Abstract
In the present report and for the first time in the international literature, the impact of the addition of NaCl upon growth and lipid production on the oleaginous yeast Rhodosporidium toruloides was studied. Moreover, equally for first time, lipid production by R. toruloides was performed under nonaseptic conditions. Therefore, the potentiality of R. toruloides DSM 4444 to produce lipid in media containing several initial concentrations of NaCl with glucose employed as carbon source was studied. Preliminary batch‐flask trials with increasing amounts of NaCl revealed the tolerance of the strain against NaCl content up to 6.0% w/v. However, 4.0% w/v of NaCl stimulated lipid accumulation for this strain, by enhancing lipid production up to 71.3% w/w per dry cell weight. The same amount of NaCl was employed in pasteurized batch‐flask cultures in order to investigate the role of the salt as bacterial inhibiting agent. The combination of NaCl and high glucose concentrations was found to satisfactorily suppress bacterial contamination of R. toruloides cultures under these conditions. Batch‐bioreactor trials of the yeast in the same media with high glucose content (up to 150 g/L) resulted in satisfactory substrate assimilation, with almost linear kinetic profile for lipid production, regardless of the initial glucose concentration imposed. Finally, fed‐batch bioreactor cultures led to the production of 37.2 g/L of biomass, accompanied by 64.5% w/w of lipid yield. Lipid yield per unit of glucose consumed received the very satisfactory value of 0.21 g/g, a value among the highest ones in the literature. The yeast lipid produced contained mainly oleic acid and to lesser extent palmitic and stearic acids, thus constituting a perfect starting material for “second generation” biodiesel.
Keywords: Fed‐batch culture, Microbial lipids, NaCl, Pasteurized conditions, Rhodosporidium toruloides
Abbreviations
- DCW
dry cell weight
- DO
dissolved oxygen
- SCO
single cell oil
1. Introduction
Environmental concerns have driven scientific research toward alternative energy resources, as means of disengagement from fossil oil 1, 2. Biodiesel consists one of the major renewable transportation fuels, deriving by trans‐esterification process of long chain fatty acids of plant or animal origin. However, constant rising demand of biodiesel production competes with the availability of existing raw materials and as a result, other nonconventional oil resources are explored, mainly of nonedible nature 2. In this light, scientific interest on microbial lipids as alternative source of oil has gain momentum the last decades.
Microbial oil production can be carried out by a number of heterotrophic (mostly yeast and fungi) or phototrophic (algae) organisms that are found to accumulate oil up to 80% of their dry weight 1, 3. This lipid, namely single cell oil (SCO), is mainly composed of neutral fractions (principally triacylglycerols‐TAGs and to lesser extent steryl‐esters) 4, while these lipid‐accumulating microorganisms are called “oleaginous” 1, 2, 3, 4. It has been well established that when culture is performed on sugars or similarly metabolized compounds (e.g. polysaccharides, glycerol, etc.), the conditions required to trigger lipid production are met in a culture environment with carbon excess and (at least) one essential nutrient depletion (usually nitrogen) 1, 3, 4, 5. As indicated, SCOs could constitute the starting material for the synthesis of the “2nd” or the “3rd generation” biodiesel 2, 6. Nevertheless, and despite the huge upsurge of interest concerning the production of microbial oils amenable to be converted into biodiesel 2, 4, the first industrial applications related with the utilization of oleaginous microorganisms referred to the production of specialty (and expensive) lipids, rarely found in the plant or animal kingdom, like the cocoa‐butter substitutes 1, 2, 3, 4, 7, 8. In any case though, the feasibility of sustainable bioprocess development for SCO production is determined by the cost of both raw materials and (mainly) the fermentation process 1, 2, 3, 9, 10, 11, 12. Yeast strains belonging to the genera Cryptococcus sp., Lipomyces sp., Rhodotorula sp., Rhodosporidium sp., and Trichosporon sp. are among those reported as possible biodiesel producers 13, 14, 15, 16, 17, 18, 19. Among those, Rhodosporidium toruloides Y4 has been reported capable of producing 106.5 g/L of biomass containing 67.5% w/w of oil, during cultivation in a 15‐L bioreactor under fed‐batch mode 20, designated as the highest oil production from the particular strain so far.
One important aspect developed in several industrial fermentations, refers to the potential of the accomplishment of the microbial conversion under nonaseptic conditions, due to obvious process cost reduction 6. While this is feasible in fermentations in which inhibiting (for any contaminant microorganisms) metabolites are accumulated into the production media (e.g. ethanol during the alcoholic fermentation processes; see Sarris and Papanikolaou 6), generation of such extra‐cellular metabolites inhibiting contaminant cells is not obvious during the fermentation of SCO production. Thus, addition of such a “hurdle” compound into the medium should be considered. Preliminary works have identified the potential of the microorganism R. toruloides DSM 4444 to grow on media containing NaCl (an important inhibiting agent against contaminant microorganisms) quantities, while in some previous studies it has been demonstrated that among other yeasts, Rhodosporidium sp. strains have been isolated from hyper‐saline habitats 13 and, therefore, could potentially grow on media presenting relatively high salinity. The objective of the study, thus, was double: to evaluate the performance of this yeast strain on glucose‐based media supplemented with different initial quantities of NaCl and to perform SCO production by this yeast under nonaseptic conditions, due to the addition of salt into the medium.
2. Materials and methods
2.1. Microorganism and media
Rhodosporidium toruloides DSM 4444, provided by the DSMZ culture collection (Leibniz, Germany), was maintained on yeast peptone dextrose agar, supplemented with malt extract, at T = 4°C and subcultured every month in order to maintain its viability. Precultures of the strain contained glucose, yeast extract, and peptone at 10 g/L each. The synthetic medium used had the following salt composition (g/L): KH2PO4, 7.0; Na2HPO4, 2.5; MgSO4·7H2O, 1.5; CaCl2, 0.15; FeCl3·6H2O, 0.15; ZnSO4·7H2O, 0.02; MnSO4·H2O, 0.06. Peptone and yeast extract were used as nitrogen sources in concentrations of 0.75 g/L and 0.5 g/L, respectively. Unless otherwise stated, cultures were supplemented with NaCl at concentrations of 0.5, 1.0, 1.5, 2.5, 4.0, and 6.0% w/v. Commercial glucose provided by the “Hellenic Industry of Sugar SA” (Thessaloniki, Greece) was used as carbon source in the fermentations performed [purity c. 95%, w/w, impurities composed of maltose (2%, w/w), malto‐dextrines (0.5%, w/w), water (1.5%, w/w), and salts (1.0%, w/w)]. The initial pH for all media before and after sterilization (121°C/20 min) was 6.0 ± 0.1. Glucose was added at different concentrations into the medium before heat sterilization. In all trials, initial glucose (Glci) concentration was measured after the sterilization. Assay of glucose before and after the sterilization demonstrated very small glucose destruction (c. 2%) due to the heat sterilization.
2.2. Culture conditions
Batch‐flask cultures were conducted in 250‐mL conical flasks, containing 50 ± 1 mL of growth medium, previously sterilized (121°C/20 min) and inoculated with 1 mL of a 24‐h exponential preculture (c. 3 × 107 cells, initial biomass concentration at the flasks at c. 0.12 ± 0.02 g/L). Cultures were performed in an orbital shaker (Lab‐Line, Illinois‐USA) at an agitation rate of 185 ± 5 rpm and incubation temperature T = 26 ± 1°C. It was desirable to maintain a medium pH >5.2; to this end, an appropriate volume of KOH (5 M) was periodically and aseptically added into the flasks when needed, in order to maintain the pH value at 6.0 ± 0.2 19.
Batch‐flask cultures were also realized under previously pasteurized media by subjecting the flasks filled with the fermentation media at 100°C for 7 min. Then 3 mL of 24‐h preculture (c. 9 × 107 cells, initial biomass concentration at the flasks at ∼0.36 ± 0.06 g/L) were used as inoculum. A Jenway 3020 pH‐meter was used for pH‐measurements of cultures. Dissolved oxygen (DO) concentration was determined using a selective electrode (OXI 96, B‐SET, Germany). Before harvesting, the shaker was stopped and the probe was placed into the flask. Then, the shaker was switched on and the measurement was taken after DO equilibration (usually within 10 min). Oxygen saturation was kept above 20% v/v during all growth phases.
Batch‐bioreactor experiments were carried out in a 3.5‐L bioreactor (Infors HT, Labfors 5), with a working volume of 2.0 L. A 7.5% v/v inoculum was employed using 24‐h exponential yeast preculture. The stirrer speed was on cascade mode, automatically varying from 200 to 500 rpm to maintain a DO concentration above 20% v/v of saturation. Aeration and temperature were maintained at 1.0 vvm and T = 26°C, respectively. The pH was maintained at 6.0 ± 0.1 by automatic addition of 5 M KOH. Fed‐batch fermentations were initiated in batch mode and when glucose concentration was reduced to 10 g/L, a volume of concentrated glucose solution (60%, w/v) was added in the bioreactor. Samples were taken periodically from the bioreactor throughout fermentation for subsequent analysis as described in the next section.
2.3. Analytical methods
The whole content of flasks (c. 50 mL) or bioreactor samples (c. 20 mL) were periodically collected and cells were harvested by centrifugation (9000 × g/15 min at 10°C) in a Hettich Universal 320R (Germany) centrifuge and washed twice with distilled water. Biomass (X, g/L) was determined by means of dry cell weight (DCW) (95 ± 2°C/24 h). Consumed glucose was determined by 3,5‐dinitrosalicylic acid assay 21. Total cellular lipid (L, g/L) was extracted from the dry biomass with a mixture of chloroform/methanol 2/1 v/v and was determined gravimetrically. Cellular lipids were converted to their methyl‐esters in a two‐step reaction 19. FAMEs were analyzed according to Fakas et al. 21 and were identified by reference to standards. In some of the performed trials, in order to investigate whether glycerol (or other polyols) was secreted into the culture medium, HPLC analysis 21 of the supernatant obtained after centrifugation was performed. All experiments were performed in duplicate by using different inocula. All of the experimental points presented in the tables and the figures are the mean value of two independent determinations, with standard error ≤10%.
3. Results
This study was focused on the evaluation of the Rhodosporidium toruloides yeast ability to grow on media containing glucose as carbon source, supplemented with various amounts of NaCl. Special attention was paid to the evolution of lipid production and the potential effect of NaCl on yeast metabolism, in batch‐flask trials as well as batch‐ and fed‐batch bioreactor experiments.
3.1. Effect of NaCl concentration on Rhodosporidium toruloides cultures
The yeast strain R. toruloides DSM 4444 was cultivated in batch‐flask trials, in media containing 50 g/L glucose supplemented with increasing NaCl concentrations. Cultures were done under nitrogen‐limited conditions (carbon‐to‐nitrogen ratio equal to 106 mol/mol) in order to stimulate lipid accumulation. Moreover, an experiment without NaCl addition was included that served as control. The obtained results with regard to the impact of NaCl upon the physiological behavior of R. toruloides are depicted in Table 1. Generally, it should be stressed that only at increased initial NaCl concentration (e.g. NaCl at 60 g/L or higher) some negative effect upon the biomass and lipid produced was observed (Table 1). Likewise, by taking into consideration the specific growth rate for the fermentations in the media with different initial salinity imposed, similar μmax values (0.09 ± 0.01 h−1) were recorder for all trials performed except for the fermentation presenting the highest initial NaCl quantity (=60 g/L), in which the μmax value slightly declined (see Table 1). Moreover, analysis of the supernatant at the end of all trials was performed in order to demonstrate whether secretion of glycerol or other polyols was performed due to the increasing osmotic pressure into the medium; it was seen that up to the threshold of 1.5% w/v no polyols were identified into the medium. Thereafter, very low glycerol accumulation into the medium occurred (the maximum quantity of glycerol, c. 1.5 g/L, was obtained at the trial with initial concentration of NaCl imposed of 40 g/L). No other polyol was synthesized as response to the osmotic stress situation by R. toruloides. On the other hand, for NaCl concentrations varying from 0.5 to 2.5% w/v, microbial growth as well as lipid production was maintained in similar levels; specifically, 88–97% of initial glucose concentration was consumed within 120–168 h of fermentation, whereas biomass production ranged between 8.2 and 8.9 g/L. In terms of lipid production, cells accumulated 60.6–62.4% w/w of oil. However, initial NaCl concentration of 4.0% w/v was found to positively affect biomass and, specifically, lipid production, yielding 9.4 and 6.7 g/L, respectively. Consequently, lipid quantity per DCW increased to 71.3% w/w. Higher NaCl additions exerted inhibitory effects on yeast growth, as only 6.3 g/L of biomass were synthesized, with concomitant impact on lipid production and yield (Table 1). On the other hand, for all the above‐mentioned trials, the yield of total biomass produced per glucose consumed (YX/Glc) was c. 0.20 g/g, ranging between 0.19 and 0.21 g/g.
Table 1.
Quantitative data of Rhodosporidium toruloides DSM 4444 originated from kinetics in media with six different initial NaCl concentrations with the same initial glucose concentration (50 g/L)
| Entry | NaCl (%, w/v) | Time (h) | μmax (h−1) | Glccons (g/L) | X (g/L) | L (g/L) | YL/X (%, w/w) |
|---|---|---|---|---|---|---|---|
| 1 | 0.0 | 168 | 0.091 | 48.8 | 8.9 | 4.9 | 55.1 |
| 2 | 0.5 | 168 | 0.088 | 43.6 | 8.7 | 5.4 | 62.1 |
| 3 | 1.0 | 144 | 0.101 | 48.7 | 8.2 | 5.1 | 62.2 |
| 4 | 1.5 | 144 | 0.093 | 44.5 | 8.5 | 5.3 | 62.4 |
| 5 | 2.5 | 120 | 0.084 | 47.9 | 8.9 | 5.4 | 60.6 |
| 6 | 4.0 | 192 | 0.090 | 48.6 | 9.4 | 6.7 | 71.3 |
| 7 | 6.0 | 168 | 0.070 | 30.0 | 6.3 | 2.8 | 44.4 |
Representation consumed glucose (Glccons, g/L), produced biomass (X, g/L), lipid content (L, g/L), and lipid in dry weight (YL/X, % w/w) when the maximum quantity of lipid (in g/L) was achieved. The maximum‐specific growth (μmax, h−1), was calculated for every of the trials performed by fitting the equation within the early exponential growth phase of the respective culture. Culture conditions: growth in 250‐mL flasks at 185 rpm, initial pH = 6.0 ± 0.1, DO > 20% v/v, incubation temperature T = 26°C. Two lots of independent cultures were conducted by using different inocula. In all of the determinations, standard error calculated was less than 15%.
Taking into account the satisfactory performance of the strain at NaCl supplementation of 4.0% w/v, subsequent batch‐flasks cultures were carried out with the same NaCl addition and increased glucose concentration (Glci≈100 g/L). In the same manner, a control experiment without NaCl addition was included. In the absence of NaCl, elevated glucose concentrations prolonged the course of the fermentation up to 433 h. At that time, c. 88% of initial carbon source concentration (≈93 g/L) was finally consumed by the strain, leading to 17.5 g/L of biomass. The μmax in the above‐mentioned culture, calculated at the early exponential growth phase by fitting the equation on the experimental data within this phase, was found to be ≈0.08 h−1 (slightly lower than that observed on the respective trial with Glci = 50 g/L, potentially due to slight inhibition exerted by the increased initial concentration of glucose). However, biomass production per substrate consumption yield (YX/Glc) was ≈0.19 g/g, the same value as in trials with Glci = 50 g/L. Furthermore, the higher Glci concentration employed and the increased carbon‐to‐nitrogen ratio (C/N = 211 mol/mol) seemed to enhance lipid production in terms of absolute values, reaching a maximum SCO production of 8.1 g/L. On the other hand, and despite the significant increase of lipid in absolute values compared with the respective trial in which Glci was adjusted to c. 50 g/L (see Table 1 entry 1), total lipid in DCW (YL/X) value was lower than the one obtained in the trial with Glci = 50 g/L (c. 46.3% against 55.1% w/w). The presence of 4.0% w/v NaCl in media with 100 g/L of glucose, extended the fermentation duration to more than 500 h, a time point in which c. 80% of the initial glucose was consumed. Biomass synthesis was slightly reduced to 16.1 g/L compared with the culture with Glci ≈ 100 g/L and no NaCl addition was performed. Nonetheless, YX/Glc and μmax values were unaffected (=0.20 g/g and 0.08 h−1 respectively) by the addition of salt into the medium. On the other hand, lipid accumulation in terms of both absolute (g/L) and relative (% in DCW) values was noticeably higher compared to the equivalent experiment (Glci≈100 g/L) in which no NaCl addition occurred (L = 9.2 g/L, YL/X = 57.1% w/w). Surprisingly enough, R. toruloides accumulated oil in an almost linear manner, whereas shortly after virtual exhaustion of the assimilable nitrogen from the culture medium (i.e. c. 50 h after inoculation) lipid in DCW almost reached its plateau (see Fig. 1).
Figure 1.
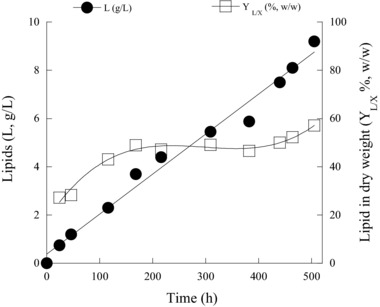
Kinetics of lipid production (L; g/L) and lipid in dry cell weight (YL/X; % w/w) during growth of Rhodosporidium toruloides DSM 4444 on glucose‐based media (100 g/L) supplemented with 4% w/v NaCl. Culture conditions as in Table 1. Two lots of independent cultures were conducted by using different inocula. In all of the determinations, standard error calculated was less than 10%.
Table 2 shows the FA profiles of R. toruloides cellular lipids, during growth on media with increasing NaCl concentrations. In every case, the predominant fatty acid of the yeast was oleic (Δ9C18:1), followed by palmitic (C16:0) stearic (C18:0) and linoleic acid (Δ9,12C18:2). The implementation of NaCl did not seem to affect the amounts of individual fatty acids in the composition of the accumulated lipids. On the contrary, the unsaturated nature of lipids increased during the course of fermentation, as indicated by the SFA/UFA ratio. This fact could be mainly attributed to the increased amounts of the unsaturated oleic and linoleic acids and the declining percentage of stearic acid that occurred in the late growth phase.
Table 2.
Fatty acid composition of cellular lipids of Rhodosporidium toroloides DSM 4444 on glucose‐based media (Glci = 50 g/L), containing various NaCl concentrations
| Fatty acid composition (%, w/w) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NaCl (%, w/v) | Growth phase | C14:0 | C16:0 | Δ9C16:1 | C18:0 | Δ9C18:1 | Δ9,12C18:2 | Δ9,12,15C18:3 | C20:0 | C22:0 | aSFA/UFA |
| 0.0 | VE | 1.1 | 28.2 | 0.8 | 11.5 | 48.6 | 7.7 | 2.1 | — | — | 0.69 |
| E | 0.8 | 24.1 | 1.5 | 9.9 | 51.2 | 9.1 | 2.4 | — | — | 0.54 | |
| L | 0.6 | 22.2 | 1.4 | 7.1 | 54.5 | 11.5 | 3.0 | — | — | 0.43 | |
| 0.5 | VE | 1.4 | 27.5 | 1.2 | 10.0 | 49.0 | 8.6 | 2.1 | — | — | 0.68 |
| E | 1.2 | 25.5 | 0.8 | 12.5 | 48.8 | 7.8 | 2.3 | 0.3 | 0.7 | 0.67 | |
| L | 1.1 | 24.6 | 1.2 | 10.0 | 49.0 | 10.4 | 2.8 | 0.6 | 0.57 | ||
| 1.0 | VE | 1.6 | 23.8 | 0.9 | 16.7 | 45.7 | 6.6 | — | — | 4.3 | 0.87 |
| E | 1.6 | 25.5 | 1.1 | 10.9 | 49.3 | 8.1 | 2.3 | 0.3 | 0.2 | 0.63 | |
| L | 1.0 | 23.3 | 0.8 | 8.6 | 52.2 | 11.2 | 2.2 | 0.2 | 0.2 | 0.50 | |
| 1.5 | VE | 1.1 | 26.8 | ‐ | 10.0 | 51.3 | 8.6 | 1.9 | — | — | 0.61 |
| E | 1.2 | 25.8 | 0.5 | 11.2 | 50.7 | 7.3 | 2.1 | 0.3 | 0.5 | 0.56 | |
| L | 0.9 | 24.5 | 1.2 | 9.8 | 51.5 | 8.0 | 2.6 | — | — | 0.56 | |
| 2.5 | VE | 1.2 | 26.9 | 1.2 | 10.5 | 51.3 | 7.0 | 1.6 | — | — | 0.63 |
| E | 1.1 | 25.8 | 0.7 | 9.6 | 50.5 | 9.0 | 2.3 | 0.2 | 0.3 | 0.59 | |
| L | 0.9 | 24.0 | 1.5 | 9.0 | 53.0 | 8.0 | 2.7 | — | — | 0.52 | |
| 4.0 | VE | 1.4 | 23.4 | 0.4 | 15.5 | 47.2 | 9.7 | 2.6 | 0.4 | 0.4 | 0.68 |
| E | 1.2 | 26.3 | 0.4 | 10.3 | 50.4 | 8.2 | 2.4 | — | 0.5 | 0.62 | |
| L | 0.8 | 25.5 | 1.5 | 8.5 | 51.8 | 8.9 | 2.8 | — | — | 0.54 | |
Ratio of saturated to unsaturated fatty acids.
Very early (VE) growth phase is that in which the incubation time is between 20–30 h. Early (E) growth phase is that in which the incubation time is between 50 and 70 h. Late growth phase is that in which incubation time is c. 150 h. Culture conditions as in Table 1.
3.2. Trials of R. toruloides on pasteurized media supplemented with NaCl
Based on evidence of the tolerance against noticeable amounts of NaCl (e.g. 4.0% w/v) shown by the employed yeast strain, it was decided to further investigate the stability of microbial growth and lipid production under pasteurized conditions and assess whether the presence of 4.0% w/v NaCl could reduce the probability of culture contamination. It is evident that a successful accomplishment of SCO production in unsterile media can reduce the cost of the process when a scale‐up is envisaged. To this end, batch‐flask trials were carried out with c. 50 and 100 g/L of glucose as substrate, in media supplemented with 4% w/w NaCl. At Glci≈50 g/L, substrate exhaustion occurred around 160 h after inoculation, yielding 11.7 g/L of biomass production (Table 3A). However, lipid accumulation was lower than in the experiment in which the medium had been previously subjected to heat sterilization (L = 5.9 g/L against 6.7 g/L). Microscopy observations revealed the presence of bacterial contamination (rods), accounting for c. 8% of the total microbial population. When higher Glci concentrations were employed in pasteurized media, equally some bacterial contamination occurred (c. 5% of the total microbial population). As in the trial with Glci ≈ 50 g/L, glucose assimilation in the pasteurized medium was more rapid than in the aseptic fermentation, possibly due to this contamination. Equally, biomass formation was enhanced in the pasteurized medium in comparison with the aseptic culture, reaching a DCW value of 17.9 g/L that contained 9.1 g/L of oil (lipid in DCW of c. 51% w/w) while in the aseptic culture the respective values were for DCW 16.1 g/L and for lipid 9.2 g/L (see Table 3A). The kinetics of biomass produced, lipid accumulated, and glucose assimilated for the trials with Glci≈100 g/L are seen in Fig. 2.
Table 3.
Quantitative data of Rhodosporidium toruloides DSM 4444 originated from kinetics in shake‐flask experiments in sterilized and pasteurized media, supplemented with 4.0% w/v NaCl and 50 and 100 g/L initial glucose concentration (A) and from kinetics in media containing 50, 100, and 150 g/L of glucose and 4% w/v NaCl in batch‐bioreactor experiments (B)
| A) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Culture mode and heat‐treatment | Glci (g/L) | Time (h) | Glccons (g/L) | X (g/L) | L (g/L) | YL/X (%,w/w) | YL/Glc (g/g) | YX/Glc (g/g) |
| Flasks, sterilized | ≈50 | 192 | 48.6 | 9.4 | 6.7 | 71.3 | 0.14 | 0.19 |
| Flasks, pasteurized | ≈50 | 160 | 48.0 | 11.7 | 5.9 | 50.4 | 0.12 | 0.24 |
| Flasks, sterilized | ≈100 | 505 | 93.0 | 16.1 | 9.2 | 57.1 | 0.10 | 0.18 |
| Flasks, pasteurized | ≈100 | 311 | 80.0 | 17.9 | 9.1 | 50.8 | 0.11 | 0.22 |
| B) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Culture mode | Glci (g/L) | Time (h) | Glccons (g/L) | X (g/L) | L (g/L) | YL/X (%,w/w) | YL/Glc (g/g) | YX/Glc (g/g) |
| Batch‐bioreactor | ≈50.0 | 72 | 44.5 | 12.7 | 8.1 | 63.8 | 0.18 | 0.29 |
| ≈100.0 | 160 | 90.9 | 25.2 | 14.2 | 56.3 | 0.16 | 0.28 | |
| ≈150.0 | 312 | 110.3 | 36.2 | 23.6 | 65.1 | 0.21 | 0.33 | |
Representation of initial glucose (Glci, g/L), consumed glucose (Glccons, g/L), produced biomass (X, g/L), produced lipid (L, g/L), lipid in dry weight (%, w/w), lipid yield per consumed substrate (YL/Glc, g/g), and biomass yield per consumed substrate (YX/Glc, g/g). Culture conditions for the shake flasks: growth in 250‐mL flasks at 185 rpm, initial pH = 6.0 ± 0.1, DO > 20% v/v, incubation temperature T = 26°C; for the bioreactor: agitation speed 200–500 rpm, pH = 6.0 ± 0.1, DO > 20% v/v, temperature T = 26°C. Two lots of independent cultures were conducted by using different inocula. In all of the determinations, standard error calculated was less than 10%.
Figure 2.
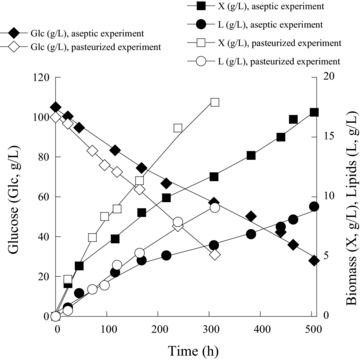
Kinetics of residual glucose (Glc; g/L), biomass production (X; g/L) and lipid accumulated (L; g/L), during Rhodosporidium toruloides DSM 4444 growth in previously sterilized (filled symbols) and pasteurized (open symbols) media with 100 g/L of glucose and 4% w/v NaCl. Culture conditions as in Table 2. Two lots of independent cultures were conducted by using different inocula. In all of the determinations, standard error calculated was less than 10%.
Table 4A shows the FA profiles of the produced cellular lipids for the previously pasteurized media, in which Glci concentration was adjusted to c. 50 and 100 g/L and constant NaCl quantity added. Despite the fact that the cultures were not axenic (as stated, some contamination by bacilli existed), the FA composition presented significant similarities with the trials in which growth and lipid accumulation occurred in previously sterilized media (see and compare Tables 2 and 4A). In any case, lipid produced through the nonaseptic experiments was rich in the FA Δ9C18:1, constituting, thus, a perfect fatty material amenable to be converted into biodiesel 2, 22.
Table 4.
Fatty acid composition of cellular lipids of Rhodosporidium toroloides DSM 4444 growing in shake flasks in previously pasteurized media presenting increasing initial glucose (Glci) concentration and constant NaCl concentration (A) and fatty composition of lipids of the same microorganism growing in batch‐bioreactor experiments in media presenting increasing initial glucose (Glci) concentration and constant NaCl concentration (B)
| A) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Glci (g/L) | Growth phase | C14:0 | C16:0 | Δ9C16:1 | C18:0 | Δ9C18:1 | Δ9,12C18:2 | Δ9,12,15C18:3 |
| ≈50 | VE | 0.6 | 26.4 | 0.7 | 8.4 | 54.4 | 5.9 | 2.3 |
| L | 1.1 | 23.0 | 0.5 | 7.9 | 57.4 | 6.9 | 2.6 | |
| ≈100 | VE | 1.2 | 24.8 | — | 9.6 | 52.9 | 6.5 | 2.7 |
| L | 1.6 | 22.0 | 1.5 | 8.2 | 55.0 | 6.8 | 2.2 | |
| B) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Glci (g/L) | Growth phase | C14:0 | C16:0 | Δ9C16:1 | C18:0 | Δ9C18:1 | Δ9,12C18:2 | Δ9,12,15C18:3 |
| ≈50 | VE | 1.5 | 25.2 | — | 12.0 | 50.2 | 8.4 | 2.0 |
| E | 1.0 | 23.8 | 1.1 | 10.1 | 54.7 | 9.3 | — | |
| L | 1.2 | 22.1 | 1.5 | 10.8 | 55.7 | 6.5 | 1.4 | |
| ≈100 | VE | — | 23.8 | — | 12.2 | 53.7 | 6.7 | 1.5 |
| E | — | 22.1 | 2.5 | 11.9 | 55.4 | 5.8 | 2.0 | |
| L | — | 20.9 | 3.0 | 9.1 | 54.9 | 8.9 | 2.5 | |
In the flask experiments, for Glci≈50 g/L, very early (VE) growth phase is that in which the incubation time is between 10 and 20 h and late growth phase is that in which incubation time is c. 150 h. For Glci≈100 g/L, VE growth phase is that in which the incubation time is between 20 and 30 h and late growth phase is that in which incubation time is c. 300 h. In the bioreactor experiments, for Glci≈50 g/L, VE growth phase is that in which the incubation time is between 10 and 20 h, early (E) growth phase is that in which the incubation time is between 30 and 40 h and late growth phase is that in which incubation time is c. 70 h. For Glci≈100 g/L, VE growth phase is that in which the incubation time is between 20 and 30 h, early (E) growth phase is that in which the incubation time is between 50 and 80 h and late growth phase is that in which incubation time is c. 150 h.
3.3. Batch‐bioreactor cultures of R. toruloides
The next step in the experimental process involved the realization of batch‐bioreactor cultures of the yeast with increasing glucose concentrations and NaCl supplementation, aiming to promote, if possible, lipid production. Previously sterilized fermentation media containing c. 50, 100, and 150 g/L of glucose and 4.0% w/v NaCl were used in bench top bioreactor cultures. Table 3B summarizes the quantitative data of R. toruloides trials in bioreactor experiments. At 50 g/L of glucose, the strain exhibited rapid substrate assimilation within 72 h. Biomass production was notably enhanced, as opposed to the respective batch‐flask experiment, yielding 12.7 g/L with 8.1 g/L of oil. Increasing amounts of carbon source did not seem to drastically negatively affect the microbial metabolism, while in all cases, it is interesting to indicate that glucose was linearly consumed (Fig. 3). On the other hand, the more the Glci concentration (and, hence, the initial molar ratio C/N of the medium) increased, the more the glucose consumption rate decreased; for Glci adjusted at c. 50 g/L, rGlc was =0.62 g/L/h decreasing to 0.54 g/L/h for Glci≈100 g/L. Finally, at Glci≈150 g/L, rGlc value eventually dropped to 0.34 g/L/h (Fig. 3). During these trials, lipid accumulation process demonstrated remarkable stability; in accordance with the trial performed in shake flasks, as depicted in Fig. 3, the evolution of lipids’ kinetic profile was almost linear, regardless of the applied initial glucose concentration. Maximum biomass production was achieved at 150 g/L of glucose equal to 34.1 g/L, containing 65.1% w/w of oil. It is interesting to indicate that under the present culture conditions, growth was not inhibited by the increment of Glci concentration up to the threshold of 150 g/L; this assumption can be justified by the fact that the yields YX/Glc and YL/Glc presented their higher values at the trial in which the concentration of carbon substrate had been adjusted at c. 150 g/L (=0.33 and 0.21 g/g, respectively), being clearly the higher ones obtained in all of the previously performed trials (including fermentations in shake‐flasks or bioreactor). In addition, specifically the yield YL/Glc value obtained in the bioreactor experiment with Glci≈150 g/L (=0.21 g/g) was a value very close to the maximum achievable one of 0.22–0.23 g per g of consumed sugar that has been achieved so far in the international literature 1, 3, 11, 21, 23, 24, suggesting, once more, the absence of inhibitory phenomena of increased Glci concentrations upon the growth of R. toruloides under the present culture conditions.
Figure 3.
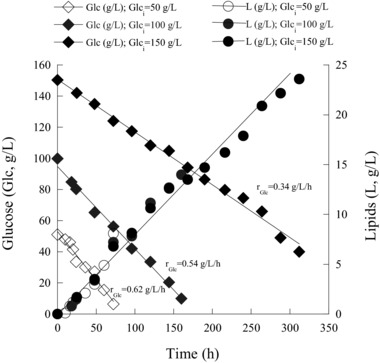
Kinetics of residual glucose (Glc; g/L) and lipid accumulated (L; g/L) during batch‐bioreactor cultures of Rhodosporidium toruloides DSM 4444 in media containing 50 g/L (open symbols), 100 g/L (gray symbols) and 150 g/L (filled symbols) of glucose, supplemented with 4.0% w/v of NaCl. Culture conditions as in Table 4B. Two lots of independent cultures were conducted by using different inocula. In all of the determinations, standard error calculated was less than 10%.
Table 4B shows the FA profiles of R. toruloides cellular lipids, during growth on bioreactor cultures in media presenting increasing Glci concentrations and constant NaCl quantity added. As in the shake‐flask trials, the predominant FA of the yeast was the Δ9C18:1, followed by the C16:0, C18:0, and Δ9,12C18:2. Moreover, as in the case of the trials with the increasing initial NaCl quantities into the medium, the increment of Glci concentration did not seem to have serious impact upon the FA composition of the strain, while cellular FAs were slightly more saturated at the beginning of the fermentation (Table 4B).
3.4. Fed‐batch bioreactor culture of R. toruloides
In an attempt to further investigate lipid production potential of the yeast R. toroloides and to reduce the time of the fermentation (as seen in the previous paragraph, the more the Glci concentration increased, the more the time of the fermentation rose) fed‐batch cultures were performed in bench top bioreactor, in media containing 4.0% w/v NaCl. Trials were initiated batch‐wise (Glci≈50 g/L) and when the glucose level dropped below 10 g/L, a volume of concentrated glucose solution was aseptically introduced to the culture. In every case, it was desirable to maintain the feeding of glucose to concentrations ≤50 g/L, in order to increase the uptake rate of glucose. In the first cycle of the fed‐batch operation (0–72 h), the rGlc was =0.63 g/L/h and the respective specific glucose consumption rate was ≈0.1 g/g/h. In the second cycle (72–192 h), rGlc was ≈0.40 g/L/h and qGlc was ≈0.06 g/g/h. In the third cycle (192–272 h) rGlc was =0.51 g/L/h and qGlc was ≈0.08 g/g/h. During 272 h of the fermentation, feeding pulses were done twice resulting in the total consumption of c. 127 g/L of glucose (Fig. 4A). Maximum biomass achieved was 37.2 g/L with 64.5% w/w of accumulated oil. (Lmax≈24 g/L). Furthermore, overall yields for lipid and biomass production per consumed substrate in fed‐batch process were 0.21 and 0.33 g/g, respectively (Fig. 4B). Compared to the batch‐bioreactor cultures with high initial glucose concentration (Glci≈150 g/L), both biomass formation and lipid accumulation were slightly improved during the fed‐batch culture mode, whereas the fermentation was accomplished more rapidly in the later case than in the former one, thus the lipid volumetric productivity achieved in the fed‐batch experiment was improved compared with the batch process presenting high Glci concentration (0.088 against 0.075 g/L/h).
Figure 4.
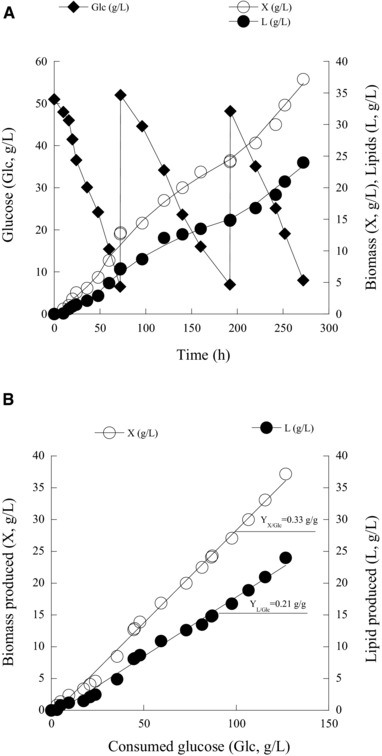
Kinetics of residual glucose (Glc; g/L), biomass production (X; g/L) and lipid accumulation (L; g/L) (A) and representation of biomass production (X; g/L) and lipid production (L; g/L) per substrate consumed (B) during fed‐batch bioreactor cultures of Rhodosporidium toruloides DSM 4444, in media supplemented with 4.0% w/v NaCl. Culture conditions as in Table 4B. Two lots of independent cultures were conducted by using different inocula. In all of the determinations, standard error calculated was less than 10%.
4. Discussion
The oleaginous nature of R. toruloides has been a topic of interest for many studies in the international literature. Origin of carbon or nitrogen sources, nutrient limitation, and feeding strategy has been assessed for the optimization of SCO production by the particular yeast. Lipid accumulation by R. toruloides has been shown to improve in the presence of organic nitrogen sources 25, 26. Although nitrogen limitation has long been recognized as a determinant factor for de novo lipid synthesis in oleaginous microorganisms 1, 2, 3, 4, 5, phosphorus‐ and sulfate‐limitation conditions have been also investigated as lipid inducing factors for R. toruloides strains 26, 27, 28. In terms of carbon sources, strains of the particular yeasts are reported to withstand carbon‐rich media and under certain conditions, to promote high density cell cultures 20. On the other hand, in earlier studies, strains of R. toruloides were flask‐cultured in media composed of pure stearic acid or blends of pure stearic acid, glucose, and glycerol and tailor‐made lipids presenting similarities with the cocoa‐butter were synthesized 8, 29. Equally in early studies, strains of this species had been cultivated on glucose‐based media in which Δ9 and Δ12 natural or artificial desaturase inhibitors had been added into the culture medium in order to suppress the desaturation reactions inside R. toruloides cells, so as finally, again to synthesize lipids presenting compositional similarities with the cocoa‐butter 30, 31. More recently Zhu et al. 32 have carried out a massive study based on genomic sequencing of R. toruloides, in an attempt to unravel lipid accumulation process on a genetic level, spent cell mass hydrolysates used as nutrients and spent water from lipid production process were used as substrates by R. toruloides for SCO production 33 while other species belonging to the genus Rhodosporidium (e.g. R. kratochvilovae, R. babjevae, R. diobovatum) have been successfully used as cell factories used for SCO on several waste streams or low‐cost materials 34, 35, 36.
One major objective of the study was to identify the effect of NaCl addition into the culture medium upon the process of lipid accumulation of R. toruloides DSM 4444. To this end, batch‐flask cultures of the microorganism were performed in media containing increasing amounts of NaCl up to 6.0% w/v. The particular strain can be categorized as halotolerant, due to its ability to grow sufficient in the presence or absence of salt 13, 37. Furthermore, NaCl concentrations up to 4.0% w/v were found to promote optimum growth of the yeast and thus, according to Larsen 38 the strain can be designated as moderate halotolerant. This feature is commonly encountered in microorganisms isolated from marine environments, possessing unique adaptation mechanisms in high salinity conditions 13, 39, 40, 41, 42. A major part of the mechanisms involved in osmotic balance regulation for halotolerant microorganisms represents the production and accumulation of solutes, such as glycerol, trehalose, mannitol, and erythritol, etc. 43. In the current investigation and after a threshold NaCl value, low (but not negligible) glycerol quantities were found into the medium, apparently as response to the osmotic stress imposed. Under this optic, the last years there has been a number of reports in which the polymorphic yeast Y. lipolytica has been cultivated in media composed of high initial concentrations of (pure or biodiesel‐derived) glycerol supplemented with increased initial concentrations of NaCl, and enhancement of production of erythritol, the concentration of which in some cases reached in indeed very high levels (e.g. >45 g/L or even c. 200 g/L) has been reported when glycerol has been employed as fermentation substrate by wild or mutant Y. lipolytica strains 44, 45, 46, 47. Likewise, besides mannitol and erythritol, due to their high osmotic tolerance, halotolerant yeasts have been proposed as candidates for bioethanol production 48, enzyme production 49 as well as xylitol biosynthesis 50. However, none of these microorganisms has ever been reported as oleaginous. Surprisingly enough, in the current study the addition of NaCl not only did not suppress microbial growth, but on the contrary was found to enhance lipid accumulation, as such was the case in media containing 4.0% w/v NaCl. Under these conditions, lipid accumulation increased to c. 29% compared to the control experiment (see Table 1). Positive correlation between salt and lipid production has been shown for oleaginous microalgae strains belonging to the genera of Dunaliella sp. and Nannochloropsis sp. 51, 52.
Another aspect of the study was the realization of microbial lipid production in the presence of NaCl under pasteurized conditions. In batch‐flask trials with 50 or 100 g/L of initial glucose concentration, signs of bacterial contamination were noted, despite the use of a more concentrated inoculum. However, bacterial presence was less than 5% of the total microbial population and was not found to be detrimental for yeast growth and lipid formation. In the case of microbial conversions, pasteurized grape must has been used for alcoholic fermentation 53, while the effect of pasteurized whey‐based medium on propionic acid production has also been evaluated 54. Additionally, the application of completely nonaseptic conditions has been tested for microbial solvent production such as ethanol and 1,3‐propanediol 55, 56, 57, 58, as means of energy and operation cost reduction. However, in all of the above‐mentioned cases (e.g. production of bio‐ethanol and 1,3‐propanediol), it was the main metabolic compound‐target (the bio‐alcohol) that was synthesized rapidly and in high concentrations that hindered the microbial contamination with undesirable microorganisms, fact that does not happen during the SCO fermentation. As far as the SCO bioprocess is concerned, in order to perform a relatively successful nonaseptic culture, a “hurdle” should be added into the medium; i.e. Moustogianni et al. 59 have successfully produced SCO by using oleaginous Zygomycetes grown on glycerol when essential oils or antibiotics had previously been added into the fermentation medium. In any case, the current study is one of the first in the literature that deals with the production of SCO under nonaseptic conditions.
During batch‐bioreactor trials, the yeast R. toruloides exhibited notable tolerance against high substrate concentrations. The particular strain grew satisfactorily without obvious substrate inhibition being exerted in media containing up to 150 g/L of glucose, while of importance was the almost linear profile of lipid accumulation, regardless of the initial substrate concentration employed into the medium. Tolerance against high substrate (e.g. sugar or glycerol) input is essential, in order to achieve high‐density cultures that have been proved as effective cultivation strategy in the case of microbial lipid production. In a similar manner, Li et al. 14 demonstrated that the yeast R. toruloides Y4 grew well in media containing glucose up to 150 g/L, a fact directly correlated with the tolerance of the yeast against osmotic stress. On the other hand, for several oleaginous yeasts, substrate (e.g. sugar or glycerol) concentrations at c. 100 g/L or even lower (e.g. 60–70 g/L) have been reported as threshold, above which microbial cell growth was repressed significantly 60, 61, 62. In contrast, oleaginous fungi seem more resistant in high initial substrate (sugar or glycerol) quantities, since initial concentrations ranging between 80 and 100 g/L have been considered as ideal in order to enhance the process of lipid accumulation for the species Mortierella isabellina, Thamnidium elegans, and Cunninghamella echinulata 21, 24, 63, 64, 65.
R. toruloides DSM 4444 presented remarkable cell growth and lipid production in both shake‐flask and bioreactor experiments. As indicated in the previous paragraphs, strains of this particular species have been employed already from early studies in order for SCO production to be performed. Initially (mid 80s) these strains had been employed as microbial cell factories in order to produce lipids that mimicked the composition of cocoa‐butter 2, 4, 8, 29, 30, 31. The last decade, with the potentiality of the replacement of edible oils by nonconventional fatty substances (e.g. yeast oils) as starting materials in order for biodiesel precursors to be synthesized has been assessed, R. toruloides has been considered as a microorganism of importance amenable to be used in the conversion of low‐cost hydrophilic materials (e.g. lignocellulosic sugars, glucose, sorghum extracts, glycerol, etc.) into SCOs. Characteristic results concerning production of lipid in several fermentation configurations are depicted in Table 5.
Table 5.
Experimental results of Rhodosporidium toruloides strains producing microbial lipid during growth on several carbon sources under various fermentation configurations and their comparisons with the present study
| Strain | Substrate | Culture mode | X (g/L) | YL/X | Reference |
|---|---|---|---|---|---|
| (% w/w) | |||||
| R. toruloides a | Pure stearic acid | Shake flasks | 11.7 | 35.0 | Gierhart 8 |
| R. toruloides AS2.1389 | Glycerol | Shake flasks | 19.2 | 47.7 | Xu et al. 9 |
| R. toruloides AS2.1389 | Glycerol | Batch bioreactor | 26.7 | 69.5 | ” |
| R. toruloides AS2.1389 | Glucose | Shake flasks | 18.3 | 76.0 | Li et al. 14 |
| R. toruloides CCT 0783 | Sweet sorghum extract | Shake flasks | 41.7 | 33.1 | Matsakas et al. 15 |
| R. toruloides DSM 444 | Glycerol/sunflower meal hydrolysate blend | Shake flasks | 27.9 | 29.0 | Leiva‐Candia et al. 18 |
| R. toruloides DSM 444 | Glycerol/sunflower meal hydrolysate blend | Fed‐batch bioreactor | 37.4 | 51.3 | ” |
| R. toruloides NRRL Y‐27012 | Biodiesel‐derived glycerol | Shake flasks | 30.1 | 40.0 | Tchakouteu et al. 19 |
| R. toruloides Y4 | Glucose | Fed‐batch bioreactor | 106.5 | 67.5 | Li et al. 20 |
| R. toruloides Y4 | Glucose (phosphate‐limited trial) | Shake flasks | 20.6 | 51.4 | Wu et al. 27 |
| R. toruloides Y4 | Glucose (phosphate‐limited trial) | Shake flasks | 19.4 | 62.4 | ” |
| R. toruloides Y4 | Glucose (sulphate‐limited trial) | Shake flasks | 23.0 | 20.8 | Wu et al. 28 |
| R. toruloides Y4 | Glucose (sulfate‐limited trial) | Shake flasks | 14.2 | 55.6 | ” |
| R. toruloides Y4 | Glucose (sulfate‐limited trial) | Shake flasks | 17.8 | 55.7 | ” |
| R. toruloides a) | Glucose/pure stearic acid | Shake flasks | 9.8 | 46.1 | Gierhart 29 |
| R. toruloides CBS14 | Glucose | Shake flasks | 12.3 | 30.8 | Moreton 30 |
| R. toruloides CBS14 | Glucose | Shake flasks | 8.0 | 42.5 | Moreton 66 |
| R. toruloides CBS14 | Fructose | Shake flasks | 7.9 | 25.3 | ” |
| R. toruloides CBS14 | Glycerol | Shake flasks | 5.8 | 34.6 | ” |
| R. toruloides CBS14 | Glucose | Batch bioreactor | 12.5 | 42.9 | ” |
| R. toruloides CBS14 | Fructose | Batch bioreactor | 8.7 | 39.8 | ” |
| R. toruloides CBS14 | Xylose | Batch bioreactor | 8.3 | 42.2 | ” |
| R. toruloides Y4 | Jerusalem artichoke extracts | Shake flasks | 25.5 | 40.0 | Zhao et al. 67 |
| R. toruloides Y4 | Jerusalem artichoke hydrolysates | Fed‐batch bioreactor | 70.1 | 56.5 | ” |
| R. toruloides CBS14 | Glucose | Fed‐batch bioreactor | 35.0 | 71.4 | Wiebe et al. 68 |
| R. toruloides CBS14 | Glucose/xylose/arabinose blend | Fed‐batch bioreactor | 27.0 | 55.5 | ” |
| R. toruloides Y4 | Biodiesel‐derived glycerol | Batch bioreactor | 35.3 | 46.0 | Uçkun Kiran et al. 69 |
| R. toruloides 2F5 | Inulin | Shake‐flasks | 15.8 | 62.1 | Wang et al. 70 |
| R. toruloides 2F5 | Inulin | Batch bioreactor | 15.6 | 70.4 | ” |
| R. toruloides CCT 0783 | Glucose/xylose blend | Batch bioreactor | 13.3 | 42.0 | Bonturi et al. 71 |
| R. toruloides DSM 444 | Glucose | Batch bioreactor | ∼22 | ∼40 | Bommareddy et al. 72 |
| R. toruloides DSM 444 | Glycerol | Batch bioreactor | ∼15 | ∼57 | ” |
| R. toruloides AS2.1389 | Glucose | Single‐stage continuous bioreactor | 8.7 | 61.8 | Shen et al. 73 |
| R. toruloides AS2.1389 | Glucose | Single‐stage continuous bioreactor | 5.7 | 53.1 | ” |
| Rhodosporidium toruloides Y4 | Biodiesel‐derived glycerol | Shake flasks | 24.9 | 48.9 | Yang et al. 74 |
| R. toruloides DSM 444 | Glucose (NaCl‐enriched culture) | Fed‐batch bioreactor | 37.2 | 64.5 | Present study |
No indication of the strain.
In the current investigation and specifically in the bioreactor experiments, conversion yields of the order of c. 0.21 g of lipid produced per g of glucose consumed have been seen. The stoichiometry of glucose (and similar sugars such as lactose and fructose) metabolism indicates that about 1.1 moles of acetyl‐CoA are generated from 100 g of glucose 1, 3, 5. Assuming that all the acetyl‐CoA produced is used for lipid synthesis, the maximum theoretical yield of SCO produced per glucose consumed is around 0.32 g/g 3, 5. This value ranges between 0.31 and 0.34 g/g in the case of xylose and is around 0.30 g/g in the case of glycerol 1, 3. However, even under ideal conditions for SCO production (e.g. highly aerated bioreactor cultures), lipid yield on glucose consumed rarely reaches values higher than 0.22–0.23 g/g 5, 11, 23. It may be assumed therefore that in the current investigation one of the highest conversion yields of SCO produced per sugar consumed has been achieved. As previously indicated 5, 23 cultivation in highly aerated bioreactors was considered as an important prerequisite in order to achieve such high conversion yields. However, in some cases, in shake‐flask experiments equally high lipid yields can be achieved; in trials with T. elegans grown on sucrose in shake flasks, the conversion yield of lipid produced per sugar consumed was c. 0.24 g/g, while utilization of other sugars (glucose or fructose) equally resulted in exceptional conversion yields, i.e. >0.20 g/g 24. Likewise, maximum conversion yields of the same magnitude compared with growth of T. elegans on sucrose (c. 0.23 g/g) have been reported for Cunninghamella echinulata cultivated on xylose in shake‐flask experiments 21.
Fatty acid analysis was carried out in lipids produced during R. toruloides batch‐flask cultures with increasing NaCl content. The main fatty acids detected in yeast oil composition were oleic (Δ9C18:1), palmitic (C16:0), linoleic (Δ9,12C18:2) as well as stearic acid (C18:0). NaCl presence did not affect the concentration of individual fatty acids, whereas microbial lipid became generally more unsaturated, during lipid accumulation phase. This is attributed to the increase of oleic acid as major constituent of accumulated triglycerides 61. The distribution of R. toruloides fatty acids is similar to oil profiles obtained by other oleaginous yeasts 10, 17, 18, 19, 75. Recent studies have shown the suitability of microbial oil as starting material for biodiesel production, through its direct transformation from microbial biomass 21.
Practical application
The yeast strain Rhodosporidium toruloides DSM 4444 was found capable of producing sufficient lipid amounts when grown in media supplemented with NaCl. This particular feature of the strain, combined with its tolerance against relatively high amounts of glucose, could denote the feasibility of microbial oil production without the need of stringent sterile conditions. The utilization of agro‐industrial residues such as salty or brackish waste‐water streams deriving from fisheries or olives production establishments as fermentation media could reduce the cost of microbial oil production by the particular yeast, whereas waste bio‐remediation could offer an additional environmental benefit in the process. Likewise, the accomplishment of microbial lipid fermentation by this strain in substrates supplemented with NaCl and without previous sterilization of the culture medium can further reduce the cost of the bioprocess.
The authors have declared no conflict of interest.
Acknowledgments
(1) State Scholarship Foundation (Athens ‐ Greece) is gratefully acknowledged for the scholarship attributed to Sidoine Sadjeu Tchakouteu; (2) Financial support was attributed by the research project entitled “Development of novel bioprocesses for the production of biofuels from food industry waste streams” (Acronym: “Nutri‐Fuel”) (09SYN‐32‐621), implemented within the National Strategic Reference Framework (NSRF) 2007–2013 and cofinanced by National (Greek Ministry of Higher Education and Religious Affairs—General Secretariat of Research and Technology) and Community (E.U.—European Social Fund) Funds.
5 References
- 1. Papanikolaou, S. , Aggelis, G. Lipids of oleaginous yeasts. Part I: Biochemistry of single cell oil production. Eur. J. Lipid Sci. Technol. 2011, 113, 1031–1051. [Google Scholar]
- 2. Papanikolaou, S. , Aggelis, G. Lipids of oleaginous yeasts. Part II: Technology and potential applications. Eur. J. Lipid Sci. Technol. 2011, 113, 1052–1073. [Google Scholar]
- 3. Ratledge, C. , Biochemistry, stoichiometry, substrates and economics, in: Moreton R. S. (Ed.), Single Cell Oil, Longman Scientific &Technical, Harlow, UK: 1988, pp. 33–70. [Google Scholar]
- 4. Papanikolaou, S. , Aggelis, G. , Yarrowia lipolytica: A model microorganism used for the production of tailor‐made lipids. Eur. J. Lipid Sci. Technol. 2010, 112, 639–654. [Google Scholar]
- 5. Ratledge, C. , Wynn, J. , The biochemistry and molecular biology of lipid accumulation in oleaginous microorganisms. Adv. Appl. Microbiol. 2002, 51, 1–51. [DOI] [PubMed] [Google Scholar]
- 6. Sarris, D. , Papanikolaou, S. , Biotechnological production of ethanol: Biochemistry, processes and technologies. Eng. Life Sci. 2016, in press DOI: 10.1002/elsc.201400199. [DOI] [Google Scholar]
- 7. Papanikolaou, S. , Chevalot, I. , Komaitis, M. , Aggelis, G. , Marc, I. , Kinetic profile of the cellular lipid composition in an oleaginous Yarrowia lipolytica capable of producing a cocoa‐butter substitute from industrial fats. Antonie Van Leeuwenhoek 2001, 80, 215–224. [DOI] [PubMed] [Google Scholar]
- 8. Gierhart, D. I. , US Patent 4485, 173, 1984.
- 9. Xu, J. , Zhao, X. , Wang, W. , Du, W. , Liu, D. , Microbial conversion of biodiesel byproduct glycerol to triacylglycerols by oleaginous yeast Rhodosporidium toruloides and the individual effect of some impurities on lipid production. Biochem. Eng. J. 2012, 65, 30–36. [Google Scholar]
- 10. Sestric, R. , Munch, G. , Cicek, N. , Sparling, R. , Levin, D. B. , Growth and neutral lipid synthesis by Yarrowia lipolytica on various carbon substrates under nutrient‐sufficient and nutrient‐limited conditions. Bioresour. Technol. 2014, 164, 41–46. [DOI] [PubMed] [Google Scholar]
- 11. Ykema, A. , Verbree, E. C. , Kater, M. M. , Smit, H. , Optimization of lipid production in the oleaginous yeast Apiotrichum curvatum in whey permeate. Appl. Microbiol. Biotechnol. 1988, 29, 211–218. [Google Scholar]
- 12. Koutinas, A. , Chatzifragkou, A. , Kopsahelis, N. , Papanikolaou, S. , Kookos, I. K. , Design and techno‐economic evaluation of microbial oil production as a renewable resource for biodiesel and oleochemical production. Fuel 2014, 116, 566–577. [Google Scholar]
- 13. Butinar, L. , Santos, S. , Spencer‐Martins, I. , Oren, A. , Gunde‐Cimerman, N. , Yeast diversity in hypersaline habitats. FEMS Microbiol. Lett. 2005, 244, 229–234. [DOI] [PubMed] [Google Scholar]
- 14. Li, Y.H. , Liu, Bo. , Zhao, Z.B. , Bai, F.W. , Optimization of culture conditions for lipid production by Rhodosporidium toruloides . Chin. J. Biotechnol. 2006, 22, 650–656. [PubMed] [Google Scholar]
- 15. Matsakas, L. , Bonturi, N. , Alves Miranda, E. , Rova, U. , Christakopoulos, P. , High concentrations of dried sorghum stalks as a biomass feedstock for single cell oil production by Rhodosporidium toruloides . Biotechnol. Biofuel. 2015, 8, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Galafassi, S. , Cucchetti, D. , Pizza, F. , Franzosi, G. et al., Lipid production for second generation biodiesel by the oleaginous yeast Rhodotorula graminis . Bioresour. Technol. 2012, 111, 398–403. [DOI] [PubMed] [Google Scholar]
- 17. Tsakona, S. , Kopsahelis, N. , Chatzifragkou, A. , Papanikolaou, S. et al., Formulation of fermentation media from flour‐rich waste streams for microbial lipid production by Lipomyces starkeyi . J. Biotechnol. 2014, 189, 36–45. [DOI] [PubMed] [Google Scholar]
- 18. Leiva‐Candia, D. E. , Tsakona, S. , Kopsahelis, N. , García, I. L. et al., Biorefining of by‐product streams from sunflower‐based biodiesel production plants for integrated synthesis of microbial oil and value‐added co‐products. Bioresour. Technol. 2015, 190, 57–65. [DOI] [PubMed] [Google Scholar]
- 19. Tchakouteu, S. S. , Kalantzi, O. , Gardeli, C. , Koutinas, A. A. et al., Lipid production by yeasts growing on biodiesel‐derived crude glycerol: Strain selection and impact of substrate concentration on the fermentation efficiency. J. Appl. Microbiol. 2015, 118, 911–927. [DOI] [PubMed] [Google Scholar]
- 20. Li, Y. H. , Zhao, Z. B. , Bai, F. W. , High‐density cultivation of oleaginous yeast Rhodosporidium toruloides Y4 in fed‐batch culture. Enzyme Microb. Technol. 2007, 41, 312–317. [Google Scholar]
- 21. Fakas, S. , Papanikolaou, S. , Batsos, A. , Galiotou Panayotou, M. et al., Evaluating renewable carbon sources as substrates for single cell oil production by Cunninghamella echinulata and Mortierella isabellina . Biomass Bioenerg. 2009, 33, 573–580. [Google Scholar]
- 22. Papanikolaou, S. , Aggelis, G. , Biotechnological valorization of biodiesel derived glycerol waste through production of single cell oil and citric acid by Yarrowia lipolytica . Lipid Technol. 2009, 21, 83–87. [Google Scholar]
- 23. Ratledge, C. , Cohen, Z. , Microbial and algal lipids: Do they have a future for biodiesel or as commodity oils? Lipid Technol. 2008, 20, 155–160. [Google Scholar]
- 24. Papanikolaou, S. , Diamantopoulou, P. , Chatzifragkou, A. , Philippoussis, A. , Aggelis, G. , Suitability of low‐cost sugars as substrates for lipid production by the fungus Thamnidium elegans . Energy Fuel. 2010, 24, 4078–4086. [Google Scholar]
- 25. Evans, C.T. , Ratledge, C. , Influence of nitrogen metabolism on lipid accumulation in oleaginous yeasts. J. Gen. Microbiol. 1984, 130, 1693–1704. [Google Scholar]
- 26. Evans, C. T. , Ratledge, C. , Influence of nitrogen metabolism on lipid accumulation by Rhodosporidium toruloides CBS 14. J. Gen. Microbiol. 1984, 130, 1705–1710. [Google Scholar]
- 27. Wu, S. , Hu, C. , Jin, C. , Zhao, X. , Zhao, Z. B. , Phosphate‐limitation mediated lipid production by Rhodosporidium toruloides . Bioresour. Technol. 2010, 101, 6124–6129. [DOI] [PubMed] [Google Scholar]
- 28. Wu, S. , Zhao, X. , Shen, H. , Wang, Q. , Zhao. Z. B. , Microbial lipid production by Rhodosporidium toruloides under sulfate‐limited conditions. Bioresour. Technol. 2011, 102, 1803–1807. [DOI] [PubMed] [Google Scholar]
- 29. Gierhart, D. I. , US Patent 4485, 172, 1984.
- 30. Moreton, R. S. , Modification of fatty acid composition of lipid accumulating yeasts with cyclopropene fatty acid desaturase inhibitors. Appl. Microbiol. Biotechnol. 1985, 22, 41–45. [Google Scholar]
- 31. Moreton, R.S. , Clode, M. , US Patent 4778 630, 1988.
- 32. Zhu, Z. , Zhang, S. , Liu, H. , Shen, H. et al., A multi‐omic map of the lipid‐producing yeast Rhodosporidium toruloides . Nat. Commun. 2012, 3, 1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang, X. , Jin, G. , Gong, Z. , Shen, H. et al., Recycling microbial lipid production wastes to cultivate oleaginous yeasts. Bioresour. Technol. 2015, 175, 91–96. [DOI] [PubMed] [Google Scholar]
- 34. Patel, A. , Pravez, M. , Deeba, F. , Pruthi, V. et al., Boosting accumulation of neutral lipids in Rhodosporidium kratochvilovae HIMPA1 grown on hemp (Cannabis sativa Linn) seed aqueous extract as feedstock for biodiesel production. Bioresour. Technol. 2014, 165, 214–222. [DOI] [PubMed] [Google Scholar]
- 35. Patel, A. , Sindhu, D. K. , Arora, N. , Singh, R. P. et al., Biodiesel production from non‐edible lignocellulosic biomass of Cassia fistula L. fruit pulp using oleaginous yeast Rhodosporidium kratochvilovae HIMPA1. Bioresour. Technol. 2015, 197, 91–98. [DOI] [PubMed] [Google Scholar]
- 36. Munch, G. , Sestric, R. , Sparling, R. , Levin, D. B. , Cicek, N. , Lipid production in the under‐characterized oleaginous yeasts, Rhodosporidium babjevae and Rhodosporidium diobovatum, from biodiesel‐derived waste glycerol. Bioresour. Technol. 2015, 185, 49–55. [DOI] [PubMed] [Google Scholar]
- 37. Margesin, R. , Schinner, F. , Biodegradation and bioremediation of hydrocarbons in extreme environments. Appl. Microbiol. Biotechnol. 2001, 56, 650–663. [DOI] [PubMed] [Google Scholar]
- 38. Larsen, H. , Halophilic and halotolerent microorganisms: An overview historical perspective. FEMS Microbiol. Rev. 1986, 39, 3–7. [Google Scholar]
- 39. Zaky, A. S. , Tucker, G. A. , Daw, Z. Y. , Du, C. , Marine yeast isolation and industrial application. FEMS Yeast Res. 2014, 14, 813–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kutty, S. N. , Philip, R. , Marine yeasts—a review. Yeast 2008, 25, 465–483. [DOI] [PubMed] [Google Scholar]
- 41. Prista C., Loureiro‐Dias M. C., Montiel, V. , García, R. , Ramos, J. , Mechanisms underlying the halotolerant way of Debaryomyces hansenii . FEMS Yeast Res. 2005, 5, 693–701. [DOI] [PubMed] [Google Scholar]
- 42. Rengpipat, S. , Lowe, S. E. , Zeikus J. K., Effect of extreme salt concentrations on the physiology and biochemistry of Halobacteroides acetoethylicus . J. Bacteriol. 1988, 170, 3065–3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Breuer, U. , Harms, H. , Debaryomyces hansenii‐an extremophilic yeast with biotechnological potential. Yeast 2006, 23, 415–437. [DOI] [PubMed] [Google Scholar]
- 44. Tomaszewska, L. , Rywińska, A. , Gładkowski, W. , Production of erythritol and mannitol by Yarrowia lipolytica yeast in media containing glycerol. J. Ind. Microbiol. Biotechnol. 2012, 39, 1333–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tomaszewska, L. , Rakicka, M. , Rymowicz, W. , Rywinska, A. , A comparative study on glycerol metabolism to erythritol and citric acid in Yarrowia lipolytica yeast cells. FEMS Yeast Res. 2014, 14, 966–976. [DOI] [PubMed] [Google Scholar]
- 46. Rywińska, A. , Juszczyk, P. , Wojtatowicz, M. , Robak, M. et al., Glycerol as a promising substrate for Yarrowia lipolytica biotechnological applications. Biomass Bioenerg. 2013, 48, 148–166. [Google Scholar]
- 47. Rywińska, A. , Tomaszewska, L. , Rymowicz, W. , Erythritol biosynthesis by Yarrowia lipolytica yeast under various culture conditions. Afr. J. Microbiol. Res. 2013, 7, 3511–3516. [Google Scholar]
- 48. Obara, N. , Ishida, M. , Hamada‐Sato, N. , Urano, N. , Efficient bioethanol production from scrap paper shredder by a marine Saccharomyces cerevisiae derived C‐19. Studies Sci. Technol. 2012, 1, 127–132. [Google Scholar]
- 49. Chen, L. , Chi, Z. M. , Chi, Z. , Li, M. , Amylase production by Saccharomycopsis fibuligera A11 in solid‐state fermentation for hydrolysis of Cassava starch. Appl. Biochem. Biotechnol. 2010, 162, 252–263. [DOI] [PubMed] [Google Scholar]
- 50. Misra, S. , Raghuwanshi, S. , Saxena, R. K. , Fermentation behavior of an osmotolerant yeast D. hansenii for xylitol production. Biotechnol. Prog. 2012, 28, 1457–1465. [DOI] [PubMed] [Google Scholar]
- 51. Takagi, M. , Karseno, and Yoshida, T. , Effect of salt concentration on intracellular accumulation of lipids and triacylglyceride in marine microalgae Dunaliella cells. J. Biosci. Bioeng. 2006, 101, 223–226. [DOI] [PubMed] [Google Scholar]
- 52. Bartley, M. L. , Boeing, W. J. , Corcoran, A. A. , Holguin, F.O. , Schaub, T. , Effects of salinity on growth and lipid accumulation of biofuel microalga Nannochloropsis salina and invading organisms. Biomass Bioenerg. 2013, 54, 83–88. [Google Scholar]
- 53. Sarris, D. , Kotseridis, Y. , Linga, M. , Galiotou‐Panayotou, M. , Papanikolaou, S. , Enhanced ethanol production, volatile compound biosynthesis and fungicide removal during growth of a newly isolated Saccharomyces cerevisiae strain on enriched pasteurized grape musts. Eng. Life Sci. 2009, 9, 29–37. [Google Scholar]
- 54. Anderson, T. M. , Bodie, E. A. , Goodman, N. , Schwartz, R. D. , Inhibitory effect of autoclaving whey‐based medium on propionic acid production by Propionibacterium shermanii . Appl. Environ. Microbiol. 1986, 51, 427–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chatzifragkou, A. , Papanikolaou, S. , Dietz, D. , Doulgeraki, A. I. et al., Production of 1,3‐propanediol by Clostridium butyricum growing on biodiesel‐derived crude glycerol through a non‐sterilized fermentation process. Appl. Microbiol. Biotechnol. 2011, 91, 101–112. [DOI] [PubMed] [Google Scholar]
- 56. Metsoviti, M. , Zeng, A. P. , Koutinas A. A., Papanikolaou S., Enhanced 1,3‐propanediol production by a newly isolated Citrobacter freundii strain cultivated on biodiesel‐derived waste glycerol through sterile and non‐sterile bioprocesses J. Biotechnol. 2013, 163, 408–418. [DOI] [PubMed] [Google Scholar]
- 57. Dietz, D. , Zeng, A. P. Efficient production of 1,3‐propanediol from fermentation of crude glycerol with mixed cultures in a simple medium. Bioproc. Biosyst. Eng. 2014, 37, 225–233. [DOI] [PubMed] [Google Scholar]
- 58. Sarris, D. , Matsakas, L. , Aggelis, G. , Koutinas, A. A. , Papanikolaou, S. , Aerated vs non‐aerated conversions of molasses and olive mill wastewaters blends into bioethanol by Saccharomyces cerevisiae under non‐aseptic conditions. Ind. Crops Prod. 2014, 56, 83–93. [Google Scholar]
- 59. Moustogianni, A. , Bellou, S. , Triantaphyllidou, I. E. , Aggelis, G. , Feasibility of raw glycerol conversion into single cell oil by zygomycetes under non‐aseptic conditions. Biotechnol. Bioeng. 2015, 112, 827–831. [DOI] [PubMed] [Google Scholar]
- 60. Zhang, J. , Fang, X. , Zhu, X. L. , Li, Y. et al., Microbial lipid production by the oleaginous yeast Cryptococcus curvatus O3 grown in fed‐batch culture. Biomass Bioenerg. 2011, 35, 1906–1911. [Google Scholar]
- 61. Meesters P. A. E. P, Huijiberts, G. N. M. , Eggink, G. Hight‐cell‐density cultivation of the lipid accumulating yeast Cryptococcus curvatus using glycerol as a carbon source. Appl. Microbiol. Biotechnol. 1996, 45, 575–579. [Google Scholar]
- 62. Liang, Y. N. , Cui, Y. , Trushenski, J. , Blackburn, J. W. , Converting crude glycerol derived from yellow grease to lipids through yeast fermentation. Bioresour. Technol. 2010, 101, 7581–7586. [DOI] [PubMed] [Google Scholar]
- 63. Papanikolaou, S. , Komaitis, M. , Aggelis, G. , Single cell oil (SCO) production by Mortierella isabellina grown on high‐sugar content media. Bioresour. Technol. 2004, 95, 287–291. [DOI] [PubMed] [Google Scholar]
- 64. Chatzifragkou, A. , Makri, A. , Belka, A. , Bellou, S. et al., Biotechnological conversions of biodiesel derived waste glycerol by yeast and fungal species. Energy 2011, 36, 1097–1108. [Google Scholar]
- 65. Zikou, E. , Chatzifragkou, A. , Koutinas, A. A. , Papanikolaou, S. , Evaluating glucose and xylose as cosubstrates for lipid accumulation and γ‐linolenic acid biosynthesis of Thamnidium elegans . J. Appl. Microbiol. 2013, 114, 1020–1032. [DOI] [PubMed] [Google Scholar]
- 66. Moreton, R.S. , Physiology of lipid accumulating yeasts, in: Moreton R.S. (Ed.), Single Cell Oil, Longman Scientific &Technical, Harlow, UK: 1988, pp. 1–32. [Google Scholar]
- 67. Zhao, X. , Wu, S. , Hu, C. , Wang, Q. et al., Lipid production from Jerusalem artichoke by Rhodosporidium toruloides Y4. J. Ind. Microbiol. Biotechnol. 2010, 37, 581–585. [DOI] [PubMed] [Google Scholar]
- 68. Wiebe, M. G , Koivuranta, K. , Penttilä, M. , Ruohonen, L. , Lipid production in batch and fed‐batch cultures of Rhodosporidium toruloides from 5 and 6 carbon carbohydrates. BMC Biotechnol. 2012, 12, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Uçkun Kiran, E. , Trzcinski, A. , Webb, C. , Microbial oil produced from biodiesel by‐products could enhance overall production. Bioresour. Technol. 2013, 129, 650–654. [DOI] [PubMed] [Google Scholar]
- 70. Wang, Z. P. , Fu, W. J. , Xu, H. M. , Chi, Z. M. , Direct conversion of inulin into cell lipid by an inulinase‐producing yeast Rhodosporidium toruloides 2F5. Bioresour. Technol. 2014, 161, 131–136. [DOI] [PubMed] [Google Scholar]
- 71. Bonturi, N. , Matsakas, L. , Nilsson, R. , Christakopoulos, P. et al., Single Cell Oil producing yeasts Lipomyces starkeyi and Rhodosporidium toruloides: Selection of extraction strategies and biodiesel property prediction. Energies 2015, 8, 5040–5052. [Google Scholar]
- 72. Bommareddy, R. R. , Sabra, W. , Maheshwari, G. , Zeng, A. P. , Metabolic network analysis and experimental study of lipid production in Rhodosporidium toruloides grown on single and mixed substrates. Microb. Cell Fact. 2015, 14, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Shen, H. , Gong, Z. , Yang, X. , Jin, J. et al., Kinetics of continuous cultivation of the oleaginous yeast Rhodosporidium toruloides . J. Biotechnol. 2013, 168, 85–89. [DOI] [PubMed] [Google Scholar]
- 74. Yang, X. , Jin, G. , Gong, Z. , Shen, H. et al., Recycling biodiesel‐derived glycerol by the oleaginous yeast Rhodosporidium toruloides Y4 through the two‐stage lipid production process. Biochem. Eng. J. 2014, 91, 86–91. [Google Scholar]
- 75. Zhao, X. , Kong, X. , Hua, Y. , Feng, B. , Zhao, Z. B. , Medium optimization for lipid production through co‐fermentation of glucose and xylose by the oleaginous yeast Lipomyces starkeyi . Eur. J. Lipid Sci. Technol. 2008, 110, 405–412. [Google Scholar]


