Abstract
The aim of this study was to formulate silica and alginate hydrogels for immobilization of β‐glucosidase. For this purpose, enzyme kinetics in hydrogels were determined, activity of immobilized enzymes was compared with that of free enzyme, and structures of silica and alginate hydrogels were characterized in terms of surface area and pore size. The addition of polyethylene oxide improved the mechanical strength of the silica gels and 68% of the initial activity of the enzyme was preserved after immobilizing into tetraethyl orthosilicate–polyethylene oxide matrix where the relative activity in alginate beads was 87%. The immobilized β‐glucosidase was loaded into glass–silicon–glass microreactors and catalysis of 4‐nitrophenyl β‐d‐glucopyranoside was carried out at various retention times (5, 10, and 15 min) to compare the performance of silica and alginate hydrogels as immobilization matrices. The results indicated that alginate hydrogels exhibited slightly better properties than silica, which can be utilized for biocatalysis in microfluidic platforms.
Keywords: Enzyme, Hydrogels, Immobilization, Microreactor
Abbreviations
- EGMS
ethylene glycol modified silane
- pNPG
4‐nitrophenyl β‐d‐glucopyranoside
- pNP
4‐nitrophenyl
- PEO
polyethylene oxide
- SEM
scanning electron microscopy
- TEOS
tetraethyl orthosilicate
1. Introduction
Hydrogels can be used for many different applications within the area of life sciences. Particularly, three‐dimensional (3D) nanostructures have received significant attention in the field of biomedical devices as a fast growing field of research 1, 2.
Inorganic silica‐based sol–gels can, for instance, be applied in microextractions, separation columns as well as immobilization of biomolecules 3, 4. The latter case is of particular interest, as the entrapment of enzymes or other bioactive compounds, such as whole cells or immunoglobulins, in sol–gel matrices comes with advantages such as decreased consumption of the often expensive biocomponent as well as more controlled reaction conditions 5, 6, 7, 8.
Apart from the widely used silica‐based sol–gels, also organic alternatives are available. A prominent example is alginate, a biopolymer gained from brown algae consisting two sugar acid monomers 9. Alginate can form gels in the presence of divalent metal ions 10, 11. The main advantage of alginate as immobilization matrix in comparison to silica sol–gels is that entrapment of biomolecules is carried out at very mild conditions, so that the sensitive compounds are protected from aggressive agents such as ethanol that can be found in silica gels. However, alginate gels itself is susceptible to degradation in the presence of molecules with a high affinity for cross‐linking ions 12.
This study aims to compare the suitability of silica and alginate hydrogels for β‐glucosidase immobilization. For this purpose, kinetic parameters of free and immobilized enzyme were determined in different buffer solutions. Additionally, the effects of β‐glucosidase and different buffer solutions on the stability of alginate hydrogels were tested. As a characterization approach, structure of silica and alginate hydrogels is compared with each other, including surface area measurements and pore size analysis.
2. Materials and methods
2.1. Preparation of hydrogels
2.1.1. Preparation of silica monoliths
Silica monoliths were prepared by the modified sol–gel method 13. Silica precursors, tetraethyl orthosilicate (TEOS), and ethylene glycol modified silane (EGMS) were mixed with dilute HCl solution and hydrolyzed at room temperature for 150 min. Subsequently, the sols were mixed with buffered enzyme solution both in the presence and absence of polyethylene oxide (PEO, MW 100 000) and 3‐aminopropyltriethoxysilane at ratios (v/v) of 2:1 and 1:1 for TEOS and EGMS gels, respectively. The obtained gels were aged in pure TEOS solution at 4°C.
2.1.2. Preparation of alginate beads
Alginate beads were produced using the internal gelation method. For this purpose, calcium carbonate was added to a 3 w/w sodium alginate solution in a ratio of 0.1825 g CaCO3 per gram dissolved alginate. For complete homogenization, the dispersion was mixed vigorously using an Ultraturrax (∼10 000 rpm) for about 3 min. In order to remove air bubbles from the viscous liquid, it was treated in an ultrasonic bath for 5 min. To ensure homogenization of the mixture, it was stirred with a magnetic stirrer for at least 15 min. The alginate beads were prepared by dropping the dispersion into 0.2 M acetic acid solution (pH 2.7) and kept cooled in the acetic acid solution for at least 6 h to allow for complete gelation. For the loading of the microreactors, the dispersion was introduced into the microreactor, which was completely immersed in acetic acid (2 M) and the gelation was completed after 10 h.
2.2. Enzyme immobilization to silica and alginate matrices
Buffered enzyme solution was directly added to hydrolyzed silica precursor solution (the sol) at desired concentration. The entire solution was mixed rapidly and left for gelation.
A stock solution of β‐glucosidase (Sigma‐Aldrich 49290; 7.4 U/mg) enzyme solubilized in Ca‐Na acetate buffer (pH 4.8) was added to the alginate/CaCO3 dispersion prior dropping the dispersion in acetic acid and mixed for at least 15 min. The concentration of enzyme amounted to 107 μg/mL alginate solution. Due to the low amount of enzyme required, the dilution of the alginate solution by the addition of the stock solution is negligible. The immobilization efficiency was assumed as 100% for both inorganic and organic matrices.
2.3. Supercritical drying of hydrogels
Supercritical drying of monoliths was carried out at SFE 100 System (Thar Instruments, Inc., UK, 2006). Prior to drying, the hydrogels were subjected to solvent exchange due to the large miscibility gap of water and carbon dioxide. Hydrogels were directly immersed in ethanol and left for 3 days. Each 12 h solvent exchange media was replaced with fresh ethanol. At the end of solvent exchange process, the gels were dried at 120 bar, 40°C, and a CO2 flow rate of 10 g/min for 4 h 14.
2.4. Characterization of hydrogels
2.4.1. Aging of hydrogels
β‐Glucosidase‐immobilized silica monoliths were aged for a 5 day duration and the activities were measured daily to compare with the activity of free enzyme. The experiments were carried out in duplicates and residual activities of aged monoliths were expressed as percentages.
The alginate gels were produced as beads were aged in acetic acid for 6 h. The droplets solidified completely after 6 h, which could be seen by the loss of turbidity. The loss of turbidity can be explained by dissolving of CaCO3 out of the gel.
2.4.2. Changes in hydrogel volume
Shrinkage and swelling of the hydrogels were determined by the change in the gel volume. The hydrogels were stored in aging mediums and the diameter and height of the gels were measured with a caliper before and after storage. The volume change was calculated as follows:
| (1) |
2.4.3. Stability of alginate beads
Alginate beads are prone to degradation caused by solubilization of Ca2+ ions into the buffer solution, hence destabilizing the gel. Furthermore, immobilized β‐glucosidase can potentially hydrolyze the β‐1,4 glycosidic bonds in the alginate. In order to investigate the stability of alginate, alginate beads were added to two different buffer solutions, 50 mM Na‐acetate and Ca‐Na acetate (ratio 1:4), both at pH 4.8. For each buffer, samples were prepared with immobilized enzyme (4 μg), as well as without enzyme. After 24 h and 48, alginic acid content in the buffer solution was analyzed with the phenol‐sulfuric acid method 15.
2.4.4. Scanning electron microscopy
The morphology of the aerogels was examined using scanning electron microscopy. Samples were coated with gold‐palladium under a vacuum at Emitech K550X sputter‐coater. The scanning electron microscopy (SEM) photographs were taken using a scanning electron microscope (FEI Quanta 250 FEG).
2.4.5. Pore size distribution and surface areas of aerogels
The specific area, pore volume, and average pore diameter of macroporous dry gels were determined by ASAP 2020 (Micromeritics, USA) surface area and porosity analyzer.
2.4.6. FT infrared analysis of aerogels
FTIR spectra of silica and alginate aerogels were recorded by Perkin Elmer Spectrum 100. This test was performed to confirm the existence of Si‐O‐Si and Si‐CH3 bonds for silica aerogels and carbonyl, carboxyl and carboxylate groups for alginate aerogels.
2.5. Enzyme kinetics
2.5.1. Enzyme activity assay
The enzymatic activities of both free and immobilized enzyme were assayed with 4‐nitrophenyl β‐d‐glucopyranoside (pNPG) as a substrate. The reaction medium contained buffer solution (100 mM citrate buffer or 50 mM Ca‐Na acetate buffer, pH 4.8), 4 μg β‐glucosidase enzyme, and 20 mM pNPG (Sigma‐Aldrich). The reactions were carried out at 37°C rotating at 100 rpm for 15 min. After incubation, the reaction was stopped by adding 100 mM Na2CO3. The amount of 4‐nitrophenyl (pNP) produced was measured at 420 nm by UV‐vis spectroscopy.
2.5.2. Determination of kinetic parameters
The Michaelis–Menten constant (KM) and maximal reaction rate (V max) were determined for free and immobilized enzyme in TEOS‐PEO monoliths. The amount of β‐glucosidase was kept constant (4 μg) while pNPG concentration was in the range of 2–100 mM for free enzyme and 10–110 mM for immobilized enzyme. Additionally, to investigate the effect of buffer systems on enzyme kinetics, experiments were carried out in two different buffer systems (100 mM citrate buffer and 50 mM Ca‐Na acetate buffer).
2.6. Flow through enzymatic reactions in microfluidic systems
Flow‐through enzymatic reactions focused on the determination of flow rate dependent conversion of pNPG to pNP were carried out at 37°C. The microreactors containing immobilized enzymes were installed in a metal housing that connects the in‐ and outlet of the microreactor with the syringe pump and collection of reaction products. The flow‐through enzymatic reactions were carried out at 2.5, 5, and 7.5 μL/min flow rates corresponding to 15, 10, and 5 min residence time, respectively. Furthermore, long‐term activity of the enzymes was determined for 48 h along with leaching trials for 30 min.
3. Results and discussion
3.1. Characterization of hydrogels
3.1.1. Aging of hydrogels
TEOS‐PEO and EGMS‐PEO gels were aged in TEOS for 3 days and activities of immobilized enzymes were assayed each day. The maximum activities were observed as 144% and 122% relative activities after 1 day aging for both TEOS‐PEO and EGMS‐PEO gels, respectively. However, the aging durations longer than 2 days cause significant activity lost (82% for TEOS‐PEO gels and 110% for EGMS‐PEO gels). Although the aging process strengthens the gel matrix and improves the enzyme activity, the intensive network created by Si‐O‐Si bonds around enzyme structure can cause hindrances as a result of condensation process during aging.
3.1.2. Changes in hydrogel volume
TEOS‐PEO and EGMS‐PEO gels were aged in pure TEOS and alginate gels were aged in 0.2 M acetic acid and 2 M acetic acid to determine the shrinkage/swelling characteristics. Both TEOS‐PEO and EGMS‐PEO gels shrank during aging; however, there was no significant difference between two silica monoliths (9.5% and 8% volume loss for TEOS‐PEO and EGMS‐PEO gels, respectively). In contrast with silica gels, alginate gels showed swelling behavior. The volume change in alginate gels in 0.2 M acetic acid and 2 M acetic acid was measured as 51.1 and 84.4%, respectively (Fig. 1A).
Figure 1.
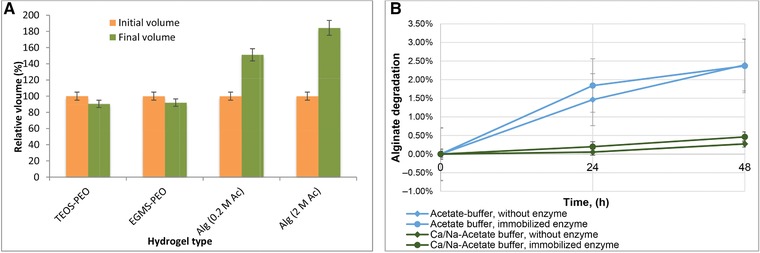
Volumetric change in hydrogels (A), and degradation of alginate in the presence of different buffers and enzymes (B). The experiments were performed in duplicates and the results are given as ± SEM.
3.1.3. Stability of alginate beads
The degradation of alginate over a 48‐h period was measured by detection of solubilized sodium alginate in the respective buffer solutions (Fig. 1B). Without immobilized enzymes, the acetate buffer without Ca2+ ions leads to a degradation of around 2.5% after 48 h, one order of magnitude higher than the degradation in the Ca‐Na acetate buffer (0.25–0.5%). The presence of Ca2+ ions in the buffer solution significantly decreases the leaching of Ca2+ ions from the alginate hydrogel due to the lower concentration gradient.
It is also apparent that the degradation of alginate with immobilized enzymes is slightly higher than without enzymes, indicating that the β‐glucosidase in fact hydrolyzes the glycosidic bonds between the alginic acid monomers. However, the absolute degradation, particularly for the Ca‐Na acetate buffer, only takes very low values, such that when considering the usual operation conditions and no significant impact on alginate stability is to be expected.
3.1.4. SEM analysis
As for SEM images of TEOS‐PEO, EGMS‐PEO monoliths, and alginate beads (Fig. 2), morphological structure of TEOS‐PEO gel was porous (Fig. 2A), while EGMS‐PEO gel structure was spherical (Fig. 2B), which are similar with the results of previous studies in literature 14, 16. Although EGMS‐PEO‐derived gels have higher surface area compared to TEOS‐PEO‐derived gels, obtained enzymatic activity was lower, possibly as a result of this spherical structure that limits the interaction between enzyme and substrate. The addition of PEO in gels led to the formation of interconnected macropores as seen in SEM pictures of the TEOS‐PEO and EGMS‐PEO gels. The pores of these gels must be large enough to prevent denaturation of the entrapped protein or enzyme and to allow rapid diffusion of small analyte molecules, besides they must be sufficiently small to ensure permanent entrapment of the enzyme molecules without leakage 17.
Figure 2.
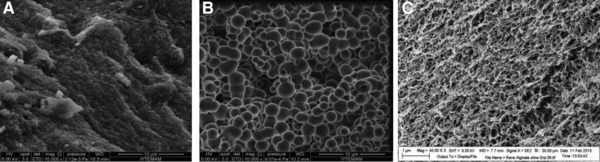
SEM images of TEOS‐PEO (A) and EGMS‐PEO (B) monoliths and alginate beads (C).
Alginate beads possessed a fibrillar structure with both meso‐ and macropores (Fig. 2C), which is in agreement with the literature. For instance, Gurikov et al. 18 prepared alginate aerogels by using high‐pressure CO2. In this study, both alginate hydrogels and aerogels were reported to possess fibrillar morphology with distinct macroporosity. The SEM images of alginate aerogels showed similar morphology to alginate beads in our study.
3.1.5. Pore size distribution and surface area of aerogels
Surface area, pore volume, and pore diameter of TEOS‐PEO and EGMS‐PEO monoliths were analyzed by BET, where surface areas of EGMS‐PEO monoliths were almost equal to the surface area of TEOS‐PEO monoliths (Table 1). Comparing these results with the literature, pore diameter is larger; however, surface area is smaller for both TEOS‐PEO and EGMS‐PEO monoliths, which showed that these monoliths have limited number of pores with large pore diameters. It is known that the type and concentration of catalyst that are used for hydrolysis of precursors and the ratio between water and precursors are the most significant parameters on the pore morphology of monolith type gels 19, 20. It is possible to improve the pore morphology of the gels by altering these parameters. On the other hand, macropore structures have advantages such as minimizing back pressure during the continuous flow and provide good mass transfer in the microsystems 21.
Table 1.
Pore size distribution and surface area of TEOS‐PEO, EGMS‐PEO, and alginate aerogels
| TEOS‐PEO | EGMS‐PEO | Alginate | |
|---|---|---|---|
| BET surface area (m²/g) | 10.480 | 9.9588 | 384.787 |
| Slope (g/cm³) | 316.442 | 0.426870 | 8.986 |
| C value | 20.938 | 42.643774 | 139.303 |
| Correlation factor | 0.982534 | 0.9997573 | 0.999969 |
| BJH desorption pore volume (cm3/g) | 0.1077 | 0.145732 | 2.310 |
Zhang et al. 22 measured the surface area of sodium alginate and sodium alginate‐NaCMC beads to determine the effect of surface properties on the enzymatic activity, and the surface area of Na‐alginate beads was found as 43.34 m2 g−1. Khoo et al. 23 compared polyvinyl alcohol and calcium alginate beads as immobilization matrices where surface area of calcium alginate was reported as 6.25 m2 g−1. In our study, BET surface area of alginate beads was found as 384.787 m2 g−1 (Table 1), which is a higher value than those reported. Additionally, Deze et al. 24 characterized calcium alginate aerogel beads for heavy metal sorption from aqueous solution and BET surface area of calcium alginate aerogel beads was calculated as 419 m2 g−1, which is quite close to the value obtained in our study. Based on these results, alginate can serve as a better monolith for immobilization of enzymes due to the large surface area and higher porosity, which can yield better interaction.
3.1.6. FT infrared analysis
FT infrared analysis was carried out to confirm the formulation and reaction of all stages such as hydrolysis, condensation, surface modification, and drying. In Fig. 3A, the major peak at 1065 and 1066 cm−1 can be assigned to Si‐O‐Si bonds, and the peaks at 954 and 796 cm−1, and 957 and 880 cm−1 can be assigned to Si‐CH3 bonds 25, 26. Major peak in the spectrum belongs to Si‐O‐Si bond, this indicates that proper formulation was applied and condensation reaction was completed.
Figure 3.
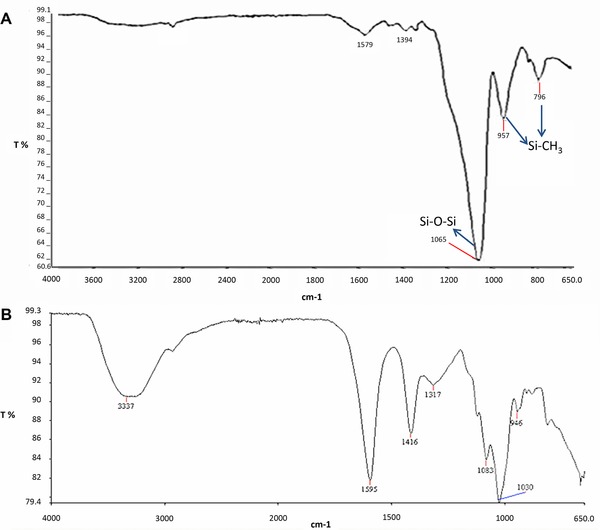
FT infrared (FTIR) spectrums of TEOS‐PEO monolith (A) and alginate beads (B).
Various distinct peaks of alginate such as hydroxyl at 3337 cm−1, carbonyl at 1595 cm−1, and carboxyl and carboxylate at about 1030–1416 cm−1 can be easily seen from the FT infrared spectrum of alginate aerogels (Fig. 3B).
3.2. Enzyme immobilization in hydrogels
3.2.1. Enzyme immobilization in silica monoliths
In this study, TEOS and EGMS were used as precursors. Four different gels, both with and without PEO, were prepared by two‐step sol–gel process, which uses pH difference between sol and reactive solutions for rapid gelation and results in more stable gels. PEO was added in order to strengthen the structure, increase permeability, and reduce shrinkage of the mesoporous gels. After 1‐day aging, β‐glucosidase‐immobilized TEOS and EGMS monoliths were crushed in order to eliminate diffusion limitations and determine possible denaturation from the sol–gel process. The obtained activities were compared with the activity of free enzyme and expressed as relative activity. The highest relative activity was obtained from the TEOS‐PEO gel formulation (Fig. 4A). The results showed that for both TEOS and EGMS monoliths, PEO had a positive effect on β‐glucosidase activity and the immobilization matrix derived from TEOS provided a higher enzymatic activity. De Souza et al. 27 reported that immobilized lipase activity could be increased from 33.98 to 89.91 U/mg by the addition of PEO to the gel structure. Likewise, Yesil‐Celiktas et al. 28 suggested that the addition of PEO improves the relative enzyme activities by strengthening the gel skeleton and reducing the possible gel damages that occur during the aging process.
Figure 4.
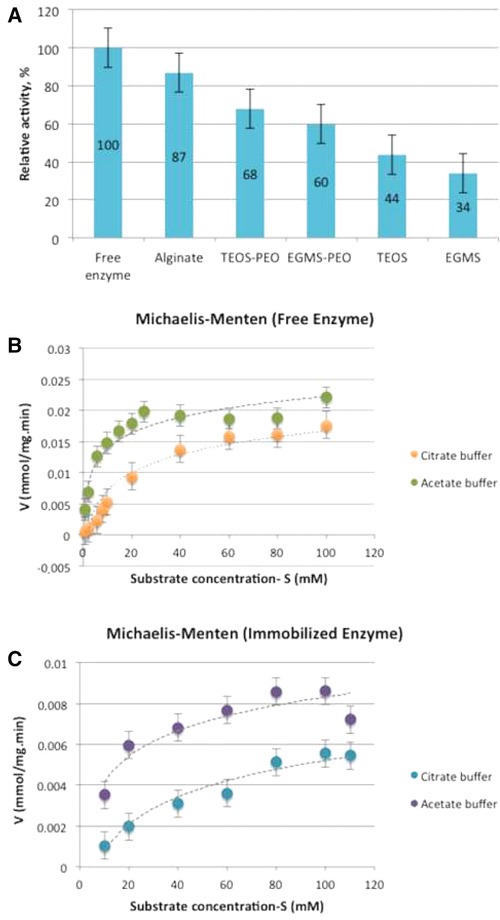
Activity of β‐glucosidase enzyme immobilized in different type of gels (A); Michaelis–Menten graphs for free enzyme (B); and immobilized enzyme in silica monoliths (C). The experiments were performed in duplicates and the results are given as ± SEM.
3.2.2. Enzyme immobilization in alginate beads
For activity tests, alginate beads were cut into very small pieces in order to decrease diffusion limitations. Compared to free enzyme, 87% relative enzymatic activity was obtained from immobilized enzyme in alginate beads (Fig. 4A), which was higher than the activities attained with the silica monoliths. This enhancement of enzymatic activity could be attributed to the higher BET surface area of alginate beads, which is quite higher than silica‐based monoliths. As indicated in Table 1, the pore volume of alginate beads is also higher than silica monoliths. This could cause easier diffusion of substrate in gel matrix as well.
3.3. Enzyme kinetics
3.3.1. Enzyme kinetics for free enzyme in different buffer systems
The activity of free β‐glucosidase enzyme was detected for different substrate concentrations ranging between 2 and 110 mM (Fig. 4B) in both citrate and Ca‐Na acetate buffers. Maximum velocity values (V max) for free enzyme were determined as 0.018 and 0.021 mmol mg−1 min−1, where the Michaelis–Menten constants (KM) were calculated as 29.8 and 4.18 mM in citrate and Ca‐Na acetate buffers, respectively.
3.3.2. Enzyme kinetics for TEOS‐PEO monoliths in different buffer systems
One‐day aged noncrushed TEOS‐PEO monoliths were used to investigate diffusion limitation effect on enzyme activity. Nonlinear regression analysis was used in order to determine kinetic values. The relative enzyme activity obtained from crushed gel (68%) was higher than the activity of uncrushed gel (50%), which shows the significant effect of diffusion limitations on enzyme activity. While maximum velocity values (V max) of immobilized enzyme were similar (0.0094 mmol mg−1 min−1 for citrate buffer and 0.0098 mmol mg−1 min−1 for Ca‐Na acetate buffer) in two buffer systems, the main difference was Michaelis–Menten constant (KM), which were 79 mM for citrate buffer and 16.88 mM for Ca‐Na acetate buffer (Fig. 4C). For immobilized enzyme, higher K m value than the free enzyme was expected due to limitations of substrate diffusion through pore matrix, apart from electrostatic and steric effects of gel matrix on enzyme activity. Additionally, nonhomogenous distribution of H+ ions might be associated with decreased enzyme activity caused by heterogeneous pH distribution 29. The results showed that both buffer systems are suitable for β‐glucosidase enzyme. However, lower substrate affinity (higher KM) and dissolution of alginate beads in citrate buffer make Ca‐Na acetate buffer more advantageous.
3.4. Flow through enzymatic reactions in microfluidic systems
In order to directly compare the TEOS‐PEO and alginate hydrogels as immobilization matrices, same amount of enzyme was loaded to each matrix and the enzymatic activity was determined at three different flow rates, corresponding to residence times of 5, 10, and 15 min inside the microreactor (Fig. 5A). The results indicated that longer residence times were correlated with higher yields (Fig. 5B). For residence times of 10 and 15 min, the product yields were slightly higher for the alginate systems, while 5 min TEOS‐PEO gel showed a higher activity. This leads to the conclusion that the enzymatic activity might be altered by the type of immobilization matrices. When comparing the conversion yields obtained from on‐the‐bench and flow‐through experiments, it is clearly seen that the elimination of external diffusion limitations resulted in higher conversion yields (Fig. 5B), which is in accordance with similar enzymatic bioconversions in microfluidic systems 30, 31, 32. Consequently, the microfluidic systems become much more advantageous than the classical immobilization systems.
Figure 5.
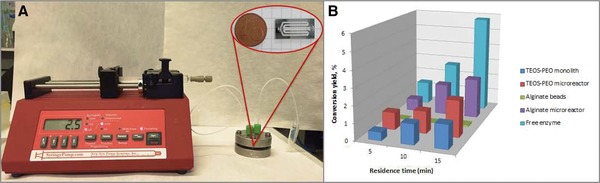
Experimental set‐up for flow‐through enzymatic reactions (A), conversion yields for free enzyme, immobilized enzyme in hydrogels, and immobilized enzyme within microreactors (B).
The enzymatic long‐term activity was determined by continuously pumping buffered substrate through the microreactors with a flow‐rate correlating a residence time of 15 min. After the activity measurements, the substrate solution was replaced by pure buffer and the microreactor was stored at 37°C. The results showed that the enzymatic activity decreased comparatively fast over time. One day after the first experiment, it was only about 68% and 63% of the initial activity and another day later, it remained at 31% and 32% for TEOS‐PEO and alginate hydrogels, respectively (Table 2). There was not a significant difference between TEOS‐PEO and alginate hydrogels. Potential reasons for the deactivation over time were the continuous exposure to elevated temperatures leading to denaturation of the enzymes or leaching of enzyme from the immobilization matrix. As for the free enzyme, an increased sensitivity to elevated temperatures was observed when β‐glucosidase was solubilized in Ca‐Na acetate buffer during storage at 4 and 37°C. The enzyme lost more than 60% of its initial activity even when stored at 4°C for 1 day. This value increased to more than 80% when stored at elevated temperatures. The cold‐stored enzyme retained a constant remaining activity of between 20 and 30% for the rest of the time, while that of the warm‐stored enzyme dropped below 5% on day 4 (Table 2). The obtained data indicated that the storage of the enzyme in buffer rather than in lyophilized form leads to a significant deactivation, which is significantly increased when the enzyme is exposed to higher temperatures.
Table 2.
Long‐term activity values of free and immobilized enzyme
| Time (h) | Relative activity (%) | |||
|---|---|---|---|---|
| Free enzyme | Immobilized enzyme | |||
| 4°C | 37°C | TEOS‐PEO | Alginate | |
| 0 | 100 | 100 | 100 | 100 |
| 24 | 37 ± 2 | 18 ± 3 | 68 ± 3 | 63 ± 2 |
| 48 | 23 ± 1 | 9 ± 2 | 31 ± 2 | 32 ± 2 |
Loss of enzymatic activity due to leaching was also investigated by conducting the standard activity assay with the purged buffer that potentially contained the leached enzymes. Ca‐Na acetate buffer was pumped through the microreactors containing alginate and TEOS‐PEO gels as well as immobilized enzymes at a flow rate of 37.5 μL/min. The samples of the purged product were collected with 5 min time intervals. The obtained conversions of pNPG to pNP has not indicated a certain pattern but rather fluctuated within a range of 0.1% and 0.4%. The flow rate applied during the leaching experiments was significantly higher than in the normal experiments, which potentially increased the amount of enzymatic activity lost due to leaching. Therefore, it can be concluded that the leaching occurring in both organic and inorganic matrices can be neglected.
4. Concluding remarks
This study was focused on the development of suitable matrices for immobilization of β‐glucosidase in microreactors. For this purpose, silica and alginate hydrogels were compared in terms of activity and gel properties. The immobilization in hydrogels proved to be an effective method to increase the temperature stability and long‐term activity of the enzymes. The results of this study indicated that alginate hydrogels exhibited slightly better properties than silica. But it is worth to mention that if the microreactors are intended to be used for several times after cleaning, then alginate hydrogels can be employed as promising immobilization matrices for catalysis reactions in microfluidic systems.
Practical application
Enzymes are biological catalysts and they have numerous applications in life sciences. It is important to immobilize enzymes to prevent them from harsh environmental conditions and also for their repeated use. The immobilization of enzymes in sol–gel matrices and their applications in microfluidic systems have several advantages such as decreased consumption of valuable catalysts and reagents, controllable reaction conditions, high mass and heat transfer, and high surface‐to‐volume ratio. In this paper, inorganic silica and organic alginate gels were prepared to immobilize β‐glucosidase enzyme for microfluidic systems. The characterization of these gels was carried out, and the performances of silica and alginate gels as immobilization matrices were compared. The results obtained in this study are applicable to other enzymes and their applications in microfluidic systems.
The authors have declared no conflict of interest.
Acknowledgments
Financial supports provided by The Research and Technological Council of Turkey (TUBITAK) (113M050) and Bundesministerin für Bildung und Forschung (BMBF) (01DL14002) are highly appreciated.
5 References
- 1. Zhao, Y. , Liu, B. , Pan, L. , Yu, G. , 3D nanostructured conductive polymer hydrogels for high‐performance electrochemical devices. Energy Environ. Sci. 2013, 6, 2856–2870. [Google Scholar]
- 2. Pan, L. , Yu, G. , Zhai, D. , Lee, H. R. et al., Hierarchical nanostructured conducting polymer hydrogel with high electrochemical activity. Proc. Natl. Acad. Sci. USA 2012, 109, 9287–9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kato, M. , Sakai‐Kato, K. , Toyo'oka, T. , Silica sol‐gel monolithic materials and their use in a variety of application. J. Sep. Sci. 2005, 28, 1893–1908. [Google Scholar]
- 4. Vera‐Avila, L. E. , García‐Salgado, E. , García de Llasera, M. P. , Pena‐Alvarez, A. , Binding characteristics of bovine serum albumin encapsulated in sol‐gel glasses: An alternative for protein interaction studies. Anal. Biochem. 2008, 373, 272–280. [DOI] [PubMed] [Google Scholar]
- 5. Hellner, G. , Boros, Z. , Tomin, A. , Poppe, L. , Novel sol‐gel lipases by designed bioimprinting for continuous‐flow kinetic resolutions. Adv. Synth. Catal. 2011, 353, 2481–2491. [Google Scholar]
- 6. Sheldon, R. A. , Enzyme immobilization: The quest for optimum performance. Adv. Synth. Catal. 2007, 349, 1289–1307. [Google Scholar]
- 7. Escobar, S. , Illanes, A. , Wilson, L. , Bernal, C. et al., In situ immobilization of β‐galactosidase from Bacillus circulans in silica by sol‐gel process: Application in prebiotic synthesis. Eng. Life Sci. 2016, 16, 396–404. [Google Scholar]
- 8. Hübner, J. , Brakowski, R. , Wohlgemuth, J. , Brenner‐Weiß, G. et al., Compartmented microfluidic bioreactor system using magnetic enzyme immobilisates for fast small‐scale biotransformation studies. Eng. Life Sci. 2015, 15, 721–726. [Google Scholar]
- 9. Augst, A. D. , Kong, H. J. , Mooney, D. J. , Alginate hydrogels as biomaterials. Macromol. Biosci. 2006, 6, 623–633. [DOI] [PubMed] [Google Scholar]
- 10. Junter, G. A. , Vinet, F. , Compressive properties of yeast cell‐loaded Ca‐alginate hydrogel layers: Comparison with alginate–CaCO3 microparticle composite gel structures. Chem. Eng. J. 2009, 145, 514–521. [Google Scholar]
- 11. Wiedemeier, S. , Ehrhart, F. , Mettler, E. , Gastrock, G. et al., Encapsulation of Langerhan’ islets: Microtechnological developments for transplantation. Eng. Life Sci. 2011, 11, 165–173. [Google Scholar]
- 12. Lee, K. Y. , Mooney, D. J. , Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smirnova, I. , Arlt, W. , Synthesis of silica aerogels: Influence of the supercritical CO2 on the sol‐gel process. J. Sol‐Gel. Sci. Technol. 2003, 28, 175–184. [Google Scholar]
- 14. Rao, A. V. , Bhagat, S. D. , Synthesis and physical properties of TEOS‐based silica aerogels prepared by two step (acid‐base) sol‐gel process. Solid State Sci. 2004, 6, 945–952. [Google Scholar]
- 15. Nielsen, S. S. , Phenol‐sulfuric acid method for total carbohydrates, in: Nielsen S. S. (Ed.), Food Analysis Laboratory Manual, Springer, West Lafayette: 2010, pp. 47–53. [Google Scholar]
- 16. Reetz, M. T. , Zonta, A. , Simpelkamp, J. , Efficient immobilization of lipases by entrapment in hydrophobic sol‐gel materials. Biotechnol. Bioeng. 1996, 49, 527–534. [DOI] [PubMed] [Google Scholar]
- 17. Cumana, S. , Ardao, I. , Zeng, A. P. , Smirnova, I. , Glucose‐6‐phosphate dehydrogenase encapsulated in silica‐based hydrogels for operation in a microreactor. Eng. Life. Sci. 2014, 14, 170–179. [Google Scholar]
- 18. Gurikov, P. , Raman, S. P. , Weinrich, D. , Frickeb, M. et al., A novel approach to alginate aerogels: Carbon dioxide induced gelation. RSC Adv. 2015, 5, 7812–7818. [Google Scholar]
- 19. Pope, E. J. A. , Mackenzie, J. D. , Sol‐gel processing of silica: II. The role of the catalyst. J. Non‐Cryst. Solids 1986, 87, 185–198. [Google Scholar]
- 20. Fardad, M. A. , Catalysts and the structure of SiO2 sol‐gel films. J. Mater. Sci. 2000, 35, 1835–1841. [Google Scholar]
- 21. He, P. , Greenway, G. , Haswell, S. J. , Development of enzyme immobilized monolith micro‐reactors integrated with microfluidic electrochemical cell for the evaluation of enzyme kinetics. Microfluid. Nanofluidicis 2009, 8, 565–573. [Google Scholar]
- 22. Zhang, Y. , Sun, G. , Wang, X. , Wang, L. et al., Efforts on membrane properties and enzymes by adding divalent cations and sodium carboxymethyl cellulose. Carbohydr. Polym. 2014, 106, 94–100. [DOI] [PubMed] [Google Scholar]
- 23. Khoo, K. M. , Ting, Y. P. , Biosorption of gold by immobilized fungal biomass. Biochem. Eng. J. 2001, 8, 51–59. [DOI] [PubMed] [Google Scholar]
- 24. Deze, E. G. , Papageorgiou, S. K. , Favvas, E. P. , Katsaros, F. K. , Porous alginate aerogel beads for effective and rapid heavy metal sorption from aqueous solutions: Effect of porosity in Cu2+ and Cd2+ ion sorption. Chem. Eng. J. 2012, 209, 537–546. [Google Scholar]
- 25. Omranpour, H. , Motahari, S. , Effects of processing conditions on silica aerogel during aging: Role of solvent, time and temperature. J. Non Cryst. Solids 2013, 379, 7–11. [Google Scholar]
- 26. Hilonga, A. , Kim, J. K. , Sarawade, P. B. , Kim, H. T. , Low‐density TEOS‐based silica aerogels prepared at ambient pressure using isopropanol as the preparative solvent. J. Alloys Compd. 2009, 487, 744–750. [Google Scholar]
- 27. De Souza, R. L. , De Faria, E. L. P. , Figueiredo, R. T. , Freitas, L. D. S. et al., Protic ionic liquid as additive on lipase immobilization using silica sol‐gel. Enzyme Microb. Technol. 2013, 52, 141–150. [DOI] [PubMed] [Google Scholar]
- 28. Yesil‐Celiktas, O. , Cumana, S. , Smirnova, I. , Silica‐based monoliths for enzyme catalyzed reactions in microfluidic systems with an emphasis on glucose 6‐phosphate dehydrogenase and cellulase. Chem. Eng. J. 2013, 234, 166–172. [Google Scholar]
- 29. Delouise, L. A. , Miller, B. L. , Enzyme Immobilization in porous silicon: Quantitative analysis of the kinetic parameters for glutathione‐S‐transferases. Anal. Chem. 2005, 77, 1950–1956. [DOI] [PubMed] [Google Scholar]
- 30. Wang, S. , Su, P. , Yang, Y. , Online immobilized enzyme microreactor for the glucose oxidase enzymolysis and enzyme inhibition assay. Anal. Biochem. 2012, 427, 139–143. [DOI] [PubMed] [Google Scholar]
- 31. Iqbal, J. , An enzyme immobilized microassay in capillary electrophoresis for characterization and inhibition studies of alkaline phosphatases. Anal. Biochem. 2011, 414, 226–231. [DOI] [PubMed] [Google Scholar]
- 32. Tušek, A. , Šalic, A. , Kurtanjek, Ž. , Zelić, B. , Modelling and kinetic parameter estimation of alcohol dehydrogenase‐catalyzed hexanol oxidation in a microreactor. Eng. Life Sci. 2012, 12, 49–56. [Google Scholar]


