Abstract
Recombinant protein production in Escherichia coli usually leads to accumulation of the product inside the cells. To capture the product, cells are harvested, resuspended, and lysed. However, in cases where the product is transported to the periplasm, selective disruption of the outer membrane leads to much purer crude extracts compared to complete cell lysis, as only 4–8% of the native E. coli host cell proteins are located in the periplasmic space. A variety of different strategies to enable selective release of the product from the periplasm is available. However, in most of these studies cells are harvested before they are resuspended in permeabilization agent and no differentiation between leakiness and lysis is made. Here, we tested and compared different strategies to trigger leakiness. In contrast to other studies, we performed these experiments during cultivation and quantified both leakiness and lysis. In summary, we recommend incubation with 350 mM TRIS at constant pH for several hours followed by a mild heat treatment up to 38°C to trigger leakiness with only minimal lysis. This study represents a comparative summary of different strategies to trigger E. coli leakiness and describes a solid basis for further experiments in this field.
Keywords: Escherichia coli, Lysis monitoring, Outer membrane integrity, Recombinant protein production, Selective periplasmic release
Abbreviations
- AP
alkaline phosphatase
- β‐gal
β‐galactosidase
- BaCl
benzalkonium chloride
- DC
during cultivation
- FCM
flow cytometry
- HRP
horseradish peroxidase
- OM
outer membrane
- PH
post harvesting
- qs,glu
specific glucose uptake rate
1. Introduction
Escherichia coli is one of the most widely used hosts for recombinant protein production as it can be cultivated on cheap minimal media to high cell densities, straight forward cloning procedures are available and high productivities can be achieved 1, 2, 3, 4, 5. As E. coli usually does not secrete proteins to the culture medium, recombinant products are usually located inside the cell 6. For product recovery, cells have to be harvested, resuspended, and lysed. However, cell lysis does not only release the product but also unwanted impurities such as DNA, host cell proteins, and proteases 7.
Due to the intrinsic feature of having disulfide bonds, some proteins need to be exported to the oxidative environment of the E. coli periplasm. In such a case, selective disruption of the outer membrane (OM) leads to much purer crude extracts compared to complete cell lysis, as only 4–8% of the native E. coli host cell proteins are located in the periplasmic space 8. Different approaches to destabilize the OM of E. coli, either during cultivation (DC) or post harvesting (PH), are summarized in Table 1.
Table 1.
Summary of approaches to release periplasmic proteins from E. coli
| DC/PH | Permeabilization agent/ method | Comments | Reference |
|---|---|---|---|
| PH | Guanidine HCl and/or Triton | Resuspension of cells in TRIS after harvesting, different combinations of Triton X‐100 and guanidine HCl | 9, 10 |
| PH | Cernitrate | Resuspension of cells in saline after harvesting | 11 |
| PH | Benzalkonium chloride | Salmonella typhimurium | 12 |
| PH | Glycol ethers | E. coli and P. fluorescens, two phase extraction with different glycol ethers | 13 |
| PH | Chloroform | Salmonella TA831, resuspension of cells in TRIS after harvesting | 14 |
| PH | TRIS | E. coli D280 and F515 | 15 |
| DC | 1% Glycine | α‐Amylase release from E. coli HB101/pHI30 | 16 |
| PH | Polyethylenimine | E. coli, P. aeruginosa, and S. typhimurium | 17 |
| DC | Polyethylenimine | Addition during cultivation aiming at improving protein/DNA ratio | 18 |
| DC/PH | Urea/10 mM DTT | Two‐phase extraction with polymer and salt at pH of 10 | 19 |
| PH | Mild heat shock + TRIS | Resuspension of E. coli cells in TRIS buffer in combination with heat shock | 20, 21, 22 |
| PH | Osmotic shock | Harvesting, washing, equilibrating, and resuspending | 7 |
DC, during cultivation; PH, post harvesting.
As shown in Table 1, several strategies to selectively release proteins from the periplasm were tested. The treatment with guanidine HCl and Triton in varying concentrations described a successful strategy leading to a release of up to 90% of periplasmic proteins 9, 10. However, all the studies shown in Table 1 lack information about the integrity of the cells and do not differentiate between complete cell lysis and selective periplasmic release. Furthermore, in most of these studies cells were harvested from the broth, before they were resuspended in the respective lysis agent. As shown in Table 1, osmotic shock is a commonly used method for extracting periplasmic protein from E. coli with high yields 7. However, sample preparation is cumbersome—harvesting, washing, equilibrating in hypertonic solution, resuspending in distilled water—and thus not possible in situ in a bioreactor. Only a few studies on releasing the periplasmic proteins directly to the cultivation broth are available to date 16, 18, 19. Such a strategy is advantageous, since product can be obtained DC and cell harvesting can be omitted. Furthermore, this approach might be promising in continuous bioprocessing as cells only get leaky but are still viable. Product is continuously harvested and cultivation can be prolonged, as lysis is kept at a minimum. However, to our knowledge, no study that analyzes and compares methods to selectively release periplasmic protein DC without significant cell lysis is available to date.
Here, we performed a systematic and comparative study of how to permeabilize the OM during E. coli cultivation processes both in shake flasks and in the controlled environment of a bioreactor. We monitored leakiness and analyzed lysis to be able to distinguish between these two states. To monitor cell integrity, alkaline phosphatase (AP), a marker protein for periplasmic leakiness 23, 24, was measured and cell lysis was monitored using the ß‐galactosidase assay 25 and flow cytometry (FCM) 26. Finally, we combined the addition of a permeabilizing agent with heat treatment, which is known to lead to leakiness of the OM in Gram‐negative bacteria 20, 27, 28, 29, resulting in a release of nearly 30% of the periplasmic content of the cells while keeping cell lysis at a minimum.
2. Materials and methods
2.1. Chemicals for permeabilizing experiments
We used the following chemicals: TRIS and Pufferan 99.3% (Carl Roth, Karlsruhe, Germany), urea 99.5% (Carl Roth), Triton X‐100 (Sigma Aldrich, St. Louis, USA), guanidine hydrochloride 98% (Sigma Aldrich), and benzalkonium chloride (BaCl) 95% (Sigma Aldrich).
2.2. Strain
Escherichia coli BL21(DE3) (Lucigen, Middleton, USA) was used for the periplasmic production of the recombinant model enzyme HRP C1A. The gene encoding HRP was condon optimized from GenScript USA Inc. (NJ, USA) and integrated in a pET39b+ vector (Novagen, San Diego, USA). Translocation of the product to the periplasmic space was accomplished using the DsbA pathway.
2.3. Media
Defined DeLisa minimal medium 30 supplemented with 0.02 g/L kanamycin and different amounts of glucose and lactose were used for all cultivations.
2.4. Shake flask cultivation
Screening experiments to test the impact of different permeabilization agents and their concentration ranges on cell integrity were performed in shake flasks. Precultures were grown in 500 mL of DeLisa medium supplemented with 8 g/L glucose in 2.5‐L ultra‐yield flasks at 37°C and 160 rpm for 20 h. Then, 5 mL of preculture were aseptically transferred into 500‐mL Erlenmeyer flasks containing 45 mL of DeLisa medium supplemented with 8 g/L glucose and 5 g/L lactose to induce recombinant production of HRP at 160 rpm and 30°C. After 4 h, permeabilization agents (Fig. 1 and Supporting Information Table S2) were added as solids and the cultivation was continued overnight.
Figure 1.
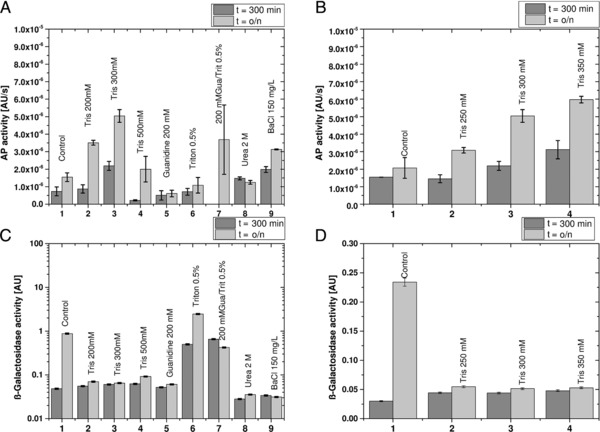
Shake flask experiments for screening of permeabilization agents for their ability to selectively release periplasmic proteins: (A) AP assay indicating periplasmic release for shake flask experiment 1; (B) β‐galactosidase assay indication cell lysis for shake flask experiment 1; 1, no addition of agent; 2, TRIS 200 mM; 3, TRIS 300 mM; 4, TRIS 500 mM; 5, guanidine HCl 2 M; 6, Triton 0.5%; 7, 200 mM guanidine HCl + 0.5% Triton; 8, urea 2 M; 9, benzalkonium chloride 150 mg/L; (C) AP assay indicating periplasmic release for shake flask experiment 2; and (D) β‐galactosidase assay indication cell lysis for shake flask experiment 1; 1, no addition of agent; 2, 250 mM TRIS; 3, 300 mM TRIS; 4, 350 mM TRIS.
2.5. Bioreactor cultivations
The impact of heat treatment in combination with permeabilization agents on cell integrity was investigated in bioreactor cultivations. The preculture for bioreactor cultivations was identical to the shake flask experiments.
A stainless steel Sartorius Biostat Cplus bioreactor (Sartorius, Göttingen, Germany) with a working volume of 10 L, containing 5000 mL DeLisa medium, supplemented with 20 g/L glucose, was inoculated with 500 mL of preculture. The bioreactor was aerated with a mixture of pressurized air and pure oxygen at 1.5 vvm and agitated constantly at 1000 rpm. Dissolved oxygen was monitored with a fluorescence dissolved oxygen electrode Visiferm DO425 (Hamilton, Reno, NV, USA) and kept above 40% throughout all cultivations by varying the ratio of pressurized air to pure oxygen. pH was monitored with an Easyferm electrode (Hamilton) and maintained constant at pH 7.2 by the addition of NH4OH (12.5%). Base consumption was determined gravimetrically. CO2 and O2 concentrations in the off‐gas were monitored by a DASGIP gas analyzer (Eppendorf, Hamburg, Germany). All process parameters were adjusted and logged by the process information management system Lucullus (Biospectra, Schlieren, Switzerland).
The batch phase was conducted at 35°C and yielded a biomass concentration of 8–9 g dry cell weight per liter. After depletion of glucose, visible by a drop in the CO2 off‐gas signal, a fed‐batch cultivation to generate biomass was conducted. The feed rate during this phase was adjusted to maintain a constant specific glucose uptake rate (q s,glu) of 0.2 g/g/h.
When the dry cell weight reached 25 g/L, the culture was induced by one point addition of IPTG (0.05 mM) or lactose, which was supplied continuously by a mixed feed containing glucose and lactose. Detailed information about the specific uptake rates of glucose and lactose in the different experiments are given in Supporting Information Table S1. After 10 h of induction, periplasmic release was triggered by heat treatment and/or addition of TRIS. Detailed information about the temperature ramps and time points of TRIS addition can be found in the result section in Fig. 2, 3, 4.
Figure 2.
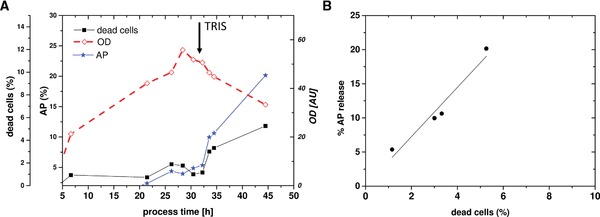
Cultivation 1 for permeabilization of the OM by the addition of 350 mM TRIS: (A) optical density at 600 nm (OD), extracellular AP activity and ratio of dead cells monitored by FCM; (B) scatter plot showing dead cells (x‐axis) versus periplasmic leakiness (AP release, y‐axis).
Figure 3.
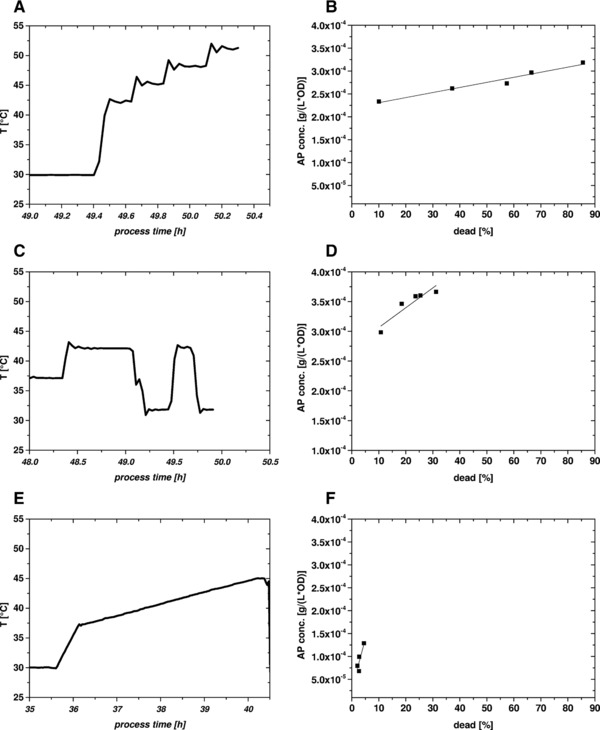
Cultivations 2–4 for permeabilization of the OM by heat treatment; left side: temperature profiles: (A) cultivation 2, stepwise increase (42–51°C); (C) cultivation 3, oscillating temperatures (32–41°C); (E) cultivation 4, ramp (30–42°C); right side: scatter plot showing dead cells (x‐axis) versus periplasmic leakiness (AP release, y‐axis); (B) cultivation 2; (D) cultivation 3; and (E) cultivation 4. As in cultivations 2 and 3 lysis was very pronounced, the samples of these cultivations were not homogenized. Therefore, AP values are not given in (%) but as normalized values in [g/(L⋅OD)].
Figure 4.
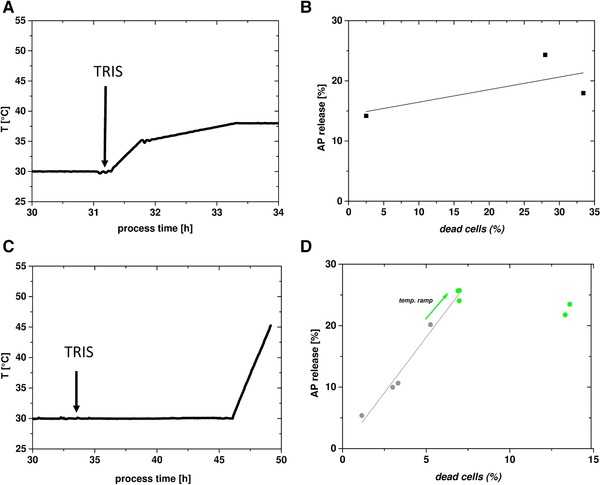
Cultivations 5 and 6 for permeabilization of the OM by combining TRIS addition and heat treatment; left side: temperature profiles and TRIS addition: (A) cultivation 5, (C) cultivation 6; right side: scatter plot showing dead cells (x‐axis) versus periplasmic leakiness (AP release, y‐axis), (B) cultivation 5, and (D) cultivation 6.
2.6. Analytics
2.6.1. AP assay
AP, a native periplasmic protein of E. coli, serves as a tracer protein for the periplasmic space and was used for the analysis of leakiness 23, 24. A photometric assay using a 96‐well plate was carried out in an Infinite 200 Pro plate reader (Tecan, Zürich, Switzerland). One hundred microliters of centrifuged culture broth (5000 g, 10 min) were mixed with 100 μL of para‐nitrophenylphosphate disodium salt hexahydrate solution (5 g/L) in TRIS buffer at pH 8.5. A kinetic cycle recorded the absorbance at 405 nm for 80 min. Calibration was performed using commercially available AP from bovine intestinal mucosa (Sigma Aldrich) exhibiting 3730 U/mg.
To quantify 100% of periplasmic leakiness, cells were homogenized in an EmusiflexC3 Homogenizer (Avestin, Ottowa, ON, USA) at 1500 bar for five passages. After centrifugation, AP was measured in the supernatant.
2.6.2. β‐Galactosidase assay
In shake flask experiments, ß‐galactosidase, a native cytoplasmic E. coli enzyme, was used for monitoring of cell lysis. Fifty microliters of centrifuged culture broth (5000 g, 10 min) were added to 50 μL 2× Assay Buffer (200 mM Na3PO4, 2 mM MgCl2, 100 mM ß‐mercaptoethanol, and 1.33 mg/mL ONPG). After 30 min of incubation at 37°C in the plate reader Infinite 200 Pro (Tecan, Zürich, Switzerland), the reaction was stopped by the addition of 150 μL 1 M Na2CO3 and absorption was measured at 420 nm.
2.6.3. Flow cytometry
FCM was used for lysis monitoring during the cultivations and the permeabilization experiments according to Langemann et al. 26. We used a CyFlow® Cube 8 flow cytometer (Partec, Münster, Germany). Data were collected using the software CyView Cube 15 and analyzed with the software FCS Express V4 (DeNovo Software, Los Angeles, CA, USA).
3. Results and discussion
The goal of this study was to selectively trigger leakiness of the periplasm by process technological means to release recombinant products during E. coli cultivation without cell lysis. We (i) tested the applicability of cell integrity monitoring assays for the used medium and permeabilizing agents, (ii) tested different permeabilization agents regarding their ability to selectively trigger leakiness in shake flask cultivations, (iii) applied the most promising results from shake flask experiments in bioreactor cultivations, (iv) tested the impact of heat treatment on selective periplasmic release, and (v) combined the addition of a permeabilization agent and heat treatment to trigger the release of periplasmic proteins while keeping cell lysis at a minimum.
3.1. Applicability of cell integrity monitoring assays
For cell integrity monitoring, different assays are described in the literature. Extracellular DNA content (e.g. by Nanodrop and Picogreen), the presence of the cytosolic enzyme ß‐galactosidase, and FCM measurements can be used to monitor cell lysis. The presence of extracellular AP, a native E.coli marker protein for the periplasmic space, can be used to monitor leakiness. An increase in the total extracellular protein content (e.g. by bicinchoninic acid and Bradford assay) can result from both lysis and leakiness.
All assays mentioned above but FCM are photometric assays and thus different matrices can severely affect the results of the assays. Therefore, sensitivity of the assays toward the permeabilizing agents was tested. As FCM relies on another measurement principle where no interactions with permeabilizing agents are expected, this assay was not investigated in this respect. Table 2 shows an overview of the performed experiments to evaluate the sensitivity of the assays toward the permeabilization agents.
Table 2.
Overview of interferences of cell integrity assays with permeabilization agents
| Assay | Control DeLisa | Tris 200 mM | Tris 300 mM | Tris 400 mM | Tris 500 mM | Guanidine 200 mM | Triton 0.5 wt% | Urea 2 M | BaCl 60 mg/L | BaCl 300 mg/L |
|---|---|---|---|---|---|---|---|---|---|---|
| Nanodrop (DNA) | − | − | − | − | − | − | − | − | − | − |
| PicoGreen (DNA) | + | + | + | + | + | n/a | n/a | n/a | n/a | n/a |
| CP marker (β‐Gal) | + | +/red | +/red | +/red | +/red | + | +/inc | + | − | + |
| PP marker (AP) | +/red | +/red | +/red | +/red | +/red | +/red | +/red | +/red | +/red | +/red |
| PP product (HRP) | + | +/red | +/red | +/red | +/red | + | + | +/inc | +/inc | +/inc |
| Total protein (bicinchoninic acid) | − | − | − | − | − | − | − | − | − | − |
| Total protein (Bradford) | + | +/inc | +/inc | +/inc | +/inc | + | − | + | − | +/inc |
+, no interference; −, strong interference; red, slightly reduced signal; inc, slightly increased signal; n/a, not available; BaCl, benzalkonium chloride.
Nanodrop showed high interferences with DeLisa medium and was thus not applicable. PicoGreen showed good results for DNA quantification but, as reagents are expensive, this assay was not used in this study. Furthermore, DNA is constantly being digested by released DNases. Without knowledge of digestion rates, DNA quantification results are highly uncertain and cannot be used for proper lysis monitoring.
Beta‐galactosidase showed slightly reduced signals in the presence of TRIS and slightly increased signals in the presence of Triton. As this assay is cheap, fast, and robust, it was used to determine cell lysis in shake flask experiments. However, FCM was used for lysis monitoring in bioreactor cultivations.
Minor effects of medium and permeabilization agents were observed for the AP and HRP assays. As HRP needs incorporation of the cofactor heme to be active, we chose the AP assay to monitor integrity of the periplasm.
For total protein determination, the bicinchoninic acid assay was not suitable in combination with DeLisa medium due to high background noise. Bradford measurements, using Coomassie Brilliant Blue G‐250, showed only slight increase when TRIS and BaCl were present. However, as the determination of total extracellular protein content does not allow differentiation between leakiness and lysis, we decided to use AP measurements to monitor leakiness and β‐galactosidase measurements and FCM to monitor lysis in this study.
3.2. Shake flask screening of permeabilization agents
All shake flasks experiments comprised a phase for biomass generation, an induction phase for recombinant protein production, and a phase for releasing periplasmic proteins by the addition of different permeabilization agents. The agents were chosen according to removability, toxicity and price. The results for periplasmic release, measured by the AP assay, and complete lysis, measured by the β‐galactosidase assay, are shown in Fig. 1 and Supporting Information Table S2.
In the first shake flask experiment, the agents TRIS, guanidine HCl, Triton, urea, BaCl, and combinations thereof were tested (Fig. 1A and B). TRIS (300 mM), a combination of guanidine HCl (200 mM) + Triton (0.5%) and BaCl (150 mg/L) were found to be the most promising candidates regarding periplasmic release (Fig. 1A). Observed lysis in the shake flask where no agent was added (Fig. 1B) was probably a result of substrate depletion as these cultures showed much higher uptake of substrate and faster growth compared to cultures containing permeabilization agent. In fact, in cultures containing permeabilization agent hardly any growth was observed and the cells appeared to be “viable but nonculturable”.
As guanidine HCl (200 mM) + Triton (0.5%) led to increased lysis (Fig. 1B) and BaCl is a biocide, we decided to perform periplasmic release with TRIS as this permeabilization agent (i) showed highest periplasmic release, (ii) triggered a low degree of lysis, and (iii) is a cheap chemical that is often used as it is easily removable and nontoxic.
In a second experiment, the impact of different TRIS concentrations on leakiness and lysis was investigated (Fig. 1C and D). TRIS at a final concentration of 350 mM showed the best results regarding periplasmic release with only minor lysis of less than 10% of all cells. Similar results were also found by Irvin and Hancock 15, 31.
3.3. Permeabilization of cells in bioreactor cultivation using 350 mM TRIS
As the addition of 350 mM TRIS was most suitable for permeabilizing the cells with only low levels of lysis in shake flasks, this approach was also tested in the controlled environment of a bioreactor. The cultivation comprised (i) a batch phase, (ii) a fed‐batch phase for biomass generation, (iii) an induction phase for recombinant periplasmic protein production, and (iv) a phase for OM permeabilization (cultivation 1; Fig. 2 and Supporting Information Table S1).
Dissolved TRIS stock solution (3.5 M) in deionized water with a pH of 7.8 was added to the bioreactor to a final concentration of 350 mM and cells were subsequently incubated for 12 h. The drop in the OD signal at around 30 h process time is the result of dilution by the addition of TRIS. The further decrease in the OD is the result of dilution by feeding as cells are viable but nonculturable. The same effect of growth inhibition by the addition of TRIS was observed in shake flask experiments. A strong increase in AP release was seen within 1 h after the addition of TRIS, which further increased over the next 12 h. The maximum amount of extracellular protein of about 22% was observed after 12 h of incubation with only about 5% lysis (Fig. 2B). Those results looked promising but still the majority of periplasmic protein was inside the cells. We also tested incubation with TRIS at high basic pH values, but obtained high lysis rates and did not investigate this further (data not shown).
3.4. Permeabilization of cells in bioreactor cultivation using heat treatment
To further increase the selective release of periplasmic proteins, heat treatment was tested. Within this study, we tried three different temperature profiles ranging between 30 and 51°C (Fig. 3).
As in cultivations 2 and 3 lysis was very pronounced, the samples of these cultivations were not homogenized. Therefore, AP values are not given in (%) but as normalized values in (g/(L⋅OD)) in Fig. 3.
In cultivation 2, we increased the temperature stepwise from 30 to 51°C. However, these conditions were too harsh and resulted not only in leakiness but also in pronounced lysis of almost 40% after the first temperature step. Accompanying release of proteases probably degraded the AP and thus resulted in a reduced AP signal. Therefore, in cultivation 3 a lower temperature range was tested. Changing the temperature between 32 and 41°C resulted in lower lysis and increased the slope in the AP versus lysis plot.
In cultivation 4, a fast ramp to 37°C with a subsequent linear ramp of 1°/h up to 42°C was applied. This rather mild treatment showed an increase in leakiness up to 7.5% within 4 h while keeping lysis below 5% and thus was the most promising approach. Similar results are shown in 29, where higher temperatures led to a significant increase in cellular lipopolysaccharides in the supernatant when temperature was above 48°C. Nevertheless, in those experiments there was a high degree of cell lysis.
In summary, we found that a rather mild heat treatment using linear ramps increased OM leakiness with only minor cell lysis. However, when temperature exceeded 40°C, pronounced lysis was detected.
3.5. Combination of permeabilizing agents and heat treatment
As treatment with 350 mM TRIS caused leakiness of around 20% and heat treatment with a moderate linear temperature ramp led to 7.5% leakiness, those conditions were combined in cultivations 5 and 6 (Fig. 4). The aim of these cultivations was to achieve a synergetic effect of both applied methods to increase leakiness.
In cultivation 5 (Fig. 4A and B), temperature was increased using a two‐step linear gradient right after addition of 350 mM TRIS. After the first temperature ramp, we already detected a high amount of cells lysis of about 25%. After the second ramp, the amount of lysed cells was already close to 35%. Apparently, the addition of 350 mM TRIS and a concomitant increase in temperature was too harsh for the cells.
Thus, in cultivation 6 the cells were incubated for 18 h with TRIS before applying a mild heat treatment (Fig. 4C). The heat treatment in cultivation 6 resembled the temperature profile of cultivation 4 (Fig. 3E) but without the steep ramp in the beginning to give the cells time to adapt. When the temperature was then increased to 38°C, nearly 30% of periplasmic proteins were released and only around 5% of the cells lysed. At higher temperatures, we only found an increase in cell lysis but no significant increase in leakiness. Thus, to trigger leakiness with only minimal lysis, we recommend incubation with 350 mM TRIS for several hours followed by a mild heat treatment up to 38°C.
4. Concluding remarks
In this study, we screened different methods for releasing periplasmic products from recombinant E. coli to the cultivation supernatant to simplify downstream processing. By the developed strategy, about 30% of the periplasmic content containing the target product was released without significant cell lysis (<5%). As cells are still viable after product release, this approach combined with suitable induction strategies might be an interesting method for continuous bioprocessing. In summary, this study represents a comparative study of different strategies to trigger E. coli leakiness and describes a solid basis for further experiments in this field.
Practical application
Recovery of recombinant product from E. coli is usually performed by harvesting the cells and subsequent cell disruption. This causes release of not only target protein but also a vast amount of impurities. In this work, we describe a comparative approach to selectively release periplasmic product from E. coli during bioreactor cultivation without triggering cell lysis. This strategy leads to less impurities in the crude product, simplifies the downstream process, and might pave the way for continuous bioprocessing.
The authors have declared no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
5 References
- 1. Elvin, J. G. , Couston, R. G. , van der Walle, C. F. , Therapeutic antibodies: Market considerations, disease targets and bioprocessing. Int. J. Pharm. 2013, 440, 83–98. [DOI] [PubMed] [Google Scholar]
- 2. Lee, Y. J. , Jeong, K. J. , Challenges to production of antibodies in bacteria and yeast. J. Biosci. Bioeng. 2015, 120, 483–490. [DOI] [PubMed] [Google Scholar]
- 3. Liu, J. K. , The history of monoclonal antibody development—Progress, remaining challenges and future innovations. Ann. Med. Surg. (Lond.) 2014, 3, 113–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rodrigues, M. E. , Costa, A. R. , Henriques, M. , Azeredo, J. et al., Technological progresses in monoclonal antibody production systems. Biotechnol. Prog. 2010, 26, 332–351. [DOI] [PubMed] [Google Scholar]
- 5. Walsh, G. , Biopharmaceutical benchmarks 2014. Nat. Biotechnol. 2014, 32, 992–1000. [DOI] [PubMed] [Google Scholar]
- 6. Baneyx, F. , Recombinant protein expression in Escherichia coli . Curr. Opin. Biotechnol. 1999, 10, 411–421. [DOI] [PubMed] [Google Scholar]
- 7. Balasundaram, B. , Harrison, S. , Bracewell, D. G. , Advances in product release strategies and impact on bioprocess design. Trends Biotechnol. 2009, 27, 477–485. [DOI] [PubMed] [Google Scholar]
- 8. French, C. , KeshavarzMoore, E. , Ward, J. M. , Development of a simple method for the recovery of recombinant proteins from the Escherichia coli periplasm. Enzyme Microb. Technol. 1996, 19, 332–338. [Google Scholar]
- 9. Hettwer, D. , Wang, H. , Protein release from Escherichia coli cells permeabilized with guanidine‐HCl and Triton X100. Biotechnol. Bioeng. 1989, 33, 886–895. [DOI] [PubMed] [Google Scholar]
- 10. Naglak, T. J. , Wang, H. Y. , Recovery of a foreign protein from the periplasm of Escherichia coli by chemical permeabilization. Enzyme Microb. Technol. 1990, 12, 603–611. [DOI] [PubMed] [Google Scholar]
- 11. Aimei, C. , Qingshan, S. , Jin, F. , Ouyang, Y. et al., Dissociation of outer membrane for Escherichia coli cell caused by cerium nitrate. J. Rare Earth. 2010, 28, 312–315. [Google Scholar]
- 12. Vaara, M. , Agents that increase the permeability of the outer‐membrane. Microbiol. Rev. 1992, 56, 395–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Allen, J. R. , Patkar, A. Y. , Frank, T. C. , Donate, F. A. et al., Use of glycol ethers for selective release of periplasmic proteins from gram‐negative bacteria. Biotechnol. Prog. 2007, 23, 1163–1170. [DOI] [PubMed] [Google Scholar]
- 14. Ames, G. F. , Prody, C. , Kustu, S. , Simple, rapid, and quantitative release of periplasmic proteins by chloroform. J. Bacteriol. 1984, 160, 1181–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Irvin, R. T. , MacAlister, T. J. , Costerton, J. W. , Tris(hydroxymethyl)aminomethane buffer modification of Escherichia coli outer membrane permeability. J. Bacteriol. 1981, 145, 1397–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ariga, O. , Watari, T. , Andoh, Y. , Fujishita, Y. et al., Release of thermophilic α‐amylase from transformed Escherichia coli by addition of glycine. J. Ferment. Bioengineer. 1989, 68, 243–246. [Google Scholar]
- 17. Helander, I. M. , Alakomi, H. L. , LatvaKala, K. , Koski, P. , Polyethyleneimine is an effective permeabilizer of Gram‐negative bacteria. Microbiology 1997, 143, 3193–3199. [DOI] [PubMed] [Google Scholar]
- 18. Voulgaris, I. , Finka, G. , Uden, M. , Hoare, M. , Enhancing the selective extracellular location of a recombinant E. coli domain antibody by management of fermentation conditions. Appl. Microbiol. Biotechnol. 2015, 99, 8441–8453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hart, R. A. , Lester, P. M. , Reifsnyder, D. H. , Ogez, J. R. et al., Large scale, in situ isolation of periplasmic IGF‐I from E. coli . Nat. Biotechnol. 1994, 12, 1113–1117. [DOI] [PubMed] [Google Scholar]
- 20. Russell, A. D. , Lethal effects of heat on bacterial physiology and structure. Sci. Prog. 2003, 86, 115–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tsuchido, T. , Katsui, N. , Takeuchi, A. , Takano, M. et al., Destruction of the outer membrane permeability barrier of Escherichia coli by heat treatment. Appl. Environ. Microbiol. 1985, 50, 298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tsuchido, T. , Aoki, I. , Takano, M. , Interaction of the fluorescent dye lN‐phenylnaphthylamine with Escherichia coli cells during heat stress and recovery from heat stress. Microbiology 1989, 135, 1941–1947. [DOI] [PubMed] [Google Scholar]
- 23. Witte, A. , Lubitz, W. , Biochemical characterization of phi X174‐protein‐E‐mediated lysis of Escherichia coli . Eur. J. Biochem. 1989, 180, 393–398. [DOI] [PubMed] [Google Scholar]
- 24. Dubose, R. F. , Hartl, D. L. , Evolutionary and structural constraints in the alkaline‐phosphatase of Escherichia coli in: Evolution at the Molecular Level. 1991, pp. 58–76. [Google Scholar]
- 25. Lederberg, J. , The beta‐d‐galactosidase of Escherichia coli, strain K‐12. J. Bacteriol. 1950, 60, 381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Langemann, T. , Mayr, U. B. , Meitz, A. , Lubitz, W. et al., Multi‐parameter flow cytometry as a process analytical technology (PAT) approach for the assessment of bacterial ghost production. Appl. Microbiol. Biotechnol. 2016, 100, 409–418. [DOI] [PubMed] [Google Scholar]
- 27. Rinas, U. , Hoffmann, F. , Selective leakage of host‐cell proteins during high‐cell‐density cultivation of recombinant and non‐recombinant Escherichia coli. Biotechnol. Prog. 2004, 20, 679–687. [DOI] [PubMed] [Google Scholar]
- 28. van Die, I. M. , Bergmans, H. E. , Hoekstra, W. P. , Transformation in Escherichia coli: studies on the role of the heat shock in induction of competence. J. Gen. Microbiol. 1983, 129, 663–670. [DOI] [PubMed] [Google Scholar]
- 29. Hitchener, B. J. , Egan, A. F. , Outer‐membrane damage in sublethally heated Escherichia coli K‐12. Can. J. Microbiol. 1977, 23, 311–318. [DOI] [PubMed] [Google Scholar]
- 30. DeLisa, M. P. , Li, J. C. , Rao, G. , Weigand, W. A. et al., Monitoring GFP‐operon fusion protein expression during high cell density cultivation of Escherichia coli using an on‐line optical sensor. Biotechnol. Bioeng. 1999, 65, 54–64. [PubMed] [Google Scholar]
- 31. Hancock, R. E. , Alterations in outer membrane permeability. Annu. Rev. Microbiol. 1984, 38, 237–264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information


