Abstract
The global proteome response toward recombinant protein production in Escherichia coli BL21 (DE3) grown in complex and defined medium was analyzed. Overproduction of human basic fibroblast growth factor (hFGF‐2), a difficult‐to‐fold protein, led to a reconstruction of the bacterial proteome. For example, heat shock chaperones were highly upregulated, especially when production occurred during fast growth in complex medium. Although heat shock chaperones increased to higher levels in complex medium more hFGF‐2 accumulated within inclusion bodies indicating that the capacity to chaperone protein folding was not sufficient for high speed production. In both types of media, cellular proteins from substrate transport systems, central metabolic pathways, and by‐product uptake (e.g. acetate) were downregulated. This downregulation was connected to growth inhibition and metabolic perturbations. For example, during production in complex and defined medium acetate reassimilation and glucose uptake, respectively, were severely hampered. Cellular proteins for degradation of less favorable substrates, elimination of reactive oxygen species, and DNA protection were also downregulated in response to hFGF‐2 production. The decrease of proteins involved in transport, central metabolic pathways, and general cell protection was more pronounced in the fast producing culture in complex medium than in the slow producing culture in defined medium. In general, production of hFGF‐2 seems to interfere with the adaptation process to changing growth conditions, in this case the adaptation from exponential growth to stationary phase.
Keywords: Complex medium, Defined medium, Metabolic burden, Stress response, Two‐dimensional gel electrophoresis
Abbreviations
- hFGF‐2
human basic fibroblast growth factor
- LB
Luria Bertani
- ROS
reactive oxygen species
- RPM
relative protein mass
- TCA
tricarboxylic acid
1. Introduction
Recombinant DNA techniques in combination with bioprocess engineering allow high level protein production using genetically modified microorganisms, such as Escherichia coli. Using strong inducible promoters, e.g. T7 promoter, heterologous proteins are typically produced at high levels resulting in physiological stress named metabolic burden often connected to the aggregation of the recombinant protein into inclusion bodies 1, 2, 3, 4, 5, 6.
The metabolic burden caused by overexpression of heterologous genes has been reported many times 7, 8, 9: it can lead to a decrease of the specific growth rate 1, 10, 11, 12, 13 and biomass yield 11, 12, 13, and can result in an increase in maintenance energy 10 and enhanced acetate excretion 11. The metabolic burden or the cellular response toward recombinant protein production in E. coli varies greatly for different target proteins and production conditions. Many reports state enhanced levels of heat shock proteins 14, 15 or increased expression of heat shock genes in response to recombinant protein production 16, 17, 18, 19. However, there are also reports that did not notice an increase of heat shock proteins 20, 21 or enhanced transcript levels of heat shock genes in response to protein overproduction 22. Also, enhanced expression of ribosomal genes 17 but also lower levels of ribosomal proteins were reported as a result of recombinant protein overproduction 21, 22. Most studies describe a general downregulation of central carbon metabolism, e.g. lower levels of enzymes involved in the pentose phosphate pathway 15, the glycolytic pathway 20, the tricarboxylic acid (TCA) cycle 20, and ATP synthase 14, 20 as well as decreased fluxes through the pentose phosphate pathway and the TCA cycle 23. However, there are also reports describing enhanced levels of TCA cycle proteins 14, 15 or a nonuniform regulation, e.g. simultaneous up‐ and downregulation of individual TCA cycle genes 22 or proteins 24 in response to recombinant protein overproduction. Contradicting findings also exist regarding transport systems. Baig et al., observed downregulation of genes related to transport of carbohydrates, amino acids, and other substrates in response to recombinant protein production 17. On the other hand, increased levels of proteins involved in carbohydrate 20 and peptide transport 15 have also been reported as a consequence of recombinant protein overproduction. A comparative examination of these seemingly contradicting findings appears impossible as above results were obtained with different proteins and host strains as well as different production conditions. Thus, a profound understanding of the cellular response toward recombinant protein production is still missing.
For recombinant protein production two different types of media are employed: complex medium (also referred to as rich medium) and defined mineral salt medium 25, 26, 27, 28, 29, 30. Complex media are popular for the daily lab routine but lot‐to‐lot variations of raw materials (e.g. yeast extract, peptone, or tryptone) can lead to poor production reproducibility in industrial scale cultures 31, 32. Moreover, the usage of defined medium is also a prerequisite in fed‐batch cultures to reach cell densities above 100 g/L dry cell mass 33. Thus, chemically defined medium properly adjusted to the needs of the producing organism is gaining more and more popularity 34, 35, 36.
In the present study, human basic fibroblast growth factor (hFGF‐2) was chosen as a “difficult‐to‐fold” model protein for production in shaken cultures using complex medium (Luria Bertani broth) and glucose‐supplemented mineral salt medium with E. coli BL21 (DE3) as expression host. hFGF‐2 is a cytokine that can be produced in E. coli in form of inclusion bodies but also as a correctly folded protein 3, 37. During production of hFGF‐2 in both types of media, a quantitative proteome analysis was performed for hFGF‐2 producing cells and also for nonproducing controls cells grown under identical conditions to get a deeper understanding of the production‐associated metabolic perturbations.
2. Materials and methods
2.1. Strain, media, and cultivation conditions
E. coli BL21 (DE3) with the plasmid pET‐29c‐hFGF‐2 was used in this study 37. The composition of Luria Bertani (LB) medium was as follows: 10 g/L tryptone, 5 g/L yeast extract, and 5 g/L NaCl. The composition of Defined Noninducing Broth was as follows: 10.91 g/L glucose, 4 g/L (NH4)2HPO4, 13.3 g/L KH2PO4, 1.55 g/L citric acid, 0.59 g/L MgSO4, 100.8 mg/L Fe(III) citrate, 2.1 mg/L Na2MoO4.2H2O, 2.5 mg/L CoCl2.6H2O, 15 mg/L MnCl2.4H2O, 1.5 mg/L CuCl2.2H2O, 3 mg/L H3BO3, 33.8 mg/L Zn(CH3COOH)2.2H2O, 14.1 mg/L Titriplex III. The pH of all media was adjusted to pH 6.8 using NaOH before sterilization. After sterilization, 50 mg/L kanamycin was added. Details of medium preparation are described elsewhere 35, 36.
Cultivations were carried out in duplicate using 1.8 L Fernbach flasks with three baffles containing 200 mL medium at 30°C and 180 rpm. When the culture reached the midexponential phase, IPTG was added to a final concentration of 0.25 mmol/L for starting hFGF‐2 production. Nonproducing control cultures were grown under identical conditions without the addition of IPTG. Culture samples were centrifuged at 17 000 g and 4°C for 3 min. After removal of the supernatant, cell pellets as well as supernatants were stored at −80°C until further analysis. Sampling points are indicated in the figure captions.
2.2. Analysis of cell density, acetate, and glucose concentrations, and SDS PAGE analysis
Cell growth was monitored by measuring the absorbance at 600 nm (OD600). Acetate was analyzed using an acetic acid kit (Cat. No. K‐ACETRM, Megazyme, Ireland). Glucose was determined by YSI 2300 STAT Plus (YSI Life Sciences, USA). For protein production SDS‐PAGE analysis was performed in Mini‐PROTEAN 3 Cell (Bio‐Rad, USA) according to standard procedures and instructions from the manufacturer. BugBusterTM protein extraction reagent with rLysozyme and Benzonase (Novagen, USA) was used to generate cell extracts and to prepare soluble and insoluble fractions according to the instructions from the manufacturer. After electrophoresis, proteins were visualized by Coomassie blue staining 38. Densitometry was carried out using ImageJ software (National Institutes of Health, USA).
2.3. Two‐dimensional gel electrophoresis
Cell pellets were disrupted by BugBuster™ protein extraction reagent. The whole cell protein in the BugBuster suspension was precipitated as described previously 39. The protein pellets were resolubilized in rehydration solution with IPG buffer (GE Healthcare, UK). About 280 μg protein for each sample were loaded onto Immobiline DryStrip gel (pH gradient 3–10NL, GE Healthcare, UK). The first‐dimension using IEF and the second dimension using SDS‐PAGE (10–15% gradient acrylamide gel) were run with the IPGphor™ Isoelectric Focusing System and Hoefer ™ DALT System (GE Healthcare, UK), respectively. The detailed protocol has been described previously 40. For each sample, 2D gels were made in triplicate and the best two gels analyzed using Proteomweaver™ 4.0 software. Each spot's intensity was normalized by the whole spots intensity of the same 2D gel. The corresponding average intensity of the spot (or the sum of several spots representing the same protein in case of spot multiplicity) taken from the two duplicate gels was used to determine this protein's portion (%) of the relative protein mass (RPM).
2.4. Protein identification and classification
Proteins were identified by MALDI‐TOF MS. Protein spots were excised from the 2D gels. After washing, reduction, and alkylation, in‐gel digestion was carried out by incubation with sequencing grade trypsin (Promega, USA). Obtained peptides were extracted and purified with reversed‐phased ZipTips C18 (Millipore, USA). The resulting peptide solutions were mixed with a saturated matrix solution (acetonitrile 40%, α‐cyano‐4‐hydroxycinnamic acid 1%, and trifluoroacetic acid 0.06%) and spotted onto a 384 MTP target and dried at room temperature. A Bruker Ultraflex time‐of‐flight mass spectrometer (Bruker Daltonics GmbH, Germany) was used to obtain peptide mass fingerprints. Detailed experimental procedures have been described previously 40, 41. The MASCOT search program (Matrix Science, UK) was used for protein identification with the annotated E. coli genome Uniprot (http://www.uniprot.org/) serving as database. All proteins with a Mowse score greater than 54 were regarded as significant (p < 0.05). For classification of identified proteins the EcoCyc (http://ecocyc.org/) 42 and KEGG (http://www.genome.jp/kegg/) databases 43 were used.
3. Results
3.1. Overproduction of hFGF‐2 in complex and defined medium
hFGF‐2 production was carried out at 30°C in complex LB medium and defined glucose‐supplemented mineral salt medium. Control cultures without production were grown under identical conditions without addition of IPTG. Cell growth prior to induced production of hFGF‐2 was more rapid in complex medium than in defined medium (Fig. 1A and C, Table 1). After induction, growth inhibition was observed in both media, however, the production‐related growth inhibition was more severe in complex medium (Fig. 1A and C). SDS‐PAGE analyses indicated more rapid hFGF‐2 production in complex medium connected to a higher level of inclusion body formation (Fig. 2, Table 1).
Figure 1.
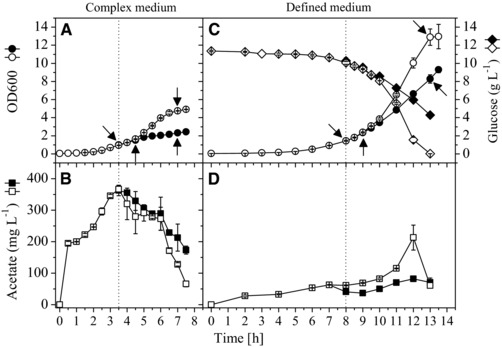
Growth performance of E. coli BL21 (DE3) during hFGF‐2 production in complex and defined medium. hFGF‐2 producing (closed symbols) and nonproducing control cells (open symbols) were grown in complex (LB, A and B) and defined glucose‐supplemented mineral salt medium (Defined Noninducing Broth, DNB, C and D) at 30°C. The time‐point of IPTG addition to the hFGF‐2 producing culture is indicated by dotted lines. Sampling points for 2D proteome analysis are indicated by arrows (see also Supporting Information Table S1).
Table 1.
hFGF‐2 production in defined and complex medium
| Medium | Specific growth rate before induction | Biomass before inductionb | Final biomass without inductionb | End of productiona | |||
|---|---|---|---|---|---|---|---|
| Biomassb | Insoluble hFGF‐2 | Specific and volumetric hFGF‐2 concentrationsc | |||||
| h−1 | g DCM/L | g DCM/L | g DCM/L | % | mg/g DCW | mg/L | |
| Complex | 0.92 | 0.37 | 1.78 | 0.85 | 55 | 45 | 38 |
| Defined | 0.52 | 0.53 | 4.77 | 3.07 | 46 | 38 | 117 |
End of production: 5 and 3.5 h after IPTG addition in defined (DNB) and complex (LB) medium, respectively.
Biomass: One unit of OD600 corresponds to 0.37 g dry cell mass per liter for E. coli BL21(DE3) 35.
Specific and volumetric hFGF‐2 concentrations were calculated assuming a constant cell protein content of 550 mg protein per gram cell dry mass 53.
Figure 2.
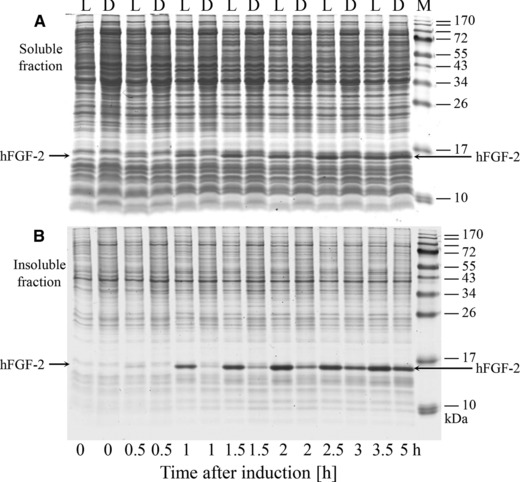
Production of hFGF‐2 in complex and defined medium. Soluble (A) and insoluble cell fractions (B) of hFGF‐2 producing cells growing in complex (L = LB) and defined medium (D = DNB) were subjected to SDS‐PAGE analysis. Time after induction is indicated.
Acetate formation was mainly observed during rapid growth in complex medium (Fig. 1B). During entry into stationary phase acetate reassimilation occurred in producing and nonproducing control cultures, however, acetate uptake was more delayed in the hFGF‐2‐producing culture (Fig. 1B). In defined medium, growth inhibition in the hFGF‐2 producing culture became apparent through inhibition of glucose uptake and not through enhanced acetate formation (Fig. 1B and D).
3.2. Proteome analysis during recombinant protein production
For a better understanding of the production associated metabolic perturbations in complex and defined medium a detailed comparative analysis of the bacterial proteome of producing and nonproducing control cells was carried out using 2D gel electrophoresis.
A first brief visual examination of 2D gels revealed that heat shock proteins accumulated in both media to higher levels in hFGF‐2 producing cells compared to nonproducing control cells (Fig. 3). In contrast, many metabolic enzymes were present in lower amounts in producing cells compared to noninduced control cells (Fig. 3). A lumped quantitative analysis of the abundance level of all identified proteins in hFGF‐2 producing cells as well as in respective control cells grown under identical conditions is given in Fig. 4. Following more detailed insights are given to hFGF‐2 production associated alterations of the cellular proteome with respect to (folding) stress and protein synthesis associated processes as well as metabolic processes such as substrate transport, central carbon metabolism, and metabolite degradation (for more detailed data refer also to the Supporting Information Table S1).
Figure 3.
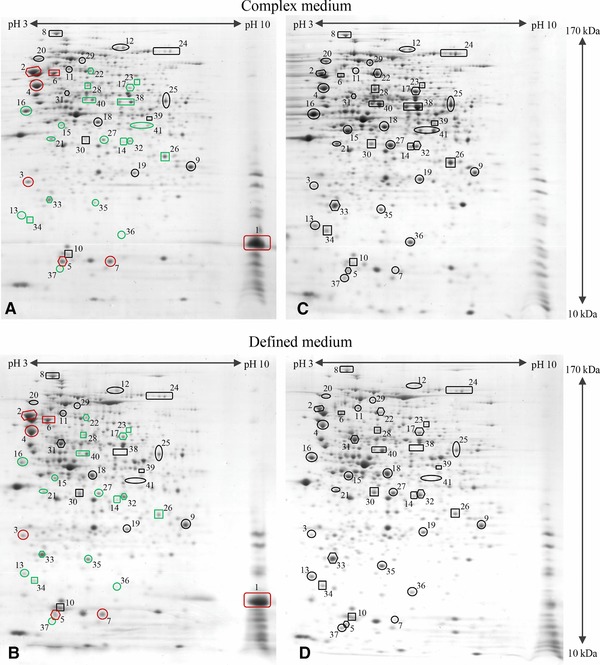
Proteomic fingerprint of hFGF‐2 producing cells. The proteome of hFGF‐2 producing cells at the end of production in complex (A, 3.5 h postinduction) and defined medium (B, 5 h postinduction) in comparison to nonproducing control cells grown under identical conditions to the same sampling time point in complex (C) and defined medium (D), respectively. Gel images indicating representative proteins are shown: hFGF‐2 1; chaperones, DnaK 2, GrpE 3, GroEL 4, GroES 5, HtpG 6, IbpA 7; transcription, RpoB 8; translation, RpsB 9, RpsF 10, GlyS 11, InfB 12; sugar uptake systems, Crr 13, ManX 14, MalE 15, LamB 16; peptide transport, OppA 17; upper glycolysis, FbaA 18; lower glycolysis, GpmA 19, PpsA 20; pentose phosphate pathway, TalB 21; by‐product assimilation, Acs 22, PoxB 23, AdhE 24; TCA cycle: GltA 25, SucD 26, Mdh 27; anaplerotic reactions, PckA 28, MaeB 29; amino acid biosynthesis, IlvE 30, IlvC 31, CysK 32; removal of ROS, AhpC 33, Tpx 34, SodB 35; DNA protection, Dps 36, UspA 37; amino acid degradation, TnaA 38, AstA 39; metabolite degradation, GatZ 40, GatD, 41. Red and green colors indicate higher or lower levels, respectively, of the corresponding proteins in hFGF‐2 producing than in nonproducing control cells. Gel images indicating all identified proteins and representing other sampling times points (e.g. directly before and 1 h after induction of hFGF‐2 synthesis) are shown in the Supporting Information Figs. S1–S8.
Figure 4.
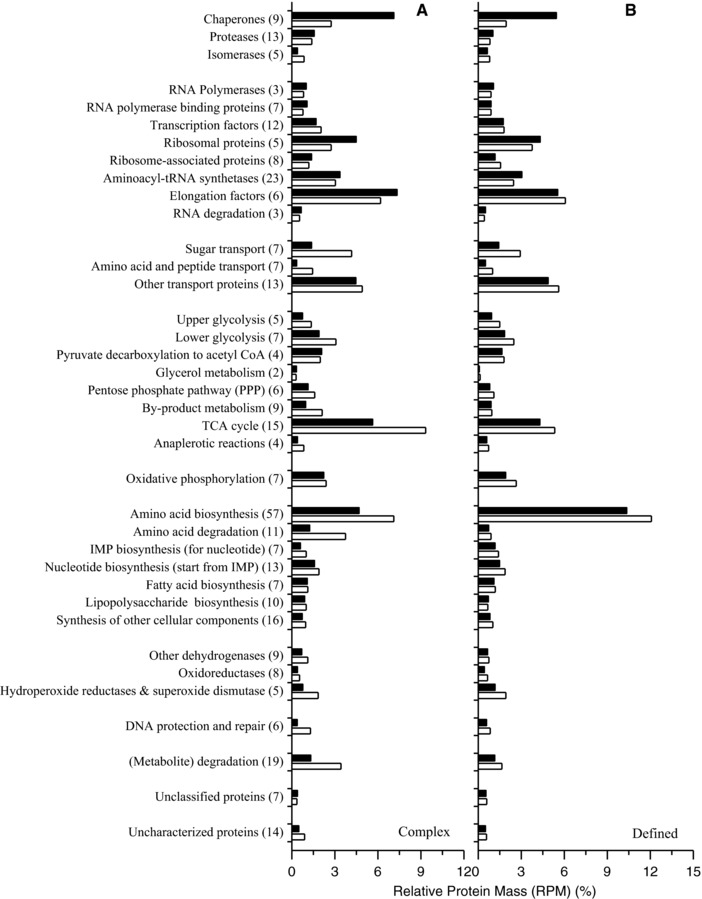
Functional quantitative overview of the proteomic response to hFGF‐2 production. Lumped quantitative proteome data sorted into functional categories comparing the proteome of E. coli BL21 (DE3) producing hFGF‐2 (black bars) in complex (A, 3.5 h postinduction) and defined medium (B, 5 h postinduction) with nonproducing control cells grown under identical conditions to the same sampling time point (open bars). Numbers in brackets after each functional category are the number of proteins that were identified within this category. The entire dataset is given in the Supporting Information Table S1.
3.2.1. Protein folding and protein synthesis associated processes
3.2.1.1. Protein folding and heat shock chaperones
Heat shock chaperones are crucially involved in helping protein folding by preventing protein misfolding and aggregation. The identified heat shock chaperones, DnaK, GrpE, GroEL/ES, HtpG, ClpB, and IbpA increased in abundance in both media after induction of hFGF‐2 synthesis (Figs. 3, 4, 5). Lumped abundances of identified chaperones reached 7.1 and 5.5% of the RPM of hFGF‐2 producing cells in complex and defined medium, respectively (Fig. 4). Nevertheless, higher amounts of chaperones in hFGF‐2 producing cells growing in complex medium did not lead to higher amounts of soluble hFGF‐2 (Fig. 2A). On the contrary, more hFGF‐2 aggregated in form of inclusion bodies during production in complex medium than during production in defined medium (Fig. 2B). In addition, the increase in the abundance of heat‐shock chaperones was not only higher, it occurred also more rapidly during hFGF‐2 production in complex medium (Supporting Information Figs. S9 and S13). The heat shock proteases HslU/V also increased strongly in abundance in response to hFGF‐2 production in both media (Fig. 5) suggesting that they may also play important roles in quality control of recombinant proteins.
Figure 5.
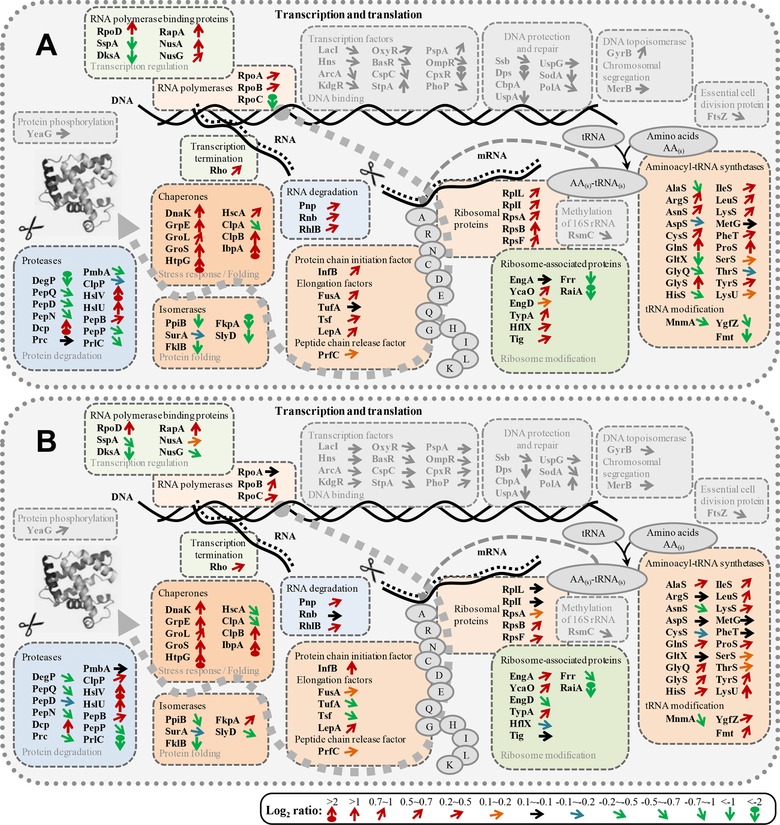
Transcription, translation, and protein folding: proteomic response to hFGF‐2 production. Details of the subproteome related to transcription, translation, and protein folding of hFGF‐2 producing cells in complex (A, 3.5 h postinduction) and defined medium (B, 5 h postinduction) in direct comparison to nonproducing control cells grown under identical conditions to the same sampling time point. Differences of the single protein abundances are expressed as Log2 (producing/nonproducing) ratios using different color coded arrows for visualization [code on bottom, e.g. Log2 ratio > 2, indicated with a red circle with up‐pointing arrow signifies that the abundance of this protein is at least four times higher in the hFGF‐2 producing culture than in the respective control culture]. The entire comparative proteomic (pathway) data are visualized in Supporting Information Figs. S9–S16 and the corresponding values are given in the Supporting Information Table S1. The list of abbreviations is found at the end of the Supporting Information Fig. S16.
Peptidyl prolyl cis‐trans isomerases (PPIases) are important folding catalysts interconverting cis/trans peptide bonds adjacent to proline 44, 45. In both media, the identified PPIases (e.g. PpiB, SurA, FklB, and SlyD) decreased in abundance in response to hFGF‐2 production (Fig. 5). The decrease was more pronounced in complex medium (with faster recombinant protein production) suggesting a potential bottleneck for correct folding.
3.2.1.2. Transcription and translation
Transcription and translation are the processes whereby cells generate new proteins. The amounts of identified proteins involved in these processes, e.g. RNA polymerase, ribosomal proteins, aminoacyl‐tRNA synthetases, and elongation factors, revealed in both media a slight increase in response to hFGF‐2 production compared to the respective nonproducing control cultures (Figs. 3, 4, 5). Again, this increase was slightly higher during production in complex medium (see Supporting Information Table S1).
3.2.2. Metabolic processes
3.2.2.1. Substrate transport systems
Substrates need to be transported into cells prior to their catabolic breakdown or employment for anabolic purposes. During growth in both types of media, proteins belonging to transport systems were present in lower amounts in hFGF‐2 producing cells than in the respective control cells grown under identical conditions (Figs. 3, 4, and 6). This difference in abundance was more prominent for proteins from specific transport systems such as the sugar transporting phosphotransferase system (PTS) (Crr and ManX), and the maltose (MalE/K and LamB) and peptide transport systems (OppA/D) than for proteins belonging to unspecific transport channels of the outer membrane (OmpA/F/X, Supporting Information Figs. S11 and S15). Thus, lower amounts of specific transport proteins point to a decreased capacity for substrate uptake after induction of hFGF‐2 synthesis.
Figure 6.
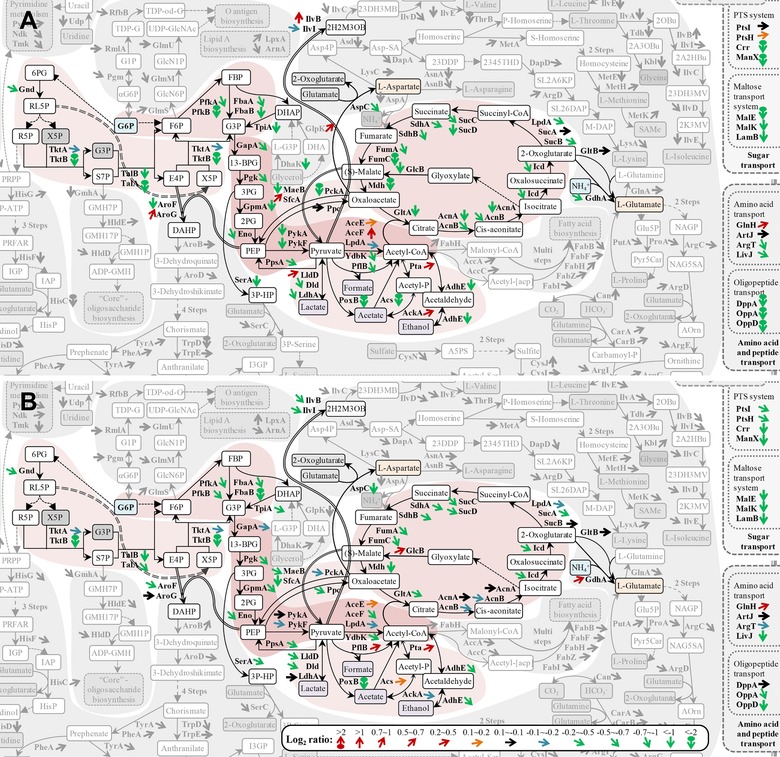
Transport systems and central metabolic pathways: proteomic response to hFGF‐2 production. Details of the subproteome related to transport and central metabolic pathways of hFGF‐2 producing cells in complex (A, 3.5 h postinduction) and defined medium (B, 5 h postinduction) in direct comparison to nonproducing control cells grown under identical conditions to the same sampling time point. Differences of the single protein abundances are expressed as Log2 (producing/nonproducing) ratios using different color‐coded arrows for visualization [code on bottom, e.g. Log2 ratio < –2, indicated with a green circle with down‐pointing arrow signifies that the abundance of this protein is at least four times lower in the hFGF‐2 producing culture than in the respective control culture]. The entire comparative proteomic (pathway) data are visualized in Supporting Information Figs. S9–S16 and the corresponding values are given in the Supporting Information Table S1. The list of abbreviations is found at the end of the Supporting Information Fig. S16.
3.2.2.2. Glycolysis, pentose phosphate pathway, and pyruvate dehydrogenase
After entering into the cell, carbon substrates (e.g. glucose) are catabolized mainly through the glycolytic and the pentose phosphate pathways. In both types of media, enzymes belonging to these pathways decreased to lower levels in hFGF‐2 producing cells compared to control cells grown under identical conditions (Figs. 4 and 6). Interestingly, pyruvate dehydrogenase, the enzyme connecting the glycolytic pathway to the TCA cycle, did not reveal lower levels in hFGF‐2 producing compared to the respective control cells (Figs. 4 and 6).
3.2.2.3. Formation of acetate and other by‐products
Acetate formation occurs mainly from acetyl‐CoA through the Pta‐AckA pathway 46 and to minor amounts directly from pyruvate by oxidative decarboxylation through PoxB 47. Acetate assimilation is exclusively catalyzed by Acs 46.
In complex medium, the enzymes of the Pta‐AckA pathway were slightly higher in the hFGF‐2 producing culture than in the control culture not producing hFGF‐2 (Fig. 6A). However, the acetate forming PoxB and, more importantly, Acs, the enzyme responsible for acetate utilization, reached considerably lower levels in the hFGF‐2 producing cells than in the nonproducing cells growing under identical conditions (Fig. 6A). Both enzymes accumulated to less than five times lower level in the hFGF‐2 producing culture than in the nonproducing control (Supporting Information Table S1). In defined medium, the enzymes of the Pta‐AckA pathway as well as Acs were at the same level in the producing as well as in the noninduced control culture (Fig. 6B). Only PoxB was present in lower amounts in the hFGF‐2 producing culture than in the nonproducing control (Fig. 6B).
Also, enzymes involved in the formation and degradation of lactate, formate, and ethanol were found in both media at lower level in hFGF‐2 producing cells than in the noninduced controls (Fig. 6).
3.2.2.4. TCA cycle and oxidative phosphorylation
Major functions of the TCA cycle are the generation of biosynthetic precursors (e.g. for amino acid synthesis) and reducing equivalents (e.g. NADH) that are subsequently channeled into the oxidative phosphorylation pathway for the production of ATP. In both media, enzymes of the TCA cycle were present at lower level in hFGF‐2 producing cells compared to noninduced control cells (Figs. 3, 4, and 6). Again, this difference was more prominent in complex medium than in defined medium. Lumped abundances of identified TCA cycle enzymes were around 1.7 times (or 40%) and 1.2 times (or 20%) lower in complex and defined medium, respectively, at end of production phase compared to the control culture grown under identical conditions (Fig. 4, Supporting Information Table S1). Moreover, identified subunits of NADH dehydrogenase I and ATP synthase F1 complex decreased in abundance during production of hFGF‐2 in both media compared to control cultures growing under identical condition, but the decrease was stronger in defined medium (Fig. 4).
3.2.3. Amino acids biosynthesis
Amino acids are the building blocks of proteins. In both media, enzymes of amino acid synthesis pathways (e.g. AroF: aromatic amino acids, Asd: lysine, AsnA: asparagine, CysK: cysteine, GlyA: glycine, IlvE: branched‐chain amino acids, SerA: serine), were present at lower level in hFGF‐2 producing cells compared to nonproducing control cells (Supporting Information Figs. S11 and S15). The observed difference was again more significant in complex medium than in defined medium. Lumped abundances of identified amino acids biosynthesis enzymes were present at around 1.5 times (or 34%) and 1.2 times (or 14%) lower level in complex and defined medium, respectively, at end of production phase compared to the control culture grown under identical conditions (Fig. 4, Supporting Information Figs. S11 and S15 as well as Table S1). These findings show that the synthesis of protein building blocks is impaired by overproduction of the recombinant protein, especially when production occurs in complex medium.
3.2.4. Utilization of less favorable substrates
Enzymes for better nutrient scavenging and degradation of less favorable substrates (e.g. arginine, trehalose, galactitol) are required for starvation survival. Enzymes involved in these substrate utilization pathways (e.g. AstA/B/D, DadX, GatD/Z, MalP, and TreA) also reached lower levels in hFGF‐2 producing cells in both media compared to the respective nonproducing control cultures (Fig. 4, Supporting Information Figs. S11 and S15). The observed difference was again more pronounced in complex medium than in defined medium (Supporting Information Table S1). Thus, the scavenging capacity for rare nutrients is impaired by the overproduction of the recombinant protein in particular during growth in complex medium.
3.2.5. Other hFGF‐2 production‐related stress responses
Elimination of reactive oxygen species (ROS) and DNA protection are very important for E. coli especially during starvation survival. Many important enzymes for removal of ROS (e.g. AhpC, Bfr, CueO, Tpx, and SodB) and DNA protection (e.g. Dps, SodA, and UspA) were present in lower amounts in hFGF‐2 producing cells in both media compared to the respective control cultures growing under identical conditions (Figs. 3 and 4, Supporting Information Figs. S11 and S15). Again, the difference was more prominent in complex medium than in defined medium (Supporting Information Table S1). These findings indicate that recombinant protein overproduction may diminish the cellular capacity for general cell protection.
4. Discussion and conclusions
The metabolic perturbations in E. coli BL21 (DE3) caused by hFGF‐2 production in complex Luria Bertani and defined glucose‐supplemented mineral salt medium were studied during batch growth in shaken cultures. Thus, protein production associated growth perturbations were investigated at conditions of initially nonlimited nutrient supply. It was found that growth and production were more rapid and the growth inhibition more severe in complex medium (Table 1). The production associated stress response became also apparent in the reconstruction of the host cell proteome. In comparison to nonproducing control cells grown under identical conditions strongly increased levels of heat shock proteins but also slightly higher levels of translation associated proteins (e.g. ribosomal proteins) were found in cells producing hFGF‐2 with a more prominent increase during production in complex medium. An increase in heat shock protein synthesis in response to recombinant protein production is not unexpected and has been reported numerous times, e.g. 48, 49, 50. However, larger amounts of heat shock chaperones in complex medium than in defined medium did not lead to higher amounts of soluble hFGF‐2. On the contrary, more hFGF‐2 was accumulating within inclusion bodies indicating that the capacity to chaperone protein folding was not sufficient for high speed production.
E. coli BL21 is known as a robust strain as it produces less acetate compared to E. coli K12 strains 51, 52. Significant acetate formation was only observed during exponential growth in complex medium, followed by acetate reassimilation during entry into stationary phase (Fig. 1). However, acetate reassimilation was delayed in the hFGF‐2 producing culture compared to the nonproducing control. This observation was consistent with five times lower levels of Acs, the enzyme required for acetate uptake, in the culture producing hFGF‐2. During production in defined medium uptake of glucose was severely hampered in hFGF‐2 producing cells consistent with a more than two times lower level of the specific sugar transport porin LamB and sugar transporting phosphotransferase system than in the nonproducing control cells. In addition to sugar transport proteins, other specific transport systems (e.g. peptide transport), but also enzymes of central carbon metabolism (e.g. TCA cycle) and amino acid biosynthesis were finally found in lower level in hFGF‐producing cells than in the nonproducing control cells. These findings point to a reduced capacity to generate building blocks required for protein synthesis and cell growth in producing cells. Also, enzymes involved in nutrient scavenging and stress protection (e.g. ROS, DNA damage) revealed lower levels in hFGF‐producing cells than in nonproducing control cells. Again, the difference to nonproducing control cells was more prominent during production in complex medium. Interestingly, many of those proteins found at lower level in hFGF‐2 producing cells usually increase during transition of E. coli BL21 (DE3) from exponential to stationary phase 46. This involves specific transport proteins, but also enzymes of central metabolic pathways and those involved in degradation of less favorable substrates, ROS elimination, and DNA protection. Thus, the stress response toward production of hFGF‐2 mimics – at least partly – an anti‐stationary phase response. In this line, proteins involved in transcription and translation decrease during transition of E. coli BL21 (DE3) from exponential to stationary phase 46. During production of hFGF‐2 they do not show this decline and are finally present at higher level than in the nonproducing control cells grown under identical conditions.
In summary, production of hFGF‐2 in E. coli BL21 (DE3) leads to the induction of the typical unfolded protein response, the enhanced synthesis of heat shock proteins. Under conditions employed during this study – induction of protein production during exponential growth in shaken cultures – it also leads to a kind of anti‐stationary phase response in particular under conditions of rapid production in complex medium. The production of hFGF‐2 seems to interfere with the adaptation process to changing growth conditions, in this case the adaptation from exponential growth to stationary phase in shaken cultures.
Practical application
This study helps to understand the cellular stress response toward recombinant protein production using E. coli as expression host. It provides important information to further improve recombinant protein production using genetic and bioprocess engineering approaches.
The authors have declared no conflict of interest.
Supporting information
Figures S1–S8. Images of 2D gels of the proteome of E. coli BL21 (DE3) growing in complex and defined medium during production of hFGF‐2 and of non‐producing control cells grown under identical conditions. Identified proteins are marked and “clickable” to get access to further protein information (http://www.uniprot.org). Figures S8–S16: Detailed comparative scheme of the (proteomic) pathway regulation during hFGF‐2 production in complex and defined medium (1 h after IPTG induction versus pre‐induction, end of production versus pre‐induction, end of production versus non‐producing control at stationary phase, stationary phase versus exponential phase without induction). Table S1: Quantitative data of individual proteins of E. coli BL21 (DE3) growing in defined and complex medium during production of hFGF‐2 and of nonproducing control cells grown under identical conditions.
Table S1: Quantitative data of individual proteins of E. coli BL21 (DE3) growing in defined and complex medium during production of hFGF‐2 and of nonproducing control cells grown under identical conditions.
Acknowledgments
Partial financial support from the German Ministry of Education and Research (BMBF) through the FORSYS‐Partner program (grant FKZ 0315285) and from the German Research Council (DFG) through the Cluster of Excellence “Rebirth” EXC62 is gratefully acknowledged.
This manuscript is dedicated to Prof. Dr. Karl Schügerl on the occasion of his 90th birthday.
5 References
- 1. Bentley, W. E. , Mirjalili, N. , Anderson, D. C. , Davis, R. et al., Plasmid‐encoded protein: the principal factor in the "metabolic burden" associated with recombinant bacteria. Biotechnol. Bioeng. 1990, 35, 668–681. [DOI] [PubMed] [Google Scholar]
- 2. Hoffmann, F. , Rinas, U. , Stress induced by recombinant protein production in Escherichia coli . Adv. Biochem. Eng. Biotechnol. 2004, 89, 73–92. [DOI] [PubMed] [Google Scholar]
- 3. Rinas, U. , Hoffmann, F. , Betiku, E. , Estape, D. et al., Inclusion body anatomy and functioning of chaperone‐mediated in vivo inclusion body disassembly during high‐level recombinant protein production in Escherichia coli . J. Biotechnol. 2007, 127, 244–257. [DOI] [PubMed] [Google Scholar]
- 4. de Groot, N. S. , Espargaro, A. , Morell, M. , Ventura, S. , Studies on bacterial inclusion bodies. Fut. Microbiol. 2008, 3, 423–435. [DOI] [PubMed] [Google Scholar]
- 5. Wang, L. , Maji, S. K. , Sawaya, M. R. , Eisenberg, D. et al., Bacterial inclusion bodies contain amyloid‐like structure. PLoS Biol. 2008, 6, e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Villaverde, A. , Carrio, M. M. , Protein aggregation in recombinant bacteria: biological role of inclusion bodies. Biotechnol. Lett. 2003, 25, 1385–1395. [DOI] [PubMed] [Google Scholar]
- 7. Gill, R. T. , Valdes, J. J. , Bentley, W. E. , A comparative study of global stress gene regulation in response to overexpression of recombinant proteins in Escherichia coli . Metab. Eng. 2000, 2, 178–189. [DOI] [PubMed] [Google Scholar]
- 8. Oh, M. K. , Liao, J. C. , DNA microarray detection of metabolic responses to protein overproduction in Escherichia coli . Metab. Eng. 2000, 2, 201–209. [DOI] [PubMed] [Google Scholar]
- 9. DeLisa, M. P. , Valdes, J. J. , Bentley, W. E. , Quorum signaling via AI‐2 communicates the "metabolic burden" associated with heterologous protein production in Escherichia coli . Biotechnol. Bioeng. 2001, 75, 439–450. [DOI] [PubMed] [Google Scholar]
- 10. Bhattacharya, S. K. , Dubey, A. K. , Metabolic burden as reflected by maintenance coefficient of recombinant Escherichia coli overpressing target gene. Biotechnol. Lett. 1995, 17, 1155–1160. [Google Scholar]
- 11. Rozkov, A. , Avignone‐Rossa, C. A. , Ertl, P. F. , Jones, P. et al., Characterization of the metabolic burden on Escherichia coli DH1 cells imposed by the presence of a plasmid containing a gene therapy sequence. Biotechnol. Bioeng. 2004, 88, 909–915. [DOI] [PubMed] [Google Scholar]
- 12. Hoffmann, F. , Rinas, U. , On‐line estimation of the metabolic burden resulting from the synthesis of plasmid‐encoded and heat‐shock proteins by monitoring respiratory energy generation. Biotechnol. Bioeng. 2001, 76, 333–340. [DOI] [PubMed] [Google Scholar]
- 13. Ow, D. S. W. , Nissom, P. M. , Philp, R. , Oh, S. K. W. et al., Global transcriptional analysis of metabolic burden due to plasmid maintenance in Escherichia coli DH5 alpha during batch fermentation. Enzyme Microb. Technol. 2006, 39, 391–398. [Google Scholar]
- 14. Dürrschmid, K. , Reischer, H. , Schmidt‐Heck, W. , Hrebicek, T. et al., Monitoring of transcriptome and proteome profiles to investigate the cellular response of E. coli towards recombinant protein expression under defined chemostat conditions. J. Biotechnol. 2008, 135, 34–44. [DOI] [PubMed] [Google Scholar]
- 15. Cheng, C. H. , Lee, W. C. , Protein solubility and differential proteomic profiling of recombinant Escherichia coli overexpressing double‐tagged fusion proteins. Microb. Cell Fact. 2010, 9, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mairhofer, J. , Scharl, T. , Marisch, K. , Cserjan‐Puschmann, M. et al., Comparative transcription profiling and in‐depth characterization of plasmid‐based and plasmid‐free Escherichia coli expression systems under production conditions. Appl. Environ. Microbiol. 2013, 79, 3802–3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baig, F. , Fernando, L. P. , Salazar, M. A. , Powell, R. R. et al., Dynamic transcriptional response of Escherichia coli to inclusion body formation. Biotechnol. Bioeng. 2014, 111, 980–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Smith, H. E. , The transcriptional response of Escherichia coli to recombinant protein insolubility. J. Struct. Funct. Genomics 2007, 8, 27–35. [DOI] [PubMed] [Google Scholar]
- 19. Lesley, S. A. , Graziano, J. , Cho, C. Y. , Knuth, M. W. et al., Gene expression response to misfolded protein as a screen for soluble recombinant protein. Protein Eng. 2002, 15, 153–160. [DOI] [PubMed] [Google Scholar]
- 20. Lee, D. H. , Kim, S. G. , Park, Y. C. , Nam, S. W. et al., Proteome analysis of recombinant Escherichia coli producing human glucagon‐like peptide‐1. J. Chromatogr. B 2007, 849, 323–330. [DOI] [PubMed] [Google Scholar]
- 21. Aldor, I. S. , Krawitz, D. C. , Forrest, W. , Chen, C. et al., Proteomic profiling of recombinant Escherichia coli in high‐cell‐density fermentations for improved production of an antibody fragment biopharmaceutical. Appl. Environ. Microbiol. 2005, 71, 1717–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haddadin, F. T. , Harcum, S. W. , Transcriptome profiles for high‐cell‐density recombinant and wild‐type Escherichia coli . Biotechnol. Bioeng. 2005, 90, 127–153. [DOI] [PubMed] [Google Scholar]
- 23. Wittmann, C. , Weber, J. , Betiku, E. , Kromer, J. et al., Response of fluxome and metabolome to temperature‐induced recombinant protein synthesis in Escherichia coli . J. Biotechnol. 2007, 132, 375–384. [DOI] [PubMed] [Google Scholar]
- 24. Hoffmann, F. , Weber, J. , Rinas, U. , Metabolic adaptation of Escherichia coli during temperature‐induced recombinant protein production: 1. Readjustment of metabolic enzyme synthesis. Biotechnol. Bioeng. 2002, 80, 313–319. [DOI] [PubMed] [Google Scholar]
- 25. Wang, C. H. , Koch, A. L. , Constancy of growth on simple and complex media. J. Bacteriol. 1978, 136, 969–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reiling, H. E. , Laurila, H. , Fiechter, A. , Mass culture of Escherichia coli: medium development for low and high density cultivation of Escherichia coli B/r in minimal and complex media. J. Biotechnol. 1985, 2, 191–206. [Google Scholar]
- 27. Nancib, N. , Branlant, C. , Boudrant, J. , Metabolic roles of peptone and yeast extract for the culture of a recombinant strain of Escherichia coli . J. Ind. Microbiol. 1991, 8, 165–169. [DOI] [PubMed] [Google Scholar]
- 28. Baev, M. V. , Baev, D. , Radek, A. J. , Campbell, J. W. , Growth of Escherichia coli MG1655 on LB medium: determining metabolic strategy with transcriptional microarrays. Appl. Microbiol. Biotechnol. 2006, 71, 323–328. [DOI] [PubMed] [Google Scholar]
- 29. Baev, M. V. , Baev, D. , Radek, A. J. , Campbell, J. W. , Growth of Escherichia coli MG1655 on LB medium: monitoring utilization of amino acids, peptides, and nucleotides with transcriptional microarrays. Appl. Microbiol. Biotechnol. 2006, 71, 317–322. [DOI] [PubMed] [Google Scholar]
- 30. Baev, M. V. , Baev, D. , Radek, A. J. , Campbell, J. W. , Growth of Escherichia coli MG1655 on LB medium: monitoring utilization of sugars, alcohols, and organic acids with transcriptional microarrays. Appl. Microbiol. Biotechnol. 2006, 71, 310–316. [DOI] [PubMed] [Google Scholar]
- 31. Zhang, J. , Reddy, J. , Buckland, B. , Greasham, R. , Toward consistent and productive complex media for industrial fermentations: studies on yeast extract for a recombinant yeast fermentation process. Biotechnol. Bioeng. 2003, 82, 640–652. [DOI] [PubMed] [Google Scholar]
- 32. Studier, F. W. , Protein production by auto‐induction in high density shaking cultures. Protein Exp. Purif. 2005, 41, 207–234. [DOI] [PubMed] [Google Scholar]
- 33. Korz, D. J. , Rinas, U. , Hellmuth, K. , Sanders, E. A. et al., Simple fed‐batch technique for high cell density cultivation of Escherichia coli . J. Biotechnol. 1995, 39, 59–65. [DOI] [PubMed] [Google Scholar]
- 34. Zhang, J. , Greasham, R. , Chemically defined media for commercial fermentations. Appl. Microbiol. Biotechnol. 1999, 51, 407–421. [Google Scholar]
- 35. Li, Z. , Kessler, W. , van den Heuvel, J. , Rinas, U. , Simple defined autoinduction medium for high‐level recombinant protein production using T7‐based Escherichia coli expression systems. Appl. Microbiol. Biotechnol. 2011, 91, 1203–1213. [DOI] [PubMed] [Google Scholar]
- 36. Li, Z. , Nimtz, M. , Rinas, U. , Optimized procedure to generate heavy isotope and selenomethionine‐labeled proteins for structure determination using Escherichia coli‐based expression systems. Appl. Microbiol. Biotechnol. 2011, 92, 823–833. [DOI] [PubMed] [Google Scholar]
- 37. Hoffmann, F. , van den Heuvel, J. , Zidek, N. , Rinas, U. , Minimizing inclusion body formation during recombinant protein production in Escherichia coli at bench and pilot plant scale. Enzyme Microb. Technol. 2004, 34, 235–241. [Google Scholar]
- 38. Candiano, G. , Bruschi, M. , Musante, L. , Santucci, L. et al., Blue silver: a very sensitive colloidal Coomassie G‐250 staining for proteome analysis. Electrophoresis 2004, 25, 1327–1333. [DOI] [PubMed] [Google Scholar]
- 39. Wessel, D. , Flugge, U. I. , A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 1984, 138, 141–143. [DOI] [PubMed] [Google Scholar]
- 40. Lu, X. , Sun, J. , Nimtz, M. , Wissing, J. et al., The intra‐ and extracellular proteome of Aspergillus niger growing on defined medium with xylose or maltose as carbon substrate. Microb. Cell Fact. 2010, 9, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang, W. , Sun, J. , Nimtz, M. , Deckwer, W. D. et al., Protein identification from two‐dimensional gel electrophoresis analysis of Klebsiella pneumoniae by combined use of mass spectrometry data and raw genome sequences. Proteome Sci. 2003, 1, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Keseler, I. M. , Collado‐Vides, J. , Gama‐Castro, S. , Ingraham, J. et al., EcoCyc: a comprehensive database resource for Escherichia coli . Nucleic Acids Res. 2005, 33, D334–D337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kanehisa, M. , Goto, S. , KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stymest, K. H. , Klappa, P. , The periplasmic peptidyl prolyl cis‐trans isomerases PpiD and SurA have partially overlapping substrate specificities. FEBS J. 2008, 275, 3470–3479. [DOI] [PubMed] [Google Scholar]
- 45. Schmid, F. X. , Mayr, L. M. , Mucke, M. , Schonbrunner, E. R. , Prolyl isomerases: role in protein folding. Adv. Protein Chem. 1993, 44, 25–66. [DOI] [PubMed] [Google Scholar]
- 46. Li, Z. , Nimtz, M. , Rinas, U. , The metabolic potential of Escherichia coli BL21 in defined and rich medium. Microb. Cell Fact. 2014, 13, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chang, Y. Y. , Cronan, J. E., Jr. , Genetic and biochemical analyses of Escherichia coli strains having a mutation in the structural gene (poxB) for pyruvate oxidase. J. Bacteriol. 1983, 154, 756–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jürgen, B. , Ying Lin, H. , Riemschneider, S. , Scharf, C. et al., Monitoring of genes that respond to overproduction of an insoluble recombinant protein in Escherichia coli glucose limited fed‐batch fermentations. Biotechnol. Bioeng. 2000, 70, 217–224. [DOI] [PubMed] [Google Scholar]
- 49. Hoffmann, F. , Rinas, U. , Kinetics of heat‐shock response and inclusion body formation during temperature‐induced production of basic fibroblast growth factor in high‐cell‐density cultures of recombinant Escherichia coli . Biotechnol. Prog. 2000, 16, 1000–1007. [DOI] [PubMed] [Google Scholar]
- 50. Hoffmann, F. , Rinas, U. , Roles of heat‐shock chaperones in the production of recombinant proteins in Escherichia coli . Adv. Biochem. Eng. Biotechnol. 2004, 89, 143–161. [DOI] [PubMed] [Google Scholar]
- 51. Yoon, S. H. , Han, M. J. , Jeong, H. , Lee, C. H. et al., Comparative multi‐omics systems analysis of Escherichia coli strains B and K‐12. Genome Biol. 2012, 13, R37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shiloach, J. , Rinas, U. , Glucose and acetate metabolism in E. coli – system level analysis and biotechnological applications in protein production processes, in: Lee S. Y. (Ed.), Systems Biology and Biotechnology of Escherichia coli, Springer, New York, Heidelberg: 2009, pp. 377–400. [Google Scholar]
- 53. Ingraham, J. L. , Maaloe, O. , Neidhardt, F. C. , Composition, organization, and structure of the bacterial cell, in: Growth of the Bacterial Cell, Sinauer Associates Inc., Sunderland, Massachusetts: 1983, pp. 1–48. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1–S8. Images of 2D gels of the proteome of E. coli BL21 (DE3) growing in complex and defined medium during production of hFGF‐2 and of non‐producing control cells grown under identical conditions. Identified proteins are marked and “clickable” to get access to further protein information (http://www.uniprot.org). Figures S8–S16: Detailed comparative scheme of the (proteomic) pathway regulation during hFGF‐2 production in complex and defined medium (1 h after IPTG induction versus pre‐induction, end of production versus pre‐induction, end of production versus non‐producing control at stationary phase, stationary phase versus exponential phase without induction). Table S1: Quantitative data of individual proteins of E. coli BL21 (DE3) growing in defined and complex medium during production of hFGF‐2 and of nonproducing control cells grown under identical conditions.
Table S1: Quantitative data of individual proteins of E. coli BL21 (DE3) growing in defined and complex medium during production of hFGF‐2 and of nonproducing control cells grown under identical conditions.


