Abstract
This review aims to present current knowledge of the fungi involved in lignocellulose degradation with an overview of the various classes of lignocellulose‐acting enzymes engaged in the pretreatment and saccharification step. Fungi have numerous applications and biotechnological potential for various industries including chemicals, fuel, pulp, and paper. The capability of fungi to degrade lignocellulose containing raw materials is due to their highly effective enzymatic system. Along with the hydrolytic enzymes consisting of cellulases and hemicellulases, responsible for polysaccharide degradation, they have a unique nonenzymatic oxidative system which together with ligninolytic enzymes is responsible for lignin modification and degradation. An overview of the enzymes classification is given by the Carbohydrate‐Active enZymes (CAZy) database as the major database for the identification of the lignocellulolytic enzymes by their amino acid sequence similarity. Finally, the recently discovered novel class of recalcitrant polysaccharide degraders‐lytic polysaccharide monooxygenases (LPMOs) are presented, because of these enzymes importance in the cellulose degradation process.
Keywords: Biological pretreatment, Carbohydrate active enzymes, Fungi and fungal enzymes, Lignocellulose degradation, Plant cell wall
Abbreviations
- AA
auxiliary activity
- CAZy
carbohydrate active enzymes database
- CAZymes
carbohydrate active enzymes
- CDH
cellobiose dehydrogenase
- GH
glycoside hydrolase
- LiP
lignin peroxidases
- LPMO
lytic polysaccharide monooxygenase
- MnP
manganese‐dependent peroxidases
1. The composition of lignocellulose‐containing raw materials
Cellulose, hemicellulose, and lignin are major constituents of lignocellulose‐containing raw materials, but a small amount of pectin, nitrogen compounds, and mineral residues are also present in these feedstocks 1. Depending on the origin, the amounts of the indicated constituents differ. The structure of the lignocellulose is compact with different bonding among cellulose, hemicellulose, and lignin that makes lignocellulose a very complex substrate for enzymes. Those constituents are mainly coupled by hydrogen bonds. Between hemicellulose and lignin are chemical bonds that primarily refer to the chemical bonds between galactose and arabinose residues and carbohydrates. Several factors responsible for lignocellulose‐containing raw materials recalcitrance are: lignin content that protects cellulose; cellulose interweaving by hemicellulose; high crystallinity and degree of polymerization of cellulose and low accessible surface area of cellulose with strong fiber strength 2. Cellulose is a linear homopolymer composed of d‐glucospyranose subunits linked by β‐1,4‐glycosidic bonds. The repeating unit is the disaccharide, cellobiose, since the single glucose units are rotated 180° relative to each other 3. The cellulose strands form micro‐fibrils that are stabilized by intra‐ and intermolecular hydrogen bonds and van der Waals forces 4. Hemicellulose is a polysaccharide formed from monomeric sugars and sugars acids: d‐xylose, d‐mannose, d‐galactose, d‐glucose, l‐arabinose, 4‐O‐methyl‐glucuronic, d–galacturonic, and d‐glucuronic acids linked together by β‐1,4‐ and β‐1,3‐glycosidic bonds. Xylan is the main carbohydrate in hemicellulose. Hemicellulose is characterized by branches with short lateral chains consisting of different sugars while cellulose by easily hydrolysable lower molecular weight oligomers, respectively 4. Lignin is a complex amorphous heteropolymer with a three‐dimensional structure composed of phenylpropane derivates linked to each other by the irregular coupling of C–C and C–O bonds. It includes three basic structural monomers: p‐phenyl monomer (H type) derived from coumaryl alcohol, guaiacyl monomer (G type) derived from coniferyl alcohol and syringyl monomer (S type) derived from sinapyl alcohol 5. Lignin is linked to both hemicellulose and cellulose and it main function in the plant wall is to give structural support, impermeability, and resistance against microbial attack and oxidative stress 4.
2. Lignocellulose degrading fungi
Fungal strategies for lignocellulose depolymerization are substantially very complex, due to the high complexity of the raw materials. Recalcitrance to saccharification is a major limitation for the enzymatic conversion of lignocellulose‐containing raw materials to get valuable end products. The combination of hemicellulose and lignin forms a protective barrier around the cellulose, which must be modified (or removed) before the hydrolysis of cellulose. But also crystalline structure of cellulose makes it insoluble and resistant for enzyme decomposition. Nevertheless, the removal of lignin is a key challenge to increase enzyme access to hemicellulose and cellulose 6.
Plant cell wall‐degrading filamentous fungi have an important role in recycling nutrients in forest ecosystem. They are known to produce a broad variety of extracellular enzymes with diverse catalytic activities for the hydrolysis of renewable lignocellulose‐containing raw materials.
Predominantly responsible for lignocellulose degradation are wood‐degrading fungi. They decompose and assimilate the most recalcitrant organic polymers, which is mainly attributed to their highly adaptive lifestyles, reflected by a large phylogenetic and phenotypic diversity 7. If they want to overcome the physical and chemical stability of lignocellulose, the fungi have to employ large sets of enzymes, which they release into the environment during their growth. Fungi have two types of degradation systems: intracellular, together with the outer cell envelope layer, and extracellular, important for polysaccharide degradation. Furthermore, the extracellular enzymatic system includes two types of enzymes: hydrolytic, responsible for polysaccharide degradation; and oxidative, which degrade lignin and open phenyl rings. Three groups of fungi, with different effects and degradation mechanisms onto the lignocellulose, have been described: soft‐rot, brown‐rot, and white‐rot fungi 4.
Soft‐rot fungi are mostly ascomycete fungi that can degrade polysaccharides in the surface layers of plants. Degradation leads to darkening and softening of the wood by the produced laccases and peroxidases involved in lignin modifications. These enzymes are unspecific and more limited in function then those isolated from white‐rot and brown‐rot fungi. The soft‐rot fungi belong to genera Aspergillus and Neurospora. Still, little is known about the degradation mechanisms of lignocellulose by soft‐rot fungi 8.
Brown‐rot fungi are basidiomycetes that rapidly metabolize cellulose and hemicellulose while only slightly modifying lignin. They have no lignin degrading enzymes except small molecule reactive species to depolymerize lignin. At an advanced stage of degradation, wood residue exhibits cube‐shape and has a brownish color due to the predominant presence of oxidized lignin. Disruption of the lignocellulose matrix by brown‐rot fungi can be demonstrated using iron‐dependent Fenton chemistry known as chelator‐mediated Fenton system (CMF). The CMF system is a unique substrate deconstruction system based on oxygen radical chemistry that allows nonenzymatic deconstruction of the cellulose. Briefly, brown‐rot fungal hyphae during the growth in the lumen area of plant cells produce oxalic acid, iron‐reducing compounds (RC), and hydrogen peroxide (H2O2). The oxalic acid binds to a Fe3+ ion formatting the complex that diffuses into cell wall along with H2O2 and RC. With the pH change, RC sequesters Fe3+ from the Fe‐oxalate complex and reduces it to Fe2+. Fe2+ then reacts with H2O2 (Fenton reaction) and produces hydroxyl radicals (‐OH). Upon attack of ‐OH radicals, lignocellulose matrix is disrupted. Models for the study of brown‐rot fungi are Gloephyllum trabeum, Coniophora puteana, and Postia placenta 9.
White‐rot fungi are able to decompose all lignocellulose constituents: lignin, cellulose, and hemicellulose. Degradation of lignin is more efficient than in the case of brown‐rot and soft‐rot fungi, because they possess an unique ability to its complete mineralization to CO2 10. Therefore, white‐rot basidiomycetes could be an interesting source of lignocellulose‐active enzymes to supplement the commercial cocktails of hemicellulases and cellulases originated from ascomycetes such as Aspergillus niger or Trichoderma reesei 4. Some of the white‐rot fungi capable of causing selective delignification of wood are Phanerochaete chrysosporium, Phanerochaete carnosa, Pleurotus ostreatus, Pycnoporus cinnabarinus, Botrytis cinerea, Stropharia coronilla, and Trametes versicolor.
3. Degradation of lignocellulose‐containing raw materials by fungal enzymes
Biological degradation of lignocellulose‐containing raw materials employs fungi, mainly belonging to the group of white‐rot and brown‐rot basidiomycetes. It requires long application periods with the rate of fungal degradation that is too low for industrial use and consumes a fraction of the plant polysaccharides 11. More convenient than fungal is enzymatic degradation, which is very selective and fast but also expensive at a large scale. The enzymatic degradation of lignocellulose‐containing raw materials is achieved through the multiple carbohydrate‐active enzymes, usually acting together with complementary, synergistic activities, and modes of action 12. In the following sections, the main enzymes and enzymatic degradation processes of the main lignocellulose constituents are described.
3.1. Lignin degradation by ligninolytic enzymes system
Lignin degradation is an oxidative process mainly attributed to the secondary metabolism, or to restricted availability of carbon, nitrogen, or sulphur, and it is normally not degraded as sole carbon and energy sources 13. In nature, it is generally attributed to the metabolism of basidiomycetes white‐rot fungi, since they degrade lignin more rapidly and extensively than other microorganisms 8. Some white‐rot fungi species that preferentially attack lignin more readily than hemicellulose and cellulose are Ceriporiopsis subvermispora, Phellinus pini, Phlebia sp., Pleurotus sp., Phanerochaete chrysosporium, Trametes versicolor, Heterobasidion annosum, and Irpex lacteus. These fungi produce a set of ligninolytic enzymes that catalyse the oxidation of an array of aromatic substrates, producing aromatic radicals and changing the structure of the lignocellulose‐containing raw materials and lignin.
Lignin consumption is mainly accomplished by laccases, manganese‐dependent peroxidases (MnP), lignin peroxidases (LiP), and versatile peroxidases (VP) that are the major groups of ligninolytic enzymes produced by the white‐rot fungi 7.
Laccases (EC 1.10.3.2; CAZy AA1) are blue multicopper enzymes able to oxidize a variety of phenolic and nonphenolic compounds. From a molecular point of view, they are monomeric, dimeric, or tetrameric glycoproteins, which usually contain four copper atoms per monomer distributed in three redox sites named T1, T2, and T3. In the resting enzymes, all four copper ions are in the 2+ oxidation state. Phenolic compounds, including lignin, polyphenols, methoxy‐substituted phenols, or diamines, are oxidized by one‐electron abstraction that leads to formation of radicals that can repolymerize or cause depolymerization 14. In the presence of a mediator (2,20‐azinobis‐3‐ethylbenzthiazoline‐6‐sulfonate (ABTS)), that behaves as an electron shuttle, these enzymes can oxidize also nonphenolic compounds 15. Because of these characteristics, laccases can be employed for delignification and removal of phenolic compounds in various fields such as biofuels and food production, pulp, and paper treatments, textile industry, nanobiotechnology, soil bioremediation, synthetic chemistry, and cosmetics 16. The most laccases are produced by white‐rot fungi that are secreted into the medium by the mycelium of filamentous fungi 17. Some examples of fungi that produce laccase with high activity are Trametes pubescens (740 000 U/L) 18, Coriolus hirsutus (83 830 U/L) 19, Trametes hirsuta (19 400 U/L) 20, Trametes versicolor (16 000 U/L) 21, Pycnoporus cinnabarinus (10 000 U/L) 22, Neurospora crassa (10 000 U/L) 23, and Pleurotus ostreatus (80 000 U/L) 24.
Besides laccases, other important enzymes that can be used for delignification are peroxidases. Peroxidases by itself are too large to penetrate the dense lignocellulosic matrix. They are thought to generate small molecular radical species that catalyze the oxidation of the lignin 25. Lignin peroxidases (LiPs; EC 1.11.1.14; CAZy AA2), known as peroxide oxidoreductases, oxidizing nonphenolic methoxyl‐substituted lignin units (>90% of the lignin) in the presence of H2O2 26. Manganese‐dependent peroxidases (MnPs; EC 1.11.1.13; CAZy AA2) oxidize a bound Mn2+ ion to Mn3+ in the presence of hydrogen peroxide generating an intermediate redox couple Mn2+/Mn3+. The product Mn3+ is released from the active site in the presence of a chelator (mostly oxalate and malate) that stabilizes it against disproportionation to Mn2+ and insoluble Mn4+. The complex Mn3+ ion can diffuse into the lignified cell wall, where it oxidizes phenolic or nonphenolic lignin components 15.
Versatile peroxidases (VPs; EC 1.11.1.16; CAZy AA2) combine the substrate‐specificity characteristics of the two other ligninolytic peroxidases (LiP and MnP). Unlike these two enzymes, it can oxidize phenolic and nonphenolic substrates including veratryl alcohol, methoxybenzenes, and lignin model compounds 26.
Peroxidases such as HRPs (EC 1.11.1.7, CAZy AA2), secreted by fungus Phanerochaete chrysosporium, are potential biocatalysts for bioremediation of environment polluted by harmful compounds (e.g. endocrine disrupting compounds: 17α‐ethinylestradiol (EE2)) 27. HRPs have also been implicated in the cell wall biosynthesis, indole‐3‐acetic acid (plant growth hormone) catabolism and oxidation of toxic compounds 28.
The lignin degradation can be further enhanced by the action of other enzymes such as: aryl alcohol oxidases (AAO; EC 1.1.3.7.; CAZy AA3) that oxidize many primary alcohols containing an aromatic ring and is described in Pleurotus eryngii 29, glyoxylate oxidase (GOx, EC 1.2.3.5) 30, pyranose 2‐oxidase (glucose 1‐oxidase; EC 1.1.3.4, CAZy AA3), and cellobiose dehydrogenase (CDH, EC 1.1.99.18; CAZy AA3). Fungal aryl‐alcohol dehydrogenases (AAD; EC 1.1.1.90) and quinone reductases (QR, EC 1.6.99.2), tyrosinases (EC.1.14.18.1) and catechol oxidases (EC 1.10.3.1) are also involved in lignin degradation 31, 32, 33. Table 1 shows an overview of fungi and enzymes involved in lignin disruption.
Table 1.
Overview of lignin‐degrading fungi and enzymes involved in lignin modification and degradation 4, 27, 33, 34, 35, 36
| Fungi | Enzyme | EC number | CAZy family |
|---|---|---|---|
| Trametes pubescens, Coriolus hirsutus, Trametes hirsute, Trametes versicolor, Pycnoporus cinnabarinus, Neurospora crassa, Pleurotus ostreatus, Botrytis cinerea | Laccase | EC 1.10.3.2 | AA1 |
| Phanerochaete chrysosporium, Mucor racemosus, Aspergillus sclerotiorum, Cladosporium cladosporioides, Stropharia coronilla, Bjerkandera adusta, Pleurotus eryngii | Manganese peroxidase | EC 1.11.1.13 | AA2 |
| Phanerochaete chrysosporium, Mucor racemosus, Aspergillus sclerotiorum, Cladosporium cladosporioides | Lignin peroxidase | EC 1.11.1.14 | AA2 |
| Pleurotus ostreatus, Pleurotus eryngii | Versatile peroxidase | EC 1.11.1.16 | AA2 |
| Phanerochaete chrysosporium, Pleurotus ostreatus, Pleurotus eryngii | Horseradish peroxidase | EC 1.11.1.7 | AA2 |
| Pleurotus sp.(P. cornucopiae, P. eryngii, P. floridanus, P. pulmonarius, P. ostreatus) | Aryl‐alcohol dehydrogenase | EC 1.1.1.90 | AAD |
| Phanerochaete chrysosporium | Glyoxylate oxidase | EC 1.2.3.5 | AA5 |
| Phanerochaete chrysosporium | Cellobiose dehydrogenase | EC 1.1.99.18 | AA3 |
| Gloeophyllum trabeum | Quinone reductase | EC 1.6.5.2 | QR |
| Pleurotus eryngii | Alcohol oxidase | EC 1.1.3.7 | AA3 |
| Peniophora sp. | Pyranose 2‐oxidase (glucose 1‐oxidase) | EC 1.1.3.4 | AA3 |
| Myceliophthora thermophile, Agaricus bisporus, Pycnoporus sanguinensiss, Trichoderma reesei | Tyrosinases | EC.1.14.18.1 | not defined |
| Aspergillus oryzae | Catechol oxidases | EC 1.10.3.1 | not defined |
Significant amounts of peroxidases have been produced by fungi in the submerged or solid state fermentation. In the submerged culture, the fungus Mucor racemosus produced lignin peroxidases (75 376 U/L) and manganese peroxidases (4484 U/L) 34 while in a solid‐state fermentation of steam exploded wheat straw by Phanerochaete chrysosporium manganese peroxidases (1375 U/L) were observed 35. Submerged fermentation of versatile peroxidases (7300 U/L) was realized by genetically modified Pleurotus ostreatus 36.
3.2. Hemicellulose degradation by hemicellulolytic enzymes system
Hemicellulose hydrolysis demands cooperative action of several types of enzymes working at different levels of the hemicelluloytic matrix. This synergistic activity is necessary not only because of hemicellulose complexity but also because of its connection with the other plant cell wall components. According to their action on distinct substrates two types of enzymes are predominantly involved in hemicellulose degradation: endo‐1,4‐β‐xylanase (EC 3.2.1.8) and exo‐1,4‐β‐xylosidase (EC 3.2.1.37; alternative names: xylan β‐1,4‐xylosidase, 1,4‐β‐D‐xylan xylohydrolase, β‐xylosidase, or xylobiase) 4. Endo‐1,4‐β‐xylanases hydrolyse β‐1,4‐xylan chains, and generate xylo‐oligosaccharides. Most of them belong to CAZy families GH10 and GH11 and some to GH5, GH7, GH8, and GH43 families. Xylan β‐1,4‐xylosidases cleave xylobiose and xylo‐oligosaccharides releasing xylose 4.
Mannan, as the major component of hemicellulose in softwood, is comprised of mannose residues or a combination of mannose and glucose residues also known as glucomannan. β‐Mannanases (endo‐β‐1,4‐mannanase; EC 3.2.1.78) are endohydrolases that hydrolyse mannan fibers by cleaving β‐1,4 bonds and producing new reducing and nonreducing ends. Depending on the active site organization most β‐mannanases are active on oligosaccharides consisting of three or four monomeric units. The hydrolytic action of β‐mannanases on mannan is supported with β‐mannosidase enzymes (exo‐β‐1,4‐mannosidase; EC 3.2.1.25) that carry out hydrolysis of terminal, nonreducing β‐d‐mannose residues. In case of glucomannan degradation, β‐glucosidases can cleave the bond between one mannose and one glucose residue. One should be aware that the action of these enzymes strongly depends on the number and pattern formed by the substituted galactoses and other substitutions and on the action of other enzymes 37.
To efficiently hydrolyse wood xylans and mannans hemicellulose degradation is supported with the help of the other enzymes whose acting synergistically. These accessorial enzymes are acetylxylan esterase (EC 3.1.1.72), feruloyl esterase (EC 3.1.1.73) and p‐coumaroyl esterase (EC 3.1.1.B10), α‐l‐arabinofuranosidase (EC 3.2.1.55), xylan α‐1,2‐glucuronosidase (EC 3.2.1.131), and α‐glucuronidase (EC 3.2.1.139). For the complete degradation of oligomer arabinoxylan, which is one of the components of wheat straw, it is necessary to employ enzyme α‐l‐arabinofuranosidase (EC 3.2.1.55). This enzyme hydrolyses covalent bonds between l‐arabinose and d‐xylose and removes residues substituted at C2 and C3 position of xylose residues 38. Table 2 summarizes the fungi and enzymes involved in hemicellulose disruption.
Table 2.
Overview of fungi and enzymes involved in hemicellulose degradation 4
| Fungi | Enzyme | EC number | CAZy family |
|---|---|---|---|
| Trichoderma longibrachiatum | Endo‐1,4‐β‐xylanase | EC 3.2.1.8 | GH5, GH7, GH8, GH10, GH11, GH43 |
| Aspergillus nidulans | Exo‐1,4‐β‐xylosidase | EC 3.2.1.37 | GH3, GH39, GH43, GH52, GH54 |
| Sclerotium rolfsii | Endo‐β‐1,4‐mannanase | EC 3.2.1.78 | GH5, GH26, GH113 |
| Aspergillus niger | Exo‐β‐1,4‐mannosidase | EC 3.2.1.25 | GH1, GH2, GH5 |
| Aspergillus niger | Feruloyl esterase | EC 3.1.1.73 | CE1 |
| Neocallimastix sp. | p‐Coumaroyl esterase | EC 3.1.1.B10 | CE1 |
| Aspergillus niger, Phanerochaete chrysosporium, | |||
| Rhizomucor miehei | Endo‐α‐1,5‐arabinanase | EC 3.2.1.99 | GH43 |
| Aspergillus niger | α‐L‐arabinofuranosidase | EC 3.2.1.55 | GH43, GH51, GH62 |
| Phanerochaete chrysosporium | α‐Glucuronidase | EC 3.2.1.139 | GH67 |
| Aspergillus fumigatus, Phlebia radiate, Pleurotus ostreatus, Trichoderma sp. (T.hamatum, T. harzianum, T. viride, T. longibrachiatum) | Xylan α‐1,2‐glucuronosidase | EC 3.2.1.131 | GH67 |
| Mortierella vinacea | α‐Galactosidase | EC 3.2.1.22 | GH4, GH27, GH36, GH57, GH97, GH110 |
| Aspergillus nidulans, Aspergillus niger, Aspergillus oryzae | Endo‐galactanase | EC 3.2.1.89 | GH53 |
| Humicola insolvens | β‐Glucosidase | EC 3.2.1.21 | GH1, GH3 |
| Trichoderma reesei | Acetyl esterase | EC 3.1.1.6 | CE16 |
| Aspergilus sp., Schizophyllum commune, Trichoderma reesei | Acetylxylan esterase | EC 3.1.1.72 | CE1, CE2, CE3, CE4, CE5, CE6, CE7, CE12, CE16 |
| Acremonium alcalophilum, Phanerochaete chrysosporium, Trichoderma reesei | Glucuronyl methyl esterase | EC 3.1.1 | CE15 |
3.3. Cellulose degradation by cellulolytic enzymes system
In order to enable utilization of insoluble cellulose as such, multiple enzymatic activities are required. Fungi able to degrade cellulose produce an array of enzymes with different specificities. Different characteristics of the cellulose, like the degree of polymerization (DP), crystallinity, particle size and surface area, influence the efficiency of enzymatic hydrolysis. Fungal cellulose degradation is accomplished by a set of glycoside hydrolase (GH) enzymes with complementary catalytic activities. These enzymes are listed in the carbohydrate‐active enzymes (CAZy) database that provides compilation of carbohydrate‐degrading or ‐modifying enzymes and describes families of structurally related enzymes.
Cellulases have different specificities to hydrolyse the β‐1,4‐glycosidic linkages bonds that connect glucose units in the cellulose fiber. Most of them have an independently folded carbohydrate binding module (CBM) connected to the catalytic domain by a flexible linker. The CBM is responsible for binding the enzyme to the crystalline cellulose. They are divided into three major classes: endoglucanases (endo‐1‐4‐β‐glucanase; EC 3.2.1.4), exoglucanases (EC 3.2.1.91), and β‐glucosidase (EC 3.2.1.21).
Endoglucanases (EC 3.2.1.4) internally cleave β‐1,4‐glycosidic bonds in the amorphous regions of cellulose thereby releasing reducing and nonreducing chain ends. Because of the artificial substrate, used for their detection, they are often called carboxymethylcellulases (CMCase) 39. They belong to CAZy families GH5, GH6, GH7, GH9, GH12, GH45, and GH74. Exoglucanases (EC 3.2.1.91) also known as cellobiohydrolases (CBH), remove dimers (cellobiose) from the end of the cellulose chain. Some CBHs are able to work only on reducing ends while others cleave cellobiose units only at the nonreducing ends 10. They belong to families GH5 and GH6. The main product of cellobiohydrolases is the disaccharide cellobiose, which is cleaved into glucose units by the enzyme β‐glucosidase 7. β‐Glucosidases (EC 3.2.1.21) hydrolyse glucose dimers and in some cases gluco‐oligosaccharides to glucose. They belong to families GH1 and GH3. Except for the above, the role of other enzymes engaged in cellulose degradation is also important (Table 3).
Table 3.
| Fungi | Enzyme | EC number | CAZy family |
|---|---|---|---|
| Trichoderma reesei, Trichoderma harzianum, Aspergillus niger, Pesralotiopsis sp., Phanerochaete chrysosporium, Fomitopsis palustris, Neocallimastix frontalis | Endoglucanase | EC 3.2.1.4 | GH5,GH6, GH7, GH9, GH12, GH45, GH74 |
| Trichoderma reesei, Trichoderma harzianum, Pesralotiopsis sp., Phanerochaete chrysosporium, Fomitopsis palustris | Exoglucanase | EC 3.2.1.91 | GH5, GH6, GH9 |
| Trichoderma reesei, Phanerochaete chrysosporium, Fomitopsis palustris | β‐Glucosidase | EC 3.2.1.21 | GH1, GH2, GH3, GH5, GH9, GH30, GH39, GH116 |
| Penicillium brefeldianum | 1,6‐β‐d‐Glucosidase | EC 3.2.1.75 | GH5, GH30 |
| Rhizopus chinensis | 1,3‐β‐d‐Glucosidase | EC 3.2.1.39 | GH5, GH16, GH17, GH55, GH64,GH81, GH128 |
| Achlya bisexuals | exo‐1,3‐β‐Glucanase | EC 3.2.1.58 | GH3, GH5, GH16, GH17, GH55 |
| Orpinomyces sp. | 1,3‐1,4‐β‐d‐Glucosidase | EC 3.2.1.73 | GH16, GH50, GH86, GH118 |
The synergistic action of these enzymes is essential in the hydrolysis of lignocellulose. It is revealed that except cellulases the other enzymes may have an important role in the degradation (Fig. 1). The recent discovery of oxidative enzymatic processes that augment cellulose degradation prompted the introduction of a new CAZy family, termed as “auxiliary activity (AA)” (before GH61 family) 40. They are known to remarkably improve the hydrolysis of lignocellulose by acting in synergy with other cellulolytic enzymes. Especially the cellulose‐active lytic polysaccharide monooxygenases (LPMOs; CAZy: AA9) attracted attention due to their ability to directly oxidize crystalline substrate surfaces, which extremely enhances the overall degradability of cellulose 41, 42, 43. Several studies have demonstrated that LPMOs are oxidative enzymes acting in synergy with cellobiose dehydrogenases (CDH) that gives a new view on a cellulose degradation 7, 44, 45, 46.
Figure 1.
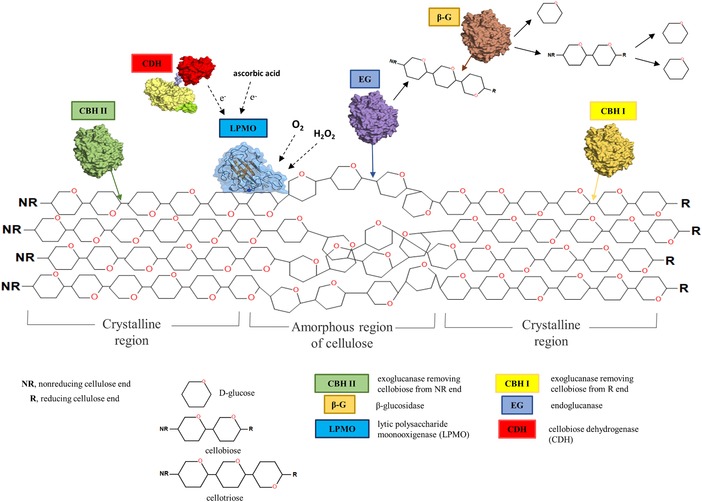
Scheme of the enzymatic degradation of cellulose chain via synergistic interaction of cellulases (endoglucanase, exoglucanase, and β‐glucosidase) and LPMO (AA9 or 10) enzymes.
4. Fungal delignification strategies
Examples of lignin degrading fungi and their enzymes used in various processes are listed in the following section.
A study of Kerem et al. 47 compared lignocellulose degradation ability of two fungi: Pleurotus ostreatus and Phanerochaete chrysosporium, during solid state fermentation on cotton stalks as a substrate. The growth of P. chrysosporium resulted in the loss of 55% of the initial dry organic matter within 15 days of fermentation, while the lignin loss equalled to 35% of the initial lignin content in the substrate. The growth of P. ostreatus resulted in the loss of only 20% of original dry organic matter, while the lignin loss was 45% of the initial lignin content in the substrate.
Li et al. 48 analyzed compositional changes of cottonseed hull substrate during P. ostreatus growth. After 45 days of incubation, lignin content decreased from an initial 17% to a final of 11% of dry matter. Moreover, they performed delignification of wheat straw by Fusarium concolor that is able to produce laccase, LiP and MnP enzymes when grow on a lignocellulosic medium. After 5 days of incubation they observed removal of 13.07% of the lignin and loss of 7.62% of the total polysaccharide fraction 49.
Herpöel et al. 50 investigated wheat straw pulp degradation combining commercial xylanases and laccases from Pycnoporus cinnabarinus, separately, followed by alkaline treatment. This two stage treatment was effective to remove 60% of lignin in wheat straw pulp. Also, xylanases and laccases, previously used for enzyme‐aided bleaching, contribute improvements in the following chemical delignification step 51. The same fungus was used in delignification of lignocellulose‐containing raw materials (Prosopis juliflora/Lantana camara) reported by Gupta et al. 52. The results showed that lignin removal improves the saccharification of cellulose. The fungal delignification was the highest during the first 15 days. An increase of 21.1–25.1 % sugar release was obtained when fungal treated substrates were enzymatically hydrolysed as compared to the hydrolysis of untreated substrates.
During a solid state cultivation on wood chips, the main oxidative enzyme produced by Cereporiopsis subvermispora is manganese peroxidase (MnP). This fungus also secretes a number of low molecular mass compounds including oxalic acid and several fatty acids 53. MnP‐initiated lipid peroxidation reactions can explain degradation of nonphenolic lignin substructures by C. subvermispora. Cellulose is more resistant to the attack by this fungus and their enzymes because this fungus tends to remove lignin in advance of cellulose and hemicellulose. The lack of cellobiohydrolases (CBHs) and the presence of systems able to suppress Fenton's reaction in the cultures can explain nonefficient cellulose degradation by this fungus. Although the low permeability of the wood cell walls to endo‐cellulases, the cellulose depolymerization, induced by low‐molecular weight agents as reported for brown‐rot fungi, should not be excluded. The abilities of C. subvermispora, used for biochemical pulping of agricultural residues (rice, wheat, and barley straws), was compared with chemical process 54. Although the delignification of rice, wheat, and barley straws was not as efficient as chemical process, the quality of papers produced by biochemical pulping of straws was high and satisfactory. The reduction of the amount of chemicals and the mechanical energy used in the process was lower what makes this process a better choice. The same fungus was used in a large‐scale biopulping process of wood chips 55.
A two‐stage fungal biopulping method, studied by Giles et al. 56, was investigated to improve enzymatic hydrolysis of wood for ethanol production. In a two‐stage wood treatment a liquid culture suspension, consisting of white‐rot fungus C. subvermispora and brown‐rot fungus Postia placenta, was used. The treatments resulted in 6 ± 0.5% mass loss and increased the yield of enzymatic hydrolysis by 67–119% 56.
The delignification of wood (Eucalyptus globulus) and nonwood (Pennisetum purpureum) feedstocks employing laccase from Trametes villosa, mediator 1‐hydroxybenzotriazole (HBT) and alkaline extraction was examined by Gutiérrez et al. 57. When using 50 U/g laccase and 2.5% HBT they aimed to remove 48 and 32% of the lignin from E. globulus and P. purpureum, respectively. The enzymatic pretreatment (25 U/g) increased the glucose yields by 61 and 12% in 72 hours and ethanol yields by 4 and 2 g/L in 17 hours, respectively.
Several studies have demonstrated the effectiveness of ligninolytic fungal enzymes to delignify lignocellulose‐containing raw materials. Very promising results have been obtained using ligninolytic enzymes in processes such as delignification and bleaching systems. Unfortunately, there is still no real concept capable to fully implement such enzymes into industry because of its high costs of application, limitations in performance, and technical feasibility, which depends on the enzymatic system. Possible alternatives to these existing enzymatic concepts could be:
oxidation system mediated with lipases, special ketone compounds, fatty acid or fat compounds, and H2O2
new enzymatic approaches with methods that generate reactive oxygen species (ROS) or reactive nitrogen species (RNS) 58.
Call and Call 59 reported a new generation of enzymatic systems for delignification and bleaching, as already mentioned. They developed two systems: one mediated oxidoreductase system is capable to delignify with the aid of the active components: peroxynitrous acid (PNA) or dicyclopentadienyl transition metal complexes (ferrocene) for obtained peroxide activation and another with special generated organosulphonic peracids or enzymatically activated sulphite that can generate in combination with ketones dioxirane 8.
5. Carbohydrate active enzymes (CAZymes)
The large diversity of monosaccharides, the multiple types of intersugar linkages and the fact that almost all organic macromolecules can be glycosylated results in an enormous amount of carbohydrate structures and conjugates 60. Furthermore, since all such carbohydrates must both be synthesized and broken down, the amount and especially the complexity of enzymes performing such activities is enormous. Enzymes that are involved in the synthesis, modification, or breakdown of glycoconjugates or complex polysaccharides are summarized as called Carbohydrate‐Active enZymes (CAZymes; the CAZy database http://www.cazy.org/) 61. The CAZy classification groups the proteins in families according to amino acid sequence similarity and was introduced to obtain a classification system that was more meaningful than the EC system, which is solely based on the reaction mechanism. Due to the modular structure of many CAZymes it is possible to find one protein in several families 62.
In 2008, the CAZy database covered approximately 300 protein families divided into five classes: glycoside hydrolases, glycosyltransferases, polysaccharide lyases, carbohydrate esterases, and noncatalytic carbohydrate‐binding modules. This database grows progressively and is constantly updated with new sequence information, 3D structures and biochemical characterizations 62. In 2013, a novel enzyme class was introduced, covering redox‐enzymes that work in concert with CAZymes, which have been named Auxiliary Activites 40, 62. Currently, the CAZy database holds about 400 protein families divided into six classes and provides a consistent nomenclature for CAZymes.
Glycosyltranferases (GTs) are responsible for the enzymatic formation of glycosidic bonds using an activated donor sugar substrate with a phosphate leaving group. Other sugars or lipids, proteins, nucleic acids, and small molecules can act as the acceptor substrate 63. GTs show great diversity in donor, acceptor, and product specificity and can potentially generate an infinite number of glucoconjugates, oligo‐, and polysaccharides 64.
Carbohydrate esterases (CEs) are a class of CAZymes that remove ester‐based modifications by de‐O or de‐N acylation of a substituted saccharide in a hydrolytic manner.
Polysaccharide lyases (PLs) use β‐elimination instead of a hydrolytic mechanism to cleave uronic acid containing polysaccharides. PLs form a complimentary strategy to the degradation of C‐6 carbohylated polysaccharides by glycoside hydrolases 65.
Glycoside hydrolases (GHs) form the enzyme class with most families, comprising 153 at present. These enzymes are responsible for the hydrolysis of glycosidic bonds between two carbohydrate moieties or one carbohydrate and one noncarbohydrate moiety. The variation of activities in the GH family is large, including enzymes that predominantly target insoluble substrates, soluble oligosaccharides of variable or strictly defined chain length and branch points of branched polysaccharides. GH activity on polysaccharides can be endo‐ or exo‐, reffering to their ability to cleave the polysaccharide chain randomly or from the chain end.
A noncatalytic class of proteins found in the CAZy database are the carbohydrate‐binding modules (CBMs). CBMs are connected with other CAZymes in multimodular structures and promote association with the substrate. By recognizing and binding the target structure, the catalytic domain is brought in close proximity to the substrate that may potentiate catalysis 61. CBMs recognize their target structure within their natural context, e.g. the plant cell wall 66. Interestingly, binding to nonsubstrate polysaccharides in an intact plant cell wall, potentiates degradation of the substrates as well by means of the proximity effect 67.
As already mentioned, AAs are the latest addition to the CAZy database. AAs involve proteins that are potentially able to aid other CAZymes in degrading a complex substrate. Hence, they comprise a wide array of enzymes that are active on polysaccharides and nonpolysaccharides like lignin, which, without exception is found in combination with polysaccharides in the plant cell wall 40. This class of enzymes includes laccases, cellobiose dehydrogenases (CDHs), copper radical oxidases, and other enzymes that utilize a redox mechanism. LPMOs are enzymes that were previously classified as family 61 of the GHs and family 33 of the carbohydrates‐binding modules. The finding that these proteins were oxidative enzymes acting on chitin 68 or cellulose 44, 69, 70 was one major reason for extending the CAZy database in order to reclassify these proteins. These enzymes work in synergy with many GHs and stimulate their activity by increasing the accessibility to the substrate 42.
6. Lytic polysaccharide monooxygenase enzymes
LPMOs are copper‐dependent enzymes that catalyze the oxidative cleavage of a glycosidic bond in the presence of hydrogen peroxide or dioxygen and an external electron donor 71. They are able to degrade insoluble polysaccharides such as crystalline cellulose, chitin and starch 71, 72, 73, 74. Also, they depolymerise noncrystalline or soluble hemicellulosic substrates such as xyloglucan, xylan, and beta‐glucans 75. LPMOs are important in biomass conversion because they act in synergy with glycoside hydrolase (GH), thereby enhancing overall polysaccharide conversion efficiency. Although these enzymes have been intensely investigated since their discovery in 2010, several aspects of their catalytic mechanism and their mode of action remain unclear. LPMOs are abundant and show high sequence diversity, which suggests functional roles beyond biomass degradation.
They are currently categorized as families 9, 10, 11, 13 14, and 15 of the auxiliary activities. Family AA9 contains only fungal enzymes that were previously referred to as GH61. Family AA10 proteins (previously CBM33) are found in all domains of life, namely archaea, bacteria, and eukaryote. Family AA11 has mainly fungal members while AA13 exclusively comprises fungal LPMOs. The latest addition to the auxiliary activities was when Couturier et al. identified the existence of a previously unknown family of LPMOs. This new family, named AA14, is distantly related to other LPMO families with the main activity to cleave xylan with oxidation of C‐1, which has been demonstrated for two AA14 LPMOs from fungus Pycnoporus coccineus 75. Likewise, another new family was recently created in CAZy database after Sabbadin et al. showed the copper‐dependent LPMO activity of two AA15 enzymes from Thermobia domestica 76.
The broad occurrence of LPMOs indicates biologically important roles that may include tasks beyond breaking down cellulosic and chitinous materials. Genomes of biomass degrading fungi usually encode several LPMO genes with numbers up to over 40. The transcription and expression of fungal LPMOs are influenced by the growth conditions of the organism and seem to be upregulated in the presence of biomass 76, 77, 78.
6.1. Substrate preferences
The first LPMO activity discovered was the oxidative cleavage of crystalline β‐chitin by CBP21 (chitin‐binding protein) 68. Another study on this enzyme revealed activity on crystalline α‐chitin, yet to a lower extent, and that synergy with Serratia marcescens chitinases decreased with a lowering degree of crystallinity of the substrate 79. Further studies uncovered activity of an AA10 from Streptomyces coelicolor and an array of AA9s on cellulose 44, 46, 69. In subsequent studies, LPMO activity on additional substrates has been revealed. For example, NcLPMO9C from Neurospora crassa exhibits not only activity on crystalline cellulose but also on soluble cello‐oligomers 80. The same LPMO was found to cleave β‐1,4‐glucan bonds in hemicellulose, in particular xyloglucan, showing its ability to accept substitutions in various positions in the β‐glucan backbone 81. Later, it was shown that an AA9 from Myceliophora thermophila (MtLPMO9A) is active on xylan‐coated cellulose and cleaves the β‐1,4‐xylosyl bonds in xylan as well as the β‐1,4‐glucosyl bonds in cellulose 82. All these substrates show a common feature, namely the β‐1,4 linkages connecting the single sugar moieties in the backbone. Vu et al. 83 discovered that LPMOs were not only restricted to cleave β‐1,4 linkages by showing activity of an N. crassa family AA13 LPMO toward starch (i.e. α‐1,4 bonds). Later, a starch active LPMO was also identified from the fungus Aspergillus nidulans 84. Thus, LPMO substrates are indeed more diverse that initially assumed. A fast and sensitive spectrophotometric (using 2,6‐dimethoxyphenol as chromogenic substrate and H2O2 as cosubstrate) assay for LPMO activity determination was developed to follow the production and purification as well as to study enzyme‐binding constants or thermal stability 85.
7. Concluding remarks
Fungi involved in complex lignocellulose‐containing raw materials degradation express a broad spectrum of enzymes. According to the enzyme composition and degradation mechanisms, three groups of fungi are described: soft‐rot, brown‐rot, and white‐rot fungi. A huge effort has been done and is still ongoing to classify and group these fungi enzymes in a central database called the Carbohydrate‐Active enzyme database (or short CAZy). The CAZy classification groups the proteins in families according to amino acid sequence similarity.
In the last few years, investigation attention was focused on the synergistic action of the fungal enzymes involved in the lignocellulose degradation. One of the key players in those actions are LPMOs. These enzymes work in synergy with glycoside hydrolases (GHs) and stimulate their activity by increasing the accessibility to the substrate and enhancing overall polysaccharide conversion efficiency. In addition, LPMOs are able to degrade insoluble polysaccharides such as crystalline cellulose and soluble cello‐oligomers. Importance of these enzymes is also confirmed by the phylogenetic investigations. Genomes of lignocelluloses degrading fungi usually encode several LPMOs gens.
Many cultivation strategies were studied to enhance efficiency of lignocellulose‐containing raw materials degradation whereas delignification was indicated as the main barrier for the successful lignocellulose utilization. To remove lignin and enhance hemicellulose and cellulose degradation, one of the strategies was the solid state cultivation of brown‐rot and white‐rot fungi. This strategy approved the hypotheses of the synergistic action of the fungal enzymes and enhancement of the delignification efficiency. However, for the industrial utilization of the fungi and their enzymes more collaborative effort and synergy has to be attained between molecular and genetic biotechnologies, enzymologist, and bioprocess engineers.
The authors have declared no conflict of interest.
Acknowledgments
This work was financially supported by the Croatian Science Foundation under the project No. 9158 “Sustainable production of bioethanol and biochemicals from agricultural waste lignocellulosic raw materials.”
8 References
- 1. Stech, M. , Hust, M. , Schulze, C. , Dübel, S. and Kubick, S. , Cell‐free eukaryotic systems for the production, engineering, and modification of scFv antibody fragments. Eng. Life Sci. 2014, 14, 387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schirmaier, C. , Jossen, V. , Kaiser, S. C. , Jüngerkes, F. et al., Scale‐up of adipose tissue‐derived mesenchymal stem cell production in stirred single‐use bioreactors under low‐serum conditions. Eng. Life Sci. 2014, 14, 292–303. [Google Scholar]
- 3. Cocinero, E. J. , Gamblin, D. P. , Davis, B. G. , Simons, J. P. , The building blocks of cellulose: the intrinsic conformational structures of cellobiose, its epimer, lactose, and their singly hydrated complexes. J. Am. Chem. Soc. 2009, 131, 11117–11123. [DOI] [PubMed] [Google Scholar]
- 4. Sánches, C. , Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnol. Adv. 2009, 27, 185–194. [DOI] [PubMed] [Google Scholar]
- 5. Chen, H. , Chemical composition and structure of natural lignocellulose, in: Chen H. (Ed.), Biotechnology of Lignocellulose: Theory and Practice, Springer Science, Netherlands: 2014, pp. 25–71. [Google Scholar]
- 6. Jaramillo, P. M. D. , Gomes, H. A. R. , Monclaro, A. V. , Silva, C. O. G. et al, Lignocellulosedegrading enzymes: An overview of the global market, in: Gupta V. K., Mach R. L. and Sreenivasaprasad S. (Eds.), Fungal Biomolecules: Sources, Applications and Recent Developments, John Wiley & Sons, Ltd., Chichester, UK: 2015, pp. 73–85. [Google Scholar]
- 7. Kracher, D. , Ludwig, R. , Cellobiose dehydrogenase: An essential enzyme for lignocellulose degradation in nature—A review. J. Land. Manag. Food Enviro. 2016, 67, 145–163. [Google Scholar]
- 8. Woiciechowski, A.L. , Porto de Souza Vandenberghe, L. , Karp, S. G. et al., The pretreatment step in lignocellulosic biomass conversion: Current systems and new biological systems, in: Faraco V. (Ed.), Lignocellulose Conversion: Enzymatic and Microbial Tools for Bioethanol Production, Springer, Verlag Berlin Heidelberg: 2013. [Google Scholar]
- 9. Arantes, V. , Goodell, B. , Current understanding of brown‐rot fungal biodegradation mechanisms: A review, in: Schultz T. P., Goodell B. and Nicholas D. D. (Eds.), Deterioration and Protection of Sustainable Biomaterials, American Chemical Society, Mississippi, 2014, pp. 4–21. [Google Scholar]
- 10. Couturier, M. , Berrin, J.‐G. , The saccharification step: The main enzymatic components, in: Faraco V. (Ed.), Lignocellulose Conversion: Enzymatic and Microbial Tools for Bioethanol Production, Springer, Verlag Berlin Heidelberg: 2013. [Google Scholar]
- 11. Salvachúa, D. , Prieto, A. , López‐Abelairas, M. , Lu‐Chau, T. , et al, Fungal pretreatment: An alternative in second‐generation ethanol from wheat straw. Bioresour. Technol. 2011, 102, 7500–7506. [DOI] [PubMed] [Google Scholar]
- 12. Payne, C.M. , Knottm B.C., Mayes, H.B. , Hansson H, et al., Fungal cellulases. Chem. Rev. 2015, 115, 1308–1448. [DOI] [PubMed] [Google Scholar]
- 13. Silva, I. S. , Menezes, C. R. , Franciscon, E. , Santos, E. C. , et al, Degradation of lignosulfonic and tannic acids by ligninolytic soil fungi cultivated under microaerobic conditions. Braz. Arch. Biol. Techn. 2010, 53, 693–699. [Google Scholar]
- 14. Dwivedi, P. , Vivekanand, V. , Pareek, N. , Sharma, A. , et al, Co‐cultivation of mutant Penicillium oxalicum SAUE‐3.510 and Pleurotus ostreatus for simultaneous biosynthesis of xylanase and laccase under solid‐state fermentation. New Biotechnol. 2011, 28, 616–626. [DOI] [PubMed] [Google Scholar]
- 15. Cragg, S. M. , Beckham, G. T. , Bruce, N. C. , Bugg, T. D. H. et al., Lignocellulose degradation mechanisms across the Tree of Life. Curr. Opin. Chem. Biol. 2015, 29, 108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Couto, S. R. , Herrera, J. L. T. , Industrial and biotechnological applications of laccases: A review. Biotechnol Adv. 2006, 24, 500–513. [DOI] [PubMed] [Google Scholar]
- 17. Couto, S. R. , Herrera, J. L. T. , Laccase production at reactor scale by filamentous fungi. Biotechnol. Adv. 2007, 25, 558–569. [DOI] [PubMed] [Google Scholar]
- 18. Galhaup, C. , Wagner, H. , Hinterstoisser, B. , Haltrich, D. , Increased production of laccase by the wood‐degrading basidiomycete Trametes pubescens . Enzyme. Microb. Technol. 2002, 30, 529–536. [Google Scholar]
- 19. Koroleva, O. V. , Gavrilova, V. P. , Stepanova, E. V. , Lebedeva, V. I. et al., Production of lignin modifying enzymes by cocultivated white‐rot fungi Cerrena maxima and Coriolus hirsutus and characterization of laccase from Cerrena maxima . Enzyme Microb Technol. 2002, 30, 573–580. [Google Scholar]
- 20. Rodríguez‐Couto, S. , Rodríguez, A. , Paterson, R. R. M. , Lima, N. , et al, High laccase activity in a 6 L airlift bioreactor by free cells of Trametes hirsuta . Lett. Appl. Microbiol. 2006, 42, 612–616. [DOI] [PubMed] [Google Scholar]
- 21. Font, X. , Caminal, G. , Gabarrell, X. , Romero, S. et al, Black liquor detoxification by laccase of Trametes versicolor pellets. J. Chem. Technol. Biotechnol. 2003, 78, 548–554. [Google Scholar]
- 22. Meza, J. C. , Sigoillot, J. C. , Lomascolo, A. , Navarro, D. et al., New process for fungal delignification of sugar‐cane bagasse and simultaneous production of laccase in a vapor phase bioreactor. J. Agric. Food Chem. 2006, 54, 3852–3858. [DOI] [PubMed] [Google Scholar]
- 23. Luke, A. K. , Burton, S. G. , A novel application for Neurospora crassa: Progress from batch culture to a membrane bioreactor for the bioremediation of phenols. Enzyme. Icrob. Technol. 2001, 29, 348–356. [Google Scholar]
- 24. Lettera, V. , Del Vecchio, C. , Piscitelli, A. , Sannia, G. , Low impact strategies to improve ligninolytic enzyme production in filamentous fungi: The case of laccase in Pleurotus ostreatus . Comptes. Rendus. Biol. 2011, 334, 781–788. [DOI] [PubMed] [Google Scholar]
- 25. Cullen, D , Kersten, P. J. , Enzymology and molecular biology of lignin degradation in: Brambl R., Marzluf G.A. (Eds.), The Mycota III. Biochemistry and Molecular Biology, 2nd edn Springer, New York, 2004, pp. 249–273. [Google Scholar]
- 26. Wong, D. W. S. , Structure and action mechanism of ligninolytic enzymes. Appl. Biochem. Biotech. 2009, 157, 174–209. [DOI] [PubMed] [Google Scholar]
- 27. Rathner, R. , Petz, S. , Tasnádi, G. , Koller, M. et al. Monitoring the kinetics of biocatalytic removal of the endocrine disrupting compound 17a‐ethinylestradiol from differently polluted wastewater bodies. J. Environ. Chem. Engin. 2017, 5, 1920–1926. [Google Scholar]
- 28. Falade, A. O. , Nwodo, U. U. , Iweriebor, B. C. , Green, E. et al., Lignin peroxidase functionalities and prospective applications. Microbiol. Open. 2017, 6, e00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guillén, F. , Martinez, A. T. , Martinez, M. J. , Substrate specificity and properties of the aryl‐alcohol oxidase from the ligninolytic fungus Pleurotus eryngii . Eur. J. Biochem. 1992, 209, 603–611. [DOI] [PubMed] [Google Scholar]
- 30. Kersten, P. , Cullen, D. , Extracellular oxidative systems of the lignin‐degrading Basidiomycete Phanerochaete chrysosporium . Forest. Genet. Biol. 2007, 44, 77–87. [DOI] [PubMed] [Google Scholar]
- 31. Guillén, F. , Martínez, M. J. , Muñoz, C. , Martínez, A. T. , Quinone redox cycling in the ligninolytic fungus Pleurotus eryngii leading to extracellular production of superoxide anion radical. Arch. Biochem. Biophys. 1997, 339, 190–199. [DOI] [PubMed] [Google Scholar]
- 32. Gutiérrez, A. , Caramelo, L. , Prieto, A. , Martínez, M. J. , et al., Anisaldehyde production and aryl‐alcohol oxidase and dehydrogenase activities in ligninolytic fungi of the genus Pleurotus.Appl. Environ. Microbiol. 1994, 60, 1783–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Frommhagen, M. , Mutte, S. K. , Westphal, A. H. , Koetsier, M. J. et al., Boosting LPMO driven lignocellulose degradation by polyphenol oxidase activated lignin building blocks. Biotechnol. Biofuels 2017, 10, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bonugli‐Santos, R. C. , Durrant, L. R. , Silva, M. , Sette, L. D. , Production of laccase, manganese peroxidase and lignin peroxidase by Brazilian marine‐derived fungi. Enzyme. Microb. Technol. 2010, 46, 32–37. [Google Scholar]
- 35. Fujian, X. , Hongzhang, C. , Zuohu, L. , Solid state production of lignin peroxidase (LiP) and manganese peroxidase (MnP) by Phanerochaete chrysosporium using steam‐exploded straw as substrate. Bioresour. Technol. 2001, 80, 149–151. [DOI] [PubMed] [Google Scholar]
- 36. Tsukihara, T. , Honda, Y. , Sakai, R. , Watanabe, T. et al., Exclusive overproduction of recombinant versatile peroxidase MnP2 by genetically modified white‐rot fungus, Pleurotus ostreatus . J. Biotechnol. 2006, 126, 431–439. [DOI] [PubMed] [Google Scholar]
- 37. Moreira, L. R. , Filho, E. X. , An overview of mannan structure and mannan‐degrading enzyme systems. Appl. Microbiol. Biotechnol. 2008, 79, 165–178. [DOI] [PubMed] [Google Scholar]
- 38. De Vries, R. , Kester, H. , Poulsen, C. , Benen, J. et al., Synergy between enzymes from Aspergillus involved in the degradation of plant cell wall polysaccharides. Carbohydr. Res. 2000, 327, 401–410. [DOI] [PubMed] [Google Scholar]
- 39. Dashtban, M. , Schraft, H. , Qin, W. , Fungal bioconversion of lignocellulosic residues; opportunities & perspectives. Int. J. Biol. Sci. 2009, 5, 578–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Levasseur, A. , Drula, E. , Lombard, V. , Coutinho, P. M. , et al., Expansion of the enzymatic repertoire of the CAZy database to integrate auxiliary redox enzymes. Biotechnol. Biofuels 2013, 6, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bey, M. , Zhou, S. , Poidevin, L. , Henrissat, B. et al., Cello‐oligosaccharide oxidation reveals differences between two lytic polysaccharide monooxygenases (Family GH61) from Podospora anserina . Appl. Environ. Microbiol. 2013, 79, 488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Horn, S. J. , Vaaje‐Kolstad, G. , Westereng, B. , Eijsink, V. G. , Novel enzymes for the degradation of cellulose. Biotechnol. Biofuels 2012, 5, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dimarogona, M. , Topakas, E. , Christakopoulos, P . Cellulose degradation by oxidative enzymes. Comp. Struct. Biotech. J. 2012, 2, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Quinlan, R. J. , Sweeney, M. D. , Lo Leggio, L. , Otten, H. et al., Insights into the oxidative degradation of cellulose by a copper metalloenzyme that exploits biomass components. Proc. Natl. Acad. Sci. USA 2011, 108, 15079–15084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Westereng, B. , Ishida, T. , Vaaje‐Kolstad, G. , Wu, M. et al., The putative endoglucanase PcGH61D from Phanerochaete chrysosporium is a metal‐dependent oxidative enzyme that cleaves cellulose. PLoS ONE 2011, 6, e27807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Langston, J. A. , Shaghasi, T. , Abbate, E. , Xu, F. et al., Oxidoreductive cellulose depolymerization by the enzymes cellobiose dehydrogenase and glycoside hydrolase 61. Appl. Environ. Microbiol. 2011, 77, 7007–7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kerem, Z. , Friesem, D. , Hadar, Y. , Lignocellulose degradation during solid‐state fermentation: Pleurotus ostreatus versus Phanerochaete chrysosporium . Appl. Environ. Microbiol. 1992, 58, 1121–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li, X. , Pang, Y. , Zhang, R. , Compositional changes of cottonseed hull substrate during P. ostreatus growth and the effects on the feeding value of the spent substrate. Bioresour. Technol. 2001, 80, 157–161. [DOI] [PubMed] [Google Scholar]
- 49. Li, L. , Li, X. Z. , Tang, W. Z. , Zhao, J. et al., Screening of a fungus capable of powerful and selective delignification on wheat straw. Soc. Appl. Microbiol. Lett. Appl. Microbio. 2008, 47, 415–420. [DOI] [PubMed] [Google Scholar]
- 50. Herpoël, I. , Jeller, H. , Fang, G. , Petit‐Conil, M. et al., Efficient enzymatic delignification of wheat straw pulp by a sequential xylanaselaccase mediator treatment. J. Pulp. Pap. Sci. 2002, 28, 3. [Google Scholar]
- 51. Viikari, L. , Kantelinen, A. , Sundquist, J. , Linko, M. , Xylanases in bleaching: from an idea to the industry. FEMS Microbiol. Rev. 1994, 13, 335–350. [Google Scholar]
- 52. Gupta, R. , Mehta, G. , Khasa, Y. P. , Kuhad, R. C. , Fungal delignification of lignocellulosic biomass improves the saccharification of cellulosics. Biodegradation 2011, 22, 797–804. [DOI] [PubMed] [Google Scholar]
- 53. Aguiar, A. , Souza‐Cruz, P. B. , Ferraz, A. , Oxalic acid, Fe3+‐reduction activity and oxidative enzymes detected in culture extracts recovered from Pinus taeda wood chips biotreated by Ceriporiopsis subvermispora . Enzyme. Microb. Technol. 2006, 38, 873–878. [Google Scholar]
- 54. Yaghoubi, K. , Pazouki, M. , Shojaosadati, S. A. , Variable optimization for biopulping of agricultural residues by Ceriporiopsis subvermispora . Bioresour. Technol. 2008, 99, 4321–4328. [DOI] [PubMed] [Google Scholar]
- 55. Ferraz, A. , Guerra, A. , Mendonça, R. , Masarin, F. et al., Technological advances and mechanistic basis for fungal biopulping. Enzyme Microb. Tech. 2008, 43, 178–185. [Google Scholar]
- 56. Giles, R. L. , Galloway, E. R. , Elliott, G. D. , Parrow, M. W. , Two stage fungal biopulping for improved enzymatic hydrolysis of wood. Bioresour. Technol. 2011, 102, 8011–8016. [DOI] [PubMed] [Google Scholar]
- 57. Gutiérrez, A. , Rencoret, J. , Cadena, E. M. , Rico, A. et al., Demonstration of laccase‐based removal of lignina from wood and non‐wood plant feedstocks. Bioresour. Technol. 2012, 119, 114–122. [DOI] [PubMed] [Google Scholar]
- 58. Call, H. P. , New developments in enzyme assisted delignification and bleaching, in: Proceedings/Abstracts, 8th International Conference On Biotechnology In The Pulp And Paper Industry, Helsinki, 2001.
- 59. Call, H. P. , Call, S. , New generation of enzymatic delignification and bleaching. Pulp. Pap. Can. 2005, 106, 45–48. [Google Scholar]
- 60. Laine, R. A. , A calculation of all possible oligosaccharide isomers both branched and linear yields 1.05 × 1012 structures for a reducing hexasaccharide: the isomer barrier to development of single‐method saccharide sequencing or synthesis systems. Glycobiology 1994, 4, 759–767. [DOI] [PubMed] [Google Scholar]
- 61. Cantarel, B. L. , Coutinho, P. M. , Rancurel, C. , Bernard, T. et al., The carbohydrate‐active enzymes database (cazy): An expert re‐ source for glycogenomics. Nucleic Acids Res. 2009, 37, 233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lombard, V. , Ramulu, H. G. , Drula, E. , Coutinho, P. M. , et al., The carbohydrate‐active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lairson, L. L. , Henrissat, B. , Davies, G. J. , Withers, S. G. , Glycosyltransferases: Structures, functions, and mechanisms. Annu. Rev. Biochem. 2008, 77, 521–555. [DOI] [PubMed] [Google Scholar]
- 64. Coutinho, P. M. , Deleury, E. , Davies, G. J. , Henrissat, B. , An evolving hierarchical family classification for glycosyltransferases. J. Mol. Biol. 2003, 328, 307–317. [DOI] [PubMed] [Google Scholar]
- 65. Lombard V, Bernard T, Rancurel C, Brumer H, Coutinho, P. M. , Henrissat B. A hierarchical classification of polysaccharide lyases for glycogenomics. Biochem. J. 2010,. 432, 437–444. [DOI] [PubMed] [Google Scholar]
- 66. Boraston, A. B. , Bolam, D. N. , Gilbert, H. J. , Davies, G. J. , Carbohydrate‐binding modules: Fine‐tuning polysaccharide recognition. Biochem. J. 2004, 382, 769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hervé, C. , Rogowski, A. , Blake, A. W. , Marcus, S. E. et al., Carbohydrate‐binding modules promote the enzymatic deconstruction of intact plant cell walls by targeting and proximity effects. Proc. Natl. Acad. Sci. USA 2010, 107, 15293–15298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Vaaje‐Kolstad, G. , Westereng, B. , Horn, S.J. , Liu, Z. et al., An oxidative enzyme boosting the enzymatic conversion of recalcitrant polysaccharides. Science 2010, 330, 219–222. [DOI] [PubMed] [Google Scholar]
- 69. Forsberg, Z. , Vaaje‐Kolstad, G. , Westereng, B. , Bunæs, A. C. et al., Cleavage of cellulose by a CBM33 protein. Protein Sci. 2011, 20, 1479–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Phillips, C. M. , Beeson, W. T. , Cate, J. H. , Marletta, M. A. , Cellobiose dehydrogenase and a copper‐dependent polysaccharide monooxygenase potentiate cellulose degradation by Neurospora crassa . ACS Chem. Biol. 2011, 6, 1399–1406. [DOI] [PubMed] [Google Scholar]
- 71. Sabbadin, F. , Hemsworth, G. R. , Ciano, L. , Henrissat, B. et al. An ancient family of lytic polysaccharide monooxygenases with roles in arthropod development and biomass digestion. Nat. Comm. 2018, 9, 756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bissaro, B. , Røhr, Å. K. , Müller, G. , Chylenski, P. et al., Oxidative cleavage of polysaccharides by monocopper enzymes depends on H2O2. Nat. Chem. Biol. 2017, 13, 1123–1128. [DOI] [PubMed] [Google Scholar]
- 73. Vaaje-Kolstad, G. , Forsberg, Z. , Loose, J. S. , Bissaro, B. et al., Structural diversity of lytic polysaccharide monooxygenases. Curr. Opinion Struc. Biol. 2017, 44, 67–67. [DOI] [PubMed] [Google Scholar]
- 74. Hemsworth, G. R. , Johnston, E. M. , Davies, G. J. , Walton, P. H. et al., Lytic Polysaccharide Monooxygenases in Biomass Conversion. Trends Biotechnol. 2015, 33, 747–761. [DOI] [PubMed] [Google Scholar]
- 75. Couturier, M. , Ladevèze, S. , Sulzenbacher, G. , Ciano, L. et al., Lytic xylan oxidases from wood‐decay fungi unlock biomass degradation. Nat. Chem. Biol. 2018, 14, 306–310. [DOI] [PubMed] [Google Scholar]
- 76. Eastwood, D. C. , Floudas, D. , Binder, M. , Majcherczyk, A. et al., The plant cell wall decomposing machinery underlies the functional diversity of forest fungi. Science 2011, 333, 762–765. [DOI] [PubMed] [Google Scholar]
- 77. Berka, R. M. , Grigoriev, I. V. , Otillar, R. , Salamov, A. et al., Comparative genomic analysis of the thermophilic biomass‐degrading fungi Myceliophthora thermophila and Thielavia terrestris . Nat. Biotechnol. 2011, 29, 922–927. [DOI] [PubMed] [Google Scholar]
- 78. Yakovlev, I. , Vaaje‐Kolstad, G. , Hietala, A. M. , Stefanczyk, E. et al Substrate‐specific transcription of the enigmatic GH61 family of the pathogenic white‐rot fungus Heterobasidion irregulare during growth on lignocellulose. Appl. Microbiol. Biot. 2012, 95, 979–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Nakagawa, Y. S. , Eijsink, V. G. H. , Totani, K. , Vaaje‐Kolstad, G. , Conversion of alpha‐chitin substrates with varying particle size and crystallinity reveals substrate preferences of the chitinases and lytic polysaccharide monooxygenase of Serratia marcescens . J. Agric. Food Chem. 2013, 61, 11061–11066. [DOI] [PubMed] [Google Scholar]
- 80. Isaken, T. , Westereng, B. , Aachmann, F. L. , Agger, J. W. et al., A C4‐oxidizing lytic polysaccharide monooxygenase cleaving both cellulose and cello‐oligosaccharides. J. Biol. Chem. 2014, 289, 2632–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Agger, J. W. , Isaken, T. , Várnai, A. , Vidal‐Melgosa, S. et al., Discovery of LPMO activity on hemicelluloses shows the importance of oxidative processes in plant cell wall degradation. Proc. Natl. Acad. Sci. USA 2014, 111, 6287–6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Frommhagen, M. , Sforza, S. , Westphal, A. H. , Visser, J. et al., Discovery of the combined oxidative cleavage of plant xylan and cellulose by a new fungal polysaccharide monooxygenase. Biotechnol. Biofuels 2015, 8, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Vu, V. V. , Beeson, W. T. , Phillips, C. M. , Cate, J. H. , Marletta, M. A. , Determinants of regioselective hydroxylation in the fungal polysaccharide monooxygenases. J. Am. Chem. Soc. 2014, 136, 562–565. [DOI] [PubMed] [Google Scholar]
- 84. Lo Leggio, L. , Simmons, T. J. , Poulsen, J. C. N. , Frandsen, K. E. H. et al., Structure and boosting activity of a starch‐degrading lytic polysaccharide monooxygenase. Nat. Comm. 2015, 6, 596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Breslmayr, E. , Hanžek, M. , Hanrahan, A. , Leitner, C. , Kittl, R. , Šantek, B. , Oostenbrink, C. , Ludwig, R. , A fast and sensitive activity assay for lytic polysaccharide monooxygenase. Biotechnol. Biofuels 2018, 11, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]


