Abstract
We have developed an agarose‐based biocompatible drug delivery vehicle. The vehicle is in the form of thin, transparent, strong and flexible films. The biocompatibility and haemocompatibility of the films is confirmed using direct and indirect contact biological assay. Contact angle measurement exhibits hydrophilic nature of the films, and protein adsorption test shows low protein adsorption on the film surface. Drugs, antibiotics and antiseptics, retain their potency after their incorporation into the films. Our bioplastic films can be a versatile medium for drug delivery applications, especially as wound and surgical dressings where a fast drug release rate is desired.
Keywords: Agarose bioplastic, Biocompatibility, Drug delivery, Haemocompatibility, Swelling
Abbreviations
- FTIR
FT infrared
- TCPS
tissue culture polystyrene
1. Introduction
A wound is an injury to a living tissue, and it is usually classified into acute and chronic wounds 1, 2. Acute wounds are caused by cuts, burns or impacts, whereas possible reasons for chronic wounds are pressure ulcers, vascular ulcers or diseases such as diabetes and cancer. Obesity and aging demography are exacerbating the risk factors for chronic wounds 3. At present, an estimated 2–3% of the total healthcare budget of developed countries like Denmark is being used for treatment of wounds 4. Less developed nations lacking facilities and resources, on the other hand, show higher mortality figures 5.
Infection of wounds by microorganisms is one major reason for delayed or even no healing 6. A number of aerobic and anaerobic microorganisms (viz. peptococci, enterococci, Staphylococcus aureus, clostridia, Escherichia coli, etc.) have been isolated from various non‐healing wounds 7, 8, 9, 10, 11, 12, 13, 14. To counter the situation, the standard modus operandi for wounds has been to clean them of any debris and sanitise them with antiseptics. In addition, antibiotics and growth factors are prescribed for serious wounds 15. To facilitate drug administration locally, drug delivery patches and medicated dressings have been developed recently 16, 17, 18, 19, 20, 21, 22, 23, 24. Essentially, the drug delivery vehicles should be cheap and easy to synthesise, have good biocompatibility in the absence of sensitisation, haemocompatibility and no toxicity, and have compatible release characteristics and encapsulation stability for the drug concerned. Agar/agarose‐based biomaterial has shown potential in packaging material as well in wound dressing application. However, agar/agarose has never been used as a lone matrix in the previously reported studies 25, 26, 27.
In this paper, we studied agarose bioplastic as a novel drug delivery vehicle for wound dressings. Agarose is a polysaccharide polymer of d‐galactose and 3,6‐anhydro‐l‐galactopyranose. It is extracted from seaweed and is cheaply and abundantly available. Apart from its use in electrophoresis and chromatography, it is also used in mammalian cell cultures and highly biocompatible 28, 29, 30, 31. It has been used for experimental drug release in the form of coated beads 32, 33. However, these applications have involved low concentrations of drugs. Since agarose is derived from agar, and agar plates for bacterial culture can have high concentrations of drugs incorporated throughout the matrix without loss of potency, we expected that bioplastics made from agarose should also have similar properties. Our aim was to fabricate and investigate agarose bioplastic film as a drug delivery vehicle.
In this report, we synthesised agarose bioplastic films with different concentrations of glycerol as plasticiser. FT infrared (FTIR) spectra of the films confirmed that no new covalent bonds were formed in our agarose films with glycerol. Tensile testing was performed on agarose–glycerol films to optimise the concentration of glycerol for adequate strength and suppleness. Films with 20% glycerol with respect to weight of agarose were selected for further experiments. Swelling behaviour of the films was tested at three different temperatures. Surface properties of the films were characterised through contact angle measurements, study of protein adsorption on the surface and cell culture assays. Direct and indirect contact tests were used to establish biocompatibility of the bioplastic films, along with tests for haemo compatibility. Antibiotic and antiseptic molecules were incorporated into the bioplastic films and their potency was established by the zones of inhibition generated by them when used on bacterial culture plates.
2. Materials and methods
2.1. Materials
Agarose (DNase, RNase free), was purchased from Merck Specialities Private Limited, Mumbai, and used without further modifications. Glycerol (99%) was purchased from Qualigens Fine Chemicals, Mumbai, Dettol from Reckitt Benckiser and Savlon from Johnson and Johnson. Kanamycin sulphate, Luria Bertani broth and agar powder were purchased from HiMedia Laboratories Pvt. Ltd, Mumbai. Ampicillin sodium salt, Triton‐X 100, sodium dodecyl sulphate, BSA powder, DMEM, Corning 10 cm tissue culture treated culture dishes, Corning 24‐well cell culture plates and Corning ultralow attachment 24‐well cell culture plates were procured from Sigma‐Aldrich (USA) and FBS was procured from Life Technologies (USA). Tetracycline hydrochloride samples (from Duchefa Biochemie) were received as a kind gift from Dr. K. Subramaniam at IIT Kanpur, and DH5α E. coli and DF‐1 chicken fibroblast cells were received as a kind gift from Dr. A. Bandyopadhyay of IIT Kanpur.
2.2. Fabrication of bioplastic
In 250‐mL Erlenmeyer flasks, 2 g of agarose powder was added to 30 mL deionised water. Different amounts of glycerol were added to give a final glycerol concentration varying from 0 to 50% (v/w) with respect to agarose. Deionised water was further added to make the final volume up to 50 mL 34, 35. Thus, the final concentration of agarose was 4% w/v in final volume of 50 mL solution. The mixture was boiled in a microwave oven set at 800 W for 4 min, which turned agarose into a viscous solution. The solution was allowed to cool at approximately 60°C and then manually swirled before casting into films. To make bioplastic films, 20 mL of the solution was poured into 90 mm Petri plates, cooled and dried at 25°C for 72 h at 50% relative humidity.
To incorporate drugs (antibiotics and antiseptics) into the bioplastic films, the drugs were added into the agarose–glycerol solution just before casting. The drugs were not added until immediately prior to casting to prevent any degradation due to the step involving high temperature. The addition of drugs was accompanied by continuous swirling to facilitate thorough mixing of the drug with the bioplastic.
2.3. FTIR spectroscopy
Samples of the films were dried for 5 days in a vacuum desiccator lined with anhydrous silica gel, before being ground into a powder. FTIR spectra of the powders were taken using a Perkin Elmer Spectrum Two spectrometer in the wave number ranging from 400 to 4000 cm−1.
2.4. Testing for mechanical properties
The thicknesses of the films were measured using Digimatic micrometre (Mitutoyo Corporation, Japan) and found to lie between 130 and 170 μm. ASTM D 1708–59 T standard was followed to measure the tensile properties of the samples. Briefly, the samples were cut into dog‐bone shaped specimens using EpilogLaser CO2 laser engraving and cutting machine. They were incubated at 25°C and 50% relative humidity for 72 h, and then tested on Instron 1195 tensile testing machine with an initial grip separation of 0.9 inches and cross‐head speed of 1 mm/min as per the above ASTM standard. Multiple specimens were tested for each sample, and the average ultimate tensile strength was recorded.
2.5. Study of swelling behaviour
Swelling behaviour of bioplastic films at three temperatures, 4, 25 and 37°C, was studied as follows. Water baths containing deionised water for each of the above temperatures were set. Small strips of bioplastic films (size 1 cm × 3 cm) were cut and weighed. A strip of the bioplastic film was immersed in each water bath. After every 15 min, the strips were taken out, wiped with filter paper, weighed and then put back into their respective water baths. The measurements were continued for a little over 2 h, by which time the successive readings had equalised. The test was performed in triplicates.
2.6. Contact angle measurements
Contact angle measurements were performed using an OCA 35 video‐based automatic contact angle measuring device obtained from Data Physics Instruments GmbH (Filderstadt, Germany). Briefly, 20 μL drop of deionised water was put on the surface of the bioplastic film (stored hydrated in PBS, pH 7.4 and blotted dry before use), allowed to equilibrate for around 1 min, and then imaged. A horizontal line was defined in the software module, which then measured the angle made by the water drop with the horizontal line. The software calculated two readings for each sample, left and right. The average of multiple readings obtained by repeating these steps on several surfaces of the bioplastic was recorded as the water contact angle.
2.7. Quantification of protein adsorption
The amount of protein adsorbed on the bioplastic surface vis‐à‐vis a control surface of tissue culture polystyrene was measured through a method of differences using BSA as a model protein. Briefly, 2 mL sample from a stock solution of 50 μg/mL BSA in normal saline was added to each of three 10 cm tissue culture polystyrene Petri dishes (control group, triplicate) and similar three 10 cm Petri dishes overlaid with a layer of bioplastic (experimental group, triplicate). The solution was swirled in all the six Petri dishes to coat the surface, and the Petri dishes were kept on a rocker for 20 min at 35°C. The amount of protein in the supernatant solution in both sets of Petri dishes was estimated through Bradford assay 36. Subtracting these values from 100 μg gave the amount of protein adsorbed on the respective surfaces. The amount of protein adsorbed per unit area was calculated by dividing the previous value by the surface area of the Petri dishes.
2.8. Cell culture assays
For direct contact assay, 300 μL molten bioplastic was added to cover the bottom surface of six wells of a 24‐well tissue culture polystyrene (TCPS) plate. These wells were then cultured with chicken fibroblasts (DF‐1) in DMEM supplemented with 10% serum medium at 37°C for 36 hours in a humidified incubator with 5% carbon‐dioxide environment. As a negative control, chicken fibroblasts were cultured under similar conditions in the wells of ultra‐low attachment 24‐well culture plates.
For indirect control assay, 50 μL of molten bioplastic was added to the sides of six wells of a 24‐well tissue‐culture polystyrene (TCPS) plate in such a way that more than half of the bottom surface of these wells remained uncoated. These wells were then cultured with chicken fibroblasts in DMEM supplemented with 10% serum medium at 37°C for 36 h in a humidified incubator with 5% carbon dioxide environment. As a negative control, chicken fibroblasts were cultured under similar conditions in other wells of the same plate that were coated with gelatin to mimic adherent surface. These wells did not have any molten bioplastic added to their sides.
For positive control of both assays, fibroblast cells were cultured under similar conditions in the wells of a 24‐well TCPS plate, with the exception that 0.1% Triton‐X 100, a known non‐biocompatible chemical, was added to the wells to induce cell death 37.
At the end of culture period, the cells were observed and imaged in an inverted microscope (model: Leica DMIL LED) under differential interference contrast settings to investigate their morphology. 3‐(4,5‐Dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT) assay for investigation of metabolic activity of the cells was also performed. The test was performed in triplicates.
2.9. Study of haemolytic behaviour
The test was performed using goat blood as described earlier 38. For the test, 50 mL blood was collected with 1 mL of 0.5 M EDTA as anticoagulant and centrifuged at 3100 rpm (700 × g) for 10 min. The red blood cells pellet was washed thrice with cold PBS pH 7.4 by centrifugation at the same rpm. The pellet was resuspended in cold PBS and stored at 4°C for later use. The RBC suspension was used within 24 h and was always freshly prepared for the test. The polymer samples were incubated with 200 μL cold PBS for few minutes in Eppendorf tubes before adding 800 μL RBC suspension to overcome the change in osmotic pressure, which can lead to haemolysis. The samples were then incubated at 37°C for 90 min. For positive control, 2 μL Triton X‐100 was added to the RBC suspension for complete haemolysis and RBC suspension was used as negative control. After incubation, samples were centrifuged at 700 g for 10 min, and absorbance of supernatant was measured at 540 nm. The test was performed in triplicate and was repeated twice. The percentage of haemolysis was calculated as
2.10. Study of potency of released drugs
The preparation of films for studying the drug release rate is explained in Section 2.3. Circular samples of 10 mm diameter were cut from drug‐loaded films. The concentration of antibiotics in agarose solution was 100 μg/mL, while dettol and savlon were diluted to 1 in 20 and 1 in 15 concentrations, respectively, as suggested by the respective manufacturers. Samples cut from control films did not contain any drug. Three hundred million colony forming units of E. coli (strain: DH5α) were spread on LB agar plates. The cut samples of the drug‐loaded films were placed on top of these plates, and the plates were incubated at 37°C for 12 h. The maximum diameter of the zone of inhibition was measured for each film, and images were recorded.
3. Results and discussion
3.1. Initial observations of bioplastic films
The cast films of agarose and glycerol were transparent (Fig. 1A). Their thickness increased from 130 to 170 μm, as the amount of glycerol was increased from 0 to 50% (v/w), relative to the weight to agarose. Films with lower concentrations of the plasticiser (glycerol) were relatively inflexible; however, with increasing concentrations of glycerol, the films became softer and appeared succulent. Inflexible films are good for applications that require structural rigidity including packaging boxes. These films, however, are not ideal for wound drug delivery applications, as these are relatively hard and may scratch or cut the skin. In addition, since many body parts are not flat, the dressings should be flexible enough to wrap around body parts. Soft, succulent films (with higher concentrations of plasticiser) were found to be better for such applications, since they were quite flexible and could be bent or wrapped (Fig. 1B and C). In addition to being soft and flexible, the films for drug delivery applications must also be strong enough to resist wear and tear during application and bodily movements, and thus we studied the mechanical properties of the films.
Figure 1.
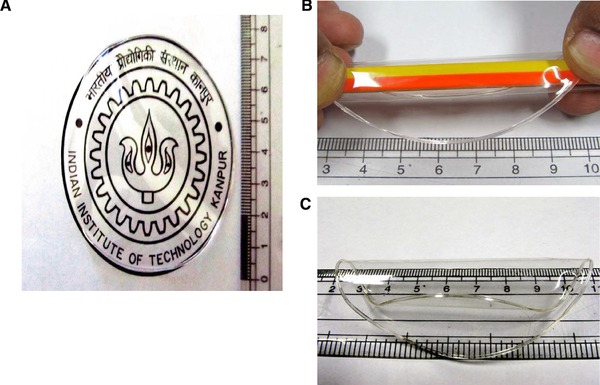
Representative images of agarose bioplastic films. The films are visually transparent (A), strong and flexible (B, C). In (A), the film was put over a logo of IIT Kanpur.
3.2. FT infrared
Although agarose and glycerol are well‐known biomaterials, it is important to ensure that during the fabrication of bioplastic films, no new covalent bonds were formed. This is because new chemical moieties formed during the processing stages may make our material non‐biocompatible. We performed a preliminary analysis through FTIR spectroscopy. The FTIR spectrum of pure agarose film (Fig. 2) is similar to that described in the literature 39. The large trough seen at 3500–3200 cm−1 is indicative of the large number of hydroxyl groups present both in agarose as well as in glycerol. Other troughs indicative of 3,6‐anhydrogalactose (930 and 1070 cm−1), C–H stretching (3000–2850 cm−1) and other functional groups are also present. It is observed that the spectra of agarose films without glycerol and those with 20% glycerol are similar with no obvious difference in troughs (Fig. 2). While this was expected since the bonds present in glycerol (‒C–H, ‒C–O, ‒O–H, ‒C–C) are also present in agarose, the emergence of no new troughs also indicates that no new covalent bonds are formed by the presence of glycerol.
Figure 2.
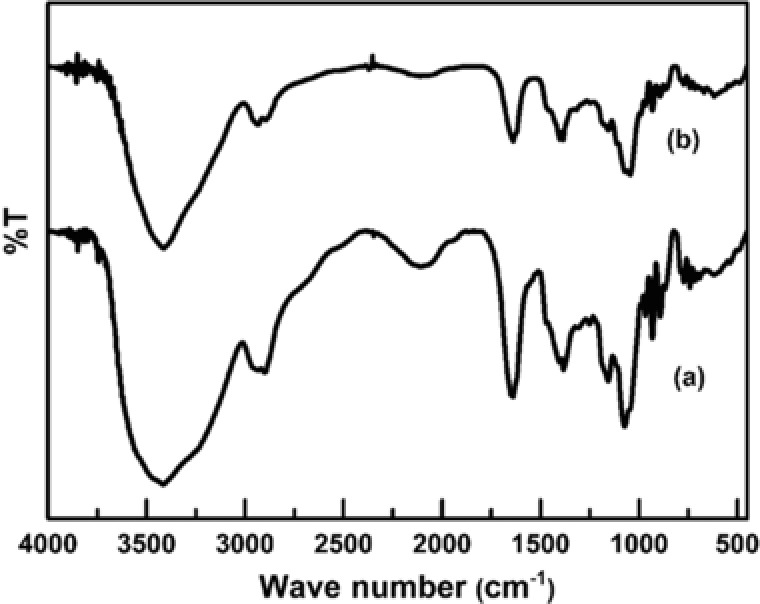
Representative IR spectra of (a) agarose bioplastic films without glycerol (continuous line; lower curve) and (b) with 20% glycerol. Similarity in the curves shows that no new covalent bonds are formed in the film with glycerol, and that the constituents exist as a physical mixture, without reacting chemically.
Thus, the FTIR results revealed that the film constituents remained in the form of a mixture. Since agarose and glycerol are both well‐known biomaterials, and no new chemical bonds were formed during fabrication of the films, we believed that the bioplastic would show good biocompatibility. More comprehensive biocompatibility assays were also performed, which are discussed in the following sections.
3.3. Mechanical properties of bioplastic films
The curve obtained was a typical graph obtained in case of polymer material. The non‐linear region indicates the plastic nature of the polymer 40. The ultimate tensile strength of films reduced from around 66 MPa to a little over 10 MPa, with increasing concentration of glycerol from 0 to 50% (v/w, relative to the amount of agarose) (Fig. 3). With higher plasticiser content, glycerol fills the spaces between agarose chains, thus increasing their distances and reducing their entanglement. Besides, glycerol is hygroscopic and introduces water into the matrix that in addition to increasing the distances between agarose chains also reduces their hydrophilic interactions. Both these factors result in a reduction in rigidity and strength of the films.
Figure 3.
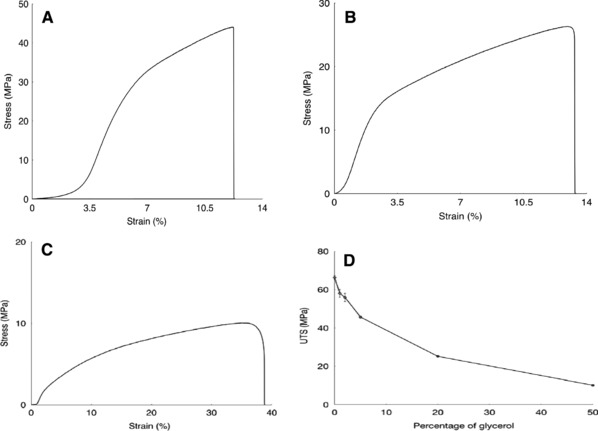
Tensile properties of bioplastic films are a function of the concentration of plasticiser. Representative stress–strain curves for films with 0% (A), 20% (B) and 50% (C) glycerol show that with increasing amounts of plasticiser, the ultimate tensile strength (UTS) decreases, while the strain at break increases. (D) UTS of bioplastic films as a function of concentration of glycerol. With increasing amounts of the plasticiser, the UTS of the films decreased. Further tests were performed on agarose –20% glycerol films for which they had appreciable strength while also having flexibility and suppleness. Multiple samples for each set were tested where number of samples (n = 5). Error bar represents standard error of mean.
The films with low concentrations of glycerol were found to be very strong, substantiating their potential as structural materials. The films with very high concentrations of glycerol were found to be very weak, though they were soft and could provide good comfort. For further studies, films with 20% glycerol were chosen following Goldilocks principle, for these films had acceptable values with three desirable properties – strength, flexibility and suppleness for drug delivery and some packaging applications.
3.4. Swelling characteristics
Many materials when exposed to water absorb it and gain mass; this phenomenon is called swelling. If a material is meant for drug delivery, then swelling is desirable, since it facilitates the release of drug. When a matrix absorbs water and swells, the average pore sizes increases, facilitating the release of the incorporated drugs.
In case of drug delivery devices, the rate of swelling could be manipulated to orchestrate the rate of release of drugs. Keeping these in mind, swelling studies of our agarose–glycerol films in water were performed for up to 135 min at three different temperatures, 4, 25 and 37°C, to mimic the conditions of cold weather, normal weather and body temperature, respectively. The percentage of swelling (SW) is defined by the following equation:
It was observed that the rate of absorption of water was highest in the beginning for all temperatures, and then gradually decreased with time, as the films became saturated (Fig. 4). The saturation was completed in 120 min. The amount of water absorbed by the films increased with temperature. At 37°C, the amount of water absorbed was more than 700% of the weight of the film. On the other hand, absorption of water at 4°C was around 500% of the original weight of the film. The reason is that at higher temperatures, the agarose chains within the matrix have more energy, and thus higher mobility. They are able to move apart with ease, generating more space that is taken up by water molecules. It is also worth mentioning that significant swelling occurred within 15 min. The large amount and fast rate of swelling indicated that the chains are relatively unhindered in their movement. The fast rate of swelling suggested the suitability of our agarose–glycerol films for applications that demand a quick release of drugs, such as drug delivery to wounds.
Figure 4.
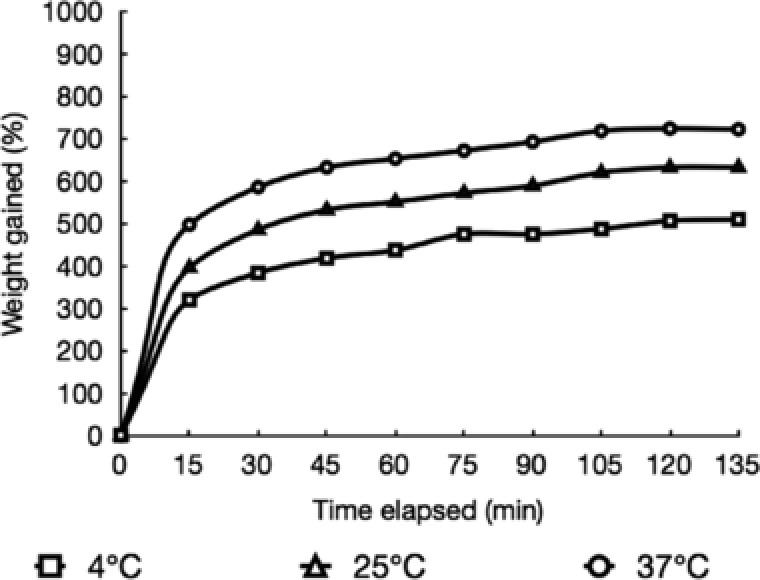
Swelling curves of agarose –20% glycerol bioplastic films at 4, 25 and 37°C as a function of time. The amount of water absorbed increases with time and temperature till a plateau is reached.
3.5. Contact angle measurements
We used sessile drop technique to estimate the contact angle made by water on the agarose bioplastic surface. It was found that the average contact angle made by water on the bioplastic surface was 11.4° (Fig. 5), thus indicating the highly hydrophilic nature of the agarose bioplastic surface.
Figure 5.
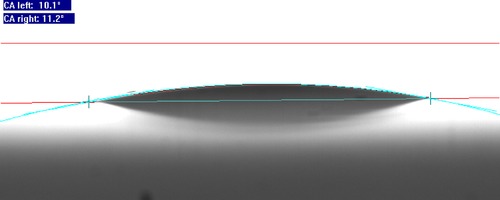
Water contact angle measurement of agarose bioplastic surface. When a drop of water was placed over the bioplastic surface, it spread. The contact angle made by the interaction of water with the bioplastic surface was measured using software. The low value of contact angle indicated that the material is hydrophilic. This surface property regulates adsorption of proteins over the surface of the biomaterial. Thus, we can expect that little amounts of proteins will be adsorbed over the bioplastic, when it comes in contact with bodily fluids.
A hydrophilic surface is warranted when we do not want cells to adhere to the surface 41, as in the present application where the drug delivery film must be removed with ease after its use. Most hydrophilic surfaces interact strongly with water molecules, effectively shielding themselves from proteins and hence are non‐fouling surfaces. Moreover, since hydrophobic interactions are the dominant way through which proteins adhere to surfaces, hydrophilicity ensures that any binding that does occur is weak 42. Since cells can attach to surfaces only through mediation of proteins, weakly adsorbed proteins guarantee that cell binding is absent or decrepit 43. It is, however, important to note that very highly hydrophobic materials can also resist cell adhesion due to alleviation of Vroman effect and denaturation of adsorbed proteins.
The cause of hydrophilic behaviour of the bioplastic lies in its chemical structure. The structure of agarose is comprised of a plethora of hydroxyl groups, with three hydroxyl groups per agarobiose unit. Owing to the large difference in electro negativities of hydrogen (electronegativity = 2.1) and oxygen (electronegativity = 3.5), the covalent bond between these atoms in the –OH group becomes highly polar. This polarity allows it to interact, via hydrogen bonding, with polar water molecules, thus giving the compound a strongly hydrophilic nature.
3.6. Quantification of protein adsorption
The quantification of protein in solution was done using Bradford assay, which is extremely sensitive towards BSA, the model protein used for the investigation. The use of BSA has other advantages too. It is one of the most abundant proteins found in blood, and is the first to adhere to surfaces. While it does not directly contribute to cell binding, it plays an important role by allowing easy replacement by fibronectin, the most prominent protein facilitating cell binding, and by changing molecular packing of fibronectin on the surface, allowing expression of its biologically active conformation for binding of cells 44.
We used the method of differences to calculate the amount of protein adsorbed on the agarose bioplastic surface. In this method, a known quantity of protein in solution was allowed to incubate with the sample under fixed conditions of time and temperature, after which the amount of protein remaining in the supernatant was calculated. The difference between these values of protein in the supernatant (initial amount – final amount) gave a measure of the amount of protein adsorbed on the surface.
It was found that half as much protein bound per unit surface of the bioplastic as did on TCPS (Fig. 6), the most common substrate used for adherent cell cultures. Ratner and colleagues have argued that “surfaces that strongly adsorb proteins will generally bind cells, and surfaces that resist protein adsorption will also resist cell adhesion” 42, 45. Thus, we expected the agarose bioplastic surface to have less cell adhesion than TCPS surface. This fact was substantiated in the next study using direct contact assay.
Figure 6.

Adsorption of BSA on agarose bioplastic and tissue‐culture polystyrene (TCPS, control) surfaces. The amount of protein adsorbed over the bioplastic was roughly half of that over the TCPS. Since protein adsorption guides host reaction to biomaterial, it can be hypothesised that the bioplastic shall initiate abated host responses when used as a drug delivery vehicle. Experiment was performed in triplicates and error bar represents standard error of mean.
3.7. Cell culture assays
Contact assays are in vitro methods to assess cytotoxicity of materials using cell cultures. As the name suggests, in direct contact assay, the cells come in direct contact with the test material. On the other hand, in indirect contact assay, either an elution from the material is added to cell cultures, or the material is separated from the cells by way of a barrier, while ensuring that any eluates leaching out from the material reach the cells. In both the assays, direct and indirect, the effect of the material on the morphology and physiology of the cells is evaluated.
It was observed that in both the contact assays, direct and indirect, the morphology of DF‐1 chicken fibroblast cells resembled that of their respective controls. The cells placed in indirect contact with the bioplastic (Fig. 7A) and those cultured with adherent surface positive controls (Fig. 7B) divulged the classic morphology of fibroblasts. On the other hand, the cells placed in direct contact with the bioplastic (Fig. 7C) and those cultured with ultralow attachment surface positive controls (Fig. 7D) revealed rounded morphology without any spreading. A number of cells remained in the cell culture suspension in these cases. For positive control, cells were cultured with non‐biocompatible Triton‐X 100 (0.1%). This detergent induces cell death.
Figure 7.
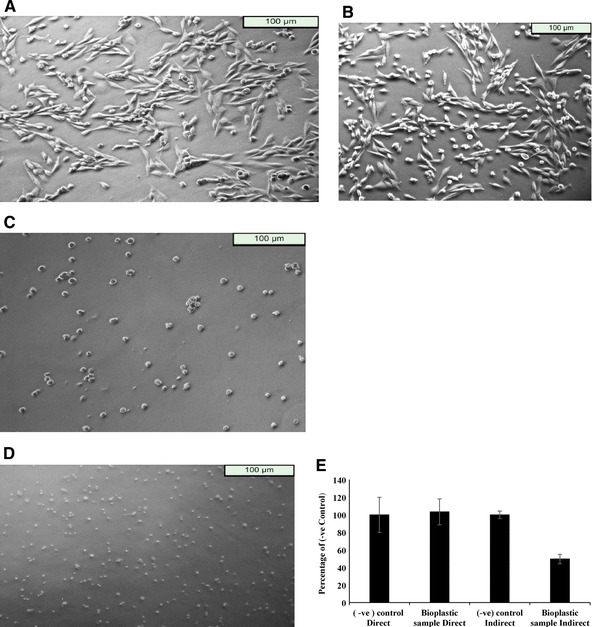
Direct and indirect contact cell culture assays for assessment of the cytocompatibility of agarose bioplastic. The morphology of cells in indirect contact with the bioplastic (A) closely resembled that of cells cultured directly on adherent surface (B). These cells were well spread over the surface and revealed the classic morphology of fibroblasts. Similarly, the morphology of cells in direct contact with the bioplastic (C) mirrored that of cells cultured directly on a non‐adherent surface (D). These cells were either unattached to the surface or revealed spherical morphology without any spreading. All these were starkly different from the morphology of dead cells while dead cells did not show any metabolic activity in MTT assay, the metabolic indices of cells cultured in direct and indirect contact with the bioplastic resembled those of their respective positive controls (E). These assays showed that the bioplastic did not produce any morphological or metabolic changes in the cells under our experimental conditions. Experiment was performed in triplicates and error bar represents standard error of mean.
For an investigation into the physiology of the cells in culture, the MTT assay 46 was chosen as a proxy. The MTT assay is a colorimetric assay, in which NAD(P)H‐dependent cellular oxidoreductase enzymes reduce the MTT dye, giving a purple precipitate. This precipitate is solubilised and quantified to provide an indication of the amount of cellular oxidoreductase enzymes. The amount of enzymes is dependent on the number of cells present in the culture and their metabolic states, with active cells having larger amounts of the enzyme than resting cells. The OD of the negative control is considered to be 100%, and reading from experimental sample is reported as percent of negative control.
The positive and negative controls were in accordance with ISO 10993‐5 cytotoxicity standard. For direct assay, wells in which cell death is induced by the addition of Triton X‐100 served as positive control. Cell seeded on other wells of same 24‐well plate served as negative control.
For indirect assays, controls were similar to direct assay with exception that wells were coated with gelatin in order to mimic adherent surface. As observed on conversion of MTT reading to percent of its negative control (Fig. 7E) in direct contact assay, the number of alive cells was comparable to that in negative control. However, in indirect assay, when MTT readings are converted to percent of its respective negative control, we observe a decrease in cell viability (OD becomes 60% of the respective negative control). DF‐1 chicken fibroblast cells are adherent cell type and require attachment to substrate for proliferation. Negative control in indirect assay was coated with gelatin and hence provided optimum conditions for cell proliferation. On the other hand, the bioplastic is non‐adherent and does not support cell attachment. Therefore, a decrease in OD was observed because of cell proliferated more in control as compared to sample and not due to cell death in the presence of bioplastic. The results from both direct and indirect contact assay suggest that the glycerol‐incorporated agarose bioplastics do not exhibit cytotoxicity, even when cells are not attached to the surface. The cells in direct contact with bioplastic remain in cell suspension form. The decrease in OD in indirect assay was because the adherent cells require attachment for proliferation and hence cells proliferated more on gelatin‐coated TCPS 47; this can also be seen in the form of lesser confluency in the DIC images (Fig. 7A and B vs. C and D).
Thus, the direct and indirect contact assays exhibited that there was no change in cellular morphology or physiology under the described experimental conditions in the presence of agarose bioplastic. This proved that the agarose bioplastic is highly cytocompatible.
3.8. Study of haemolytic behaviour
If a material is used for surgical dressing applications, blood and blood components would be amongst the first bodily materials to come into its contact. Therefore, it is necessary that the effect of the material or its exudates be investigated on blood cells to ascertain that there are no negative effects on blood.
Blood is a fluid connective tissue that is composed of several components, including red blood cells, white blood cells, platelets and plasma. Among these, the red blood cells are the most numerous, occurring at around 5 million cells per microlitre in human blood. They are especially fragile and upon lysis release the stored haemoglobin that can easily be quantified using UV–Vis spectrophotometry. Thus, they serve as good models to study haemolytic behaviour of substances. For positive control, we needed a procedure that lysed cells. A common method is to use detergents such as Tween 20 or Triton‐X 100, which disrupt cellular membrane and cause cell lysis.
The haemolysis assay results (Fig. 8) demonstrated that the amount of haemolysis occurring in the presence of bioplastic was equivalent to that occurring in negative control conditions (PBS pH 7.4). The values for haemolysis in the presence of bioplastic and negative control were 6.505 and 3.11%, respectively. Thus, the bioplastic is haemocompatible and safe to use in applications where it comes in contact with blood. Next, we wanted to check if the material can be used as a drug delivery vehicle, for which we studied the rate of drug release and the potency of the incorporated drug.
Figure 8.
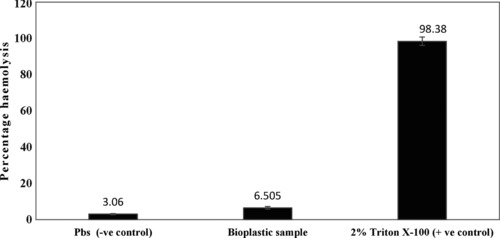
Haemocompatibility assay of agarose bioplastic sample vis‐à‐vis PBS pH 7.4 (negative control) and 2% Triton‐ X 100 (positive control). The percent haemolysis seen in the bioplastic sample was negligible, similar to that seen in the negative control. In the positive control, large amount of haemolysis occurred. The results showed that the agarose bioplastic is highly haemocompatible. Experiment was performed in triplicates and error bar represents standard error of mean.
3.9. Study of potency of released drugs
Since the drugs were added at a temperature of around 60˚C, there was a possibility, albeit slight, that they got denatured. To ascertain this, the potencies of several drugs released from the film were tested through standard agar diffusion tests. It was found that while the control discs without any antibacterial drug did not inhibit the growth of bacteria (Fig. 9A), those with drugs had a distinct zone of inhibition around them in which there was no perceptible bacterial growth (Fig. 9B–F). This showed that the drugs, even after their incorporation in the agarose bioplastic, retained their potencies.
Figure 9.
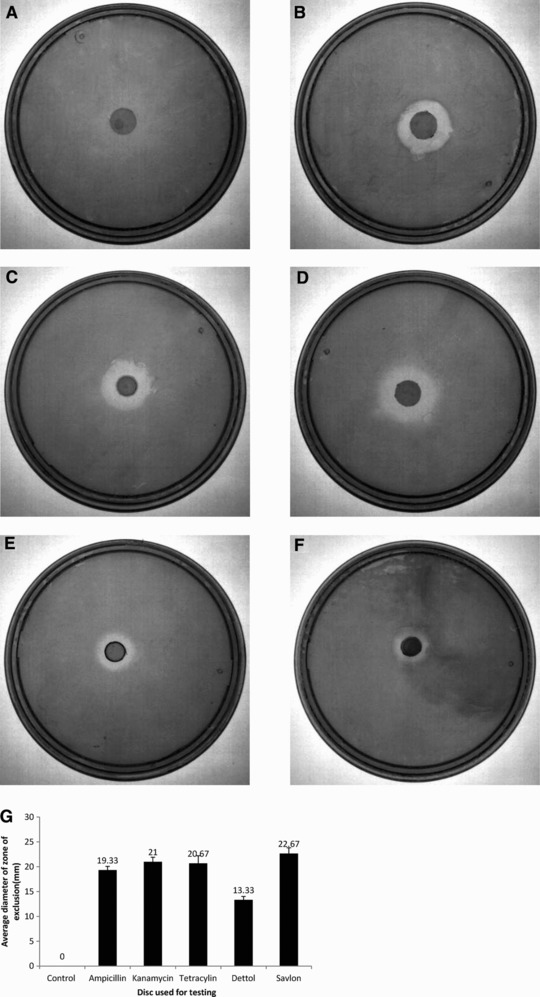
Study of potencies of drugs incorporated in agarose bioplastic, as determined through agar diffusion assay. Agar diffusion assays were performed on control films without any antibacterial drugs (A), and on films loaded with ampicillin (B), kanamycin (C), tetracycline (D), dettol (E) and savlon (F). The films loaded with drugs had a distinct zone of inhibition around them where bacterial growth was impeded, whereas the control films did not hamper the growth of bacteria. The sizes of the zones of inhibition for the drugs are also depicted (G). The experiment was just indicative to show potency of drug hence done only once.
4. Concluding remarks
In the present work, a bioplastic made up of agarose with glycerol as plasticiser was synthesised and tested for drug delivery application. The bioplastic film was shown to be transparent, strong, flexible and with swelling properties that aid drug delivery. The FTIR curves displayed that the constituents of the film, glycerol and agarose, did not form any chemical bonds and remained in the form of a mixture. Hence, they did not lose their biocompatibility. The bioplastic films prepared in this report exhibited desired properties for use as drug delivery vehicles in applications where fast release of drugs is required. The films were hydrophilic and showed low adsorption of proteins on their surface. Direct and indirect contact assays revealed that the films were non‐cytotoxic and non‐adherent, and haemolysis assay showed that the films were haemocompatible. Many drugs were incorporated in the bioplastic, and they were found to be unaffected by the processing steps. They exhibited their potencies by displaying their characteristic bacteriostatic and bactericidal properties. All these properties strongly posit the agarose bioplastic as a suitable drug delivery vehicle for surgical dressings.
In addition to possible applications in surgical dressings where incorporation of drugs are required, in future, agarose bioplastic can also be modified for use in sustained drug release applications, such as transdermal patches.
Practical application
A wide variety of drug delivery systems have been in vogue, since antiquity, for surgical dressings and wound dressings: from simple cotton wool and cotton‐based gauzes to extensively designed, engineered and evaluated poly(lactic‐co‐glycolic acid) films. This variety is imperative, principally because the multifariousness of wounds requisites the concoction of an assorted arsenal of dressing materials, with distinct properties with regard to their conformation, configuration, ease of drug incorporation, economics and facileness of their preparation and obtainability. This paper demonstrates a novel drug delivery system in the form of agarose bioplastic films that is thin, transparent, flexible and biocompatible, and maintains drug potency and low temperature processing, permitting substantial incorporation of even heat‐labile antibiotics. We are of the opinion that such a vehicle will generate its own niche in the arsenal of surgical and wound dressing materials.
The authors declare competing financial interest. A.A. and V.V. have filed for an Indian patent with application number 906/DEL/2015 dated March 31, 2015 and an international patent with application number PCT/IB2015/053216 on May 02, 2015. The work demonstrated here is part of the patents.
Acknowledgments
We thank Dr. Amitabha Bandyopadhyay and Dr. K. Subramaniam for kindly providing E. coli DH5α strain and tetracycline samples, respectively, and Dr. Pradip Sinha for providing access to Nanodrop facilities. The research was supported by DST‐SERB (SR/S3/CE/038/2012) and DBT (BT/PR14121/BRB/10/813/201). Laser cutting was done at 4i Laboratories, mechanical testing was performed at Advanced Center for Materials Sciences and imaging of Petri plates was done at Biological Sciences and Bioengineering department, IIT Kanpur. We are thankful to Dr. Amitabha Bandyopadhyay for kindly providing facilities for cell culture studies and Dr. Krishnacharya for contact angle measurements. Mr. Sankalp Verma aided in the scanning electron microscopy of film samples. A.A. is supported by institute fellowship from IIT Kanpur. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
5 References
- 1. Demidova‐Rice, T.N. , Hamblin, M.R. , Herman, I.M. , Acute and impaired wound healing: Pathophysiology and current methods for drug delivery, part 2: Role of growth factors in normal and pathological wound healing: Therapeutic potential and methods of delivery. Adv. Skin Wound Care 2012, 25, 349–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Demidova‐Rice, T.N. , Hamblin, M.R. , Herman, I.M. , Acute and impaired wound healing: Pathophysiology and current methods for drug delivery, part 1: Normal and chronic wounds: Biology, causes, and approaches to care. Adv. Skin Wound Care 2012, 25, 304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sen, C.K. , Gordillo, G.M. , Roy, S. , Kirsner, R. et al., Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009, 17, 763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gottrup, F. , Apelqvist, J. , Bjarnsholt, T. , Bjansholt, T. et al., EWMA document: Antimicrobials and non‐healing wounds. Evidence, controversies and suggestions. J. Wound Care 2013, 22, S1–S89. [DOI] [PubMed] [Google Scholar]
- 5. Mock, C.N. , Jurkovich, G.J. , nii Amon‐ Kotei, D. , Arreola‐Risa, C. et al., Trauma mortality patterns in three nations at different economic levels: Implications for global trauma system development. J. Trauma 1998, 44, 804–812. [DOI] [PubMed] [Google Scholar]
- 6. Howell‐Jones, R.S. , Wilson, M.J. , Hill, K.E. , Howard, A.J. et al., A review of the microbiology, antibiotic usage and resistance in chronic skin wounds. J. Antimicrob. Chemother. 2005, 55, 143–149. [DOI] [PubMed] [Google Scholar]
- 7. Sapico, F.L. , Ginunas, V.J. , Thornhill‐Joynes, M. , Canawati, H.N. et al., Quantitative microbiology of pressure sores in different stages of healing. Diagn. Microbiol. Infect. Dis. 1986, 5, 31–38. [DOI] [PubMed] [Google Scholar]
- 8. Sopata, M. , Luczak, J. , Ciupinska, M. , Effect of bacteriological status on pressure ulcer healing in patients with advanced cancer. J. Wound Care 2002, 11, 107–110. [DOI] [PubMed] [Google Scholar]
- 9. Citron, D.M. , Goldstein, E.J.C. , Merriam, C.V. , Lipsky, B.A. et al., Bacteriology of moderate‐to‐severe diabetic foot infections and in vitro activity of antimicrobial agents. J. Clin. Microbiol. 2007, 45, 2819–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gerding, D.N. , Foot infections in diabetic patients: The role of anaerobes. Clin. Infect. Dis. 1995, 20, S283–S288. [DOI] [PubMed] [Google Scholar]
- 11. Johnson, S. , Lebahn, F. , Peterson, L.R. , Gerding, D.N. , Use of an anaerobic collection and transport swab device to recover anaerobic bacteria from infected foot ulcers in diabetics. Clin. Infect. Dis. 1995, 20, S289–S290. [DOI] [PubMed] [Google Scholar]
- 12. Louie, T.J. , Aerobic and anaerobic bacteria in diabetic foot ulcers. Ann. Intern. Med. 1976, 85, 461–463. [DOI] [PubMed] [Google Scholar]
- 13. Davies, C.E. , Hill, K.E. , Wilson, M.J. , Stephens, P. et al., Use of 16S ribosomal DNA PCR and denaturing gradient gel electrophoresis for analysis of the microfloras of healing and nonhealing chronic venous leg ulcers. J. Clin. Microbiol. 2004, 42, 3549–3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Davies, C.E. , Hill, K.E. , Newcombe, R.G. , Stephens, P. et al., A prospective study of the microbiology of chronic venous leg ulcers to reevaluate the clinical predictive value of tissue biopsies and swabs. Wound Repair Regen. 2007, 15, 17–22. [DOI] [PubMed] [Google Scholar]
- 15. Mustoe, T. , Understanding chronic wounds: A unifying hypothesis on their pathogenesis and implications for therapy. Am. J. Surg. 2004, 187, S65–S70. [DOI] [PubMed] [Google Scholar]
- 16. Sofokleous, P. , Stride, E. , Edirisinghe, M. , Preparation, characterization, and release of amoxicillin from electrospun fibrous wound dressing patches. Pharm. Res. 2013, 30, 1926–1938. [DOI] [PubMed] [Google Scholar]
- 17. Boateng, J.S. , Matthews, K.H. , Stevens, H.N.E. , Eccleston, G.M. , Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [DOI] [PubMed] [Google Scholar]
- 18. Yamane, T. , Nakagami, G. , Yoshino, S. , Muramatsu, A. et al., Hydrocellular foam dressing promotes wound healing along with increases in hyaluronan synthase 3 and PPARα gene expression in epidermis. PLoS One 2013, 8, e73988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dumville, J.C. , Deshpande, S. , O'Meara, S. , Speak, K. , Hydrocolloid dressings for healing diabetic foot ulcers. Cochrane Database Syst. Rev. 2012, 2, CD009099. [DOI] [PubMed] [Google Scholar]
- 20. Dumville, J.C. , O'Meara, S. , Deshpande, S. , Speak, K. , Alginate dressings for healing diabetic foot ulcers. Cochrane Database Syst. Rev. 2013, 6, CD009110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. O'Meara, S. , Martyn‐St James, M. , Alginate dressings for venous leg ulcers. Cochrane Database Syst. Rev. 2013, 4, CD010182. [DOI] [PubMed] [Google Scholar]
- 22. Liakos, I. , Rizzello, L. , Bayer, I.S. , Pompa, P.P. et al., Controlled antiseptic release by alginate polymer films and beads. Carbohydr. Polym. 2013, 92, 176–183. [DOI] [PubMed] [Google Scholar]
- 23. Tsuchiya, S. , Ichioka, S. , Sekiya, N. , Tajima, S. et al., The effect of a hydrocolloid dressing containing ceramide‐2 on split‐thickness wounds in a laser‐induced erosion model. Adv. Skin Wound Care 2013, 26, 224–229. [DOI] [PubMed] [Google Scholar]
- 24. Pereira, R. , Carvalho, A. , Vaz, D.C. , Gil, M.H. et al., Development of novel alginate based hydrogel films for wound healing applications. Int. J. Biol. Macromol. 2013, 52, 221–230. [DOI] [PubMed] [Google Scholar]
- 25. Kickhöfen, B. , Wokalek, H. , Scheel, D. , Ruh, H. , Chemical and physical properties of a hydrogel wound dressing. Biomaterials 1986, 7, 67–72. [DOI] [PubMed] [Google Scholar]
- 26. Higa, O. , Rogero, S. , Machado, L.D. , Mathor, M. et al., Biocompatibility study for PVP wound dressing obtained in different conditions. Radiat. Phys. Chem. 1999, 55, 705–707. [Google Scholar]
- 27. Tian, H. , Xu, G. , Yang, B. , Guo, G. , Microstructure and mechanical properties of soy protein/agar blend films: Effect of composition and processing methods. J. Food Eng. 2011, 107, 21–26. [Google Scholar]
- 28. Carlsson, J. , Physical and nutritional factors in gel culture of mammalian cells. In Vitro 1978, 14, 860–867. [DOI] [PubMed] [Google Scholar]
- 29. Natarajan, S. , Chow, T.T. , Shay, J.W. , Wright, W.E. , Replica plating of mammalian cells using low melt agarose. Cytotechnology 2007, 54, 145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kurkus, J. , Nilsson, R. , Lindén, O. , Schönström, N. et al., Biocompatibility of a novel avidin‐agarose adsorbent for extracorporeal removal of redundant radiopharmaceutical from the blood. Artif. Organs 2007, 31, 208–214. [DOI] [PubMed] [Google Scholar]
- 31. Fernández‐Cossío, S. , León‐Mateos, A. , Sampedro, F.G. , Oreja, M.T.C. , Biocompatibility of agarose gel as a dermal filler: Histologic evaluation of subcutaneous implants. Plast. Reconstr. Surg. 2007, 120, 1161–1169. [DOI] [PubMed] [Google Scholar]
- 32. Sloan, A.J. , Smith, A.J. , Stimulation of the dentine‐pulp complex of rat incisor teeth by transforming growth factor‐beta isoforms 1‐3 in vitro. Arch. Oral Biol. 1999, 44, 149–156. [DOI] [PubMed] [Google Scholar]
- 33. Berezhna, L.G. , Ivanov, A.E. , Leistner, A. , Lehmann, A. et al., Structure and biocompatibility of poly(vinyl alcohol)‐based and agarose‐based monolithic composites with embedded divinylbenzene‐styrene polymeric particles. Prog. Biomater. 2013, 2, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brill, A. , Dashevsky, O. , Rivo, J. , Gozal, Y. et al., Platelet‐derived microparticles induce angiogenesis and stimulate post‐ischemic revascularization. Cardiovasc. Res. 2005, 67, 30–38. [DOI] [PubMed] [Google Scholar]
- 35. Iacoviello, M. P. , Rubin, S. A. , Sterile preparation of antibiotic‐selective LB agar plates using a microwave oven. Biotechniques 2001, 30, 963–965. [DOI] [PubMed] [Google Scholar]
- 36. Bradford, M.M. , A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Anal. Biochem. 1976, 72, 248–254. [DOI] [PubMed] [Google Scholar]
- 37. Koley, D. , Bard, A.J. , Triton X‐100 concentration effects on membrane permeability of a single HeLa cell by scanning electrochemical microscopy (SECM). Proc. Natl. Acad. Sci. USA 2010, 107, 16783–16787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fischer, D. , Li, Y. , Ahlemeyer, B. , Krieglstein, J. et al., In vitro cytotoxicity testing of polycations: Influence of polymer structure on cell viability and hemolysis. Biomaterials 2003, 24, 1121–1131. [DOI] [PubMed] [Google Scholar]
- 39. Samiey, B. , Ashoori, F. , Adsorptive removal of methylene blue by agar: Effects of NaCl and ethanol. Chem. Cent. J. 2012, 6, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Callister, W.D. , David, J. R. , Rethwisch, G. , Materials Science and Engineering: An Introduction, 9th ed., John Wiley & Sons Inc, 2014. [Google Scholar]
- 41. Horbett, T.A. , Waldburger, J.J. , Ratner, B.D. , Hoffman, A.S. , Cell adhesion to a series of hydrophilic‐hydrophobic copolymers studied with a spinning disc apparatus. J. Biomed. Mater. Res. 1988, 22, 383–404. [DOI] [PubMed] [Google Scholar]
- 42. Ratner, B.D. et al, Biomaterials Science: An Introduction to Materials in Medicine, Elsevier Academic Press, Amsterdam: 2012. [Google Scholar]
- 43. Enderle, J.D. , Bronzino, J.D. , Introduction to Biomedical Engineering, Academic Press, 2012. [Google Scholar]
- 44. Grinnell, F. , Feld, M.K. , Fibronectin adsorption on hydrophilic and hydrophobic surfaces detected by antibody binding and analyzed during cell adhesion in serum‐containing medium. J. Biol. Chem. 1982, 257, 4888–4893. [PubMed] [Google Scholar]
- 45. Ratner, B.D. , Hoffman, A.S. , Schoen, F.J. , Lemons, J.E. et al., Biomedical Engineering e‐Mega Reference, Elsevier: Academic Press, 2009. [Google Scholar]
- 46. Mosmann, T. , Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [DOI] [PubMed] [Google Scholar]
- 47. Lodish, H. , Berk, A. , Zipursky, S.L. , Matsudaira, P. et al., Mol. Cell Biol. W H Freeman & Co 2000. [Google Scholar]


