Abstract
d‐Lactic acid production is gaining increasing attention due to the thermostable properties of its polymer, poly‐d‐lactic acid . In this study, Lactobacillus coryniformis subsp. torquens, was evaluated for its ability to produce d‐lactic acid using Dried Distiller's Grains with Solubles (DDGS) hydrolysate as the substrate. DDGS was first subjected to alkaline pretreatment with sodium hydroxide to remove the hemicellulose component and the generated carbohydrate‐rich solids were then subjected to enzymatic hydrolysis using cellulase mixture Accellerase® 1500. When comparing separate hydrolysis and fermentation and simultaneous saccharification and fermentation (SSF) of L. coryniformis on DDGS hydrolysate, the latter method demonstrated higher d‐lactic acid production (27.9 g/L, 99.9% optical purity of d‐lactic acid), with a higher glucose to d‐lactic acid conversion yield (84.5%) compared to the former one (24.1 g/L, 99.9% optical purity of d‐lactic acid). In addition, the effect of increasing the DDGS concentration in the fermentation system was investigated via a fed‐batch SSF approach, where it was shown that the d‐lactic acid production increased to 38.1 g/L and the conversion yield decreased to 70%. In conclusion, the SSF approach proved to be an efficient strategy for the production of d‐lactic acid from DDGS as it reduced the overall processing time and yielded high d‐lactic acid concentrations.
Keywords: DDGS, d‐Lactic acid, Separate hydrolysis and fermentation (SHF), Simultaneous saccharification and fermentation (SSF)
Abbreviations
- μmax
maximum specific growth rate
- DDGS
dried distiller's grains with solubles
- EMP
Embden‐Meyerhof‐Parnas pathway
- MRS
deMan Rogosa Sharpe
- PLA
polylactic acid
- SSF
simultaneous saccharification and fermentation
- SHF
separate hydrolysis and fermentation
1. Introduction
Lactic acid (C3H6O3) is considered as one of the most useful chemical products and has attracted a great attention worldwide due to its widespread applications in food, chemical, cosmetic, textile, and pharmaceutical sectors. It has also emerged into the bioplastics industry, where lactic acid serves as the building block for polylactic acid (PLA) synthesis. PLA is a biodegradable polymer that holds great potential in replacing petroleum‐based polymers. Because of its degradability and biocompatibility, PLA is extensively used in the biomedical field as a surgical suture, drug‐delivery material and bone fixation material 1, 2. In addition, PLA received a Generally Recognized as Safe (GRAS) status from the US Food and Drug Administration (FDA) in 2002, which allowed the expansion of its applications within the food industry. PLA can be utilised as a food contact material, e.g. for the production of cutlery, cups, plates, and containers, or as food packaging material 2, 3. At the moment, the PLA market demand accounts for 11.4% of the total bioplastic production worldwide, and is equal to approximately 180,000 metric tonnes per year. In addition, the PLA demand is estimated to grow significantly, by 28% per year until 2025, as a result of the expansion of the bioplastics market 4, 5.
PLA can be manufactured by utilizing either the d‐ or l‐ forms of lactic acid, or its racemic mixture 6, 7. Poly‐l‐lactic acid polymer has a low melting point (180°C) and low crystallisation ability 8. On the other hand, polymer blends of purified poly‐l‐lactic acid and purified poly‐d‐lactic acid produce racemic crystals called stereo‐complexes which have higher melting point (230°C) and distortion temperatures, and as such, offer significant advantages for a number of applications such as high heat packaging materials 9, 10, 11, 12. The optical purity of lactic acid is one of the crucial factors towards the production of highly crystalline PLA. To this end, the microbial lactic acid production route offers advantages compared to the chemical production route, as specific isomers of d‐ or l‐ lactic acid can be produced depending on the selected bacterial strain.
Over 90% of the commercially produced lactic acid is derived from microbial fermentation utilizing glucose, sucrose, or corn starch as carbon sources 13. However, the relatively high cost of pure sugars has driven research on industrial fermentation towards the use of alternative resources, which can be obtained through the valorization of cheap, renewable agricultural biomass 13, 14, 15, 16, 17. Apart from no interference with food industry, the other advantages of utilizing renewable sources is the possibility of producing cheaper fermentation medium at higher nutrient content to support bacterial growth. Specifically, agricultural residues such as corn stover 18, 19, rice bran 20, peanut meal 21, broken rice 22, and unpolished rice 23 have been studied as potential carbon sources for lactic acid production. However, the hydrolysis of biomass materials, that are rich in cellulose/hemicellulose, produces a mixture of sugar monomers such as glucose, xylose, mannose, arabinose, and galactose. Most of the homofermentative d‐lactic acid producers (i.e., Lactobacillus sp. and Sporolactobacillus sp.) are unable to ferment sugars other than glucose, thus, leave the non‐utilized sugar to accumulate in the fermentation broth at the end of fermentation process 18, 19. Besides, fermentation broths derived from renewable sources also contain a mixture of compounds, including a variety of sugars and proteins, degraded compound from pretreatment, polyphenols and organic acids, and thus require an effective downstream processing for the recovery of the lactic acid 24, 25. Several methods such as precipitation, extraction, crystallization, ion exchange, adsorption, membrane filtration, distillation, and nanofiltration can be used to recover lactic acid from fermentation broth 17, 25.
Agricultural biomass needs to be treated either chemically (with acid or alkali) or enzymatically hydrolyzed in order to be converted into fermentable sugars. The enzymatic approach is preferred to chemical hydrolysis, as the reactions are more specific and less hazardous 25, 26. The overall production process can consist either of the two steps operated sequentially, i.e. separate hydrolysis and fermentation (SHF) or concurrently, i.e. simultaneous saccharification and fermentation (SSF) 25. In SHF, enzymatic hydrolysis and fermentation take place separately, and each process is conducted at its optimal conditions. The major disadvantage of SHF is that the accumulation of sugars after hydrolysis can reduce the activity of enzymes, particularly cellulase and β‐glucosidase, by 60–75% 26, 27, 28. In contrast, in SSF, enzymatic hydrolysis and fermentation process are carried out simultaneously, allowing for the direct assimilation of monomeric sugars by the microbial cells, thus reducing the risk of sugar accumulation in the medium. Additional advantages of SSF include shorter production times for the targeted product, higher production yields (% g product/g of substrate) and lower production costs due to the lower amount of energy and labor required 26, 29, 30.
Dried distiller's grains with solubles (DDGS) is a by‐product of bioethanol production from wheat or corn, as well as of the distillery industry, and is currently used as animal feed due to its high protein (29–38%) and fibre content (40‐46%) 31, 32, 33. However, DDGS has to compete with other protein sources such as soybean meal and rapeseed meal within the animal feed market which are considered of a better quality 31. Moreover, the possible high levels of mycotoxins (three‐fold compared to the original sources, i.e. wheat or corn grains) in DDGS have become a concern for the farming industry 34. In terms of fibre composition, DDGS is primarily composed of cellulose and hemicellulose, which mainly consist of the monosaccharides glucose, xylose and arabinose as the main sugars. As DDGS is also characterized by a high fibre content, it could potentially be used as an alternative carbon source for lactic acid fermentation 35. In commercial lactic acid production, glucose and corn starches have been widely used as substrates for fermentation. However, this is economically unfavorable as pure sugars have a higher economic value than the lactic acid produced 13, 36.
The main objective of this study was to develop a fermentation process for the production of optically pure d‐lactic acid from wheat DDGS hydrolysates using Lactobacillus coryniformis subsp. torquens. Two fermentation approaches, SHF and SSF, were evaluated in terms of lactic acid yield, productivity, and purity.
2. Materials and methods
2.1. Microorganisms and culture conditions
Lactobacillus coryniformis subsp. torquens (DSM 20004) was obtained from the German Collection of Microorganisms and Cell Cultures (DSMZ). The stock of the bacterial culture was kept in mixtures of commercial deMan Rog (MRS) broth and glycerol and stored at −80°C. Bacterial strains were cultivated in 250 mL Erlenmeyer flasks containing 50 mL MRS broth at 37°C and 150 rpm agitation speed for 18 h, and were subsequently used as inoculum. The cell growth was monitored by OD using a Biomate 3 UV/VIS Spectrophotometer (Thermo Spectronic, Rochester, NY) at 600 nm wavelength.
2.2. Raw material and enzyme
DDGS was supplied from a bioethanol plant (Vivergo, Yorkshire, UK). It was ground using a coffee grinder (DeLonghi, Australia) into a fine powder (particles smaller than 0.85 mm) and stored at room temperature (25°C) prior to alkaline pretreatment. The commercial cellulase mixture Accellerase® 1500 was kindly provided by Danisco US Inc. (Genencor, Leiden, Netherlands); it consisted of multiple enzyme activities including endoglucanase (2200–2800 CMC U/g), β‐glucosidase (450–775 pNPG U/g), exoglucanase, and hemicellulase. The enzyme was kept at 4°C before use.
2.3. Growth of L. coryniformis in semi‐defined MRS‐based media
The microbial growth of L. coryniformis was initially studied in a 100 mL fermentation vessel containing 50 mL of MRS as the basal medium [glucose, 16 g/L (commercially present in MRS media); casein peptone, 10.0 g/L; meat extract, 10.0 g/L; yeast extract, 5.0 g/L; tween 80, 1.0 g/L; potassium phosphate dibasic (K2HPO4), 2.0 g/L; sodium acetate (CH3COONa) 5.0 g/L; di‐ammonium hydrogen citrate (C6H6O7), 2.0 g/L; magnesium sulfate heptahydrate (MgSO4 × 7H2O), 0.2 g/L; and manganese (ll) sulfate monohydrate (MnSO4 × H2O), 0.05 g/L]. The fermentation system consisted of a 100 mL glass vessel connected to a temperature controlled water bath (GD 120, Grant, Cambridge) set at 37°C, a FerMac 260 pH controller (Electrolab, Hertfordshire) and a Stuart stirrer; no nitrogen or air addition was included in the system. The strain was inoculated at a similar starting OD (OD∼0.05) for all fermentations. Three fermentation runs were conducted: (i) with the pH controlled at pH 5, (ii) with the pH controlled at pH 6, and (iii) with uncontrolled pH. The maximum specific growth rate (μmax) was calculated from the slope of the plot depicting the natural logarithm (ln) of the OD against time. Using the best pH for L. coryniformis growth, the strains were then cultivated at 37°C in modified MRS basal medium (outsources carbon) supplemented with a single carbon source (20 g/L of glucose, xylose, or arabinose) as well as mixed sugars (10 g/L glucose and 10 g/L xylose). For all fermentations, samples were taken at regular time intervals and analyzed for cell growth by OD measurement, lactic acid and acetic acid concentration, residual sugar, and optical purity of d‐lactic acid (%), as described in Section 2.7.
2.4. Alkaline pretreatment of DDGS
DDGS was pretreated with 5% (w/v) NaOH at 121°C (∼16 psi) for 15 min at 10% (w/v) DDGS loading. After pretreatment, the mixture was cooled down to room temperature and centrifuged at 17,105 x g (Heraeus Multifuge X3R, Thermo Fisher, USA) for 20 minutes at 4°C. The obtained solids were extensively washed with distilled water until the pH reached around 8, and the pH neutralised with HCl (6 M) to a final pH between 5–5.5. The solids were frozen (−20°C), freeze‐dried (VisTis Sentry 2.0, Warminster, PA) and stored in a closed container at room temperature (25°C) until further use.
2.5. SHF of DDGS hydrolysate
Alkaline pretreated DDGS solids (3.3 g) were hydrolysed with Accellerase® 1500 (5 mL) at a ratio of 1 mL enzyme: 0.33 g cellulose; the cellulose content of DDGS pretreated solids was approximately 50% w/w. The enzymatic hydrolysis was conducted at 50°C for 24 h at 300 rpm in a shaking incubator (SciQuip, Shropshire, UK), followed by heat inactivation at 95°C for 10 min. The mixture was centrifuged at 17,105 x g for 20 min (4°C) and the supernatant was collected and filter sterilised using 0.22 μm sterile vacuum filter (EMD Milipore StericupTM). 5 mL of sterile concentrated yeast extract (200 g/L) was then added aseptically into the 100 mL fermentation vessel (final concentration 20 g/L). L. coryniformis was inoculated into 50 mL of DDGS hydrolysate at a similar starting OD of ∼0.05. Lactic acid fermentation was carried out at 37°C for 54 h; no nitrogen or air was passed through the fermentation medium. The pH of the cultures was maintained at 6 through the addition of NaOH (2 M). Samples were taken at regular time intervals and kept at −20 °C until further analysis.
2.6. SSF of DDGS hydrolysate
The SSF experiments were conducted in a 100 mL fermentation vessel using the fermentation system described in Section 2.3; the pH was controlled at 5 at 37°C. 3.3 g of alkaline pretreated DDGS were steam‐sterilised inside the fermentation vessel by autoclaving the vessel at 121°C for 15 min. After cooling, sterile distilled water and yeast extract (20 g/L) were added into the fermentation vessel. The SSF process was initiated by the addition of Accellerase® 1500 into the DDGS hydrolysate at a loading rate of 1: 0.33 (mL enzyme: g cellulose), followed by inoculation with L. coryniformis at a starting OD of approximately 0.05. In certain runs, 1.1 g of pretreated DDGS (resulting in 11 and 22 g/L of glucose, respectively) were aseptically added, with the aid of a portable Bunsen burner, when the glucose in the fermentation medium was depleted (fed‐batch SSF); this was approximately after 24 h of fermentation. No nitrogen or air was passed through the fermentation medium. For all SSF experiments, samples were taken at regular time intervals and kept at −20°C for further analysis.
The SSF process was also studied in a 2 L stirred tank bioreactor (Biostat B, Sartorious, Germany) with 1.5 L working volume. 99 g of alkaline pretreated DDGS solids were added into the bioreactor and steam‐sterilised by autoclaving at 121°C for 30 min. After cooling the bioreactor, 1500 mL of sterile distilled water was added along with yeast extract (final concentration 20 g/L). Accellerase® 1500 was added at a loading rate of 1:0.33 (mL enzyme: g cellulose), followed by the addition of L. coryniformis inoculum at a starting OD of ∼0.05. The fermentation was carried out at 37°C with an initial agitation speed of 250 rpm. The minimum dissolved oxygen (DO) level was kept at 20% by controlling automatically the stirrer speed and the pH was maintained at 5 with 5 M NaOH and HCl. Antifoam 204 (10%, v/v, Sigma) was added to prevent foaming during fermentation.
2.7. Analytical methods
Sugars (xylotriose, xylobiose, glucose, xylos,e and arabinose) and organic acids (lactic acid and acetic acid) were analysed by HPLC in an Agilent Infinity 1260 system (Agilent Technologies, USA) equipped with an Aminex HPX‐87H column (Bio‐rad, Hercules, CA) at 0.6 mL/min flow rate, with 5 mM H2SO4 as mobile phase. The temperature of the column was set at 65°C and the sugars and organic acids were detected using a refractive index detector. The presence of d‐ and l‐lactic acid in the fermentation broth was determined by using the d‐ and l‐lactate dehydrogenase enzyme kit (K‐DLATE 07/14, Megazyme, Ireland). The optical purity (%) of d‐lactic acid was calculated as follows 21, 37:
The nitrogen consumption during fermentation was determined by the Free amino nitrogen method as described by Lie 38 with some modifications. 0.5 mL of diluted sample was mixed with 0.25 mL of color reagent (49.71 g of Na2HPO4·2H2O, 5 g of ninhydrin, 3 g of fructose, and ∼40 g of KH2PO4 dissolved in 1 L of distilled water; pH 6.6–6.8) in a 2 mL Eppendorf tube. The mixture was heated at 100°C in a thermal block (Grant, Cambridge) for 16 min and immediately cooled in an ice bath. 1.5 mL of dilution reagent (2 g potassium iodate, KIO3, in 616 mL distilled water and 384 mL 96% ethanol) was added and the free amino nitrogen content was measured at 570 nm. A calibration curve was constructed using glycine as standard at various concentrations (0.25–2 mg/L).
3. Results and discussion
3.1. Effect of growth conditions on L. coryniformis fermentation in MRS‐based media
The results from the growth experiments of L. coryniformis in the MRS‐based medium with glucose as the carbon source under pH‐controlled (pH 5 and 6) and non pH‐controlled conditions are shown in Table 1. The optimum pH value for the cell growth of Lactobacillus sp., including the species used in this work is normally between 5 and 7 39, 40. The results are in line with the above as good cell growth was obtained in the pH values tested. In the uncontrolled pH culture, the pH dropped to ∼4.1 after 24 h of growth, and the μmax for L. coryniformis was 0.30 h−1. Approximately, 3 g/L glucose was left unutilized in the media after fermentation. When the pH of the medium was controlled, the μmax increased to 0.36 h−1 at both pH 5 and 6, indicating improved microbial growth with all glucose being utilized during fermentation. This indicated that pH is one of the controlling factors that promote high lactic acid production 36.
Table 1.
Growth parameters of L. coryniformis in MRS‐based media
| Conditions | OD 600 nm * | Maximum specific growth rate (μmax, h−1) | Lactic acid (g/L) | YLac/S (%, w/w)a |
|---|---|---|---|---|
| Glucose (16 g/L) Uncontrolled pH | 4.5 ± 0.06 | 0.30 | 12.5 ± 0.10 | 97.63 |
| Glucose (16 g/L) pH 5 | 7.0 ± 0.50 | 0.36 | 15.7 ± 0.47 | 98.13 |
| Glucose (16 g/L) pH 6 | 6.4 ± 0.16 | 0.36 | 15.6 ± 1.85 | 97.50 |
| Glucose (20 g/L) pH 6 | 8.2 ± 0.04 | 0.38 | 19.7 ± 0.02 | 99.9 |
| Xylose (20 g/L) pH 6 | 0.5 ± 0.01 | ‐ | ‐ | ‐ |
| Arabinose (20 g/L) pH 6 | 0.4 ± 0.17 | ‐ | ‐ | ‐ |
| Glucose and Xylose (10 g/L each) pH 6 | 4.5 ± 0.18 | 0.29 | 10.5 ± 0.97 | 52.50 |
YLac/S (%, w/w) = (g lactic acid produced/g sugar consumed) x 100
Data based on three independent fermentation trials and are shown as mean ± SD
Another factor that plays an important role for lactic acid fermentation is the type of carbon source used. Glucose, xylose, and arabinose (at 20 g/L) were tested as the carbon source in a MRS‐based medium (Table 1), as these sugars are most likely present in the DDGS hydrolysates. Among the sugars, L. coryniformis only consumed glucose, with 98–99.9% of glucose being converted to lactic acid and did not consume xylose and arabinose. This finding shows that L. coryniformis is a homolactic acid producer which utilizes the Embden‐Meyerhof‐Parnas pathway (EMP) to convert one molecule of glucose into two molecules of lactic acid 39, 41. Lactobacillus species can produce l‐or d‐ lactic acid, depending on the type of lactate dehydrogenase (nLDH) present in their cluster (ldhL or ldhD, respectively) 42, 43, 44. In the case where both d‐ and l‐ lactic acid are produced from a single strain, this is likely due to the presence of both ldhD and ldhL 45. Moreover, in all the growth experiments L. coryniformis produced exclusively d‐lactic acid (99.9% optical purity) which is in line with previous studies with this particular microorganism 9 .
3.2. SHF of L. coryniformis on DDGS hydrolysate
Alkaline pretreated DDGS consisted of 52.6 g glucose, 25.0 g xylose, 10.3 g arabinose, and 0.04 g protein per 100 g of dried material. In the first part of the work, alkaline treated DDGS solids were hydrolyzed to simple sugars using the Accellerase® 1500 enzyme at 50°C for 24 h. The sugar composition in the DDSG hydrolysate was: glucose ∼27.0 g/L, xylose ∼6.1 g/L, xylobiose ∼5.9 g/L, and arabinose ∼0.8 g/L. 86.5% cellulose was successfully hydrolyzed to glucose during hydrolysis. The hydrolysate was then used as fermentation medium for lactic acid production by L. coryniformis. The fermentation pH for L. coryniformis was set to 6, based on the results in Table 1.
Figure 1 shows the fermentation characteristics (cell growth, nitrogen and sugar consumption, lactic acid production) of L. coryniformis in the DDGS hydrolysate as a function of time. Result shows that L. coryniformis grew very well in the hydrolysate, with a maximum OD600 of 8.7 and a lactic acid concentration of 24.0 g/L, being obtained after 18 h fermentation, the time point at which almost all of the available glucose was depleted. The concentration of xylobiose, xylose, and arabinose remained unchanged throughout the fermentation, which is in line with the results obtained in the semi‐defined media (Table 1). An increase in acetic acid production was observed after glucose depletion, reaching 2.0 g/L after 30 h fermentation. According to Yáñez, et al. 46 and Hofvendahl and Hahn–Hägerdal 47, under glucose limitation, homofermentative lactic acid bacteria tend to produce other by‐products such as formic or acetic acid through alternative pyruvate catabolic pathways, whereas Slavica, et al. 39 also reported an increase in acetic acid when glucose was depleted in a MRS fermentation medium. Interestingly, between 18 and 30 h, the concentration of lactic acid slightly decreased to 22.9 g/L, most likely due to degradation of lactic acid to acetic acid. Although lactic acid bacteria, especially lactobacilli, are classified as homofermentative or heterofermentative according to their ability to produce lactic acid through the EMP pathway, many are able to degrade lactic acid, especially if oxygen is available as an electron acceptor. The lactic acid that is initially formed from the EMP pathway can be converted to acetic acid after glucose depletion under aerobic conditions. This has been reported for L. brevis 48 and L. plantarum 49, although no works have shown this for L. coryniformis up to now.
Figure 1.
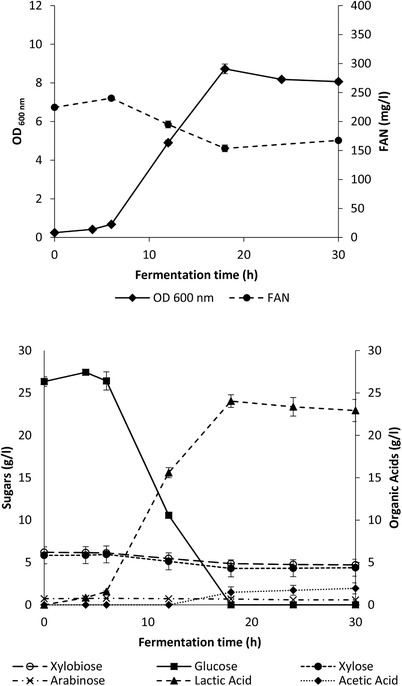
Fermentation profile of L. coryniformis in pretreated DDGS hydrolysate in a 100 mL bioreactor (temperature: 37°C, pH: 6). OD: OD at 600 nm; Free amino nitrogen. Data based on two independent fermentation trials and reported as mean ± SD.
In order for lactic acid to be used as a monomer for PLA synthesis, optical purity is one of the most important factors to be considered. The optical purity of d‐ or l‐ lactic acid has to be >90% in order to be used for the synthesis of crystalline PLA 9, 50. The optical purity (%) of d‐lactic acid obtained with L. coryniformis in the DDGS hydrolysate was 99.9% indicating that the produced d‐lactic acid could be potentially used for the production of crystalline poly‐d‐lactic acid if the purity levels of d‐lactic acid obtained after purification of the fermentation broth are high, i.e. >90% 50.
3.3. SSF of L. coryniformis on DDGS hydrolysate
Figure 2 depicts the fermentation data for the SSF of L. coryniformis in alkaline pretreated DDGS medium. Accellerase® 1500 was used to hydrolyze DDGS during the SSF process. SSF offers several advantages compared to the SHF, performed previously, as it combines enzymatic hydrolysis and fermentation in a single step process, resulting in reduced overall processing times and capital costs 51. Moreover, SSF also reduces the potential of cellulase inhibition due to the high concentration of glucose in the hydrolysate 9. However, compatible operating temperatures and pH for both the processes (hydrolysis and fermentation) should be carefully selected to ensure high lactic acid production. Previous research works have reported that temperature range from 37 to 40°C and a pH around 5 is appropriate for the production of lactic acid production from biomass by lactobacilli. For example, lactic acid was produced via a SSF approach from cassava bagasse 52, rice bran by L. delbrueckii 20, and from curcuma longa (tumeric) biomass of by L. paracasei and L. coryniformis 53.
Figure 2.
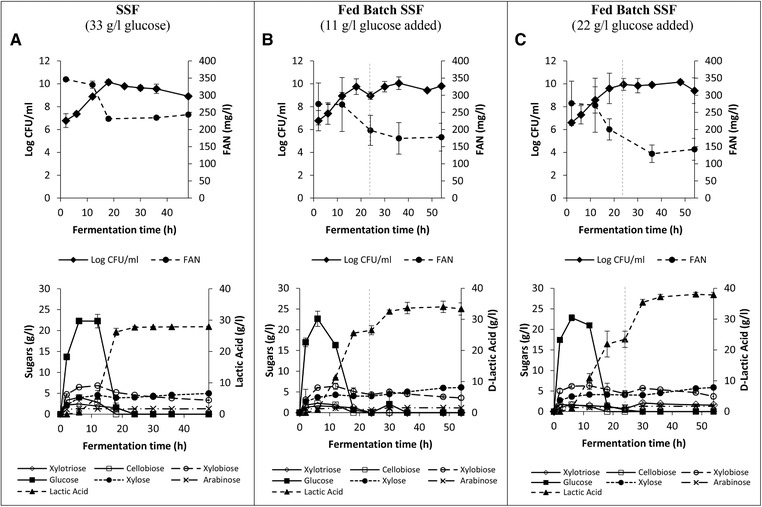
SSF of alkaline pretreated DDGS by L. coryniformis in a 100 mL bioreactor (temperature 37°C, pH 5) under different fermentation regimes. 3a) DDGS concentration: 33 g/L; 3b) addition of 11g/L DDGS after 24 hours; 3c) addition of 22 g/L DDGS after 24 hours. Data based on two independent fermentation trials and reported as mean ± SD.
In this study, the SSF process was carried out in 100 mL bioreactor containing 33 g/L of glucose from alkaline pretreated DDGS at 37°C, with the pH being controlled at 5 throughout the process. Since L. coryniformis could grow well in media with both pH 5 and 6, the former pH was selected for SSF since it was the optimum pH for cellulase activity as stated by the manufacturer. During the first 6 h ∼68% of the cellulose present in the alkaline pretreated DDGS (22 g/L) was converted to glucose and was not utilized by L. coryniformis, as the strain was still in the lag phase; as a result, low production of lactic acid (0.6 g/L) was detected during this period. L. coryniformis started to consume glucose after 12 h, with the highest lactic acid and viable cell concentrations obtained after 24 h, i.e. 28 g/L of lactic acid (99.9% optical purity of d‐ lactic acid) and 9.8 CFU/mL, respectively after 24 h (Figure 2a). Unlike SHF, in SSF no reduction in the lactic acid concentration was observed after glucose depletion (24 to 48 h). This might be due to the action of the cellulose enzyme of Accellerase® 1500 that was still actively converting the remaining traces of cellulose in the DDGS to glucose, albeit in very small amounts, which was most likely rapidly consumed by L. coryniformis. At the end, around 84.5% of the cellulose present in the pretreated DDGS was converted to lactic acid, demonstrating the efficient utilization of DDGS during the SSF process.
In an attempt to increase lactic acid production, the effect of increasing the amount of pretreated DDGS loading in the SFF process was investigated. However, increasing the substrate loading results in highly viscous suspensions, which reduces the efficiency of enzymatic hydrolysis 54. One way to overcome this problem is via multi‐step feeding or fed‐batch SSF. In this approach, additional cellulosic biomass substrate is added at a particular point during the process; as a result free water is liberated, which reduces the viscosity and stiffness of the suspension 55, 56. In this study, when the glucose concentration reached <0.5 g/L (at 24 h), alkaline pretreated DDGS was added at two levels, 11 g/L glucose (Figure 2b) and 22 g/L glucose (Figure 2c); 34.0 and 38.1 g/L of lactic acid (99.9% optical purity of d‐lactic acid) were produced respectively, after 48 h of fermentation. However, a reduction in the conversion yield of glucose to lactic acid (76% vs 70%, respectively) was observed with the higher substrate loading. This might be due to inadequate stirring at the higher solid content, which resulted in insufficient mass transfer and thus reduced the adsorption capacity of cellulase to cellulose and the efficiency of the enzymatic digestion of DDGS solids 54, 57.
A comparison of the fermentation characteristics obtained by employing different fermentation approaches is shown in Table 2. Overall, SSF demonstrated better fermentation characteristics compared to SHF; more specifically high d‐lactic acid concentration (27.9 g/L), productivity (1.46 g/L/h), glucose conversion yield (84.5%) and d‐lactic acid yield (42.3%) were observed when SSF process was employed. When DDGS solids were added using the SSF fed‐batch approach, the d‐lactic acid concentration increased up to ∼38 g/L (when adding 22 g/L glucose from alkaline pretreated DDGS), but in this case the other fermentation characteristics decreased. On the other hand, the fermentation characteristics in the case of adding 11 g/L glucose during the SSF fed‐batch approach were deemed overall optimal and demonstrate the potential of using this approach at a commercial large scale operation.
Table 2.
Overall fermentation characteristics of different fermentation processes for the production of d‐lactic acid by L. coryniformis cultivation
| Fed batch SSF | ||||
|---|---|---|---|---|
| Paramete | SHF 33 g/L glucosea | SSF 33 g/L glucosea | Addition of 11 g/L glucose* | Addition of 22g/L glucosea |
| Lactic acid production (g/L) | 24.1 | 27.9 | 34.0 | 38.1 |
| Lactic acid productivity (g/L/h) | 1.3 | 1.5 | 0.7 | 0.8 |
| Glucose conversion (%)b | 72.9 | 84.5 | 76.1 | 70.0 |
| Lactic acid yield (%)c | 32.1 | 42.3 | 40.1 | 35.0 |
present in alkaline treated DDGS solids
Lactic acid (mg)/glucose in alkaline pretreated DDGS (mg) × 100
Lactic acid (mg)/pretreated DDGS (mg) × 100
Data based on two independent fermentation trials and are shown as mean ± SD
The feasibility of the SSF process was evaluated in a 2‐L stirred tank bioreactor with 1.5 L working volume (Figure 3). The data obtained were very similar to those obtained for the SSF in the 100 mL bioreactor, i.e. the maximum d‐lactic acid concentration was 26.4 g/L (produced after 18 h), the glucose conversion yield was ∼80%, the productivity was 1.47 g/L/h, the lactic acid yield was ∼40%, and the d‐lactic acid optical purity was 99.9%. The key difference compared to the smaller scale SSF process was the fact that after glucose depletion (18 h), the lactic acid concentration decreased (from 26.4 g/L at 18 h to 22.6 g/L at 30 h) and acetic acid was produced (from 1.3 g/L at 18 h to 4.0 g/L after 30 h). This phenomenon could be due the ability of L. coryniformis to convert lactic acid to acetic acid under aerobic conditions, once glucose was depleted as shown in fermentation of L. plantarum 49. In order to avoid the accumulation of acetic acid in the fermentation medium, which may interfere with the purification process, it is important that the optimum time for stopping the fermentation and the optimal aeration conditions are identified.
Figure 3.
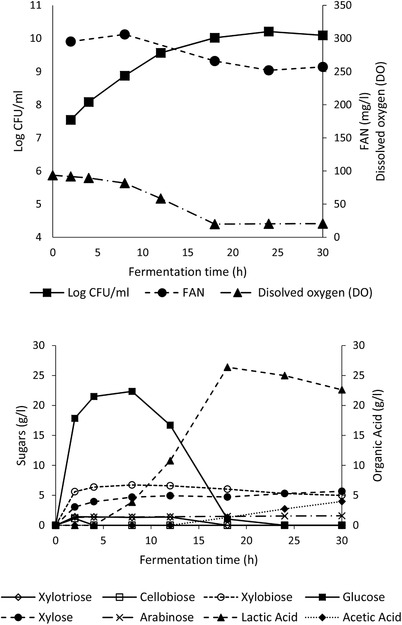
SSF of alkaline pretreated DDGS by L. coryniformis in a 2 L bioreactor (temperature 37°C, pH 5); Free amino nitrogen.
4. Concluding remarks
This study highlights the potential of producing d‐lactic acid with high optical purity from alkaline pretreated DDGS, which is produced in large amounts by the bioethanol industry. The SSF approach using L. coryniformis resulted in faster and higher production of optically pure d‐lactic acid (99.9%), with a higher conversion yield of glucose to lactic acid (84.5%) compared to conventional SHF (72.9%). The d‐lactic acid production could be further enhanced by employing a fed‐batch SSF process where the fermentation medium is fed with DDGS during the process, however, further work is needed to identify the operating conditions that result to increase the substrate conversion yields from 70–75% to above 85%. The SSF process demonstrated good scalability as similar fermentation characteristics were obtained between the small (100 mL) and larger scale (2‐L) fermentation vessels.
Practical application
d‐lactic acid is an important monomer for the synthesis of biodegradable plastics, where mixtures of poly‐d‐lactic acid and poly‐l‐lactic acid generate heat stable polylactic acid (PLA) suitable for high temperature processing applications. However, research on d‐lactic acid is limited whereas the optical purity of lactic acid is one of the crucial factors towards the production of highly crystalline PLA. This study demonstrated the potential of producing d‐lactic acid with high optical purity from alkaline pretreated DDGS, a cheap and renewable resource produced in large amounts by the bioethanol industry. The SSF approach resulted in faster and higher production of d‐lactic acid, with a higher conversion yield of glucose to lactic acid (84.5%) compared to conventional SHF (72.9%). The SSF process demonstrated good scalability as similar fermentation characteristics were obtained between the small (100 mL) and larger scale (2‐L) fermentation vessels.
The authors have declared no conflicts of interest.
Highlights
DDGS is a suitable substrate for L. coryniformis growth and d‐lactic acid production
The SSF approach resulted in a high glucose to d‐lactic acid conversion yield (84.5%).
A fed batch SSF led to high d‐lactic acid although with lower conversion yields (70–75%).
Acknowledgement
The authors would like to acknowledge the Ministry of Education Malaysia (MOE) and the National University of Malaysia, Bangi (UKM), for providing a doctoral sponsorship and financial support to carry out this research.
5 References
- 1. Nampoothiri, K. M. , Nair, N. R. , John, R. P. , An overview of the recent developments in polylactide (PLA) research, Bioresour. Technol. 2010, 101, 8493–8501. [DOI] [PubMed] [Google Scholar]
- 2. Conn, R. E. , Kolstad, J. J. , Borzelleca, J. F. , Dixler, D. S. , et al., Safety assessment of polylactide (PLA) for use as a food‐contact polymer, Food Chem. Toxicol. 1995, 33, 273–283. [DOI] [PubMed] [Google Scholar]
- 3. Jamshidian, M. , Tehrany, E. A. , Imran, M. , Jacquot, M. , Desobry, S. , Poly‐lactic acid: Production, applications, nanocomposites, and release studies, Compr. Rev. Food Sci. Food Saf. 2010, 9, 552–571. [DOI] [PubMed] [Google Scholar]
- 4. Ltd., C. , Market: Lactic Acid, Polylactic Acid, Ethyl Lactate, 2015. http://cellulac.co.uk/en/market/. (Accessed 29th September 2015). [Google Scholar]
- 5. Aeschelmann, F. , Carus, M. , Bio‐based Building Blocks and Polymers in the World‐Capacities, Production and Applications: Status Quo and Trends towards 2020, Nova‐Institut GmbH, Michael Carus (V.i.S.d.P), 2015.
- 6. Yañez, R. , Moldes, A. B. , Alonso, J. L. , Parajo, J. C. , Production of D(−)‐lactic acid from cellulose by simultaneous saccharification and fermentation using Lactobacillus coryniformis subsp. torquens , Biotechnol. Lett. 2003, 25, 1161–1164. [DOI] [PubMed] [Google Scholar]
- 7. Klotz, S. , Kuenz, A. , Prüße, U. , Nutritional requirements and the impact of yeast extract on the d‐lactic acid production by Sporolactobacillus inulinus, Green Chemistry 2017, 19, 4633–4641. [Google Scholar]
- 8. Xu, H. , Teng, C. , Yu, M. , Improvements of thermal property and crystallization behavior of PLLA based multiblock copolymer by forming stereocomplex with PDLA oligomer, Polymer 2006, 47, 3922–3928. [Google Scholar]
- 9. Nguyen, C. M. , Choi, G. J. , Choi, Y. H. , Jang, K. S. , J.‐C. Kim, d‐ and l‐lactic acid production from fresh sweet potato through simultaneous saccharification and fermentation, Biochem. Eng. J. 2013, 81, 40–46. [Google Scholar]
- 10. Ishida, N. , Suzuki, T. , Tokuhiro, K. , Nagamori, E. , et al., d‐lactic acid production by metabolically engineered Saccharomyces cerevisiae , J. Biosci. Bioeng. 2006, 101, 172–177. [DOI] [PubMed] [Google Scholar]
- 11. Ikada, Y. , Jamshidi, K. , Tsuji, H. , Hyon, S. H. , Stereocomplex formation between enantiomeric poly(lactides), Macromolecules 1987, 20, 904–906. [Google Scholar]
- 12. Kim, S. , Gu, S.‐A. , Kim, Y. H. , Kim, K.‐J. , Crystal structure and thermodynamic properties of d‐lactate dehydrogenase from Lactobacillus jensenii , Int. J. Biol. Macromol. 2014, 68, 151–157. [DOI] [PubMed] [Google Scholar]
- 13. Buyondo, J. P. , Liu, S. , Lactic acid production by Lactobacillus pentosus from wood extract hydrolysates, J‐FOR‐Journal of Science and Technology for Forest Products and Processes 2011, 1, 38. [Google Scholar]
- 14. Dusselier, M. , Van Wouwe, P. , Dewaele, A. , Makshina, E. , Sels, B. F. , Lactic acid as a platform chemical in the biobased economy: the role of chemocatalysis, Energy Environ. Sci. 2013, 6, 1415–1442. [Google Scholar]
- 15. Van Wouwe, P. , Dusselier, M. , Vanleeuw, E. , Sels, B. , Lactide Synthesis and Chirality Control for Polylactic acid Production, ChemSusChem 2016, 9, 907–921. [DOI] [PubMed] [Google Scholar]
- 16. Abdel‐Rahman, M. A. , Tashiro, Y. , Sonomoto, K. , Recent advances in lactic acid production by microbial fermentation processes, Biotechnol. Adv. 2013, 31, 877–902. [DOI] [PubMed] [Google Scholar]
- 17. Klotz, S. , Kaufmann, N. , Kuenz, A. , Prusse, U. , Biotechnological production of enantiomerically pure d‐lactic acid, Appl Microbiol Biotechnol 2016, 100, 9423–9437. [DOI] [PubMed] [Google Scholar]
- 18. Zhang, Y. , Vadlani, P. V. , d‐Lactic acid biosynthesis from biomass‐derived sugars via Lactobacillus delbrueckii fermentation, Bioprocess. Biosyst. Eng. 2013, 36, 1897–1904. [DOI] [PubMed] [Google Scholar]
- 19. Bai, Z. , Gao, Z. , He, B. , Wu, B. , Effect of lignocellulose‐derived inhibitors on the growth and d‐lactic acid production of Sporolactobacillus inulinus YBS1‐5, Bioprocess. Biosyst. Eng. 2015, 38, 1993–2001. [DOI] [PubMed] [Google Scholar]
- 20. Tanaka, T. , Hoshina, M. , Tanabe, S. , Sakai, K. , et al., Production of d‐lactic acid from defatted rice bran by simultaneous saccharification and fermentation, Bioresour. Technol. 2006, 97, 211–217. [DOI] [PubMed] [Google Scholar]
- 21. Wang, L. , Zhao, B. , Li, F. , Xu, K. , et al., Highly efficient production of d‐lactate by Sporolactobacillus sp. CASD with simultaneous enzymatic hydrolysis of peanut meal, Appl. Microbiol. Biotechnol. 2011, 89, 1009–1017. [DOI] [PubMed] [Google Scholar]
- 22. Nakano, S. , Ugwu, C. U. , Tokiwa, Y. , Efficient production of d‐(‐)‐lactic acid from broken rice by Lactobacillus delbrueckii using Ca(OH)2 as a neutralizing agent, Bioresour. Technol. 2012, 104, 791–794. [DOI] [PubMed] [Google Scholar]
- 23. Lu, Z. , Lu, M. , He, F. , Yu, L. , An economical approach for d‐lactic acid production utilizing unpolished rice from aging paddy as major nutrient source, Bioresour. Technol. 2009, 100, 2026–2031. [DOI] [PubMed] [Google Scholar]
- 24. Binder, J. B. , Raines, R. T. , Fermentable sugars by chemical hydrolysis of biomass, Proceedings of the National Academy of Sciences 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cubas‐Cano, E. , González‐Fernández, C. , Ballesteros, M. , E. Tomás‐Pejó, Biotechnological advances in lactic acid production by lactic acid bacteria: lignocellulose as novel substrate, Biofuels, Bioprod. Biorefin. 2018, 12, 290–303. [Google Scholar]
- 26. Binod, P. , Janu, K. U. , Sindhu, R. , Pandey, A. , Hydrolysis of Lignocellulosic Biomass for Bioethanol Production, in: Pandey A., Larroche C., Ricke S. C., Dussap C. G., Gnansounou E. (Eds.), Biofuels. Alternative feed stock and conversion processes, Academic press, Burlington, 2011, pp. 229–250. [Google Scholar]
- 27. Jönsson, L. J. , Alriksson, B. , Nilvebrant, N.‐O. , Bioconversion of lignocellulose: inhibitors and detoxification, Biotechnol. Biofuels. 2013, 6, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Limayem, A. , Ricke, S. C. , Lignocellulosic biomass for bioethanol production: current perspectives, potential issues and future prospects, Prog. Energy Combust. Sci. 2012, 38, 449–467. [Google Scholar]
- 29. Marques, S. , Santos, J. A. L. , Gírio, F. M. , Roseiro, J. C. , Lactic acid production from recycled paper sludge by simultaneous saccharification and fermentation, Biochem. Eng. J. 2008, 41, 210–216. [Google Scholar]
- 30. Zhang, Z. Y. , Jin, B. , Kelly, J. M. , Production of lactic acid from renewable materials by Rhizopus fungi, Biochem. Eng. J. 2007, 35, 251–263. [Google Scholar]
- 31. Villegas‐Torres, M. F. , Ward, J. M. , Lye, G. J. , The protein fraction from wheat‐based dried distiller's grain with solubles (DDGS): extraction and valorization, N. Biotechnol. 2015, 32, 606–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Widyaratne, G. P. , Zijlstra, R. T. , Nutritional value of wheat and corn distiller's dried grain with solubles: Digestibility and digestible contents of energy, amino acids and phosphorus, nutrient excretion and growth performance of grower‐finisher pigs, Can. J. Anim. Sci. 2007, 87, 103–114. [Google Scholar]
- 33. Chatzifragkou, A. , Prabhakumari, P. C. , Kosik, O. , Lovegrove, A. , et al., Extractability and characteristics of proteins deriving from wheat DDGS, Food Chem. 2016, 198, 12–19. [DOI] [PubMed] [Google Scholar]
- 34. Zhang, Y. , Caupert, J. , Survey of mycotoxins in US distiller's dried grains with solubles from 2009 to 2011, J. Agric. Food Chem. 2012, 60, 539–543. [DOI] [PubMed] [Google Scholar]
- 35. Chatzifragkou, A. , Kosik, O. , Prabhakumari, P. C. , Lovegrove, A. , et al., Biorefinery strategies for upgrading Distillers’ Dried Grains with Solubles (DDGS), Process Biochem. 2015, 50, 2194–2207. [Google Scholar]
- 36. Ahring, B. K. , Traverso, J. J. , Murali, N. , Srinivas, K. , Continuous fermentation of clarified corn stover hydrolysate for the production of lactic acid at high yield and productivity, Biochem. Eng. J. 2016, 109, 162–169. [Google Scholar]
- 37. Prasad, S. , Srikanth, K. , Limaye, A. M. , Sivaprakasam, S. , Homo‐fermentative production of d‐lactic acid by Lactobacillus sp employing casein whey permeate as a raw feed‐stock, Biotechnol. Lett. 2014, 36, 1303–1307. [DOI] [PubMed] [Google Scholar]
- 38. Lie, S. , The EBC‐Ninhydrin method for determination of free alpha amino nitrogen, J. I. Brewing 1973, 79, 37–41. [Google Scholar]
- 39. Slavica, A. , Trontel, A. , Jelovac, N. , Kosovec, Ž. , et al., Production of lactate and acetate by Lactobacillus coryniformis subsp. torquens DSM 20004T in comparison with Lactobacillus amylovorus DSM 20531T , J. Biotechnol. 2015, 202, 50‐59. [DOI] [PubMed] [Google Scholar]
- 40. Kim, J. H. , Shoemaker, S. P. , Mills, D. A. , Relaxed control of sugar utilization in Lactobacillus brevis, Microbiology 2009, 155, 1351–1359. [DOI] [PubMed] [Google Scholar]
- 41. Nguyen, C. M. , Kim, J. S. , Song, J. K. , Choi, G. J. , et al., d‐Lactic acid production from dry biomass of Hydrodictyon reticulatum by simultaneous saccharification and co‐fermentation using Lactobacillus coryniformis subsp. torquens , Biotechnol. Lett. 2012, 34, 2235–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Garvie, E. I. , Bacterial lactate dehydrogenases, Microbiol. Rev. 1980, 44, 106–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zheng, Z. , Sheng, B. , Ma, C. , Zhang, H. , et al., Relative catalytic efficiency of ldhl‐ and ldhd‐encoded products is crucial for optical purity of lactic acid produced by Lactobacillus strains, Appl. Environ. Microbiol. 2012, 78, 3480–3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gu, S.‐A. , Jun, C. , Joo, J. C. , Kim, S. , et al., Higher thermostability of l‐lactate dehydrogenases is a key factor in decreasing the optical purity of d‐lactic acid produced from Lactobacillus coryniformis , Enzyme. Microb. Technol. 2014, 58–59, 29–35. [DOI] [PubMed] [Google Scholar]
- 45. Wright, A. , Axelsson, L. , Lactic acid bacteria: An introduction, in: Lahtinen S., Ouwehand A. C., Salminen S., von Wright, A. (Eds.), Lactic acid bacteria: Microbiological and functional aspects, CRC Press, Boca Raton, FL, 2012. [Google Scholar]
- 46. Yáñez, R. , Alonso, J. L. , J.C. Parajó, d‐Lactic acid production from waste cardboard, J. Chem. Technol. Biotechnol. 2005, 80, 76–84. [Google Scholar]
- 47. Hofvendahl, K. , B. Hahn–Hägerdal, Factors affecting the fermentative lactic acid production from renewable resources 1, Enzyme. Microb. Technol. 2000, 26, 87–107. [DOI] [PubMed] [Google Scholar]
- 48. Guo, T. , Zhang, L. , Xin, Y. , Xu, Z. , et al., Oxygen‐Inducible Conversion of Lactate to Acetate in Heterofermentative Lactobacillus brevis ATCC 367, Appl. Environ. Microbiol. 2017, 83, e01659‐01617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Quatravaux, S. , Remize, F. , Bryckaert, E. , Colavizza, D. , Guzzo, J. , Examination of Lactobacillus plantarum lactate metabolism side effects in relation to the modulation of aeration parameters, J. Appl. Microbiol. 2006, 101, 903–912. [DOI] [PubMed] [Google Scholar]
- 50. Niaounakis, M. , Chapter 1 ‐ Introduction, Biopolymers: Processing and Products, William Andrew Publishing, Oxford, 2015, pp. 1–77. [Google Scholar]
- 51. Zhang, L. , Li, X. , Yong, Q. , Yang, S.‐T. , et al., Simultaneous saccharification and fermentation of xylo‐oligosaccharides manufacturing waste residue for l‐lactic acid production by Rhizopus oryzae, Biochem. Eng. J. 2015, 94, 92–99. [Google Scholar]
- 52. John, R. P. , Nampoothiri, K. M. , Pandey, A. , Simultaneous saccharification and fermentation of cassava bagasse for l‐(+)‐lactic acid production using Lactobacilli, Appl. Biochem. Biotechnol. 2006, 134, 263–272. [DOI] [PubMed] [Google Scholar]
- 53. Nguyen, C. M. , Kim, J.‐S. , Thanh Ngoc, N. , Kim, S. K. , et al., Production of l‐ and d‐lactic acid from waste Curcuma longa biomass through simultaneous saccharification and cofermentation, Bioresour. Technol. 2013, 146, 35–43. [DOI] [PubMed] [Google Scholar]
- 54. Triwahyuni, E. , Muryanto, Yni. , Abimanyu, H. , The Effect of Substrate Loading On Simultaneous Saccharification And Fermentation Process For Bioethanol Production from Oil Palm Empty Fruit Bunches, Energy Procedia 2015, 68, 138–146. [Google Scholar]
- 55. Moldes, A. B. , Alonso, J. L. , J.C. Parajó, Multi‐step feeding systems for lactic acid production by simultaneous saccharification and fermentation of processed wood, Bioprocess Engineering 2000, 22, 175–180. [Google Scholar]
- 56. Elliston, A. , Collins, S. R. A. , Wilson, D. R. , Roberts, I. N. , Waldron, K. W. , High concentrations of cellulosic ethanol achieved by fed batch semi simultaneous saccharification and fermentation of waste‐paper, Bioresour. Technol. 2013, 134, 117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Varga, E. , Klinke, H. B. , Réczey, K. , Thomsen, A. B. , High solid simultaneous saccharification and fermentation of wet oxidized corn stover to ethanol, Biotechnol. Bioeng. 2004, 88, 567–574. [DOI] [PubMed] [Google Scholar]


