Abstract
The use of nanotechnology and biotechnology to improve the production of plant bioactive compounds is growing. Hyoscyamus reticulatus L. is a major source of tropane alkaloids with a wide therapeutic use, including treatment of Parkinson's disease and to calm schizoid patients. In the present study, hairy roots were obtained from two‐week‐old cotyledon explants of H. reticulatus L. using the A7 strain of Agrobacterium rhizogenes. The effects of different concentrations of the signaling molecule nano‐zinc oxide (ZnO) (0, 50, 100 and 200 mg/L), with three exposure times (24, 48 and 72 h), on the growth rate, antioxidant enzyme activity, total phenol contents (TPC), tropane alkaloid contents and hyoscyamine‐6‐beta‐hydroxylase (h6h) gene expression levels were investigated. Growth curve analysis revealed a decrease in fresh and dry weight of ZnO‐treated hairy roots compared to the control. ANOVA results showed that the antioxidant activity of the enzymes catalase, guaiacol peroxidase and ascorbate peroxidase was significantly higher in the ZnO‐treated hairy roots than in the control, as was the TPC. The highest levels of hyoscyamine (37%) and scopolamine (37.63%) were obtained in hairy roots treated with 100 mg/L of ZnO after 48 and 72 h, respectively. Semi‐quantitative RT‐PCR analysis revealed the highest h6h gene expression was in hairy roots treated with 100 mg/L of ZnO after 24 h. It can be concluded that ZnO is as an effective elicitor of tropane alkaloids such as hyoscyamine and scopolamine due to its enhancing effect on expression levels of the biosynthetic h6h gene.
Keywords: Agrobacterium rhizogenes, Antioxidant enzymes, Hyoscyamine, Scopolamine, Nano‐zinc oxide
Abbreviations
- APX
ascorbate peroxidase
- CAT
catalase
- GPX
guaiacol peroxidase
- h6h
hyoscyamine‐6‐beta‐hydroxylase
- PCR
polymerase chain reaction
- TPC
total phenol content
- ZnO
nano‐zinc oxide
1. Introduction
There is a growing demand for plant secondary metabolites for various applications, including pharmaceutical and cosmetic, and as additives and preservatives in the food industry. However, the cultivation of plants per hectare for bioactive compound production has dropped significantly worldwide, because of pressure to feed growing populations and climate change. There is therefore an urgent need to find alternative means of production 1, especially because the chemical synthesis of these valuable metabolites is often complex and their production levels in nature are low. According to the United Nations Comtrade Statistics, the estimated size of the global market for bioactive compounds in 2013 was US $26 billion, increasing by 8.1% over the past five years. The market for herbal dietary supplements in the United States has reached an estimated total of $6.4 billion, increasing by 6.85% in 2014 compared with the previous year (http://www.portal.nifa.usda.gov).
Hyoscyamine and scopolamine, the main tropane alkaloids produced in Hyoscyamus reticulatus L., which act as inhibitors of the parasympathetic nerve system 2, have been targeted in biotechnological systems to increase yields and reduce production costs. Approaches to improve plant metabolite production include cell or organ in vitro cultures, genetic engineering and the use of biotic and abiotic elicitors, which can enhance yields and secretion to the media 3. Hairy root cultures as a potential source of valuable secondary metabolites have been established by different strains of A. rhizogenes 4, 5.
Nanotechnology, or atom and molecular technology, is an emerging technology of this century. Research on nanoparticles has attracted considerable interest, with potential applications in many fields. Although there is concern about their side effects on the environment, these can be minimized by limiting the concentration of nanoparticle solutions. Recent studies have shown that nanoparticle applications can significantly affect plant physiology and gene expression in plant cell or organ cultures, although their impact on metabolite production is still ambiguous. The easy penetration of ultra‐fine nanoparticles into the plant cell wall and vacuoles disturbs metabolic activity. A few studies on the production of secondary metabolites in response to nanoparticle treatment have provided insights into the exploitation of these submicron‐size particles as a new class of elicitor. For instance, the artemisinin content was increased in a hairy root culture of Artemisia annua upon treatment with silver nanoparticles 6. In Glycyrrhiza glabra, nanoparticles of zinc oxide and copper oxide increased the content of glycyrrhizin, total phenols, flavonoids, anthocyanin and proline in comparison to the control 7. In H. reticulatus, the highest hyoscyamine and scopolamine production (an increase of about five‐fold compared to the control) was achieved with 900 and 450 mg/L FeNPs at 24 and 48 h of exposure time, respectively 8. The application of silver nanoparticles in Calendula officinalis resulted in a decrease in the anthocyanin and flavonoid contents and an increase in carotenoids and saponins 9. Treatment of Trigonella foenum‐graecum L. seedlings with biosynthesized silver nanoparticles (Ag‐NPs) was found to have a significant impact on growth parameters such as leaf number, root and shoot length, and wet weight. HPLC‐based analysis revealed that Ag‐NP‐treated seedlings had an increased production of diosgenin (214.06 ± 17.07 μg/mL) 10. Multi‐walled carbon nanotubes stimulated the biosynthesis of secondary metabolites and the antioxidant capacity of the medicinal plant Satureja khuzestanica grown in vitro 11. In H. perforatum cell suspension cultures, zinc and iron nano‐oxides stimulated hypericin and hyperforin production at low concentrations 13. However, no reports are available on the use of nano‐zinc oxide as an elicitor in H. reticulatus hairy root cultures.
In the context of a continuously growing market for tropane alkaloids, especially scopolamine, and the need for an improvement in current production techniques, in this study we explored the effect of ZnO on tropane alkaloid yield and h6h gene expression in H. reticulatus L. hairy roots.
2. Materials and methods
2.1. Place of research
Hyoscyamus reticulatus L. cultivation experiments and morphological and biochemical analysis were performed at the Horticultural Sciences Laboratory of Urmia University. Phytochemical analysis of tropane alkaloids and molecular studies were carried out at the Institute Center for Research on Biotechnology of Urmia University.
2.2. Plant materials
H. reticulatus L. seeds were obtained from Pakan Bazr Company (Esfahan, Iran) and identified by Dr. Heidari (expert in medicinal plants) in an agriculture research center in west Azarbaijan, Iran. They were surface‐sterilized in 70% (v/v) ethanol for one min and in 50% (v/v) NaOCl for 10 min, and then washed three times with sterile distilled water. The seeds were then cultivated in MS medium (Murashige and Skoog) 14 supplemented with 3% (w/v) sucrose, 7 g/L agar (Duchefa, the Netherlands) and 0.1 g/L myo‐inositol. The pH was adjusted to 5.8 before adding agar. The culture medium was autoclaved at 121°C for 20 min. Cultures were kept in artificial light (fluorescent tube giving light intensity of 3500 Lux) for 16 h followed by 8 h in the dark at 25°C.
2.3. Preparation of A. rhizogenes strain and hairy root induction
A. rhizogenes A7 strain was grown on solid LB medium 15 supplemented with 50 mg/L rifampicin and incubated at 28°C for 48 h. One‐week‐old cotyledons from in vitro H. reticulatus L. seedlings were used for infection. Acetosyringone was not used in any of the infection experiments. The infected cotyledon explants were placed on the MS medium under similar culture conditions. After 2 days, cotyledon explants were placed on MS medium supplemented with 200 mg/L filter‐sterilized cefotaxime. After 10 days, the hairy roots were induced at the wound sites and sub‐cultured every 10 days. Hairy roots at every stage of the sub‐culture were cultured on fresh MS medium with reduced concentrations of cefotaxime (100–50 mg/L) until the bacteria were completely eradicated. The cultures were then transferred to 250 mL Erlenmeyer flasks containing 30 mL hormone‐free liquid MS media, grown at 25°C on a rotary shaker (120 rpm) in the dark and sub‐cultured every 10 days.
2.4. Polymerase chain reaction analysis
The presence of the A. rhizogenes rol B gene in H. reticulatus hairy roots was evaluated through polymerase chain reaction (PCR). PCR amplification was performed on DNA isolated from hairy roots and untransformed roots using the specific primers
Forward 5′‐ATGGATCCCAAATTGCTATTCCCCCACGA‐3′
Reverse 5′‐TTAGGCTTCTTTCATTCGGTTTACTGCAGC‐3′
The PCR was performed under the following conditions: 94°C for 5 min, 35 cycles of three steps including denaturation at 94°C for 50 s, annealing at 60°C for 1 min, and extension at 72°C for 1 min and 10 s, and 72°C for 10 min for the final extension using a thermocycler. Based on the primer sequence used, the amplicon fragment size of rolB gene was around 780 bp. The amplicons were analyzed by electrophoresis on 1% (w/v) agarose gel with a 1 kb DNA marker.
2.5. Elicitation experiments
As there are few studies of the effects of nano materials on secondary metabolite production, finding optimum concentrations of nano elicitors is not straightforward. The concentrations in this study were selected based on our previous experience 8 and a review of the few published papers in this field. The ZnO used in this research, in the size of 30–50 nm, was prepared by Jahan Sany Toos Nano Materials. To obtain different concentrations of ZnO (0, 50, 100, 200 mg/L), a 1000 mg/L stock solution was prepared. The fastest growing hairy root line was used in the elicitation experiments, and the optimum time (day 21) for adding the elicitor was selected based on the growth curve (Fig. 1). All ZnO concentrations were applied with three exposure times (24, 48, and 72 h). For the elicitation 2 g hairy roots (fresh weight) in 250 mL Erlenmeyer flasks containing 10 mL MS basal media was used.
Figure 1.
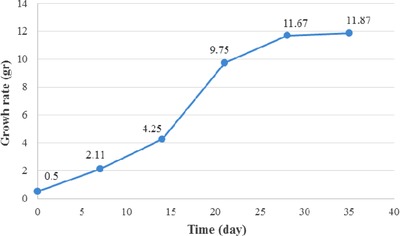
Growth curve of Hyoscyamus reticulatus hairy roots grown in liquid MS media fortified with 3 or 1% sucrose on a shaker (120 rpm) for 35 days.
2.6. Extraction and GC‐MS analysis of tropane alkaloids
Tropane alkaloids were extracted from the hairy roots by the method of Kamada et al. 16. Five hundred milligrams of powdered plant material from the hairy roots was homogenized in CHCl3, MeOH and NH4OH (25%) (15:5:1), sonicated for 30 min and kept at room temperature for 1 h in darkness. The homogenate was filtered through a paper filter and washed twice with 1 mL of CHCl3. Then, the solvent was evaporated and dried in a rotary evaporator. 5 mL of CHCl3 and 2 mL of 1 N H2SO4 were added and mixed thoroughly. The CHCl3 fraction was removed and the solvent was adjusted to pH 10 with 28% NH4OH on ice. Alkaloids were extracted once with 2 mL, and twice with 1 mL of CHCl3. After the addition of anhydrous Na2SO4, the solvent was filtered through filter paper and the residue was washed with 1 mL of CHCl3. Finally, the powder was dissolved in 500 μl MeOH following evaporation. The obtained extract was analyzed by Gas Chromatography‐Mass spectrometry using the method of Gharari et al. 17. GC analysis was carried out on a Hewlett‐Packard (HP, Palo Alto, CA, USA) HP 7890A GC equipped with a split/splitless injector and 5975C mass selective detector system. Chromatographic separation was carried out in a HP‐5 capillary column (30 m × 0.25 mm, 0.25 μm in film thickness). The MS was operated in the EI mode (70 eV). The GC‐MS interface, ion source, and quadrupole temperatures were set at 280, 230, and 150°C, respectively. The injector temperature was set at 270°C. For GC analysis, the column temperature program started at 60°C for 3 min, increased by 5°C/min to 250°C, and was held for 10 min. Helium (99.999%) was used as the carrier gas. The GC analysis was performed with 1 μL injection volume without any derivatization and in full scan mode. There is no calibration curve and identification of spectra was carried out by comparing mass spectra fragmentation with those stored in the Wiley 7n version. Peak area normalization was used to determine the composition percentage.
2.7. Preparation of enzyme extracts
The 0.5 g roots were ground in 2.5 mL extraction buffer (50 mM Tris‐HCl (pH 7.5) for 5 min using a pestle and mortar on ice, followed by centrifugation at 12 000 rpm and 4°C for 20 min. The supernatant was used as the crude extract to assay antioxidant enzyme activity.
2.8. Enzyme assay
Catalase (CAT) (EC 1.11.1.6) activity was assayed by measuring the consumption of H2O2 at 240 nm 18. The reaction mixture contained 2.5 mM of 50 mM potassium phosphate buffer (pH = 7) and 20 μL of 3% hydrogen peroxide and 20 μL enzyme extract. Guaiacol peroxidase (GPX) (EC 1.11.1.7) activity was determined by measuring increases in absorbance at 470 nm due to the oxidation of guaiacol 19. The reaction mixture contained 25 μL of enzyme extract, 2.777 μL of 50 mM potassium phosphate buffer with a pH of 7, 100 μL of hydrogen peroxide 1%, and 100 μL of guaiacol 4%. Ascorbate peroxidase (APX) (EC 1.11.1.11) activity was determined by measuring the reduction in absorbance at 290 nm due to the oxidation of ascorbate 20. The reaction mixture contained 2 mL of 50 mM potassium phosphate buffer (pH = 7), 0.2 mL of 50 μM ascorbate, 0.1 mL enzyme extract and 0.2 mL of 3% hydrogen peroxide.
2.9. Determination of total phenol content (TPC)
The method of Hajimahdipour et al. 21 was used, and the absorbance was measured at 760 nm. First, 500 mg of hairy roots was placed in the cold mold. Then, 10 mL of 80% methanol was added and completely washed down. After centrifuging for 15 min at about 10 000 rpm at 4°C, 50 μL of plant extract was mixed with 10% folin (1200 μL) in a glass tube. After 5 min, 960 μL of 7.5% sodium carbonate was added. Finally, 180 μL of distilled water was added and the glass tube was placed in the dark for 40 to 30 min.
2.10. Analysis of the h6h gene expression
2.10.1. Storing the gene sequence in databases and primer design
The h6h gene plays a key role in the biosynthesis of tropane alkaloids in H. reticulatus L. The specific primer of the h6h gene was designed based on the gene coding sequence in the NCBI database. Fast PCR software was used to design the primers. The specific primer sequences used for gene expression analysis were:
h6h F: TGGCTACTTTTGTGTCGAACTGG
h6h R: CTTGGGTCTGGGCATGGTG
18S F: ATGATAACTCGACGGATCGC
18S R: CTTGGATGTGGTAGCCGTTT
2.10.2. RNA extraction
RNA extraction from hairy root samples was performed using the RNAX‐Plus extraction kit (Cinaclon Co., Iran) according to the manufacturer's instructions.
2.10.3. cDNA synthesis
The First Strand cDNA Synthesis kit (Cinaclon Co., Iran) was used for the synthesis of cDNA according to the manufacturer's instructions. The cDNA was amplified using the 18s‐rRNA gene primers in the PCR process with a final volume of 20 μL. To carry out the PCR reaction from 12.5 μL PCR Master Mix (Pishgaman Co. Iran), 0.5 μL of each primer and 2 μL of cDNA were used. The initial denaturation temperature for the 18s‐rRNA gene was 94°C for 5 min, followed by 35 cycles of three steps: denaturation at 94°C for 30 s, annealing at 60°C for 30 s, and 72°C for 40 s, and for the final extension 72°C for 5 min using a thermocycler. To conduct the PCR reaction from 12.5 μL PCR Master Mix (Pishgaman Co. Iran), 0.5 μL of each return primer, 2 μL of diluted cDNA (1:50) and 4.5 μL of DNA‐free water were used. The temperatures used for the h6h gene were 94°C for 5 min, followed by 35 cycles of three steps: denaturation at 94°C for 1 min, annealing at 66°C for 1/55 min and 72°C for 1/40 min, and for the final extension 72°C for 10 min using a thermocycler. Amplification products were separated by electrophoresis on 0.8% agarose gel. To test the h6h gene amplification accuracy, negative control reactions (‐RT) and a no‐template negative control (NTC) were used.
2.11. Statistical analysis
The experiment was conducted as a completely randomized design with three replicates. Analysis of variance (ANOVA) of the results was performed using one‐way ANOVA. The means were compared using Duncan's multiple range test at 99% confidence level (p ≤ 0.01) using SAS 9.1 software. PIXCAVATOR 6.0 software was also used to analyze RT‐PCR results and find correlations between gene expression analysis and the phytochemical analysis.
3. Results
3.1. PCR analysis
The presence of the rol B gene in the genomic DNA of putative transgenic roots was confirmed by PCR using specific primers yielding fragments of 780 bp. No amplification was observed in the control roots. All transformed hairy roots showed the presence of 780 bp rol B product amplicons (Fig. 2).
Figure 2.
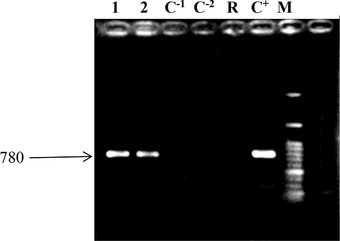
Confirmation of transgenic hairy roots using rol‐B specific primers by PCR reaction. 0.8% agarose gel electrophoresis of PCR‐ amplified products; Lane M: Marker (1 kbp), Lane C+: Agrobacterium A7 Strain as positive control, Lane R, Non‐transgenic Hyoscyamus reticulatus roots as negative control, Lane C−1 and C−2, water as negative control.
3.2. Effects of ZnO on hairy root growth
The effects of the ZnO concentration and treatment duration on the growth of H. reticulatus hairy roots were significant (Table 1). Mean comparisons indicate that the highest hairy root fresh weight (11.03 g) was obtained after 24 h exposure to 100 mg/L of ZnO and the lowest (8.108 g) after 72 h exposure to 200 mg/L of ZnO (Fig. 3C). The highest dry weight (690 +/–0.05 mg) was observed in the control and the lowest (390+/–0.03 mg) in roots treated with 200 mg/L of ZnO (Fig. 3A and B). The results therefore show that the hairy root biomass gradually declined with increasing elicitor concentration. The decrease in growth indicates that at high concentrations nanoparticles are toxic to H. reticulatus hairy roots (Fig. 4).
Table 1.
Variance analysis of the effect of concentrations and exposure times of ZnO on the growth of Hyoscyamus reticulatus hairy roots
| Means of squares (MS) | |||
|---|---|---|---|
| Treatments | df | Fresh weight (g) | Dry weight (g) |
| Elicitor concentration (a) | 3 | 2.88* | 0.14** |
| Treatment time (b) | 2 | 2.28** | 0.057** |
| Reaction (a × b) | 6 | 2.28* | 0.002ns |
| Error | 24 | 0.762 | 0.002 |
| Coefficient of variation (%) | 9.12 | 9.849 | |
ns non‐significant
* significant at p ≤ 0.05
** significant at p ≤ 0.01
Figure 3.
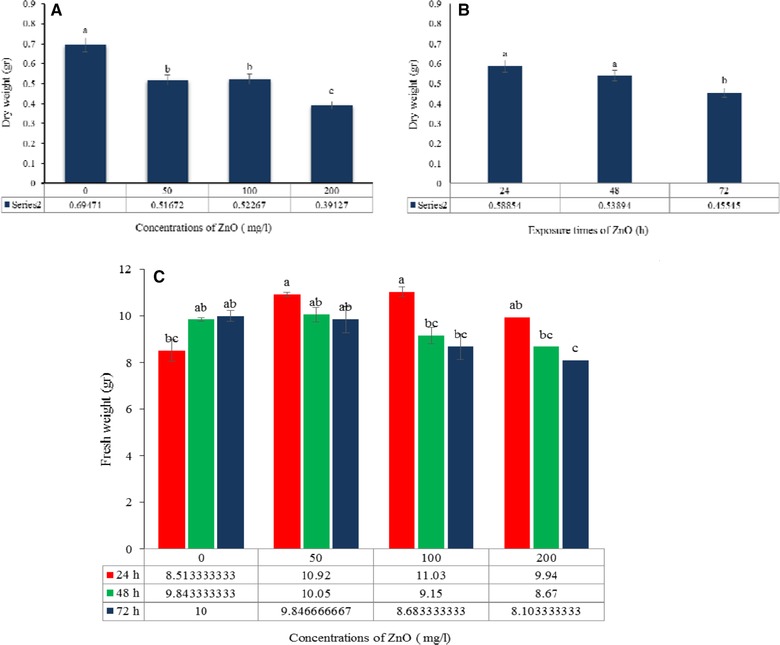
The simple effects of ZnO concentration (A) and exposure time (B) on dry weight; and interaction effects of elicitor concentration and exposure time on fresh weight (C) in Hyoscyamus reticulatus hairy roots. The same letters indicate that there is no significant difference at the 1 and 5% probability level based on the Duncan test
Figure 4.
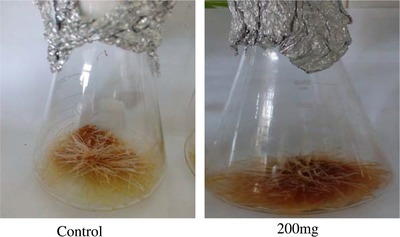
The effects of ZnO concentrations on hairy root growth. Control hairy roots (A), and elicited hairy roots with 200 mg/L ZnO (B).
3.3. Effects of ZnO on antioxidant enzyme activity
The effects of ZnO concentration and treatment duration on the free radicals hydrogen peroxide (H2O2) and super oxide (O−2) in the H. reticulatus hairy roots are presented in Table 2. In comparison with the control, significant interaction effects of exposure time and elicitor concentration were observed on the activity of the CAT enzyme (p≤0.05) in hairy roots, (Fig. 5A), but not for GPX and APX, so the results for the latter enzymes are presented separately (Fig. 5B). Maximum CAT, GPX, and APX activity was observed in roots treated with ZnO at 100, 200 and 100 mg protein/min at 48, 72 and 48 h of exposure time, respectively, and minimum activity in the non‐elicited control hairy roots at 72, 24 and 24 h, respectively (Fig. 5C and D).
Table 2.
Variance analysis of the effect of concentrations and exposure times of ZnO on total phenol content (TPC) and antioxidant enzyme activity in Hyoscyamus reticulatus hairy roots
| Means of squares (MS) | |||||
|---|---|---|---|---|---|
| Treatments | Df | TPC | CAT | GPX | APX |
| Elicitor concentration (a) | 3 | 1.306** | 1991.96** | 875.71** | 1093.7** |
| Treatment time (b) | 2 | 0.847** | 398.91** | 228.24** | 142.79ns |
| Reaction (a × b) | 6 | 0.064* | 106.73* | 26.44ns | 28.38ns |
| Error | 24 | 0.0011 | 41.73 | 29.08 | 44.22 |
| Coefficient of variation (%) | 11.007 | 20.08 | 10.08 | 14.38 | |
ns non‐significant
* significant at p ≤ 0.05
** significant at p ≤ 0.01
Figure 5.
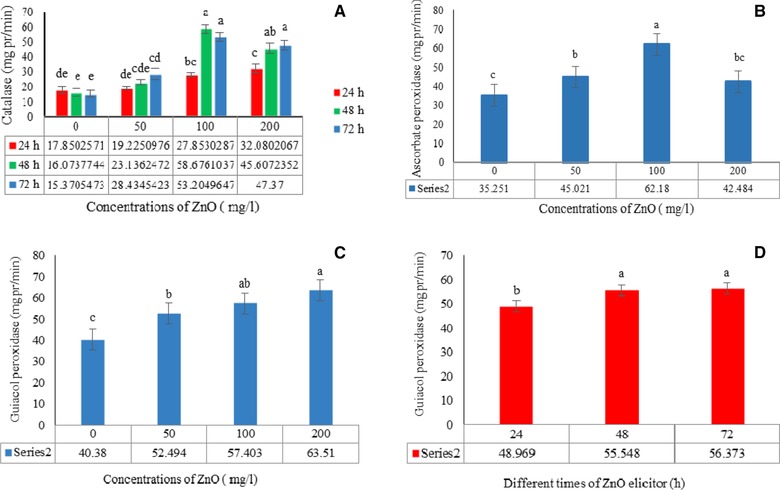
The effects of concentration and exposure time of ZnO on catalase (A), ascorbate peroxidase (B) and guaiacol peroxidase (C and D) in Hyoscyamus reticulatus hairy roots. The same letters indicate that there is no significant difference at the 1 and 5% probability level based on the Duncan test.
3.4. Effects of ZnO on total phenol content
Statistical analysis revealed significant differences (p ≤ 0.01) in the TPC of the H. reticulatus hairy root extracts treated with various concentrations and exposure times of ZnO (Table 2). The TPC increased significantly when hairy roots were transferred to the media containing ZnO and continued to increase with higher concentrations and longer exposure times, except at the highest concentration (200 mg/L). The highest TPC (1.67 mg of gallic acid/g fresh weight) was obtained in hairy roots elicited by 100 mg/L ZnO for 72 h, whereas the lowest TPC (0/41 mg of gallic acid /g fresh weight) was found in the control hairy roots at 24 h (Fig. 6).
Figure 6.
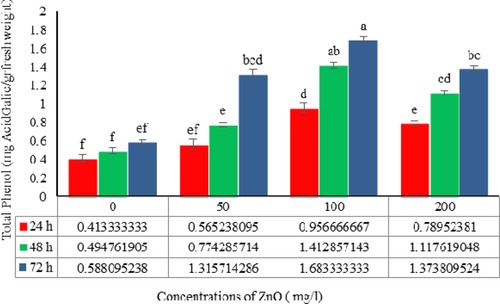
The effects of ZnO concentration and exposure time on the total phenol content in Hyoscyamus reticulatus hairy roots. The same letters indicate that there is no significant difference at the 1% probability level based on the Duncan test.
3.5. Effects of ZnO on tropane alkaloid production
The effects of ZnO concentration and treatment duration on the production of hyoscyamine and scopolamine are presented in Table 3. The addition of ZnO to the culture medium in the lag phase of hairy root growth stimulated tropane alkaloid production (Fig. 7). The control hairy roots were able to naturally produce at least 6.25% hyoscyamine. The highest amount of hyoscyamine (37%) was obtained by treatment with 100 mg/L ZnO for 48 h, which was 4.61‐fold higher than in the control hairy roots after the same time (8.01%) and 5.92‐fold higher than in control hairy roots at 24 h (6.25%) (Table 3 and Fig. 7A). The highest amount of scopolamine (37.63%) was obtained by treatment with 100 mg/L ZnO for 72 h, which was 3.2‐fold higher than in the control hairy roots (16.35%) at the same time; the lowest amount of scopolamine (11%) was recorded in the control hairy roots after 24 h (Fig. 7B). Other substances such as aposcopolamine, pyrrolidine, cyclohexanol, eucalyptol, tropigline, hygrine were detected in the H. reticulatus hairy root cultures (Table 4).
Table 3.
Variance analysis of the effect of concentrations and exposure times of ZnO on h6h gene expression and tropane alkaloid production in Hyoscyamus reticulatus hairy roots
| Means of squares (MS) | ||||
|---|---|---|---|---|
| Treatments | Df | h6h Expression | Hyoscyamine (%) | Scopolamine (%) |
| Elicitor concentration (a) | 3 | 77.75** | 748.73** | 570.41** |
| Treatment time (b) | 2 | 7.13* | 180.09** | 174.12** |
| Reaction (a × b) | 6 | 5.15* | 22.76* | 35.50* |
| Error | 24 | 1.88 | 8.103 | 13.13 |
| Coefficient of variation (%) | 17.23 | 16.34 | 16.48 | |
ns non‐significant
* significant at p ≤ 0.05
** significant at p ≤ 0.01
Figure 7.
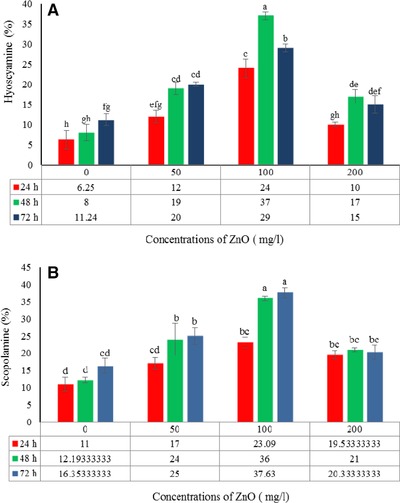
The effects of ZnO concentration and exposure time on hyoscyamine (%) (A) and scopolamine (%) (B) in Hyoscyamus reticulatus hairy roots. The same letters indicate that there is no significant difference at the 1 and 5% probability level based on the Duncan test.
Table 4.
Effect of ZnO concentrations and exposure time on some substances detected by GC‐MS in Hyoscyamus reticulatus hairy roots
| Treatment | Aposcopolamine (%) | Pyrrolidine (%) | Cyclohexanol (%) | Eucalyptol (%) | Tropigline (%) | Hygrine (%) | Scopolamine (%) | Hyoscyamine (%) |
|---|---|---|---|---|---|---|---|---|
| C1 T1 | 3.27 | 1.28 | 0.32 | 1.64 | 7.3 | 32 | 11 | 6.25 |
| C1 T2 | 4.12 | 1.31 | 0.56 | 1.45 | 9 | 24 | 12.19 | 8.01 |
| C1 T3 | 6.12 | 1.37 | 0.71 | 1.12 | 4.6 | 42.01 | 16.35 | 11.24 |
| C2 T1 | 6.49 | 1.26 | 0.62 | 1.39 | 4.01 | 20.3 | 17 | 12.02 |
| C2 T2 | 6.72 | 1.22 | 0.69 | 1.27 | 6.4 | 17.2 | 24.02 | 19.1 |
| C2 T3 | 7.01 | 1.19 | 0.67 | 1.12 | 8.01 | 31 | 25.09 | 20.03 |
| C3 T1 | 7.9 | 1.11 | 0.82 | 1.35 | 7.4 | 39.06 | 23.09 | 24.09 |
| C3 T2 | 8.2 | 1.42 | 0.84 | 1.30 | 6.8 | 20.62 | 36 | 37 |
| C3 T3 | 9.1 | 1.58 | 0.89 | 1.22 | 8.2 | 15.07 | 37.63 | 29.04 |
| C4 T1 | 6.79 | 1.31 | 0.49 | 1.21 | 8.7 | 40.4 | 19.52 | 10 |
| C4 T2 | 6.92 | 1.47 | 0.38 | 1.12 | 8.401 | 46 | 21 | 17.02 |
| C4 T3 | 7.1 | 1.21 | 0.31 | 0.89 | 7.03 | 37.7 | 20.23 | 15 |
3.6. Effects of ZnO on h6h gene transcripts
The expression of the h6h gene, involved in the biosynthetic pathway of hyoscyamine and scopolamine, in the H. reticulatus hairy roots increased significantly after the addition of ZnO to the culture media (p≤0.05) (Table 3). The maximum expression level was observed in hairy roots treated with 100 mg/L ZnO over a period of 48 h, being up to 4.02‐fold higher than in the control hairy roots. The lowest expression was obtained in hairy roots elicited with 200 mg/L ZnO after 24 h exposure time (Figs. 8 and 9).
Figure 8.
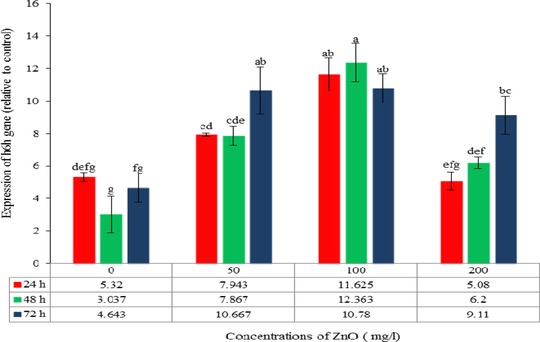
Comparison of the mean interactions of ZnO concentration and exposure time on the h6h gene expression in Hyoscyamus reticulatus hairy roots. The same letters indicate that there is no significant difference at the 5% probability level based on the Duncan test.
Figure 9.
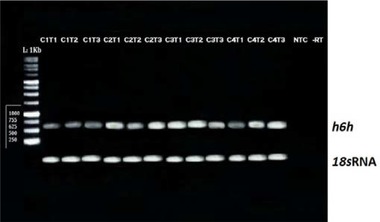
RT‐PCR analysis of the expression of the h6h gene and the 18s internal control under the influence of different ZnO concentrations (0, 50, 100 and 200 mg/L); C1: Control, C2: 50 mg/L, C3: 100 mg/L C4: 200 mg/L, T1: 24 h, T2: 48 h, T3: 72 h, NTC: negative control reaction, RT: negative control reaction
3.7. Correlation of the scopolamine accumulation with relative overexpression of the h6h gene under ZnO treatment
RT‐PCR analysis showed that ZnO elicitation led to the overexpression of the h6h gene. A significant correlation was found between scopolamine accumulation and h6h gene expression at a probability level of 5% (Fig. 10). Normality of data was assessed by SPSS v. 21, and the scroll plate was drawn. Both correlation and scatter plots show a positive linear correlation (Fig. 11). These results therefore demonstrate that overexpression of the h6h gene had a positive effect on the production of tropane alkaloids, especially scopolamine.
Figure 10.
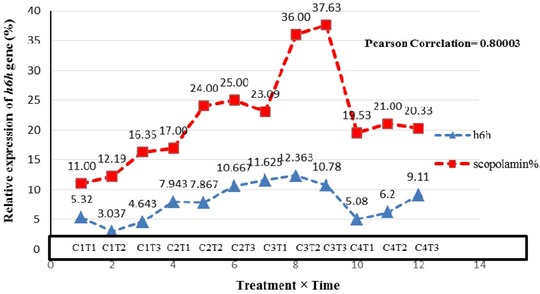
Relationship between scopolamine accumulation and the relative expression of the h6h gene in hairy roots elicited by ZnO (C1 = 0, C2 = 50, C3 = 100 and C4 = 200 mg/L) at different exposure times (T1 = 24, T2 = 48 and T3 = 72 h). Pearson correlation coefficient between two variables is significant.
Figure 11.
Positive linear correlation between scopolamine accumulation and h6h gene expression.
4. Discussion
Few studies have been carried out to determine the effects of ZnO on plants. Zinc is an essential micronutrient for organisms and plays an important role in plant processes 22, 23, being necessary for lipid metabolites, nucleic acid, RNA metabolism, stability and DNA simulation and gene expression regulation. Zinc also plays an important role in cell proliferation 24. It tends to form tetragonal complexes with O, N, and especially S ligands, which affects the metabolic activity. As a catalyzer, zinc has an activating or building role in many plant enzymes 25, being involved in the structure of more than 300 enzymes and the only metal present in all six classes of enzymes 26.
The obtained results show that the growth capacity of H. reticulatus hairy roots was negatively affected by ZnO treatment, in agreement with the results reported by Zheng et al 27 in rice roots. A negative effect on growth was also observed after aluminum oxide nanoparticle treatment, which inhibited the root length of corn, cucumber, soybean, cabbage, and carrot 28. Inhibition of root growth varies greatly among plants and according to the type and concentration of nanoparticles applied 29. The negative impact of the ZnO treatment could be the result of a reduced permeability of the root cell membrane, since heavy metals interact with the sulfide groups in vacuoles, preventing water from penetrating the plant tissues 30. Therefore, weight loss of ZnO‐elicited hairy roots could be due to a loss of water content in the roots and its effect on related physiological processes.
Increasing the concentration of microelements is known to significantly affect the antioxidant activity of enzymes such as CAT, GPX and APX, which are the major scavengers of reactive oxygen species (ROS) in plant cells and organs. The enhanced antioxidant enzyme activity in this study was probably due to the increase of ROS such as H2O2, as reported in studies with Corylus avellana cell suspension cultures treated with silver nano particles 31. In a study on tomato seedlings, ZnO induced oxidative stress by promoting antioxidant enzyme activities, as salinity stress induces anti‐oxidative activity 32, 33. Elevated concentrations of ZnO (10–2000 mg/L) induced an abnormal defense system against ROS in Fagopyrum esculentum 21.
Even under optimal conditions, many metabolic processes produce ROS, and the production of toxic oxygen is increased as a result of all types of environmental stresses. To scavenge ROS, plant cells possess an antioxidant system consisting of low molecular‐weight antioxidants, such as ascorbate, α‐tocopherol, glutathione, and carotenoids (nonenzymatic antioxidants), as well as antioxidant enzymes such as SOD, CAT, and APX 34.
CAT is an important enzyme in the plant defense system, converting H2O2 to H2O and O2, and preventing the production of ROS. Hosseini et al. 35 and Lee et al. 36 showed that the activity of the antioxidant enzyme CAT in Zea mays increased with higher zinc concentrations in the culture media. Similarly, CAT activity in buckwheat herbage increased after treatments with up to 100 mg/L ZnO 37, the same concentration that produced the maximum CAT activity in the present study. The activity of APX in the H. reticulatus hairy roots was increased by ZnO concentrations of up to 100 mg/L, but decreased at 200 mg/L. The activity of GPX, an oxidative enzyme of phenolic compounds 38, was significantly enhanced by ZnO elicitation, especially at 200 mg/L, but was not affected by different exposure times (24, 48 and 72 h). Also, the application of high concentrations of ZnO is reported to induce phytotoxicity by decreasing Zn bioavailability and accumulation and reducing ROS production 39. These results confirm that ZnO elicitation can stimulate ROS production in plants. An increase in H2O2 levels was observed in cell suspension cultures of Scrophularia kakudensis in the initial hour after treatment with biotic elicitors, methyl jasmonate, salicylic acid and sodium nitroprusside, which in turn activated the antioxidant system, thereby increasing the activity of CAT and GPX 40.
The metabolism of phenolic compounds is usually due to environmental factors or stressful conditions 41. The TPC in extracts of H. reticulatus hairy roots increased after elicitation with the various ZnO concentrations and exposure times in comparison with non‐elicited hairy roots. The highest TPC was observed in the media after treatment with 100 mg/L ZnO for 72 h. Phenolic compound production can be increased by exposure to heavy metals, due to the enhanced activity of antioxidant enzymes 42. The few published studies on plant metabolite production in response to nanoparticle elicitation diverge in the effects of submicron‐size particles on natural metabolites in cell and organ cultures 7. Bota and Deliu 43 report that nano‐copper increased the production of flavonoids in Digitalis lanata cell suspension cultures, which is consistent with the increased production of phenolic compounds in the current study.
In addition to producing defensive responses, elicitation can induce the accumulation of secondary metabolites, including tropane alkaloids 44. Elicitation with ZnO caused an increase in hyoscyamine and scopolamine content at all exposure times compared to the control hairy roots, although the maximum level of alkaloids was detected with 100 mg/L ZnO. Moharrami et al. 8 reported maximum scopolamine levels (20.3%) in media containing FeNPs (450 mg/L) after 48 h. Ghorbanpour et al. 45 revealed that application of nano‐sized titanium dioxide particles enhanced hyoscyamine and scopolamine production in H. niger L. plants.
The biosynthetic pathway of tropane alkaloids is not fully understood and there are still some enzymes to be described along this route, although the final two steps leading to the production of scopolamine from hyoscyamine are well‐defined 3, 46. To increase tropane alkaloid production, various techniques have been applied, such as genetic engineering targeting key enzymes in the biosynthetic pathway. For example, engineered Atropa belladonna L hairy roots harboring a transgenic hyoscyamine‐6β‐hydroxylase gene showed a five‐fold higher scopolamine production compared to native roots 47. The induction of h6h gene expression in plants is known to increase the scopolamine content 48. Yang et al. 49 revealed that overexpression of pmt and h6h genes in A. belladonna L. caused a huge increase (11‐ and 24‐fold, respectively) in hyoscyamine content in elicited hairy roots compared to control and native roots. The physical and chemical properties of nanoparticles, for example, their surface‐to‐volume ratio, high surface reactivity, and high ability to exchange electrons, can be harnessed to promote secondary metabolite production in plant cultures 50. In this study, the highest h6h gene expression was observed when hairy roots were treated with 100 mg/L ZnO after 48 h, being 4.2‐fold higher than in the non‐elicited hairy roots. Similarly, cobalt and zinc nanoparticles have been shown to increase the expression of genes associated with the artemisinin biosynthetic pathway and have been proposed as elicitors to enhance artemisinin production 51.
Nanoparticles can also affect the nitrogen metabolism in plants by increasing the activity of nitrate reductase and glutamate dehydrogenase, and stimulate plant growth and photosynthesis 52, 53. By increasing nitrogen metabolism, nanoparticles also increase protein levels and stimulate gene expression in plant cells. It is therefore likely that elicitation with ZnO increases the expression of H6H and PMT enzymes 49, 52. In a biotechnological process, the most important effect of elicitors is the stimulation of the expression of genes involved in the biosynthesis of a target secondary metabolite. In this work, it seems that ZnO enhanced the production of hyoscyamine and scopolamine through a higher transcription of the h6h gene. Analysis of the correlation of scopolamine accumulation with the expression of the h6h gene under the influence of ZnO revealed a significant relationship with a probability level of 5%, both factors showing a similar trend throughout the experiment.
The distribution of tropane alkaloids varies among Hyoscyamus species and plant parts. Scopolamine is the predominant tropane alkaloid in H. pusillus, H. niger and H. kurdicus 53. Ionkova 54 obtained a high content of scopolamine (46%) and hyoscyamine (35%) in a high scopolamine‐producing clone (Hr‐3) of H. reticulatus. In this study, the highest contents of scopolamine (37.63%) and hyoscyamine (37%) were obtained with different elicitor concentrations and exposure times (Fig. 12, 13, 14, 15).
Figure 12.
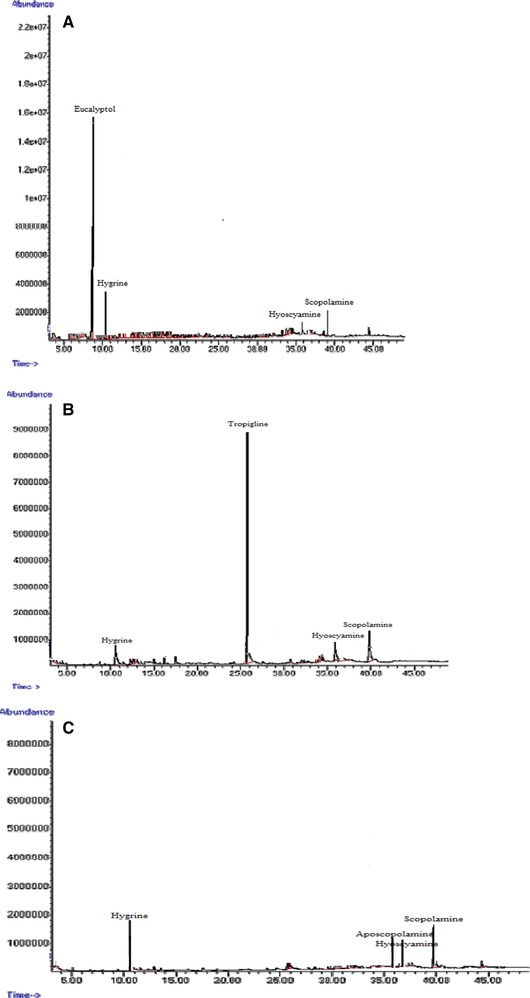
GC‐MS chromatograms of tropane alkaloids in Hyoscyamus reticulatus control hairy roots. 24 h (A); 48 h (B); 72 h (C).
Figure 13.
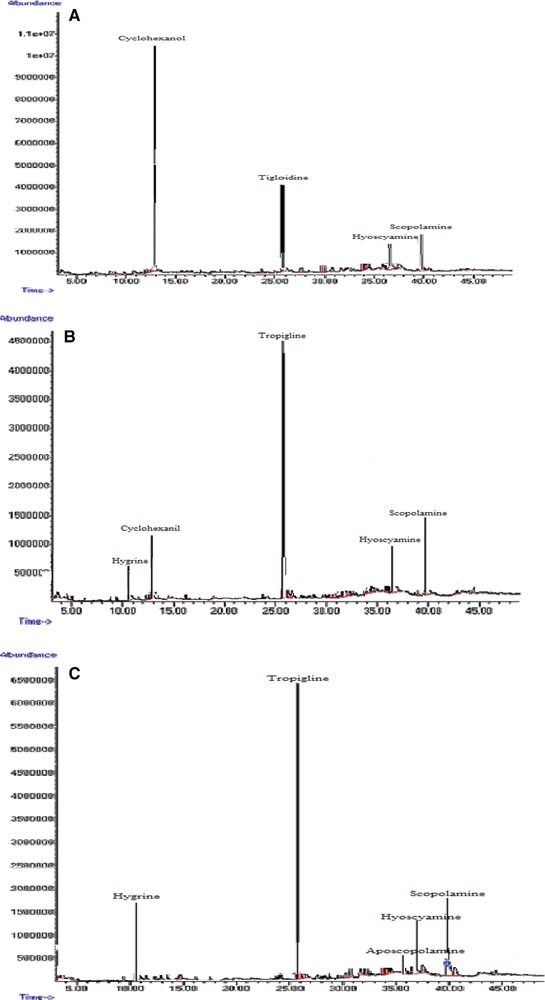
GC‐MS chromatograms of tropane alkaloids in Hyoscyamus reticulatus hairy roots elicited with 50 mg/L ZnO. 24 h (A); 48 h (B); 72 h (C).
Figure 14.
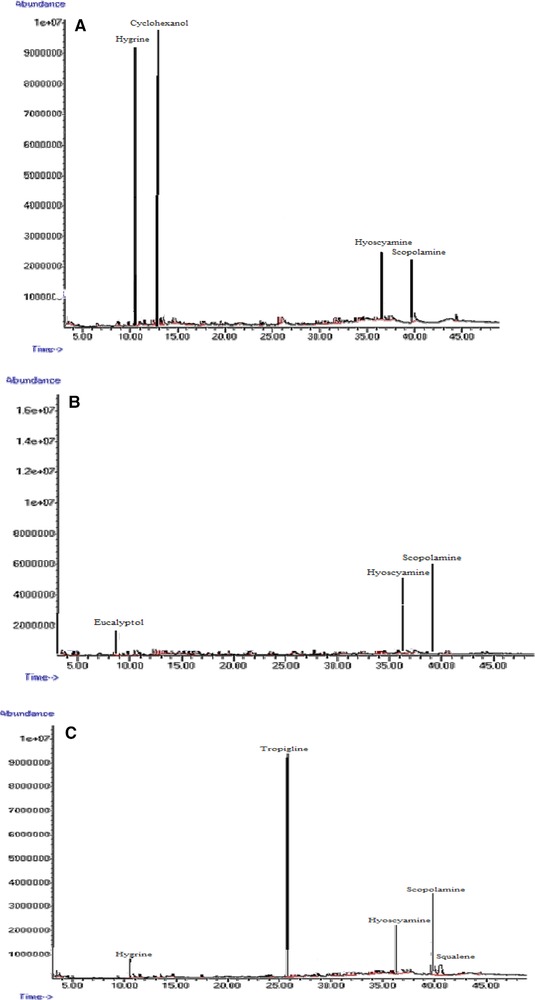
GC‐MS chromatograms of tropane alkaloids in Hyoscyamus reticulatus hairy roots elicited with 100 mg/L ZnO. 24 h (A); 48 h (B); 72 h (C).
Figure 15.
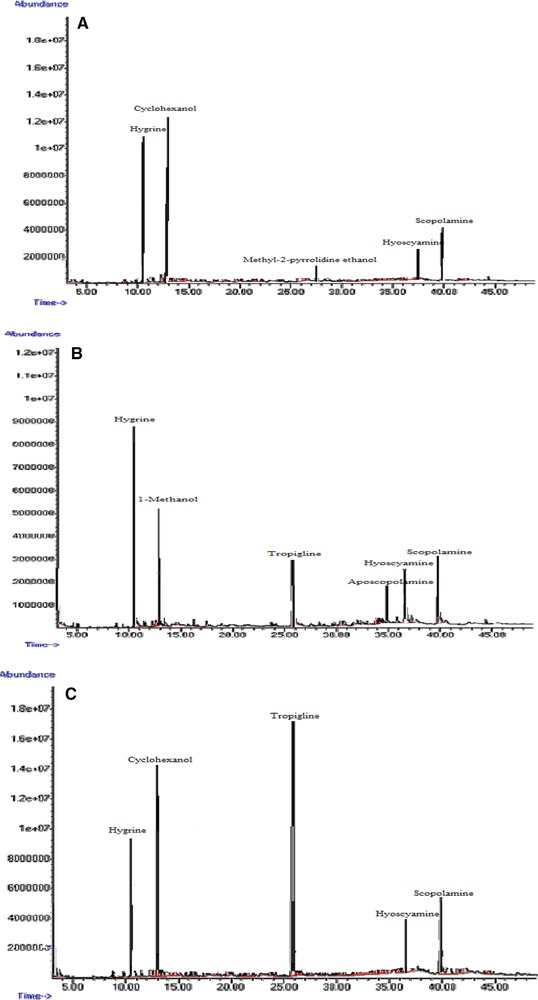
GC‐MS chromatograms of tropane alkaloids in Hyoscyamus reticulatus hairy roots elicited with 200 mg/L ZnO. 24 h (A); 48 h (B); 72 h (C).
The results of this study are consistent with those reported by Vakili et al. 12 in Atropa belladonna L and Moharrami et al. 8 in lattice henbane. Also, the treatment of Glycyrrhiza glabra with CuO and ZnO nanoparticles increased the content of glycyrrhizin 7. Silver nanoparticles have been used to promote the production of artemisinin in Artemisia annua 6 and atropine in Datura metel 55. Thus, the results of the current and previous studies suggest that nano‐sized elicitors could be used to increase the target alkaloid accumulation and reduce the costs of production systems.
5. Concluding remarks
The results of this study suggest that ZnO is absorbed by hairy roots. The negative impact of high ZnO concentrations on the hairy root biomass requires further research. The ZnO treatment (100 mg/L for 48 h) of H. reticulatus hairy roots enhanced the antioxidant activity of the CAT, APX and GPX enzymes and improved tropane alkaloid production and accumulation. Based on these results, ZnO seems a promising elicitor to increase the production of target secondary metabolites in plants. Further research is necessary to explore in more depth the effects of nanoparticles on the production and accumulation mechanisms of valuable metabolites in plants.
Practical application
A large number of metabolites produced by plants are not essential for growth and development but play an important role in plant adaptation to diverse environmental conditions. These secondary metabolites show a wide range of structures and functions, and have extensive application in different industries, including foods, fragrance, and pharmaceuticals. However, due to a natural low production, high extraction costs, and the complexity of their chemical synthesis, the demand for some of these valuable metabolites outstrips the supply, especially those with anticancer, antiviral and antibacterial properties. Plant cells and organs used as factories can constitute alternative production systems. Tropane alkaloids are valuable plant metabolites used extensively in the pharmaceutical industry. Hyoscyamine and scopolamine, mainly produced in Hyoscyamus reticulatus L., are parasympathetic nerve inhibitors. In this study, the scopolamine content in hairy roots treated with 100 mg/L of nano‐zinc oxide was enhanced by 37.63% after 72 h.
The authors have declared no conflict of interest
Author contributions
Kamal Rashidi Asl and Bahman Hosseini are responsible for the hairy root induction and elicitation experiments, sampling, enzyme analysis and statistical analyses presented in this paper. Ali Sharafi is responsible for the extraction of alkaloids and GC/MS analysis. Javier Palazon is responsible for the final revision and editing. All authors have read and approved the final manuscript
Acknowledgments
We acknowledge the staff of the plant tissue culture laboratory of the Horticulture Department, Biotechnology Center of Urmia University, for their skillful technical assistance. This research was completed by a grant of the Biotechnology Center of Urmia University.
6 References
- 1. Vidal‐Limon, H. , Sanchez‐Muñoz, R. , Khojasteh, A. , Moyano, E. , et al., Taxus cell cultures, an effective biotechnological tool to enhance and gain new biosynthetic insights into taxane production, in: Pavlov A., Bley T. (Eds.), Bioprocessing of Plant In vitro Systems, Reference Series in Phytochemistry, Springer, Cham: 10.1007/978-3-319-32004-5_5-1 [DOI] [Google Scholar]
- 2. Zayed, R. and Wink, M. , Induction of tropane alkaloid formation in transformed root cultures of Brugmansia suaveolens (Solanaceae). Z. Naturforsch. 2004, 59, 863–867. [DOI] [PubMed] [Google Scholar]
- 3. Palazón, J. , Navarro‐Ocaña, A. , Hernandez‐Vazquez, L. and Mirjalili, M. H. , Application of metabolic engineering to the production of scopolamine. Molecules 2008, 13, 1722–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Valimehr, S. , Sanjarian, F. , Sohi, H. H. , Sabouni, F. and Sharafi, A. , A reliable and efficient protocol for inducing genetically transformed roots in medicinal plant Nepeta pogonosperma . Physiol. Mol. Biol. Plants 2014, 20, 351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zeynali, Z. , Hosseini, B. , Rezaei, E. , Effect of elicitation on antioxidant activity and production of tropane alkaloids in Hyoscyamus reticulatus hairy root cultures. Res. J. Pharm. 2016, 3, 43–53. [Google Scholar]
- 6. Zhang, B. , Zheng, L. P. , Yi Li, W. and Wen Wang, J. , Stimulation of artemisinin production in Artemisia annua hairy roots by Ag‐SiO2 core‐shell nanoparticles. Curr. Nanosci. 2013, 9, 363–370. [Google Scholar]
- 7. Oloumi, H. , Soltaninejad, R. and Baghizadeh, A. , The comparative effects of nano and bulk size particles of CuO and ZnO on glycyrrhizin and phenolic compounds contents in Glycyrrhiza glabra L. seedlings. Indian J. Plant. Physiol. 2015, 20, 157–161. [Google Scholar]
- 8. Moharrami, F. , Hosseini, B. , Sharafi, A. and Farjaminezhad, M. , Enhanced production of hyoscyamine and scopolamine from genetically transformed root culture of Hyoscyamus reticulatus L. elicited by iron oxide nanoparticles. In vitro Cell. Dev. Biol., Plant. 2017, 53, 104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ghanati, F. , Bakhtiarian, S. , Effect of methyl jasmonate and silver nanoparticles on production of secondary metabolites by Calendula officinalis L (Asteraceae). Trop. J Pharm. Res. 2014, 13, 1783–1789. [Google Scholar]
- 10. Jasim, B. , Roshmi, T. , Jyothis, M. , Radhakrishnan, E. K. , Plant growth and diosgenin enhancement effect of silver nanoparticles in Fenugreek (Trigonella foenum‐graecum L.). Saudi Pharm. J. 2017, 25, 443–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ghorbanpour, M. , Hadian, J. , Multi‐walled Carbon nanotubes stimulate callus induction, secondary metabolites biosynthesis and antioxidant capacity in medicinal plant Satureja khuzestanica grown in vitro. Carbon, 2015; 94, 749–759. [Google Scholar]
- 12. Vakili, B. , Karimi, F. , Sharifi, M. , and Behmanesh, M. , Chromium‐induced tropane alkaloid production and h6h gene expression in Atropa belladonna L. (Solanaceae) in vitro‐propagated plantlets. Plant Physiol. Bioch. 2012, 52, 98–103. [DOI] [PubMed] [Google Scholar]
- 13. Sharafi, E. , Khayam Nekoei, S. M. , Fotokian, M. H. , Davoodi, D. , et al. Improvement of hypericin and hyperforin production using zinc and iron nano‐oxides as elicitors in cell suspension culture of St John's wort (Hypericum perforatum L.). J. Med. Plants By‐Prod. 2013, 2, 177–184. [Google Scholar]
- 14. Murashige, T. and Skoog, F. , A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plantarum. 1962, 15, 473–497. [Google Scholar]
- 15. Bertani, G. , Studies on lysogenesis I. The mode of phage liberation by lysogenic Escherichia coli . J. Bacteriol. 1951, 62, 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kamada, H. , Okamura, N. , Satake, M. , Harada, H. and Shimomura, K. , Alkaloid production by hairy root cultures in Atropa belladonna . Plant Cell Rep. 1986, 5, 239–242. [DOI] [PubMed] [Google Scholar]
- 17. Gharari, H. , Farjaminezhad, M. , Marefat, A. , Fakhari, A. R. , All‐in‐one solid‐phase microextraction: development of a selective solid‐phase microextraction fiber assembly for the simultaneous and efficient extraction of analytes with different polarities. J. Sep. Sci. 2016, 39, 1709–1716. [DOI] [PubMed] [Google Scholar]
- 18. Aebi, H. I. and Bergmeyer, H. , Methods of enzymatic analysis. Academic Press, New York: 1974, pp. 674–684. [Google Scholar]
- 19. Zhang, J. , Liu, H. , Sun, Y. , Wang, X. , et al., Responses of the antioxidant defenses of the Goldfish Carassius auratus, exposed to 2, 4‐dichlorophenol. Environ. Toxicol. Pharmacol. 2005, 19, 185–190. [DOI] [PubMed] [Google Scholar]
- 20. Nakano, Y. and Asada, K. , Hydrogen peroxide is scavenged by ascorbate‐specific peroxidase in spinach chloroplasts. Plant Cell Physiol.. 1981, 22, 867–880. [Google Scholar]
- 21. Hajimahdipour, H. , Khanavi, M. , SHekarchi, M. , Abedi, Z. and Pirali Hamedani, M. , Study the best method of extraction of phenolic compounds in Echinacea purpurea . J. Med. Plant. 2009, 4, 145–152. [Google Scholar]
- 22. Alloway, B. J. , Zinc in soils and crop nutrition. Second edition International zinc Association and International Fertilizer Industry Association. Brussels, Belgium and Paris, France. 2008.
- 23. Stoyanova, Z. , Doncheva, S. , The effect of Zinc supply and succinate treatment on plant growth and mineral uptake in pea plant”, Braz. J. Plant Physiol. 2002, 14, 111–116. [Google Scholar]
- 24. Cakmak, I. , Welch, R. M. , Erenoglu, B. , Romheld, V. , et al., “Influence of varied Zinc supply on re‐translocation of cadmium (109Cd) and rubidium (86Rb) applied on mature leaf of durum wheat seedlings”, Plant Soil, 2000, 219, 279–284. [Google Scholar]
- 25. Prasad, T. N. V. K. V. , Sudhakar, P. , Sreenivasulu, Y. , Latha, P. , et al., Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut, J. Plant Nutr. 2012, 35, 905–927. [Google Scholar]
- 26. Ozturk, L. , Yazici, M. A. , Yucel, C. , Torun, A. , et al., Concentration and localization of Zinc during seed development and germination in wheat”, Physiol. Plant. 2006, 128, 144–152. [Google Scholar]
- 27. Zheng, L. , Hong, F. , Lu, S. , and Liu, C. , Effect of nano‐TiO2 on strength of naturally aged seeds and growth of spinach. Biol. Trace Elem. Res. 2005, 104, 83–91. [DOI] [PubMed] [Google Scholar]
- 28. Lin, D. , and Xing, B. , Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250. [DOI] [PubMed] [Google Scholar]
- 29. Yang, Z. , Chen, J. , Dou, R. , Gao, X. , et al., Assessment of the phytotoxicity of metal oxide nanoparticles on two crop plants, Maize (Zea mays L.) and rice (Oryza sativa L.). Int. J. Environ. Res. and Public Health. 2015, 12, 15100–15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shah, K. and Dubey, R. , Cadmium elevates level of protein, amino acids and alters activity of proteolytic enzymes in germinating rice seeds. Acta Physiol. Plant. 1998, 20, 189–196. [Google Scholar]
- 31. Jamshidi, M. , Ghanati, F. , Rezaei, A. and Bemani, E. , Change of antioxidant enzymes activity of hazel (Corylus avellana L.) cells by AgNPs. Cytotechnol. 2016, 68, 525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li, M. , Ahammed, G. J. , Li, C. , Bao, X. , et al., Brassinosteroid ameliorates zinc oxide nanoparticles‐induced oxidative stress by improving antioxidant potential and redox homeostasis in tomato seedling. Front. Plant. Sci. 2016, 7, 615 10.3389/fpls.2016.00615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rajput, V. D. , Chen, Y. , Ayup, M. , Effects of high salinity on physiological and anatomical indices in the early stages of Populus euphratica growth. Russ. J. Plant. Physiol. 2015, 62, 229–236. [Google Scholar]
- 34. Noctor, G. , Foyer, C. H. , Ascorbate and glutathione: keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol., 1998, 49, 249–279. [DOI] [PubMed] [Google Scholar]
- 35. Hosseini, Z. and Poorakbar, L. , Zinc toxicity on antioxidative response in (Zea mays L.) at two different pH. J. Stress Physiol. Bioch. 2013, 9, 66–73. [Google Scholar]
- 36. Lee, S. , Kim, S. , Kim, S. and Lee, I. , Assessment of phytotoxicity of ZnO NPs on a medicinal plant, Fagopyrum esculentum. Environ. Sci. Pollut. Res. 2013, 20, 848–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pandey, N. , Gupta, B. and Pathak, G. , Antioxidant responses of pea genotypes to zinc deficiency. Russ. J. Plant Physiol. 2012, 59, 198–205. [Google Scholar]
- 38. Agarwal, S. and Pandey, V. , Antioxidant enzyme responses to NaCl stress in Cassia angustifolia . Biologia. Plantarum. 2004, 48, 555–560. [Google Scholar]
- 39. Wang, F. , Liu, X. , Shi, Z. , Tong, R. , et al., Arbuscular mycorrhizaealleviate negative effects of zinc oxide nanoparticle and zinc accumulation in maize plants – a soil microcosm experiment. Chemosphere, 2016, 147, 88–97. [DOI] [PubMed] [Google Scholar]
- 40. Manivannan, A. , Soundararajan, P. , Park, Y. G. and Jeong, B.R. , Chemical elicitor‐induced modulation of antioxidant metabolism and enhancement of secondary metabolite accumulation in cell suspension cultures of Scrophularia kakudensis Franch. Int. J. Mol. Sci.. 2016. 17, 399 10.3390/ijms17030399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sakihama, Y. , Cohen, M. F. , Grace, S. C. and Yamasaki, H. , Plant phenolic antioxidant and prooxidant activities: phenolics‐induced oxidative damage mediated by metals in plants. Toxicology 2002, 177, 67–80. [DOI] [PubMed] [Google Scholar]
- 42. Michalak, A. , Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol. J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- 43. Bota, C , Deliu, C . The effect of copper sulphat on the production of flavonoids in Digitalis lanata cell cultures. Farmacia. 2011, 59, 113–118. [Google Scholar]
- 44. Kai, G. , Zhang, A. , Guo, Y. , Li, L. , et al., Enhancing the production of tropane alkaloids in transgenic Anisodus acutangulus hairy root cultures by over‐expressing tropinone reductase I and hyoscyamine‐6β‐hydroxylase. Mol. BioSyst.. 2012, 8, 2883–2890. [DOI] [PubMed] [Google Scholar]
- 45. Ghorbanpou, r M , Hatami, M . and Hatami, M. , Activating antioxidant enzymes, hyoscyamine and scopolamine biosynthesis of Hyoscyamus niger L. plants with nano‐sized titanium dioxide and bulk application. Acta Agric. Slov. 2015, 105, 23–32. [Google Scholar]
- 46. Kim, Y. D. , Kang, S. M. , Min, J. Y. , Choi, W. K. , et al., Production of tropane alkaloids during de‐differentiation of Scopolia parviflora calli . J. Nat. Prod. 2010, 73, 147–150. [DOI] [PubMed] [Google Scholar]
- 47. Hashimoto, T. , Yun, D. , Yamada, Y . Production of tropane alkaloids in genetically engineered root cultures. Phytochem. 1993, 32, 713–718. [Google Scholar]
- 48. Yun, D.‐J. , Hashimoto, T. and Yamada, Y. , Metabolic engineering of medicinal plants: transgenic Atropa belladonna with an improved alkaloid composition. Proc. Natl. Acad. Sci. USA. 1992, 89, 11799–11803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yang, C. , Chen, M. , Zeng, L. , Zhang, L. , et al., Improvement of tropane alkaloids production in hairy root cultures of' Atropa belladonna' by overexpressing pmt and h6h Genes. Plant Omics 2011, 4, 29–33. [Google Scholar]
- 50. Mukherjee, M. and Mahapatra, A. , Catalytic effect of silver nanoparticle on electron transfer reaction: reduction of [Co (NH 3) 5 Cl] (NO 3) 2 by iron (II). Colloids and Surfaces A: Colloids Surf . A Physicochem Eng. Asp. 2009, 350, 1–7. [Google Scholar]
- 51. Ghasemi, B. , Hosseini, R. , Dehghani Nayeri, F. , Effects of cobalt nanoparticles on artemisinin production and gene expression in Artemisia annua . Turk J Bot. 2015, 39, 769–777. [Google Scholar]
- 52. Fan, Y. , Chao, Liu . Fengqing, G. , Mingyu, S. , et al., The improvement of spinach growth by Nano‐anatase TiO2 treatment is related to nitrogen photoreduction. Biol. Trace Elem. Res.. 2007, 119, 77–88. [DOI] [PubMed] [Google Scholar]
- 53. Bahmanzadegan, A. , Sefikon, F. , and Sonboli, A. , Determination of hyoscyamine and scopolamine in four hyoscyamus species from Iran. Iran J. Pharm. Res. 2009, 8, 65–70. [Google Scholar]
- 54. Ionkova, I. , In vitro Culture and the Production of Secondary Metabolites in Hyoscyamus reticulatus L. in: Nagata T., Ebizuka Y. (eds) Medicinal and Aromatic Plants XII. Biotechnology in Agriculture and Forestry. 2002, 51, 75–94. [Google Scholar]
- 55. Shakeran, Z. , Keyhanfar, M. , Asghari, G. and Ghanadian, M. , Improvement of atropine production by different biotic and abiotic elicitors in hairy root cultures of Datura metel . Turk J. Biol. 2015, 39, 111–118. [Google Scholar]


