Abstract
The aim of this study was to improve l‐lactic acid production of Lactobacillus thermophilus SRZ50. For this purpose, high efficient heavy‐ion mutagenesis technique was performed using SRZ50 as the original strain. To enhance the screening efficiency for high yield l‐lactic acid producers, a scale‐down from shake flask to microtiter plate was developed. The results showed that 24‐well U‐bottom MTPs could well alternate shake flasks for L. thermophilus cultivation as a scale‐down tool due to its a very good comparability to the shake flasks. Based on this microtiter plate screening method, two high l‐lactic acid productivity mutants, A59 and A69, were successfully screened out, which presented, respectively, 15.8 and 16.2% higher productivities than that of the original strain. Based on fed‐batch fermentation, the A69 mutant can accumulate 114.2 g/L l‐lactic acid at 96 h. Hence, the proposed traditional microbial breeding method with efficient high‐throughput screening assay was proved to be an appropriate strategy to obtain lactic acid‐overproducing strain.
Keywords: Heavy ion mutagenesis, High productivity, High‐throughput screening, Lactobacillus thermophilus, l‐lactic acid
Abbreviations
- HIRFL
heavy ion research facility in Lanzhou
- LET
linear energy transfer
- MTP
microtiter plate
- PLA
poly lactic acid
1. Introduction
Traditionally, petroleum derivatives have gained tremendous demand as feedstock for the production of plastics 1. However, the rapid depletion of fossil fuels and the concerns for the environment problems have stimulated researchers worldwide to develop green methods for producing recyclable and biodegradable plastics using renewable resources as feed stocks 2. Currently, poly lactic acid (PLA), a biodegradable polymer, is increasingly applied as an environmentally friendly alternative to petroleum‐based plastics 3. Optically pure l‐ and d‐lactic acids are a necessary basis for producing biodegradable PLA that drives the global expansion of lactic acid production 4. The global demand for lactic acid is estimated to 367300 metric tons by 2017, and over one million tons by 2020 5. Thus, the efficient production of lactic acid is crucial for fitting the worldwide requirements.
Fermentation at high temperature above 50°C using thermophilic bacteria such as Lactobacillus thermophilus is the most important factor for industrial biotechnology for l‐lactic acid due to minimizing contamination by other microbial contamination 6. Although L. thermophilus is very commercially attractive for l‐lactic acid production, their l‐lactic acid titer and productivity still need to be further improved to meet the commercial requirements. In addition to process optimizing, mutation breeding for excellent lactic acid strains via random mutagenesis has been suggested as a valuable option, such as ultraviolet mutagenesis 7, atmospheric and room temperature plasma treatment 8, ethylmethanesulfonate 9, and low‐energy ion beam irradiation 10. Recently, some novel and powerful mutagenesis tools for microbial strain breeding have been developed, such as heavy ion beam mutagenesis, atmospheric, and room temperature plasma mutation system, and nanosecond pulsed electric fields 11, 12, 13. Of these, heavy ion beam mutagenesis technique attracts wide attention. A biologically important characteristic of the accelerated heavy ions (MeV/μ) can deposit its energy into a highly dense region with a localized area 14, which can result in clustered damage on DNA molecules in irradiated microbial cells 15. For example, in the Heavy Ion Research Facility in Lanzhou (HIRFL), the linear energy transfer (LET; the energy transferred per unit length, KeV/μ) values of heavy ions for use in biological breeding research can range from 30 to 150 KeV/μ 16, which are much higher than Low‐LET radiation mutagens such as γ‐rays and X‐rays (below 5 KeV/μ) 14. Such high LET of heavy ion beam could lead to a large number of DNA strand breaks and then cause high mutation efficiency and wide variety 17, which is beneficial for microbial mutation breeding. Moreover, the LET value of high energy heavy ion beams is controllable, and several physical parameters combination (such as different LET and different irradiated ion particles) by heavy ion beams can be supplied for microbial breeding in HIRFL. This mutagenesis technique can effectively mutate microalgae 18, 19, 20, bacteria 21, fungi 22, 23, 24, yeast 25, and actinomycetes 26, etc. In previous study, the screen processing for robust lactic acid strain consisted of mutant library construction, agar plate preliminary screening, flask fermentation rescreening, and HPLC identification for lactic acid. Such screen process based on shake flasks for obtaining positive mutants was time‐consuming and expensive because the large number of strains needed to be tested. Therefore, it is important and preferential to establish a simple, fast, low‐cost, and high throughput screening (HTS) method to obtain high‐production lactic acid strains after treated by random mutagenesis methods. Currently, the screening platform based on microtiter plates has become an attractive alternative to shake flasks as a rapid screening tool for high‐productivity microbial mutant screening after treated by random mutagenesis methods (containing filamentous fungi, bacteria, microalgae, and actinomycetes) in bioindustry 27, 28.
The aim of this study was to obtain L. thermophilus mutants with better production profiles for l‐lactic acid after treated by 80 MeV/μ heavy ions irradiation with the LET of 40 KeV/μ. A high‐throughput screening process based on microtiter plate was also developed. Based on this HTS screening method, the mutant strains A59 and A69 were successfully screened from the heavy ion mutagenesis libraries. The productivity and genetic stability of the typical mutants were also examined.
2. Materials and methods
2.1. Strain and media
Original L. thermophilus SRZ50, which can produce l‐lactic acid, was obtained from heavy ion irradiation and provided by biophysics lab of IMP, CAS. The strain was maintained on MRS agar slant at 4°C. The MRS agar slant medium contained (L−1): glucose 20 g, peptone 10 g, beef extract 5 g, yeast extract 5 g, K2HPO4 2 g, (NH4)3C6H5O7 2 g, CH3COONa 5 g MgSO4·7H2O 0.2 g, MnSO4·4H2O 0.05 g, Agar 15 g, tween‐80 1 mL, and pH 6.0–6.4. The selection agar plates had the same compositions except that 2.5 g/L CaCO3 and 0.1 g/L bromocresol purple were added. The seed medium contained (L−1): glucose 20 g, peptone 10 g, beef extract 10 g, yeast extract 5 g, K2HPO4 2 g, (NH4)3C6H5O7 2 g, CH3COONa 5 g, MgSO4·7H2O 0.58 g, MnSO4·4H2O 0.25 g, tween‐80 1 mL, and pH 6.2–6.4. The fermentation medium contained (L−1): glucose 100 g, peptone 10 g, beef extract 10 g, yeast extract 5 g, sodium acetate 5 g, K2HPO4 2 g, (NH4)3C6H5O7 2 g, CH3COONa 5 g, MgSO4·7H2O 0.58 g, MnSO4·4H2O 0.25 g, tween‐80 1 mL, CaCO3 70 g and pH 6.7–7.0.
2.2. Establishment of the HTS procedure based on 24‐well U‐bottom microtiter plates
24‐well U‐bottom microtiter plates: Single original L. thermophilus SRZ50 colonies on the slant plates were transferred to 24‐well U‐bottom microtiter plates (MTPs) containing 3 mL of the seed culture, and cultivated on a rotary incubator (Yiheng Co., Ltd, Shanghai, China) at 50°C and 100 rpm for 24 h, Then, 300 μL of the seed culture was transferred to 24‐well U‐bottom MTPs containing 3 mL of fermentation culture, and fermented for different fermentation period at 50°C, with shaking at 100 rpm.
Traditional shake flask: Single original L. thermophilus SRZ50 colonies on the slant plates were transferred to 50 mL shake flasks with 25 mL seed medium, and cultivated on a HZQ 300 rotary incubator (Yiheng Co., Ltd, Shanghai, China) at 50°C and 100 rpm for 24 h, Then, 2.5 mL of the seed culture was transferred to 50 mL shake flasks with 25 mL fermentation medium, and fermented for different fermentation period at 50°C, with shaking at 100 rpm.
2.3. Irradiation and screening process
Original L. thermophilus SRZ50 was incubated on the MRS agar slants at 50°C for 24 h, and L. thermophilus SRZ50 from slants was diluted with sterile saline water. Then 1.0 mL suspension liquid was transferred into 35 mm irradiation dish, respectively. Bacteria suspension in irradiation dish was subjected to mutagenesis by 80 MeV/μ carbon ions with the LET of 40 KeV/μ in HIRFL. The irradiation doses were set as 75 Gy and 100 Gy. After irradiation, the mutagen‐treated and untreated aliquots of L. thermophilus SRZ50 cells were properly diluted and spread on selective plate medium, followed by cultivated at 50°C for 24 h. The colonies showing large yellow halos on selective plate medium were selected as the primary screening, and then cultivated in 24‐well U‐bottom MTPs for l‐lactic acid accumulation as the second round screening.
2.4. The analysis of fermentation characteristics between original L. thermophilus SRZ50 and mutants
Both original L. thermophilus SRZ50 and its mutants were precultured in 50 mL shake flasks with 25 mL seed medium on a HZQ 300 rotary incubator (Yiheng Co., Ltd, Shanghai, China) at 50°C and 100 rpm for 24 h. Then, 2.5 mL of the seed culture was transferred to 50 mL shake flasks with 25 mL fermentation medium, and fermented for different fermentation period at 50°C, with shaking at 100 rpm.
2.5. Fed‐batch fermentation in flasks
Fed‐batch fermentations were first performed in a 50 mL flask containing 25 fresh medium at 50°C and 100 rpm. The initial concentration of the total glucose was set at 92.8 g/L. After 16 h cultivation, 2 mL of mixed liquid (containing 60 g of glucose and 42 g of calcium carbonate) was repeatedly added to bring the sugar concentration to approximately 101.3 g/L. Then the mutant A69 fermented for different fermentation period at 50°C, with shaking at 100 rpm.
2.6. Analytical methods
The biomass was diluted by seven times volume of diluted hydrochloric acid, then the optical density at wavelength of 620 nm (OD620) of the biomass was measured using an epoch spectrophotometer (Times Legend Bio‐Scientific Co. Ltd., Shanxi, China). The concentrations of l‐lactic acid were determined by SBA‐40D biosensor analyzer based on the immobilized oxidases technology (Institute of biology, Shandong academy of Sciences, Shandong, China). The concentrations of glucose were determined by Fehling reagent. The pH of fermentation broth was determined by the Mettler Toledo pH meter (Gießen, Germany). All experiments were conducted in triplicates.
3. Results
3.1. Establishing high throughput screening method based on 24‐deep MTPs
To enhance the screening efficiency, use of the 24‐well U‐bottom microplate as high throughput screening method was developed. In previous studies, the microplate method was at least comparable to shake flask methods as batch fermentation in terms of reproducibility and variation in order to evaluate the microbial cultivation performances 29. Hence, in present study, fermentation process parameters such as OD, pH, l‐lactic acid accumulation, and glucose consumption of L. thermophilus SRZ50 were present in both 24‐well U‐bottom MTPs and shake flasks cultivations between the two different fermentation scales (3 and 25 mL). Figure 1 shows that similar behaviors of fermentation process parameters between 24‐deep MTPs and shake flasks cultivations were obtained. The correlation coefficients of these fermentation process parameters between 24‐deep MTPs and shake flasks were all better than 0.93 by statistical analysis (Fig. 2). These results suggested that 24‐well U‐bottom MTPs could be applied well as alternate to shake flasks for L. thermophilus cultivation as a scale‐down tool.
Figure 1.
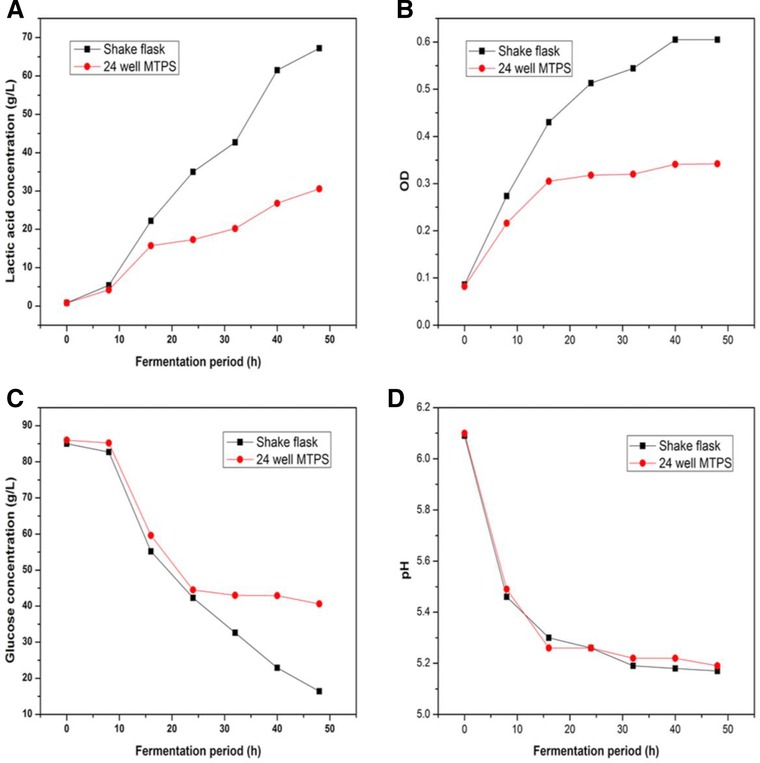
Comparison of L. thermophilus SRZ50 culture performance between shake flasks and 24‐deep MTPs.
Figure 2.
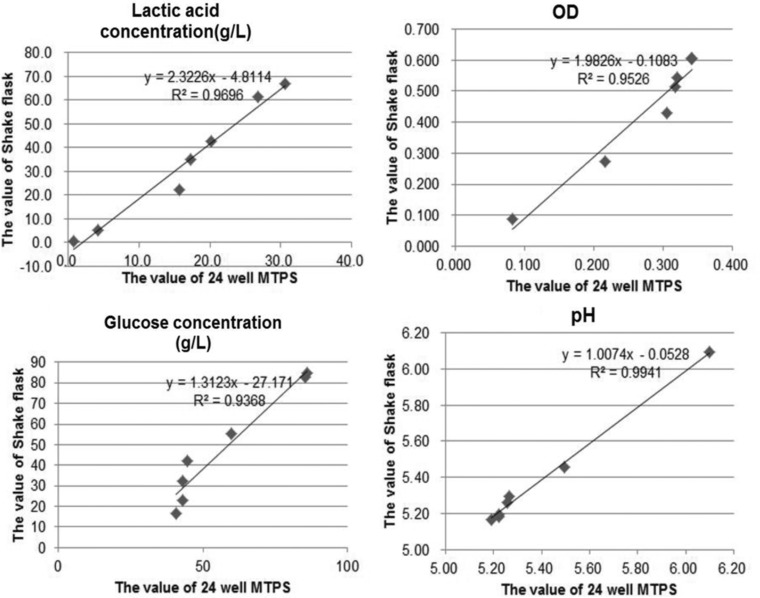
The correlation coefficients of these fermentation process parameters between 24‐deep MTPs and shake flasks.
3.2. Selection of mutants with high l‐lactic acid productivity based on 24‐deep MTPs after 80 MeV/u carbon ion mutagenesis
In order to select efficient mutants, a color zone plate containing bromocresol purple were also chosen as the preliminary selective method 30 as bromocresol purple can convert from purple to yellow when the pH changes from 6.8 to 5.2. The modified industrial producer L. thermophilus SRZ50 was then treated by the 80 MeV/μ heavy ion beams as described to build a mutant library. The treated cell suspension was then cultivated on solid plate containing bromocresol purple for 24 h cultivation. Ninety colonies exhibited larger large yellow halos compared with original strain, and were then tested with 24‐deep MTPs fermentation. A number of mutants showed relative increase in l‐lactic acid accumulation than that of the original strain (Fig. 3A) and 16 mutants were further tested with 24‐deep MTPs fermentation as the second round screening. Based on these multiple rounds of screening, four mutants showed obviously higher lactic acid accumulation than original strain (p < 0.05), especially the two mutants (A59 and A69) showed the better lactic acid accumulation (Fig. 3B). These results indicated that heavy ion mutagenesis method combination of 24‐deep MTPs was an appropriate strategy to obtain lactic acid‐overproducing strain.
Figure 3.
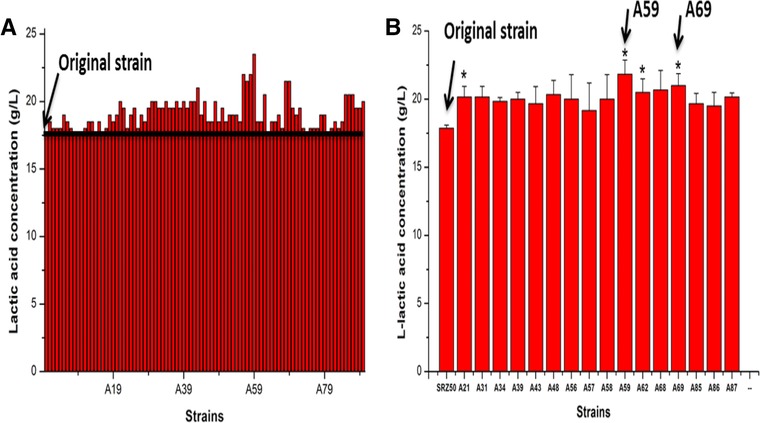
(A) The first round mutation screening l‐lactic acid high‐production mutants based on 24‐deep MTPs. (B) The second round screening based on 24‐deep MTPs. The error bars in the figure indicate the standard deviations of three parallel replicates, and p < 0.05 indicated a significant difference and p < 0.01 indicated a highly significant difference.
3.3. Comprehensive fermentation characteristics between original strain and mutants
The two mutants (A59 and A69) were then inoculated for 48 in 50‐mL flasks containing 25 mL of fermentation to test its stability in producing l‐lactic acid. The original strain was set as the control, and the experiments were conducted in triplicates. In particular, several fermentation indexes were included: lactic acid concentration, glucose consumption, growth rate, and pH. As shown in Fig. 4A, both the mutant A59 and A69 exhibited significant increase in lactic acid accumulation at 48 h compared to the concentration observed for the original strain. The maximal lactic acid production by A69 mutant reached 67.5 g/L at 48 h, which was 16.2% higher than the original strain under the same cultivation conditions. This indicated that heavy ion mutagenesis can promoted lactic acid production in L. thermophilus. The OD values and the glucose consumption of mutant strains and original strain in the stationary phase were both measured. The results were shown in Fig. 4B and Fig. 4C. It was also obtained that the glucose consumption and growth rate of both the mutant A59 and A69 were faster than those of the original strain. During the whole fermentation period, the fermentation broth pH of both the mutant A59 and A69 were lower than those of the original strain, indication that the mutant A59 and A69 showed stronger tolerance for low pH environment than the original strain (Fig. 4D).
Figure 4.
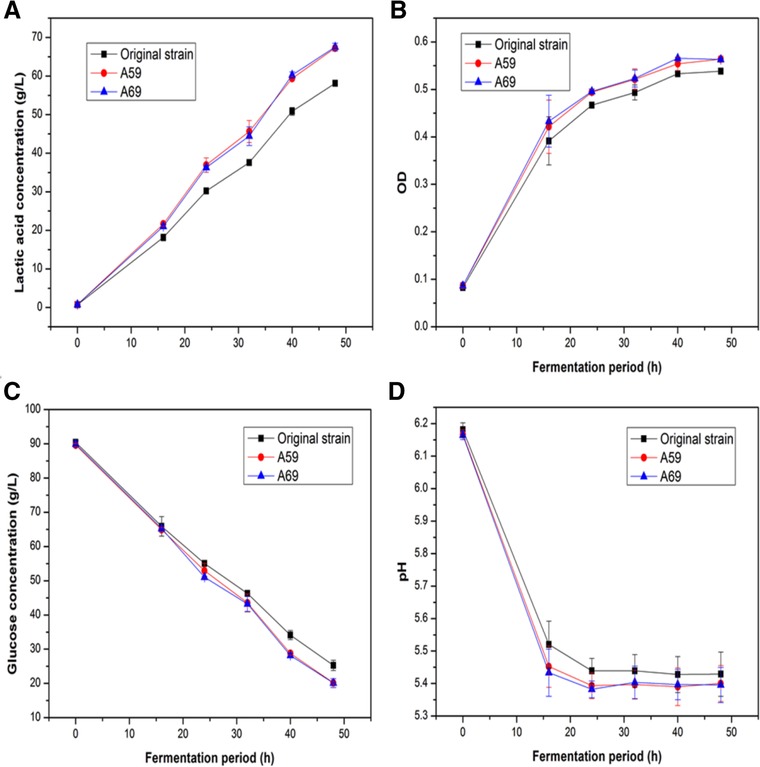
Comparison of glucose consumption and l‐lactic acid production among original strain SRZ50, mutant A59 and A69. The error bars in the figure indicate the standard deviations of three parallel replicates.
The genetic stability of the mutant A59 and A69 for l‐lactic acid accumulation were also evaluated by continuous cultivation. The results showed that these two mutants exhibited good genetic stability for l‐lactic acid accumulation (Fig. 5). Based on previous studies, Aurantiochytrium sp. mutant T‐99 20 and Aspergillus niger mutant H4002 23 also exhibited high genetic stability after heavy ion mutagenesis. These results indicated that heavy ion mutagenesis is a promising tool for generating genetically stable microbial mutants with enhanced metabolite accumulation ability.
Figure 5.
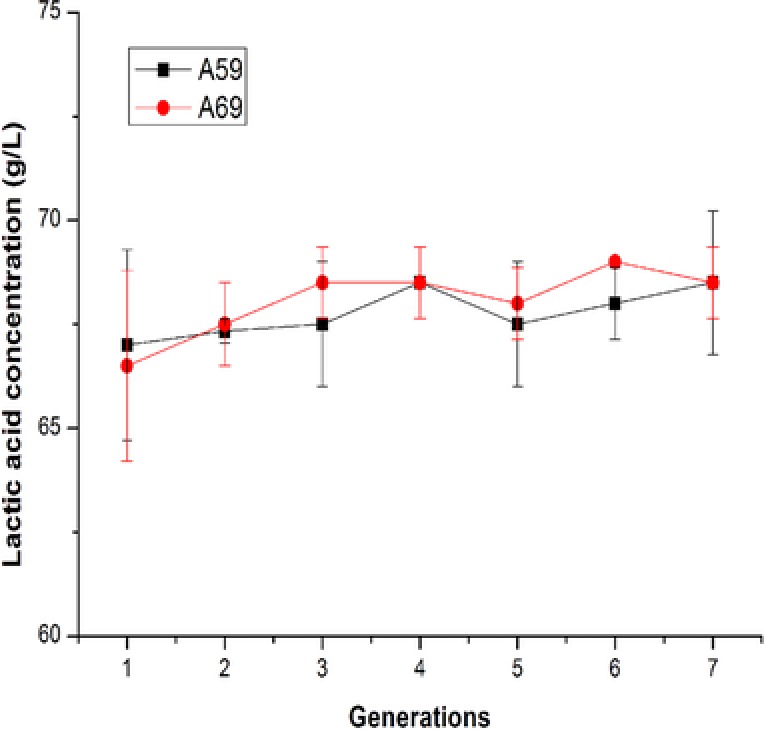
The genetic stability of the mutant A59 and A69 for l‐lactic acid accumulation. The error bars in the figure indicate the standard deviations of three parallel replicates.
3.4. l‐lactic acid production of A69 mutant in fed‐batch fermentation
As the mutant strain A69 exhibited the highest capacity for producing l‐lactic acid, fed‐batch fermentations were carried out to further enhance production. To investigate the effect of initial glucose concentration on the production of l‐lactic acid, multiple batch fermentations were conducted. As shown in Fig. 6A and B, when the initial glucose concentration was increased from 72.4 to 111.5 g/L, the relative lactic acid concentration by A69 increased gradually. Using an initial total glucose concentration of 92.4 g/L, 84.9 g/L l‐lactic acid was produced by mutant strain A69 after 80 h of fermentation. When the initial glucose concentration was set at 111.5 g/L, mutant strain A69 only accumulated 91.2 g/L l‐lactic acid. The reason may be due to high initial glucose concentration inhibition effect. Therefore, 92.4 g/L was chosen as the initial total glucose concentration in fed‐batch fermentations. The fermentation curves of mutant strain A69 were shown in Fig. 7. The production of l‐lactic acid terminated at 96 h with a final lactic acid concentration of 114.2 g/L when the residual reducing sugars were 13.2 g/L. The l‐lactic acid productivity of mutant strain A69 was 1.19 g/L/h.
Figure 6.
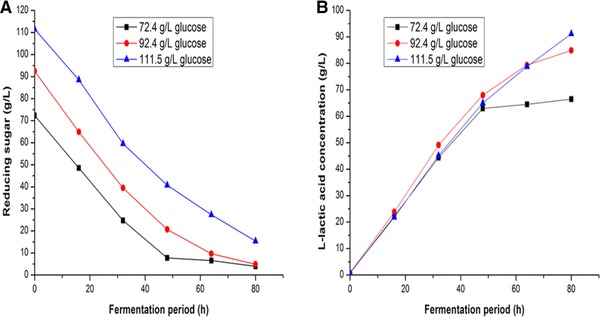
Effects of different initial concentrations of total glucose on l‐lactic acid production by strain A69. (A) Total reducing sugars consumption, (B) l‐lactic acid production.
Figure 7.
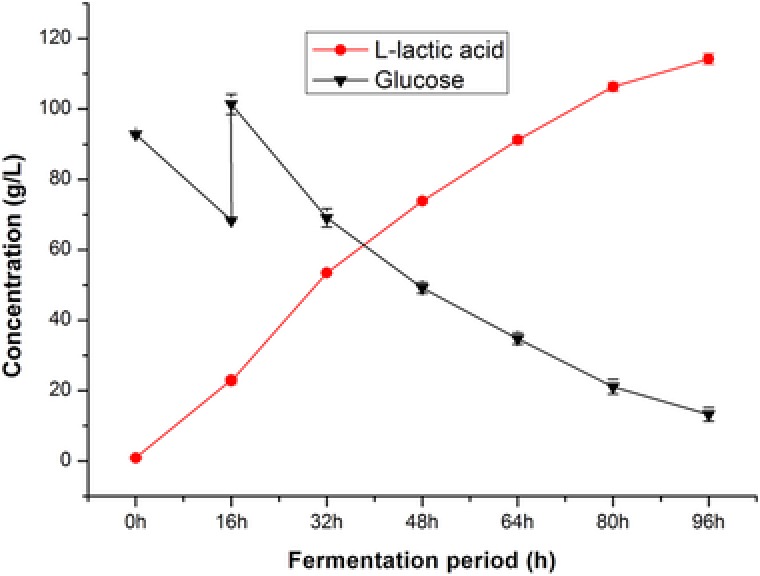
Fed‐batch fermentation by mutant A69 in a 50 mL flask. The error bars in the figure indicate the standard deviations of three parallel replicates.
4. Discussion
Industrial microbial strains are a promising approach for production of commercially valuable products from renewable carbon sources. In nature, microbial strains have low productivity. Therefore, artificial strategies are developed to improve microbial strains with desired phenotype including two major approaches: “rational metabolic engineering” and “random mutagenesis and screening” 31. Although engineering microbial strains for improving microbial productivity has become preferred option, rapid development of HTS methods also prompt wide application of some novel and powerful mutagenesis technologies to boost microbial productivity. Meanwhile, several novel and powerful mutagenesis technologies have been reported to obtain robust microbial strains effectively, such as heavy ion mutagenesis and ARTP mutagenesis. Therefore, this study was carried out to obtain a promising industrial strain of L. thermophiles with high productivity for l‐lactic acid via heavy ion mutagenesis.
After treated by heavy ion mutagenesis, thousands of lactic acid‐producing mutants can be generated due to its high LET value, and how to rapidly screen these lactic acid‐producing mutants with enhanced lactic acid accumulation is the major challenge in the next step experiment. Generally, traditional screening method based on shake flask for high‐yield lactic acid‐producing mutants is usually time‐consuming and ineffective 32. In order to overcome the throughput limitation, developing HTS method for screening robust lactic acid‐producing mutants after treated by heavy ion mutagenesis could be a valuable option.
In comparison to the traditional shake flask screening methods, the similar behaviors of fermentation process parameters between 24‐deep MTPs and shake flasks cultivations were obtained in present study, indicating that 24‐well U‐bottom MTPs could be applied well as alternate to shake flasks for L. thermophilus cultivation as a scale‐down tool. Meanwhile, the main advantage of 24‐deep MTPs method developed was the high‐throughput characteristics for screening robust L. thermophilus mutants with enhanced lactic acid accumulation. To the best of our knowledge, this is the first report on the efficient screening strategy based on 24‐deep MTPs for obtaining l‐lactic acid strains with good performance after treated by heavy ion mutagenesis. Based on this HTS screening method, two high l‐lactic acid productivity mutants, A59 and A69, were successfully screened out, which presented, respectively, 15.8 and 16.2% higher productivities than that of the original strain. Especially, the A69 mutant with the highest yield of lactic acid was obtained by novel heavy ion mutagenesis‐screening method. Based on fed‐batch fermentation, the A69 mutant can accumulate 114.2 g/L l‐lactic acid at 96 h.
Although the cell growth and lactic acid production capacity in A69 mutant were significantly enhanced by heavy ion mutagenesis in this study, high‐yield molecule mechanism by which the A69 mutant increased its lactic acid production have not been completely understood. These mechanisms should be further elucidated by means of omics methods, such as comparative genomics and transcriptomics 33, which have become extremely useful tools for analysis of gene function in industrial microbial strains. Key genes related to the improved lactic acid titer between the original strain and the A69 mutant will be correspondingly parsed by comparative genomics and transcriptomics analyses. These omics analyses results could not only reveal high‐yield molecule mechanism in desired L. thermophilus mutants, but they could also provide theoretical foundation for rationally improving lactic acid production by genetically and metabolically engineering L. thermophilus in the future.
Practical application
An efficient screening strategy, which combined high throughput screening (HTS) screening process based on microtiter plates and heavy ion mutagenesis, was established for screening L. thermophilus mutants with high yield lactic acid in present study, indicating that traditional random mutagenesis methods such as heavy ion mutagenesis become more efficient to obtain improved industrial microbial strains with desired phenotype cross‐linking modern HTS techniques in the future. We also believe that heavy‐ion mutagenesis technique could greatly contribute to the fermentation industry as a promising and convenient mutation tool in the future.
The authors have declared no conflict of interest.
Acknowledgement
The study was supported financially by the National Natural Science Foundation of China (No. 11605259) and (Y706030XB0).
5 References
- 1. Juturu, V. , Wu, J. C ., Microbial production of lactic acid: the latest development. Crit. Rev. Biotechnol. 2016, 36, 967–977. [DOI] [PubMed] [Google Scholar]
- 2. Abdel‐Rahman, M. A. , Tashiro, Y. , Sonomoto, K ., Recent advances in lactic acid production by microbial fermentation processes. Biotechnol. Adv. 2013, 31, 877–902. [DOI] [PubMed] [Google Scholar]
- 3. Wang, Y. , Tashiro, Y. , Sonomoto, K ., Fermentative production of lactic acid from renewable materials: recent achievements, prospects, and limits. J. Biosci. Bioeng. 2015, 119, 10–18. [DOI] [PubMed] [Google Scholar]
- 4. Sauer, M. , Porro, D. , Mattanovich, D. , Branduardi, P ., Microbial production of organic acids: expanding the markets. Trends Biotechnol. 2008, 26, 100–108. [DOI] [PubMed] [Google Scholar]
- 5. Upadhyaya, B. P. , DeVeaux, L. C. , Christopher, L. P ., Metabolic engineering as a tool for enhanced lactic acid production. Trends Biotechnol. 2014, 32, 637–644. [DOI] [PubMed] [Google Scholar]
- 6. Ou, M. S. , Ingram, L. O. , Shanmugam, K. T ., l(+)‐Lactic acid production from non‐food carbohydrates by thermotolerant Bacillus coagulans . J. Ind. Microbiol. Biot. 2011, 38, 599–605. [DOI] [PubMed] [Google Scholar]
- 7. Joshi, D. S. , Singhvi, M. S. , Khire, J. M. , Gokhale, D. V ., Strain improvement of Lactobacillus lactis for d‐lactic acid production. Biotechnol. Lett. 2010, 32, 517–520. [DOI] [PubMed] [Google Scholar]
- 8. Sun, J. D. , Wang, Y. , Wu, B. , Bai, Z. Z. , et al. Enhanced production of d‐lactic acid by Sporolactobacillus sp Y2‐8 mutant generated by atmospheric and room temperature plasma. Biotechnol. Appl. Bioc. 2015, 62, 287–292. [DOI] [PubMed] [Google Scholar]
- 9. Demirci, A. , Pometto, A. L ., Enhanced production of d‐lactic acid by mutants of Lactobacillus delbrueckii ATCC 9649. J. Industrial Microbiol. 1992, 11, 23–28. [Google Scholar]
- 10. Xu, T. T. , Bai, Z. Z. , Wang, L. J. , He, B. F ., Breeding of d‐lactic acid high producing strain by low‐energy ion implantation and preliminary analysis of related metabolism. Appl. Biochem. Biotech. 2010, 160, 314–321. [DOI] [PubMed] [Google Scholar]
- 11. Hu, W. , Li, W. , Chen, J ., Recent advances of microbial breeding via heavy‐ion mutagenesis at IMP. Lett. Appl. Microbiol. 2017, 65, 274–280. [DOI] [PubMed] [Google Scholar]
- 12. Zhang, X. , Zhang, X. F. , Li, H. P. , Wang, L. Y. et al., Atmospheric and room temperature plasma (ARTP) as a new powerful mutagenesis tool. Appl. Microbiol. Biot. 2014, 98, 5387–5396. [DOI] [PubMed] [Google Scholar]
- 13. Guo, J. S. , Ma, R. N. , Su, B. , Li, Y. L. et al. Raising the avermectins production in Streptomyces avermitilis by utilizing nanosecond pulsed electric fields (nsPEFs). Sci. Rep. 2016, 6, 25949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kazama, Y. , Ma, L. Q. , Hirano, T. , Ohbu, S. et al. Rapid evaluation of effective linear energy transfer in heavy‐ion mutagenesis of Arabidopsis thaliana . Plant Biotechnol. Nar. 2012, 29, 441–445. [Google Scholar]
- 15. Asaithamby, A. , Chen, D. J ., Mechanism of cluster DNA damage repair in response to high‐atomic number and energy particles radiation. Mutat. Res. Fund. Mol. M. 2011, 711, 87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou, L. , Li, W. , Yu, L. , Li, P. et al., Linear energy transfer dependence of the effects of carbon ion beams on adventitious shoot regeneration from in vitro leaf explants of Saintpaulia ionahta . Int. J. Radiat. Biol. 2006, 82, 473–481. [DOI] [PubMed] [Google Scholar]
- 17. Kazama, Y. , Ishii, K. , Hirano, T. , Wakana, T. et al., Different mutational function of low‐ and high‐linear energy transfer heavy‐ion irradiation demonstrated by whole‐genome resequencing of Arabidopsis mutants. Plant J. 2017, 92, 1020–1030. [DOI] [PubMed] [Google Scholar]
- 18. Ma, Y. B. , Wang, Z. Y. , Zhu, M. , Yu, C. J. et al., Increased lipid productivity and TAG content in Nannochloropsis by heavy‐ion irradiation mutagenesis. Bioresource Technol. 2013, 136, 360–367. [DOI] [PubMed] [Google Scholar]
- 19. Hu, G. R. , Fan, Y. , Zhang, L. , Yuan, C. et al., Enhanced lipid productivity and photosynthesis efficiency in a Desmodesmus sp mutant induced by heavy carbon ions. Plos One 2013, 8, e60700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cheng, Y. R. , Sun, Z. J. , Cui, G. Z. , Song, X. et al., A new strategy for strain improvement of Aurantiochytrium sp. based on heavy‐ions mutagenesis and synergistic effects of cold stress and inhibitors of enoyl‐ACP reductase. Enzyme Microb Technol. 2016, 93–94, 182–190. [DOI] [PubMed] [Google Scholar]
- 21. Zhou, X. , Lu, X. H. , Li, X. H. , Xin, Z. J. et al., Radiation induces acid tolerance of Clostridium tyrobutyricum and enhances bioproduction of butyric acid through a metabolic switch. Biotechnol. Biofuels 2014, 7, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li, S. W. , Li, M. , Song, H. P. , Feng, J. L. et al., Induction of a High‐yield lovastatin mutant of Aspergillus terreus by 12C6+ heavy‐ion beam irradiation and the influence of culture conditions on lovastatin production under submerged fermentation. Appl. Biochem. Biotech. 2011, 165, 913–925. [DOI] [PubMed] [Google Scholar]
- 23. Hu, W. , Liu, J. , Chen, J. H. , Wang, S. Y. ,et al., A mutation of Aspergillus niger for hyper‐production of citric acid from corn meal hydrolysate in a bioreactor. J. Zhejiang Univ. Sc. B 2014, 15, 1006–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li, Z. Z. , Chen, X. J. , Li, Z. L. , Li, D. M. et al., Strain improvement of Trichoderma viride for increased cellulase production by irradiation of electron and 12C6+ ion beams. Biotechnol. Lett. 2016, 38, 983–989. [DOI] [PubMed] [Google Scholar]
- 25. Wang, J. F. , Li, R. M. , Lu, D. , Ma, S. et al., A quick isolation method for mutants with high lipid yield in oleaginous yeast. World J. Microb. Biot. 2009, 25, 921–925. [Google Scholar]
- 26. Wang, S. Y. , Chen, J. H. , Li, W. J. , Liang, J. P. et al., Influence of 12C6+ ion irradiation on mutant avermitilis. Chinese Phys. C. 2012, 36, 1140–1144. [Google Scholar]
- 27. Duetz, W. A., Microtiter plates as mini‐bioreactors: miniaturization of fermentation methods. Trends Microbiol. 2007, 15, 469–475. [DOI] [PubMed] [Google Scholar]
- 28. Hevekerl, A. , Kuenz, A. , Vorlop, K. D ., Filamentous fungi in microtiter plates‐an easy way to optimize itaconic acid production with Aspergillus terreus . Appl. Microbiol. Biot. 2014, 98, 6983–6989. [DOI] [PubMed] [Google Scholar]
- 29. Sohoni, S. V. , Bapat, P. M. , Lantz, A. E ., Robust, small‐scale cultivation platform for Streptomyces coelicolor . Microb. Cell Fact. 2012, 11, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim, M. J. , Jung, S. W. , Park, H. R. , Lee, S. J ., Selection of an optimum pH‐indicator for developing lactic acid bacteria‐based time‐temperature integrators (TTI). J. Food Eng. 2012, 113, 471–478. [Google Scholar]
- 31. Liu, Y. N. , Li, Q. G. , Zheng, P. , Zhang, Z. D. et al., Developing a high‐throughput screening method for threonine overproduction based on an artificial promoter. Microb. Cell Fact. 2015, 14, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Linde, T. , Hansen, N. B. , Lubeck, M. , Lubeck, P. S ., Fermentation in 24‐well plates is an efficient screening platform for filamentous fungi. Lett. Appl. Microbiol. 2014, 59, 224–230. [DOI] [PubMed] [Google Scholar]
- 33. Jia, N. , Ding, M. Z. , Du, J. , Pan, C. H. et al., Insights into mutualism mechanism and versatile metabolism of Ketogulonicigenium vulgare Hbe602 based on comparative genomics and metabolomics studies. Sci. Rep. 2016, 6, 23068. [DOI] [PMC free article] [PubMed] [Google Scholar]


