Enzyme Microb. Technol. 1997, 21, 137‐142. [Google Scholar]
- 90. Fakas, S. , Galiotou‐Panayotou, M. , Papanikolaou, S. , Komaitis, M. et al., Compositional shifts in lipid fractions during turnover in Cunninhamella echinulata . Enzyme Microb. Technol. 2007, 40, 1321–1327. [Google Scholar]
- 91. Zikou, E. , Chatzifragkou, A. , Koutinas, A. A. et al., Evaluating glucose and xylose as cosubstrates for lipid accumulation and γ‐linolenic acid biosynthesis of Thamnidium elegans . J. Appl. Microbiol. 2013, 114, 1020–1032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Microscopic observation of Mortierella isabellina ATHUM 2935 during growth on glucose, in the fermentation point in which the highest lipid production was observed. Magnification ×100
Figure S2. Kinetics of glycerol (Glol, g L–1), mannitol (Ml, g L–1) and erythritol (Ery, g L–1) of Yarrowia lipolytica LFMB 20 during growth on industrial glycerol, at initial glycerol concentration (Glol0) of c. 100 g L–1, under nitrogen‐limited conditions. Each point is the mean value of two independent measurements (SE<10%).


