Abstract
Thermostable enzymes (thermozymes) have been recognized as extremophilic compounds with a greatest biotechnological importance in different industrial areas. Quite recently exopolysaccharides (EPSs) synthesized by thermophiles became an object of increased research interest due to their unique properties appropriate for some specific industrial needs. Thermophilic producers of biotechnologically valuable enzymes and novel EPS were isolated by our group from Bulgarian thermal springs with a diverse geotectonic origin and different water properties. Laboratory reactor processes for their production were developed in batch and continuous cultures. Some of the synthesized thermostable enzymes were among the first described in their groups, for example, the single known thermostable gellan lyase that demonstrated specific activity higher than that of the mesophilic enzymes. Isolated by us thermostable xylanase was able to degrade more than 60% of beechwood xylan in a coprocess with an archaeal β‐xylosidase. Lipase purified by us was active between 55 and 90°C with an optimum at 75–80°C in a large pH range. It was able to degrade a broad range of substrates. Isolates from Bulgarian hot springs synthesized EPS with novel composition and high thermostability. Thus, Bulgarian hot springs harbor a wide set of thermophilic producers of novel enzymes and EPS with potential for a large number of biotechnological applications.
Keywords: Biotechnological significance, Exopolysaccharides, Reactor processes, Thermophilic bacteria, Thermostable enzymes
Abbreviation
- EPSs
exopolysaccharides
1. Introduction
Thermophiles are a type of extremophiles, growing at relatively high temperatures, that is, between 45 and 122°C 1. Extremophiles not only survive but are also functionally active in some of the most extreme conditions found on Earth, under which most organisms such as human cannot survive 2. The following groups of thermophiles are commonly suggested based on their optimal growth temperature: moderate thermophiles growing between 50 and 70°C with an optimum of 55–65°C, extreme thermophiles growing optimally at temperatures between 65 and 80°C, and hyperthermophiles with optimum growth higher than 80°C 3. These microorganisms have developed unique metabolic and physiological capabilities to thrive in extreme habitats and to produce particular metabolites that are rarely present in microbes of modest environments. Such compounds significantly contribute to biotechnological advancements in recent years 4, 5, 6. Among compounds involved in thermophilic adaptation, enzymes and exopolysaccharides (EPSs) are of special biotechnological interest due to their unusual stability not only to high temperatures but also to detergents, organic solvents, high salt concentrations. The high stability of enzymes and EPSs allows for their use in harsh industrial conditions.
Despite some studies on thermostable enzymes isolated from mesophilic and even psychrophilic microorganisms, thermophiles are a natural and most promising source of thermostable enzymes. Thermophiles demonstrate many advantages for industrial use such as high growth rates, which accelerate fermentation processes two to three times over those with mesophilic producers, reduced risk of microbial contamination, higher diffusion rate and mass turnover, respectively, decreased viscosity of culture liquids, and better solubility of polymeric substrates and fats that are generally poorly soluble compounds. Thermostable enzymes are more resistant to proteolysis and chemical denaturation. Hence, stability of these molecules slows down the process of enzyme “aging,” which is particularly important in commercial preparations and allows their storage at room temperature with a longer half‐life. They can be used in the presence of mixed solvent systems since the stability of the protein molecules predetermines their resistance to denaturing agents such as organic solvents and detergents. Also, they are less impacted by enzyme purification that is required for some processes and is, generally, inevitably accompanied by some loss of enzymatic activity.
Despite the large biotechnological potential of thermophilic microorganisms, their industrial application is still limited to specific processes. In general, the difficulties for wide exploration of thermophiles and their enzymes are due to technical difficulties in their cultivation, reduced oxygen solubility resulting in a lower biomass and productivity yield in aerobic organisms, greater sensitivity of enzyme synthesis to substrate or product inhibition, reduced specific enzyme activity due to the increased number of bonds limiting the rate of formation of the enzyme–substrate complex, and energy costs to maintain high temperatures.
Bulgaria is a country rich in mineral springs: There are over 700 springs from about 140 natural deposits. Cold water springs are predominant in northern Bulgaria, while warm (temperature of 37–60°C) and hot (over 60°C) springs are typical for southern Bulgaria. Hyperthermal mineral waters are located predominantly in the valley of the rivers Struma and Mesta and in the Rhodopes mountain. The hottest spring in Europe, Sapareva Banya (103°C at the drilling site), the big Rupi basin (94.2 ha), and 56 springs on the territory of the town Velingrad are among the most renowned. These springs are from different geotectonic origins, and have different temperatures and pH (the latter varying in the range of 6.5–9.5). They belong to the continental low‐temperature springs connected with nonactive volcanos with hydrothermal activity and therefore belong to the neutral and slightly alkaline group of waters according to the existing bimodal pH distribution of hot water 7. They also differ in the degree of mineralization and the concentrations of different ions (hydrocarbonaceous, sulfide, sulfate, radon, chloride, nitrate, ammonium, and carbonaceous). Thermal springs located in southeastern Europe are generally poorly studied ecological niches. Insufficient knowledge of the thermophilic diversity in general, the lack of similar studies for Bulgarian hot springs, and a promising future for the industrial use of thermostable enzymes and EPSs motivate the scientific activity of researchers from the Laboratory of Extremophilic Bacteria, Institute of Microbiology, Bulgarian Academy of Sciences. This review covers investigations on biotechnological processes for the production of selected thermostable enzymes and EPSs synthesized by moderate thermophilic bacteria isolated from Bulgarian hot springs, their properties, and their possible applications in various industries.
2. Thermostable enzymes
2.1. Polysaccharide degrading enzymes produced by Bulgarian thermophilic isolates
Organoheterotrophic bacteria are capable of utilizing various carbohydrates and lipids as sources for energy and carbon. The high temperature of solubilization of these natural polymers determines the special biotechnological interest toward thermophilic enzymes that are usually synthesized extracellularly. The choice of a taxonomic group suitable for enzyme production is determined both by the advantages of the respective group and by the industrially exploited hosts for enzymatic expression. Thermophilic bacilli are the main industrially used bacterial producers of extracellular thermostable enzymes (proteases, amylases, lipases, pullulanases, xylanases, etc.) 8. Glycosyl hydrolases are enzymes capable of hydrolyzing glycosidic bonds between two or more carbohydrate components or between a carbohydrate and a noncarbohydrate component of the molecule. The structural modification of plant and microbial polysaccharides is associated with a change in their properties, which can increase their commercial value and broaden the scope of their application. Carbohydrate degrading enzymes represent the second largest group of industrially produced enzymes after proteases 9.
We have investigated the phylogenetic diversity of culturable bacteria from a genus Bacillus and related genera, isolated from 18 Bulgarian hot springs with respect to their functional diversity 10. Sixty‐seven thermophilic strains were isolated under aerobic conditions at 60°C. One of the strains belonged to the genus Thermoactinomyces and 66 to eight species in four genera from Bacillus group: Anoxybacillus, Geobacillus, Brevibacillus, and Bacillus. Based on a phylogenetic analysis, four strains belonged to groups representing potentially novel species (<97% sequence similarity of 16S rRNA gene). Later, they were described as new species Anoxybacillus rupiensis 11 and Anoxybacillus bogrovensis 12. Producers of carbohydrases, degrading 12 from the tested 13 substrates were isolated. About half of the isolates degraded amylose by exo‐ or endomechanism of action of their enzymes. Strains degrading hemicellulose components such as arabinan, arabinoxylan, β‐glucan, galactan, galactomannan, and xyloglucan were isolated. Some of the microorganisms were able to uptake microbial polysaccharides such as curdlan and gellan as the only carbon source. Our results revealed that Bulgarian hot springs hosted a diversity of Bacillus group microorganisms capable of using a variety of carbohydrates.
2.1.1. Gellan lyase produced by Geobacillus stearothermophilus 98
Gellan is a microbial heteropolysaccharide used as a gelling and suspending agent, sealant and stabilizer (E418) in a number of food products: sauces, dessert creams, ice creams and jellies, puddings, glazes, marmalades, ready‐to‐cook foods. It is frequent ingredient of cosmetic products such as lotions, creams, and toothpastes; matrices for immobilization of various cell lines in cell culture and tissues; inert carriers in the manufacturing of drugs; or impregnates in the paper industry 13. Gelrite is used for the preparation of microbiological media or cell cultures, especially in the cultivation of thermophilic microorganisms, since it is the only gelling agent that can be successfully used for solid nutrient media at temperatures above 70°C.
The industrial use of gellan is still limited due to the forming of highly viscous solutions. Being able to control its viscosity will result in expanding its use, especially in the food industry. Gellan lyase is an endoenzyme able to modify the molecular weight and hence the viscosity of gellan solutions by cleavage of the linear gellan molecule to its tetrasaccharide units (D‐glucose–D‐glucuronic acid–D‐glucose–L‐rhamnose), which is accompanied by the formation of a double bond in the glucuronic residue. Despite the increasingly widespread use of gellan in the food industry, an effective enzymatic method for its depolymerization has not yet been developed on an industrial scale. Thus far, only mesophilic bacilli growing at temperatures 28–30°C have been described as gellan lyase producers 14, 15. Their enzymes hydrolyze gellan at temperatures below 45°C, while this polysaccharide is soluble above 50°C, hence limiting their industrial use. The need for a thermostable gellan lyase motivated us to seek for a thermophilic producer of an enzyme that is active at temperatures at which the polymer is soluble.
A thermophilic bacterium able to grow at high temperature (50–82°C) with an optimum at 55°C was isolated from the Rupi basin, Bulgaria, and identified as a strain of G. stearothermophilus 16. This strain was able to grow and synthesize the enzyme in a pH range of 5.5–8.5 with an optimum at 7.5, the optimal temperature for synthesis was 55°C. Despite the growth of G. stearothermophilus 98 in all tested media, the enzyme was synthesized only in the presence of a specific carbon source, gellan, suggesting a strong inductive nature of the synthesis. An activity of 79 U/L was reached in a mineral medium with 0.2% gellan in a laboratory reactor during the late exponential phase and the whole process continued only 15 h. In comparison, the fermentation processes of mesophilic gellan lyase producers last for several days. Our experiments on enzyme synthesis in continuous cultures showed about 10‐fold higher enzyme activity relative to batch cultures (Fig. 1). The maximum enzymatic activity of the culture fluid (700–720 U/L) was observed in the range of low dilution rates (D) = 0.05–0.1 h−1. Since the growth of the strain in batch and continuous cultures was similar (about 0.5 g/L), the specific activity of gellan lyase was also ten‐fold greater (192.6 U/g for batch and 1931.7 U/g for continuous cultures). The specific activity of the enzyme in the culture supernatant was even higher than that reported for the mesophilic enzyme from Bacillus sp. GL1 (1390 U/g) 17 despite the generally accepted belief that the rigidity of thermostable molecule limits enzyme activity 18. Maximum enzyme productivity of 64.8 U/(L h) was observed at D = 0.1 h−1.
Figure 1.
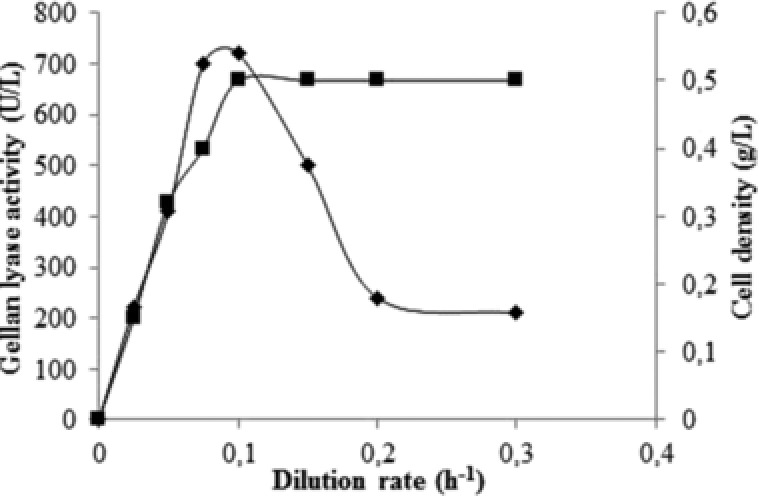
Growth (▪) and gellan lyase activity (♦) in continuous cultures of G. stearothermophilus 98 in MSM at 55°C, pH 7.5, 300 rpm, and aeration 1.0 vvm (volume air per volume medium per minute).
The purified gellan lyase showed a molecular weight of about 220 kDa and was active in the temperature range 55–80°C, showing maximum activity at 70°C 19. Its temperature optimum is more than 25°C higher than that for other known gellan lyases 17, 20. The residual activity remained at 100% after 24 h at 60°C or 30 min at 70°C. The half‐life of the enzyme at 70°C was 45 min in the absence of a substrate, while in its presence the enzyme molecule was further stabilized and the residual activity was 100% after 2.5 h. The purified enzyme was active at a wide pH range (pH 4–8), which is the broadest pH range compared to other gellan lyases typically active at pH 6.5–7.5. Retaining enzyme activity after 3‐h treatment at the corresponding pH should motivate future applications in industrial processes conducted at a different pH. Its stability in the acidic pH range is of particular importance for the food industry, especially in the preparation of fruit milk and desserts. The enzymatic modification of viscous gellan solutions at low pH and high temperature allows for a cost‐effective, one‐step process. Lineweaver–Burk's plot showed linear regression and follows the kinetics of Michaels–Menten equation. The relatively low kinetic constant K m of 0.21 μM, the reciprocal value of which reflects the enzyme affinity to the substrate, indicated high affinity to the gellan. The enzyme was sensitive to all inhibitors used with the highest degree of inhibition toward N‐bromosuccinimide, suggesting an important role of tryptophan/histidine at the active site of the enzyme.
Analysis of the products of the purified enzyme revealed only one product identified as a tetrasaccharide. Additional spots of glucose and rhamnose were observed when unpurified enzyme fraction (supernatant) was used for gellan degradation. These results revealed consequent enzyme action on gellan‐ ((divis)) initial degradation by gellan lyase, followed by hydrolysis of the tetrasaccharide by exoenzymes that provide complete degradation to monosaccharides. β‐D‐glucosidase and α‐L‐rhamnosidase activities were established in both the supernatant and intracellular fraction by the corresponding p‐nitrophenyl glycoside substrates. These results were consistent with the results reported by other authors who had found that the digestion of gellan was accomplished by the sequential action of three enzymes: gellan lyase, β‐D‐glucosidase, and α‐L‐rhamnosidase, the synthesis of which is inducible in the presence of gellan 20, 21.
Laboratory process of enzyme treatment (0.81 U/mL) showed a gradual decrease in the viscosity of 1% gellan solution and the residual amount of gellan was only 12% after 24 h. A slight decrease in the gellan viscosity has been observed for the mesophilic enzyme at the same concentration and 38% residual gellan was registered after 25 h of incubation at 30°C 21. The results from the gellan treatment with a thermostable gellan lyase proved a very effective process of partial depolymerization for a relatively short period of time, resulting in a strongly reduced viscosity at a high temperature.
2.1.2. Xylanase produced by Anoxybacillus flavithermus BC
Lignocellulose is a potential alternative source of fuel, because it is (1) the most common renewable natural resource; (2) it is a waste product from a number of industries such as forestry, woodworking, straw, plant growing, juice production, and others; (3) not directly usable for human consumption. The need in effective degradation of the huge plant biomass composed mainly of cellulose and hemicellulose to cheap fermentable sugars could be used for different purposes such as further conversion into ethanol, production of dietary fibers, improving the nutritional value of animal feed, or the production of xylitol that is used as a sweetener in the food industry. A main component of hemicellulose is xylan, the second most abandon polysaccharide (20‐35%). Thermostable and alkaline xylanases are especially useful for the pulp whitening in the paper industry without using environmentally harmful chlorinating agents 22. The use of thermoactive and thermostable xylanase allows for simultaneous pretreatment and enzymatic action at high temperatures, shortening the process steps and reducing the duration of the process. Current limiting factors for using thermophilic xylanases, however, are the low yield and low specific activity of these enzymes 23. The majority of the well‐studied xylanases are optimally active at mesophilic temperatures (40–60°C) and at neutral or low acidic рН. Hence, the preliminary treatment of the substrates at elevated temperature followed by lowering of temperature for enzyme treatment is required. The lack of effective biocatalysts active in harsh industrial environments have motivated scientific interest toward high‐yield synthesis of enzymes that operate at temperatures around and above 80°C.
As a result of our efforts, a thermophilic producer of thermostable alkali‐resistant xylanase was isolated from a hot spring Dolni Bogrov, Sofia region and identified as a strain of the genus Anoxybacillus 24. Fermentation processes were performed in batch and in continuous cultures in a laboratory reactor at 60°C. They revealed that production in continuous cultures was about two‐fold higher and 3.5‐ to 4.6‐fold faster than in batch cultures. Xylanase activity was a function of the dilution rate in continuous cultures; a maximum activity was observed at a dilution rate of 0.2 h−1. The xylanase showed high thermostability comparable to most thermostable enzymes — it retained 100% of its activity after 40 h at 70°C in six‐fold concentrated enzyme supernatant 25. The enzyme from Bacillus sp., which lost 11% of its activity after 2 h at 70°C, has been previously reported as the most thermostable xylanase 26. Bataillon et al. 27 have observed maintenance of activity after 4 h at 70°C of the thermostable enzyme from Bacillus sp. SPS‐0. Among the most thermostable enzymes reported subsequently have been those from anaerobic thermophiles such as Caldicellulosiruptor saccharolyticus 28 and C. bescii 29. The half‐life of inactivation of xylanase from Dictyoglomus thermophilum at 80°C and 500 MPa has been over 30 h 30. The observed two active bands, one strong and one weak, in the zymogram of the culture supernatant revealed the synthesis of several enzyme forms observed also by other authors 31.
The high thermal stability of A. flavithermus BC xylanase provides the opportunity of an effective process of xylan hydrolysis by a combined action of this enzyme and exoenzyme β‐xylosidase/α‐arabinosidase from the hyperthermophilic archaeum Sulfolobus solfataricus Oα 32. Xylans from different plant sources were hydrolyzed with xylanase and β‐xylosidase/α‐arabinosidase separately and in coaction 33. The xylan degradation with xylanase from A. flavithermus BC alone showed no efficient conversion to xylo‐oligosaccharides in all three substrates tested. The highest degree of degradation after 2 h incubation was achieved using beechwood xylan (26.1%), while the corresponding values for birch wood and oat spelts xylans were 10.2 and 11.6%, respectively. An increase of the reaction time to 4 h showed an enhance in the rate of hydrolysis to 32.8% for beechwood xylan (Table 1), while a decrease for oat spelts xylan was observed probably due to a withdrawal of the reaction in the opposite direction. Depolymerization of the polysaccharide with other xylanases is also a slow process and leads to a partial hydrolysis. One of the most effective enzymes, namely that of Thermomonospora fusca BD25, has degraded 28% of the oat spelts xylan at a reaction time of 3 h 34, a rate similar to that obtained for the enzyme by A. flavithermus BC. The incubation of xylans with β‐xylosidase/α‐arabinosidase from S. solfataricus Oα alone resulted in poor hydrolysis and beechwood xylan showed the highest hydrolysis (6%) after 6 h at 70°C. Simultaneous action of xylanase from A. flavithermus BC and β‐xylosidase/α‐arabinosidase from S. solfataricus Oα significantly increased the efficiency of the hydrolysis process. Two‐fold increase in the amount of the reducing sugars was observed after 2‐h incubation of beechwood and birch wood xylans, indicating the cooperative action of the enzymes in substrate degradation. An additional increase was observed after an elongation of the reaction time up to 3 and 4 h with beechwood xylan, reaching a degree of hydrolysis of 63.6% after 4 h. Overall, the xylanase isolated by our group was shown to participate in an effective xylan degradation processes that achieved more than 60% hydrolysis. The high degree of beechwood xylan hydrolysis suggested that a combined application of the enzymes could be very useful in industrial processes for possible sugar and fuel production from wood. A similar coprocess with different xylanolytic enzymes has been described for Bacillus thermoantarcticus, and an effectiveness of only 30% xylan conversion has been reported for the same reaction time and temperature 35. Coaction of xylanase and β‐xylosidase from Thermotoga maritima has resulted in almost complete hydrolysis of the polymer, but at longer incubation time (12 h) at 90°C 36.
Table 1.
Action of xylanase from A. flavithermus and recombinant β‐xylosidase/α‐arabinosidase on beechwood xylan
| Enzyme(s) | Incubation time (h) | Reducing sugars yield (mg xylose equivalents per mL) | Xylan hydrolysis (%) |
|---|---|---|---|
| Xylanase |
|
|
|
| β‐Xylosidase/α‐arabinosidase | 6 | 0.60 | 6.0 |
| Xylanase + β‐xylosidase/α‐arabinosidase |
|
|
|
| Control | 4 | 0.006 | 0.06 |
Chromatography (TLC and HPLC) based identification of the products released from the hydrolysis of different xylans with xylanase from A. flavithermus BC alone showed the presence of xylo‐oligosaccharides with various Degree of polymerization DP after 2‐h incubation. Xylotetraose was the main hydrolysis product from beechwood xylan, while xylotriose was the major oligosaccharide released from oat spelts and birch wood xylans. Variation in DP of the final products has been explained by the different behavior of the xylanases with respect to the xylan structures 37. Only xylose was identified after 6 h of incubation of different xylans with β‐xylosidase/α‐arabinosidase alone, and xylo‐oligosaccharides were not observed. In the synergistic action by both enzymes, xylose was recorded as a main product of the beechwood xylan hydrolysis, with negligible traces of xylobiose after 2 h of incubation (Fig. 2) confirming that the β‐xylosidase/α‐arabinosidase degraded the xylo‐oligosaccharides released by the xylanase. Normally, degradation of polysaccharides is a multistage process due to the action of different enzymes, often accompanied by a change of operative conditions for the next enzyme used in the process. Mutual action of enzymes in one stage at the same operative conditions would simplify significantly industrial processes of carbohydrate utilization and could have a great economic benefit.
Figure 2.
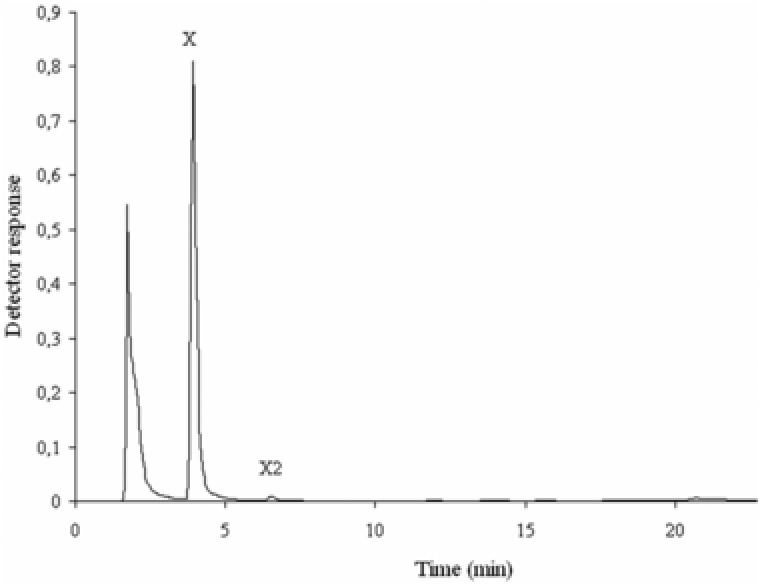
HPLC profile of degradation products from beechwood xylan at 70°C by xylanase and β‐xylosidase/α‐arabinosidase after 2 h (C). X, xylose; X2, xylobiose.
2.2. Lipase produced by G. stearothermophilus MC7
Microbial lipases have been of particular interest for several decades not only because of their ability to hydrolyze lipids by cleavage of the ester bond in acylglycerols but also because of their ability to form acyl glycerols from glycerol and free fatty acids. These enzymes are characterized by selectivity to the type and position of the fatty acids in the esters, enantiospecificity (specificity in stereoisomer synthesis), and ability to catalyze heterogeneous reactions between two phases — water‐soluble and water‐insoluble. They are used for protection of functional groups, for separation of racemic mixtures in the production of enantioisomers, for transesterification in the field of fine organic synthesis, and are hence useful for the pharmaceutical, cosmetic, and chemical industries 38. Methyl and ethyl esters with long chain fatty acids are valuable for the production of fuel, detergents, and lubricants. Other applications include fat hydrolysis in the dairy industry, removal of noncellulosic impurities in raw cotton prior to further processing in dyed and finished products, removal of subcutaneous fat in the tanning industry, wastewater treatment, modification of fats and oils for higher nutritional value and for the synthesis of structural fats as food additives, and improvement of texture taste and aroma of the food products. The majority of animal fats are solid at room temperature and their increased solubility at high temperatures dictates the industrial need for lipases active at high temperatures and stable to organic solvents.
The lipase isolated by our group from Bacillus sp. MC7 was among the first reported thermostable lipases 39. The enzyme producer was isolated from a hot spring in the basin Marikostinovo using the method of continuous cultivation. Samples containing water, soil particles and algae were used to inoculate a reactor containing only triolein as a carbon source. Based on its phenotypic properties and 16S rDNA analysis, the strain was identified as a member of the species G. stearothermophilus. Maximum enzyme amounts were synthesized at 55°C and pH 8.5. Enzyme synthesis was growth‐associated and a maximal level of lipase activity was observed in the late exponential phase after only 10 h of cultivation. Lipase was not synthesized in media without lipids as a carbon source, suggesting an inducible nature of enzyme synthesis. Saturated fatty acids (butyric, capric, palmitic, and stearic) almost completely inhibited lipase synthesis. While most of the lipases are unstable at pH above 9.0, purified lipase from G. stearothermophilus MC 7 was stable at a significantly wider pH range of 7.0–11.0 with the highest activity in pH range 7.5–9.0, making it suitable for use in detergents, leather processing, and in the chemical industry. The enzyme was active between 55 and 90°C with an optimum at 75–80°C, which is another advantage over mesophilic lipases, the activity of which abruptly decreases at temperatures above 40°C. The half‐life of the electrophoretically homogeneous enzyme at 70°C was 30 min 40. The enzyme from G. stearothermophilus MC7 was one of the most thermostable alkaline lipases at the time of its publication. The thermostability was significantly increased in the presence of substrates such as olive oil and tributyltin (Fig. 3) as well as sorbitol. A similar effect of the substrate has been also reported for a lipase from Bacillus sp. J33 41. The authors explain this effect by a hindered dehydration of enzyme molecule in the presence of lipophilic compounds and polyvalent alcohols, which is particularly important for industrial processes in organic solvents media. Like most other lipases, the catalytic activity of the enzyme from G. stearothermophilus MC7 increased slightly up to about 110% in the presence of Ca2+ due to a lack of product inhibition as the released fatty acids formed insoluble Ca‐salts. Enzyme activity was strongly influenced by the presence of thiol inhibitor p‐CMB, which was an evidence for the important role of SH‐groups in the catalysis mechanism. The enzyme showed an ability to hydrolyze both water‐soluble and water‐insoluble emulsified substrates 40. The broad range of detected substrates comprised triglycerides from C4 to C18, with the highest affinity for tributyltin. Similar to one of the most active lipases, namely that of Pseudomonas aeruginosa MB 5001 42, the lipase of G. stearothermophilus MC 7 hydrolyzed glycerides with short chain fatty acids faster than those with long chains. Triglycerides were preferred over monoglycerides. The rate of hydrolysis of saturated (tristearin, C18) and unsaturated (triolein, C18:1) fatty acid esters was similar. Despite the demonstrated activity to triacetin and different types of Tweens (Tween 20, 60, and 80), its ability to hydrolyze olive oil, insoluble triesters, and p‐NP long chain fatty acid esters characterized the enzyme as a lipase with some esterase activity. The lipase from G. stearothermophilus MC 7 was robust to a number of organic solvents and polyalcohols widely used in the leather industry, in pharmacy for preparation of racemic mixtures, and in the development of structural lipids in the food industry.
Figure 3.
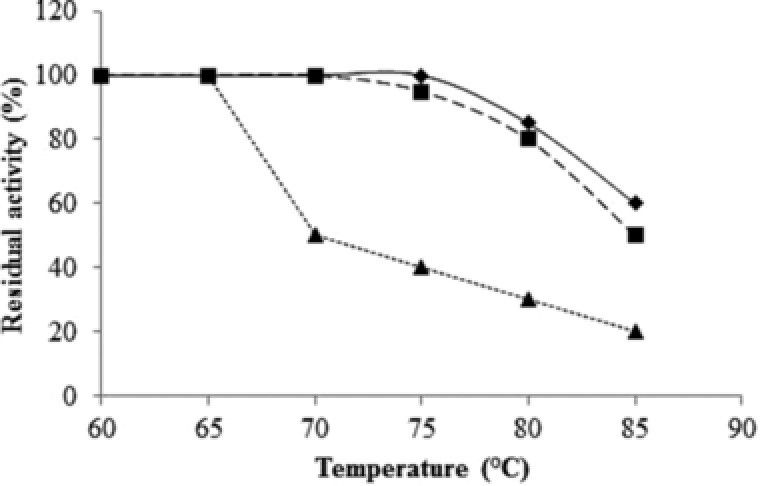
Substrate effect on lipase thermostability. ♦ 1% Tributhyrin; ▪ 1% olive oil; ▴, without substrate. The lipase solution was incubated for 30 min at different temperatures. The residual activity was determined by the pH stat method at 60°C, pH 8.5.
The effectiveness of the immobilization process is an important advantage for industrial applications of this enzyme in continuous processes. In order to assess long‐term use, G. stearothermophilus MC 7 lipase was immobilized on various carriers. The highest rate of enzyme activity (86.1%) and enzyme binding (100%) were observed using DEAE‐cellulose for immobilization 40. The enzyme thermostability was not significantly affected by the immobilization process: when immobilized on DEAE cellulose the enzyme showed a half‐life of 37 min at 70°C. The suitability of the immobilized enzyme for efficient lipid transformation processes as well as the influence of nonionic inhibitors on enzyme activity was observed in a number of follow‐up studies. All studied additives slowed down the process of thermal denaturation; an increased enzyme stability was observed for a large number of modifiers applied within a broad concentration range with the highest degree of activation when PEG 6000 was applied (up to 2.3‐fold increase) 43. A beneficial effect on the operational and thermal stability of lipase MC7 immobilized on nanostructured tin dioxide was observed in two ionic liquids 44. Stearyl stearate production was achieved at a much higher reaction rate in the presence of 3‐methyl‐1‐octyl imidazolium chloride compared to solvent‐free medium and 95% conversion of the substrates was achieved for 5 h at 65°C. The biocatalyst was almost fully active after eight synthetic cycles in this green solvent. MC7 lipase immobilized on DEAE cellulose was successfully applied for the synthesis of dioleoylpalmitoylglycerol, a structured triglyceride used in healthy food 45. The degree of substitution of palmitic acid with oleic acid exceeded 50% after 48 h. The immobilized enzyme exhibited a high operational thermostability with a half‐life of 50 days at 60°C in a solvent‐free system. Four different polymer carriers were used for acidolysis of triolein with caprylic acid in a selective transesterification reaction 46. The highest degree of conversion was achieved in the presence of lipase immobilized on polypropylene and only monosubstitution in the glycerol backbone was obtained as a result.
3. Exopolysaccharides produced by Bulgarian thermophilic isolates
Microbial EPSs have many industrial applications as viscosity‐increasing agents, gelling agents, stabilizers in multiphase solutions, or contributing to food quality as lubricants, flocculants, or flavor enhancers in the food industry; emulsion stabilizers, film formers, binders, viscosity increasing agents, and skin conditioning agents in the cosmetic industry; for remediation of environmental effluents produced by the mining and metallurgy industries, etc. The observed variety in properties of these EPSs is a result of different compositions and structures 47. EPS from extremophiles are of particular interest due to the industrial need for new microbial polysaccharides with higher emulsifying and flocculating activities, better rheological characteristics, resistance to high temperature and to solvents and reduced toxicological effects. Although many efforts have been spent in exploring thermophilic bacilli as sources for thermostable enzymes, knowledge of their ability to produce EPSs is still limited. As of today, only a few EPS‐secreting thermophilic bacteria and archaea have been described 48. Representatives of two thermophilic bacilli genera have been reported as EPSs producers: a genus Bacillus with the species B. thermoantarcticus 49 and Bacillus licheniformis 50, 51, 52, and a genus Geobacillus with the species G. thermodenitrificans 50.
Geobacillus tepidamans V264 was isolated from Mizinka hot spring, Velingrad, Bulgaria 53. Maltose and (NH4)2HPO4 were chosen as best carbon and nitrogen sources in concentrations of 30 and 3 g/L, respectively. The utilization of inorganic nitrogen source for EPS production by our isolate is a promising opportunity for a further industrial design of a cost‐effective medium. Unlike mesophilic processes that last for several days, this strain reached a maximum EPS level for only 8 h of cultivation in a reactor at optimal temperature of 60°C and pH of 7.0. Analysis of the monosaccharide composition of this EPS revealed it was glucan containing ˃98% glucose and small quantities of galactose, fucose, and fructose. Similarly, glucose has accounted for more than 90% of the polymer produced from Thermotoga maritime; however, additional sugars have been ribose and mannose 54. EPSs isolated from two Geobacillus sp. strains have contained glucose, galactose and mannose in different proportions. Glucosamine and arabinose together with galactose and mannose have been included in the EPS from the third strain 50.
EPS synthesis from Aeribacillus pallidus 418, isolated from the Rupi basin, Bulgaria was investigated by our group in batch and continuous cultures. A maximum EPS yield of 170 μg/mL was measured in a single impeller monoagitator system type Narcissus at agitation speed 900 rpm and aeration rate 0.5 vvm 55. Optimization of the conditions for EPS synthesis in a laboratory reactor revealed more pronounced influence of the agitation speed than of the aeration rate. Optimal agitation and aeration rates were reached at values resulting in a short‐time oxygen starvation of the culture, sufficient to provoke EPS synthesis as a protective reaction, and not sufficient to hinder culture growth (Fig. 4). Two oxygen transfer parameters were determined by the unsteady stop‐gassing measurement technique for bioprocess extrapolation and scale‐up: namely, oxygen uptake rate and oxygen mass transfer coefficient (K Lα). The K Lα values obtained were found to be comparable with the range of values reported for bench‐scale mass transfer under oxygen consumption. Oxygen uptake rate was most pronounced at 900 rpm and 0.5 vvm, for example, at conditions corresponding to the most viable biomass producing the highest EPS yields. On the other hand, as expected K Lα depended strongly on the viability of the culture, being higher at the higher growth rates. K Lα increased with an increase in both, mixing intensity (i.e. input power) and aeration rate.
Figure 4.
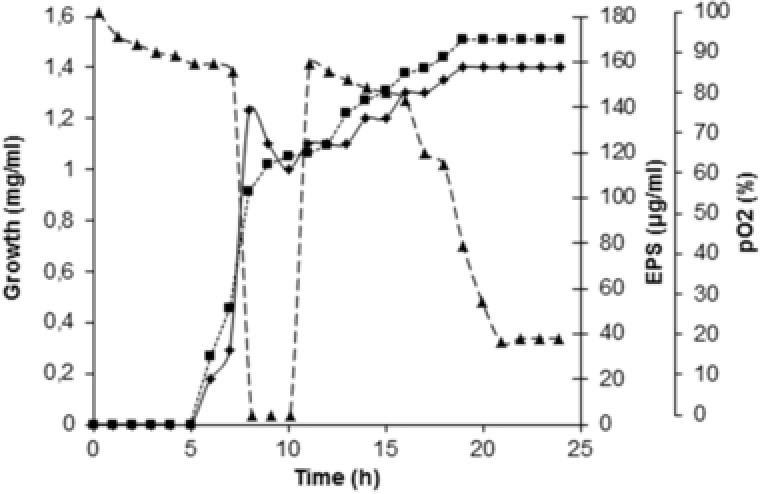
Biomass yield and EPS production by A. pallidus 418 in a stirred‐tank reactor at agitation rate 900 rpm and aeration of 0.5 vvm. Symbols: (♦), growth (mg/mL); (▪), EPS (μg/mL); (▴), dissolved oxygen level (%).
A slight increase in EPS production was observed in continuous cultures at low dilution rate (D = 0.06) 56. Although the obtained value for EPS (180 μg/mL) was not significantly higher than that of batch culture (170 μg/mL), the ability to use the advantages of continuous cultivation suggest the possibility of large scale production of EPS by A. pallidus 418 in a continuous cultivation process. Almost pure EPS was obtained after precipitation of the liquid from continuous cultures — it represented 95.3–97% of the precipitated polymer fraction obtained in continuous cultivation, while in batch cultivation it only reached 80.5%. Two heteropolysaccharides were obtained after purification of the polymer fraction, namely, electroneutral EPS 1 and negatively charged EPS 2. Their relative weight ratio was 3:2.2 and they consisted of an unusually high variety of sugars (six for EPS 1 and seven for EPS 2) 57. The main sugar in EPS 1 was mannose (69.3 %); further identified were smaller quantities of glucose (11.2%), galactosamine (6.3%), glucosamine (5.4%), galactose (4.7%), and ribose (2.9%). The main sugar in EPS 2 was also mannose (33.9%), followed by galactose (17.9%), glucose (15.5%), galactosamine (11.7%), glucosamine (8.1%), ribose (5.3%), and arabinose (4.9%). Both polymers showed high thermostability and among the highest molecular masses reported for microbial EPS: 700 kDa for EPS1 and over 1000 kDa for EPS 2. Hence, these EPSs could be used to achieve high viscosity in industrial products by a comparatively low‐quantity polymer used. Similarly, a molecular mass higher than 300 kDa was reported for EPSs from thermophilic bacilli 50.
Analysis of surface tension and foaming ability showed that the increased concentration of EPS resulted in a decreased surface tension σ determined according to the equation
where а is a constant (mN/m), b is a constant (m3 kg), and c denotes concentration (kg/m3). This effect was especially observed at lower EPS concentrations. For example, low surface tension is particularly important for a good rub of cosmetic creams. The synthesized EPS showed a high ability to form foam and a high stability of the obtained foam phase. Significant foam (80%) was observed even at low EPS concentration (0.5%) and it increased up to 100% (expressed as a ratio of the foam volume to the volume of the water solution) at 1.5% EPS content. Valuable functional properties of EPS from A. pallidus 418 motivated us to interrogate the changes in the properties of a cosmetic cream after EPS inclusion in the cream composition. Addition of the polysaccharide to the basal cream led to an increase in the viscosity at low shear rates and pseudoplastic behavior of the cream. The yield stress of the cream was determined as a maximum value in the shear stress‐time dependence at a constant shear rate 1 s−1. The shear stress‐shear rate dependences were approximated to the Oswald de Waele power law:
where ẏ is the shear rate and K is the flow consistency (texture) index of the sample, which coincides with the dynamic viscosity at power index n = 1 for Newtonian fluids. The experimental results from the mechanical hysteresis test of the cream clearly demonstrated its increase after the addition of EPS assuming that EPS create a polymer network in the film, the destruction of which requires additional energy. The mechanical hysteresis for the cream containing 1% or 2% EPS remained constant, suggesting that 1% EPS was sufficient for the creation of such a stable network and that the strength of the additional network did not depend significantly on the EPS concentration.
Brevibacillus thermoruber strain 423 was isolated by our group from a hot spring Gradechnitsa, Blagoevgrad region, southwest Bulgaria, and its EPS synthesis was investigated 58. Highest EPS production levels by B. thermoruber 423 were reached at 55°C and pH 6.5 in the presence of maltose and peptone in ratio 18:1. The enhanced production in abundance of carbon source and minimal nitrogen source reaching values up to 18:1 has been reported as typical for the EPS production processes 59. EPS from B. thermoruber 423 showed highest EPS production (897 mg/L) at early stationary phase in a reactor. The yield and productivity reached were comparable with these from mesophiles. Chemical characterization of EPS from B. thermoruber strain 423 by TLC, GC‐MS, FT‐IR, and NMR suggested a heteropolymer structure with the following sugar composition (%): glucose/galactose/mannose/galactosamine/mannosamine (57.7/16.3/9.2/14.2/2.4).
Essential genes potentially associated with EPS biosynthesis were identified through genome annotation of the full genome of B. thermoruber strain 423, and together with the experimental evidence, a hypothetical mechanism for EPS biosynthesis was generated 60.
4. Concluding remarks
In conclusion, the reported results revealed that Bulgarian hot springs harbor a high diversity of thermophilic bacteria that could be promising sources of novel thermostable enzymes and EPSs. Thermostable enzymes described by our group were among the first reported in the corresponding enzymatic groups. They demonstrated high thermostability and activity in wide pH ranges, which are clear advantages for several industrial processes that depend on enzymes with such properties. Development of laboratory technologies for their synthesis in optimized media and parameters for cultivation could favor a transition to semiscale and industrial‐scale processes. EPSs synthesized by thermophilic bacteria showed novel compositions, high thermostability, and improved rheological properties that were exemplarily and successfully explored in the development of novel cosmetic cream emulsions.
Practical application
Over the years, we have isolated variety of thermophilic bacteria from Bulgarian hot springs that produce biotechnologically valuable enzymes. The special interest toward thermostable glycosyl hydrolases and lipase was determined by the low solubility of polysaccharides and animal fats at mesophilic temperatures. In particular, a gellan lyase synthesized by an isolate from Bulgarian hot spring is the only known thermostable enzyme that is able to modify the properties of the polysaccharide gellan. Gellan is largely used as a gelling and suspending agent, sealant and stabilizer in food industry, cosmetics, and medicine. High thermostability of a xylanase isolated by us determined a successful conversion of xylan to almost pure xylose in an effective coprocess with archaeal β‐xylosidase. The activity of a lipase at high temperatures, its stability to the action of organic solvents and polyalcohols, wide pH range of activity, and successful immobilization are valuable traits for use in detergents, leather processing, chemical industry, and production of structural foods. Exopolysaccharides synthesized by thermophilic bacteria show high biological activity and improve the rheological properties of cosmetic creams.
The author has declared no conflict of interest.
5 References
- 1. Takai, K. , Nakamura, K. , Toki, T. , Tsunogai, U. et al., Cell proliferation at 122°C and isotopically heavy CH4 production by a hyperthermophilic methanogen under high‐pressure cultivation. Proc. Natl. Acad. Sci. USA 2008, 105, 10949–10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. MacElroy, R. D. , Some comments on the evolution of extremophiles. Biosystems 1974, 6, 74–75. [Google Scholar]
- 3. Bonch‐Osmolovskaya, E. , Atomi, H. , Editorial overview: Extremophiles: From extreme environments to highly stable biocatalysts. Curr. Opin. Microbiol. 2015, 25, vii–viii. [DOI] [PubMed] [Google Scholar]
- 4. Elleuche, S. , Schäfers, C. , Blank, S. , Schröder, C. , Antranikian G., Exploration of extremophiles for high temperature biotechnological processes. Curr. Opin. Microbiol. 2015, 25, 113–119. [DOI] [PubMed] [Google Scholar]
- 5. DeCastro, M. E. , Rodríguez‐Belmonte, E. , González‐Siso, M. I. , Metagenomics of thermophiles with a focus on discovery of novel thermozymes. Front. Microbiol. 2016, 7, 10.3389/fmicb.2016.01521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saxena, R. , Dhakan, D. B. , Mittal, P. , Waiker, P. et al., Metagenomic analysis of hot springs in Central India reveals hydrocarbon degrading thermophiles and pathways essential for survival in extreme environments. Front. Microbiol. 2017, 7, 10.3389/fmicb.2016.02123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marini, L. , Zuccolini, M. V. , Saldi, G. , The bimodal pH distribution of volcanic lake waters. J. Volcanol. Geotherm. Res. 2003, 121, 83–98. [Google Scholar]
- 8. Haki, G. D. , Rakshit, S. K. , Developments in industrially important thermostable enzymes: A review. Bioresour. Technol. 2003, 89, 17–34. [DOI] [PubMed] [Google Scholar]
- 9. Bhat, S. G. , Sukumaran, R. K. , Enzyme technologies for bioconversion of food processing by‐products, in: Chandrasekaran M. (Ed.), Valorization of Food Processing By‐Products, CRC Press, Boca Raton, FL: 2012, pp. 233–266. [Google Scholar]
- 10. Derekova, A. , Mandeva, R. , Kambourova, M. , Phylogenetic diversity of thermophilic carbohydrate degrading bacilli from Bulgarian hot springs. World J. Microbiol. Biotechnol. 2008, 24, 1697–1702. [Google Scholar]
- 11. Derekova, A. , Sjøholm, C. , Mandeva, R. , Kambourova, M. , Anoxybacillus rupiensis sp. nov., a novel thermophilic bacterium isolated from Rupi basin (Bulgaria). Extremophiles 2007, 11, 577–583. [DOI] [PubMed] [Google Scholar]
- 12. Atanassova, M. , Derekova, A. , Mandeva, R. , Sjøholm, C. and Kambourova, M. , Anoxybacllus bogrovensis sp. nov., a novel thermophilic bacterium isolated from Dolni Bogrov's hot spring, Bulgaria. Int. J. Syst. Evol. Microbiol. 2008, 58, 2330–2335. [DOI] [PubMed] [Google Scholar]
- 13. Kambourova, M. , Derekova, A. , Developments in thermostable gellan lyase, in: Satyanarayana T., Littlechild J., Kawarabayasi Y. (Eds.), Thermophilic Microbes in Environmental and Industrial Biotechnology: Biotechnology of Thermophiles, Springer, Berlin: 2013, pp. 711–730. [Google Scholar]
- 14. Mikolajczak, M. J. , Thorne, L. , Pollock, T. J. , Armentrout, R. W. , Sphinganase, a new endoglycanase that cleaves specific members of the gellan family of polysaccharides. Appl. Environ. Microbiol. 1994, 60, 402–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hashimoto, W. , Sato, N. , Kimura, S. , Murata, K. , Polysaccharide lyase: Molecular cloning of gellan lyase gene and formation of the lyase from a huge precursor protein in Bacillus sp. GL1. Arch. Biochem. Biophys. 1998, 354, 31–39. [DOI] [PubMed] [Google Scholar]
- 16. Derekova, A. , Sjoholm, C. , Mandeva, R. , Michailova, L. , Kambourova, M. , Biosynthesis of a thermostable gellan lyase by newly isolated and characterized strain of Geobacillus stearothermophilus 98. Extremophiles 2006, 10, 321–326. [DOI] [PubMed] [Google Scholar]
- 17. Hashimoto, W. , Maesaka, K. , Sato, N. , Kimura, S. et al., Microbial system for polysaccharide depolymerization: Enzymatic route for gellan depolymerization. Arch. Biochem. Biophys. 1997, 339, 17–23. [DOI] [PubMed] [Google Scholar]
- 18. Schweizer, L. , Mueller, L. , Protein conformational dynamics and signaling in evolution and pathophysiology, in: Arley B. (Ed.), Biased Signaling in Physiology, Pharmacology and Therapeutics, Academic Press, San Diego, CA: 2014, pp. 209–249. [Google Scholar]
- 19. Derekova, A. , Atanassova, M. , Christova, P. , Tchorbanov, B. , et al., Physicochemical characteristics of a thermostable gellan lyase from Geobacillus stearothermophilus 98. Zeitschrift fur Naturforschung C 2010, 65, 231–238. [DOI] [PubMed] [Google Scholar]
- 20. Jung, Y. , Park, C. S. , Lee, H. G. , Cha, J. , Isolation of a novel gellan‐depolymerizing Bacillus sp. strain YJ‐1. J. Microbiol. Biotechnol. 2006, 16, 1868–1873. [Google Scholar]
- 21. Kennedy, L. , Sutherland, I. W. , Gellan lyases—Novel polysaccharide lyases. Microbiology 1994, 140, 3007–3013. [DOI] [PubMed] [Google Scholar]
- 22. Walia, A. , Guleria, S. , Mehta, P. , Chauhan, A. , et al., Microbial xylanases and their industrial application in pulp and paper biobleaching: A review. 3 Biotech. 2017, 7, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Walia, A. , Mehta, P. , Chauhan, A. , Kulshrestha, S. , et al., Purification and characterization of cellulase‐free low molecular weight endo b‐1, 4 xylanase from an alkalophilic Cellulosimicrobium cellulans CKMX1 isolated from mushroom compost. World J. Microbiol. Biotechnol. 2014, 30, 2597–2608. [DOI] [PubMed] [Google Scholar]
- 24. Emanuilova, E. I. , Dimitrov, P. L. , Mandeva, R. D. , Kambourova, M. S. , et al., Extracellular xylanase production by two thermophilic alkali‐tolerant Bacillus strains in batch and continuous cultures. Z. Naturforsch. C 2000, 55c, 66–69. [DOI] [PubMed] [Google Scholar]
- 25. Dimitrov, P. , Kambourova, M. , Mandeva, R. , Emanuilova E., Isolation and characterization of xylan‐degrading alkali‐tolerant thermophiles. FEMS Microbiol. Lett. 1997, 157, 27–30. [Google Scholar]
- 26. Cordeiro, C. A. M. , Martins, M. L. L. , Luciano, A. B. , da Silva, R. F. , Production and properties of xylanase from thermophilic Bacillus sp. Brazilian Arch. Biol. Technol. 2002, 45, 413–418. [Google Scholar]
- 27. Bataillon, M. , Nunes‐Cardinali, A. P. , Castillon, N. , Duchiron, F. , Purification and characterization of a moderately thermostable xylanase from Bacillus sp. strain SPS‐0. Enzyme Microbiol. Technol. 2000, 26, 187–192. [DOI] [PubMed] [Google Scholar]
- 28. VanFossen, A. L. , Ozdemir, I. , Zelin, S. L. , Kelly, R. M. , Glycoside hydrolase inventory drives plant polysaccharide deconstruction by the extremely thermophilic bacterium Caldicellulosiruptor saccharolyticus . Biotechnol. Bioeng. 2011, 108, 1559–1569. [DOI] [PubMed] [Google Scholar]
- 29. Su, X. , Mackie, R. I. , Cann, I. K. , Biochemical and mutational analyses of a multidomain cellulase/mannanase from Caldicellulosiruptor bescii . Appl. Environ. Microbiol. 2012, 78, 2230–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li, H. , Voutilainen, S. , Ojamo, H. , Turunen, O. , Stability and activity of Dictyoglomus thermophilum GH11 xylanase and its disulphide mutant at high pressure and temperature. Enzyme Microb. Technol. 2015, 70, 66–71. [DOI] [PubMed] [Google Scholar]
- 31. Dhiman, S. S. , Sharma J., Rattan B., Industrial applications and future prospects of microbial xylanases: A review. BioResources 2008, 3, 1377–1402. [Google Scholar]
- 32. Cannio, R. , di Prizito, N. , Rossi, M. , Morana, A. , A xylan‐degrading strain of Sulfolobus solfataricus: Isolation and characterization of the xylanase activity. Extremophiles 2004, 8, 117–124. [DOI] [PubMed] [Google Scholar]
- 33. Kambourova, M. , Mandeva, R. , Fiume, I. , Maurelli, L. et al., Hydrolysis of xylan at high temperature by co‐action of the xylanase from Anoxybacillus flavithermus BC and the beta‐xylosidase/alpha‐arabinosidase from Sulfolobus solfataricus Oalpha . J. Appl. Microbiol. 2007, 102, 1586–1591. [DOI] [PubMed] [Google Scholar]
- 34. Tuncer, M. , Ball, A. S. , Co‐operative actions and degradation analysis of purified xylan‐degrading enzymes from Thermomonospora fusca BD25 on oat spelts xylan. J. Appl. Microbiol. 2003, 96, 1030–1035. [DOI] [PubMed] [Google Scholar]
- 35. Lama, L. , Calandrelli, V. , Gambacorta, A. , Nicolaus, B. , Purification and characterization of a thermostable xylanase and β‐xylosidase by the thermophilic bacterium Bacillus thermoantarcticus . Res. Microbiol. 2004, 155, 283–289. [DOI] [PubMed] [Google Scholar]
- 36. Xue, Y. , Shao, W. , Expression and characterization of a thermostable β‐xylosidase from the hyperthermophile, Thermotoga maritima . Biotechnol. Lett. 2004, 26, 1511–1515. [DOI] [PubMed] [Google Scholar]
- 37. Liab, K. , Azadi, P. , Collins, R. , Tolan, J. et al., Relationships between activities of xylanases and xylan structures. Enzyme Microb. Technol. 2000, 27, 89–94. [DOI] [PubMed] [Google Scholar]
- 38. Bhardwaj, K. K. , Gupta, R. , Synthesis of chirally pure enantiomers by lipase. J. Oleo Sci. 2017, 66, 1073–1084. [DOI] [PubMed] [Google Scholar]
- 39. Emanuilova, E. , Kambourova, M. , Dekovska, M. , Manolov, R. , Thermoalkalophilic lipase‐producing Bacillus selected by continuous cultivation. FEMS Microbiol. Lett. 1993, 108, 247–250. [Google Scholar]
- 40. Kambourova, M. , Kirilova, N. , Mandeva, R. , Derekova, A. , Purification and properties of thermostable lipase from a thermophilic Bacillus stearothermophilus MC 7. J. Mol. Catal. B 2003, 22, 307–313. [Google Scholar]
- 41. Nawani, N. , Kaur, J . Purification, characterization and thermostability of lipase from a thermophilic Bacillus sp. J33. Mol. Cell. Biochem. 2000, 206, 91–96. [DOI] [PubMed] [Google Scholar]
- 42. Chartain, M. , Katz, L. , Marcin, C. , Thien, M. et al., Purification and characterization of a novel bioconverting lipase from Pseudomonas aeruginosa MB 5001. Enzyme Microb. Technol. 1993, 15, 575–580. [Google Scholar]
- 43. Guncheva, M. , Zhiryakova, D. , Radchenkova, N. , Kambourova, M. , Effect of nonionic detergents on the activity of a thermostable lipase from Bacillus stearothermophilus MC7. J. Mol. Catal. B 2007, 49, 88–91. [Google Scholar]
- 44. Guncheva, M. , Dimitrov, M. , Kambourova, M. , Excellent stability and synthetic activity of lipase from B. stearothermophilus MC7 immobilized on tin dioxide in environmentally friendly medium. Biotechnol. Biotechnol. Equip. 2013, 27, 4317–4322. [Google Scholar]
- 45. Guncheva, M. , Zhiryakova, D. , Radchenkova, N. , Kambourova, M. , Acidolysis of tripalmitin with oleic acid catalyzed by a newly isolated thermostable lipase. J. Am. Oil Chem. Soc. 2008, 85, 129–132. [Google Scholar]
- 46. Guncheva, M. , Zhiryakova, D. , Radchenkova, N. , Kambourova, M. , Properties of immobilized lipase from Bacillus stearothermophilus MC7. Acidolysis of triolein with caprylic acid. World J. Microbiol. Biotechnol. 2009, 25, 727–731. [Google Scholar]
- 47. Kumar, A. S , Mody, K. , Jha, B. , Bacterial exopolysaccharides—A perception. J. Basic Microbiol. 2007, 47, 103–117. [DOI] [PubMed] [Google Scholar]
- 48. Nicolaus, B. , Kambourova, M. , Oner, E. T. , Exopolysaccharides from extremophiles: From fundamentals to biotechnology. Environ. Technol. 2010, 31, 1145–1158. [DOI] [PubMed] [Google Scholar]
- 49. Manca, M. C. , Lama, L. , Improta, R. , Esposito, E. et al., Chemical composition of two exopolysaccharides from Bacillus thermoantarcticus . Appl. Environ. Microbiol. 1996, 62, 3265–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nicolaus, B. , Moriello, V. , Maugeri, T. , Gugliandolo, C. , et al., Bacilli from shallow Mediterranean marine vents producers of exopolysaccharides. Recent Res. Dev. Microbiol. 2003, 7, 197–208. [Google Scholar]
- 51. Ramanathan, T. , Ahmad, A. , Ahmad, A. S. , Kalimutho, M. , Taxonomical identity and polysaccharide produced by Bacillus species isolated from old aged medicinal decoctions. J. Sports Sci. Med. 2011, 6, 2–9. [Google Scholar]
- 52. Spanò, A. , Gugliandolo C., Lentini, V. , Maugeri, T. L. et al., A novel EPS‐producing strain of Bacillus licheniformis isolated from a shallow vent of Panarea Island (Italy). Curr. Microbiol. 2013, 67, 21–29. [DOI] [PubMed] [Google Scholar]
- 53. Kambourova, M. , Mandeva, R. , Dimova, D. , Poli, A. et al., Production and characterization of a microbial glucan, synthesized by Geobacillus tepidamans V264 isolated from Bulgarian hot spring. Carbohydr. Polym. 2009, 77, 338–343. [Google Scholar]
- 54. Vanfossen, A. L. , Lewis, D. L. , Nichols, J. D. , Kelly R. M., Polysaccharide degradation and synthesis by extremely thermophilic anaerobes. Ann. N. Y. Acad. Sci. 2008, 1125, 322–337. [DOI] [PubMed] [Google Scholar]
- 55. Radchenkova, N. , Vassilev, S. , Martinov, M. , Kuncheva, M. et al., Optimization of aeration and agitation speed on exopolysaccharide production by Aeribacillus palidus 418 and emulsifying properties of the product. Process Biochem. 2014, 49, 576–582. [Google Scholar]
- 56. Radchenkova, N. , Panchev, I. , Vassilev, S. , Kuncheva, M. et al., Continuous cultivation of a thermophilic bacterium Aeribacillus pallidus 418 for production of an exopolysaccharide applicable in cosmetic creams. J. Appl. Microbiol. 2015, 119, 1301–1309. [DOI] [PubMed] [Google Scholar]
- 57. Radchenkova, N. , Vassilev, S. , Panchev, I. , Anzelmo, G. et al., Production and properties of two novel exopolysaccharides synthesized by a thermophilic bacterium Aeribacillus pallidus 418. Appl. Biochem. Biotechnol. 2013, 171, 31–43. [DOI] [PubMed] [Google Scholar]
- 58. Yasar Yildiz, S. , Anzelmo, G. , Ozer, T. , Radchenkova, N. et al., Brevibacillus themoruber: A promising microbial cell factory for exopolysaccharide production. J. Appl. Microbiol. 2014, 116, 314–324. [DOI] [PubMed] [Google Scholar]
- 59. Banik, R. M. , Kanari, B. , Upadhyay S. N., Exopolysaccharide of the gellan family: Prospects and potential. World J. Microbiol. Biotechnol. 2000, 16, 407–414. [Google Scholar]
- 60. Yildiz, S. Y. , Radchenkova, N. , Arga, K. Y. , Kambourova, M. , et al., Genomic analysis of Brevibacillus thermoruber 423 reveals its biotechnological and industrial potential. Appl. Microbiol. Biotechnol. 2015, 99, 2277–2289. [DOI] [PubMed] [Google Scholar]


