Abstract
Anaerobic oxidation of methane (AOM) coupled to sulfate reduction is a microbially mediated unique natural phenomenon with an ecological relevance in the global carbon balance and potential application in biotechnology. This study aimed to enrich an AOM performing microbial community with the main focus on anaerobic methanotrophic archaea (ANME) present in sediments from the Ginsburg mud volcano (Gulf of Cadiz), a known site for AOM, in a membrane bioreactor (MBR) for 726 days at 22 (± 3)°C and at ambient pressure. The MBR was equipped with a cylindrical external ultrafiltration membrane, fed a defined medium containing artificial seawater and operated at a cross flow velocity of 0.02 m/min. Sulfide production with simultaneous sulfate reduction was in equimolar ratio between days 480 and 585 of MBR operation, whereas methane consumption was in oscillating trend. At the end of the MBR operation (day 726), the enriched biomass was incubated with 13C labeled methane, 13C labeled inorganic carbon was produced and the AOM rate based on 13C‐inorganic carbon was 1.2 μmol/(gdw d). Microbial analysis of the enriched biomass at 400 and 726 days of MBR operation showed that ANME‐2 and Desulfosarcina type sulfate reducing bacteria were enriched in the MBR, which formed closely associated aggregates. The major relevance of this study is the enrichment of an AOM consortium in a MBR system which can assist to explore the ecophysiology of ANME and provides an opportunity to explore the potential application of AOM.
Keywords: Anaerobic methanotrophs (ANME), Anaerobic oxidation of methane (AOM), Desulfosarcina, Membrane bioreactor (MBR), Sulfate reduction (SR)
Abbreviations
- ANME
anaerobic methanotrophs
- AOM
anaerobic oxidation of methane
- AOM‐SR
anaerobic oxidation of methane coupled to sulfate reduction
- CARD‐FISH
Catalyzed reporter deposition‐fluorescence in situ hybridization
- DIC
dissolved inorganic carbon
- MBR
membrane bioreactor
- SRB
sulfate reducing bacteria
1. Introduction
The anaerobic oxidation of methane (AOM) coupled to sulfate reduction is a prominent methane consuming microbial process occurring in anoxic marine sediments 1, 2, 3, 4. From a thermodynamic view point, AOM coupled to sulfate reduction (AOM‐SR) yields relatively low amounts of Gibbs free energy (∼16.5 KJ/mol). The AOM‐SR process is common in sites with methane seeping or diffusing to the sediment surface, such as at methane seeps 5, 6, methane hydrates 7, 8, 9, hydrothermal vents 10, 11 and methanogenic sediments 4, 12, 13. In those specific niches, few phylogenetically distinct microbial clades, that is, anaerobic methanotrophic archaea (ANME) and their associated sulfate reducing bacteria (SRB), are able to grow via energy obtained from AOM‐SR 1.
Three groups of ANME have been distinguished so far, of which ANME‐1 and ANME‐2 are the most abundant and geographically widespread groups 1, 14, 15, 16, whereas ANME‐3 has been retrieved from a few marine mud volcanoes 16. The Desulfosarcina/Desulfococcus group and Desulfobulbus clades of the Deltaproteobacteria are well known sulfate reducing partners of ANME. There is still a major knowledge gap in AOM studies about the mechanisms and the ecophysiology of ANME. Some of the studies have postulated independent AOM performed solely by ANME 3, 17, whilst other studies have postulated that a consortia of ANME and SRB act together for AOM by interspecies electron transfer 18.
Several AOM rate determination and ANME cultivation studies have been performed in batch and continuous bioreactors using several ANME enrichments, often with largely varying results. For instance, AOM‐SR rates were observed in a wide range of 0.375‐286 μmol per gram dry weight per day (μmol/(gdw d)) in continuous systems 19, 20, 21, 1–20 μmol/(gdw d) in batch systems 10, 22, 23 and up to 230 μmol/(gdw d) in a fed‐batch system 24. In a batch system inoculated with sediment from the Gulf of Mexico, the AOM rate was 8.75 (± 1.05) μmol/(gdw d), i.e. almost 25 times lower than in a fed‐batch incubation system 22. The AOM‐SR rate obtained from southern Hydrate Ridge (Offshore Oregon) was 20 to 230 μmol/(gdw d) when this sediment was incubated in a fed‐batch bioreactor, while the highest AOM‐SR rate (230 μmol/(gdw d)) was obtained at elevated methane partial pressures of 1.4 MPa 24, 25. In another recent study, ANME‐2c growth was observed when the biomass from Eckernförde Bay (Baltic Sea) was incubated at 10 MPa pressure in high pressure capsules with the AOM rate of 24 μmol/(gvss d) 26.
A major hindrance for studies pertaining to the AOM mechanism is the difficulty to culture or enrich ANME, imputable to the requirements of rigorous anaerobic conditions and constant methane availability as well as the extremely slow growth rate of the ANME. A robust bioreactor design is thus required for their enrichment and cultivation. Anaerobic membrane bioreactors (MBR) allow complete biomass retention and are commonly applied at the industrial scale for wastewater and waste gas treatment 27. In the field of AOM, at the laboratory scale, MBR have been used to enrich slow growing anaerobes such as ANME‐SR 21 or ANME‐denitrification 28. In a continuously operated submerged membrane MBR, inoculated with estuarine sediment from Eckernförde Bay (Baltic Sea), a maximum AOM rate of 286 μmol/(gdw d) was achieved after 400 days of operation 21. In that study, the highest AOM rate was observed only when the MBR was operated at 15°C (similar to the in situ temperature) and ambient pressure. Another type of MBR, the continuous hollow‐fiber membrane reactor, has also been tested for ANME‐2d enrichment inoculated with a bioreactor sludge enriched for ANNAMOX and ANME‐2d, performing methane‐dependent denitrification 28. These previous studies with MBR have shown that the continuous supply of methane and the biomass retention capacity of the MBR reactor configuration enable enrichment of ANME and achieving high AOM rates 21, 28. Nevertheless, none of the studies have yet attempted to enrich AOM hosting in a deep sea sediment from a cold seep at ambient temperature and pressure.
The objective of this study was, therefore, to enrich an anaerobic methanotrophic community in sediments collected from a cold seep environment, i.e. from Ginsburg mud volcano (Gulf of Cadiz), in a MBR operated at room temperature (22 ± 3°C) and ambient pressure. Cold seep sediments from the Gulf of Cadiz show active AOM‐SR in high pressure bioreactors operated at 8 MPa and 15°C 29. These operating conditions differ significantly from the in situ conditions (temperature 12°C and pressure 10 MPa), but were chosen to check if ANME can proliferate in an MBR as from a logistic, economical and safety view point, bioreactors operating at ambient conditions are preferred over those operated at high pressures. This study, therefore, evaluates the AOM activity at ambient pressure and temperature, while continuous bubbling of methane in the MBR. This mimics the in situ habitat of the Ginsburg mud volcano inoculum with diffusive methane supply, whereas the membrane (pore size of < 2 μm) retains the biomass. AOM rates were measured by performing activity assays with 13C labeled methane and the results were compared with other AOM‐SR bioreactors. The microbial community performing the AOM was visualized using catalyzed reporter deposition‐fluorescence in situ hybridization (CARD‐FISH).
2. Materials and methods
2.1. Origin of the inoculum
Sediments obtained from the Ginsburg mud volcano (Gulf of Cadiz, Spain) at a depth of 53–153 cm below the sea floor were used as the inoculum for the MBR. Ginsburg mud volcano is one of the noted sites for AOM among the mud volcanoes in the Gulf of Cadiz 16. The sampling location was in the crater of Ginsburg mud volcano (35o 22.431′N; 07o 05.291′W) at a water depth of ca. 910 m 30. The sediment core was immediately capped and stored under anaerobic conditions at 4°C, as described previously by Zhang et al. 30. Then, the sediment core was diluted 2 times with artificial seawater and a homogenized wet sediment mixture was prepared as soon as it was brought to the laboratory. The wet sediment mixture was stored at 4°C under methane atmosphere for almost 4 years until it was inoculated in the MBR. Prior to the inoculation in the MBR, the inoculum was reactivated in two 500 mL serum bottles by diluting it three times using anaerobic artificial sea water and applying 2 bar methane pressure for 30 days at room temperature (22 ± 3°C).
2.2. Mineral medium
The artificial seawater medium was prepared according to the composition recommended in a previous study 30, in per liter of demineralised water: NaCl (26 g), KCl (0.5 g) MgCl2ˑ6H2O (5 g), NH4Cl (0.3 g), CaCl2ˑ2H2O (1.4 g), Na2SO4 (1.43 g), KH2PO4 (0.1 g), trace element solution (1 mL), 1 M NaHCO3 (30 mL), vitamin solution (1 mL), thiamin solution (1 mL), vitamin B12 solution (1 mL), 0.5 g/L resazurin solution as a redox indicator (1 mL) and 0.5 M Na2S solution (1 mL). The trace element and vitamin solutions were prepared according to Widdel and Bak 31. The pH was adjusted to 7.0 with sterile 1 M Na2CO3 or 1 M H2SO4 solutions.
2.3. MBR design and operation
The MBR (Fig. 1) was made of a cylindrical glass column (length‐65 cm, diameter‐100 mm). It was operated for more than two years (i.e. 726 days) to study AOM‐SR and enrich the microbial community responsible for AOM. The MBR was sealed gas‐tight to prevent any leakage or air intrusion during its operation. The reactor was equipped with a gas diffuser for sparging methane, a smart thermal mass flow controller (Brooks Instrument, Model SLA5850, Veenendaal, the Netherlands), one way valves and sampling ports (Fig. 1). A hollow fiber ultrafiltration membrane (Pentair X‐Flow, Enschede, the Netherlands) with < 2 μm pore size was placed externally in the recirculation loop and operated with a cross flow velocity of 0.02 m/min. The hollow fiber membrane allowed the permeate to pass through, while it retained the biomass in the reactor. The tubings, joints, connectors and the reactor column were cleaned with ethanol and dried under nitrogen atmosphere, and then washed with sterilized water prior to the start‐up of the MBR.
Figure 1.
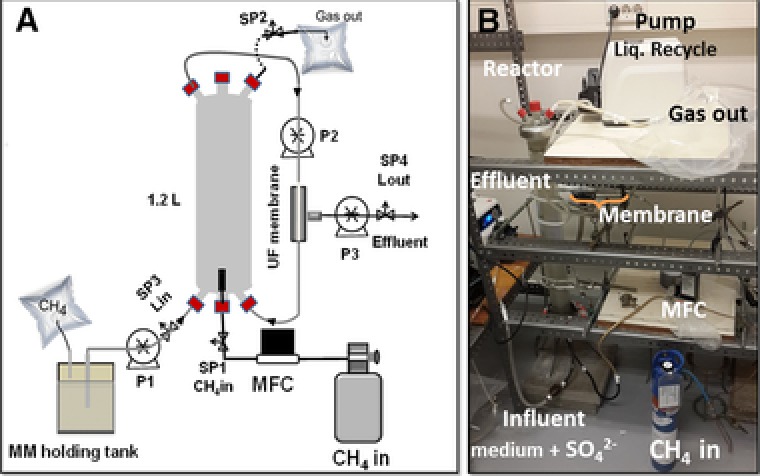
Membrane bioreactor with external ultrafiltration membrane for the anaerobic oxidation of methane and enrichment of anaerobic methanotrophs: (A) Schematic of the MBR, and (B) Photograph of the MBR set‐up in the laboratory. SP: sampling port, UF: ultrafiltration, MM: artificial seawater medium and MFC: mass flow controller.
Methane was bubbled at a constant rate of 0.5 mL/min and the reactor was operated at atmospheric pressure and a temperature of 22 (± 3)°C throughout the experimental period. The anaerobic artificial seawater medium was continuously recirculated in the MBR and passed from the bottom of the bioreactor to the ultrafiltration membrane and back to the bioreactor at a rate of 20 mL/min. The seawater medium was intermittently fed to the MBR. The MBR effluent was sampled twice per week for sulfate, sulfide and volatile fatty acids (VFA) analysis. The inlet and outlet gas composition was also measured twice a week for estimating the carbon dioxide and methane concentrations. Biomass samples were collected on day 400 and at the end of the MBR operation (day 726) for microbial analysis by CARD‐FISH. The details of the mode of bioreactor operation are provided in the supporting information.
2.4. Activity assay with labeled methane
AOM activity tests with 13C labeled methane were performed as described by Scheller et al. 3, in triplicate with duplicate controls under nitrogen atmosphere (without methane). After determination of the exact weight and volume of the 118 mL serum bottles, they were closed with butyl rubber stoppers and caps, and the gas phase was replaced several times with nitrogen gas and made vacuum thereafter. Subsequently, 20 mL of the biomass suspension collected from the MBR was transferred to each serum bottle using hypodermic needles and 40 mL of artificial seawater was added to the biomass suspension. The headspace of the bottles was flushed with methane for 8 min and an estimated equivalent amount of 5% of the headspace was taken out and filled again with the same amount of 13C labeled methane. The bottles were incubated on an orbital shaker at 100 rpm and 22 (± 3)°C for 50 days. Liquid samples for sulfate and sulfide measurements and gas samples for the measurement of the carbon isotopic fraction were collected once a week in these batch assays. During each sampling, ∼2 mL of liquid sample was collected periodically for the estimation of pH, total dissolved sulfide and sulfate concentrations and almost 0.2 mL of headspace gas for methane and carbon dioxide analysis.
The stable carbon isotope composition of methane and carbon dioxide was determined using a Gas Chromatography‐Isotope Ratio Mass Spectrometer (GC‐IRMS, Agilent 7890A) and the carbon isotopic fraction (13C/12C) was estimated as described previously 32. Measurements were performed in triplicate and the standard deviation was smaller than 0.5 δ‐units. Standard gas mixtures of methane and carbon dioxide were measured together with the entire isotopic analysis to assure the quality of the measurements. The AOM‐SR rate was estimated on the basis of the total dissolved inorganic carbon (DIC) produced during the activity assay incubation as described by Scheller et al. 3 and detailed in supporting information.
2.5. Microbial analysis
Microbial analysis of MBR biomass was performed mainly by CARD‐FISH on enriched biomass from the MBR after 400 days of operation and the biomass obtained at the end of reactor operation (726 days). Prior to microbial visualization by CARD‐FISH, the biomass was screened with FISH by using three different ANME specific probes, i.e. ANME‐1 350 7, ANME‐2 538 33, ANME‐3 1249 16 and fluorescence was only obtained from the ANME‐2 specific probe (data not shown). Therefore, CARD‐FISH was performed only for the ANME‐2 clade and its associated sulfate reducing partner (Desulfosarcina). CARD‐FISH was performed according to standard protocol as described previously 34. Archaeal and bacterial horseradish peroxidase labeled oligonucleotide probes ARCH915 35 and EUB338‐I‐III 36, respectively, were used for hybridization at a formamide concentration of 35% (v/v hybridization buffer). The probes DSS658 7 and ANME‐2 538 33 were used for the detection of, respectively, Desulfosarcina‐Desulfococcus sp. and ANME‐2. Oligonucleotide probes were purchased from Biomers (Ulm, Germany). All cells were stained with 4′, 6′‐diamidino‐2‐phenylindole (DAPI) and analyzed using an epifluorescence microscope (Carl Zeiss, Oberkochen, Germany).
For dual‐CARD‐FISH, peroxidases of initial hybridizations were inactivated according to the procedure described by Holler et al. 10. Tyramide amplification was performed using the fluorochromes Alexa Fluor 488 and Alexa Fluor 594, prepared according to the procedure described by Pernthaler et al. 37. Note that the double hybridization for ANME‐2 and Desulfosarcinales was not performed for the biomass obtained on day 400 from the MBR.
2.6. Chemical analysis
A total of 5 mL of liquid sample was collected from the MBR for the analysis of different parameters. The pH was measured using a pH meter (Metrohm Applikon B.V, Schiedam, the Netherlands) fitted with a pH electrode (SenTix WTW, Amsterdam, the Netherlands). Sulfate was analyzed using an Ion Chromatograph system (Dionex‐ICS‐1000 integrated with a AS‐DV sampler), as described previously by Villa‐Gomez et al. 38. Total dissolved sulfide was analyzed spectrophotometrically using the methylene blue method at a wavelength of 670 nm 39 and the sample for sulfide measurement was prepared as described in Bhattarai et al. 4. The sulfate reduction rate and sulfide production rate was estimated as described in previous study 21 and detailed in supporting information.
Methane and carbon dioxide concentrations in the inlet and outlet of the MBR were measured by gas chromatography (GC 3800, VARIAN, Middelburg, the Netherlands) as described previously in Bhattarai et al. 4. Acetate was measured along with other VFA by gas chromatography (GC 340, VARIAN, Middelburg, the Netherlands). The gas chromatograph was equipped with a flame ionization detector (FID) and CP WAX 58 (FFAP) column having the following dimensions: 25 m × 0.32 mm × 0.2 μm. The carrier gas was helium at a flow rate of 78 mL/min. The oven was set at 105°C and the FID temperature was maintained at 300°C. The injection volume was 1 μL, injected by an auto sampler which was set for duplicate measurements for each sample. Prior to the measurements, the samples were acidified and the internal standard mixture (50 μL) of formic/propionic acid was added. For each measurement, VFA standards with known concentrations were measured in order to ensure quality assurance.
3. Results
3.1. Development of AOM activity in the MBR
The start‐up period of the MBR lasted for 150 days. During this period of MBR operation, the reactor showed non‐steady state profiles of sulfate and methane consumption and the total amount of sulfide produced was negligible (lower than 0.5 mM). Methane was not consumed in the MBR during this period (Fig. 2). The sulfate consumption increased between days 350 and 400 to 8 mM accompanied by the simultaneous production of 5 mM sulfide (Fig. 2B). Then, the sulfate profile showed a continuous consumption between days 400 to 580, while sulfide was present in low amounts between days 400 to 500. However, the amount of sulfide in the MBR increased abruptly on day 585. A maximum sulfide concentration of 5.5 mM and minimum sulfate concentration of 1.5 mM were reached in the MBR between days 480 and 585, which showed the active reduction of the sulfate concentration from 6.5 to 1.5 mM, with the stoichiometric production of sulfide of 5.5 mM according to the equation of AOM.
Figure 2.
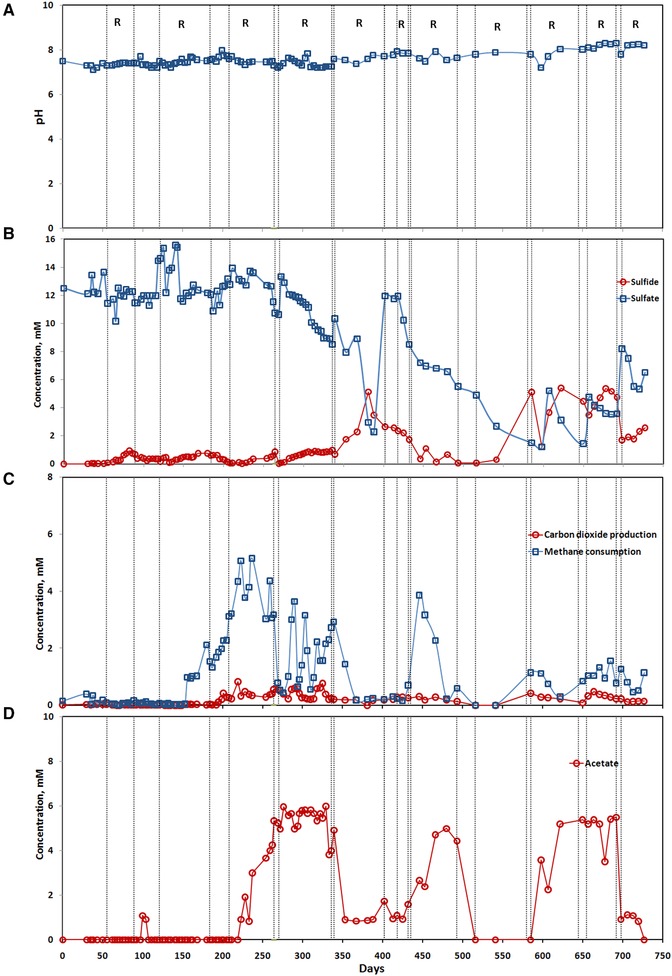
Performance of the membrane bioreactor during long term operation of 726 days: (A) pH, and (B) sulfate and sulfide concentrations, (C) carbon dioxide and methane concentrations, and (D) acetate concentration. R in the figure represents to recirculation mode of MBR operation, that is, distinguished by the dotted line in each plot, which alternates with the continuous mode of MBR operation.
The pH increased to values > 8.0 in the medium and it was neutralized to 7.0–8.0 by the manual addition of sulfuric acid. After 600 days, the pH increment was rapid and reached up to 8.3 (Fig. 2A). These pH changes in the bioreactor suspension are typical for the redox mechanism (AOM‐SR) which produces hydrogen sulfide and bicarbonate.
Methane consumption showed a rather fluctuating trend throughout the MBR operation, with methane consumption peaks of 2–5 mM. Methane consumption was almost undetectable during the first 150 days of reactor operation. Thereafter, methane consumption increased up to 5 mM between days 200 to 223 and remained in a range of 3–5 mM up to day 265 (Fig. 2C). Later on, the methane consumption was in oscillating trend with the abrupt rise to 3–4 mM around day 400. During this operational period, the carbon dioxide production rate was 5–20 times lower than the methane consumption rate. However, the increment in the carbon dioxide production was often observed simultaneously with the increment in methane consumption.
Acetate production was observed intermittently during different periods of MBR operation, especially between days 200 and 520 (Fig. 2D). The acetate production followed a similar trend as that of the methane consumption, i.e. the acetate production increased whenever there was an increase in the methane consumption. In some instances, acetate production was even higher than the methane consumption as it reached > 5 mM between days 260 and 330, whereas the methane consumption was < 4 mM during that period.
3.2. Methane oxidation and sulfate reduction rates from batch activity assays
13C labeled methane was consumed by the microbial community present in the MBR suspension sampled on day 726, as shown by the simultaneous 13C DIC and sulfide production (Fig. 3). However, the sulfate reduction and sulfide production were ∼20 times higher than the 13C DIC production (Fig. 3A–C). In control incubations with nitrogen instead of methane in the headspace, no DIC production was observed, suggesting that the 13C DIC production was solely due to the 13C methane consumption.
Figure 3.
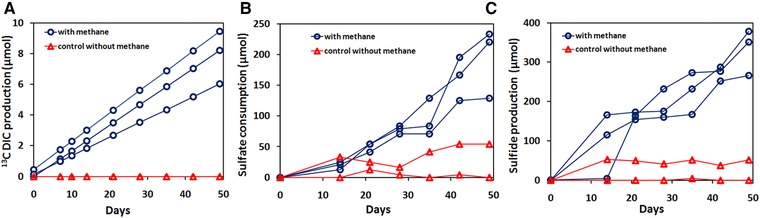
Estimated (A) 13C DIC production, (B) sulfate consumption and (C) sulfide production profiles in batch activity assays (5% 13C methane) using biomass collected at the end of the MBR operation (726 days).
The sulfate consumption was < 50 μmol of sulfate for the control incubations, whereas the cumulative consumption of sulfate for the incubations with 13C methane was 200 μmol of sulfate (Fig. 3B). The cumulative sulfide production was < 30 μmol during the incubation period for control experiments, whereas in the case of incubations with 13C methane, the cumulative sulfide production reached values between 300 and 400 μmol.
On the basis of the total DIC production, the estimated average AOM rate for the incubations with 13C methane was 1.2 μmol/(gdw d). Furthermore, the sulfate reduction and sulfide production rates amounted to 29.5 μmol/(gdw d) and 40.5 μmol/(gdw d), respectively, in the incubations with 13C methane.
3.3. CARD‐FISH analysis of the microbial community
ANME‐2 cells were observed by CARD‐FISH in both biomass samples investigated, i.e. the samples collected on days 400 (Fig. 4) and 726 (Fig. 5) from the MBR. In most of the CARD‐FISH observations, the total amount of ANME‐2 cells was ∼20–25% of the total amount of cells stained by DAPI in the biomass collected on day 400 (Fig. 4B and D). Figure 4C shows that the ANME‐2 cells were < 5% in some of the CARD‐FISH analysis from the biomass obtained on day 400 of MBR operation. The observed ANME‐2 cells were cocci shaped with ∼1.0–1.5 μm in size. Some of those ANME‐2 cells appeared as circular discs with no fluorescence signal from the center of the cell (Fig. 4B). Desulfosarcinales cells, a common sulfate reducing partner of ANME‐2, were also visualized along with other cells that were stained by DAPI (Fig. 4E and F). Most of these Desulfosarcinales were rod shaped cells with a size of ∼1 μm. Approximately, 20–30% of the total cells were estimated to be Desulfosarcinales from the microscopic observations.
Figure 4.
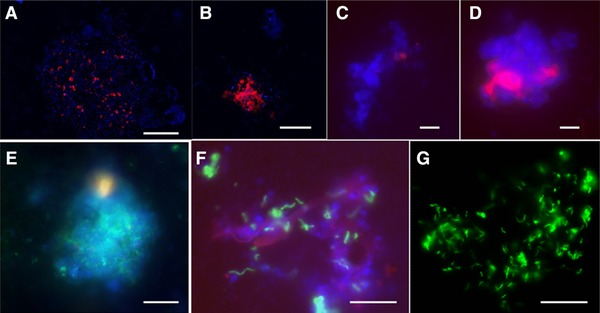
Photomicrographs staining of the microbial cells, ANME‐2 and Desulfosarcinales cells visualized using CARD‐FISH present in biomass enriched after 400 days of MBR operation. Upper panel photomicrographs (A–D) represent large cluster of ANME‐2 hybridized with ANME‐2 538 probes (red) and all cells stained by DAPI (blue). Lower panel photomicrographs (E–G) represent clusters of Desulfosarcinales hybridized with DSS 658 (green) and all cells stained by DAPI (blue). The white scale bar represents 10 μm.
Figure 5.
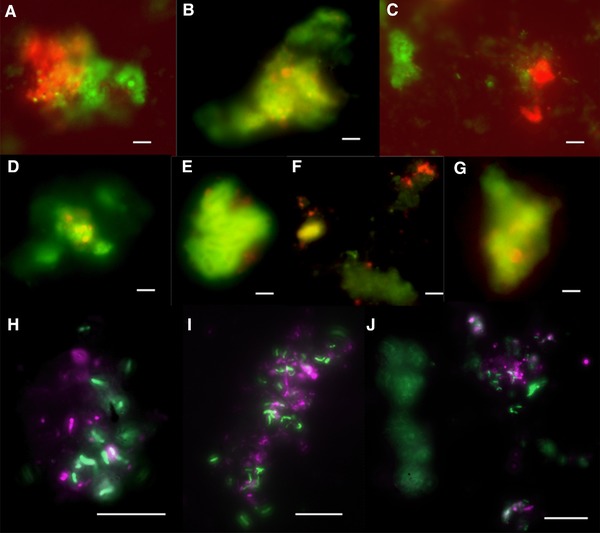
Photomicrographs of all the microbial cells, ANME‐2 and Desulfosarcinales visualized using CARD‐FISH present in biomass enriched at the end of the batch activity assays (50 days), performed with biomass sampled on day 726 from the MBR. Photomicrographs (A–J) were obtained from 13C methane activity assays (presented in Fig. 3), in which (A–G) represents the tight consortia of ANME‐2 hybridized with ANME‐2 538 (red) and Desulfosarcina hybridized with DSS 658 (green). (H–J) represents the cell clusters of Desulfosarcinales hybridized with DSS 658 (green) and other bacterial cells hybridized with EUB338‐I‐III (violet). The white scale bar represents 10 μm.
For the biomass obtained at the end of the MBR operation, i.e. on day 726, aggregates of ANME‐2 cells and Desulfosarcina were observed (Fig. 5A–G). For the majority of the observations by CARD‐FISH, ANME‐2 and Desulfosarcina were clustered together at a ratio of 1:2 (Fig. 5B–F). However, in some observations, the amount of ANME‐2 and Desulfosarcinales cells was nearly equal (Fig. 5A and G). The amount of ANME‐2 cells compared to the total amount of cells stained by DAPI was ∼35%. ANME‐2 cells were also observed in the incubations with 13C methane (Fig. 5A–F). However, the ANME‐2 cells could not be clearly distinguished in the control incubations under nitrogen atmosphere (50 days). The rod shaped Desulfosarcinales cells were detected mostly in concurrence with other bacterial cells (Fig. 5H–J). The biomass obtained at the end of the MBR operation showed the amount of Desulfosarcinales cells was in a 1:1 ratio with other bacterial cells by double hybridization CARD‐FISH analysis.
4. Discussion
4.1. AOM and sulfate reduction in the MBR
This study showed that AOM‐SR was achieved in a MBR operated at ambient pressure and 22 (± 3)°C, inoculated with the Ginsburg Mud volcano sediment (in situ pressure 10 MPa, temperature 15°C). The achieved AOM activity and rate after 200 days of the MBR operation was in the range reported for in situ conditions from seep and mud volcano samples 1. Moreover, the AOM rate obtained in this study is also comparable to those reported in a hanging sponge bioreactor fed with high sulfate loads (30 mmol/(L d)) and operated at 10°C for 2013 days 19. However, it was lower than the rates reported for high pressure incubations and high pressure bioreactor systems 26, 29, 40.
As methane is poorly soluble in marine water at ambient pressure 41, 42, the AOM reaction is highly influenced by the methane partial pressure. An effective and continuous methane supply to the anaerobic methanotrophs is thus an important factor for the enrichment of ANME. Similar to a previously reported MBR with an internal polysulfone membrane 21, the methane was continuously bubbled in the MBR (Fig. 1) to ensure the continuous availability of methane to the AOM enrichment. However, the AOM rate estimated by 13C methane incubation in this MBR was almost 100 times lower than the AOM rate achieved in the MBR operated by Meulepas et al. 21 for 800 days, inoculated with sediment from Eckernförde Bay at a water depth of 28 m. The MBR used in this study was inoculated with the cold seep sediment from Ginsburg mud volcano obtained at a water depth of 910 m and hence the in situ pressure of the original sediment biomass was ∼10 MPa. This confirms the nature, composition and environmental conditions of the inoculum are important factors influencing the AOM rate 30. Further, the activity of the inoculated biomass was probably negatively affected by the long storage period prior to MBR start‐up. The AOM‐SR rates increased after 150 days of reactor operation, showing that the deep‐sea biomass can regain its AOM activity after a long storage period (for > 4 years) at 4°C under methane atmosphere, after which the AOM‐SR increased exponentially (Fig. 2B).
AOM activity was evidenced by the DIC production in the batch activity tests which were supplied with methane in the headspace, while the control batches without methane did not show any DIC production (Fig. 3A). Nevertheless, slight sulfate reduction and simultaneous sulfide production was observed in these control batches, but almost 5 times less than for the batches with added methane. The small amount of sulfate reduction in the control batches was possibly due to sulfate reduction with endogenous organic matter or residual methane which mainly occurred in the initial 15 days of the experiment. A decoupling between the AOM and sulfate reduction was observed in the activity assays tested in batch incubations as the sulfate consumption rate was much higher than the 13C DIC production rate when incubated with 13C methane as electron donor (Fig. 3). The CARD‐FISH analysis supported this observation as the amount of sulfate reducing Desulfosarcinales cells was nearly twice compared to the amount of ANME clusters (Fig. 5). Sulfate reduction rates are often higher than the AOM rates in ANME habitats, such as in Black sea microbial mats with two times higher sulfate reduction rates than AOM rates 43, Gulf of Mexico hydrate with 20 times higher sulfate reduction than AOM rates 44, 45 and mud volcanoes from Gulf of Cadiz with 2 times higher sulfate reduction rate than the AOM rate 16. In this study, almost 15 mM of sulfate and methane was added initially to the batch incubation, which provides an environment suitable for both sulfate reducing bacteria and ANME. The DIC concentration could have been underestimated as the increasing pH from the AOM conversion causes a large fraction of the produced DIC to remain in its dissolved form and the estimation of DIC was based on the headspace 13C fraction, whereas the dissolved DIC species (as CO2 (aq.), H2CO3, HCO3 − or CO3 2−) were not measured 3. Moreover, when the highly enriched biomass from a continuously operated MBR inoculated with Eckernförde Bay sediment was incubated in batches, the amount of methane consumption was more than ten times to the amount of carbon dioxide produced 21. Some of the carbon dioxide could be reduced by methanogenesis, which could co‐occur with AOM in the MBR and thus lower the amount of carbon dioxide detected, as discussed in previous studies 21, 23. CARD‐FISH analysis for methanogens or high‐throughput sequencing of enrichment biomass may confirm the presence of methanogens in the MBR biomass, which are possibly performing the carbon dioxide reduction.
4.2. MBR selects the ANME‐2 dominated enrichment from Ginsburg mud volcano
This study shows that ANME‐2 and Desulfosarcinales were enriched in the MBR equipped with an external ultrafiltration membrane (Fig. 5). These two clades are usually observed together and typical for many in situ AOM studies as well as in AOM enrichments in bioreactors 21, 26, 29. Although the ANME clades were below the detection limit of the Illumina Miseq analysis in the inoculum (Bhattarai et al. unpublished data), this study evidenced the AOM and enrichment of ANME‐2 in the MBR mixed liquor. In contrast, in a biotrickling filter (BTF) inoculated with the same Ginsburg Mud Volcano inoculum, ANME‐1 (40%) was mainly enriched after 248 days of operation, whereas only 10% of the total archaeal community was affiliated to ANME‐2 (Bhattarai et al. unpublished data). Both bioreactor configurations, i.e. the BTF and the MBR, were operated by continuously recirculating the mineral medium. In the MBR, however, the microbial biomass was mainly in suspension, whereas it was attached as a biofilm onto the polyurethane foam packing material in the BTF. The operational conditions and the hydrodynamics of the MBR resemble conditions prevailing in the methane bubbling sediment environment, such as the Eckernförde bay in the Baltic sea 13, 21, methane hydrates of the Gulf of Mexico (less saline part) 45, 46, cold seeps from the Gulf of Cadiz 16 and mud volcanoes 47, 48, 49, 50. In most of these diffusive sediments bearing continuously methane bubbling, ANME‐2/Desulfosarcina consortia are dominant. In contrast, the conditions in the BTF resemble those of a carbonate chimney, e.g. in the Black sea microbial mat, where ANME‐1 is dominant 51, 52. ANME‐1 is more frequently observed in attached form within these carbonate chimneys and nodules, which provide a porous matrix for ANME proliferation.
In most of the ANME habitats, ANME‐2 appeared to be present in consortia with Desulfosarcinales clades of SRB, except for a few habitats with discrete ANME cells 52, 53. The CARD‐FISH analyses showed that the AOM performing microbial community in the MBR, i.e. ANME‐2 and Desulfosarcinales, were closely associated to each other. Syntrophy between ANME‐2 and Desulfosarcinales to sustain the coupling of the AOM reaction with the sulfate reduction has also been reported previously for AOM consortia present in Eel river hydrate 15, 54, Eckernförde Bay sediment 21 and Gulf of Cadiz sediment 16. Cocci shaped ANME‐2 and Desulfosarcinales cells were observed during CARD‐FISH. Nevertheless, further studies on the characterization and evaluation of enriched consortium can be performed by microscopic tools such as CARD‐FISH in combination with nanometer scale secondary ion mass spectrometer (NanoSIMS) 17, scanning (SEM) or transmission (TEM) electron microscopy or atomic force microscopy (AFM) 55 and genomic analysis in combination with other microscopic techniques 56.
4.3. Role of acetate production in the MBR
Acetate formation was observed in the MBR, intermittently, even though acetate was not fed to the MBR and methane was the sole supplied electron donor (Fig. 2D). The release of acetate from the bioreactor material was unlikely as the MBR was constructed using a glass vessel and fitted with non‐degradable Tygon tubings. Acetate formation in a reactor or batch incubation inoculated with Ginsburg mud volcano sediment, when fed solely with methane, has so far not been reported. A possible reason could be the degradation of organic matter present in the sediment and the production of acetate by acetogens. This is, however, highly unlikely as the organic matter commonly degrades within the first 100 days of bioreactor operation and the acetate production was detected only after 200 days (Fig. 2D). The production of acetate from methane is also highly unlikely, as it is thermodynamically not favorable at standard conditions (i.e. Gibbs free energy of the reaction is ΔGo = +31 kJ/mol). However, genetic analysis has shown that the ANME clade genome contains all the required enzymes for the reverse methanogenesis step, including the genes involved in the acetogenesis process 56, 57, 58. Therefore, the intermittent production of acetate in the MBR suggests the ANME produced the acetate. This hints to a possible role of acetate in the AOM mechanism, i.e. acetate being intermediate of the AOM‐SR reaction. Indeed, an earlier hypothesis already suggested acetate being an intermediate for electron transfer between ANME and SRB 59, 60, 61. Acetate was assumed to be the favorable electron shuttle in high methane pressure environments 61 and even detected in high pressure incubations of the ANME‐2a clades (Cassidy et al., unpublished data). Therefore, ANME may metabolize methane to acetate, which is subsequently utilized by the SRB or by the ANME themselves and finally converted to DIC with simultaneous sulfide production.
The acetate being the byproduct of acetogenesis and then used for acetoclastic methanogenesis is less likely as the process was shown to be suppressed when methane is supplied in excess 62. Moreover, methanogenic substrates other than acetate, such as hydrogen, formate, methanol and methanethiol have been hypothesized as the intermediates during AOM 59, 60, 61. There could be other potential modes of electron transfer involved in AOM‐SR, such as direct cell to cell electron transfer 18. The addition of acetate to AOM enrichments did not enhance AOM, but it favored the growth of sulfate reducers rather than ANME 13, 63, 64. Hence, further studies on the exploration of the electron transfer mechanism among ANME‐2 and Desulfosarcinales using NanoSIMS analysis 17, micro audio radiography and fluorescence in situ hybridization (MAR ‐ FISH) 65, 66 and stable isotope probing 67 using 13C labeled acetate and 13C labeled methane are required to further elucidate the role of acetate in the AOM‐SR process.
5. Conclusion
A MBR inoculated with sediment originating from a cold seep environment, i.e. Ginsburg mud volcano, was operated for 726 days to evaluate the AOM at ambient pressure and temperature. An active ANME‐2 and Desulfosarcinales consortium was enriched in the MBR, whose co‐occurrence has been reported in many natural AOM hosting sediments. The experimental set‐up with the bubbling methane was favorable for the proliferation of an active AOM consortium in the MBR. A maximum volumetric AOM induced sulfate reduction rate of 0.5 mM/d was achieved during MBR operation. During the 13C labeled experiment, the AOM rate was estimated at 1.2 μmol/(gdw d) with the consecutive sulfate reduction rate at 29.5 μmol/(gdw d). The production of acetate in the MBR suggests a potential involvement or production of acetate in the AOM‐SR process. This study has widened the prospective of applying bioreactors for the enrichment of ANME at ambient pressure and temperature.
Practical application
This study shows an anaerobic membrane bioreactor can be used for the enrichment of anaerobic methanotrophs (ANME) and sulfate reducing bacteria from a cold seep environment. This enrichment occurs at ambient pressure and temperature. The membrane bioreactor operation showed enrichment of ANME with efficient biomass retention, while methane was the sole supplied electron donor. The obtained enriched ANME biomass provides an opportunity to study the physiology and mechanism of ANME. The anaerobic oxidation of methane coupled to sulfate reduction process has a potential applicability to reduce sulfate in ground water, mining or inorganic wastewater streams by using the cheap electron donor, in other words, methane, for the sulfate reduction. This study widens the prospective of using the membrane bioreactors for the enrichment of consortia performing anaerobic oxidation of methane at ambient pressure and temperature.
The authors have declared no conflict of interest.
Supporting information
Supporting Material
Acknowledgments
We acknowledge Dr. Yu Zhang from Shanghai Jiao Tong University (Shanghai, China) for providing the sediment from Ginsburg mud volcano. The authors acknowledge the Centre for Chemical Microscopy (ProVIS) at the Helmholtz‐Centre for Environmental Research for provision of the analytical facilities, which is supported by European Regional Development Funds (EFRE and Europe funds Saxony) and the Helmholtz Association. The authors acknowledge Dr. Niculina Musat (team leader of ProVIS) for her advice and practical support during CARD‐FISH analysis. We thank Dr. Graciela Gonzalez‐Gill (UNESCO‐IHE, the Netherlands) for providing suggestions during reactor operation and Ms. Zita Naangmenyele (UNESCO‐IHE, the Netherlands) for help in building the MBR. This research was funded by the Erasmus Mundus Joint Doctorate Programme ETeCoS3 (Environmental Technologies for Contaminated Solids, Soils and Sediments) under the grant agreement FPA no. 2010‐0009.
6 References
- 1. Knittel, K. , Boetius, A. , Anaerobic oxidation of methane: Progress with an unknown process. Annu. Rev. Microbiol. 2009, 63, 311–334. [DOI] [PubMed] [Google Scholar]
- 2. Reeburgh, W. S. , Oceanic methane biogeochemistry. Chem. Rev. 2007, 107, 486–513. [DOI] [PubMed] [Google Scholar]
- 3. Scheller, S. , Yu, H. , Chadwick, G. L. , McGlynn, S. E. et al., Artificial electron acceptors decouple archaeal methane oxidation from sulfate reduction. Science. 2016, 351, 703–707. [DOI] [PubMed] [Google Scholar]
- 4. Bhattarai, S. , Cassarini, C. , Gonzalez‐Gil, G. , Egger, M. et al., Anaerobic methane‐oxidizing microbial community in a coastal marine sediment: anaerobic methanotrophy dominated by ANME‐3. Microb. Ecol. 2017, 74, 608–622. [DOI] [PubMed] [Google Scholar]
- 5. Ruff, S. E. , Arnds, J. , Knittel, K. , Amann, R. et al., Microbial communities of deep‐sea methane seeps at Hikurangi continental margin (New Zealand). PLoS One. 2013, 8, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ruff, S. E. , Kuhfuss, H. , Wegener, G. , Lott, C. et al., Methane seep in shallow‐water permeable sediment harbors high diversity of anaerobic methanotrophic communities, Elba, Italy. Front. Microbiol. 2016, 7, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boetius, A. , Ravenschlag, K. , Schubert, C. J. , Rickert, D. et al., A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature. 2000, 407, 623–626. [DOI] [PubMed] [Google Scholar]
- 8. Knittel, K. , Lösekann, T. , Boetius, A. , Kort, R. et al., Diversity and distribution of methanotrophic archaea at cold seeps. Appl. Environ. Microbiol. 2005, 71, 467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Treude, T. , Boetius, A. , Knittel, K. , Wallmann, K. et al., Anaerobic oxidation of methane above gas hydrates at Hydrate Ridge, NE Pacific Ocean. Mar. Ecol. Prog. Ser. 2003, 264, 1–14. [Google Scholar]
- 10. Holler, T. , Widdel, F. , Knittel, K. , Amann, R. et al., Thermophilic anaerobic oxidation of methane by marine microbial consortia. ISME J. 2011, 5, 1946–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vigneron, A. , Cruaud, P. , Pignet, P. , Caprais, J.‐C. et al., Archaeal and anaerobic methane oxidizer communities in the Sonora Margin cold seeps, Guaymas Basin (Gulf of California). ISME J. 2013, 7, 1595–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Treude, T. , Krause, S. , Maltby, J. , Dale, A. W. et al., Sulfate reduction and methane oxidation activity below the sulfate‐methane transition zone in Alaskan Beaufort Sea continental margin sediments: Implications for deep sulfur cycling. Geochim. Cosmochim. Ac. 2014, 144, 217–237. [Google Scholar]
- 13. Treude, T. , Krüger, M. , Boetius, A. , Jørgensen, B. B. , Environmental control on anaerobic oxidation of methane in the gassy sediments of Eckernförde Bay (German Baltic). Limnol. Oceanogr. 2005, 50, 1771–1786. [Google Scholar]
- 14. Hinrichs, K.‐U. , Summons, R. E. , Orphan, V. , Sylva, S. P. et al., Molecular and isotopic analysis of anaerobic methane‐oxidizing communities in marine sediments. Org. Geochem. 2000, 31, 1685–1701. [Google Scholar]
- 15. Orphan, V. J. , House, C. H. , Hinrichs, K. U. , McKeegan, K. D. et al., Methane‐consuming archaea revealed by directly coupled isotopic and phylogenetic analysis. Science 2001, 293, 484–487. [DOI] [PubMed] [Google Scholar]
- 16. Niemann, H. , Duarte, J. , Hensen, C. , Omoregie, E. et al., Microbial methane turnover at mud volcanoes of the Gulf of Cadiz. Geochim. Cosmochim. Ac. 2006, 70, 5336–5355. [Google Scholar]
- 17. Milucka, J. , Ferdelman, T. G. , Polerecky, L. , Franzke, D. et al., Zero‐valent sulphur is a key intermediate in marine methane oxidation. Nature 2012, 491, 541–546. [DOI] [PubMed] [Google Scholar]
- 18. McGlynn, S. E. , Chadwick, G. L. , Kempes, C. P. , Orphan, V. J. , Single cell activity reveals direct electron transfer in methanotrophic consortia. Nature 2015, 526, 531–535. [DOI] [PubMed] [Google Scholar]
- 19. Aoki, M. , Ehara, M. , Saito, Y. , Yoshioka, H. et al., A long‐term cultivation of an anaerobic methane‐oxidizing microbial community from ceep‐sea methane‐seep sediment using a continuous‐flow bioreactor. PLoS One 2014, 9, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Girguis, P. R. , Cozen, A. E. , DeLong, E. F. , Growth and population dynamics of anaerobic methane‐oxidizing archaea and sulfate‐reducing bacteria in a continuous‐flow bioreactor. Appl. Environ. Microbiol. 2005, 71, 3725–3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Meulepas, R. J. W. , Jagersma, C. G. , Gieteling, J. , Buisman, C. J. N. et al., Enrichment of anaerobic methanotrophs in sulfate‐reducing membrane bioreactors. Biotechnol. Bioeng. 2009, 104, 458–470. [DOI] [PubMed] [Google Scholar]
- 22. Kruger, M. , Wolters, H. , Gehre, M. , Joye, S. B. et al., Tracing the slow growth of anaerobic methane‐oxidizing communities by 15N‐labelling techniques. FEMS Microbiol. Ecol. 2008, 63, 401–411. [DOI] [PubMed] [Google Scholar]
- 23. Treude, T. , Orphan, V. , Knittel, K. , Gieseke, A. et al., Consumption of methane and CO2 by methanotrophic microbial mats from gas seeps of the anoxic Black Sea. Appl. Environ. Microbiol. 2007, 73, 2271–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nauhaus, K. , Albrecht, M. , Elvert, M. , Boetius, A. et al., In vitro cell growth of marine archaeal‐bacterial consortia during anaerobic oxidation of methane with sulfate. Environ. Microbiol. 2007, 9, 187–196. [DOI] [PubMed] [Google Scholar]
- 25. Nauhaus, K. , Treude, T. , Boetius, A. , Kruger, M. , Environmental regulation of the anaerobic oxidation of methane: a comparison of ANME‐I and ANME‐II communities. Environ. Microbiol. 2005, 7, 98–106. [DOI] [PubMed] [Google Scholar]
- 26. Timmers, P. H. , Gieteling, J. , Widjaja‐Greefkes, H. A. , Plugge, C. M. et al., Growth of anaerobic methane‐oxidizing archaea and sulfate‐reducing bacteria in a high‐pressure membrane capsule bioreactor. Appl. Environ. Microbiol. 2015, 81, 1286–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Skouteris, G. , Hermosilla, D. , López, P. , Negro, C. et al., naerobic membrane bioreactors for wastewater treatment: a review. Chem. Eng. J. 2012, 198, 138–148. [Google Scholar]
- 28. Shi, Y. , Hu, S. , Lou, J. , Lu, P. et al., Nitrogen removal from wastewater by coupling anammox and methane‐dependent denitrification in a membrane biofilm reactor. Environ. Sci. Technol. 2013, 47, 11577–11583. [DOI] [PubMed] [Google Scholar]
- 29. Zhang, Y. , Maignien, L. , Zhao, X. , Wang, F. et al., Enrichment of a microbial community performing anaerobic oxidation of methane in a continuous high‐pressure bioreactor. BMC Microbiol. 2011, 11, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang, Y. , Henriet, J.‐P. , Bursens, J. , Boon, N. , Stimulation of in vitro anaerobic oxidation of methane rate in a continuous high‐pressure bioreactor. Biores. Technol. 2010, 101, 3132–3138. [DOI] [PubMed] [Google Scholar]
- 31. Widdel, F. , Bak, F . Gram negative mesophilic sulfate reducing bacteria in: The Prokaryotes, Springer, New York, USA: 1992, pp. 3352–3378. [Google Scholar]
- 32. Dorer, C. , Vogt, C. , Neu, T. R. , Stryhanyuk, H. et al., Characterization of toluene and ethylbenzene biodegradation under nitrate‐, iron (III)‐and manganese (IV)‐reducing conditions by compound‐specific isotope analysis. Environ. Pollut. 2016, 211, 271–281. [DOI] [PubMed] [Google Scholar]
- 33. Treude, T. , Knittel, K. , Blumenberg, M. , Seifert, R. et al., Subsurface microbial methanotrophic mats in the Black Sea. Appl. Environ. Microbiol. 2005, 71, 6375–6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cassarini, C. , Rene, E. R. , Bhattarai, S. , Esposito, G. et al., Anaerobic oxidation of methane coupled to thiosulfate reduction in a biotrickling filter. Biores. Technol. 2017, 240, 214–222. [DOI] [PubMed] [Google Scholar]
- 35. Stahl, D. A. , Amann, R. I. , Development and application of nucleic acid probes in: Stackebrandt E., Goodfellow M. (Ed.), Nucleic Acid Techniques in Bacterial Systematics, John Wiley & Sons Ltd, Chichester, UK: 1991, pp. 205–248. [Google Scholar]
- 36. Daims, H. , Brühl, A. , Amann, R. , Schleifer, K.‐H. et al., The domain‐specific probe EUB338 is insufficient for the detection of all bacteria: development and evaluation of a more comprehensive probe set. Sys. Appl. Microbiol. 1999, 22, 434–444. [DOI] [PubMed] [Google Scholar]
- 37. Pernthaler, A. , Pernthaler, J. , Amann, R. , Kowalchuk, G. et al., Sensitive multi‐color fluorescence in situ hybridization for the identification of environmental microorganisms. Springer Netherlands, Dodrecht, the Netherlands 2004 1402021763 Contract No.: Ed. 2.
- 38. Villa‐Gomez, D. , Ababneh, H. , Papirio, S. , Rousseau, D. P. L. et al., Effect of sulfide concentration on the location of the metal precipitates in inversed fluidized bed reactors. J. Hazard. Mater. 2011, 192, 200–207. [DOI] [PubMed] [Google Scholar]
- 39. Sipma, J. , Meulepas, R. J. W. , Parshina, S. N. , Stams, A. J. M. et al., Effect of carbon monoxide, hydrogen and sulfate on thermophilic (55°C) hydrogenogenic carbon monoxide conversion in two anaerobic bioreactor sludges. Appl. Microbiol. Biotechnol. 2004, 64, 421–428. [DOI] [PubMed] [Google Scholar]
- 40. Deusner, C. , Meyer, V. , Ferdelman, T. , High‐pressure systems for gas‐phase free continuous incubation of enriched marine microbial communities performing anaerobic oxidation of methane. Biotechnol. Bioeng. 2009, 105, 524–533. [DOI] [PubMed] [Google Scholar]
- 41. Thauer, R. K. , Shima, S. , Methane as fuel for anaerobic microorganisms. Ann. N. Y. Acad. Sci. 2008, 1125, 158–170. [DOI] [PubMed] [Google Scholar]
- 42. Yamamoto, S. , Alcauskas, J. B. , Crozier, T. E. , Solubility of methane in distilled water and seawater. J. Chem. Eng. Data. 1976, 21, 78–80. [Google Scholar]
- 43. Krüger, M. , Blumenberg, M. , Kasten, S. , Wieland, A. et al., A novel, multi‐layered methanotrophic microbial mat system growing on the sediment of the Black Sea. Environ. Microbiol. 2008, 10, 1934–1947. [DOI] [PubMed] [Google Scholar]
- 44. Joye, S. B. , Boetius, A. , Orcutt, B. N. , Montoya, J. P. et al., The anaerobic oxidation of methane and sulfate reduction in sediments from Gulf of Mexico cold seeps. Chem. Geol. 2004, 205, 219–238. [Google Scholar]
- 45. Orcutt, B. , Boetius, A. , Elvert, M. , Samarkin, V. et al., Molecular biogeochemistry of sulfate reduction, methanogenesis and the anaerobic oxidation of methane at Gulf of Mexico cold seeps. Geochim. Cosmochim. Ac. 2005, 69, 4267–4281. [Google Scholar]
- 46. Lloyd, K. G. , Lapham, L. , Teske, A. , An anaerobic methane‐oxidizing community of ANME‐1b archaea in hypersaline Gulf of Mexico sediments. Appl. Environ. Microbiol. 2006, 72, 7218–7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Heijs, S. K. , Haese, R. R. , Van der Wielen, P. W. , Forney, L. J. et al., Use of 16S rRNA gene based clone libraries to assess microbial communities potentially involved in anaerobic methane oxidation in a Mediterranean cold seep. Microb. Ecol. 2007, 53, 384–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kormas, K. , Meziti, A. , Dählmann, A. , De Lange, G. et al., Characterization of methanogenic and prokaryotic assemblages based on mcrA and 16S rRNA gene diversity in sediments of the Kazan mud volcano (Mediterranean Sea). Geobiology 2008, 6, 450–460. [DOI] [PubMed] [Google Scholar]
- 49. Pachiadaki, M.G. , Lykousis, V. , Stefanou, E.G. , Kormas, K. A. , Prokaryotic community structure and diversity in the sediments of an active submarine mud volcano (Kazan mud volcano, East Mediterranean Sea). FEMS Microbiol. Ecol. 2010, 72, 429–444. [DOI] [PubMed] [Google Scholar]
- 50. Pachiadaki, M. G. , Kallionaki, A. , Dählmann, A. , De Lange, G. J. et al., Diversity and spatial distribution of prokaryotic communities along a sediment vertical profile of a deep‐sea mud volcano. Microb. Ecol. 2011, 62, 655–668. [DOI] [PubMed] [Google Scholar]
- 51. Blumenberg, M. , Seifert, R. , Reitner, J. , Pape, T. et al., Membrane lipid patterns typify distinct anaerobic methanotrophic consortia. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 11111–11116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Michaelis, W. , Seifert, R. , Nauhaus, K. , Treude, T. et al., Microbial reefs in the Black Sea fueled by anaerobic oxidation of methane. Science 2002, 297, 1013–1015. [DOI] [PubMed] [Google Scholar]
- 53. Wankel, S. D. , Adams, M. M. , Johnston, D. T. , Hansel, C. M. et al., Anaerobic methane oxidation in metalliferous hydrothermal sediments: Influence on carbon flux and decoupling from sulfate reduction. Environ. Microbiol. 2012, 14, 2726–2740. [DOI] [PubMed] [Google Scholar]
- 54. Hinrichs, K. U. , Hayes, J. M. , Sylva, S. P. , Brewer, P. G. et al., Methane‐consuming archaebacteria in marine sediments. Nature 1999, 398, 802–805. [DOI] [PubMed] [Google Scholar]
- 55. Polerecky, L. , Adam, B. , Milucka, J. , Musat, N. et al., Look@ NanoSIMS–a tool for the analysis of nanoSIMS data in environmental microbiology. Environ. Microbiol.. 2012, 14, 1009–1023. [DOI] [PubMed] [Google Scholar]
- 56. Haroon, M. F. , Hu, S. , Shi, Y. , Imelfort, M. et al., Anaerobic oxidation of methane coupled to nitrate reduction in a novel archaeal lineage. Nature 2013, 500, 567–570. [DOI] [PubMed] [Google Scholar]
- 57. Meyerdierks, A. , Kube, M. , Kostadinov, I. , Teeling, H. et al., Metagenome and mRNA expression analyses of anaerobic methanotrophic archaea of the ANME‐1 group. Environ. Microbiol. 2010, 12, 422–439. [DOI] [PubMed] [Google Scholar]
- 58. Wang, F.‐P. , Zhang, Y. , Chen, Y. , He, Y. et al., Methanotrophic archaea possessing diverging methane‐oxidizing and electron‐transporting pathways. ISME J. 2014, 8, 1069–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hoehler, T. M. , Alperin, M. J. , Albert, D. B. , Martens, S. , Field and laboratory studies of methane oxidation in an anoxic marine sediment: Evidence for a methanogen‐sulfate reducer consortium. Global Biogeochem. Cy. 1994, 8, 451–463. [Google Scholar]
- 60. Sørensen, K. , Finster, K. , Ramsing, N. , Thermodynamic and kinetic requirements in anaerobic methane oxidizing consortia exclude hydrogen, acetate, and methanol as possible electron shuttles. Microb. Ecol. 2001, 42, 1–10. [DOI] [PubMed] [Google Scholar]
- 61. Valentine, D. L. , Biogeochemistry and microbial ecology of methane oxidation in anoxic environments: a review. Anton. Leeuwen. 2002, 81, 271–282. [DOI] [PubMed] [Google Scholar]
- 62. Meulepas, R. J. , Jagersma, C. G. , Zhang, Y. , Petrillo, M. et al., Trace methane oxidation and the methane dependency of sulfate reduction in anaerobic granular sludge. FEMS Microbiol. Ecol. 2010, 72, 261–271. [DOI] [PubMed] [Google Scholar]
- 63. Meulepas, R. , Jagersma, C. , Khadem, A. , Stams, A. et al., Effect of methanogenic substrates on anaerobic oxidation of methane and sulfate reduction by an anaerobic methanotrophic enrichment. Appl. Microbiol. Biotechnol. 2010, 87, 1499–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Moran, J. J. , Beal, E. J. , Vrentas, J. M. , Orphan, V. J. et al., Methyl sulfides as intermediates in the anaerobic oxidation of methane. Environ. Microbiol. 2008, 10, 162–173. [DOI] [PubMed] [Google Scholar]
- 65. Lee, N. , Nielsen, P. H. , Andreasen, K. H. , Juretschko, S. et al., Combination of fluorescent in situ hybridization and microautoradiography‐a new tool for structure‐function analyses in microbial ecology. Appl. Environ. Microbiol. 1999, 65, 1289–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nielsen, J. , Nielsen, P. , Combined microautoradiography and fluorescence in situ hybridization (MAR‐FISH) for the identification of metabolically active microorganisms in: Handbook of Hydrocarbon and Lipid Microbiology, Springer, Berlin Heidelberg: 2010, pp. 4093–4102. [Google Scholar]
- 67. Kellermann, M. Y. , Wegener, G. , Elvert, M. , Yoshinaga, M. Y. et al., Autotrophy as a predominant mode of carbon fixation in anaerobic methane‐oxidizing microbial communities. Proc. Natl. Acad. Sci. U.S.A. 2012, 109, 19321–19326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Material


