Abstract
Currently, fossil materials form the majority of our energy and chemical source. Many global concerns force us to rethink about our current dependence on the fossil energy. Limiting the use of these energy sources is a key priority for most countries that pledge to reduce greenhouse gas emissions. The application of biomass, as substitute fossil resources for producing biofuels, plastics and chemicals, is a widely accepted strategy for sustainable development. Aquatic plants including algae possess competitive advantages as biomass resources compared to the terrestrial plants in this current global situation. Bio‐oil production from algal biomass is technically and economically viable, cost competitive, requires no capacious lands and minimal water use and reduces atmospheric carbon dioxide. The aim of this paper is to review the potential of converting algal biomass, as an aquatic plant, into high‐quality crude bio‐oil through applicable processes in Malaysia. In particular, bio‐based materials and fuels from algal biomass are considered as one of the reliable alternatives for clean energy. Currently, pyrolysis and hydrothermal liquefaction (HTL) are two foremost processes for bio‐oil production from biomass. HTL can directly convert high‐moisture algal biomass into bio‐oil, whereas pyrolysis requires feedstock drying to reduce the energy consumption during the process. Microwave‐assisted HTL, which can be conducted in aqueous environment, is suitable for aquatic plants and wet biomass such as algae.
Keywords: algal biomass, crude bio‐oil, environmental impact, microwave assisted, renewable resources
Abbreviations
- CO2
carbon dioxide
- GHGs
greenhouse gases
- HTL
hydrothermal liquefaction
- LCA
life‐cycle analysis
- PHA
polyhydroxyalkanoates
- PLA
polylactic acid
1. INTRODUCTION
One of the main challenges in the 21st century is the development of technologies that enable to sustain an ever‐increasing demand of energy, chemicals and materials without significantly affecting the environment. The increase in worldwide energy consumption together with new guidelines and ongoing concerns about global climate change have led researchers to seek renewable materials and develop more sustainable processes to produce energy and chemicals. Current technologies are heavily based on fossil materials, which not only represent a limited resource and are increasingly difficult to extract, but are also heavily contributing to anthropogenic carbon dioxide (CO2) emissions. Climate change is one of the major problems due to huge amount of CO2 in the atmosphere. This carbon overload is mainly caused by the burning of fossil fuels including coal, oil and gas, and the unrestricted deforestation. There are other heat‐trapping gases (from methane to water vapour), but CO2 puts us at the substantial risk of irreversible changes if it is continuously accumulated in the atmosphere 1, 2. CO2 remains in the atmosphere longer than the other major heat‐trapping gases emitted from human activities. It is estimated that 40% of CO2 from the emission will remain in the atmosphere for 100 years and 20% will reside for 1000 years, while the final 10% will take 10,000 years to turn over 3. An established statistics illustrate that CO2 forms around 72% of total greenhouse gases (GHGs) emission and it has steadily increased 1.6 times in recent decades 4. Hence, the necessity of GHGs reduction alternatives has been globally highlighted. Renewables offer cost‐effective and readily available solutions to address the pressing environmental issues facing humanity and the planet at both global and local levels 5.
The climate change and the depletion of fossil fuel are spurring towards a transition from conventional fossil‐based economy to a bio‐based economy. The concept of a bio‐based economy, in which biomass resources are used for the production of energy and materials instead of fossil fuels, is gaining a lot of attention. Over the past decades, the global demand for materials has grown roughly in line with the world's human population and economy 6. Plastics have become indispensable for many applications throughout society. Both fossil fuel depletion and climate change are linked to the energy‐intensive nature of plastics. Plastics production uses energy carriers not just to supply process energy (e.g. heat, steam, electricity required to run a process) but also as feedstock. In 2015, over 99% of plastics produced globally were based on fossil fuel. As the fossil fuel reserves are non‐renewable, hence it is not sustainable 7. Plastic wastes are now found throughout the natural environment, including the ‘plastic soup’ in oceans. Plastic pollution can damage wildlife through entanglement and ingestion, and enter food chains after breaking down into microplastics 8. Some of the additives used in plastics have raised health concerns, for example related to toxicity and endocrine disruption 9, 10, 11.
Furthermore, a water‐quality study disclosed that roughly 83% of testing sites across five continents and dozens of samples are contaminated with plastic fibre. That number is even higher in the United States with 94% of the samples testing positive 12. Hence, the development of bio‐based material is suggested to solve the water pollution caused by the excessive amount of plastics. Bio‐based material refers to a product's main constituent consisting of a substance, or substances, originally derived from living organisms. These substances may be natural or synthesised organic compounds that naturally exist. Many of the modern innovations use bio‐based materials to produce biodegradable products. Bioplastics are plastics made from plants, including corn, sugar cane and algae. Conventional petroleum‐based plastic takes a long time to break down. When it does break down, it just disintegrates into smaller and smaller pieces that are eventually absorbed into the environment – mostly becoming more hazardous than its first form ever was.
PRACTICAL APPLICATION
Algal biomass can potentially be used to produce crude bio‐oil that can replace existing conventional bio‐oil, either for producing polymer or upgrading into fuel. The thermochemical conversion using microwave might also become a high potential implementation to enhance the productivity of oil in more economical way.
In Malaysia, National Biomass Strategy 2020 was launched in November 2011. It aims to access how Malaysia can gain more revenue through the utilisation of biomass not only from palm oil industry, but also from forestry sector and the dedicated crops on marginal land 13. Moreover, Biomass Technical Advisory Committee has developed a vision for Bioenergy and Bio‐based Products in the United States, established a long‐term goals that 20% of transportation fuels and 25% of chemicals and materials would be produced from biomass by 2030 14. The global biopolymers market is predicted to amass benefits from the use of bio‐based raw materials. The dependence on petroleum‐based plastics is gradually reducing with the development of bioplastics in numerous applications such as packaging and domestic goods. Although new bio‐based products development is preferred to reduce GHG emissions, the related production processes must be cost‐effective. The raw materials used in the production of biopolymers are originally renewable and are available in abundance across the world, compared to petroleum and fossil‐based raw materials. Thus, the global outlook for bio‐based raw materials for polymers is significantly more potential than conventional petroleum‐based raw materials. The increasing focus on sustainable production is one of the key trends in the bioplastics and biopolymers market. The growth of biodegradable polymers market is projected to be high as a result of the growing focus on sustainability and the advent of favourable government regulations for green procurement. In fact, a recent report predicts that the biodegradable plastics market will grow from $2 billion in 2015 to $3.4 billion by 2020. It is believed that Western Europe is the main contributor this growth due to strict regulations on single‐use petroleum products comprising almost half of the global market 12.
In particular, most studies in the renewable energy and sustainability sectors focus on the electricity generation from the alternatives of conventional fossil fuel. It cannot be denied that the potential of nuclear, wind, solar, and hydrotechnologies in the electricity generation sector is high. However, their potential is limited, as the energy derived is not in the liquid form and hence, it cannot directly replace the liquid fuels derived from fossil fuels. As most of the transportation and industrial engine require liquid fuel to operate, the biofuel obtained from biomass offers a superior advantage over the others. Besides, the biofuel derived can also be further processed to produce bio‐based material. In short, biomass can be converted into different forms of energy, chemicals and materials that are conventionally derived from fossil resources. Biomass is the only renewable source of carbon that can be transformed into gas, liquid and solid products. Furthermore, biomass has the potential to reduce the CO2 level in the atmosphere through photosynthesis during their growing process. Although CO2 is released during the burning of biofuel, however, no new carbon is emitted as it is part of the carbon cycle and so, it is a renewable process. Hence, the biomass technology might have more potential on emerging sustainable products development.
2. BIOMASS RESOURCES
Biomass is a carbon‐neutral energy source. The resources are abundant and broadly available on the earth 15. The utilisation of biomass to produce bio‐oil is well accepted both as an energy source and a feedstock for chemical production. The usage of biomass as renewable resource provides significant environmental advantages as it absorbs atmospheric CO2 during its growing. This eventually offsets the increase in atmospheric CO2 that results from fuel combustion. The CO2 emitted will then again be utilised to generate more biomass feedstocks 16. Moreover, based on the fact that there are abundant crop residue and limited forestry resource, significant efforts should be taken to utilise these residues to fulfil large‐scale industrial production of bioenergy.
Biomass is usually a heterogeneous mixture of organic substances with a small amount of inorganic substances, which is typically 30–40% oxygen, 30–60% carbon and 5–6% hydrogen on a dry basis, depending on the ash content. Other inorganic elements include nitrogen, chlorine and sulphur, which together make up less than 1% of the biomass. Generally, biomass is composed of cellulose, hemicellulose, lignin and extractives including proteins, ash and pectin 17. In practical, biomass sources are abundance which include a wide range of terrestrial plants, aquatic plants, agricultural crops and animal wastes. Most of them are considered as potential sources of fuels and chemical feedstock 18. The diversity of biomass sources has gained attention, and continuous research has been conducted to investigate the potential of biomass in replacing the conventional fossil fuel.
Looking into the current biomass scope that is actively being studied, the terrestrial plants may seem to gain more attentions. Terrestrial plants get their nutrients from two main sources which are soil and air. The roots absorb the water and minerals from the soil, as well as strongly hold the plant in place. Those essential nutrients are transported to cells in leaves by a system of tubes called vascular tissue. The leaves of terrestrial plant absorb the CO2 gas from atmosphere for photosynthesis process, where the second set of vascular tissue carries the food made by the leaves to the rest of the plant once the photosynthesis is done. Land plants are also equipped with woody stems and branches that hold them upright so that the plants are able to receive plenty of sunlight for photosynthesis.
Apart from the plant living on the land, plants living in water might also be biomass resources. Oceans cover most of the earth's surface. Almost 99% of organisms, approximately five million species (most of them unclassified), live in oceans 19. As a result, oceans are significant to the well‐being of life and economies. Aquatic plants or macrophytes are commonly multicellular and some of them consist of certain features, such as roots, leaves and stems. These plants are generally characterised by the place they live, and thus, they can be divided into three categories: floating, submerged and emergent. Algae are similar to aquatic plants; however they do not have roots, leaves or stems. Both algae and aquatic plants produce their own energy through photosynthesis, which means they have the ability to fix CO2 from the atmosphere in the presence of sunlight. At the same time of making organic carbon, they also release oxygen in the process. Algae are a food source for a number of species. They can be classified as primary producers in the ecosystem, and thus, they are located at the bottom of the food chain 20.
Generally, aquatic plants obtain their nutrients, water and dissolved gases from seawater. Since the entire marine plant is surrounded with water, these dissolved nutrients simply absorbed by each cell. Hence, marine plants do not have vascular tissue for photosynthesis process or to transport the products to each cell. In addition, marine plants do not require support structures because they are held up by the buoyant force of the water. The bodies of marine plants are flexible since water in the ocean is always moving, permitting them flexibly follow that movement. Some marine plants secrete mucus to make their surfaces slick, further reducing their drag or resistance to water movement 21.
Previously, it is often claimed that the aquatic habitat is less beneficial for growth of higher plants than the terrestrial plant, especially the availability of inorganic carbon is assumed to be a limiting factor for the photosynthesis and growth. Hence, these plants have evolved a number of strategies to cope with this situation. However, a research found that there are no significant differences in the growth rates 22. Hence, the potential of accumulating the aquatic plant as biomass resources should not be ignored. Besides, the comparison between the terrestrial plants and aquatic plants can be found in Table 1.
Table 1.
Aquatic plant and terrestrial plant comparison
| Plant type | Aquatic plants and algae | Terrestrial plants | Reference |
|---|---|---|---|
| Characteristic |
|
|
23 |
| Waxy layer | Lack a waxy coating because carbon dioxide is easier to absorb without this layer | Have waxy cuticles covering the tops of their leaves to resist evaporation. | 24 |
| Photosynthesis | Conducting photosynthesis under water | Conducting photosynthesis in the air | 25 |
| Sunlight absorption |
|
May have direct sunlight absorption if less competition with surrounding plants. The competition of absorbing sunlight might happen for high crop population area. | 25 |
| Carbon dioxide absorption | Absorb carbon dioxide (CO2) diffused in the water. CO2 diffuse slower in water than in air. | Absorb CO2 from the air. CO2 diffuse faster in air than in water. | 25 |
| Nutrient and water absorption | Plants absorbs nutrient directly from the water surrounding | Plants absorb nutrients and water from the ground through their extensive root system | 26 |
| Plant growth competition |
|
High competitive to arable land | 27 |
| Available space for crop development | Lots of space available for growth or cultivation development | Limited space for crop and development | 27 |
One of the promising biomass feedstock from aquatic resources is algae. Algae have a fast growth rate compared to other terrestrial plants and higher photosynthetic efficiency. Furthermore, algae able to grow in various liquid media, giving advantage to it since less competition for arable land. Hence, due to the prolific growth rate and lipid productivity, utilisation of waste CO2, and production of fuel precursors and high‐value biochemical, algal biomass gained increased attention as a feedstock for renewable fuels and chemicals production 28, 29, 30. There is a wide range of biomass conversion processes at varying stages of technical maturity. However, it is reported that the algal‐based oil production platform is technologically immature 28. Considering the conversion technologies of algal biomass specifically into phenolic bio‐oil, thermochemical conversion process might drive towards the solution. Although the previous research found that algal bio‐oil might has a lower density than lignocellulosic bio‐oil, and a viscosity in the typical range of wood bio‐oil, the potential of algae in producing high quality of phenolic bio‐oil compound is undeniable 31. Thus, the main purpose of this paper specifically is to focus on thermochemical conversion process of the algal biomass into the phenolic compound of bio‐oil.
3. ALGAE AS A POTENTIAL BIOMASS
The term algae originally refers to aquatic plants and it is now broadly used to include a number of different groups of unrelated organisms. Algae can be either single‐cell or large‐cell, multicellular organisms. They can be found in freshwater or salt water (most seaweeds are algae) or on the moist surfaces of soil or rocks. The multicellular algae generally lack of true stems, leaves or roots, although some of them consist of tissues that may be organised into structures to serve particular functions. The cell walls of algae are generally made of cellulose and can also contain pectin, which gives algae its slimy feel.
Algal biomass, as one of the potential biomass for biofuel production, is currently gaining much attention. It is considered as the third‐generation biofuel feedstock. Unlike the first‐generation biofuel feedstock (edible crop), such as soybean, palm tree, coconut and rapeseed, algal biomass does not create conflict with the food interest 32. Besides, the utilisation of algal biomass as biofuel feedstock will also reduce the conflict arose by the second‐generation biofuel feedstock (non‐edible crop), as some of the non‐edible crops are used for commercial application 32. Apart from biofuel production, algae also play an important role in serving as the biomass feedstock for application, such as waste treatment, CO2 mitigation, cosmetic production, synthesis of drug and pigments, and act as the biofertiliser, nutrition and food additives.
One of the significant advantages of using algae as the biomass source is that it can be grown very easily, and potentially achieve higher production rates of biomass compared to land‐based crops in term of the land surface area used. Algae are fast growing eukaryotic microorganisms that convert sunlight, water and CO2 into biomass by photosynthesis, and can be cultivated with inexpensive water and nutrients, such as municipal and agricultural wastewaters 33. Wastewater which normally hinders the growth of plants instead is very effective for growing algae. Algae are typically found growing in ponds, waterways or other wetlands which receive sunlight and CO2. They can grow in any kind of the water‐based area, while utilising photosynthesis for biomass production 34, 35.
Growth of algae varies on many factors, including temperature, sunlight utilisation, pH control, fluid mechanics and others 36. Man‐made production of algae tends to replicate the natural environments to achieve ideal growth conditions 35. For algal cultivation purpose, several factors that influence the growth rate are listed as below 37.
Temperature: The culture temperature varies with algae species. The temperatures higher than 35°C can be lethal for a number of algal species, while temperatures lower than 16°C may slow down the algal growth.
Light: Algae need about one‐tenth of direct sunlight for the growth in most cultivation. Bulk algal biomass may block light from reaching into deeper water, thus light only penetrates the top 7–10 cm of water in most water systems.
Mixing: Agitation or circulation is needed to mix the algal cultures.
Nutrients: Autotrophic growth needs carbon, hydrogen, oxygen, nitrogen, phosphorous, sulphur, iron and trace elements.
pH value: Algae prefer a pH from neutral to alkaline growth medium for efficient growth.
The number of products that can be produced from algae is virtually unlimited, due to the large assortment of species where the composition can be influenced by varying the conditions of cultivation. With only a few commercial algae‐based products available, this resource is largely untapped. The market of potential microalgae products is wide, including food, protein powder and edible oils 38. However, the main factors limiting the development of algae markets is the production and processing costs of algal biomass, mostly affected by the complexity of the cultivation phase and the downstream processes required to extract the high‐value products in a biorefinery concept. Despite these critical issues, and the photosynthetic efficiency, algae can (i) be produced on marginal or degraded lands, avoiding competition with other food crops; (ii) garner a significant amounts of lipids (for biodiesel, green diesel and other processes) or carbohydrates (for bioethanol); (iii) grow effectively without pesticides; (iv) grow in saline waters, hence can avoid effecting fresh water resources; (v) fix CO2 from flue gases and (iv) be cultivated on wastewaters consisting nutrients that are needed for algal growth 39, 40.
The technology of cultivation of algae and its contribution in reducing CO2 in atmosphere is actually well established. Algae can efficiently convert CO2 into biomass by their photosynthesis process 34. Some species of algae are able to produce up to 60% of dry weight in liquid form (oil) based on previous analytical studies. The cells grow in aqueous suspension, where they can effectively acquire water, CO and nutrients and capable to produce huge amount of biomass and usable oil in either with the help of photo bioreactors or high rate algal ponds 35.
3.1. Diversity of algae species
There are numerous types of algae, which are normally differentiated by their species and also size ranges 35. Two major classifications of algae are microalgae and macroalgae. Macroalgae and microalgae can either be heterotropic or autotropic. Autotropic algae need inorganic compounds, such as CO2 and a light energy sources for growth. Heterotrophic algae are non‐photosynthetic and require an external nutrient for growth. Some of photosynthetic algae are mixontropic that acquire exogenous organic nutrients and perform photosynthesis as well 34, 41.
Macroalgae, or widely known as seaweeds, are multicellular organisms and belong to the category called 'lower plants', consisting of a leaf‐like thallus instead of leaves, stems and roots 27, 42, 43. Seaweed is an established term embracing macroscopic, multicellular, benthic marine algae which includes some major classification which are red, brown and green algae. They are photosynthetic, like plants, and 'simple' because they lack many distinct organs found in terrestrial plants 44. Typically, macroalgae consist of (i) thallus: the body; (ii) lamina/blade: a leaf‐like flattened structure; (iii) sorus: a spore cluster; (iv) fucus/air bladder: a hollow structure that is filled with gas, which can be found on the blade to help the macroalgae to float; (v) floats: organs that help the macroalgae float, and is located between blade and stipe; (vi) stipe: a stem‐like structure, might be absence; (vii) holdfast: a specialised structure that holds the macroalgae in place; (viii) haptera: finger‐like extensions of holdfast to allow the macroalgae anchor to benthic substrate. The basic structure of macroalgae is shown in Figure 1.
Figure 1.
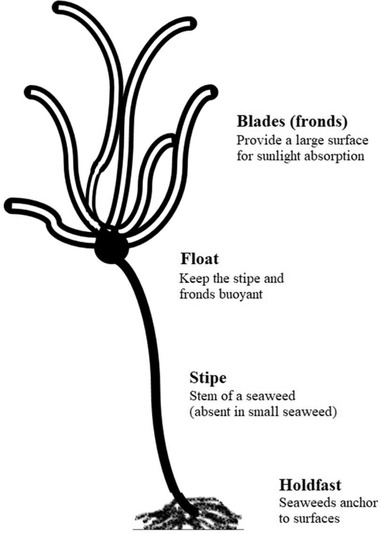
Basic structure of macroalgae
Microalgae comprise a vast group of photosynthetic, heterotrophic organisms which have an extraordinary potential for cultivation as energy crops. Microalgae are microscopic organisms that can rapidly grow in both salt and fresh water environments 45. In fact, it can also be grown in the extreme environments, such as ice or hot springs. From the previous research, microalgae are thought to be one of the most primitive life forms on the earth with a very fast growing rate 27, 46. Microalgae can survive in diverse ecological habitats. In other words, they are able to grow in flexible conditions, even at high temperatures and growth medium with high pH value. Furthermore, microalgae are able to divide their cells within 3–4 h, but mostly divide every 1 and 2 days under favourable growing conditions 47. These abilities make microalgae the most abundant living organisms on earth. For cultivation purpose, they can also be cultivated under difficult agro‐climatic conditions and are able to produce a wide range of commercially interesting by‐products 44.
Microalgae can be unicellular and multicellular microorganisms, including prokaryotic microalgae that are cyanobacteria (chloroxybacteria) and eukaryotic microalgae for example, green algae (chlorophyta) and diatoms (bacillariophuta) 35. The production of biofuel from microalgae is widely for several reasons: (i) they have higher biomass production of oil crops per unit surface area which is up to 30 times; (ii) less competition with traditional agricultural resources as they can be cultivated on non‐arable land or on wastewater 31, 48; (iii) rich oil content, around 20–50% dry weight of biomass in most of the species 49.
Although microalgae relatively gain much more attention compared to macroalgae for research purpose, macroalgae also have undeniable potential to be developed and improved for bio‐based substance production. The differences between macroalgae and microalgae are presented in Table 2.
Table 2.
| Algae classification | Macroalgae (known as seaweed) | Microalgae |
|---|---|---|
| Growth |
|
|
| Plant type |
|
|
| Main classes |
|
|
| Main commercial use |
|
Biofuel and biodiesel production |
| Example of commercially used algae species |
|
|
| Contents | Mostly carbohydrate and some protein |
|
| Advantages |
|
|
3.2. Algae potential in Malaysia
Algae species have a flexible grow and can survive over a wide range of temperatures. However, the maximum algae productivity can be obtained for some particular algae strain at specific range of temperature. The area with average ambient temperature below 15°C, which is shown outside the purple rectangle map in Figure 2, was assumed to be unpropitious for achieving high algae productivity. The regions within the blue rectangle map, however, are considered to have suitable condition and have a huge potential to maximise the algae productivity, and Malaysia is located within this area 53.
Figure 2.
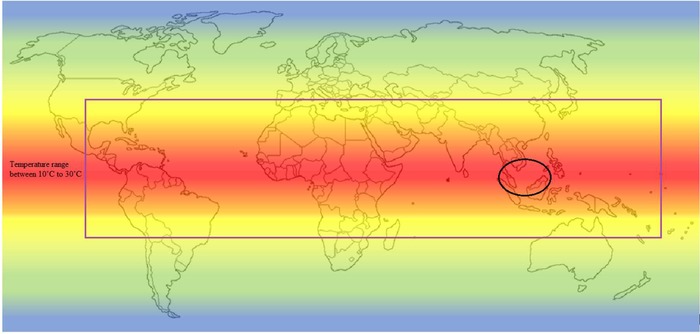
Temperature zone projected to be suitable for algal biomass production
Malaysia is located in a geographic area covering the waters adjacent to six countries (i.e. Indonesia, Malaysia, Papua New Guinea, the Philippines, the Solomon Islands and Timor‐Leste) in Southeast Asia and the Pacific, or generally known as the Coral Triangle. Malaysia is situated at the coordinate of 4.2105°N, 101.9758°E which is located near the equator line and the climate is categorised as equatorial, being hot and humid throughout the year, 250 cm (98 in.) average rainfall a year and 27°C (80.6°F) average temperature. The tropical conditions in the coastal waters of Malaysia provide a favourable environment for the growth and production of diverse types of algae species 54. Geographically, the South China Sea borders Peninsular Malaysia in the east and both Sarawak and Sabah in the north that has natural advantages for algae culture. Malaysia has various salt lakes that offer researchers advanced algae‐based technology. Hence, Malaysia region is surrounded by sea and has an extensive coastline fringed by numerous islands, providing various habitats for the proliferation of tropical algae.
Until recently, more than 100,000 algae species have been identified. According to the general estimates, around 400,000 species of algae exist worldwide 55. Malaysia also has the vast algae resources. Several studies have been carried out in Malaysia, and the reports show that 375 specific and intra‐specific taxa have been identified in Malaysia with reference to the regular collections and documentations of algae strains up to 2016 27, 56, 57. The number of known species of marine algae in Malaysia and the world are presented in Table 3 58.
Table 3.
Estimates number of known species of marine algae as reported by Mazlan et al
| Major categories | Groups | Estimate numbers in Malaysia | Estimate numbers in the world |
|---|---|---|---|
| Seaweed and other algae | Chlorophyceae | 78 | 800 |
| Rhodophyceae | 69 | 4000 | |
| Phaeophyceae | 49 | 1500 | |
| Cynophyceae | 13 | 1500 | |
| Microalgae | Diatoms | 70 | 4200 |
| Dinoflagellates | 30 | 1200 |
The use of algae as a potential biomass feedstock is still in infancy in Malaysia and has been receiving a lot of interest in recent years. Microalgae and macroalgae typically have their own development strategy, and the current cultivation development of both algae is typically exiled. A review data state that 31 countries and territories are recorded with algae farming production, and 99.6% of global cultivated microalgae production comes only from eight countries, as shown in Figures 3 and 4. Malaysia plays a role in global algal production. As one of the major microalgae‐producing country, Malaysia has contributed about 1% of annual microalgae production, which is approximately 207,900 tons compared to those other major microalgae‐producing countries 27, 59. Meanwhile, about 1% of global macroalgae seaweed production is also attributed by Malaysia.
Figure 3.
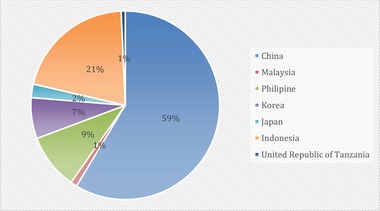
Malaysia annual microalgae production in comparison to major algae‐producing countries
Figure 4.
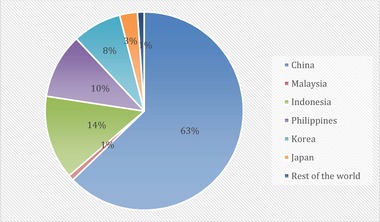
Global seaweed production up to 2018 [50]
The fundamental studies in algal research have shown in many publications that recorded the microalgae diversity in Malaysia 27. Besides microalgae, macroalgae also gained attention on some research area in Malaysia, such as research and commercial applications 60, 61. A study on red algae or scientifically known as Rhodophycaea has been reported by a group of researchers from Malaysia 62. The research found that besides being a sustainable source of energy, red algae has exciting prospects in several product markets. The primary algae project at the university lab, in fact, is creating paper from red algae. From that particular research, red algae are preferable to wood as a source material for paper products. This shows that the composition in algae has the potential to be used as a source of diverse type of application. Table 6 shows the list of several established algal species with their respective biochemical composition on dry matter basis.
Table 6.
Development, Strategies and Policy of Seaweed industry in Malaysia
| 1973 | Cultivation of seaweed Eucheuma cottonii (Webervan Bosse 1913) has been introduced to the Semporna district of eastern Sabah by The State government of Sabah 70, 71 |
| 1980 | A seaweed project has been initiated in Sabah by the Federal government. It was initially failed due to lack of support from local people, and the techniques and knowledge for cultivation were not yet properly implemented 54 |
| 1988–2001 | Seaweed production started to rise and grow |
| 2002 | Seaweed production declined to its lowest level |
| 2004 | Seaweed production gradually improved 72 |
| 2008 | Malaysia was ranked ninth among the top 10 seaweed‐producing countries (0.4% contribution of the world seaweed production 71; 111,298 ton of total growing seaweed 71 which is from |
|
|
| 2010–2015 (10th Malaysia Plan) | The promotion and development of the seaweed industry also procured importance in that plan 54 |
| 2011–2020 (Malaysian National Agro‐Food Policy) | Sea weed was considered a high‐value commodity 72 |
| By 2020, through the seaweed industrial zone development, the Ministry of Agriculture and Agro‐Based Industries aims to | |
|
|
| 2013 | Malaysia was ranked eighth, produce about 269,431 ton of seaweed (1% of the total worldwide seaweed production) 73 |
| 2014–now | Seaweed industry gradually improved |
| 2015–2020 (11th Malaysia Plan) | The plan did not directly refer to the seaweed industry, the industry was included in various key areas related to coastal development, conservation of natural resources, and improvement of the livelihood of coastal area populations 54 |
Besides, studies have been conducted to identify the suitable algal species to be a source of alternative fuel and chemical feedstock. Numerous efforts have been also undertaken mainly in Sabah and Sarawak area in order to produce algal biomass. Algae are a promising source for crude bio‐oil production as it will not undermine to the other products derived from crop plants 63. Although palm oil is one of the major raw materials for biomass in Malaysia and has been widely investigated, the production of palm oil is insufficient to meet the current demand, and also there is conflict with the food interest 27. Biochemical composition of algae expressed on a dry matter basis (%dry weight) is shown in Table 4 and some of the potential products or application of algal biomass is shown in Figure 5.
Table 4.
| Algae | Protein | Carbohydrates | Lipid |
|---|---|---|---|
| Macroalgae | |||
| Acanthophora spicifera | 12.0–13.2 | 11.6–13.2 | 10.0–12.0 |
| Boergesenia forbesii | 7.43 | 21.38 | 11.42 |
| Caulerpa fergusonii | 7.76 | 23.63 | 7.15 |
| Caulerpa peltata | 6.41 | 45.00 | 11.42 |
| Caulerpa racemosa | 11.8–12.5 | 16.0 | 9.0–10.5 |
| Chaetomorpha aerea | 10.13 | 31.50 | 8.50 |
| Chaetomorpha antennina | 10.13 | 27.00 | 11.45 |
| Codium tomentosum | 5.06 | 29.25 | 7.15 |
| Dictyosphaeria cavernosa | 6.00 | 42.75 | 10.51 |
| Enteromorpha compressa | 7.26 | 24.75 | 11.45 |
| Halimeda macroloba | 5.40 | 32.63 | 9.89 |
| Laurencia papillosa | 11.8–12.9 | 12.0–13.3 | 8.9–10.8 |
| Ulva lactuca | 11.4–12.6 | 11.6–13.2 | 9.6–11.4 |
| Ulva reticulata | 12.83 | 16.88 | 8.50 |
| Valoniopsis pachynema | 8.78 | 31.50 | 9.09 |
| Microalgae | |||
| Anabaena cylindrical | 43–56 | 25–30 | 4–7 |
| Botryococus braunii | 8–17 | 8–20 | 21 |
| Chlamydomonas rheinhardii | 48 | 17 | 21 |
| Chlorella vulgaris | 51–58 | 12–17 | 14–22 |
| Dunaliella bioculata | 49 | 4 | 8 |
| Euglena gracilis | 39‐61 | 14‐18 | 14‐20 |
| Isochrysis sp. | 31–51 | 11–14 | 20–22 |
| Neochloris oleoabundans | 20–60 | 20–60 | 35–54 |
| Porphyridium cruentum | 28–39 | 40–57 | 9–14 |
| Scenedesmus obliquus | 50–56 | 10–17 | 12–14 |
| Tetraselmis maculate | 52 | 15 | 3 |
Figure 5.
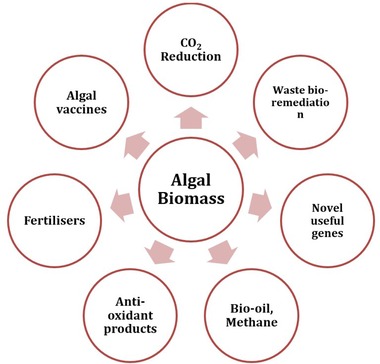
The potential products and application of algal biomass 67
The majority of the seaweed production in Malaysia comes from Semporna, which is located at the East coast of Sabah 68, 69. Hence, Sabah is the main seaweed producer in Malaysia. However, Malaysia still has huge potential in becoming the top leader player in global seaweed production as Malaysia consists of a lot of potential areas for seaweed cultivation. Table 5 records some of the macroalgae (seaweed) species and their respective location in Malaysia.
Table 5.
Location and habitat of various kinds of seaweed in Malaysia 57
| Macroalgae (Seaweed) species | Location in Malaysiaa | Habitatb |
|---|---|---|
| Acanthophora spicifera | W, E, P | C, D, R, S |
| Boergesenia forbesii | E | C, E, R |
| Caulerpa fergusonii | W | R |
| Caulerpa peltata | W, E | C, R, S |
| Caulerpa racemosa | W, E, Sb | C, M, S |
| Chaetomorpha aerea | E | ‐ |
| Chaetomorpha antennina | W | R |
| Codium tomentosum | E, P | C, R |
| Dictyosphaeria cavernosa | W, E | C, R |
| Enteromorpha compressa | W | ‐ |
| Halimeda macroloba | W, E | C, S |
| Laurencia papillosa | W, E, Sb, Sk | C, R, S |
| Ulva lactuca | W | D |
| Ulva reticulata | P, W | D |
| Valoniopsis pachynema | W | R |
Distribution: E, East Coast Peninsular Malaysia; P, Peninsular Malaysia; Sb, Sabah Malaysia; Sk, Sarawak Malaysia; W, West Coast Peninsular Malaysia.
Habitat: C, coral; D, driftweed; E, epiphyte; M, mud; R, rock, bedrock and stones; S, sand.
Sabah is geographically situated below the monsoon and typhoon belt, and, therefore, it is known as the land below the wind. Sabah is located on the Island of Borneo and well known as the only part of Malaysia where seaweed is cultivated commercially 54. The eastern coast of Sabah has a suitable environment for growing good value seaweed that includes several species from red seaweed and the green seaweed 57. Figure 6 highlights the potential locations in Sabah for seaweed growing.
Figure 6.
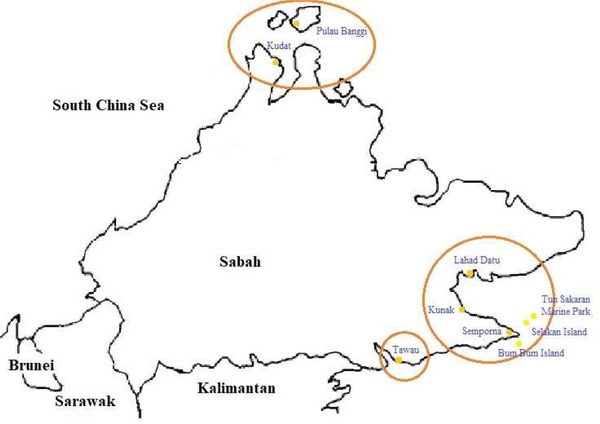
Location of seaweed growing areas in Sabah; Banggi, Kudat, Lahad Datu, Kunak, Semporna, Selakan Island, Bum Island and Tawau (extracted information from 54)
Other than as a sustainable nutrient‐dense food source, seaweed is acknowledged to have wide application potentials similar or even better than other established biomass resources, such as palm oil and cocoa. Seaweed‐based industries, including seaweed cultivation or farming had started and are gradually developing. Besides, apart from the government agencies focussing on socio‐economy development for rural peoples, there are also private local companies venturing in seaweed processing and cultivation for a larger scale in Semporna.
Cultivation of seaweed or macroalgae in Sabah can be traced back to 40 years. The development of seaweed production has been improved progressively from time to time since the early stage of development, and eventually seaweed has become an economically important natural resource for Malaysia. Table 6 shows the seaweed evolution since the early stage of development 54 and Figure 7 shows the increasing production of seaweed in metric tonnes based on its dry weight.
Figure 7.
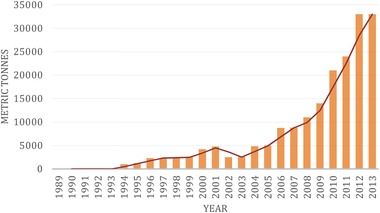
Total production of Seaweed in Sabah, Malaysia (1989–2013) based on the dry weight)
Source: Sabah Annual Fisheries Statistic 74.
Currently, there are plenty of companies, government entities, scientists and researchers actively working around the world to develop the potential products from algae. In the United States alone, there are more than 50 research institutions and over a 100 companies working on algae technologies across the value chain. These companies and institutions are working in all aspects of the algae product life‐cycle including identifying and optimising specific strains of algae, developing growth and cultivation systems, enhancing refining technologies, farming large quantities of algae and oil production. In Malaysia, there are several established company that focussing on algae development such as Algaetech International Sdn Bhd (Algaetech Malaysia) 75 and Algae International Berhad (AIB) 38. Those companies have made algae competitive with fossil fuels at current prices. This is a major inflection point for the energy industry. In short, Malaysia has the potential to be a major seaweed player in the region including infrastructure, manpower, product quality, transfer of technology, industrial support and marketing. Malaysia has reliable resources for both microalgae and macroalgae, which can be optimally utilised. It is believed that if more efforts are put into improving the seaweed industry, the seaweed production volume and value will further increase.
3.3. Conversion strategies of algae biomass
Biomass conversion from its natural solid form to liquid fuels is not an unsolicited process. The conventional liquid fossil fuels that have been harnessed on a large scale took thousands of years of geochemical processing to convert biomass into crude oil and gas. Since then, several conversion technologies of biomass have been developed to obtain liquid products for fuels and chemicals usage. The technologies of conversion are broadly classified into two categories, namely biochemical and thermochemical conversion 18. In order to obtain liquid products from different sources, the main difference between both is that thermochemical conversions are processed at several higher degrees of temperature compared to biochemical conversions in the presence of appropriate catalysts. However, thermochemical conversions are generally much rapid and commonly carried out in much shorter time than the biochemical conversions. Thermochemical conversion implied to upgrade biomass by heating under pressurised and oxygen deprived enclosure 18. Moreover, one of the main advantages is that the thermochemical conversion has the potential to be integrated into the existing petroleum‐processing infrastructure 31.
Algae biomass is a type of biomass that has high moisture content. The aqueous phase of algal feedstocks has biogenic carbon, phosphorous, nitrogen and micronutrients that can be recycled for algal cultivation purposes. In addition, by extraction and catalytic processes, high‐value chemicals such as ethanol, acetone and acetic acid can be obtained 17. Hence, the conversion process involving drying requires a large amount of thermal energy due to the high latent heat of vaporisation of water 76. From Figure 8, the thermochemical algae biomass can be processed with three conversion method categories which are transesterification, biochemical conversion and thermochemical conversion, while each category consists of several specific conversion methods. Each conversion method of algae will produce a different end product which is listed in Table 7.
Figure 8.
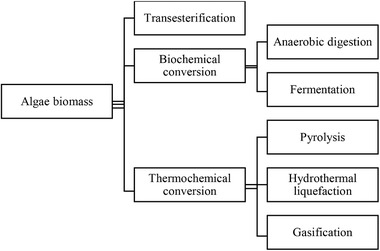
Current strategies of biofuel production from algal biomass
Table 7.
| Conversion method | Method description | Advantages | Disadvantages | Products |
|---|---|---|---|---|
| Transesterification | Converting biomass into biodiesel by the reaction of biomass feedstock catalytically with a short‐chain aliphatic alcohols 81 | No dewatering | Poor yield | Biodiesel |
| Anaerobic digestion | Biological processes in which microorganisms break down biodegradable material in the absence of oxygen |
|
High nitrogen and sodium inhibition | Methane, hydrogen |
| Fermentation | The chemical process by which molecules such as glucose are decomposed anaerobically. It can involve complete decomposition of the glucose to carbon dioxide and water (with energy) or can be adapted to produce ethanol (ethyl alcohol and energy) 82 | High carbohydrates contents | Low efficiency, mixed sugars | Ethanol |
| Pyrolysis | Biomass feedstock is subjected to high temperatures at low oxygen levels and it may be carried out under pressure |
|
High temperature and energy consumption | Bio‐oil, biochar, syngas |
| Hydrothermal liquefaction | Converting into liquid form at low temperature with high pressure thermochemical conversion process carried out in the liquid phase | Drying not required | – | Bio‐oil, biochar, syngas |
| Gasification | Converting a solid or liquid form of biomass into a gaseous fuel without leaving any solid carbonaceous residue 81 | – | – | Syngas |
In short, the selection of conversion methods depends on the desired form of the end product and techno‐economic considerations. A large number of scientific works pointed out that the biofuel production from algae is technically feasible 39, 77, even if not yet optimised where the positive economical and energy balance achievement is still under demonstration 39, 78, 79, 80.
4. THERMOCHEMICAL CONVERSION PROCESS OF ALGAL BIOMASS TO PRODUCE BIO‐OIL
Algae cultivation, algae harvesting, algal biomass to bio‐oil conversion and utilisation of co‐products from extracted algal oils are the important steps in algal bio‐oil production. Those several steps make bio‐oil production process and energy more intensive. The conversion process of algal biomass has significant techno‐economic challenges in algal bio‐oil commercialisation. The major challenges include (i) less efficiency of harvesting method; (ii) require high energy for drying algae; (iii) consumption of large amounts of hazardous chemicals in lipid extraction; (iv) extensive process of lipid separation and purification; (v) high cost of the conversion processes. Therefore, it is necessary to initiate a simple and eco‐friendly conversion that diminishes the chemical and energy consumption, and duration of overall process in bio‐oil production 83.
Thermochemical processing technologies have been employed since 18th century to convert biomass into crude bio‐oil. They are gaining prevalent interest as an alternative to accommodate the energy demands while tackling the arise concerns on environment related to increasing global warming issue and limited fossil fuel reserves 17, 76. Thermochemical conversion is the thermal decomposition of organic matter in biomass for oil production. Moreover, thermochemical conversion has the potential to be integrated into the existing petroleum‐processing infrastructure 31.
Bio‐oil production from algae is a straightforward process that consisted of growing the algae by providing necessary inputs for photosynthesis, harvesting, dewatering and oil extraction. Algae cells absorb energy in the form of photons, which convert inorganic compounds of CO and water into sugars and oxygen. The sugars are eventually converted into complex carbohydrates, starches, proteins and lipids within the algae cells. In order to extract the valuable lipids, a series of steps must be undertaken to isolate the algae cells and oil 35. However, avoiding the drying step is a significant advantage by wet extraction. In wet pathways, cell disruption can be based on mechanical approaches such as microwave, ultra‐sonication, high‐pressure stresses, sudden changes of pressure and others. Meanwhile, the biological approaches include the use of enzyme for cell disruption or osmotic stresses, or else thermochemical processing such as hydrothermal liquefaction (HTL) 39.
Pyrolysis and HTL are both technologies to maximise liquid products from biomass. These liquids produced are, however, not one‐to‐one replacements for all petroleum products. The liquids can directly replace petroleum products for heating and electricity purposes and some chemical compounds. The replacement of some other chemicals and transportation fuels requires an additional upgrading step, in which the quality of the bio‐oil/biocrude is elevated to the higher requirements of those products 84, 85, 86. The bio‐oil produced by these technologies can be used to replace petroleum in other markets as well. The scope of the research will include all those markets, as they all contribute to the technological development of Fast Pyrolysis (FP) and HTL 84. Figure 9 shows the process overview of algal bio‐oil and the application.
Figure 9.
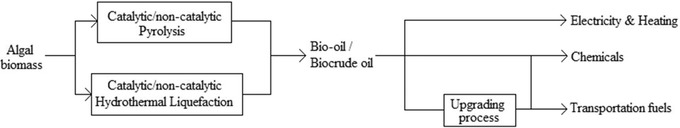
Overview of algal bio‐oil process and application
In thermochemical conversion process, these biomass pyrolysis and HTL are two comparable technologies, as they both can produce bio‐based intermediate products which is referred as crude bio‐oil. However, the complex associated reaction pathways of these technologies are not yet clear, and many researchers are at present focussing on understanding these pathways [76]. The present work examines the possible routes for thermochemical conversion of algae into liquid biofuels, distinguishing between dry processes, namely pyrolysis and wet processes (near‐critical water) using HTL 39.
In the recent years, the phenolic‐rich bio‐oil production has garnered some attentiveness to be the desirable compounds for the fuel properties of bio‐oil because their low oxygen contents notably enhance the heating value of bio‐oil. Phenolic compounds also cover a wide application range for the production of energies and fine chemicals 87. Although algal bio‐oil might has a slightly lower density than lignocellulosic bio‐oil, and a viscosity in the typical range of wood bio‐oil 31, the potential of algae in producing high quality of phenolic bio‐oil compound is undeniable.
4.1. Pyrolysis of algal biomass
Pyrolysis is one of the eco‐friendly and cost‐effective conversion technologies to extract bioenergy from biomass 88. It is the thermal disintegration process of biomass operating in the absence of oxygen. Pyrolysis is the conversion of biomass into bio‐oil, biochar and syngas (CO2, carbon monoxide, hydrogen and methane). This particular thermochemical process is commonly carried out at atmospheric pressure and high temperature range, from 300°C to 700°C and above. The yield and properties of the pyrolysis products depend on the pyrolysis temperature, heating rate, residence time and catalyst 31, 39, 89. Pyrolysis is classified into stages which are shown in Table 8.
Table 8.
Classification of Pyrolysis
| Pyrolysis | Temperature | Heating rate | Main yield |
|---|---|---|---|
| Slow pyrolysis | Low temperature (less than 450°C) | Slow heating rates | Biochar |
| Fast pyrolysis | High temperature (higher than 800°C) | Rapid heating rates | Gases |
| Intermediate pyrolysis | Intermediate temperature | Under relatively high heating rates | Bio‐oil |
Broadly, there are two main classes of pyrolysis: slow pyrolysis and fast pyrolysis 90. The difference between both is the heating rate that is used during the process. Slow pyrolysis is characterised by lower heating rates, and with the purpose of maximising the char yield. On the other hand, fast pyrolysis is heated at rapid rates and thereby maximises the liquid yields 84, 91. Usually, fast pyrolysis involves high operating temperatures, very short contact times (residence time) and fine particles 92. Moreover, pyrolysis can be catalytic, as well as non‐catalytic 84, 93. Pyrolysis processes is applicable for a broad range of biomass feedstocks. The pyrolysis process is very dependent on the moisture content of the feedstock, which should be around 10%. At higher moisture contents, high levels of water are produced and at lower levels there is a risk that the process only produces dust instead of oil. Generally, high‐moisture waste streams require drying before subjecting to pyrolysis 89.
Besides those main classes, some variations have been put forward. There is a reaction that is slightly quicker than slow pyrolysis, known as intermediate pyrolysis 94. The heating rate of intermediate pyrolysis is significantly lower than in fast pyrolysis, and residence time of intermediate pyrolysis is much longer. The derived products are more evenly distributed between liquid, char and gas compared to fast pyrolysis. In addition, a very fast pyrolysis is also introduced as flash pyrolysis, in which requires even higher temperatures and shorter residence times compared to fast pyrolysis. The products derived by flash pyrolysis contain a higher oil yield, however, there are still a lot of technological limitations. Therefore, fast pyrolysis has gained popularity in producing liquid yields 95.
4.2. Hydrothermal liquefaction of algal biomass
HTL is another propitious route of biomass conversion that recently drawn attention. HTL is actually similar to hydrous pyrolysis but is operated at lower temperatures and heating rates compared to pyrolysis 18. HTL also involves the thermochemical conversion of diverse type of biomass in the presence of hot compressed water at subcritical water conditions to produce bio‐oil 17. The HTL process convert biomass into a crude bio‐oil containing monomeric to oligomeric compounds, which mimics the natural geological process of fossil fuels production 33. The HTL of algal biomass involves liquefaction process in the presence of water, a hydrogen donor solvent at temperature below 400°C and pressure between 4 and 20 MPa for 5–30 min, where subcritical water conditions (temperature range of 250–350°C) are typically reached 84, 96, 97. Hydrothermal processing that involves thermal decomposition of biomass in hot compressed water, wherein a series of complex reactions causes changes in the physical properties of the water including density, solubility and dielectric constant 17, 98. Following HTL, energy rich bio‐oil and biochar can be obtained and easily separated.
It obviously shows that hydrothermal processing technologies have notable potential for high moisture content biomass processing. Conventional technologies is generally not an economical option for the biomass with high moisture content such as algae, as a quite large amount of energy required for drying process and incur high costs for drying and dewatering 35, even the energy required for drying may exceeds the energy used for hydrothermal processing at supercritical conditions for the moisture content higher than 30%. Thus, the use of aqueous phase organics is an option where the operating costs of the hydrothermal technology can be reduced, as using organics helps reduce wastewater treatment costs 17, 99. Therefore, HTL is suitable to accommodate the production of crude bio‐oil from various biomass resources with varying moisture content including wood, waste, and algae‐based biomass 76. HTL can directly convert wet biomass into liquid bio‐oil either with or without a catalyst 39, 100. HTL mainly produces the product in liquid form. At even higher temperatures, gasification reactions dominate, which results in the production of gas 101. The latter happens above the critical point of water 102. An overview of the main classes is given in Figure 10.
Figure 10.
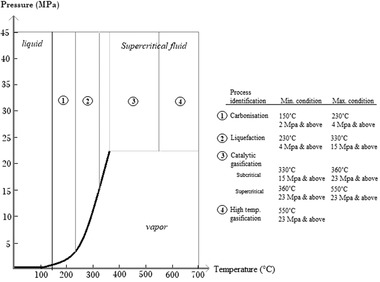
Graphical overview of hydrothermal processing variations 84, 97, 101
This study focusses on HTL to mainly produce liquids. Compared with gasification, there are more publications and reports on HTL of algae which could directly produce liquid oil. Previous research reported the yield of bio‐oil, which is estimated to be in the range of 10–50% with a heating value of 35–40 MJ kg−1, slightly lower than petroleum crude oil of 43 MJ kg−1 heating value 103, 104, 105. However, some other research found that the typical HTL oil yield reported in several studies is equal to approximately 50–60% depending also on the use of homogeneous or heterogeneous catalysts 104. The bio‐oil from HTL contains approximately 10–20% of oxygen and nitrogen, with energy density in the range of 30–37 MJ kg−1 39, 106. Meanwhile, 107 operated a continuous‐flow HTL reactor to decompose several different algae feedstocks at 350°C and obtained a crude bio‐oil yield around 38–64% 33.
The efficiency, yield and quality of algal bio‐oil by HTL conversion are influenced by several parameters such as feedstock composition, reaction temperature and residence time, retention time, biomass loading and presence of catalysts. Different from the algae‐to‐biodiesel pathway, which essentially only depends on the algae strain and lipid contents, HTL and pyrolysis can be used to convert not only the lipid fraction of microalgae but also the other organic components including proteins and carbohydrates 39, 104.
4.3. Comparison between pyrolysis and hydrothermal liquefaction
Pyrolytic bio‐oil consists of compounds with lower mean molecular weights and contains more low boiling compounds than bio‐oil produced by HTL 31.
In data, it is found that the value of the HTL bio‐oil was increasing with the temperature while it was constant for pyrolysis (Table 9). According to previous experimental research, the HTL led to bio‐oil yield decreasing from 67% mass fraction at 220°C to 59% mass fraction at 310°C, whereas the bio‐oil yield increased from 53% mass fraction at 400°C to 60% mass fraction at 550°C for pyrolysis. Energy ratios (energy produced in the form of bio‐oil divided by the energy content of the initial algae) between 66% at 220°C and 90% at 310°C in HTL were obtained, whereas it was in the range 73–83% at 400–550°C for pyrolysis. Algae cultivation in aqueous phase produced by HTL was also investigated and showed promising results 108. Furthermore, Hu et al. concluded that HTL produced higher yield of bio‐oil and lower yield of biochar for the same raw samples compared with pyrolysis process 109. The comparison between the pyrolysis and HTL process using different algal biomass is presented in Table 10.
Table 9.
| Process | Pyrolysis | Hydrothermal liquefaction |
|---|---|---|
| Operating temperature | 300–700°C | 250–350°C |
| Operating pressure |
|
Higher pressure 4–20 MPa |
| Feedstock | Suitable for dry feedstock | High‐moisture biomass |
| Process condition |
|
Absence of oxygen |
| Drying feedstock | Drying process needed | No drying process |
| Energy density | Produce a bio‐oil that contains approximately twice the energy density of pyrolysis oil | 30–37 MJ kg |
| Oil yield relative to operating temperature | Constant | Increasing with the temperature 50–60% |
| Water content (moisture of biomass) | Less than 40% | 80–85% |
| Feedstock | Low moisture content | High moisture content |
| Oil quality | Low | High |
Table 10.
Comparison between pyrolysis and hydrothermal liquefaction using different algal biomass
| Methods | Algal biomass | Temperature (°C) | Bio‐oil yield (%) | Remarks | Reference |
|---|---|---|---|---|---|
| Pyrolysis | Chlorella vulgaris | 400 | 19.7 | Non‐catalytic, fixed bed reactor | 110 |
| Enteromorpha clathrata | 500 | 41.2 | 50 L h−1 carrier gas flow | 111 | |
| 550 | 29.56 | Fixed bed reactor | 112 | ||
| Saccharina japonica | 350 | 44.99 wt | Fluidised bed reactor | 113 | |
| Sargassum natans | 500 | 33.7 | 50 L h−1 carrier gas flow | 114 | |
| Hydrothermal liquefaction | Alaria esculenta | 350 | 17.8 | Batch‐type reactor | 115 |
| Enteromorpha clathrata | 550 | 32.52 | Autoclave reactor, co‐solvent | 112 | |
| Laminaria saccharina | 350 | 35.97 | 16.5 bar, muffle furnace | 116 | |
| 350 | 13 | Batch‐type reactor | 115 | ||
| Laminaria digitata | 350 | 17.6 | Batch ‐type reactor | 115 | |
| Laminaria hyperbore | 350 | 9.8 | Batch‐type reactor | 115 | |
| Nannochloropsis Oceanica | 260 | 54 | Batch reactor, co‐solvent | 117 | |
| Oedogonium macroalgae | 300 | 25 wt | Co‐solvent, batch reactor | 118 |
4.4. Advancement in liquefaction process of algae using microwave to produce bio‐oil
The microwave‐assisted technology in organic chemistry emerges in the mid‐1980s, and there has been a significant increase in the number of publications on microwave‐assisted organic reactions since the 1990s 119, 120 because of increased valuable application associated with the process. The encouragement of microwave‐assisted reactions in organic chemistry has improved the speed, reduced energy and cost spent, brings it to become a sustainable process 121, 122 and are continuously encouraged until today's applications to reduce the non‐renewable resources as well as polluting solvent, and to minimise secondary toxic products generation and reduce harmful gases emission 123, 124, 125. Microwave‐assisted reactions in organic chemistry attain the same by faster reactions under bulk conditions and reducing the reaction time 119, 124. Scientists investigate the microwave dielectric heating mechanism and identify the advantages of the chemical synthesis 126. Table 10 shows the advantage and disadvantage of microwave reaction.
Microwave energy engages as a part of electromagnetic spectrum, characterised by being situated in the wavelength interval between 1 mm and 1 m, and frequency interval, and consists of electromagnetic radiation which operates at the high‐frequency waves, ranging between 0.3 (300 MHz) and 300 GHz 127, 128 (Table 11). Electromagnetic waves with a higher frequency are associated with higher energy and shorter wavelengths 32. In the electromagnetic spectrum, the microwave radiation region is located between infrared radiation and radiowaves. Microwaves are a form of electromagnetic energy which is a non‐ionising radiation that causes molecular motion by migration of ions and rotation of dipoles but does not cause change in molecular structure.
Table 11.
| Advantages | Rapid in reaction |
| Produce products with high purity | |
| Minimum unnecessary side‐products | |
| High product yields | |
| Synthetic procedure is simplified and improved | |
| Flexible usable operating temperature | |
| Energy‐efficient | |
| Cost‐saving | |
| Environmentally friendly | |
| Sophisticated measurement and safety technology | |
| Modular systems enable changing scale adjustment | |
| Disadvantages | Difficult control of heat force |
| Dangerous closed container – risk to burst | |
| Water evaporation |
Generally, microwave‐assisted process commonly operates at a frequency of about 2.45 GHz (12 cm), causing dielectric heating primarily by absorption of energy in water and other polar compounds available in wet biomass or in a given sample 129, 130. In order to prevent the interference with telecommunications devices, the frequency range between 2 and 8 GHz is generally used for domestic and synthetic purposes 119, 131. This may be the reason that all commercially available microwave reactors for chemical use operate at the same frequency 126 which is at 2.45 GHz. The energy of the quantum involved can be calculated by Planck's law E = hν and is found to be 0.3 cal mol–1 126.
Compared with conduction/convection heating, which is based on interfacial heat transfer, microwave uses the ability of direct heating of the target object due to applied electromagnetic field. Precisely, polar substances, such as water, are the main ingredients that allow a substance to be heated by microwaves during microwave heating process. The higher the water content of the substance, the faster the heating rate. Water in molecular level behaves exactly like a magnet. Water has two oppositely charged ends due to the presence of positively charged two hydrogen atoms and a negatively charged oxygen molecule. In other words, due to two different poles in water, water molecules rotate when microwaves oscillate. This happens because the positive end of water is attracted to the negatively charged end of microwave, while the negatively charged end of water is attracted to the positively charged end of microwave. The microwaves rotate at severely high speed of 2450 times per second which means that the water molecule also rotates 2450 times for every second a microwave rotates. This extremely high rotation rate causes molecules of water to collide with each other at very fast rate, and then creates friction between them. This friction generates heat which can flow through the substance by conduction, convection or radiation 127.
With this heating mechanism, the most renowned characteristic of microwave heating is volumetric heating, which is quite different from conventional heating and eventually becomes an advantage of microwave processing technology 127. Table 12 shows the comparison between conventional and microwave heating, and Figure 11 shows the illustration comparison between conventional heating and microwave heating mechanism.
Table 12.
Comparison between microwave heating and conventional heating 132
| Microwave heating | Conventional heating |
|---|---|
| Microwave effect | Thermal effect |
| Conversion of energy within the system | Transferring energy into the reacting system |
| In‐core volumetric and uniform heating at molecular level | Superficial heating via conduction, convection and radiation |
| Rapid heating | Slow heating |
| Volumetric heating of materials – extensive heating inside the material | Heating from outer layers of the material |
| Higher conversion efficiency | Lower conversion efficiency |
| Lower thermal inertia | Higher thermal inertia |
| Microwaves couple directly with the molecules of the entire reaction mixture, cause rapid rise in the temperature | Heat is transferred gradually from outer surface to inner surface |
Figure 11.
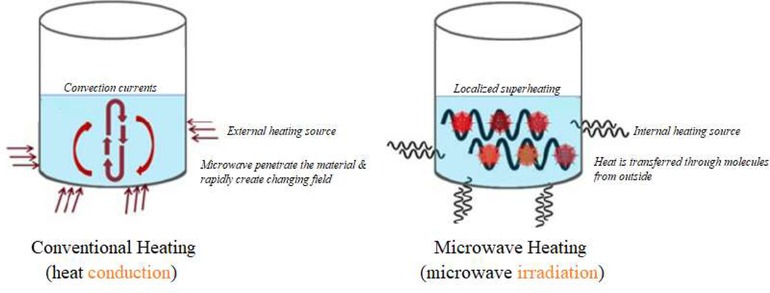
Different heating mechanisms for microwave and conventional heating (modified information from 133)
Due to the advantages and potential of microwave energy, there have been a lot of research and application using microwave – even the bio‐oil conversion from biomass. Numerous studies have been conducted on the implementation of microwave technology into the liquefaction of various biomass feedstocks, such as algae, wood and corn stover 134, 135, 136. The microwave‐assisted liquefaction process generally gives higher efficiency compared to conventional liquefaction process. Hence, it has been reported that microwave‐assisted reactor should be one of the best way to convert algal biomass into high quality of bio‐oil. Table 13 shows several previous studies associated with microwave‐assisted liquefaction. Based on the results from each study, it is proven that the microwave‐assisted liquefaction is better than conventional liquefaction process in implementation.
Table 13.
Microwave‐assisted liquefaction application from previous study
| Feedstock | Reaction condition | Catalyst | Results | References |
|---|---|---|---|---|
| Lignin |
|
No catalyst |
|
137 |
| Baggase Lignin (sugar cane) |
|
Oxalic acid |
|
138 |
| Cork |
|
2‐Ethyl hexanol/DEG and p‐toluene sulphuric acid |
|
139 |
| Corn stover | – | Sulphuric acid |
|
136 |
| Rape straw |
|
Acid | Kunak (0.3%) | 140 |
| Ulva prolifera (Algae species) |
|
Sulphuric acid | The bio‐oil obtained under the optimum conditions had a high liquefaction yield (84.81 ± 0.13%) and heating value (15.05 MJ kg−1) by microwave reaction. However, it contained large quantities of O, S and N, which would have to be removed before being used as a fuel | 134 |
| Wood | – | Sulphuric acid and phosphoric acid |
|
135 |
To further increase the depth of study of microwave‐assisted conversion of algal biomass, the energy efficiency can be described by the following equation:
| (1) |
This equation shows the heat energy supplied by the microwave system in terms of power dissipation and exposure time 83. Thermal effects caused by the microwave radiation system in the sample volume, however, can be quantified by the following equation:
| (2) |
Hence, the microwave energy efficiency is calculated by the ratio of both values:
| (3) |
5. UTILISATION OF ALGAL BIO‐OIL FOR BIOPLASTIC PRODUCTION
Nowadays, the applications of plastic and plastic‐based material are almost infinity. Plastic are flexibly used as a chemical material, including packaging purpose, automobile production, construction materials, furniture manufacture, as well as in the electronics industry and in the manufacturing of domestic equipment. According to the global statistics, the plastic consumption in 1976 is 50 million tonnes worldwide and continues to increase, which is expected to rise 330 million tonnes in 2015 141. Plastics are organic polymers, which can be processed in assorted ways. Their technical properties, such as formability, hardness, elasticity, rigidity, heat resistance and chemical resistance, can be varied across a diverse range by stipulating the correct raw materials, manufacturing process and additives.
For the current status, vast amounts of traditional plastic materials are produced from petroleum since the predominant availability of petroleum at the early of the 20th century 142. This is another significant effect to the environment by dumping the use of fossil fuel based on materials production. Petroleum‐based plastic is a non‐renewable fossil material that causes huge carbon emissions. The widespread consumption of petroleum‐based plastics has proven to be a significant source of the carbon footprints in atmosphere that leads to global climate change. The other challenges with respect to plastic are from health perspective. The elements of plastics, such as pthalates and bisphenol A, which are added to resin to reduce brittleness and promote plasticity, are two commonly cited examples of chemicals that have shown to cause a negative impact on human health. These products may remain in surrounding environment for centuries and live on in human bodies. Chemical‐ and petroleum‐derived plastics often contain allegedly harmful chemicals, such as bisphenol A and phthalates, linked to hormone disruption and developmental disorders.
The emerging market demand for green products creates problems for plastics manufacturers and suppliers to find the substitutes for petroleum‐based plastics. The abundant and reliable biomass source needs to be secured to sustain the bioplastics business model. While bioplastics have the potential to become green solutions for many environmentally harmful conventional plastics problems, being a bio‐based material does not instinctively guarantee that it is biodegradable or even environmentally friendly. Plastics have not always been produced from fossil materials, such as petroleum, which is generally known as the contributor towards the environmental negative impact. Bioplastics consist in a large part, or even completely, of renewable resources. Thus, bioplastics are bio‐based plastics. Biodegradable, but petroleum‐based plastics, are not considered as bioplastics, as shown in Figure 12. Hence, selecting the raw material to produce bio‐based should be emphasised in order to produce safe products with high quality 143.
Figure 12.
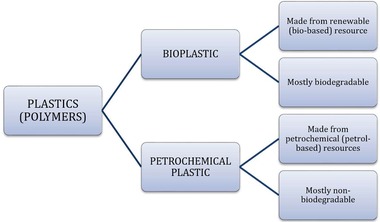
Plastics (polymer) resources and degradability
The worldwide production capacity for bioplastics is expected to rise from around 1.6 million tons in 2013 to approximately 6.7 million tons by 2018; there is a predicted increase of 83% for Europe's bioplastic production, totalling 511,480 ton 144. The strongest growth in the bioplastic industry is the bio‐based and non‐biodegradable group such as bio‐based versions of bulk plastics, polyethylene and polyethylene terephtalate, which entirely differ from their conventional counterparts in terms of their renewable raw material base, and are building up in large capacities. Meanwhile, biodegradable plastics also denote impressive growth rates, which is expected to increase their production capacity by two‐thirds by 2016. Polylactic acid (PLA) and polyhydroxyalkanoates (PHA) are the leading contributors in bioplastic growth. PLA was the most produced biodegradable biopolymer in Europe in 2013. PLA accounts for 298,000 tons (+60%), while PHA accounts for 142,000 tons (+700%) 144. It is a sustainable alternative to petroleum‐based plastics and is compostable and biodegradable. In observation, a disturbing trend of geographic distribution of production capacities should also be looked through, which is generally simplified in Table 14.
Table 14.
Geographic distribution trend for Bioplastic development
| Location | Bioplastic development |
|---|---|
| Europe and North America |
|
| South America and Asia | Establishment of new production capacities |
There are several factors for the growth in bioplastics, including consumer demand for environmentally sustainable products, the development of bio‐based feed stocks for commodity plastic resins and increasing restrictions on the use of non‐degradable plastic products, particularly plastic bags. Certainly, a key aspect in assessing the applicability of bioplastics is the impact on the environment resulting from their use during the entire life cycle – from production to final disposal. The life‐cycle analysis (LCA) is based on finding some factors considered crucial in assessing the impact that a particular product can have on the environment.
Table 15 shows a collection of LCA literature data 148, 149 in which each LCA characterises and compares the environmental impact of various bioplastics (thermoplastic starch, PLA and PHA and traditional plastics [high and low density] polyethylene, Nylon 6, polyethylene terephtalate, polystyrene, polyvinyl alcohol [PVA] and polycaprolactone) with an approach cradle to grave.
Table 15.
Energy required from non‐renewable sources and CO2 emissions for different types of plastics currently on the market
| Type of plastic | Energy requirement (MJ kg−1) | Global warming (kg CO2 eq kg−1) |
|---|---|---|
| From non‐renewable sources | ||
| HDPE | 80.0 | 4.84 |
| LDPE | 80.6 | 5.04 |
| Nylon‐6 | 120.0 | 7.64 |
| PET | 77.0 | 4.93 |
| PS | 87.0 | 5.98 |
| PVOH | 102.0 | 2.70 |
| PCL | 83.0 | 3.10 |
| From renewable sources | ||
| TPS | 25.4 | 1.14 |
| TPS + 15% PVOH | 24.9 | 1.73 |
| TPS + 60% PCL | 52.3 | 3.60 |
| PLA | 57.0 | 3.84 |
| PHA | 57.0 | Not Available |
PET, polyethylene terephtalate; PS, polystyrene; TPS, thermoplastic starch.
Overall, the data reported in Table 15 show how the production and use of bioplastics is more advantageous compared to conventional plastics from the energy demand and emissions of GHG point of view. Previous studies identified that phenolic bio‐oil, generated from renewable resources, contains chemical‐active compounds, effectuate it to successfully prepare the interior and thermal‐curing adhesives or composites 145. Bio‐based plastics can be produced from a wide range of plant‐based raw materials including algae. Thus, the production of bio‐oil from algal biomass might be one of the promising ways to be one of the alternatives to replace petroleum‐based plastic. Algae are one of the potential raw materials that can be utilised for bio‐based plastic production. Emphasising the advantage of utilising algae for this purpose, there is an established project funded by EU, which has initiated and developed a process of using seaweed as a novel base for bioplastics. Seaweed‐based plastic does not compete for land use, besides it will also save water and perhaps achieve higher productivity, biodegradable bioplastics, contributing to innovation in the bioplastic sector and the transition from petrochemistry to green chemistry. Utilising seaweed for bioplastic production gives various environmental benefit and financial advantages. Thus, the project will help to minimise the harmful environmental effects of fossil fuel based plastics, thereby helping to achieve the EU 2020 target of 10% of market plastics being bioplastics 146.
6. CONCLUDING REMARKS
Fossil fuel and fossil materials utilisation for industrial development had the negative impact to the environment. The truth is that the vast range of plastic materials used in every industry is produced from petroleum: a non‐renewable fossil fuel with a huge carbon footprint. The widespread consumption of petroleum‐based plastics has proven to be a significant source of the carbon emissions that cause global climate change. Biomass‐derived fuels with zero net CO2 emission are promising substitutes for fossil fuels. In fact, a significant reduction of non‐renewable energy consumption and CO2 emission can be accomplished. The development and exploitation of renewable and sustainable energy sources can give a significant contribution to the solutions to these problems. Since biomass is one of the potential renewable energy, the enhancements of bio‐oil production quality have been continuously researched from time to time according to the technological development while considering the economical aspect. In order to reduce the CO2 gas that can be harmful to the environment, the renewable energy and sustainable product alternatives need to be given more attention.
Algae have an abundance resource, and this type of biomass with proper quality enhancement might have high potential to replace conventional fossil fuel in future. By utilising the resources, it will relatively reduce the pollution and secure more sustainable future especially in sustainable product. There are many companies specialising in algae cultivation and development, and AIB is an established company in Malaysia. This shows that algae resources are reliable and less likely for shortage of resources in future. Algae species are broadly classified into microalgae and macroalgae. The previous studies mostly reported on the microalgae, as it is fast growing and easy to cultivate. However, the macroalgae species potential as biomass is also undeniable as it can contribute to biomass feedstock. In this particular review, two major potential thermochemical conversions are identified to produce crude bio‐oil from algae, which are HTL and pyrolysis. In fact, both conversion methods are relatively similar but different in application. HTL seems to be more suitable and effective in processing algae into crude bio‐oil because of high moisture content of the feedstock. HTL operates at lower temperature compared to pyrolysis process. Microwave‐assisted reactor for HTL shows an interactive way for crude bio‐oil production and widely established technology.
CONFLICT OF INTEREST
The authors have declared no conflict of interest.
ACKNOWLEDGMENTS
This work was supported by TNB Seed Fund URND/TNBFund‐2018 (Project Code U‐TR‐RD‐18‐11). A precious appreciation should also be given to the project team, those who attempt to complete the project successfully.
Abdul Latif NS, Ong MY, Nomanbhay S. Hydrothermal Liquefaction of Malaysia's Algal Biomass for High‐Quality Bio‐oil Production. Eng Life Sci 2019;19:246–269. 10.1002/elsc.201800144
REFERENCES
- 1. Ward, D. S. , Mahowald, N. M. , Contributions of developed and developing countries to global climate forcing and surface temperature change. Environ. Res. Lett. 2014, 9, 74008–74017. [Google Scholar]
- 2. Hansen, J. , Sato, M. , Kharecha, P. , et al., Young people's burden: Requirement of negative CO2 emissions. Earth Syst. Dynam. 2017, 8, 577–616. [Google Scholar]
- 3. Joos, F. , Roth, R. , Fuglestvedt, J. S. , Peters, G. P. , Enting, I. G. , von Bloh, W. , Carbon dioxide and climate impulse response functions for the computation of greenhouse gas metrics: A multi‐model analysis. Atmos. Chem. Phys. 2013, 13, 2793–2825. [Google Scholar]
- 4. Hosseini, S. E. , Aghili, N. , Wahid, M. A. , The scenario of greenhouse gases reduction in Malaysia. Renew. Sustain. Energ. Rev. 2013, 28, 400–409. [Google Scholar]
- 5. IRENA (2017), REthinking Energy 2017: Accelerating the global energy transformation. International Renewable Energy Agency, Abu Dhabi.
- 6. Schandl, H. , Fischer‐Kowalski, M. , West, J. , et al., Global Material Flows and Resource Productivity. An Assessment Study of the UNEP International Resource Panel. Paris, UNEP; 2016. [Google Scholar]
- 7. Weiss, M. , Haufe, J. , Carus, M. , et al., A review of the environmental impacts of biobased materials. J. Ind. Ecol. 2012, 16, 169–181. [Google Scholar]
- 8. Hammer, J. , Kraak, M. H. S. , Parsons, J. R. , Reviews of environmental contamination and toxicology. In: Whitacre D. M., editor. Plastics in the Marine Environment: The Dark Side of a Modern Gift. New York, NY, Springer; 2012, 1–44. [DOI] [PubMed] [Google Scholar]
- 9. Koch, H. M. , Calafat, A. M. , Human body burdens of chemicals used in plastic manufacture. Philos. Trans. R. Soc. Lond. 2009, 364, 2063–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meeker, J. D. , Sathyanarayana, S. , Swan, S. H. , Phthalates and other additives in plastics: Human exposure and associated health outcomes. Philos. Trans. R. Soc. Lond. B. 2009, 364, 2097–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oehlmann, J. , Schulte‐Oehlmann, U. , Kloas, W. , et al., A critical analysis of the biological impacts of plasticizers on wildlife. Philos. Trans. R. Soc. Lond. B. 2009, 364, 2047–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. FP International , Biodegradable vs. bioplastics: What's the difference? 2018. [Online]. Available from: https://minipakr.com/en/biodegradable-vs-bioplastics-whats-the-difference/
- 13. National Biomass Strategy 2020 (NBS2020) , One government agency for everything biomass in Malaysia; 2018. [Online]. Available from: http://www.nbs2020.gov.my
- 14. U.S. Department of Energy and Department of Agriculture . Biomass Research and Development Technical Advisory Committee, “Vision for Bioenergy & Biobased Products in the United States”, 2002.
- 15. Mohan, D. , Pittman, C. U. , Steele, P. H. , Pyrolysis of wood/biomass for bio‐oil: A critical review. Energy Fuels 2006, 20, 848–889. [Google Scholar]
- 16. Industrial Technology Research Institute (ITRI) , Bio‐oil produced from biomass pyrolysis technology; 2018. [Online]. Available from: https://www.itri.org.tw/eng/Content/MsgPic01/Contents.aspx?SiteID=1&MmmID=620170236661141772&MSid=620170253103470227 [Google Scholar]
- 17. Kumar, M. , Oyedun, A. O. , Kumar, A. , A review on the current status of various hydrothermal technologies on biomass feedstock. Renew. Sust. Energy Rev. 2018, 81, 1742–1770. [Google Scholar]
- 18. Gollakotaa, A. R. K. , Kishoreb, N. , Gua, S. , A review on hydrothermal liquefaction of biomass. Renew. Sust. Energy Rev. 2018, 81, 1378–1392. [Google Scholar]
- 19. Watling, L. , Fegley, J. , Moring, J. , Life Between the Tides: Marine Plants and Animals of the Northeast. Thomaston, ME, Tilbury House; 2003. [Google Scholar]
- 20. Rideau Valley Conservation Authority 2016, Algae and aquatic plant, Educational Manual. Rideau Valley Conservation Authority (RVCA); 2016.
- 21. Brigham, M. , What is the difference between terrestrial and aquatic plants? Quora; 2016.
- 22. Nielsen, S. L. , Sand‐Jensen, K. , Growth rates and morphological adaptations of aquatic and terrestrial forms of amphibious Littorella uniflora (L.) Aschers. Plant Ecol. 1997, 129, 135–140. [Google Scholar]
- 23. Canoy, W. Z. , Aquatic plants: Structure and functions; 2017.
- 24. Proflowers , Aquatic plants and flowers; 2016. [Online]. Available from: https://www.proflowers.com/blog/aquatic-plants-and-flowers
- 25. Mentzer, A. P. , Photosynthesis in aquatic plants; 2018. [Online]. Available from: https://sciencing.com/photosynthesis-aquatic-plants-5816031.html
- 26. McPhee, I. , Difference between water plants and land plants; 2017. [Online]. Available from: https://www.gardenguides.com/91053-difference-between-water-plants-land-plants.html
- 27. Rajkumar, R. , Takriff, M. S. , Prospects of algae and their environmental applications in Malaysia: A case study. J. Bioremediat. Biodegrad. 2016, 7. [Google Scholar]
- 28. Ben‐Iwo, J. , Manovic, V. , Longhurst, P. , Biomass resources and biofuels potential for the production of transportation fuels in Nigeria. Renew. Sust. Energy Rev. 2016, 63, 172–192. [Google Scholar]
- 29. Mata, T. M. , Martins, A. A. , Caetano, N. S. , Microalgae for biodiesel production and other applications: A review. Renew. Sust. Energy Rev. 2010, 14, 217–232. [Google Scholar]
- 30. Tsukahara, K. , Sawayama, S. , Liquid fuel production using microalgae. J. Jpn. Petrol. Inst. 2005, 48, 251–259. [Google Scholar]
- 31. Zhenyi, D. , Thermochemical conversion of microalgae for biofuel production; 2013. Available from: the University of Minnesota Digital Conservancy: http://hdl.handle.net/11299/144467
- 32. Nomanbhay, S. , Ong, M. Y. , A review of microwave‐assisted reactions for biodiesel production. Bioengineering 2017, 4, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liang, S. , Wei, L. , Passero, M. L. , Feris, K. , McDonald, A. G. , Hydrothermal liquefaction of laboratory cultivated and commercial algal biomass into crude bio‐oil. AIChe pp. 781–787, 2017. 10.1002/ep.12629 [DOI] [Google Scholar]
- 34. Mansoori, G. A. , Enayati, N. , Agyarko, L. B. , Algae as a potential biomass feedstock for biofuel production In: Energy Sources, Utilisation, Legislation, Sustainability, Illinois as Model State. Singapore: World Scientific; 2016, 333–338. [Google Scholar]
- 35. Abishek, M. P. , Patel, J. , Rajan, A. P. , Algae oil: A sustainable renewable fuel of future. Biotechnol. Res. Int., 2014. 10.1155/2014/272814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nochta, P. , Posten, I. C. , Simulations of light intensity variation in photobioreactors. J. Biotechnol. 2007, 131, 276–285. [DOI] [PubMed] [Google Scholar]
- 37. Dutton, J. A. , Alternative fuels from biomass sources: Algae growth and reaction conditions; 2017. [Online]. Available from: https://www.e-education.psu.edu/egee439/node/694
- 38. Algae International Berhad (AIB) , Algae International Berhad; 2015. [Online]. Available from: http://www.algaeinternational.biz/business.html
- 39. Chiaramonti, D. , Prussi, M. , Buffi, M. , Rizzo, A. M. , Pari, L. , Review and experimental study on pyrolysis and hydrothermal liquefaction of microalgae for biofuel production, Appl. Energy 2017, 185, 963–972. [Google Scholar]
- 40. Wijffels, R. H. , Barbosa, M. J. , Eppink, M. H. M. , Microalgae for the production of bulk chemicals and biofuels. Biofuel. Bioprod. Biorefin. 2010, 4, 287–295. [Google Scholar]
- 41. Brennan, L. , Owende, P. , Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co‐products. Renew. Sust. Energy Rev. 2010, 14, 557–577. [Google Scholar]
- 42. Chen, P. , Min, M. , Chen, Y. , Wang, L. , Yecong, L. , Review of the biological and engineering aspects of algae to fuels approach. Int. J. Agric. Biol. Eng. 2009, 2, 1–30. [Google Scholar]
- 43. Lobban, C. S. , Harrison, P. J. , Duncan, M. J. , The Physiological Ecological of Seaweed. Cambridge, Cambridge University Press; 1985. [Google Scholar]
- 44. Oilgae , About Algae; 2018. [Online]. Available from: http://www.oilgae.com/algae/algae.html
- 45. Carlsson, A. S. , Van beilen, J. B. , Moller, R. , Clayton, D. , Micro and Macro Algae: Utility for Industrial Applications. Newbury: CPL Press; 2007. [Google Scholar]
- 46. Falkowski, P. G. , Katz, M. E. , Knoll, A. H. , Quigg, A. , Raven, J. A. , The evolution of modern eukaryotic phytoplankton. Science 2004, 305, 354–360. [DOI] [PubMed] [Google Scholar]
- 47. Williams, P. J. L. B. , Laurens, L. M. L. , Microalgae as biodiesel & biomass feed stocks: Review & analysis of the biochemistry, energetics & economics. Energy Environ. Sci. 2010, 3, 554–590. [Google Scholar]
- 48. Schenk, P. M. , Thomas‐Hall, S. R. , Stephens, E. , et al., Second generation biofuels: High‐efficiency microalgae for biodiesel production. Bioenerg. Res. 2008, 1, 20–43. [Google Scholar]
- 49. Chisti, Y. , Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [DOI] [PubMed] [Google Scholar]
- 50. Sudhakar, K. , Mamat, R. , Samykano, M. , Azmi, W. H. , Ishak, W. F. W. , Yusaf, T. , An overview of marine macroalgae as bioresource. Renew. Sust. Energy Rev. 2018, 91, 165–179. [Google Scholar]
- 51. Wellinger, A. , Algal biomass. IEA Bioenergy 2009, 37, 1–13. [Google Scholar]
- 52. Sze, P. , A Biology of the algae, Second ed. Dubuque, IA: Wm. C. Brown Publishers; 1993. [Google Scholar]
- 53. Global CCS Institute , Climate; 2006. [Online]. Available from: https://hub.globalccsinstitute.com/publications/realistic-technology-and-engineering-assessment-algae-biofuel-production/42-climate
- 54. Hussin, H. , Khoso, A. , Seaweed cultivation and coastal communities in Malaysia: An overview. Asian Fish. Sci. 2017, 30, 87–100. [Google Scholar]
- 55. Petrick, I. , Dombrowski, L. , Kroger, M. , Beckert, T. , Kuchling, T. , Algae biorefinery – Material and energy use of algae. Report No. 16. Leipzig, Germany, DBFZ Deutsches Biomasseforschungszentrum gemeinnutzige GmbH; 2013. [Google Scholar]
- 56. Charles, S. V. , Ang, M. Y. , Palm oil mill effluent (POME) cultured marine microalgae as supplementary diet for rotifer culture. J. Appl. Phycol. 2008, 20, 603–608. [Google Scholar]
- 57. Phang, S. M. , Seaweed resource in Malaysia: Current status and future prospects. Aquat. Ecosyst. Health Manag., 2006, 9, 185–202. [Google Scholar]
- 58. Mazlan, A. G. , Zaidi, C. C. , Wan‐Lotfi, W. M. , Othman, B. H. R. , On the current status of coastal marine biodiversity in Malaysia. Indian J. Mar. Sci. 2005, 34, 76–87. [Google Scholar]
- 59. Barsanti, L. , Gualtieri, P. , Anatomy, Biochemistry and Biotechnology, Second Edition ed. Boca Raton, FL, CRC Press; 2005. [Google Scholar]
- 60. Renn, D. , Biotechnology and the red seaweed polysaccharide industry: Status, needs and prospects. Trends Biotechnol. 1997, 15, 9–14. [Google Scholar]
- 61. Radmer, R. J. , Algal diversity and commercial algal products. Bioscience 1996, 46, 263–270. [Google Scholar]
- 62. Malaysia, C. , Scientists studying algae for miracle biofuel in Malaysia; 2015. [Online]. Available from: http://cleanmalaysia.com/2015/09/05/scientists-studying-algae-for-miracle-biofuel-in-malaysia/
- 63. Ahmad, A. L. , Mat Yasin, N. H. , Derek, C. J. C. , Lim, J. K. , Optimization of microalgae coagulation process using chitosan. Chem. Eng. J. 2011, 173, 879–882. [Google Scholar]
- 64. Singh, A. , Pant, D. , Olsen, S. I. , Nigam, P. S. , Key issues to consider in microalgae based biodiesel production. Energy Educ. Sci. Technol. Part A vol. 2012, 29, 687–700. [Google Scholar]
- 65. Sydney, E. B. , Sturm, W. , de Carvalho, J. C. , Thomaz‐Soccol, V. , Larroche, C. , Potential carbon dioxide fixation by industrially important microalgae. Bioresour. Technol. 2010, 101, 5892–5896. [DOI] [PubMed] [Google Scholar]
- 66. Um, B. H. , Kim, Y. S. , Review: A chance for Korea to advance algal‐biodiesel technology., J. Ind. Eng. Chem. 2009, 15, 1–7. [Google Scholar]
- 67. Siew‐Moi, P. , Potential products from tropical algae and seaweeds, especially with. Malaysian J. Sci. 2010, 29, 160–166. [Google Scholar]
- 68. Ahmad, F. , Sulaiman, M. R. , Saimon, W. , Yee, C. F. , Matanjun, P. , Proximate compositions and total phenolic contents of selected edible seaweed from Semporna, Sabah, Malaysia. Borneo Sci. 2012, 31, 74–83. [Google Scholar]
- 69. Sade, A. , Ali, I. , Ariff, M. R. M. , The seaweed industry in Sabah, East Malaysia. J. Southeast Asian Stud. 2006, 11, 97–107. [Google Scholar]
- 70. Hurtado, A. Q. , Gerung, G. S. , Yasir, S. M. , Critchley, A. T. , Cultivation of tropical red seaweeds in the BIMPEAGA region. J. Appl. Phycol. 2014, 26, 707–718. [Google Scholar]
- 71. Kaur, C. R. , Ang, M. , Developing the seaweed aquaculture sector in Malaysia Seminar Report, Kuala Lumpur 2009. [Google Scholar]
- 72. Safari, S. , Prospects and policy review of seaweed as a high‐value commodity in Malaysia. FFTC Agricultural Policy; 2015. [Google Scholar]
- 73. FAO , FAO global aquaculture production database updated to 2013 – Summary information; 2015. [Online]. Available from: http://www.fao.org/3/a-i4899e.pdf
- 74. Department of Fisheries Sabah . Annual Fisheries Statistics, Sabah, 1989-2013.
- 75. Ministry of International Trade and Industry (MITI) , Deputy Minister (Industry) of MITI Visited Algaetech International Sdn Bhd. Ministry of International Trade and Industry; 2016.
- 76. Dimitriadis, A. , Bezergianni, S. , Hydrothermal liquefaction of various biomass and waste feedstocks for biocrude production: A state of the art review. Renew. Sust. Energy Rev. 2017, 68, 113–125. [Google Scholar]
- 77. Davis, R. , Aden, A. , Pienkos, P. T. , Techno‐economic analysis of autotrophic microalgae for fuel production. Appl. Energy 2011, 88, 3524–3531. [Google Scholar]
- 78. Tredici, M. , Photobiology of microalgae mass cultures: understanding the tools for the next green revolution. Biofuels 2010, 1, 143–162. [Google Scholar]
- 79. Lardon, L. , Helias, A. , Sialve, B. , Steyer, J.‐P. , Bernard, O. , Life‐cycle assessment of biodiesel production from microalgae. Environ. Sci. Technol. 2009, 43, 6475–6481. [DOI] [PubMed] [Google Scholar]
- 80. Razon, L. F. , Tan, R. R. , Net energy analysis of the production of biodiesel and biogas from the microalgae: Haematococcus pluvialis and Nannochloropsis. Appl. Energy 2011, 88, 3507–3514. [Google Scholar]
- 81. Singh, A. , Singh Rawat, K. , Nautiyal, O. P. , Chavdal, T. V. , Biomass to fuel: Conversion techniques In: Energy Resources: Development, Harvesting and Management. Dehradun, Uttarakhand, M/s Bishen Singh Mahendra Pal Singh; 2016. [Google Scholar]
- 82. Speight, J. G. , Industrial Organic Chemistry. Environ. Organic Chem. Eng. 2017, 87–151. [Google Scholar]
- 83. Patil, P. , Deng, S. , Microwave‐enhanced in situ transesterification of algal biomass to biodiesel In: Zheng R. L. X. Q. F., editor. Production of Biofuels and Chemicals with MIicrowave Berlin: Springer; 2015, p. 145–165. [Google Scholar]
- 84. Koks, Z. , Technological development of fast pyrolysis and hydrothermal liquefaction. Utrecht: Utrecht University; 2016. [Google Scholar]
- 85. Bridgwater, A. V. , Upgrading fast pyrolysis liquid In: Brown, R., editor. Thermochemical Processing of Biomass: Conversion into Fuels Hoboken, NJ, John Wiley & Sons; 2011. [Google Scholar]
- 86. Elliott, D. C. , Historical developments in hydroprocessing bio‐oils. Energy Fuel. 2007, 21, 1792–1815. [Google Scholar]
- 87. Zhang, Z.‐b. , Lu, Q. , Ye, X.‐N. , Li, W.‐T. , Hu, B. , Dong, C.‐Q. , Production of phenolic‐rich bio‐oil from catalytic fast pyrolysis of biomass using magnetic solid base catalyst. Energy Convers. Manag., 2015, 106, 1309–1317. [Google Scholar]
- 88. Kim, J.‐S. , Production, separation and applications of phenolic‐rich bio‐oil – A review. Biosour. Technol. 2015, 178, 90–98. [DOI] [PubMed] [Google Scholar]
- 89. Zafar, S. , 2018. BioEnergy Consult : Biomass Pyrolysis Process. [Online] Available at: https://www.bioenergyconsult.com/biomass-pyrolysis-process/
- 90. Yang, Y. , Brammer, J. G. , Mahmood, A. S. N. , Hornung, A. , Intermediate pyrolysis of biomass energy pellets for producing sustainable liquid, gaseous and solid fuels. Bioresour. Technol. 2014, 169, 794–799. [DOI] [PubMed] [Google Scholar]
- 91. Diebold, J. P. , A review of the chemical and physical mechanisms of the storage stability of fast pyrolysis bio‐oils In: Bridgwater A. V., editor. Fast Pyrolysis of Biomass: A Handbook. Newbury, UK: CPL Press; 2002. 243–292. [Google Scholar]
- 92. Demirbas, A. , Arin, G. , An overview of biomass pyrolysis. Energy Sources, 2002, 24, 471–482. [Google Scholar]
- 93. Bridgwater, A. V. , Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy, vol. 38, 2012, 68–94. [Google Scholar]
- 94. Hornung, A. , Apfelbacher, A. , Sagi, S. , Intermediate pyrolysis: A sustainable biomass‐to‐energy concept‐biothermal valorisation of biomass (BtVB) process. J. Sci. Ind. Res., vol. 70, p. 664–667. [Google Scholar]
- 95. Jahirul, M. I. , Rasul, M. G. , Chowdhury, A. A. , Ashwath, N. , Biofuels production through biomass pyrolysis – A technological review. Energies, vol. 5, p. 4952–5001, 2012. [Google Scholar]
- 96. Jegathese, S. J. , Farid, M. , Microalgae as a Renewable Source of Energy: A Niche Opportunity. J. Renew. Energy 2014. 10.1155/2014/430203 [DOI] [Google Scholar]
- 97. Peterson, A. A. , Vogel, F. , Lachance, R. P. , Fröling, M. , Antal, M. J. , Tester, J. W. , Thermochemical biofuel production in hydrothermal media: A review of sub‐ and supercritical water technologies. Energy Environ. Sci. vol. 1, p. 32–65, 2008. [Google Scholar]
- 98. Brunner, G. , Near critical and supercritical water Part 1 : Hydrolytic and Hydrothermal processes. Supercritical Fluids, no. 47, pp. 373–381, 2009. [Google Scholar]
- 99. Savage, P. E. , Levine, R. B. , Huelsman, C. M. , Chapter 8 hydrothermal processing of biomass In: Thermochemical Conversion of Biomass to Liquid Fuels and Chemicals. London: The Royal Society of Chemistry; 2010. p. 192–221. [Google Scholar]
- 100. Yu, G. , Zhang, Y. , Schideman, L. , Funk, T. L. , Wang, Z. , Hydrothermal liquefaction of low lipid content microalgae into bio‐crude oil. Am. Soc. Agric. Biol. Eng. vol. 54, p. 239–246, 2011. [Google Scholar]
- 101. Elliott, D. C. , Biller, P. , Ross, A. B. , Schmidt, A. J. , Jones, S. B. , Hydrothermal liquefaction of biomass: Developments from batch to continuous process. Bioresour. Technol., vol. 178, p. 147–156, 2015. [DOI] [PubMed] [Google Scholar]
- 102. Valdez, P. J. , Nelson, M. C. , Wang, H. Y. , Lin, X. N. , Savage, P. E. , Hydrothermal liquefaction of Nannochloropsis sp.: Systematic study of process variables and analysis of the product fractions. Biomass Bioenergy, vol. 46, p. 317–331, 2012. [Google Scholar]
- 103. Duan, P. G. , Savage, P. E. , Hydrothermal liquefaction of a microalga with heterogeneous catalysts. Ind. Eng. Chem. Res. no. 50, pp. 52–61, 2011. [Google Scholar]
- 104. Biller, P. , Ross, A. B. , Potential yields and properties of oil from the hydrothermal liquefaction of microalgae with different biochemical content. Bioresour. Technol., no. 102, pp. 215–225, 2011. [DOI] [PubMed] [Google Scholar]
- 105. Zou, S. P. , Wu, Y. L. , Yang, M. D. , Li, C. , Tong, J. M. , Thermochemical catalytic liquefaction of the marine microalgae Dunaliella tertiolecta and characterization of bio‐oils. Energy Fuels, no. 23, pp. 3753–3758, 2009. [Google Scholar]
- 106. Yang, Y. , Gilbert, A. , Charles., X. C. , Production of bio‐crude from forestry waste by hydro‐liquefaction in sub‐/super‐critical methanol. AIChE J., vol. 55, p. 807–819, 2009. [Google Scholar]
- 107. Elliott, D. C. , Hart, T. R. , Schmidt, A. J. , et al., Process development for hydrothermal liquefaction of algae feedstocks in a continuous‐flow reactor. Algal Res. vol. 2, p. 445–454, 2013. [Google Scholar]
- 108. Hognon, C. , Texier, J. , Delrue, F. , Roubaud, A. , Comparison of pyrolysis and hydrothermal liquefaction of Chlamydomonas reinhardtii. Growth studies on the recovered hydrothermal aqueous phase. Biomass Bioenergy, 2015, 73, 23–31. [Google Scholar]
- 109. Hu, Q. , Yang, H. , Yao, D. , et al., The densification of biochar: Effect of pyrolysis temperature on the qualities of pellets. Bioresour. Technol., vol. 200, pp. 521–527, 2016. [DOI] [PubMed] [Google Scholar]
- 110. Zainan, N. H. , Srivatsa, S. C. , Li, F. , Bhattacharyaa, S. , Quality of bio‐oil from catalytic pyrolysis of microalgae Chlorella vulgaris . Fuel, vol. 223, pp. 12–19, 2018. [Google Scholar]
- 111. Wang, S. , Wang, Q. , Jiang, X. , Han, X. , Jia, H. , Compositional analysis of bio‐oil derived from pyrolysis of seaweed. Energy Convers. Manage., vol. 68, pp. 273–280, 2013. [Google Scholar]
- 112. Hu, Y. , Wang, S. , Li, J. , et al., “Co‐pyrolysis and co‐hydrothermal liquefaction of seaweeds and rice husk: Comparative study towards enhanced biofuel production. J. Anal. Appl. Pyrolysis, vol. 129, pp. 162–170, 2018. [Google Scholar]
- 113. Ly, H. V. , Kim, S.‐S. , Woo, H. C. , Choi, J. H. , Suh, D. J. , Kim, J. , Fast pyrolysis of macroalga Saccharina japonica in a bubbling fluidized‐bed reactor for bio‐oil production. Energy, vol. 93, pp. 1436–1446, 2015. [Google Scholar]
- 114. Wang, S. , Wang, Q., Jiang, X. , Han, X. , Ji, H. , Compositional analysis of bio‐oil derived from pyrolysis of seaweed. Energy Convers. Manag., vol. 68, pp. 273–280, 2013. [Google Scholar]
- 115. Anastasakis, K. , Ross, A. B. , Hydrothermal liquefaction of four brown macro‐algae commonly found on the UK coasts: An energetic analysis of the process and comparison with bio‐chemical conversion methods. Fuel, vol. 139, pp. 546–553, 2015. [Google Scholar]
- 116. Bach, Q.‐V. , Sillero, M. V. , Tran, K.‐Q. , Skjermo, J. , Fast hydrothermal liquefaction of a Norwegian macro‐alga: Screening tests. Algal Res., vol. 6, pp. 271–276, 2014. [Google Scholar]
- 117. Caporgno, M. P. , Pruvost, J. , Legrand, J. , Lepine, O. , Tazerout, M. , Bengo, C. , Hydrothermal liquefaction of Nannochloropsis oceanica in different solvents. Bioresour. Technol., vol. 214, pp. 404–410, 2016. [DOI] [PubMed] [Google Scholar]
- 118. He, Y. , Liang, X. , Jazrawi, C. , et al., Continuous hydrothermal liquefaction of macroalgae in the presence of organic co‐solvents. Algal Res., vol. 17, pp. 185–195, 2016. [Google Scholar]
- 119. Jacob, J. , Microwave assisted reactions in organic chemistry: A review of recent advances. Int. J. Chem., vol. 4, pp. 29–43, 2012. [Google Scholar]
- 120. Kappe, C. O. , Stadler, A. , Microwaves in organic and medicinal chemistry. J. Am. Chem. Soc., vol. 128, pp. 1771–1772, 2006. [Google Scholar]
- 121. Tsoleridis, C. A. , Neochoritis, C. G. , Tzitzikas, T. , et al., One‐pot microwave assisted synthesis under green chemistry conditions, antioxidant screening, and cytotoxicity assessments of benzimidazole Schiff bases and pyrimido[1,2‐a]benzimidazol‐3(4H)‐ones. Eur. J. Med. Chem., vol. 46, pp. 297–306, 2011. [DOI] [PubMed] [Google Scholar]
- 122. Prasad, D. , Preetam, A. , Nath, M. , Microwave‐assisted green synthesis of dibenzo[a,j]xanthenes using p‐dodecylbenzenesulfonic acid as an efficient Bronsted acid catalyst under solvent‐free conditions. C. R. Chim., vol. 15, pp. 675–678, 2012. [Google Scholar]
- 123. Wang, S. , Cheng, C. , Wu, F. , et al., Microwave‐assisted multi‐component reaction in water leading to highly regioselective formation of benzo[f]azulen‐1‐ones. Tetrahedron, vol. 67, pp. 4485–4493, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Erdmenger, T. , Guerrero‐Sanchez, C. , Vitz, J. , Hoogenboom, R. , Schubert, U. S. , Recent developments in the utilization of green solvents in polymer chemistry. Chem. Soc. Rev., vol. 39, pp. 3317–3333, 2010. [DOI] [PubMed] [Google Scholar]
- 125. Tucker, J. , Green chemistry: Cresting a summit toward sustainability. Org. Process Res. Dev., vol. 14, pp. 328–331, 2010. [Google Scholar]
- 126. Surati, M. A. , Jauhari, S. , Desai, K. R. , A brief review: Microwave assisted organic reaction. Scholars Research Library, vol. 4, pp. 645–661, 2012. [Google Scholar]
- 127. Kalla, A. M. , Devaraju, R. , Microwave energy and its application in food industry: A review. Asian J. Dairy Food Res. vol. 36, pp. 37–44, 2017. [Google Scholar]
- 128. Gong, M. , Bassi, A. , Carotenoids from microalgae: A review of recent developments . Biotechnol. Adv., vol. 34, p. 1396–1412, 2016. [DOI] [PubMed] [Google Scholar]
- 129. Kapoore, R. V. , Butler, T. O. , Pandhal, J. , Vaidyanathan, S. , Microwave‐assisted extraction for microalgae: From Biofuels to Biorefinery,” Biology, vol. 7, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Kadam, S. U. , Tiwari, B. K. , O'Donnell, C. P. , Application of novel extraction technologies for bioactives from marine algae, J. Agric. Food Chem., vol. 61, p. 4667–4675, 2013. [DOI] [PubMed] [Google Scholar]
- 131. Horikoshi, S. , Hamamura, T. , Kajitani, M. , Yoshizawa‐Fujita, M. , Serpone, N. , Green chemistry with a novel 5.8‐GHz microwave apparatus. Prompt one‐pot solvent‐free synthesis of a major ionic liquid: The 1‐butyl‐3‐methylimidazolium tetrafluoroborate system. Org. Process Res. Dev., vol. 12, p. 1089–1093, 2008. [Google Scholar]
- 132. Nomanbhay, S. , Salman, B. , Hussain, R. , Ong, M. Y. , Microwave pyrolysis of lignocellulosic biomass – A contribution to power Africa. Energy Sust. Soc. vol. 7, pp. 1–24, 2017. [Google Scholar]
- 133. Gude, V. G. , Martinez‐Guerra, P. P. , Deng, S. , Nirmalakhandan, N. , Microwave energy potential for biodiesel production. Sust. Chem. Proc. vol. 1, p. 1, 2013. [Google Scholar]
- 134. Zhuang, Y. , Guo, J. , Chen, L. , Li, D. , Liu, J. , Ye, N. , “Microwave‐assisted direct liquefaction of Ulva prolifera for bio‐oil production by acid catalysis,” Bioresour. Technol., vol. 116, p. 133–139, 2012. [DOI] [PubMed] [Google Scholar]
- 135. Pan, H. , Zheng, Z. , Hse, C. Y. , “Microwave‐assisted liquefaction of wood with polyhydric alcohols and its application in preparation of polyurethane (PU) foam,” Eur. J. Wood Prod., vol. 70, p. 461–470, 2012. [Google Scholar]
- 136. Xiao, W. , Han, L. , Zhao, Y. , “Comparative study of conventional and microwave‐assisted liquefaction of corn stover in ethylene glycol,” Ind. Crops Prod., vol. 34, p. 1602–1606, 2011. [Google Scholar]
- 137. Gosz, K. , Kosmela, P. , Hejna, A. , Gajowiec, G. , Piszczyk, Ł. , “Biopolyols obtained via microwave‐assisted liquefaction of lignin: structure, rheological, physical and thermal properties,” Wood Sci. Technol., vol. 52, p. 599–617, 2018. [Google Scholar]
- 138. Li, Y. , Li, B. , Du, F. , Wang, Y. , Pan, L. , Chen, D. , “Microwave‐assisted hydrothermal liquefaction of lignin for the preparation of phenolic formaldehyde adhesive,” J. Appl. Polym. Sci., 2017. [Google Scholar]
- 139. dos Santos, R. G. , Bordado, J. C. , Mateus, M. M. , “Microwave‐assisted liquefaction of cork – From an industrial waste to sustainable chemicals,” Ind. Eng. Manag. vol. 4, 2015. [Google Scholar]
- 140. Huang, X.‐Y. , Li, F. , Xie, J.‐L. , et al., “Microwave‐assisted liquefaction of rape straw for the production of bio‐oils,” Bioresources, vol. 12, 2017. [Google Scholar]
- 141. Plastics Europe , “An analysis of European plastics production, demand and waste data for 2011,” N. N.: Plastics – the Facts 2012, Brussels; 2012. [Google Scholar]
- 142. Thielen, M. (Bioplastics magazine), bioplastics, Gulzow: Fachagentur Nachwachsende Rohstoffe e.V. (FNR). Agency for Renewable Resources; 2014.
- 143. Fachagentur Nachwachsende Rohstoffe e. V. (FNR) , Bioplastics, OT Gülzow, 2013.
- 144. Seabioplas , Final Report Summary – Seabioplas (Seaweeds From Sustainable Aquaculture As Feedstock For Biodegradable Bioplastics). Ireland; 2016. [Google Scholar]
- 145. Ren, X. , Cai, H. , Du, H. , Chang, J. , “The preparation and characterization of pyrolysis bio‐oil‐resorcinol‐aldehyde resin cold‐set adhesives for wood construction,” Polymers, vol. 9, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Seabioplas , “Seaweed – A sustainable source of bioplastics,” Ireland, 2017. [Google Scholar]
- 147. Elliott, D. , “IEA bioenergy Task 34 ‐ Pyrolysis,” IEA Bioenergy Welcome to Task 34, p. 37, 2015. [Google Scholar]
- 148. Patel, M. , “Life cycle assessment of synthetic and biological polyesters,” In Proceedings of the International Symposium on Biological Polyesters, Munster, Germany; 2002. [Google Scholar]
- 149. Patel, M. , “Starch‐Based Technology,” In: Handbook of Biodegradable Polymers, Shrewsbury, MA: Rapra Technology Limited, 2005, p. 431–466. [Google Scholar]


