Abstract
Modern bioprocess monitoring demands sensors that provide on‐line information about the process state. In particular, sensors for monitoring bioprocesses carried out in single‐use bioreactors are needed because disposable systems are becoming increasingly important for biotechnological applications. Requirements for the sensors used in these single‐use bioreactors are different than those used in classical reusable bioreactors. For example, long lifetime or resistance to steam and cleaning procedures are less crucial factors, while a requirement of sensors for disposable bioreactors is a cost that is reasonable on a per‐use basis. Here, we present an overview of current and emerging sensors for single‐use bioreactors, organized by the type of interface of the sensor systems to the bioreactor. A major focus is on non‐invasive, in‐situ sensors that are based on electromagnetic, semiconducting, optical, or ultrasonic measurements. In addition, new technologies like radio‐frequency identification sensors or free‐floating sensor spheres are presented. Notably, at this time there is no standard interface between single‐use bioreactors and the sensors discussed here. In the future, manufacturers should address this shortcoming to promote single‐use bioprocess monitoring and control.
Keywords: Bioprocess monitoring, Disposable bioreactors, Disposable sensors, Process analytical technology, Process control, Sensor systems, Single‐use
Abbreviations
- ATP
adenosine triphosphate
- ATR
attenuated total reflection
- CF
electrical capacitance of film material
- CHO
Chinese Hamster Ovary
- FET
field‐effect transistor
- FIA
flow injection analysis
- GMP
Good Manufacturing Practice
- HPTS
8‐hydroxypyrene‐1,3,6‐trisulfonic acid
- IR
infrared
- ISFET
ion‐sensitive field‐effect transistor
- MIR
mid‐infrared
- NAD(P)H
nicotinamide adenine dinucleotide phosphate
- NIR
near‐infrared
- pO2
partial pressure of oxygen
- pCO2
partial pressure of carbon dioxide
- PPAR
pharmaceutical process analytics roundtable
- PAT
process analytical technology
- RF
electrical resistance of film material
- RFID
radio frequency identification
- RTD
resistance temperature detectors
- STR
stirred‐tank reactor
- SUB
single‐use bioreactor
- UV/Vis
ultraviolet and visible light
1. Introduction
Single‐use bioreactors (SUBs) have become widely used in the biopharmaceutical industry 1, 2. In many production areas, especially for the production of high‐value products in small volumes, they have replaced traditional stainless steel reactor systems 3. Disposable reactor technology offers increased facility flexibility with lower investment and energy costs, and especially a simpler production when Good Manufacturing Practice (GMP) requirements are followed, because elaborate and labor‐intensive cleaning procedures become obsolete 3, 4. Today, various disposable bioreactor systems are available on the market, covering culture volumes from several mL (e.g. Micro‐24, Pall Corporation, Port Washington, USA; ambr® systems, Sartorius Stedim Biotech, Göttingen, Germany) up to 2000 L (e.g. Biostat® Cultibag STR, Sartorius Stedim Biotech, Göttingen, Germany) 5. These systems are stirred, shaken, or pneumatically blended to provide sufficient mixing of all nutrients within the reactor system and to supply an efficient gas exchange into the medium. Although primarily used for mammalian cell culture, the cultivation of yeast and other microorganisms in a disposable bag reactor has been demonstrated 4, 6, 7. Overviews of the available systems are given in review articles by Lopez et al. 8 and Eibl et al. 5, 9, 10.
Detailed knowledge of bioprocesses is necessary for improved understanding, control, and optimization. Based on the Process Analysis Technology (PAT) initiative of the US Food and Drug Administration (FDA), improved sensor concepts have been investigated over the last 20 years toward the goal of monitoring of the physical environment (e.g., temperature, shear stress), the chemical environment (e.g., substrate and product concentrations), and the biological system itself. In order to further improve the use of disposable bioreactor technology, sensor concepts for this type of bioreactor are urgently needed 11, 12.
In general, sensor application types can be classified as in‐line, at‐line, or off‐line. In‐line or in‐situ sensors are directly interfaced to the reactor and in contact with the process medium. At‐line systems rely on a sample that is withdrawn via a sampling module and analyzed outside the bioreactor. The measurement of in‐line or at‐line sensors can be considered to be on‐line if the data are recorded continuously and the response time of the sensor signal is small in comparison to the process dynamics. Every other measurement is defined as off‐line 13.
Sensors can be connected to disposable bioreactors in three ways. Single‐use, sterilizable sensors can be interfaced as in‐situ sensors into the disposable bioreactor during its production process, prior to the final sterilization step, and used only once, together with the reactor. In general, only inexpensive sensors will be used in this way. To interface a sensor directly into an already sterilized SUB, special sterile adapter technologies are needed. In this case, devices such as optical or impedance sensors could be placed external to the bioreactor, interfaced via special connector ports that form a sterile barrier and must be transparent to the sensing wavelengths so the bioreactor contents can be interrogated. Such sensors offer the advantage of being reusable and non‐invasive. Another monitoring interface method is the use of appropriate sampling devices to withdraw a representative sample under sterile conditions from the bioprocess medium. This sample stream can then be analyzed at‐line. Figure 1 gives an overview over the possible connection types.
Figure 1.
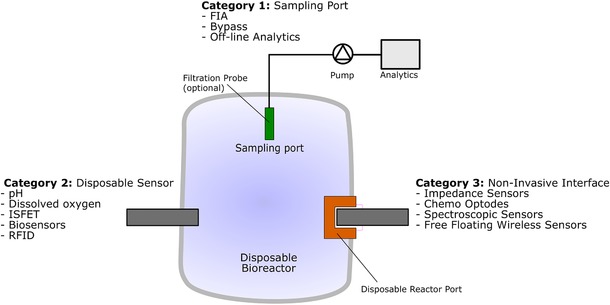
Sensor connection to disposable bioreactors. Three different categories are defined: Noninvasive interfaces, disposable sensors and special disposable sampling ports.
In principle, all sensors available for conventional bioprocess monitoring can also be used for disposable reactor technology. Such sensors are reviewed by Biechele et al 13. In contrast, this review will focus on existing sensors for SUBs, such as sensors for optical dissolved oxygen measurement or impedance spectroscopic probes and sensor concepts. These can be used in the near future for monitoring of disposable reactor cultivations.
2. Interface category 1: sampling systems
Probes for cell‐free or cell‐containing sampling allow the use of ex‐situ sensor systems to analyze samples of disposable bioreactor processes. Moreover, these sampling probes are suitable for connecting at‐line analytical systems to the bioreactor, by including a bioreactor bypass. The most important challenge with sampling ports is to retain sterility while providing a representative sample stream for frequent analysis 14.
Cell‐containing samples from disposable bioreactors are collected via integrated sampling ports. Most disposable bioreactor systems include a sampling port made of a thermoplastic welding tube or a special aseptic connector. Pre‐sterilized sampling containers are connected via welding and later are sealed by heating. Containers reaching from several mL up to 1 L exist 15. Aseptic connectors offer the advantage that no welding machine is needed, but most manufacturers use proprietary systems that limit flexibility.
After sampling, the metabolic activity of the cells in the sample must be stopped, or there might be changes in sample composition 16, 17, 18, 19, 20, 21, 22, 23. This can be accomplished by cooling or by the addition of metabolism inhibitor solutions to the samples 24. Lücking et al. 25 described an automated sampling system for cell‐containing samples that could be suitable for a SUB. In addition to the collection of the sample in a cooled rack, the system integrates a novel measurement chamber that allows the use of well‐established classical sensors in bypass mode. The modular design of the chamber enables the adoption of different probe dimensions and measurement principles.
In‐line filtration probes enable cell‐free sampling. These devices use microfiltration 26, 27, 28 or dialysis 29, 30, 31 membranes as sterile barrier. While most of the probes are designed for repeated use in classical stainless steel reactors 32, 33, 34, a fully disposable dialysis probe has recently been introduced to the market by Trace Analytics GmbH (Braunschweig, Germany). This novel probe is connected to a standard sampling port of a SUB via a standard Luer connector 35.
Another tool for at‐line measurements of specific analytes that is suitable for the use with sampling systems is flow injection analysis (FIA). Many examples for FIA‐based sensor systems have been described in literature 36, 37, 38. The advantage of FIA analysis is that a defined reaction environment is generated and that samples can be diluted and preconditioned in fully automated manner 39, 40.
At‐line sensors can be used for gas phase monitoring in bioprocesses in addition to the analysis of liquid samples. The most common analysis of this type is off‐gas monitoring because the sensors can be interfaced to the process on the non‐sterile side of the off‐gas filter. Thus, sterility is not a concern. In bioprocesses, oxygen and carbon dioxide are the most important gases to monitor. Changes in their concentration give information about cell growth, metabolism, and productivity 13. In principle, every off‐gas analyzer that is used for classical stainless steel bioreactors can be used for disposable bioreactors without any modifications. Biechele et al. 13 gives an overview over the most common gas monitoring techniques for bioprocesses.
3. Interface category 2: in‐situ disposable sensors
This interface is based on a single‐use sensing element that is integrated into the reactor during manufacturing and sterilized together with the SUB, usually by γ‐radiation. This interface requires a physical penetration through the bioreactor bag to connect the sensing element on the inside to the transmitter and detection unit outside of the reactor. Because these sensors are disposed together with the bioreactor, they must be relatively inexpensive. Another requirement of in‐situ sensors is that they cannot leach any extractable compounds into the fermentation medium, including after sterilization. Examples of sensors that belong to this class are electrochemical pH sensors, ion selective field effect transistors (ISFETs), chemo‐ and biosensors for the detection of metabolites such as glucose, glutamine, or lactate, and passive radio frequency identification (RFID) sensing elements. A method for determining O2 and CO2 concentrations in bioprocesses with in‐situ disposable sensors is described by Chatterjee et al. 41. They used an in‐situ silicone loop which is permeable for oxygen and carbon dioxide. The two gases are recirculated through gas impermeable tubing's to the respective gas sensors on the outside of the SUB. More information on measurement of oxygen and carbon dioxide in single‐use reactors is given in chapter 4.1.1.
3.1. Single‐use electrochemical pH sensor
One of the most common electrochemical sensors for bioprocess monitoring and control is the pH probe. Classical potentiometric glass electrodes are well established, reliable and robust but are too expensive to be disposed after a single cultivation run. Therefore, single‐use glass electrodes have been developed. These sensors can be integrated directly into the bioreactor during manufacturing and can be stored dry. The sensors combine both the pH and the reference electrode, and they retain high accuracy after γ‐radiation. Some probes even have a built‐in temperature sensor for the temperature compensation of the signal. Examples for the use of single‐use electrochemical pH probes are the ambr® 250 (Sartorius Stedim Biotech, Göttingen, Germany) or CerCell systems (CerCell ApS, Holte, Denmark). Nevertheless, single‐use glass electrodes are still very expensive and need optimization regarding costs.
3.2. Ion‐selective field effect transistors
Chemical field effect transistors (FETs) are most promising alternatives to common electrochemical sensor systems for disposable systems, because they are small, cheap and easily producible in large amounts. Every FET needs a power supply and wired connection to a readout unit. Thus, a penetration through the SUB is required for the wired connection, which allows the separation of the disposable FET from the reusable electrical equipment.
The measuring principle of FETs is based on the attachment of ions or molecules to an ion‐ or analyte‐sensitive layer that is coated on the gate membrane of the transistor. Two electrodes (source and drain) are grounded on a substrate and connected to a current. The attachment of the ions/analytes to the gate membrane causes an electric potential and by this a change in the current between the source and drain electrode. These changes are proportional to the ion/analyte concentrations 14. The bias point is defined by a reference electrode that is in contact with the measuring solution as well.
Different membrane coatings enable measurement of various process variables, including enzymes, proteins, or pH 42, 43, 44, 45. FETs coated with a biological component such as an enzyme, antibody, or DNA structure are called bioFETs. They can accommodate a wide variety of biological components that can be used as immobilized gate components, and thus many different analyte molecules could be detected in bioprocesses 43, 46, 47. bioFETs are a combination of FET and biosensor technology that is described in the next section.
3.3. Chemo‐ and biosensors
Both chemosensors and biosensors are versatile tools for bioprocess monitoring as they offer a targeted specificity in complex media 48. Chemosensors are based on the specific interaction of an analyte with an indicator molecule. In case of optical chemosensors, this indicator is immobilized on a tip of a light guide system and can be illuminated by an external light source (e.g., LED). Changes in the optical properties of the indicator molecules due to the interaction with the analytes are reflected in changes in the photoluminescence intensity, adsorption, or reflection. These changes are correlated with the analyte concentration and measured by an external light detector (e.g., photo diode) 49. The primary analytes for chemosensors are oxygen, carbon dioxide, and pH (hydrogen ion). While chemosensors with physical connections to a hardware unit have been evaluated, they are more commonly implemented as optical sensors interrogated through a port, and thus are discussed with other Interface Category 3 sensors.
Biosensors for many different analytes have been developed 48, 50, 51, 52. Their basic structure has three parts: A biological detection component, immobilized on a signal transducer, which is amplified by a signal conversion unit. The analytes are selectively and sensitively recognized by the bio‐component either via a catalytic mechanism (using enzymes) or through binding (usually using antibodies or nucleic acids) 48, 53, 54, 55. The transducer determines the interaction of the biological detection unit. Classical oxygen and pH electrodes are often used as transducers 56, 57.
Because of the susceptibility of the biological components to steam sterilization, biosensors are not used for in‐situ monitoring in classical stainless steel or glass reactors. But they can tolerate γ‐sterilization and therefore are appropriate for the use in disposable bioreactors.
Novel single‐use biosensors for the on‐line measurement of glucose, lactate, and glutamate are available. They use an enzymatic oxidation process and direct electron transfer from glucose to the electrode by a chemical wiring process 58. A dialysis membrane is cast over the sensing head to separate the sensor from the cultivation medium. These sensors are delivered ready to use, and can be integrated in the ventilation cap of a shake flask or directly integrated into disposable bioreactors via standard ports. Bauer et al. 58 tested this type of sensor in Chinese Hamster Ovary (CHO) cultivations successfully and demonstrated their usefulness in single‐use applications.
Further developments, that allow the measurement of at least one bioprocess parameter non‐invasively without placing sensors on the inside of the reactor are described in a patent of Rao et al. 59. This invention bases on a barrier membrane that is at least partially permeable to the analyte and in contact with the sensor. Once realized, this allows single use biosensors of interface category 3, described later in this review.
3.4. Radio‐frequency identification‐based sensors
In the pharmaceutical industry, the use of radio‐frequency identification (RFID) chips is recommended by the FDA to improve supply chain efficiency, track and trace protection, and reduced counterfeiting 60, 61.
In contrast to conventional stainless steel reactors, SUBs are made of plastics, which are transparent to the radio‐frequency radiation of an RFID device. This allows the use of RFID transponders as single‐use sensors for disposable bioreactors. Passive, battery‐free RFID chips are directly applied inside a bioreactor and sterilized as a combined system 62. Figure 2 illustrates the sensing principle. The power is transferred via an electromagnetic field of an external readout unit. Thus, no penetration through the bioreactor wall is required. The antenna of the RFID chip generates an electromagnetic field, which interacts either directly with the cultivation broth or with a functionalized sensing layer. Changes in the environment, such as conductivity or impedance, result in a change of the resonance frequency that can be analyzed with the readout unit. Since the transducing principle is independent of the sensing element, RFID chips can be used as a sensing platform for a variety of different parameters. Sensors for temperature and humidity, along with those for gas detection (e.g., ethanol or n‐propanol) have been developed 63. Pioneering work in this field has been done by Potyrailo et al. 60, 64, 65, 66, 67. They presented passive RFID sensors for physical, chemical, and biological sensing 68. Furthermore, a first study on RFID sensor performance in a disposable bioreactor was performed 65. In principle, RFID sensors are suitable for large scale and low‐cost production; however, no RFID sensor is yet on the market 63.
Figure 2.
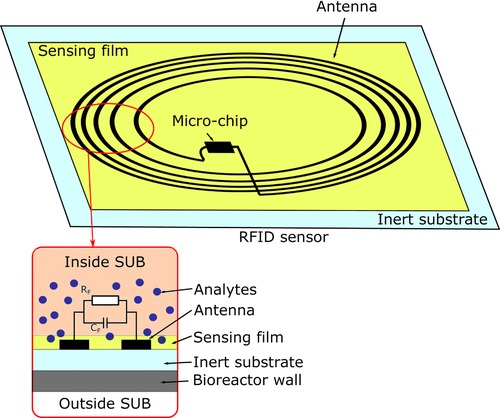
Modified RFID tag for chemo‐ or bio‐sensing via a sensing film applied on the antenna. The analyte affects the film materials resistance RF and capacitance CF between the antenna turns. Thus, the RFID tag operates as the transducer 68.
4. Interface category 3: noninvasive interface
Sensors of Interface Category 3 allow a non‐invasive connection of the sensing device to the SUB. Bioreactor temperature and weight are usually measured on‐line from the outside of the single‐use bioreactor (SUB) via surface‐mounted resistance temperature detectors (RTD) and scales integrated in the bag retainer. Other sensors belonging to Category 3 are interfaced into a pre‐sterilized disposable bioreactor via special sterile adaptors. These adaptors are either optical windows or connector ports, such as electrodes for impedance measurement. The connectors are integrated into the plastic foil of the SUB during manufacturing and sterilized together with the reactor. Because the adapter is transparent to the sensing wavelengths, the transmitting and detection unit is decoupled from the element and can be reused. This is essential for the use of sensor technology which otherwise would be too costly for single‐use applications. While devices like ultrasonic sensors or free‐floating sensor spheres do not need any connecting adapter, the entire disposable reactor must be transparent to the sensing wavelengths in this case as well.
4.1. Optical sensors
Popular examples that rely on optical windows are pO2, pH, and pCO2 chemo‐optodes, available for almost every disposable bioreactor on the market. This interface is also very well suited for spectroscopic sensors, because they need to be in direct optical contact with the culture broth. However, no spectroscopic system for disposable bioreactors is commercially available yet. For future developments, several opportunities to integrate the windows into the wall of the SUB are available, depending on the measurement principle.
The simplest setup is an optical window, integrated into the plastic wall of the bioreactor. The windows must be stable enough to withstand the pressure from culture broth inside the reactor and be highly polished on the surface in contact with the broth such that no cell attachment can occur. Most important is their ability to transmit light of the necessary wavelengths without any optical interaction (reflection, absorbance, or filtering) caused by the window material. For example, normal borosilicate (window) glass is not transparent to UV light, so quartz or sapphire glasses must be used for those wavelengths.
The optical window must be modified further if there is a need for absorption or transflection measurements, as is the case for ultraviolet and visible light (UV/Vis) and infrared (IR) spectroscopy. To create defined measurement spaces, a chamber/cuvette setup could be installed on the inner side of the SUB during manufacturing. A schematic overview of different disposable optical reactor ports is shown in Fig. 3.
Figure 3.
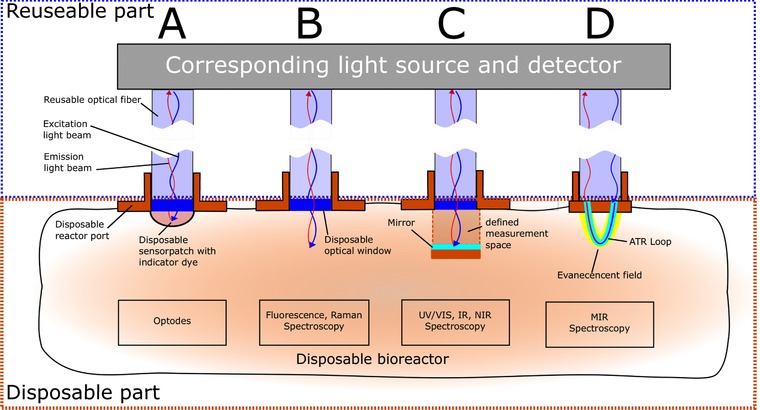
Schematic of different disposable optical ports that could be implemented in single‐use bioreactors. The boxes highlight the reusable and disposable parts of the system. Expensive equipment like light source and detector are separable from the single‐use optical windows.
4.1.1. Optical chemosensors
Optical pH and pO2 sensors can be considered standard equipment for current single‐use bioreactors because they allow non‐invasive operation of chemosensors. Usually, they are connected to the readout unit via a reusable fiber optic that is inserted into a sheath that leads to an optical window at its end. On the other side of the window, inside the bioreactor, a disposable sensor patch is mounted (Fig. 3A). This patch contains an immobilized indicator dye.
Optical measurement of the partial pressure of oxygen is based on the fluorescence quenching of an indicator molecular by oxygen 69, 70, 71. Oxygen‐sensitive indicators are complexes of ruthenium, palladium, or platinum; a widely used indicator is Tris‐4,7‐diphenyl‐1,10‐phenanthrolin‐ruthenium(II) 72, 73. The fluorescent indicator is immobilized in an oxygen‐permeable polymer matrix such as silicone 71, 74. In contrast to conventional amperometric sensors, optodes do not consume oxygen during measurement, which allows their use in small volumes and in diffusion‐limited cases at low oxygen concentrations below 5% 75. Another very interesting system for noninvasive measurement of the partial pressure of oxygen is shown by Gupta et al. 76. They demonstrated the measurement of oxygen concentration through the SUB wall itself. Oxygen diffuses through special oxygen permeable vessel wall and becomes detectable by optical oxygen sensors, placed outside the SUB wall.
Fiber‐optic pH sensors are mostly based on pH‐sensitive fluorescent indicator dyes. Frequently used indicators are based on the on the structure of fluorescein and its derivatives or pyranine like HPTS (8‐hydroxy–pyrene‐1,3,6‐trisulfonic acid) 77, 78. These indicators are immobilized on solid substrates or in polymers that are mounted directly on transparent materials (e.g., reactor wall, glass) 72, 79, 80. Thus, the instrument setup is similar to that for an oxygen optode. In comparison to single‐use electrochemical probes, pH optodes are less expensive but suffer from cross sensitivity to ionic strength, a limited dynamic range, and the loss of sensitivity during sterilization or cleaning procedures 71, 80. To increase the dynamic range, Gupta and Sharma 81 presented a long‐range fiber optic pH sensor based on evanescent wave absorption.
Most optical CO2 sensors operate in the same way as the classical Severinghaus electrodes, which measure the change of pH of an internal carbonate buffer system due to CO2 presence. They are typically based on the fluorometric or colorimetric changes of pH‐sensitive indicators that are added to the buffer system and separated from the analyte solution by a CO2‐permeable membrane 82. There have been many examples of this disposable sensing systems 83, 84, 85. The response time of the sensor is in the range of minutes because the CO2 diffusion through the membrane is slow. Optical pCO2 sensors suffer from the same drawbacks as pH optodes, including low stability at high temperatures 86.
Recently, Bradley et al. 87 published a biopharmaceutical industry perspective, based on the results of a pharmaceutical analytic roundtable meeting (PPAR) in 2015. The survey of applications reveals that there are still concerns that single‐use – especially pH – optodes are not robust enough for manufacturing operation due to factors such as lot‐to‐lot variability, long equilibration times, unknown strength of signal drift, insufficient data on leachables and extractable or difficulties recalibration the transmitter.
4.1.2. Spectroscopic sensors
Spectroscopic analyses are based upon the interaction of electromagnetic waves and molecules, molecular bonds, or particles. Common spectroscopic methods for bioprocess monitoring cover different spectral ranges from ultraviolet (UV) to mid‐infrared (MIR), including fluorescence and Raman spectroscopy. Within these spectral ranges, various bioprocess variables can be measured 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105.
Spectroscopic sensors offer the advantage that no sampling is needed (except for initial calibration) and there is essentially no interaction between the sensor and analytes. Additionally they can easily be multiplexed and deliver signals instantaneously 106. While several different process variables can be determined simultaneously in real time, elaborate chemometric data analysis and data sets to train the algorithms are required for spectroscopic bioprocess monitoring to extract relevant process information from the generated data.
For reusable bioreactors, different probes for standard ports are available, but for single‐use bioreactors, optical ports must be established to connect the spectroscopic hardware to the bioprocess. Each spectral technique has its own requirement for successful, reproducible measurements, and therefore different modifications of the optical interface have to be considered. For all optical measurements, the influence of ambient light through the transparent or translucent SUB wall has to be considered for signal‐to‐noise ratio.
4.1.2.1. Fluorescence spectroscopy
Process‐relevant molecules like proteins with aromatic amino acids, NAD(P)H (correlated with biomass), ATP, pyruvate, vitamins, pyridoxines, coenzymes, and flavins are fluorescent 101, 107, 108, 109, 110, 111. Each fluorescent compound has a specific pair of excitation and emission wavelengths at which the signal is maximized, and these are easily detected through an optical window.
Fluorescence probes can be attached to SUBs via UV‐transparent glass windows (Fig. 3B). Since fluorescence measurement has no direction, measurement can be performed easily by collecting light 180° from the input, simplifying the interface.
4.1.2.2. UV/Vis spectroscopy
UV/Vis spectroscopy operates in the ultraviolet and visible light region (200–740 nm) to excite the electrons of molecules. The observable transitions take place at unsaturated bonds, such as in aromatics 100. Different analytes, substrates, metabolites, and products absorb light in this range.
Because of the absorption measurement, it is necessary to provide a defined measurement space with a known path length, as described by the Lambert‐Beer principle. Thus, to interface this technology to single‐use systems, a disposable in‐line quartz‐cuvette or defined measurement chamber (Fig. 3C) are necessary. This inner setup could be installed and pre‐sterilized with the whole reactor during manufacturing.
4.1.2.3. Infrared spectroscopy
Infrared (IR) spectroscopy uses different spectral areas. Near infrared (NIR) spectroscopy is performed at wavelengths from 740 to 1300 nm and mid‐infrared extends to 15 000 nm. Far infrared ranges start above 15 μm but this range is rarely used for bioprocess monitoring. IR light excites different vibrational modes of molecules, whereby each organic and inorganic compound has its special spectral IR signature. The more different vibrational modes can be excited, the more specifically it is possible to determine an analyte 106, 112. IR spectroscopy is very fast, robust, and sensitive. Multi‐analyte information from the culture broth of bioprocesses can be obtained on‐line, in‐line, and in a noninvasive manner, but there are challenges in doing so as the complexity of the medium increases. The high concentration of water molecules also poses a challenge for NIR measurements.
NIR Spectroscopy: NIR spectroscopy is based on different vibrational modes, overtone and combination vibrations after excitation. Bioprocess relevant targets are the O‐H bonds of alcohols, C‐H bonds of aliphatic and aromatic carbon compounds, and N‐H bonds of proteins. The NIR range is suitable for monitoring of substrates such as glucose and lactate, biomass, and the products of a bioprocess 107. As a result of the lower energy of the NIR and the resulting overtone vibrations, the bands are much broader, often overlapping, and not as specific as in MIR spectroscopy 113. Thus, to obtain a higher signal‐to‐noise ratio, a longer path length is used 106, 113, 114. NIR spectroscopy has a more qualitative character, compared to the more precise and quantitative MIR spectroscopy. Since NIR probes measure in absorption mode, an inner setup with a defined measurement space is required on the inside of the SUB (compare to UV/Vis spectroscopy in Fig. 3).
MIR Spectroscopy: Process‐relevant molecules such as glucose, lactate, fructose, acetic acid, ammonia, and even antibodies 106 have specific characteristic absorption spectra, known as the molecular fingerprint of fundamental rotational vibrations of functional groups. Despite the high degree of water absorption appearing in MIR spectra, in‐line measurement in aqueous solutions is possible using appropriate fiber‐optic systems that incorporate attenuated total reflection (ATR) technology and Fourier transformation 98, 108. Light reflection at the phase boundary between the ATR crystal and bioprocess medium results in a very short (few μm) path length. Information about cells cannot be obtained because they are too large to enter the measuring zone. The ATR crystal can be applied to disposable systems at the inner side of the SUB and connected to external equipment 14. Due to the very high price of a diamond ATR crystal, the use of this connection for disposable reactors is unlikely.
An alternative is the use of ATR loops. A loop consists of polycrystalline infrared fibers, which are non‐toxic, very flexible, and transparent across a broad spectral region from 3 to 18 μm. Low‐cost loops are available (e.g., Art Photonics, Berlin, Germany) that could be located on the inside wall of an SUB and connected to outer reusable equipment via fiber optics (Fig. 3D).
4.1.2.4. Raman spectroscopy
Raman spectroscopy is based on shifted wavelength scattering of molecules after excitation by monochromatic light (e.g., from an adjustable laser) 13, 53. Compared to IR spectroscopy, Raman spectroscopy has less inference from water molecules in aqueous solutions and a high signal‐to‐noise ratio, but is limited by the strong fluorescence of biomolecules in the culture broth 13, 115. However, several analytes, including glucose, glutamine, glutamate, ammonia or biomass, can be monitored 116, 117. For bioprocess monitoring, commercial versions of in‐line Raman analyzers are on the market that can be connected to standard reusable bioreactors (e.g. ProcellicsTM, Resolution Spectra Systems, Meylan, France). It is likely that versions for SUBs will become available. To attach Raman probes to single‐use bioreactors, a special adaptor, composed of a glass window without inner hardware, could be used (Fig. 3B) because measurement of the light scattering takes place at an angle of 180°.
4.2. Impedance spectroscopy
Electrical impedance spectroscopy enables the measurement of the dielectric properties of materials. A sinusoidal alternating electrical field is applied between two electrodes. Inside the reactor, each viable cell is enclosed by a minimally conducting cytoplasmic membrane, which separates the cytosol (containing charge carriers) from the conductive growth medium. Due to alternating characteristics of the applied field, cells behave like small capacitors and become polarized. The electrical properties of the cells in the measurement volume are described by the capacitance / electrical permittivity and conductivity, and can be correlated to process variables.
Important parameters such as biomass concentration, cell size, viability and cell membrane integrity can be analyzed. Impedance spectroscopy operates in a noninvasive, label free manner and can readily be miniaturized. In contrast to optical density measurement, only living cells with intact plasma membranes significantly influence the observed electrical characteristics 73, 118, 119, 120. Air bubbles, non‐biological particles, and the ruptured cell membranes of dead cells do not affect the measurement. Modern impedance sensors use frequency scanning techniques in a range of 100 kHz to 20 MHz (dielectric spectroscopy) to estimate cell size and volume in addition to biomass concentration 118.
Currently, several sensors designed for standard bioreactors ports are commercially available, and new approaches for application in modern disposable reactor systems have been developed 121, 122, 123. These systems require special connectors that position the disposable electrodes inside the reactor because they need to be in direct contact with the culture broth. The connectors/ports are integrated into the SUB during production and are sterilized together with the reactor by γ‐radiation. For measurement, a reusable sensing device is connected to the electrodes via the port and the signal is obtained by an external hardware unit. To overcome this limitation, new sensor designs are in development that can measure directly through the plastic wall of the reactor without the need for a special connector. To accomplish this, the generated electrical field must be strong enough to pass through the SUB material while building up a sufficient electrical field inside the culture broth. This can be accomplished by the use of a high frequency coplanar transmission line as the sensor instead of electrodes placed inside the reactor (Fig. 4) 124.
Figure 4.
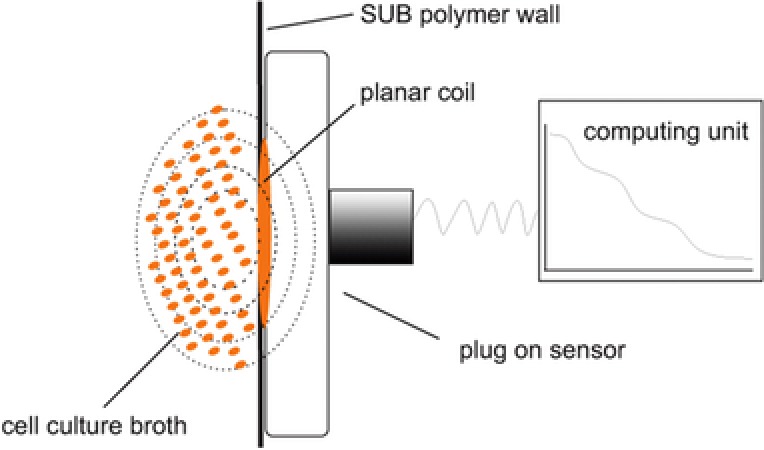
Setup of an impedance spectroscopy sensor, without the need for special connectors. Instead of two electrodes that are in direct contact with the culture broth, a planar coil is placed in direct contact to the outside of the SUB polymer wall. The electric field generated by the coil is strong enough to build up an electrical field inside the reactor, sufficient for measurement of culture permittivity.
4.3. Ultrasonic sensors
Ultrasonic signals are widely used in industry and medicine. In addition to the known applications such as medical imaging, mixing, and emulsifying, information about bioprocesses can be gathered by ultrasonic treatment. The principal parameters of measurement are the velocity of sound, the attenuation of sound, and the acoustic impedance. From these parameters, the chemical identity of pure liquids, the concentration of components in pure and mixed solutions, and particle sizes can be determined. The velocity of sound is calculated from the time difference between different echoes over a known distance, or by measuring the resonance frequency 125, 126. The attenuation of the signal is measured by the analysis of the exponential decrease of the echo amplitude. Attenuation and velocity are generally measured simultaneously because the determination of velocity is not very accurate for complex, multi‐component systems such as fermentation broth. Extensive information about process monitoring using ultrasound sensors is given by Henning and Rautenberg 127 or Hauptmann 125.
Ultrasonic sensors are particularly suitable for disposable bioreactors because they are noninvasive. Thus, the piezoelectric transmitter and receiver can be placed outside of the reactor without any modifications to the disposable bag itself (Fig. 5). However, the location and orientation of the transmitter and reflector/receiver are critical. Ultrasonic sensors do not involve any moving parts, respond rapidly, have excellent long‐term stability, and consume little power. Since the measurements are influenced by physical parameters including temperature and pressure, these must be monitored simultaneously to ensure meaningful data from an ultrasonic sensor. Air bubbles can also cause problems during measurement. Moreover, knowledge of the acoustic properties of the substances through which the sound is traveling is necessary 125.
Figure 5.
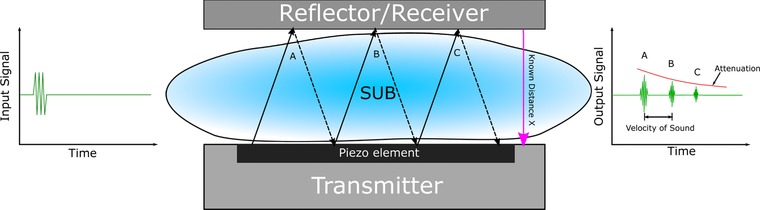
Schematic setup of an ultrasonic sensor for single‐use bioreactors.
Becker et al. 128 presented a noninvasive method based on ultrasonic velocity measurement for the determination of density during beer fermentation. Stanke et al. 129 developed a method for automated sonic velocity calculations for on‐line process monitoring. The use of ultrasonic waves for analytical protein monitoring was evaluated by Holz et al. 130. Beyond that, ultrasonic on‐line monitoring of cell mass in microbial, yeast and mammalian cultivation has been demonstrated 131, 132, 133. Cha et al. 134 showed that there is a linear correlation between the ultrasonic velocity and the concentrations of biomass, ethanol, and glucose during the cultivation of Saccharomyces cerevisiae. They developed several multivariate data analysis methods to derive quantitative information about the bioprocess on‐line.
4.4. Free‐floating wireless sensors
Recently, sensors have been developed that do not require any physical connection through the reactor bag but transmit data from wireless sensor spheres or capsules that float in the cultivation broth 135. Spheres to determine fluid dynamics are described in detail by Zimmermann et al. 136, 137, and sensor spheres to measure temperature, conductivity, pH, pressure and turbidity are also commercially available (e.g., smartCAPS, smartINST, Lyon, France). The shell of the sphere is made of sterilizable polyether ether ketone and is composed of onboard electronics, a battery, and the corresponding sensing element. The sensing element can take several forms, including ISFET technology with integrated reference electrode for pH measurement or LED/photodiode for turbidity monitoring. The data of every sphere is transmitted wirelessly to an external readout unit in real time.
Sensors floating in the cultivation broth offer the advantage that their flow follows the fluid so that any effects caused by the position of the sensor are eliminated. These sensors could be included in the SUB during production and can be delivered pre‐sterilized as well as pre‐calibrated. However, their sterilization has only been reported for steam and ethylene oxide procedures. γ‐radiation, used for sterilization of disposable bioreactors, might be problematic as it could damage the complex electronics.
5. Conclusions and outlook
Bioprocess monitoring in single‐use equipment needs novel sensors for better process understanding and optimization. Although numerous sensor systems for bioprocess analysis are available, few have been adapted to disposable reactors to date. Single‐use systems offer new application options for sensor technologies that are already available, but were difficult to apply to classical bioreactors (e.g., biosensors). In particular, noninvasive setups, such as ultrasonic or spectroscopic sensors, are favorable for transfer to disposable applications. Wireless technologies, including RFID chips or free‐floating sensor spheres, do not need any penetration through the reactor bag and thus have a great potential for future applications. Although simple translation of the novel sensors into classical systems is desired by the industry 87, it should be considered that new technologies offer the chance for improved or completely revised sensor implementation. This would allow the development of new, innovative reactor layouts and user interfaces. While scientists must further improve the accuracy, reproducibility, and durability of the sensor systems, device and SUB manufacturers should work together to develop standard interfaces, communication protocols, and the development of plug‐and‐play devices. Critical to this development is the willingness of industry to adopt new technologies.
The authors have declared no conflict of interest.
6 References
- 1. Rader, R. A. , Langer, E. S. , Upstream single‐use bioprocessing systems. Bioprocess Int. 2012, 10, 12–18. [Google Scholar]
- 2. Singh, V. , Singh, V. , Disposable bioreactor for cell culture using wave‐induced agitation. Cytotechnology 1999, 30, 149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allison, N. , Richards, J. , Current status and future trends for disposable technology in the biopharmaceutical industry. J. Chem. Technol. Biotechnol. 2014, 89, 1283–1287. [Google Scholar]
- 4. de Vries, I. , Busse, C. , Kopatz, J. , Neumann, H. , et al., Polysialic acid production using Escherichia coli K1 in a disposable bag reactor. Eng. Life Sci. 2017, 17, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eibl, R. , Kaiser, S. , Lombriser, R. , Eibl, D. , Disposable bioreactors: the current state‐of‐the‐art and recommended applications in biotechnology. Appl. Microbiol. Biotechnol. 2010, 86, 41–49. [DOI] [PubMed] [Google Scholar]
- 6. Jonczyk, P. , Schmidt, A. , Bice, I. , Gall, M. , et al., Strikt anaerobe Batch‐Kultivierung von Eubacterium ramulus in einem neuartigen Einweg‐Beutelreaktorsystem. Chemie‐Ingenieur‐Technik 2011, 83, 2147–2152. [Google Scholar]
- 7. Jonczyk, P. , Takenberg, M. , Hartwig, S. , Beutel, S. , et al., Cultivation of shear stress sensitive microorganisms in disposable bag reactor systems. J. Biotechnol. 2013, 167, 370–376. [DOI] [PubMed] [Google Scholar]
- 8. Lopes, A. G. , Single‐use in the biopharmaceutical industry: a review of current technology impact, challenges and limitations. Food Bioprod. Process 2015, 93, 98–114. [Google Scholar]
- 9. Eibl, R. , Eibl, D. , Disposable Bioreactors II, Springer New York, New York: 2014. [Google Scholar]
- 10. Eibl, R. , Eibl, D. , Disposable Bioreactors, Springer Berlin / Heidelberg, Dordrecht London, New York: 2009. [Google Scholar]
- 11. Lindner, P. , Endres, C. , Bluma, A. , Höpfner, T. , et al., Disposable Sensor Systems, in: Eible R., Eibl D. (Eds.), Single‐Use Technology in Biopharmaceutical Manufacture, John Wiley & Sons, Inc, Hoboken, NJ, USA: 2010, pp. 68–77. [Google Scholar]
- 12. Bluma, A. , Höpfner, T. , Prediger, A. , Glindkamp, A. , et al., Process analytical sensors and image‐based techniques for single‐use bioreactors. Eng. Life Sci. 2011, 11, 550–553. [Google Scholar]
- 13. Biechele, P. , Busse, C. , Solle, D. , Scheper, T. , et al., Sensor systems for bioprocess monitoring. Eng. Life Sci. 2015, 15, 1–20. [Google Scholar]
- 14. Glindkamp, A. , Riechers, D. , Rehbock, C. , Hitzmann, B. , et al., Sensors in disposable bioreactors status and trends, Adv. Biochem. Eng./Biotechnol. 2009, 115, 1–25. [DOI] [PubMed] [Google Scholar]
- 15. Bink, L. R. , Furey, J. , Using in‐line disposable pressure sensors to evaluate depth filter performance. Bioprocess Int. 2010, 8, 44–49. [Google Scholar]
- 16. Hans, M. A. , Heinzle, E. , Wittmann, C. , Quantification of intracellular amino acids in batch cultures of Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2001, 56, 776–779. [DOI] [PubMed] [Google Scholar]
- 17. Wittmann, C. , Krömer, J. O. , Kiefer, P. , Binz, T. , et al., Impact of the cold shock phenomenon on quantification of intracellular metabolites in bacteria. Anal. Biochem. 2004, 327, 135–139. [DOI] [PubMed] [Google Scholar]
- 18. Buchholz, A. , Hurlebaus, J. , Wandrey, C. , Takors, R. , Metabolomics: quantification of intracellular metabolite dynamics. Biomol. Eng. 2002, 19, 5–15. [DOI] [PubMed] [Google Scholar]
- 19. Buchholz, a , Takors, R ., Wandrey, C. , Quantification of intracellular metabolites in Escherichia coli K12 using liquid chromatographic‐electrospray ionization tandem mass spectrometric techniques. Anal. Biochem. 2001, 295, 129–137. [DOI] [PubMed] [Google Scholar]
- 20. Weuster‐Botz, D. , Sampling tube device for monitoring intracellular metabolite dynamics. Anal. Biochem. 1997, 246, 225–233. [DOI] [PubMed] [Google Scholar]
- 21. da Luz, J. A. , Hans, E. , Zeng, A. P. , Automated fast filtration and on‐filter quenching improve the intracellular metabolite analysis of microorganisms. Eng. Life Sci. 2014, 14, 135–142. [Google Scholar]
- 22. Hiller, J. , Franco‐Lara, E. , Papaioannou, V. , Weuster‐Botz, D. , Fast sampling and quenching procedures for microbial metabolic profiling. Biotechnol. Lett. 2007, 29, 1161–1167. [DOI] [PubMed] [Google Scholar]
- 23. Lange, H. C. , Eman, M. , Van Zuijlen, G. , Visser, D. , et al., Improved rapid sampling for in vivo kinetics of intracellular metabolites in Saccharomyces cerevisiae. Biotechnol. Bioeng. 2001, 75, 406–415. [DOI] [PubMed] [Google Scholar]
- 24. Hakanson, H. , Nilsson, M. , Mattiasson, B. , General sampling system for sterile monitoring of biological processes. Anal. Chim. Acta 1991, 249, 61–65. [Google Scholar]
- 25. Lücking, T. H. , Busse, C. , Lüder, C. , Bulnes‐Abundis, D. , et al., A novel measuring chamber and automation platform for mammalian cell culture processes. BMC Proc. 2015, 9, P30. [Google Scholar]
- 26. Hilmer, J. M. , Scheper, T. , A new version of an in situ sampling system for bioprocess analysis. Acta Biotechnol. 1996, 16, 185–192. [Google Scholar]
- 27. Holzhauer‐Rieger, K. , Zhou, W. , Schügerl, K. , On‐line high‐performance liquid chromatography for the determination of cephalosporin C and by‐products in complex fermentation broths. J. Chromatogr. A 1990, 499, 609–615. [DOI] [PubMed] [Google Scholar]
- 28. Wilkens, E. , Ringel, A. K. , Hortig, D. , Willke, T. , et al., High‐level production of 1,3‐propanediol from crude glycerol by Clostridium butyricum AKR102a. Appl. Microbiol. Biotechnol. 2012, 93, 1057–1063. [DOI] [PubMed] [Google Scholar]
- 29. Mattiasson, B. , Hakanson, H. , Sampling and sample handling–crucial steps in process monitoring and control. Trends Biotechnol. 1993, 11, 136–142. [DOI] [PubMed] [Google Scholar]
- 30. Mandenius, C. F. , Danielsson, B. , Mattiasson, B. , Evaluation of a dialysis probe for continuous sampling in fermentors and in complex media. Anal. Chim. Acta 1984, 163, 135–141. [Google Scholar]
- 31. Buttler, T. , Nilsson, C. , Gorton, L. , Marko‐Varga, G. , et al., Membrane characterisation and performance of microdialysis probes intended for use as bioprocess sampling units. J. Chromatogr. A 1996, 725, 41–56. [Google Scholar]
- 32. Seifert, G. K. E. , Matteau, P. P. , An automatic aseptic bioreactor sampling system. Biotechnol. Bioeng. 1988, 32, 923–926. [DOI] [PubMed] [Google Scholar]
- 33. Reda, K. D. , Thien, M. P. , Feygin, I. , Marcin, C. S. , et al., Automatic whole broth multi‐fermentor sampling. J. Ind. Microbiol. 1991, 7, 215–220. [Google Scholar]
- 34. Chong, L. , Saghafi, M. , Knappe, C. , Steigmiller, S. , et al., Robust on‐line sampling and analysis during long‐term perfusion cultivation of mammalian cells. J. Biotechnol. 2013, 165, 133–137. [DOI] [PubMed] [Google Scholar]
- 35. Hartlep, M. , Künnecke, W. , Online‐Glukoseregelung zur Kultivierung von tierischen und humanen Zelllinien, in: Bioprocessing Days 2016, Recklinghausen: 2016. [Google Scholar]
- 36. Růžička, J. , Hansen, E. H. , Flow Injection Analysis, John Wiley & Sons, New York, USA: 1988. [Google Scholar]
- 37. Scheper, T. , Brandes, W. , Maschke, H. , Plötz, F. , et al., Two FIA‐based biosensor systems studied for bioprocess monitoring. J. Biotechnol. 1993, 31, 345–356. [DOI] [PubMed] [Google Scholar]
- 38. Haouz, A. , Stieg, S. , Continuous monitoring of D‐glucose and L‐lactate by flow injection analysis. Enzyme Microb. Technol. 2002, 30, 129–133. [Google Scholar]
- 39. Forman, L. W. , Thomas, B. D. , Jacobson, F. S. , On‐line monitoring and control of fermentation processes by flow‐injection analysis. Anal. Chim. Acta 1991, 249, 101–111. [Google Scholar]
- 40. Garn, M. , Gisin, M. , Thommen, C. , Cevey, P. , Flow injection analysis system for fermentation monitoring and control. Biotechnol. Bioeng. 1989, 34, 423–428. [DOI] [PubMed] [Google Scholar]
- 41. Chatterjee, M. , Ge, X. , Uplekar, S. , Kostov, Y. , et al., A unique noninvasive approach to monitoring dissolved O2 and CO2 in cell culture. Biotechnol. Bioeng. 2015, 112, 104–110. [DOI] [PubMed] [Google Scholar]
- 42. Bergveld, P. , Thirty years of ISFETOLOGY: what happened in the past 30 years and what may happen in the next 30 years. Sensors Actuators B Chem. 2003, 88, 1–20. [Google Scholar]
- 43. Yuqing, M. , Jianguo, G. , Jianrong, C. , Ion sensitive field effect transducer‐based biosensors. Biotechnol. Adv. 2003, 21, 527–534. [DOI] [PubMed] [Google Scholar]
- 44. Zafar, S. , D´Emic, C. , Afzali, A. , Flechter, B. , et al., Optimization of pH sensing using silicon nanowire field effect transistors with HfO2 as the sensing surface. Nanotechnology 2011, 22, 405501. [DOI] [PubMed] [Google Scholar]
- 45. Knopfmacher, O. , Tarasov, A. , Fu, W. , Wipf, M. , et al., Nernst limit in dual‐gated Si‐nanowire FET sensors. Nano Lett. 2010, 10, 2268–2274. [DOI] [PubMed] [Google Scholar]
- 46. Vijayalakshmi, A. , Tarunashree, Y. , Baruwati, B. , Manorama, S. V , et al., Enzyme field effect transistor (ENFET) for estimation of triglycerides using magnetic nanoparticles. Biosens. Bioelectron. 2008, 23, 1708–1714. [DOI] [PubMed] [Google Scholar]
- 47. Lee, C.‐S. , Kim, S. K. , Kim, M. , Ion‐sensitive field‐effect transistor for biological sensing. Sensors 2009, 9, 7111–7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mulchandani, a , Bassi, a S ., Principles and applications of biosensors for bioprocess monitoring and control. Crit. Rev. Biotechnol. 1995, 15, 105–124. [DOI] [PubMed] [Google Scholar]
- 49. Scheper, T. , Hitzmann, B. , Stärk, E. , Ulber, R. , et al., Bioanalytics: detailed insight into bioprocesses. Anal. Chim. Acta 1999, 400, 121–134. [Google Scholar]
- 50. Bracewell, D. G. , Gill, A. , Hoare, M. , An in‐line flow injection optical biosensor for real‐time bioprocess monitoring. Food Bioprod. Process. 2002, 80, 71–77. [Google Scholar]
- 51. Jong Il Rhee, A. R. , Scheper, T. , On‐line monitoring and control of substrate concentrations in biological processes by flow injection analysis systems. Biotechnol. Bioprocess Eng. 2004, 9, 156–165. [Google Scholar]
- 52. Borisov, S. M. , Wolfbeis, O. S. , Biosensors Optical. Chem. Rev. 2008, 108, 423–461. [DOI] [PubMed] [Google Scholar]
- 53. Becker, T. , Hitzmann, B. , Muffler, K. , Pörtner, R. , et al., Future aspects of bioprocess monitoring. Adv. Biochem. Eng. Biotechnol. 2006, 105, 249–293. [DOI] [PubMed] [Google Scholar]
- 54. Grieshaber, D. , MacKenzie, R. , Vörös, J. , Reimhult, E. , Electrochemical biosensors ‐ sensor principles and architectures. Sensors 2008, 8, 1400–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Thévenot, D. R. , Toth, K. , Durst, R. A. , Wilson, G. S. , Electrochemical biosensors: recommended definitions and classification. Biosens. Bioelectron. 2001, 16, 121–131. [DOI] [PubMed] [Google Scholar]
- 56. Cleland, N. , Enfors, S. , Control of glucose‐fed batch cultivations of E. coli by means of an oxygen stabilized enzyme electrode. Eur. J. Appl. Microbiol. Biotechnol. 1983, 18, 141–147. [Google Scholar]
- 57. Park, J. , Kim, H. , A new biosensor for specific determination of glucose or fructose using an oxidoreductase of Zymomonas rnobilis. Biotechnol. Bioeng. 1990, 36, 744–749. [DOI] [PubMed] [Google Scholar]
- 58. Bauer, I. , Poggendorf, I. , Spichiger, S. , Spichiger‐Keller, U. E. , et al., Novel single‐use sensors for online measurement of glucose. Bioprocess Int. 2012, 10, 56–60. [Google Scholar]
- 59. Rao, G. , Lam, H. , Kostov, Y. , Optical instrumentation for bioprocess monitoring. Adv. Biochem. Eng. Biotechnol. 2010, 116, 125–142. [DOI] [PubMed] [Google Scholar]
- 60. Potyrailo, R. A. , Surman, C. , Monk, D. , Morris, W. G. , et al., RFID sensors as the common sensing platform for single‐use biopharmaceutical manufacturing. Meas. Sci. Technol. 2011, 22, 1–17. [Google Scholar]
- 61. Radiofrequency identification technology: protecting the drug supply. FDA Consum. Mag. 2005. https://www.fda.gov/Drugs/DrugSafety/ucm169918.htm. [PubMed] [Google Scholar]
- 62. Demuth, C. , Chemische sensoren in der bioprozessanalytik. Chemie Unserer Zeit 2013, 47, 2–9. [Google Scholar]
- 63. Oprea, a. , Courbat, J. , Bârsan, N. , Briand, D. , et al., Temperature, humidity and gas sensors integrated on plastic foil for low power applications. Sensors Actuators, B Chem. 2009, 140, 227–232. [Google Scholar]
- 64. Potyrailo, R. A. , Surman, C. , Sivavec, T. , Wortley, T. , Passive multivariable RFID pH sensors. 2011 IEEE Int. Conf. RFID‐Technologies Appl. RFID‐TA 2011 2011, 533–536.
- 65. Potyrailo, R. a , Wortley, T. , Surman, C. , Monk, D. , et al., Passive multivariable temperature and conductivity RFID sensors for single‐use biopharmaceutical manufacturing components. Biotechnol. Prog. 2011, 27, 875–84. [DOI] [PubMed] [Google Scholar]
- 66. Potyrailo, R. A. , Surman, C. , A passive radio‐frequency identification (RFID) gas sensor with self‐correction against fluctuations of ambient temperature. Sensors Actuators, B Chem. 2013, 185, 587–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Surman, C. , Potyrailo, R. a. , Morris, W. G. , Wortley, T. , et al., Temperature‐independent passive RFID pressure sensors for single‐use bioprocess components. IEEE Int. Conf. RFID 2011, April 12–14, 2011, 78–84. [Google Scholar]
- 68. Potyrailo, R. A. , Morris, W. G. , Multianalyte chemical identification and quantitation using a single radio frequency identification sensor. Anal. Chem. 2007, 79, 45–51. [DOI] [PubMed] [Google Scholar]
- 69. Bambot, S. B. , Holavanahali, R. , Lakowicz, J. R. , Carter, G. M. , et al., Phase fluorometric sterilizable optical oxygen sensor. Biotechnol. Bioeng. 1994, 43, 1139–1145. [DOI] [PubMed] [Google Scholar]
- 70. Harms, P. , Kostov, Y. , Rao, G. , Bioprocess monitoring. Curr. Opin. Biotechnol. 2002, 13, 124–127. [DOI] [PubMed] [Google Scholar]
- 71. Rehbock, C. , Beutel, S. , Brückerhoff, T. , Hitzmann, B. , et al., Bioprozessanalytik. Chemie Ing. Tech. 2008, 80, 267–286. [Google Scholar]
- 72. Rao, G. , Lam, H. , Kostov, Y. , Optical Instrumentation for Bioprocess Monitoring, in: Optical Sensor Systems in Biotechnology, Springer, Berlin Heidelberg, 2010, pp. 125–142. [Google Scholar]
- 73. Sonnleitner, B. , Automated measurement and monitoring of bioprocesses: key elements of the M3C strategy, in: Measurement, Monitoring, Modelling and Control of Bioprocesses, Springer, Berlin, 2013, pp. 1–33. [DOI] [PubMed] [Google Scholar]
- 74. Amao, Y. , Probes and polymers for optical sensing of oxygen. Microchim. Acta 2003, 143, 1–12. [Google Scholar]
- 75. Ulber, R. , Frerichs, J.‐G. , Beutel, S. , Optical sensor systems for bioprocess monitoring. Anal. Bioanal. Chem. 2003, 376, 342–348. [DOI] [PubMed] [Google Scholar]
- 76. Gupta, P. A. , Ge, X. , Kostov, Y. , Rao, G. , A completely noninvasive method of dissolved oxygen monitoring in disposable small‐scale cell culture vessels based on diffusion through permeable vessel walls. Biotechnol. Prog. 2014, 30, 172–177. [DOI] [PubMed] [Google Scholar]
- 77. Wolfbeis, O. S. , Materials for fluorescence‐based optical chemical sensors. J. Mater. Chem. 2005, 15, 2657–2669. [Google Scholar]
- 78. Weidgans, B. M. , Krause, C. , Klimant, I. , Wolfbeis, O.S. , Fluorescent pH sensors with negligible sensitivity to ionic strength. Analyst 2004, 129, 645–650. [DOI] [PubMed] [Google Scholar]
- 79. Lin, J. , Recent development and applications of optical and fiber‐optic pH sensors. TrAC Trends Anal. Chem. 2000, 19, 541–552. [Google Scholar]
- 80. Beutel, S. , Henkel, S. , In situ sensor techniques in modern bioprocess monitoring. Appl. Microbiol. Biotechnol. 2011, 91, 1493–1505. [DOI] [PubMed] [Google Scholar]
- 81. Gupta, B. D. , Sharma, S. , A long‐range fiber optic pH sensor prepared by dye doped sol‐gel immobilization technique. Opt. Commun. 1998, 154, 282–284. [Google Scholar]
- 82. Ali, R. , Saleh, S. M. , Meier, R. J. , Azab, H. A. , et al., Upconverting nanoparticle based optical sensor for carbon dioxide. Sensors Actuators B Chem. 2010, 150, 126–131. [Google Scholar]
- 83. Ge, X. , Kostov, Y. , Rao, G. , High‐stability noninvasive autoclavable naked optical CO2 sensor. Biosens. Bioelectron. 2003, 18, 857–865. [DOI] [PubMed] [Google Scholar]
- 84. Ge, X. , Kostov, Y. , Rao, G. , Low‐cost noninvasive optical CO2 sensing system for fermentation and cell culture. Biotechnol. Bioeng. 2005, 89, 329–334. [DOI] [PubMed] [Google Scholar]
- 85. Chatterjee, M. , Ge, X. , Uplekar, S. , Kostov, Y. , et al., A unique noninvasive approach to monitoring dissolved O2 and CO2 in cell culture. Biotechnol. Bioeng. 2014, 112, 104–110. [DOI] [PubMed] [Google Scholar]
- 86. Glindkamp, A. , Riechers, D. , Rehbock, C. , Hitzmann, B. , et al., Sensors in disposable bioreactors status and trends. Dispos. Bioreact. Adv. Biochem. Eng. / Biotechnol. 2010, 115, 145–169. [DOI] [PubMed] [Google Scholar]
- 87. Diehl, B. H. , Lapack, M. A. , Wang, T. Y. , Kottmeier, R.E. , et al., A biopharmaceutical industry perspective on single‐use sensors for biological process applications. BioPharm Int. 2015, 28, 28–53. [Google Scholar]
- 88. Rhiel, M. , Ducommun, P. , Bolzonella, I. , Marison, I. , et al., Real‐time in situ monitoring of freely suspended and immobilized cell cultures based on mid‐infrared spectroscopic measurements. Biotechnol. Bioeng. 2002, 77, 174–185. [DOI] [PubMed] [Google Scholar]
- 89. Sandor, M. , Rüdinger, F. , Bienert, R. , Grimm, C. , et al., Comparative study of noninvasive monitoring via infrared spectroscopy for mammalian cell cultivations. J. Biotechnol. 2013, 168, 636–645. [DOI] [PubMed] [Google Scholar]
- 90. Sellick, C.A. , Hansen, R. , Jarvis, R.M. , Maqsood, A. R. , et al., Rapid monitoring of recombinant antibody production by mammalian cell cultures using fourier transform infrared spectroscopy and chemometrics. Biotechnol. Bioeng. 2010, 106, 432–442. [DOI] [PubMed] [Google Scholar]
- 91. Schenk, J. , Marison, I. W. , von Stockar, U. , pH prediction and control in bioprocesses using mid‐infrared spectroscopy. Biotechnol. Bioeng. 2008, 100, 82–93. [DOI] [PubMed] [Google Scholar]
- 92. Kornmann, H. , Rhiel, M. , Cannizzaro, C. , Marison, I. , et al., Methodology for real‐time, multianalyte monitoring of fermentations using an in‐situ mid‐infrared sensor. Biotechnol. Bioeng. 2003, 82, 702–709. [DOI] [PubMed] [Google Scholar]
- 93. Mazarevica, G. , Diewok, J. , Baena, J. R. , Rosenberg, E. , et al., On‐line fermentation monitoring by mid‐infrared spectroscopy. Appl. Spectrosc. 2004, 58, 804–810. [DOI] [PubMed] [Google Scholar]
- 94. Arnold, S. A. , Crowley, J. , Woods, N. , Harvey, L. M. , et al., In‐situ near infrared spectroscopy to monitor key analytes in mammalian cell cultivation. Biotechnol. Bioeng. 2003, 84, 13–19. [DOI] [PubMed] [Google Scholar]
- 95. Rao, G. , Henriques, J. G. , Buziol, S. , Stocker, E. , et al., Monitoring mammalian cell cultivations for monoclonal antibody production using near‐infrared spectroscopy, in: Optical Sensor Systems in Biotechnology, Springer; Berlin Heidelberg, 2010, pp. 29–72. [Google Scholar]
- 96. Clavaud, M. , Roggo, Y. , Von Daeniken, R. , Liebler, A. , et al., Chemometrics and in‐line near infrared spectroscopic monitoring of a biopharmaceutical Chinese hamster ovary cell culture: prediction of multiple cultivation variables. Talanta 2013, 111, 28–38. [DOI] [PubMed] [Google Scholar]
- 97. Card, C. , Hunsaker, B. , Smith, T. , Hirsch, J. , Near‐infrared spectroscopy for rapid, simultaneous monitoring. Bioprocess Int. 2008, 6, 58–67. [Google Scholar]
- 98. Kiviharju, K. , Salonen, K. , Moilanen, U. , Meskanen, E. , et al., On‐line biomass measurements in bioreactor cultivations: comparison study of two on‐line probes. J. Ind. Microbiol. Biotechnol. 2007, 34, 561–566. [DOI] [PubMed] [Google Scholar]
- 99. Noui, L. , Hill, J. , Keay, P. J. , Wang, R. Y. , et al., Development of a high resolution UV spectrophotometer for at‐line monitoring of bioprocesses. Chem. Eng. Process. Process Intensif. 2002, 41, 107–114. [Google Scholar]
- 100. Pons, M.‐N. , Le Bonté, S. , Potier, O. , Spectral analysis and fingerprinting for biomedia characterisation. J. Biotechnol. 2004, 113, 211–230. [DOI] [PubMed] [Google Scholar]
- 101. Duysens, L. N. M. , Amesz, J. , Fluorescence spectrophotometry of reduced phosphopyridine nucleotide in intact cells in the near‐ultraviolet and visible region. Biochim. Biophys. Acta 1957, 24, 19–26. [DOI] [PubMed] [Google Scholar]
- 102. Hantelmann, K. , Kollecker, M. , Hüll, D. , Hitzmann, B. , et al., Two‐dimensional fluorescence spectroscopy: a novel approach for controlling fed‐batch cultivations. J. Biotechnol. 2006, 121, 410–417. [DOI] [PubMed] [Google Scholar]
- 103. Haack, M. B. , Eliasson, A. , Olsson, L. , On‐line cell mass monitoring of Saccharomyces cerevisiae cultivations by multi‐wavelength fluorescence. J. Biotechnol. 2004, 114, 199–208. [DOI] [PubMed] [Google Scholar]
- 104. Lee, H. L. T. , Boccazzi, P. , Gorret, N. , Ram, R. J. , et al., In situ bioprocess monitoring of Escherichia coli bioreactions using Raman spectroscopy. Vib. Spectrosc. 2004, 35, 131–137. [Google Scholar]
- 105. Cannizzaro, C. , Rhiel, M. , Marison, I. , von Stockar, U. , On‐line monitoring of Phaffia rhodozyma fed‐batch process with in situ dispersive Raman spectroscopy. Biotechnol. Bioeng. 2003, 83, 668–680. [DOI] [PubMed] [Google Scholar]
- 106. Landgrebe, D. , Haake, C. , Höpfner, T. , Beutel, S. , et al., On‐line infrared spectroscopy for bioprocess monitoring. Appl. Microbiol. Biotechnol. 2010, 88, 11–22. [DOI] [PubMed] [Google Scholar]
- 107. Vojinovic, V. , Cabral, J. M. S. , Fonseca, L. P. , Real‐time bioprocess monitoring: Part I: in situ sensors. Sensors Actuators B Chem. 2006, 114, 1083–1091. [Google Scholar]
- 108. Tartakovsky, B. , Sheintuch, M. , Hilmer, J. M. , Scheper, T. , Application of scanning fluorometry for monitoring of a fermentation process. Biotechnol. Prog. 1996, 12, 126–131. [DOI] [PubMed] [Google Scholar]
- 109. Scheper, T. H. , Lorenz, T. H. , Schmidt, W. , Schügerl, K. , On‐line measurement of culture fluorescence for process monitoring and control of biotechnological processes. Ann. N. Y. Acad. Sci. 1987, 506, 431–445. [DOI] [PubMed] [Google Scholar]
- 110. Anders, K. D. , Wehnert, G. , Thordsen, O. , Scheper, T. , et al., Biotechnological applications of fiber‐optic sensing: multiple uses of a fiber‐optic fluorimeter. Sensors Actuators B Chem. 1993, 11, 395–403. [Google Scholar]
- 111. Sato, K. , Yoshida, Y. , Hirahara, T. , Ohba, T. , On‐line measurement of intracellular ATP of Saccharomyces cerevisiae and pyruvate during sake mashing. J. Biosci. Bioeng. 2000, 90, 294–301. [PubMed] [Google Scholar]
- 112. Kessler, R. W. , Prozessanalytik, WILEY‐vch, Weinheim: 2006. [Google Scholar]
- 113. Roychoudhury, P. , Harvey, L. M. , McNeil, B. , The potential of mid infrared spectroscopy (MIRS) for real time bioprocess monitoring. Anal. Chim. Acta 2006, 571, 159–166. [DOI] [PubMed] [Google Scholar]
- 114. Scarff, M. , Arnold, S. A. , Harvey, L. M. , McNeil, B. , Near infrared spectroscopy for bioprocess monitoring and control: current status and future trends. Crit. Rev. Biotechnol. 2006, 26, 17–39. [DOI] [PubMed] [Google Scholar]
- 115. Claßen, J. , Aupert, F. , Reardon, K. F. , Solle, D. , et al., Spectroscopic sensors for in‐line bioprocess monitoring in research and pharmaceutical industrial application. Anal. Bioanal. Chem. 2017, 409, 651–666. [DOI] [PubMed] [Google Scholar]
- 116. Whelan, J. , Craven, S. , Glennon, B. , In situ Raman spectroscopy for simultaneous monitoring of multiple process parameters in mammalian cell culture bioreactors. Biotechnol. Prog. 2012, 28, 1355–1362. [DOI] [PubMed] [Google Scholar]
- 117. Berry, B. N. , Dobrowsky, T. M. , Timson, R. C. , Kshirsagar, R. , et al., Quick generation of Raman spectroscopy based in‐process glucose control to influence biopharmaceutical protein product quality during mammalian cell culture. Biotechnol. Prog. 2016, 32, 224–234. [DOI] [PubMed] [Google Scholar]
- 118. Carvell, J. , Dowd, J. , On‐line measurements and control of viable cell density in cell culture manufacturing processes using radio‐frequency impedance. Cytotechnology 2006, 50, 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Bill, R. M. , Recombinant protein production in yeast: methods and protocols. Methods Mol. Biol. 2012, 866, 1064–3745. [DOI] [PubMed] [Google Scholar]
- 120. Palmer, S. M. , Kunji, E.R. , Online monitoring of biomass accumulation in recombinant yeast cultures. Methods Mol. Biol. 2012, 866, 165–179. [DOI] [PubMed] [Google Scholar]
- 121. Logan, D. W. , Carvell, J. P. , Lee, M. P. H. , Creating new opportunities in process control through radio frequency impedance spectroscopy, in: BMC Proceedings, BioMed Central Ltd, London, UK, 2011, p. P57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Downey, B. J. , Graham, L. J. , Breit, J. F. , Glutting, N. K. , A novel approach for using dielectric spectroscopy to predict viable cell volume (VCV) in early process development. Biotechnol. Prog. 2014, 30, 479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Arnoux, A. S. , Preziosi‐Belloy, L. , Esteban, G. , Teissier, P. , et al., Lactic acid bacteria biomass monitoring in highly conductive media by permittivity measurements. Biotechnol. Lett. 2005, 27, 1551–1557. [DOI] [PubMed] [Google Scholar]
- 124. Reinecke, T. , Biechele, P. , Schulte, V. , Scheper, T. , et al., Low‐cost sensor system for noninvasive monitoring of cell growth in disposable bioreactors. Procedia Eng. 2015, 120, 548–551. [Google Scholar]
- 125. Hauptmann, P. , Lucklum, R. , Puttmer, A. , Henning, B. , Ultrasonic sensors for process monitoring and chemical analysis: state‐of‐the‐art and trends. Sensors Actuators, A Phys. 1998, 67, 32–48. [Google Scholar]
- 126. Hauptmann, P. , Hoppe, N. , Püttmer, A. , Application of ultrasonic sensors in the process industry. Meas. Sci. Technol. 2002, 13, R73–R83. [Google Scholar]
- 127. Henning, B. , Rautenberg, J. , Process monitoring using ultrasonic sensor systems. Ultrasonics 2006, 44, 1395–1399. [DOI] [PubMed] [Google Scholar]
- 128. Becker, B. T. , Mitzscherling, M. , Delgado, A. , Ultrasonic velocity ‐ a noninvasive method for the determination of density during beer fermentation. Eng. Life Sci. 2001, 1, 61–67. [Google Scholar]
- 129. Stanke, M. , Lindner, P. , Holz, S. , Hitzmann, B. , Automated sonic velocity calculation based on ultrasonic resonator measurements for on‐line process monitoring. Sensors Actuators, A Phys. 2013, 198, 69–74. [Google Scholar]
- 130. Holz, S. , Schoenbeck, I. , Stanke, M. , Hartlep, M. , et al., Onlineanalyse von Proteinen mittels Ultraschall – der „ PROTEINMONITOR “ Zusammenfassung Einleitung, in: 10. Dresdner Sensor‐Symposium, AMA, 2011, pp. 77–80.
- 131. Henning, H. B. , Lachmann, A. , Afschar, K. H. B. , Ultraschalluntersuchungen Während des Fermentationsprozesses, in: INCOM, Düsseldorf: 1996, p. 305. [Google Scholar]
- 132. Blake‐Coleman, B. C. , Clarke, D. J. , Calder, M. R. , Moody, S. C. , Determination of reactor biomass by acoustic resonance densitometry. Biotechnol. Bioeng. 1986, 28, 1241–1249. [DOI] [PubMed] [Google Scholar]
- 133. Kilburn, D. G. , Fitzpatrick, P. , Blake‐Coleman, B. C. , Clarke, D. J. , et al., On‐line monitoring of cell mass in mammalian cell cultures by acoustic densitometry. Biotechnol. Bioeng. 1989, 33, 1379–1384. [DOI] [PubMed] [Google Scholar]
- 134. Cha, Y. , Ultraschallgeschwindigkeitsmessungen zum Bioprozessmonitoring, Leibniz Universität, Hannover, 2005. [Google Scholar]
- 135. Tyndall National Institute , Invention could revolutionize production of future medicine. Science Daily 2015, https://www.sciencedaily.com/releases/2015/10/151019123748.htm. [Google Scholar]
- 136. Zimmermann, R. , Fiabane, L. , Gasteuil, Y. , Measuring Lagrangian accelerations using an instrumented particle. Arxiv Prepr. arXiv 2012, 14063, 1–8. [Google Scholar]
- 137. Zimmermann, R. , Fiabane, L. , Gasteuil, Y. , Volk, R. , et al., Characterizing flows with an instrumented particle measuring Lagrangian accelerations. New J. Phys. 2013, 15, 015018. [Google Scholar]


