Abstract
Microalgae emerge as the most promising protein sources for aquaculture industry. However, the commercial proteins production at low cost remains a challenge. The process of harnessing microalgal proteins involves several steps such as cell disruption, isolation and extraction. The discrete processes are generally complicated, time‐consuming and costly. To date, the notion of integrating microalgal cell disruption and proteins recovery process into one step is yet to explore. Hence, this study aimed to investigate the feasibility of applying methanol/potassium ATPS in the integrated process for proteins recovery from Chlorella sorokiniana. Parameters such as salt types, salt concentrations, methanol concentrations, NaCl addition were optimized. The possibility of upscaling and the effectiveness of recycling the phase components were also studied. The results showed that ATPS formed by 30% (w/w) K3PO4 and 20% (w/w) methanol with 3% (w/w) NaCl addition was optimum for proteins recovery. In this system, the partition coefficient and yield were 7.28 and 84.23%, respectively. There were no significant differences in the partition coefficient and yield when the integrated process was upscaled to 100‐fold. The recovered phase components can still be recycled effectively at fifth cycle. In conclusions, this method is simple, rapid, environmental friendly and could be implemented at large scale.
Keywords: Aqueous two‐phase system, Cell disruption, Microalgae, Process integration, Proteins recovery
Abbreviations
- ATPS
aqueous two‐phase system
- BCA
bichinchoninic acid
- BSA
bovine serum albumin
- PEG
poly(ethylene glycol)
1. Introduction
Fishmeal is an excellent foundation of aquaculture feed formulation 1 due to high quality and high digestibility of proteins 2, 3. Nonetheless, the growing demand for protein feed sources in aquaculture industry greatly affects the supply and price of fishmeal 4, 5, 6. The imbalance between supply and demand has caused an inevitable increase in the price of fishmeal 1, 4, and this could ultimately impede the sustainability of aquaculture sector 7. The other alternative protein source to replace fishmeal is soybean crops 2, 6, 8. However, soybean is not an ideal fishmeal replacer for cultured fish due to a few limitations. These include the low digestibility of protein 5, 9 and the existence of anti‐nutritional factors. Furthermore, deficiency in certain essential amino acids such as methionine, lysine and cysteine will cause significant changes in the nutritional quality of the cultured fish and thus additional investments for the addition of synthetic amino acids is required 10, 11. In view of this, it is crucial to look for a new protein sources that could supply comparable nutritional value in a sustainable and economical way for aquaculture industry 4. Recent literature reported that there is an increasing popularity of using microalgae as the most promising alternative to conventional protein sources for fish farming 1, 12, 13, 14, 15. This is mainly due to their high protein content 16, 17, 18 and nutritional quality 5 with adequate balance of essential amino acids 19. Studies on various microalgae revealed that besides lipid and carbohydrate 20, protein is also one of the major constituents of the microalgae biomass 17 and is higher than the most widely‐cultivated soybean crops 21. Microalgae can be cultivated in indoor and outdoor environments 22, whereby the indoor cultivation of microalgae is unaffected by seasonal variations 23, 24. Microalgae are tiny in size compared to the other higher plants but possess a number of attractive attributes which offer several advantages over conventional crops 14, 25. These include high photosynthetic efficiency 18, 26, high growth rate 27, 28, 29 and amenable to mass culture with minimum land space requirement 30.
Nonetheless, the mass production of microalgal proteins presents a significant challenge especially in the downstream processing. The process of proteins recovery from microalgae includes the isolation and extraction of proteins after being exposed to cell disruption 31. The conventional discrete processes are generally time‐consuming, tedious, complicated, require high energy input and would lead to difficulty for a smooth operation 32. The use of traditional separation techniques such as chromatography involves high cost, batch operation, low throughput and complex scale‐up 33, whereas the elimination of cell debris by high‐speed centrifugation or cross‐flow membrane filtration may be difficult to achieve at large scale 31. Furthermore, a higher number of processing steps would result in higher product loss due to the loss of some quantity of target biomolecules in each operational step 32, 34.
It has been reported that most of these limitations can be overcome by using aqueous two‐phase systems (ATPS) in the downstream processing 32, 35. A number of operational steps involved the use of conventional techniques can be replaced by ATPS, making the whole process more cost effective 36. ATPS enable the extraction and isolation of target product from cell debris by concentrating them into one of the phases 37. There are many other advantages of using this well‐established method in downstream processing. These include simplicity in operation, high effectiveness, low cost 32, 38, 39, rapid mass transfer and phase equilibrium, gentle due to the presence of high water content in both phases, low interfacial tension of phases 38, rapid separation with little denaturation, high selectivity, biocompatibility, low energy requirement and potential for upscaling 32, 40. Previous studies reported that ATPS able to separate intracellular proteins from cell debris 33 or other soluble cell components 34, 41. In the process of extracting papain from wet Carica papaya latex, ATPS showed higher recovery (88%) with a much shorter processing time than traditional procedure involving a two‐step salt precipitation (49%) 42. The purification of selective proteins namely photosynthetic pigment C‐phycocyanin 43 or non‐chlorophyll accessory pigments such as fucoxanthin 44 from microalgae using ATPS have also been studied recently. The efficiency of biomolecules partitioning in ATPS can be manipulated and improved significantly by altering various parameters, such as the concentration of the phase component, biomolecule size, affinity of the molecules for the phase‐forming component, pH 38 and system temperature 32, 45, 46. Various compounds such as polyethylene glycol (PEG), dextran, salts, and ionic liquids can be used for the formation of ATPS. ATPS formed by alcohol and salt is simple, low‐cost, characterized by low viscosity, short phase‐separation time and high polarity 47. Additionally, the phase‐forming components can be recovered easily for reuse after aqueous two‐phase extraction 47. Alcohol can be recovered using evaporation method whilst salt can be recycled by dilution crystallization method 48, 49. These characteristics contribute to the easy upscaling of alcohol/salt ATPS for industrial scale 48. However, extensive research concerning the recycling of the phase components from this type of ATPS is still limited.
In the recent years, the notion to implement process integration into the industrial scale of bioproducts recovery process is of great practical and economic interest 50, 51. Previous study demonstrated that the attempt to recover intracellular proteins from bakers’ yeast using process integration wherein cell disruption and ATPS were integrated into one single step was realizable 31. Integrated method offers considerable potential benefit for the recovery of intracellular proteins. Studies showed that the direct integration of cell disruption with primary recovery unit operations enable faster processing with less opportunity for target modification or degradation. This could enhance both the yield and molecular quality of protein products 31, 52. Besides reducing the number of operational steps, this method could also lessen waste, reduce energy consumption and consequently decrease the overall cost 38, 39.
Due to the growing significance of protein sources for aquaculture sector, the effort to commercially harness proteins from microalgae at large scale with minimum processing cost is garnering worldwide attention. Process integration is a promising approach towards reducing overall production cost by simplifying the total number of unit operations in downstream processing. An interesting review written by Benavides et al. in 2008 has provided comprehensive information on the potential achievement of process integration based upon ATPS strategies 50. They expressed concerns about the restricted development of ATPS‐based bioprocess, which is due to poor understanding and characterization of the effects of ATPS influential parameters on the partitioning of a particular compound 50. In view of this, this study aimed to investigate the feasibility of using methanol/potassium salt ATPS in the integrated process of cell disruption and proteins recovery from protein‐rich strain of Chlorella sorokiniana. The effects of different ATPS variables such as types of salt, the concentrations of methanol and potassium salt, the addition of sodium chloride (NaCl) on the partitioning behaviour of proteins were determined to obtain maximum proteins yield from microalgae under optimum conditions. In addition, the possibility of upscaling and the effectiveness of using the recycled phase components at each recycling step were also investigated.
2. Materials and methods
2.1. Materials
The bicinchoninic acid (BCA) protein test kit with bovine serum albumin (BSA) standards included in (71285‐3) was purchased from Merck. Chemicals such as methanol, NaCl, tripotassium phosphate (K3PO4) and dipotassium phosphate (K2HPO4) were purchased from Sigma Aldrich.
2.2. Microalgae
C. sorokiniana, which belongs to the division of Chlorophyta and Trebouxiophyceae class, was provided by National Cheng Kung University, Taiwan for this study. The microalgae were cultivated in a laboratory‐scale photobioreactor to produce maximum biomass. Next, the frozen paste of crude microalgal biomass was freeze dried for 48 h using freeze dryer. After that, the biomass was manually ground into fine powder using a mortar for the subsequent studies of cell disruption and proteins recovery in the process integration. This freeze drying process mainly served to dry the microalgal biomass and was not part of the extractive disruption integrated process. Freeze drying is broadly used for dewatering of microalgal biomass. It is a gentle processing technique which enables the preservation of all the cell constituents without causing damage to the cell wall 53, 54, 55. Besides ease of storage, the microalgal stocks were prepared and used in dried biomass form throughout the study. This was to standardize the starting materials and avoid measurement errors associated with liquid carry‐over in microalgae fresh weight 56. As all samples were being treated equally, this shall normalize the effect of freeze drying, hence the results would be strictly depends on the subsequent differential treatments for comparison.
2.3. Phase diagram
The phase diagrams for 10–55% (w/w) methanol/ 4–40% (w/w) K2HPO4 and 10–60% (w/w) methanol/ 4–40% (w/w) K3PO4 were constructed according to the turbidometric titration method which has been described in detail by Albertsson 57. In this experiment, a mixture of methanol and potassium salt of known amount was titrated drop‐wise with the appropriate amount of distilled water until the mixture turned clear. This indicated the formation of one phase. Drop‐wise titrations were carried out on an electrical balance. The mixture was mixed constantly after each droplet. The total weight of water added was measured and the resultant compositions of methanol and potassium salt at the point of transition were calculated. The above procedures were repeated to obtain sufficient binodal points. Phase diagrams were then plotted at varying methanol and salt concentrations based on the binodal points.
2.4. Process integration of ultrasonication‐assisted disruption and aqueous two‐phase extraction for microalgal proteins recovery
This section describes the procedures of process integration in which both ultrasound‐assisted disruption and aqueous two‐phase extraction were integrated into one process and carried out simultaneously. Appropriate amounts of potassium salt, water and methanol were weighed into a 15 mL centrifuge tube. Subsequently, 0.2% (w/w) or 0.02 g of dry microalgal biomass was added to each system to obtain a final total weight of 10 g. All components of the system were measured by weight. After thorough mixing by a vortex mixer, each mixture was exposed to ultrasonication for 20 min. Subsequently, the mixture was centrifuged at 4000 rpm for 5 min for the complete formation of biphasic system. The volume ratio of each system was recorded and protein concentrations in the top and bottom layer of ATPS were estimated, respectively, by BCA assay. All experiments were carried out at room temperature for at least three independent trials.
2.5. Optimization of ATPS
Optimization of ATPS was performed based on four parameters to achieve maximum protein recovery from microalgae. Parameters such as types of salt (K2HPO4 and K3PO4), salt concentrations, methanol concentrations and NaCl addition (0–5%) (w/w) were investigated in this study. The concentration of the salt and methanol were selected based on the phase diagram (above the binodal curve) constructed in Section 2.4, in which the mixture of both components able to form two immiscible aqueous phases. The volume ratio of each system was recorded and protein concentrations in the top and bottom layer of ATPS were estimated, respectively, by BCA assay. All experiments were carried out at room temperature for at least three independent trials.
2.6. Upscaling of ATPS
After optimization, the chosen system was scaled up to a final total weight of 1000g which composed of appropriate amount of potassium salt, methanol, water and microalgae. The sample preparation steps were carried out similar to the procedures mentioned in Section 2.6. The volume ratio of each system was recorded and protein concentrations in the top and bottom layer of ATPS were estimated, respectively, by BCA assay. All experiments were carried out at room temperature for at least three independent trials.
2.7. Morphological observation of microalgae cells
Microscope slides of microalgal cells before and after subjected to process integration were prepared for microscopy observation. A drop of water containing microalgal cells was placed onto a microscope slide and checked under a light microscope. Images were captured to evaluate the morphological changes of the treated cells compared with the untreated cells (control).
2.8. Recycling of phase components
The effectiveness of using recovered phase components of the integrated process was evaluated in this section. After centrifugation, the methanol‐rich top phase which was separated from the salt‐rich bottom phase was then subjected to evaporation for recycling. The recycled methanol was then mixed with the bottom phase recovered from the primary system to form a secondary two‐phase system. Separation of cell debris was performed between cycles. Appropriate amounts of fresh methanol, water and potassium salt were added in to account for phase components losses from the system, when deemed necessary. Five successive recovery operations were performed by repeating the same procedures. The volume ratio of each system was recorded and protein concentrations in the top and bottom layer of ATPS were estimated, respectively, by BCA assay. All experiments were carried out at room temperature for at least three independent trials.
2.9. Protein analysis using colorimetric method: BCA assay
The total protein concentrations were estimated by the BCA protein assay according to the user manual of Merck protein assay kit. A total of 25 μL of sample was mixed with 200 μL of the working reagent in a 96‐well plate and subsequently incubated for 30 min at 37°C. The absorbance was measured at 562 nm using microplate absorbance reader. BSA was used as a protein standard to construct a calibration curve. To eliminate interference from the phase components, samples were analyzed against a set of blanks containing the identical phase system without microalgae. All the protein content estimations were carried out in triplicate. The spectrophotometric absorbance readings were converted to protein concentrations (μg/mL) using an equation generated from the BSA standard curve.
2.10. Calculation
The partition coefficient (K) of the proteins is defined as the ratio of the proteins concentration in the two phases:
| (1) |
where A T and A B represent the equilibrium concentration of the partitioned proteins (μg/mL) in the top phase and bottom phase, respectively.
The volume ratio (Vr) was calculated according to the following equation:
| (2) |
where V T symbolizes the volume of top phase; V B denotes the volume of bottom phase
Yield of soluble proteins in top phase was determined to evaluate the recovery performance:
| (3) |
where Vr is the volume ratio; K is the partition coefficient
2.11. Statistical analysis
Results were expressed as the mean ± SD of three replicates. Data were statistically analyzed at a 95% confidence level either using one‐way analysis of variance (ANOVA) or two‐sample t‐test.
3. Results and discussion
3.1. Phase diagram
ATPS is a liquid–liquid fractionation method 32 which is composed of two liquid phases of structurally different components that are immiscible when the limiting concentrations are exceeded 36. Reliable phase diagram are beneficial to the selection of an appropriate ratio of the phase composition when designing an ATPS. Information about concentration of phase‐forming components required to form two phases, the salting‐out and phase‐separation abilities as well as the ratio of phase volumes can be extrapolated from the phase diagram 58. The distinct boundary of the phase separation presented on the diagram (Fig. 1A and B) is known as a binodal curve. It divides a region of component concentrations that will form two immiscible aqueous phases (beyond the curve) from those that will form one phase (at and below the curve) 36. Exclusion of hydrophilic solvents and salts crystallization are the two common phenomena resulted from the mutual competition between hydrophilic solvent and salt for water molecules 58.
Figure 1.
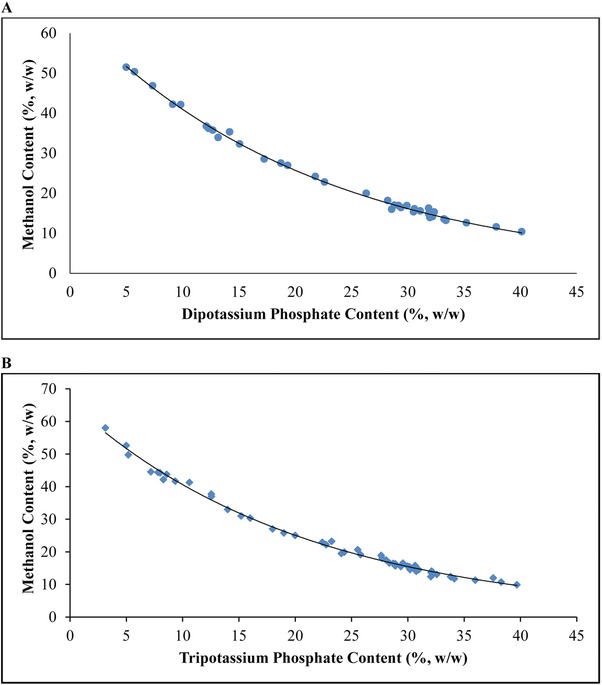
Phase diagrams of (A) methanol/dipotassium phosphate ATPS with approximate pH range 10.4–10.8; and (B) methanol/tripotassium phosphate ATPS with approximate pH range 12.8–13.6.
The two systems considered in this study were two aqueous phases of different natures: a predominant hydrophobic top phase constituted mainly of methanol and a more hydrophilic salt‐rich bottom phase. The aqueous solutions of different types of phosphate salt confer different pH values for each system, with pH range approximately 10.4–10.8 and 12.8–13.6 for K2HPO4 and K3PO4, respectively (Fig. 1A and B). Previous studies revealed that the alkaline solution was effective in serving the dual purpose of disrupting cell wall and solubilizing proteins from microalgae 59, 60. Alkaline treatment could act in synergy with the mechanical characteristics of the microalgal cell envelope, resulting in a significant increase of the protein release in the aqueous phase 13, 61. Also, the alkaline solution could improve the solubility of proteins by inducing net electrical charges on the amino acid residues 62.
By referring to the phase diagrams illustrated in Fig. 1A and B, the appropriate concentrations of methanol and potassium salt (above the binodal curve) that can induce phase separation to yield two phases were chosen for the optimization of proteins recovery from microalgae in this study. The influences of potassium salt types, salt concentrations, methanol concentrations, NaCl addition, the effectiveness of reusing the recycled phase components and the possibility of upscaling were investigated by means of partition coefficient and proteins yield.
3.1.1. Effect of salt types
Water‐miscible methanol has the ability to form ATPS with the kosmotropics salts, namely K2HPO4 and K3PO4 63. The multivalent anions PO43− and HPO42‐ have a great influence in inducing phase separation. These strong salting‐out inducing anions tend to create ion‐hydration complexes by excluding water from the alcohol‐rich phase, favouring the formation of ATPS 64. The selection of the best phase‐separation salt with a high salting‐out ability is the primary step in designing ATPS for efficient proteins recovery from microalgae. Figure 2A and B showed that the proteins partitioning behaviour in ATPS was influenced by the type of potassium salts, resulting in varying partition coefficients and yields. The influence of different salt types on proteins partitioning can be explained by the nature of the salt ions and the system's net charges 65. The non‐uniform distribution of the salt ions between two phases and the difference in the electric potential were reported to have great impacts on the movement of proteins to the other phase through electrostatic repulsion or attraction 65.
Figure 2.
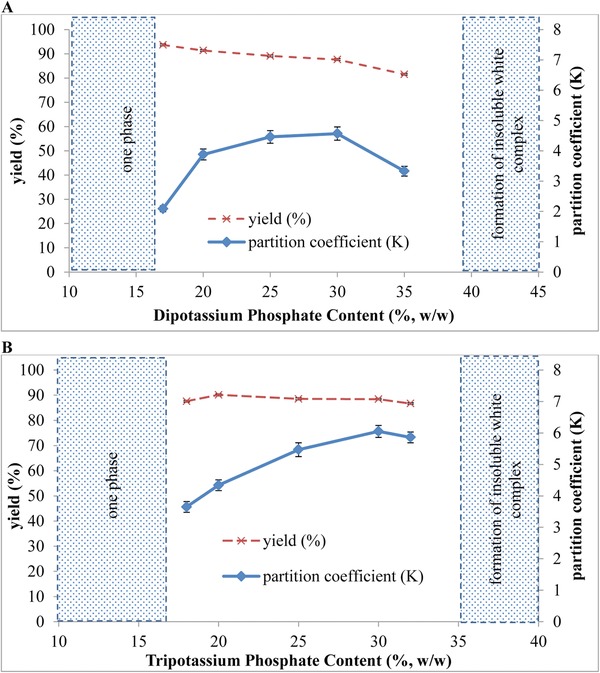
Effect of (A) dipotassium phosphate concentration; and (B) tripotassium phosphate concentration on partitioning and yield of proteins. The optimization of the system was performed by varying the potassium salt concentration. Above dipotassium phosphate concentration of 37% (w/w) and tripotassium phosphate concentration of 35% (w/w), respectively, white insoluble complex was formed upon the addition of BCA working reagent into the sample and blocked absorbance measurement. Partition coefficient and yield were calculated using Eqs. (1), and (2), (3), respectively. The results were expressed as the means of triplicate readings (mean ± SD).
Sample with higher partition coefficient value suggested that majority of the proteins were concentrated in the methanol‐rich top phase. Overall, it was found that better proteins partitioning was achieved with ATPS composed of K3PO4, whereas the proteins partition efficiency was lower in the system with K2HPO4 salt (Fig. 2A and B). For example, when comparing both salts at the same concentration of 25% (w/w), the partition coefficient of K3PO4 (5.47) was noticeably higher than K2HPO4 (4.46) (Fig. 2A and B). This phenomenon can be explained by the Hofmeister series which describes the classification of ions based on their salting‐out ability and phase separation influence in aqueous media. The relative effectiveness of salt types in promoting phase separation in this study was seen to follow the Hofmeister series: K3PO4 > K2HPO4 66, 67. As such, ATPS formed by K3PO4 was chosen for the subsequent studies.
3.1.2. Effect of salt concentration
Besides the salt types, salt concentrations also have strong effect in controlling the distribution of proteins in ATPS. Figure 2B showed that the partition coefficient was enhanced significantly from 4.34 to 6.05 (p < 0.05) when the salt concentrations of K3PO4 were increased from 20% (w/w) to 30% (w/w), while the methanol concentration remained constant at 30% (w/w). The increase in partition coefficient value could be due to the hydrophobic interaction and net charge effect which may have strong contributions to the preferential migration of proteins in ATPS 68. However, when the K3PO4 concentration of the system was increased above 35% (w/w), the bottom phase of the system was found to have incompatible issue with BCA protein assay (Fig. 2B). The formation of insoluble white complex was clearly seen upon the addition of BCA working reagent into the sample. The same incompatible issue was also observed in the ATPS containing 38% (w/w) K2HPO4 and above (Fig. 2A). Based on the results illustrated in Fig. 2B, ATPS formed by 30% (w/w) of K3PO4 and 30% (w/w) of methanol showed the highest partition coefficient of 6.05 (p < 0.05) and was thus selected for the subsequent studies.
A similar trend had also been reported in an experiment to partition fish proteins from fish processing industrial effluent using ATPS formed by 15% of PEG2000 (w/w) with increasing salt concentrations. It was explained that at higher salt concentration, the ions decreased the solubility of proteins in the salt‐rich bottom phase (salting‐out effect), with decreasing free water available in the bottom phase for the protein dissolution. The salting‐out effect of the salt over proteins increased the hydrophobic interaction between the proteins and polymer phase, leading to the partitioning of the proteins to top phase 65.
3.1.3. Effect of methanol concentration
The influence of methanol concentrations on proteins partitioning was carried out with varying concentrations, while maintaining the concentration of K3PO4 at 30% (w/w). It should be noted that according to the phase diagram of K3PO4, the minimum limit of methanol capable of forming two phases should be above 16% (w/w). Therefore, the concentrations of the methanol selected in this study were from 20% (w/w) to 40% (w/w). However, the bottom phase of the system containing 30% (w/w) K3PO4 and 40% (w/w) methanol formed insoluble white complex when reacted with the BCA working reagent.
Contrary to what was observed with the potassium salt concentration effect, decrease in the concentration of methanol led to higher partition coefficient of proteins (Fig. 3). This was a good phenomenon because the decrease in the amount of methanol can efficiently save the cost of separation 69 and proteins which preferentially partitioned to the methanol‐rich top phase can be concentrated with lower volume ratio. Similar observations were reported in the previous studies using alcohol/salt system to recover lipase 49 and human interferon alpha‐2b 70, respectively. The decrease in the partition efficiency was due to the gradual dehydration of the bottom phase when the alcohol concentration was increased in the top phase, causing an imbalance of partitioning which was not conducive for the retention of target biomolecules in the alcohol‐rich top phase 49, 70. It was found that ATPS formed by 30% (w/w) K3PO4 and 20% (w/w) methanol exhibited the maximum partitioning coefficient of 6.72 (p < 0.05) and the yield of the extraction was 80.77 % (p < 0.05) (Fig. 3). Hence, this system was chosen for the subsequent optimization studies.
Figure 3.
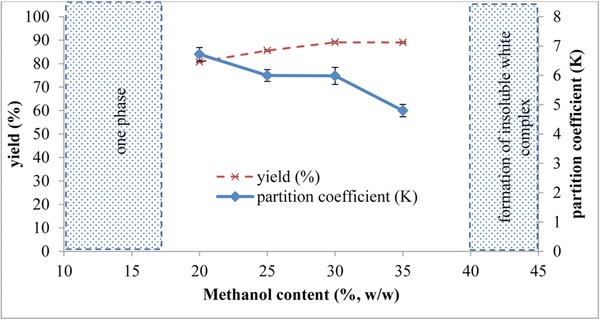
Effect of methanol concentration on partitioning and yield of proteins. The optimization of the methanol/tripotassium phosphate system was performed by varying the methanol concentration from 20–40% (w/w), while the concentration of K3PO4 was maintained at 30% (w/w). Above methanol concentration of 40% (w/w), white insoluble complex was formed upon the addition of BCA working reagent into the sample and blocked absorbance measurement. Partition coefficient and yield were calculated using Eqs. (1), and (2), (3), respectively. The results were expressed as the means of triplicate readings (mean ± SD).
3.1.4. Effect of NaCl addition
The addition of NaCl into the system could alter the proteins partitioning. In this study, the ATPS formed by 30% (w/w) of K3PO4 and 20% (w/w) of methanol was used to investigate the effect of different NaCl concentrations ranging from 0 to 5% (w/w) on the partitioning of proteins. The effect of NaCl salt on proteins partitioning expressed in partition coefficient and proteins yield is shown in Fig. 4. Based on the results, the partition coefficient of the ATPS was generally improved with increasing NaCl concentrations. However, further addition of NaCl salt from 3.5% (w/w) onwards would lead to the salt precipitation at the bottom phase. This was because the solution has reached its saturation point and can no longer dissolve any more NaCl salt. As illustrated in Fig. 4, system that contained 3% (w/w) of NaCl addition showed the highest partition coefficient (7.28) and yield (84.23%), which suggested that most of the hydrophobic proteins were migrated to the methanol‐rich top phase. Even though ANOVA analysis showed that NaCl was not statistically significant in influencing the partition behaviour of the integrated system, it was observed in this experiment that the speed of phase separation of ATPS was found to be accelerated upon the addition of NaCl and was particularly obvious at 3% (w/w). This phenomenon is favourable in the large scale process which implied that phase separation of the system is possible to be achieved without requiring the aid of centrifugation which is relatively energy‐intensive. By considering these two factors, system that contained 3% (w/w) of NaCl addition was chosen for the next experimental step.
Figure 4.
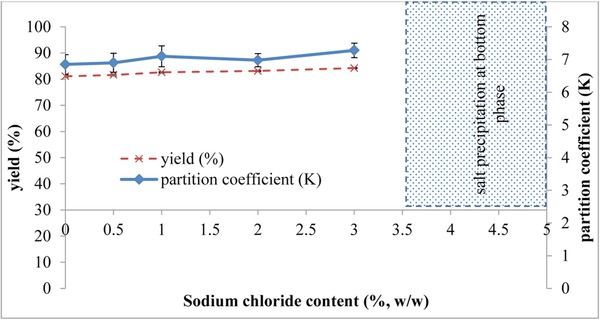
Effect of sodium chloride (NaCl) concentration on partitioning and yield of proteins. The optimization of the methanol/tripotassium phosphate system was performed by varying the NaCl concentration from 0–5% (w/w). However, precipitation at the bottom phase was observed due to salt saturation when the concentration of NaCl was added above 3.5% (w/w). Partition coefficient and yield were calculated using Eqs. (1), and (2), (3), respectively. The results were expressed as the means of triplicate readings (mean ± SD).
These findings were in agreement with several previously published literatures. Researchers agreed that ATPS added with NaCl could speed up the phase separation by affecting the phase potential and improve the hydrophobic resolution of the system due to the generation of difference in electrical potential between the two phases 36, 37, 71. In addition, specific interactions between salts and proteins were believed to be responsible for proteins partitioning efficiency 72. In the ATPS composed of PEG and poly(acrylic acid) (PAA), the partition coefficient of BSA increased from 2.75 to 6.4 with increasing NaCl concentrations (from 0 to 1M), indicating that the addition of NaCl promoted the migration of BSA to the PEG‐rich top phase 72. This can be explained by the electrostatic interaction and repulsion between two charged phases generated by the non‐uniform distribution of chloride ions 72. Another research team also reported about the preferred migration of proteins to the PEG‐rich top phase in ATPS containing PEG and potassium citrate 73.
3.2. Scale up of integrated process
The ATPS integrated process could be an attractive alternative to the traditional discrete process attributed to several advantages such as simple, rapid and cost‐effective 31, 38, 39, 52. The integrated process allowed the operation of cell disruption and proteins recovery to be carried simultaneously. Therefore, the possibility of scaling up the integrated process is undoubtedly one of the most exciting topics among researchers to determine its feasibility and applicability at commercial level. In this study, a 100‐fold increment in the total weight of ATPS was upscaled to determine the effectiveness of implementing the integrated process at larger scale. The difference between the small scale and big scale of the integrated system was analyzed statistically using t‐test. Based on the results, the values of the test statistic for both partition coefficient (–3.182 < –2.637 < 3.182) and yield (–2.777 < 0.0502 < 2.777) did not fall in the rejection region. This implied that there were no significant differences in terms of partition coefficient and yield compared to the small scale (Table 1). When the system was scaled up to 100‐fold, the partition coefficient and yield obtained from the large scale integrated process were 6.88 and 84.26%, respectively. These findings suggested that the efficiency of the integrated process can be extrapolated from the small scale system without compromising the efficiency of proteins partition and yield. Application of this process in downstream processing is thus possible.
Table 1.
ATPS integrated system containing 30% of K3PO4, 20% methanol with 3% of NaCl addition was scaled up to a final total weight of 1000 g. Partition coefficient, volume ratio and yield were calculated using Eqs. (1), (2) and (3), respectively. The results were expressed as the means of triplicate readings (mean ± SD)
| Integrated system | Small scale | Big scale |
|---|---|---|
| Final total weight (g) | 10 | 1000 |
| Volume ratio (Vr) | 0.74±0.03 | 0.74 ± 0.03 |
| Partition coefficient (K) | 7.28 ± 0.22 | 6.88 ± 0.13 |
| Yield (%) | 84.23 ± 0.22 | 84.26 ± 0.30 |
| Total recovered proteins (mg) | 3.60 ± 0.18 | 347.07 ± 3.38 |
In addition to partition coefficient, phase volume ratio is another important factor in determining the effectiveness of extraction 74. ATPS was found to be capable of concentrating target proteins into one of the phases 39. Based on the result obtained from this study, the top phase volume of the chosen system was lower than the bottom phase volume, with volume ratio not more than 0.80. Lower phase volume ratio is favourable because it could reduce the volume to be handled in subsequent purification steps 74. It has been reported that large scale of biomass separation using centrifugation and cross‐flow membrane filtration involves high energy demand, high capital investment and operating costs 75. This technical difficulty can be overcome by ATPS integrated process, attributed to its rapid phase separation characteristic. In this study, it was observed that cell debris with higher density was trapped in the lower phase whereas proteins were partitioned into the top phase, exhibited the high partition coefficient value. Similar observation was also reported previously in the process of extracting animal proteins via ATPS 76.
3.3. Microscopy observation: Cell disruption of microalgae in integrated process
Cell disruption treatment is essential prior to proteins recovery 60 as the release of proteins from microalgae is restricted by the multiple layers of recalcitrant cell wall 77, 78. The addition of both potassium salts created the alkaline medium 67, 79 which favoured the cell disruption process by inducing the hydrolysis of cell envelope and proteins solubilization process 80, 81. To further enhance the proteins release and solubility, the system was exposed to ultrasonic vibration which could result in the greater penetration of solvent into cellular components 82.
The microscopy image revealed that these microalgal cells were spherical in shape and their intracellular compartments were densely packed before treated with integrated process (Fig. 5A). In contrast, microalgal cells treated with integrated process led to the loss of the intracellular components and the cytoplasm appeared hollow (Fig. 5B). This suggested that the integrated process was capable of disrupting the microalgal cell walls without fragmentizing them into very fine pieces. This method is favourable in the large scale process whereby the cell debris can be removed easily in the subsequent purification process.
Figure 5.
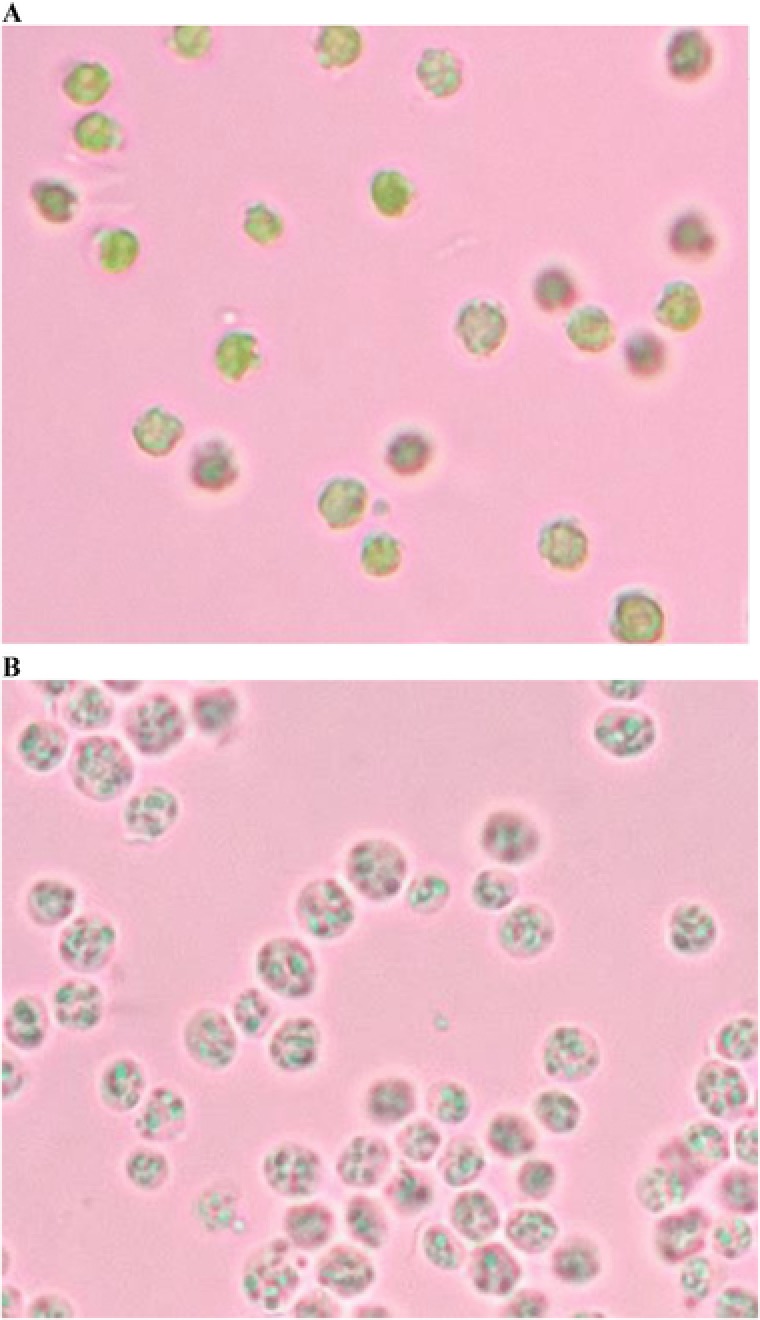
Light microscopy image of (A) untreated C. sorokiniana (control) (1000x); and (B) C. sorokiniana after treated with integrated process (1000x).
3.4. Recycling of phase components
Phase‐forming chemicals can be a considerable proportion of the cost of the ATPS integrated process. Waste disposal of those materials would also incur some costs. In the recent decades, study on the recycling of phase‐forming materials has caught the attention of researchers. This is because an effective recycling of the phase components could lower the production costs, minimize waste generation and environmental pollution 83, 84.
The possibility of recycling both top and bottom phases were investigated in this study. As stated in the methodology, the methanol from the top phase was evaporated to be recycled back and reused in the secondary system. Methanol is the cheapest and has low boiling point compare to other alcohols with longer hydrocarbon chain. This can be explained by the fact that the boiling point of alcohols increases as the length of hydrocarbon chain increases. Methanol cannot form azeotrope with water, thus it can be separated via evaporation easily for recycling in the integrated process. As a result, the energy demand and cost for methanol recycling were relatively low compare to other longer chain alcohols 69.
The recycling of the bottom phase was expected to increase the process yield by minimizing proteins loss in which some of the proteins that were retained in the bottom phase could be partitioned back into the top phase in the next recycling process. This observation was supported by the experimental evidence that the protein concentration in the top phase were generally showing a slight upward trend compared to the primary system (Fig. 6B). However, Fig. 6A showed that the partition coefficient and yield were decreasing gradually with increasing numbers of recycling cycle. This may be due to the increasingly saturation of cell debris and other contaminants in the bottom phase from each previous recycling process, resulting in a gradual decrease of the salting out effect by potassium salt after being recycled repetitively. Nonetheless, the partition coefficient and yield of the proteins obtained from the 5th recycling cycle were relatively satisfactory, with the value of 5.19 and 76.45%, respectively.
Figure 6.
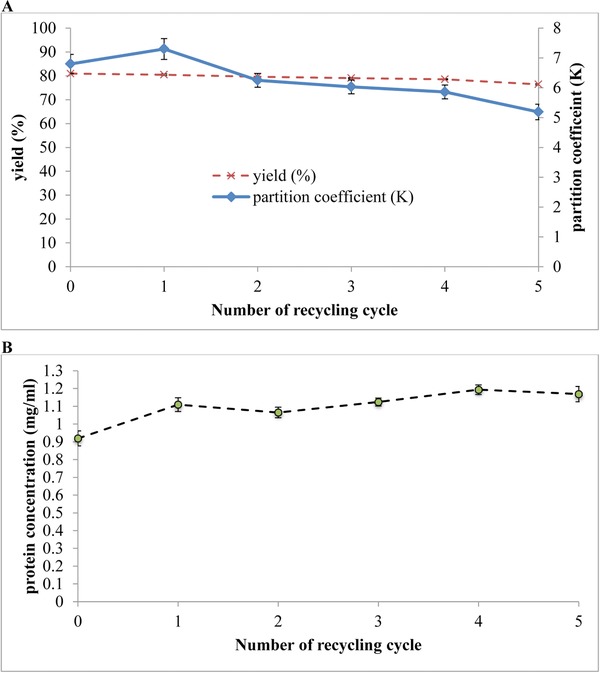
(A) Partitioning efficiency and yield of proteins using recycled phase components were investigated. The recycling processes were repeated for up to 5 times. Partition coefficient and yield were calculated using Eqs. (1), and (2), (3), respectively. (B) The influence of number of recycling cycle on the concentration of proteins in the top phase. The protein concentrations were estimated by BCA protein assay. The results were expressed as the means of triplicate readings (mean ± SD).
4. Concluding remarks
In this study, an integrated process using ATPS in disrupting cell wall and recovering proteins from microalgae simultaneously was adopted. The partitioning behaviour of proteins in the process can be manipulated by a few influential parameters such as types of salt, the concentrations of methanol and potassium salt as well as the addition of NaCl salt. It was concluded that ATPS composed of 30% (w/w) K3PO4 and 20% (w/w) methanol with the addition of 3% (w/w) NaCl showed the best capability of recovering proteins from microalgae. In this system, the partition coefficient was 7.28 and its proteins yield was 84.23%.
There were no significant differences in terms of the partition coefficient and proteins yield when the proposed integrated process was scaled up to 100‐fold. In addition, it is also less environmentally polluting whereby the phase components can still be recycled and reused effectively at the 5th cycle. Taken together, the results suggested that the ATPS‐based integrated process was simple, rapid and environmental friendly. Undoubtedly, this is a logical approach towards reducing overall cost by simplifying several downstream processing steps such as disruption, isolation, extraction and concentration and thus opens promising perspective for the application of this method at large scale. For that reason, assessing its general applicability to recover a wide range of intracellular compounds such as carbohydrate, lipid, and chlorophyll from microalgae could be an interesting topic for further study.
Practical application
The commercial production of protein sources from microalgae at low cost remains an uphill battle because the downstream processing generally involve several operational steps and are thus complicated, time‐consuming and costly. This obstacle could be overcome by adopting the ATPS‐based integrated process, which allows the process of cell disruption, isolation and extraction to be integrated and carried out simultaneously. Attributed to its rapid phase separation characteristic, the integrated process enables the separation of target proteins from cell debris to be accomplished with ease. The environmental pollution and production cost can also be minimized by recycling the phase components of the ATPS integrated process for reuse. Overall, the study suggested that this method is simple, rapid, environmental friendly, scalable and therefore opens promising perspective for large scale application.
The authors declare no financial or commercial conflict of interest.
Acknowledgments
This work was supported financially by SATU Joint Research Scheme (RU022E‐2014) from University of Malaya, Malaysia's Fundamental Research Grant Scheme (FP054‐2013B and FRGS/1/2013/SG05/UNIM/02/1), Malaysia's Ministry of Science, Technology, Innovation (SF016‐2013 and MOSTI‐02‐02‐12‐SF0256), Taiwan's Ministry of Science and Technology (104‐3113‐E‐006 ‐003 ‐ and 103‐2221‐E‐006‐190‐MY3) and Taiwan's Ministry of Education on Top University Grants.
5 References
- 1. Roy, S. S. , Pal, R. , Microalgae in aquaculture: A review with special references to nutritional value and fish dietetics. Proc. Zool. Soc. 2014, 8, 1–8. [Google Scholar]
- 2. Dersjant‐li, Y. , The use of soy protein in aquafeeds. 2002, 3, 541–558. [Google Scholar]
- 3. Ayadi, F. Y. , Rosentrater, K. A. , Muthukumarappan, K. , Alternative protein sources for aquaculture feeds. J. Aquacultre Feed Sci. Nutr. 2012, 4, 1–26. [Google Scholar]
- 4. Sirakov, I. , Velichkova, K. , Stoyanova, S. , Staykov, Y. , The importance of microalgae for aquaculture industry. Review . Int. J. Fish. Aquat. Stud. Concern. 2015, 2, 81–84. [Google Scholar]
- 5. Norambuena, F. , Hermon, K. , Skrzypczyk, V. , Emery, J. A. et al., Algae in fish feed: Performances and fatty acid metabolism in juvenile atlantic salmon. PLoS One 2015, 10, e0124042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Taelman, S. E. , De Meester, S. , Van Dijk, W. , da Silva, V. et al., Environmental sustainability analysis of a protein‐rich livestock feed ingredient in The Netherlands: Microalgae production versus soybean import. Resour. Conserv. Recycl. 2015, 101, 61–72. [Google Scholar]
- 7. Kiron, V. , Phromkunthong, W. , Huntley, M. , Archibald, I. et al., Marine microalgae from biorefinery as a potential feed protein source for Atlantic salmon, common carp and whiteleg shrimp. Aquac. Nutr. 2012, 18, 521–531. [Google Scholar]
- 8. Bhosale, S. , Bhilave, M. , Nadaf, S. , Formulation of fish feed using ingredients from plant sources. Res. J. Agric. Sci. 2010, 1, 284–287. [Google Scholar]
- 9. Li, P. , Mai, K. , Trushenski, J. , Wu, G. , New developments in fish amino acid nutrition: Towards functional and environmentally oriented aquafeeds. Amino Acids 2009, 37, 43–53. [DOI] [PubMed] [Google Scholar]
- 10. Watanabe, T. , Strategies for further development of aquatic feeds. Fish. Sci. 2002, 68, 242–252. [Google Scholar]
- 11. Stankovic, M. , Dulic, Z. , Markovic, Z. , Protein sources and their significance in carp (Cyprinus carpio L.) nutrition. J. Agric. Sci. Belgrade 2011, 56, 75–86. [Google Scholar]
- 12. Vanthoor‐Koopmans, M. , Wijffels, R. H. , Barbosa, M. J. , Eppink, M. H. M. , Biorefinery of microalgae for food and fuel. Bioresour. Technol. 2013, 135, 142–149. [DOI] [PubMed] [Google Scholar]
- 13. Safi, C. , Charton, M. , Ursu, A. V. , Laroche, C. et al., Release of hydro‐soluble microalgal proteins using mechanical and chemical treatments. Algal Res. 2014, 3, 55–60. [Google Scholar]
- 14. Li, Y. , Horsman, M. , Wu, N. , Lan, C. Q. et al., Articles: Biocatalysts and bioreactor design. Biotechnol. Prog 2008, 24, 815–820. [DOI] [PubMed] [Google Scholar]
- 15. Solana, M. , Rizza, C. S. , Bertucco, A. , Exploiting microalgae as a source of essential fatty acids by supercritical fluid extraction of lipids: Comparison between Scenedesmus obliquus, Chlorella protothecoides and Nannochloropsis salina. J. Supercrit. Fluids 2014, 92, 311–318. [Google Scholar]
- 16. Gu, N. , Lin, Q. , Li, G. , Tan, Y. et al., Effect of salinity on growth, biochemical composition, and lipid productivity of Nannochloropsis oculata CS 179. Eng. Life Sci. 2012, 12, 631–637. [Google Scholar]
- 17. Blackburn, S. I. , Volkman, J. K. , Microalgae: A renewable source of bioproducts, in: Dunford N. T. (Ed.), Food and Industrial Bioproducts and Bioprocessing, Wiley‐Blackwell, Oxford, UK; 2012, pp. 221–241. [Google Scholar]
- 18. López Barreiro, D. , Prins, W. , Ronsse, F. , Brilman, W. , Hydrothermal liquefaction (HTL) of microalgae for biofuel production: State of the art review and future prospects. Biomass and Bioenergy 2013, 53, 113–127. [Google Scholar]
- 19. Spolaore, P. , Joannis‐Cassan, C. , Duran, E. , Isambert, A. , Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [DOI] [PubMed] [Google Scholar]
- 20. Eriksen, N. T. , Riisgård, F. K. , Lønsmann Iversen, J. J. , Gunther, W. S. , On‐line estimation of O2 production, CO2 uptake, and growth kinetics of microalgal cultures in a gas‐tight photobioreactor. J. Appl. Phycol. 2007, 19, 161–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Acién Fernández, F. G. , Fernández Sevilla, J. M. , Grima, E. M. , Photobioreactors for the production of microalgae. Rev Env. Sci Biotechnol 2013, 12, 131–151. [Google Scholar]
- 22. Chisti, Y. , Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [DOI] [PubMed] [Google Scholar]
- 23. Kula, M. , Rys, M. , Skoczowski, A. , Far‐red light (720 or 740 nm) improves growth and changes the chemical composition of Chlorella vulgaris. Eng. Life Sci. 2014, 14, 651–657. [Google Scholar]
- 24. Duong, V. T. , Ahmed, F. , Thomas‐Hall, S. R. , Quigley, S. et al., High protein‐ and high lipid‐producing microalgae from northern australia as potential feedstock for animal feed and biodiesel. Front. Bioeng. Biotechnol. 2015, 3, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yeh, K.‐L. , Chang, J.‐S. , Chen, W. , Effect of light supply and carbon source on cell growth and cellular composition of a newly isolated microalga Chlorella vulgaris ESP‐31. Eng. Life Sci. 2010, 10, 201–208. [Google Scholar]
- 26. Wang, S.‐K. , Stiles, A. R. , Guo, C. , Liu, C.‐Z. , Microalgae cultivation in photobioreactors: An overview of light characteristics. Eng. Life Sci. 2014, 14, 550–559. [Google Scholar]
- 27. Taher, H. , Al‐Zuhair, S. , Al‐Marzouqi, A. H. , Haik, Y. et al., Effective extraction of microalgae lipids from wet biomass for biodiesel production. Biomass Bioenergy 2014, 66, 159–167. [Google Scholar]
- 28. Chen, C.‐Y. , Zhao, X.‐Q. , Yen, H.‐W. , Ho, S.‐H. et al., Microalgae‐based carbohydrates for biofuel production. Biochem. Eng. J. 2013, 78, 1–10. [Google Scholar]
- 29. Cheah, W. Y. , Show, P. L. , Chang, J.‐S. , Ling, T. C. et al., Biosequestration of atmospheric CO2 and flue gas‐containing CO2 by microalgae. Bioresour. Technol. 2014, 184, 190–201. [DOI] [PubMed] [Google Scholar]
- 30. Sambusiti, C. , Bellucci, M. , Zabaniotou, A. , Beneduce, L. et al., Algae as promising feedstocks for fermentative biohydrogen production according to a biorefinery approach: A comprehensive review. Renew. Sustain. Energy Rev. 2015, 44, 20–36. [Google Scholar]
- 31. Rito‐Palomares, M. , Lyddiatt, A. , Process integration using aqueous two‐phase partition for the recovery of intracellular proteins. Chem. Eng. J. 2002, 87, 313–319. [Google Scholar]
- 32. Raja, S. , Murty, V. R. , Thivaharan, V. , Rajasekar, V. et al., Aqueous two phase systems for the recovery of biomolecules—A Review. Sci. Technol. 2011, 1, 7–16. [Google Scholar]
- 33. Asenjo, J. a. , Andrews, B. a. , Aqueous two‐phase systems for protein separation: Phase separation and applications. J. Chromatogr. A 2012, 1238, 1–10. [DOI] [PubMed] [Google Scholar]
- 34. Mohammadi, H. S. , Omidinia, E. , Taherkhani, H. , Rapid one‐step separation and purification of recombinant phenylalanine dehydrogenase in aqueous two‐phase systems. Iran. Biomed. J. 2008, 12, 115–122. [PubMed] [Google Scholar]
- 35. Luo, X. , Smith, P. , Raston, C. L. , Zhang, W. , Vortex Fluidic device‐intensified aqueous two phase extraction of C‐phycocyanin from Spirulina maxima. ACS Sustain. Chem. Eng. 2016, 4, 3905–3911. [Google Scholar]
- 36. Hatti‐Kaul, R. , Aqueous Two‐Phase Systems: Methods and Protocols, Humana Press Inc, Totowa: 2000. [Google Scholar]
- 37. Leong, Y. K. , Koroh, F. E. , Show, P. L. , Chi‐Wei, J. L. et al., Optimisation of extractive bioconversion for green polymer via aqueous two‐phase system. 2015, 45, 1495–1500. [Google Scholar]
- 38. Goja, A. M. , Yang, H. , Cul, M. , Li, C. , Aqueous two‐phase extraction advances for bioseparation. J. Bioprocess. Biotech. 2013, 4, 140. [Google Scholar]
- 39. Zhao, L. , Peng, Y. L. , Gao, J. M. , Cai, W. M. , Bioprocess intensification: An aqueous two‐phase process for the purification of C‐phycocyanin from dry Spirulina platensis. Eur. Food Res. Technol. 2014, 238, 451–457. [Google Scholar]
- 40. Rito‐Palomares, M. , Practical application of aqueous two‐phase partition to process development for the recovery of biological products. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004, 807, 3–11. [DOI] [PubMed] [Google Scholar]
- 41. Gu, Z. , Recovery of recombinant proteins from plants using aqueous two‐phase partitioning systems: an outline. Methods Mol. Biol. 2014, 1129, 77–87. [DOI] [PubMed] [Google Scholar]
- 42. Nitsawang, S. , Hatti‐Kaul, R. , Kanasawud, P. , Purification of papain from Carica papaya latex: Aqueous two‐phase extraction versus two‐step salt precipitation. Enzyme Microb. Technol. 2006, 39, 1103–1107. [Google Scholar]
- 43. Sørensen, L. , Hantke, A. , Eriksen, N. T. , Purification of the photosynthetic pigment C‐phycocyanin from heterotrophic Galdieria sulphuraria. J. Sci. Food Agric. 2013, 93, 2933–2938. [DOI] [PubMed] [Google Scholar]
- 44. Gómez‐Loredo, A. , Benavides, J. , Rito‐Palomares, M. , Partition behavior of fucoxanthin in ethanol‐potassium phosphate two‐phase systems. J. Chem. Technol. Biotechnol. 2014, 89, 1637–1645. [Google Scholar]
- 45. Fu, H. , Dai, J. , Sun, Y. , Zhang, D. et al., Partition behavior of hydrophilic diols in an ethanol/ammonium sulfate salting‐out extraction system. Eng. Life Sci. 2015, 15, 797–803. [Google Scholar]
- 46. Benavides, J. , Rito‐Palomares, M. , Potential aqueous two‐phase processes for the primary recovery of colored protein from microbial origin. Eng. Life Sci. 2005, 5, 259–266. [Google Scholar]
- 47. Tianwei, T. , Qing, H. , Qiang, L. , Purification of glycyrrhizin from Glycyrrhiza uralensis Fisch with ethanol / phosphate aqueous two phase system. Biotechnol. Lett. 2002, 24, 1417–1420. [Google Scholar]
- 48. Tan, Z. J. , Li, F. F. , Xu, X. L. , Extraction and purification of anthraquinones derivatives from Aloe vera L. using alcohol/salt aqueous two‐phase system. Bioprocess Biosyst. Eng. 2013, 36, 1105–1113. [DOI] [PubMed] [Google Scholar]
- 49. Ooi, C. W. , Tey, B. T. , Hii, S. L. , Kamal, S. M. M. et al., Purification of lipase derived from Burkholderia pseudomallei with alcohol/salt‐based aqueous two‐phase systems. Process Biochem. 2009, 44, 1083–1087. [Google Scholar]
- 50. Benavides, J. , Aguilar, O. , Lapizco‐Encinas, B. , Rito‐Palomares, M. , Extraction and purification of bioproducts and nanoparticles using aqueous two‐phase systems strategies. Chem. Eng. Technol. 2008, 31, 838–845. [Google Scholar]
- 51. Fresewinkel, M. , Rosello, R. , Wilhelm, C. , Kruse, O. et al., Integration in microalgal bioprocess development: Design of efficient, sustainable, and economic processes. Eng. Life Sci. 2014, 14, 560–573. [Google Scholar]
- 52. Buyel, J. F. , Twyman, R. M. , Fischer, R. , Extraction and downstream processing of plant‐derived recombinant proteins. Biotechnol. Adv. 2015, 33, 902–913. [DOI] [PubMed] [Google Scholar]
- 53. Chen, P. , Min, M. , Chen, Y. , Wang, L. et al., Review of the biological and engineering aspects of algae to fuels approach, 2009, 2, 1–30. [Google Scholar]
- 54. Brennan, L. , Owende, P. , Biofuels from microalgae‐A review of technologies for production, processing, and extractions of biofuels and co‐products. Renew. Sustain. Energy Rev. 2010, 14, 1–21. [Google Scholar]
- 55. Guldhe, A. , Singh, B. , Rawat, I. , Ramluckan, K. et al., Efficacy of drying and cell disruption techniques on lipid recovery from microalgae for biodiesel production. Fuel 2014, 128, 46–52. [Google Scholar]
- 56. Slocombe, S. P. , Ross, M. , Thomas, N. , McNeill, S. et al., A rapid and general method for measurement of protein in micro‐algal biomass. Bioresour. Technol. 2013, 129, 51–57. [DOI] [PubMed] [Google Scholar]
- 57. Albertsson, P.‐A. , Partition of cell particles and macromolecules. 1986, 5, 233–234. [Google Scholar]
- 58. Wang, Y. , Wang, J. , Han, J. , Hu, S. et al., Liquid‐liquid equilibrium of novel aqueous two‐phase systems and evaluation of salting‐out abilities of salts. Cent. Eur. J. Chem. 2010, 8, 886–891. [Google Scholar]
- 59. Gerde, J. A. , Wang, T. , Yao, L. , Jung, S. et al., Optimizing protein isolation from defatted and non‐defatted Nannochloropsis microalgae biomass. Algal Res. 2013, 2, 145–153. [Google Scholar]
- 60. Ursu, A. V. , Marcati, A. , Sayd, T. , Sante‐Lhoutellier, V. et al., Extraction, fractionation and functional properties of proteins from the microalgae Chlorella vulgaris. Bioresour. Technol. 2014, 157, 134–139. [DOI] [PubMed] [Google Scholar]
- 61. Safi, C. , Ursu, A. V. , Laroche, C. , Zebib, B. et al., Aqueous extraction of proteins from microalgae: Effect of different cell disruption methods. Algal Res. 2014, 3, 61–65. [Google Scholar]
- 62. Damodaran, S. , Amino acids, peptides and proteins, in: Fennema O. R. (Ed.), Food Chemistry, Marcel Dekker, New York: 1996, pp. 321–430. [Google Scholar]
- 63. Wang, Y. , Han, J. , Xu, X. , Hu, S. et al., Partition behavior and partition mechanism of antibiotics in ethanol/2‐propanol–ammonium sulfate aqueous two‐phase systems. Sep. Purif. Technol. 2010, 75, 352–357. [Google Scholar]
- 64. Golubitskii, G. B. , Budko, E. V. , Basova, E. M. , Kostarnoi, a. V. et al., Stability of ascorbic acid in aqueous and aqueous‐organic solutions for quantitative determination. J. Anal. Chem. 2007, 62, 742–747. [Google Scholar]
- 65. Nagaraja, V. H. , Iyyaswami, R. , Aqueous two phase partitioning of fish proteins: Partitioning studies and ATPS evaluation. J. Food Sci. Technol. 2014, 52, 3539–3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ananthapadmanabhan, K. P. , Goddard, E. D. , A correlation between clouding and aqueous biphase formation in polyethylene oxide/inorganic salt systems. J. Colloid Interface Sci. 1986, 113, 294–296. [Google Scholar]
- 67. Reis, I. a O. , Samuel, B. , Nascimento, L. a S. , Oliveira, N. et al., Extraction of ascorbic acid using alcohol / phosphate potassium salt – based aqueous two‐phase system. Food process engineering in a changing world. Proceedings of the 11th International Congress on Engineering and Food. 2011, 1–6.
- 68. Andrews, B. a. , Schmidt, a. S. , Asenjo, J. a. , Correlation for the partition behavior of proteins in aqueous two‐phase systems: Effect of surface hydrophobicity and charge. Biotechnol. Bioeng. 2005, 90, 380–390. [DOI] [PubMed] [Google Scholar]
- 69. Li, Z. , Teng, H. , Xiu, Z. , Extraction of 1,3‐propanediol from glycerol‐based fermentation broths with methanol/phosphate aqueous two‐phase system. Process Biochem. 2011, 46, 586–591. [Google Scholar]
- 70. Lin, Y. K. , Ooi, C. W. , Tan, J. S. , Show, P. L. et al., Recovery of human interferon alpha‐2b from recombinant Escherichia coli using alcohol/salt‐based aqueous two‐phase systems. Sep. Purif. Technol. 2013, 120, 362–366. [Google Scholar]
- 71. Gu, Z. , Glatz, C. E. , Aqueous two‐phase extraction for protein recovery from corn extracts. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 845, 38–50. [DOI] [PubMed] [Google Scholar]
- 72. Settu, S. , Velmurugan, P. , Jonnalagadda, R. R. , Nair, B. U. , Extraction of bovine serum albumin using aqueous two‐phase poly ( ethylene glycol ) – poly ( acrylic acid ) system. 2015, 74, 348–353. [Google Scholar]
- 73. Ramyadevi, D. , Subathira, a. , Saravanan, S. , Protein recovery from shrimp waste using aqueous two phase system: Effect of process parameters on partitioning using response surface methodology. 2013, 5, 156–166. [Google Scholar]
- 74. Anandharamakrishnan, C. , Raghavendra, S. N. , Barhate, R. S. , Hanumesh, U. et al., Aqueous two‐phase extraction for recovery of proteins from cheese whey. Food Bioprod. Process. 2005, 83, 191–197. [Google Scholar]
- 75. Wilk, R. , Chojnacka, K. , Upstream processing in the technology of algal extracts: Biomass harvesting and preparation for extraction process, in: Marine Algae Extracts: Processes, Products, and Applications, John Wiley & Sons, Hoboken: 2015, p. 880. [Google Scholar]
- 76. Boland, M. J. , Aqueous two‐phase extraction and purification of animal proteins. Mol. Biotechnol. 2002, 20, 85–93. [DOI] [PubMed] [Google Scholar]
- 77. Günerken, E. , D'Hondt, E. , Eppink, M. H. M. , Garcia‐Gonzalez, L. et al., Cell disruption for microalgae biorefineries. Biotechnol. Adv. 2015, 33, 243–260. [DOI] [PubMed] [Google Scholar]
- 78. González‐Fernández, C. , Sialve, B. , Bernet, N. , Steyer, J. P. , Thermal pretreatment to improve methane production of Scenedesmus biomass. Biomass Bioenergy 2012, 40, 105–111. [DOI] [PubMed] [Google Scholar]
- 79. Reis, I. a O. , Santos, S. B. , Pereira, F. D. S. , Sobral, C. R. S. et al., Extraction and recovery of rutin from acerola waste using alcohol‐salt‐based aqueous two‐phase systems. Sep. Sci. Technol. 2014, 49, 656–663. [Google Scholar]
- 80. Yen, H. W. , Hu, I. C. , Chen, C. Y. , Ho, S. H. et al., Microalgae‐based biorefinery—From biofuels to natural products. Bioresour. Technol. 2013, 135, 166–174. [DOI] [PubMed] [Google Scholar]
- 81. Kim, J. , Yoo, G. , Lee, H. , Lim, J. et al., Methods of downstream processing for the production of biodiesel from microalgae. Biotechnol. Adv. 2013, 31, 862–876. [DOI] [PubMed] [Google Scholar]
- 82. Show, K.‐Y. , Lee, D.‐J. , Tay, J.‐H. , Chang, J.‐S. , Microalgal drying and cell disruption—Recent advances. Bioresour. Technol. 2014, 184, 258–266. [DOI] [PubMed] [Google Scholar]
- 83. Michalak, I. , Chojnacka, K. , Algal extracts: Technology and advances. Eng. Life Sci. 2014, 14, 581–591. [Google Scholar]
- 84. Carlsson, M. , Berggren, K. , Linse, P. , Veide, A. et al., Effects of fused tryptophan rich peptides to a recombinant protein A domain on the partitioning in polyethylene glycol‐dextran and Ucon‐dextran aqueous two‐phase systems. J. Chromatogr. A 1996, 756, 107–117. [DOI] [PubMed] [Google Scholar]


