Abstract
Heterologous production of naringenin, a valuable flavonoid with various biotechnological applications, was well studied in the model organisms such as Escherichia coli or Saccharomyces cerevisiae. In this study, a synergistic co‐culture system was developed for the production of naringenin from xylose by engineering microorganism. A long metabolic pathway was reconstructed in the co‐culture system by metabolic engineering. In addition, the critical gene of 4‐coumaroyl‐CoA ligase (4CL) was simultaneously integrated into the yeast genome as well as a multi‐copy free plasmid for increasing enzyme activity. On this basis, some factors related with fermentation process were considered in this study, including fermented medium, inoculation size and the inoculation ratio of two microbes. A yield of 21.16 ± 0.41 mg/L naringenin was produced in this optimized co‐culture system, which was nearly eight fold to that of the mono‐culture of yeast. This is the first time for the biosynthesis of naringenin in the co‐culture system of S. cerevisiae and E. coli from xylose, which lays a foundation for future study on production of flavonoid.
Keywords: Co‐culture, Heterologous expression, Naringenin, Orthogonal optimization, Xylose utilization
Abbreviations
- CHI
chalcone isomerase
- CHS
chalcone synthase
- 4CL
4‐coumaroyl‐CoA ligase
- JCAT
JAVA Codon Adaptation Tool
- LB
Lysogeny Broth
- LiAc/SS
lithium acetate/single‐stranded
- OD
optical density
- SC
synthetic complete
- SPSS
Statistical Product and Service Solutions
- TAL
L‐tyrosine ammonia lyase
1. Introduction
The utilization of biomass feedstocks, a huge potential resource, has attracted an increasing attention. Xylose is one of the most abundant sugar in the hemicellulose of hardwoods and the agricultural residues 1, 2. However, the utilization of xylose (accounting for 5–20% in hydrolytes of lignocellulosic feedstocks), still presents a challenge 3, 4. As a result, efficient utilization of xylose is one of the most important fields in the high productivity of biomass conversion, which had to be urgently settled 5, 6.
D‐xylose can be converted to some other metabolites by the wild type or the engineered Escherichia coli strains via xylose metabolic pathways 7, 8, 9. In order to convert xylose to high value‐added compounds, a secondary metabolite of plant naringenin was chosen as the final product in this study. Naringenin, a dihydro‐flavonoid, is widely dispersed in the citrus plant of rutaceae family, especially in grapefruit and bitter orange. In recent years, the identified mechanisms of action include scavenging of oxygen radicals, anti‐inflammatory, antiviral and antitumor activities 10, 11, 12, 13, 14. Naringenin not only shows the huge potential values but also is the intermediate platform compound to produce other flavonoids, such as eriodictyol, catechin, anthocyanin etc. 15, 16, 17. There are four important enzymes involved in the biosynthesis of naringenin from the endogenous metabolite, L‐tyrosine ammonia lyase (TAL), 4‐coumaroyl‐CoA ligase (4CL), chalcone synthase (CHS) and chalcone isomerase (CHI) 18, 19 (Fig. 1).
Figure 1.
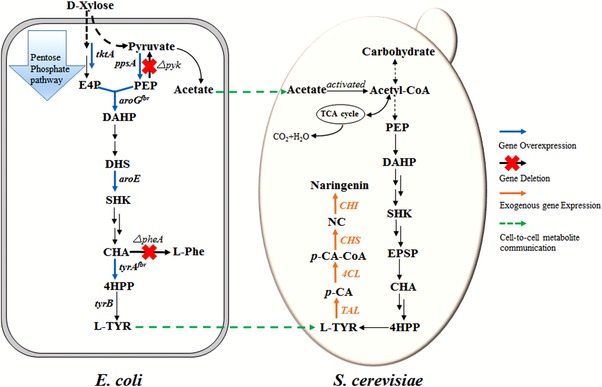
Schematic representation of metabolic pathway in the biosynthesis of naringenin via the co‐culture system. The artificial synthetic pathway to naringenin from L‐tyrosine consists of four enzymes encoded by TAL, 4CL, CHS, CHI. Abbreviations: PPP pentose phosphate pathway, PEP phosphoenolpyruvate, E4P erythrose‐4‐phosphate, DAHP 3‐deoxy‐D‐arabino‐heptulosonate‐7‐phosphate, DHS 3‐dehydroshikimic acid, SHK shikimic acid, CHA chorismic acid, 4‐HPP 4‐hydroxyphenylpyruvic acid, L‐Phe L‐phenylalanine, L‐Tyr L‐tyrosine, EPSP 5‐enolpyruvylshikimate‐3‐phosphate, p‐CA p‐coumaric acid, p‐CA‐CoA p‐coumaroyl‐CoA, NC naringenin chalcone, TCA cycle tricarboxylic acid cycle. TAL tyrosine ammonia lyase, 4CL 4‐coumarate‐CoA ligase, CHS chalcone synthase, CHI chalcone isomerase.
Generally, these potential secondary metabolites are mostly from plants. Moreover, S. cerevisiae is the eukaryotic organism, which is much more suitable than the prokaryotes to express the pathway of natural products from plants. As a result, S. cerevisiae was engineered to produce the natural products by importing the whole heterogeneous pathway. Interestingly, E. coli can metabolize xylose and excrete acetate, which is an inhibitor to its growth, while S. cerevisiae can utilize acetate as the carbon source without producing ethanol 20. The relationship between E. coli and S. cerevisiae in the artificial system lived on xylose is commensalism rather than competition when lived on glucose 21. Therefore, it is ponderable and potential to engineer microbial utilizing xylose as a carbon source.
During the last decade, several research groups have focused on engineering an attractive metabolic pathway for the production of naringenin. To our knowledge, the biosynthesis of naringenin is performed in the model laboratory and industrial microbial respectively—E. coli and S. cerevisiae. Different ways to refrain malonyl‐CoA from flowing downstream to synthesize fatty acid was implemented to increase the amount of malonyl‐CoA in E. coli 22, 23, 24, 25. In addition, a production of 200 μM (approximately 54.45 mg/L) naringenin was obtained in glucose‐grown shake‐flask cultures of S. cerevisiae by modifying the upstream synthesis of precursors 26. E. coli has been challenging to functionally express cytochrome P450 enzymes, and malonyl‐CoA is insufficient to support the heterogeneous synthetic metabolism 25. On the other hand, when it comes to S. cerevisiae, it is sophisticated and arduous to synthesize aromatic amino acids, thus an ocean of modifications to the upstream pathway were very important 27.
On the other hand, some researchers begin to engineer a co‐culture system to solve the problem that cannot be easily settled via a mono‐culture system. Since synergistic microbial communities are prevalent in nature and show attracting features, such as complicated metabolic capabilities and robustness 28. For instance, a consortium of two E. coli strains converted different sugars to succinate was developed with respective optimal sugar‐selective pathway 29. A synthetic fungal‐bacterial (Trichoderma reesei‐E. coli) consortia was designed for the production of isobutanol and the two microorganisms were responsible for different functions in this system 28. Moreover, according to Bayer's research, a symbiotic consortium composed of Actinotalea fermentans‐ S. cerevisiae was developed to synthesize methyl halides from non‐food agriculture resources 30. Furthermore, the co‐culture system was also meaningful for the production of valuable natural products with a long synthetic route 31. Based on these work, it is obviously clear that the co‐culture microbial system presents a positive effect on the production of chemicals and natural products. Zhou et al. distributed the metabolic pathway of taxanes from xylose into two parts and 33 mg/L oxygenated taxanes was gained with an engineered E. coli‐S. cerevisiae consortium 20. From the above, it is easy to see that the synthetic co‐culture system could probably offer an estimable platform for the production of natural products.
In this study, a co‐culture system composed of an engineered E. coli and S. cerevisiae was developed for the production of naringenin from D‐xylose. To our knowledge, it is the first report for the biosynthesis of naringenin from xylose via a co‐culture system. In order to achieve the optimal strain contributed to this system, a modified E. coli was developed by deletion and overexpression some relevant genes which play the important roles in the process of tyrosine biosynthesis, as shown in Fig. 1. The present study was designed to engineer a tyrosine‐producing E. coli and a naringenin‐producing S. cerevisiae by optimizing the medium components, inoculation size and inoculation ratio of two microorganisms for achieving a platform with high efficiency.
2. Materials and methods
2.1. Materials
Oligonucleotides and synthetic long DNA fragments were ordered from GenScript (Nanjing, China). Q5 DNA polymerase and Not I‐HF restriction endonuclease were purchased from New England Biolabs (MA, USA). Rapid DNA ligases were purchased from Thermo Scientific (Beijing, China). DNA purification, DNA Gel Extraction and plasmids isolation kits were purchased from Tiangen (Beijing, China). DNA sequencing was conducted by Genewiz (Beijing, China). Standards of naringenin and p‐coumaric acid were purchased from Sigma (Sigma‐Aldrich, MO, USA). E. coli DH5α competent cells were used for the propagation of recombinant plasmids.
2.2. Code optimization and plasmid construction
All the strains, plasmids and primers used in this study are summarized in Tables 1 and 2.
Table 1.
Strains and plasmids used in this study
| Description | Source | |
|---|---|---|
| Strain name | ||
| E. coli | LacI q rrnB T14ΔlacZ WJ16 hsdR514 | NBRP‐E. coli at NIG |
| BW25113 | ΔaraBAD AH33 ΔrhaBAD LD78 | This laboratory |
| BKT5 | BW25113, ∆ptsG, ∆tyrR, ∆pykA, ∆pykF, ∆pheA, with pYBT5 | This laboratory |
| S. Cerevisiae | ||
| CEN.PK2‐1C | MATa, ura3‐52, trp1‐289, leu2‐3,112, his3∆1, MAL2‐8C, SUC2 | This laboratory |
| SyBE_sc02120100 | CEN.PK2‐1C, URA3‐PGK1p‐CHI‐FBA1t‐TPI1p‐CHS‐PGK1t‐TEF1p‐4CL‐CYC1t‐TDH3p‐TAL‐TEF1t | This study |
| SyBE_sc02120101 | SyBE_sc02120100, pZW01 | This study |
| Plasmid | ||
| pBldgbrick1 | pMB1 ori with PlacUV5 and Ptrc; AmpR | This laboratory |
| pYBT5 | pBldgbrick 1 with PlacUV5 aroG fbr tyrA fbr aroE, Ptrc ppsA tktA glk | This laboratory |
| pRS425K | Multiple copies plasmid with LEU2 and Kan marker | This laboratory |
| pZW01 | pRS425K harboring cassette TEF1p‐4CL‐CYC1t | This study |
Table 2.
Primers used in module construction in this study
| Module construction | Primers | Sequences (5’‐3’) |
|---|---|---|
| CYC1t‐TDH3p‐TAL‐TEF1t | TAL module‐1 | AAGGAAAAAAGCGGCCGCCATGTAATTAGTTATGTCACGCTTAC |
| TAL module‐2 | GCAGTATTGATAATGATAAACTCGAACTGAAAGCCTTCGAGCGTCCC | |
| TAL module‐3 | GGGACGCTCGAAGGCTTTCAGTTCGAGTTTATCATTATCAATACTGC | |
| TAL module‐4 | GAAATAGAATCCAATGATGGAGCCATTTTGTTTGTTTATGTGTGTTTATTCGAAAC | |
| TAL module‐5 | GTTTCGAATAAACACACATAAACAAACAAAATGGCTCCATCATTGGATTCTATTTC | |
| TAL module‐6 | GAAAAGTCTTATCAATCTCCTTATTTTTAAGCCAACATTTTTAACAAGAC | |
| TAL module‐7 | GTCTTGTTAAAAATGTTGGCTTAAAAATAAGGAGATTGATAAGACTTTTC | |
| TAL module‐8 | AAGGAAAAAAGCGGCCGCGATAGCGCCGATCAAAGTA | |
| PGK1t‐TEF1p‐4CL‐CYC1t | 4CL module‐1 | AAGGAAAAAAGCGGCCGCATTGAATTGAATTGAAATCGATAG |
| 4CL module‐2 | GGAAGAGTAAAAAAGGAGTAGAAACATTAACGAACGCAGAATTTTCG | |
| 4CL module‐3 | CGAAAATTCTGCGTTCGTTAATGTTTCTACTCCTTTTTTACTCTTCC | |
| 4CL module‐4 | CTTTCGGGGCAACGCAGTCACCCATTTTGTAATTAAAACTTAGATTAGATTGCTATGC | |
| 4CL module‐5 | GCATAGCAATCTAATCTAAGTTTTAATTACAAAATGGGTGACTGCGTTGCCCCGAAAG | |
| 4CL module‐6 | GTAAGCGTGACATAACTAATTACATGTTACTTCGGCAGGTCGCCGCTCGC | |
| 4CL module‐7 | GCGAGCGGCGACCTGCCGAAGTAACATGTAATTAGTTATGTCACGCTTAC | |
| 4CL module‐8 | AAGGAAAAAAGCGGCCGCAAAGCCTTCGAGCGTCCC | |
| FBA1t‐TPI1p‐CHS‐PGK1t | CHS module‐1 | AAGGAAAAAAGCGGCCGCGTTAATTCAAATTAATTGATATAGTTTTTTAATG |
| CHS module‐2 | CAACCTGATGGGTTCCTAGATATAAAAGATGAGCTAGGCTTTTGTAAAAATATC | |
| CHS module‐3 | GATATTTTTACAAAAGCCTAGCTCATCTTTTATATCTAGGAACCCATCAGGTTG | |
| CHS module‐4 | CCTGTACTCTTCGACAGTAACCATTTTTAGTTTATGTATGTGTTTTTTGTAGT | |
| CHS module‐5 | ACTACAAAAAACACATACATAAACTAAAAATGGTTACTGTCGAAGAGTACAGG | |
| CHS module‐6 | CTATCGATTTCAATTCAATTCAATTTATGTTGCGACTGAGTGCAAAACG | |
| CHS module‐7 | CGTTTTGCACTCAGTCGCAACATAAATTGAATTGAATTGAAATCGATAG | |
| CHS module‐8 | AAGGAAAAAAGCGGCCGCAACGAACGCAGAATTTTCG | |
| GPDt‐PGK1p‐CHI‐FBA1t | CHI module‐1 | AAGGAAAAAAGCGGCCGCGTGAATTTACTTTAAATCTTGCATT |
| CHI module‐2 | CTTGAGTTGAAGTCAGGAATCTAAAATAGGAATCTGTGTATATTACTGCATCTAG | |
| CHI module‐3 | CTAGATGCAGTAATATACACAGATTCCTATTTTAGATTCCTGACTTCAACTCAAG | |
| CHI module‐4 | CTTAGTGACAGAGACTGGTGGAGACATTGTTTTATATTTGTTGTAAAAAGTAGATAATTAC | |
| CHI module‐5 | GTAATTATCTACTTTTTACAACAAATATAAAACAATGTCTCCACCAGTCTCTGTCACTAAG | |
| CHI module‐6 | TTAAACACCAATAACAGGAATAGTAGCATTAAAAAACTATATCAATTAATTTGAATTAAC | |
| CHI module‐7 | GTTAATTCAAATTAATTGATATAGTTTTTTAATGCTACTATTCCTGTTATTGGTGTTTAA | |
| CHI module‐8 | AAGGAAAAAAGCGGCCGCAAAGATGAGCTAGGCTTTTGTAAAAATATC |
Starting from amino acid sequence of TAL from Rhodosporidium toruloides, 4CL from Petroselinum crispum, CHS from Petunia x hybrid, CHI from Petunia x hybrid, we optimized the codon usage for gene heterologous expression in S. cerevisiae with a program named JCAT, and removed the selected restriction enzyme recognition sites.
Then the overlap extension PCR (OE‐PCR) was used to construct the mono gene expression module. Firstly, all the promoters, terminators and their own gene ORF frames were cloned with the designed primers. Then all the fragments and the relevant primers were mixed together and the complete DNA sequence, named module, was gained as we designed. Five modules delta1‐URA3‐GPDt, GPDt‐PGK1p‐CHI‐FBA1t, FBA1t‐TPI1p‐CHS‐PGK1t, PGK1t‐TEF1p‐4CL‐CYC1t, CYC1t‐TDH3p‐TAL‐TEF1t‐delta2 were built in the vector pRS425K separately with their terminator acting as homologous fragment to each other. All the sequences of promoters and terminators were determined according to Sun's work 32. Specifically, for the pRS425K‐delta1‐URA3‐GPDt, the delta1 fragment, the URA3 marker gene from Kluyveromyces lactis and the GPDt terminator were constructed by OE‐PCR. The resulting fragment was digested by Not I and then introduced into the corresponding site of vector pRS425K to form pRS425K‐delta1‐URA3‐GPDt. For pRS425K‐GPDt‐PGK1p‐CHI‐FBA1t module, the empty plasmid pRS425K‐GPDt‐PGK1p‐FBA1t was first constructed by OE‐PCR, consisted of upstream homologous fragment GPDt terminator, promotor PGK1p, and downstream terminator FBA1t into the plasmid. Then, the gene CHI from Petunia x hybrid was introduced into the empty plasmid constructed above to create pRS425K‐GPDt‐PGK1p‐CHI‐FBA1t using the Golden Gate method 33. For CYC1t‐TDH3p‐TAL‐TEF1t‐delta2, delta 2 was introduced into the second terminator as the downstream homologous region with yeast chromosome. Correct construction of the plasmid was confirmed by diagnostic PCR using primers annealing to the end of the promoter and the beginning of the terminator. In each case, the correct insertion was confirmed by vector insert sequencing.
The modules described above were digested by the corresponding restriction enzymes, the URA3 marker module and the gene modules related to the naringenin pathway were co‐transformed into yeast (CEN.PK2‐1C) for delta site integration after gel purification. Yeast was transformed using the LiAc/SS carrier DNA/PEG method 34. Gene delta site integrations were verified by diagnostic PCR.
2.3. Fermentation media and cultivation conditions
Luria–Bertani (LB) medium containing the appropriate antibiotic, 100 μg/L ampicillin or 50 μg/L kanamycin was used for cultivating the general strains for cloning or preparation of fermentation seeds. All the yeast strains engineered in this study are based on CEN.PK2‐1C (MATa). Engineered yeast strains were selected on synthetic complete (SC) medium (6.7 g/L yeast nitrogen base without amino acids, 20 g/L glucose, and 2 g/L amino acid drop‐out mix). The synthetic fermented medium contained 40 g/L xylose, 5 g/L yeast extraction, and the extra added inorganic salt (13.3 g/L KH2PO4, 4 g/L (NH4)2HPO4, 1.7 g/L citric acid, 0.0084 g/L EDTA, 0.0025 g/L CoCl2, 0.015 g/L MnCl2, 0.0015 g/L CuCl2, 0.003 g/L H3BO3, 0.0025 g/L Na2MoO4, 0.008 g/L Zn(CH3COO)2, 0.06 g/L Fe(III) citrate, 1.3 g/L MgSO4).
For shake‐flask fermentation, the engineered strains and control strains were activated on the plates, then engineered yeast was inoculated into a tube containing 5 mL SC‐ura‐leu medium for 24 h growing, then the preculture was inoculated (Initial OD600 = 0.2) into 250 mL shake‐flask containing 50 mL SC‐ura‐leu medium for overnight growing. The engineered E. coli was inoculated into a tube containing 5 mL LB with AmpR, then the preculture was inoculated (Initial OD600 = 0.2) into a 250 mL shake‐flask containing 50 mL LB AmpR. After all the strains growing to the mid‐log phase, the seeds were already prepared. The engineered E. coli and the engineered yeast were transferred to the synthetic fermented medium at an initial OD600 respectively and cultivated at 30°C, 220 rpm.
2.4. Extraction and quantification of naringenin and p‐coumaric acid
Naringenin and p‐coumaric acid were extracted twice from the culture medium and the disrupted cells of S. cerevisiae with an equal volume of ethyl acetate. The organic phase containing the target products was collected and dried by nitrogen flow, and the residue was dissolved in methanol. The above extracts were analyzed on a waters e2695 HPLC system. An Ascentis@ Express C18 column (10 cm × 2.1 mm, 2.7 μm) was used. A (methanol) and C (water containing 0.1% formic acid (v/v)) were the mobile phase and the gradient eluted program was listed in Supporting Information Table 1. The flow rate was 0.2 mL/min and the column temperature was 30°C. The injection volume was 5 μL. The wavelength for Naringenin was 290 nm and the wavelength for p‐coumaric acid was 310 nm. The products were confirmed by the retention time compared to that of authentic standards.
2.5. Determination of extracellular metabolites
Different consumption of added sugars, ethanol production, and the intermediate amount of acetic acid were determined on a waters e2695 HPLC system with a refractive index detector. An Aminex HPX‐87H column (300 × 7.8 mm, 9 μm) was used for separation at the column temperature of 65°C. 5 mM H2SO4 was used as the eluent with the flow rate of 0.6 mL/min. The injection volume was 10 μL. The fermentation supernatant was diluted 5‐fold with 5 mM H2SO4, and was filtered by 0.22 μm cellulose acetate membrane.
2.6. Quantification of E. coli and S. cerevisiae cell number
A sucrose gradient centrifugation method was used to quantify the cell number of both E. coli and S. cerevisiae in the co‐culture system. At indicated time points, 0.2 mL of cell suspension was sampled and loaded onto 0.20 mL of 15%, 30%, 45% and 60% of each in a 1.5 mL; falcon tube for the order of the sucrose concentration which was then centrifuged at 3000 rpm for 2 min. Microbes in the supernatant were exclusively E. coli and those in the pellets were mostly S. cerevisiae. The supernatant was then centrifuged at 12 000 rpm for 2 min; After this separation, the cell number of the two microbes could be quantified by measuring optical density at 600 nm.
3. Results and discussion
3.1. Simultaneous construction of naringenin heterologous biosynthesis pathway in S. cerevisiae and primary establishment of the co‐culture system of E. coli and S. cerevisiae
Naringenin biosynthetic genes were selected from different species, TAL from Rhodosporidium toruloides, 4CL from Petroselinum crispum while CHS and CHI were all from Petunia x hybrid. It has been demonstrated that TAL is a crucial gene in the whole pathway, which leads to the important intermediate product 26. So, a relative strong promoter was selected to start the gene. From our previous study, 4CL was the limited enzyme throughout the whole pathway in our previous study 35. As a result, a stronger promoter, TEF1p, was selected before 4CL gene in this study. The naringenin pathway was constructed by delta site genomic integration of TAL, 4CL, CHS, CHI in the initial S. cerevisiae strain CEN.PK2‐1C (Fig. 1). The naringenin production of the constructed strain was approximately 2 mg/L cultured in the SC medium (data not shown). For other researchers, the upstream of naringenin biosynthesis was modified, such as the overexpression of the feedback inhibition genes as well as the deletion of the bypass genes. However, it is a time consuming and painstaking work 15. In this study, we made the less modification to the strain and looked forward to get naringenin production via the co‐culture system. The initial engineered E. coli was from our previous work.
To assess the ability of single yeast to synthesize naringenin, we cultivated the strain SyBE_sc02120100 on the initial medium condition individually, 5.6 g/L acetate (12.67 g/L sodium acetate added), 5 g/L yeast extract, one‐fold inorganic salt was added into the medium, and the initial inoculation was set as OD600 = 2. As the data shown in Fig. 2, the production of naringenin was 2.37 mg/L when SyBE_sc02120100 was mono‐cultured. Meanwhile, the production of p‐coumaric acid performed at a low level. Subsequently, a new consortium was developed with SyBE_sc02120100 and a wild type E. coli without any modifications in the whole pathway of tyrosine. The E. coli was inoculated as OD600 = 0.1. And the production of naringenin in the shake flask reached 2.20 mg/L with 20 g/L xylose as the carbon source. Compared with the mono‐culture, the production of p‐coumaric acid reached to 3.06 mg/L, which increased by 45%. It was obvious to see the advantages of co‐culture system compared with the traditional mono‐culture including better relationships between the two microbes and the carbon source supply particularly.
Figure 2.
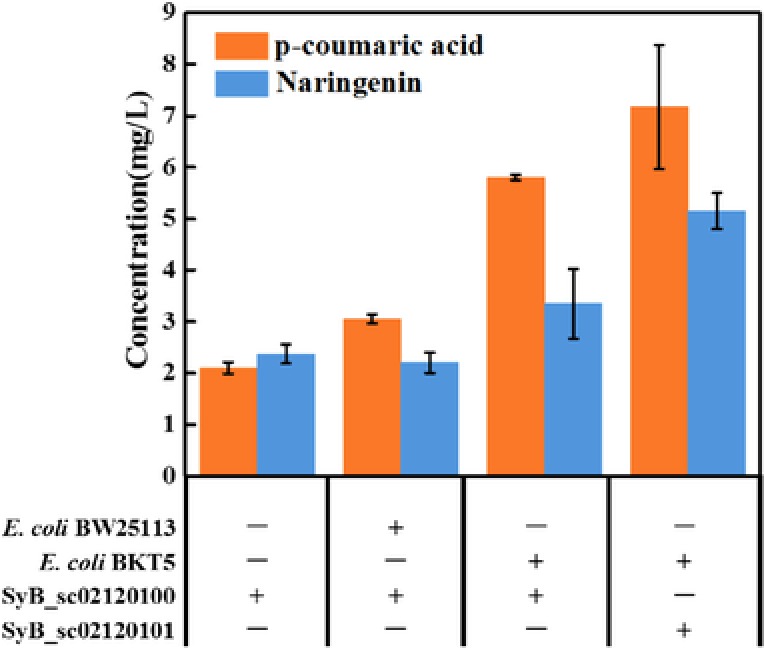
Comparison of mono culture and co‐culture system based on the initial medium condition. The mono culture of single yeast was fermented on 5.6 g/L acetate, 5 g/L yeast extract, one‐fold inorganic salt and the inoculation was OD600 = 1, the co‐culture system of S. cerevisiae and E. coli was fermented on 20 g/L xylose, the other conditions were the same as the single one. + represented the strain involved in the cultured system; – represented the opposite. All error bars indicate ± SD, n = 3.
3.2. Metabolic engineering of strains to realize the precursor L‐tyrosine improvement in E. coli and the metabolic flux amplification from p‐coumaric acid to naringenin in yeast
The E. coli strain BW25113 was modified by metabolic engineering to improve the precursor amino acid L‐tyrosine supply to the whole co‐culture system 36. The wild type E. coli in the co‐culture system was replaced by the modified one, BKT5. The production of the new consortium improved to 3.35 mg/L, increased by 52% when compared with the original consortium. Moreover, 5.80 mg/L p‐coumaric acid was produced in the new co‐culture system, nearly doubled of the previous one. It is easy to estimate that the modified E. coli had a great enhancement in the production of naringenin and p‐coumaric acid. According to our previous work, we found that the activity of 4CL was insufficient. In this work, a multi‐copy pRS425K plasmid which contains the 4CL gene expression cassette was used to increase the amount of the enzyme. Then the yeast harboring the multi‐copy plasmid was co‐cultured with the modified E. coli to enhance the metabolic flux. As the data shown in Fig. 2, the production of naringenin of this co‐culture system lead to a relevantly high level of 5.15 mg/L, which was 54% increase than that of the initial synthetic system. Meanwhile, the yield of p‐coumaric acid increased by 24%. That is, the major difference between the two consortia reflected in the production of both the naringenin and the p‐coumaric acid. The metabolic flux from p‐coumaric to naringenin also affected that of the upstream which produced more naringenin and p‐coumaric acid in turn.
3.3. Optimization to improve the naringenin production in the synthetic S. cerevisiae‐E. coli co‐culture by orthogonal experiment design
To improve the production of the co‐culture system, a series of medium optimizations were designed and implemented in the subsequent experiments. The Taguchi orthogonal array design was used for the preliminary test. The independent variables investigated were xylose (A), inorganic salt mixtures(B) yeast extract (C) and OD600 of yeast initial inoculation(D). The practical experiments were carried out in four factors and three levels according to Statistical Product and Service Solutions (SPSS) design. Nine experiments were chosen and carried out as represented by the orthogonal design while the inoculation of E. coli involved into the co‐culture system was still set as OD600 = 0.1. The low and high values for variables were selected from the results of pilot experiments.
It was found that the strain on 9th condition (40 g/L xylose, 5 g/L yeast extract, 1.5‐fold inorganic salt, yeast inoculation OD600 = 5) produced the highest production of naringenin (21.16 mg/L) after 96 h, which was 3.1‐fold increase than that of the initial cultured condition (Fig. 3). It was easy to see that the amount of xylose and the yeast inoculation size presents big influence on the production of naringenin. The amount of yeast extract showed a profile of negative correlation. Therefore, yeast extract (C) was kept at 5 g/L, xylose (A) was kept at 40 g/L and inorganic salt was kept at 1.5‐fold.
Figure 3.
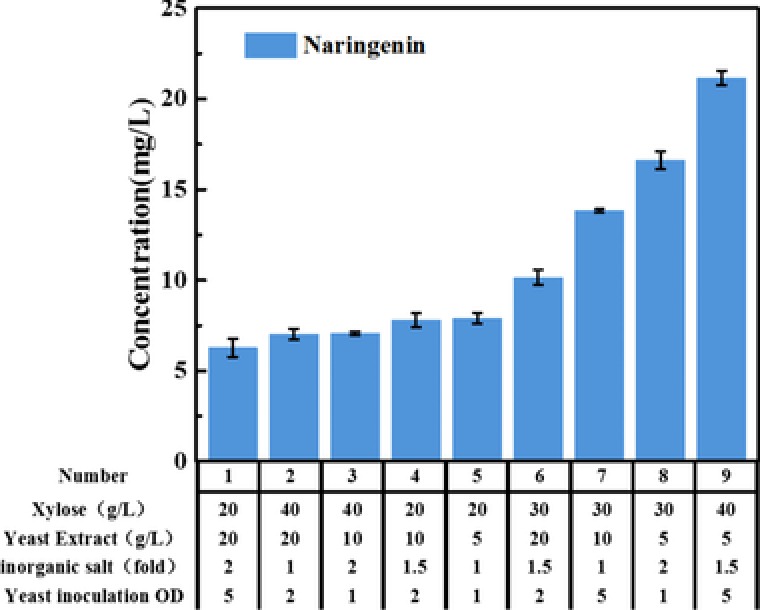
Effects of four different factors on the naringenin production through the orthogonal experiments. The inoculation of E. coli is fixed value as 0.1. Four factors (Xylose, Yeast Extract, Inorganic salt, Yeast inoculation) and three levels of each are tested in this study to find an appropriate fermentation condition. All error bars indicate ±SD, n = 3.
3.4. Effect of inoculum size and inoculum ratio of S. cerevisiae and E. coli on naringenin and p‐coumaric acid fermentation
From the above, the inoculation size of yeast was determined to influence the production of naringenin most. Hence, we decided to explore the effects of inoculum size and the inoculum ratio of the two engineered microorganism on the production of naringenin and p‐coumaric acid. Three different levels of inoculum size (OD600 = 1, 2, 3) were chosen. Moreover, under each level of inoculum, four inoculum ratios were selected according to the Zhou's study (40:1) 20. The inoculum ratio of the co‐culture system was set to over inoculation of S. cerevisiae, four ratios were 70:1, 50:1, 30:1 and 10:1 respectively.
When S. cerevisiae and E. coli were co‐cultured, the production of naringenin varied from the inoculation size and ratio (Fig. 4). However, it is obvious to see that when the inoculation ratio of S. cerevisiae to E. coli was decreased by adding E. coli and reducing S. cerevisiae under a fixed inoculum size, the naringenin production was improved. As a consequence, the ratio of 70:1 presented best. According to three levels of inoculum size, the production of naringenin was increased with the size expansion. When the inoculum size was set as 3, the production of naringenin production was 19.94 mg/L, nearly the same to the control one. Specifically, the p‐coumaric acid production reached 82.29 mg/L. Compared with the naringenin produced in S. cerevisiae without much gene manipulations in the upstream, this work had achieved a relative improvement with no precursor addition 37. Nevertheless, we would not intent to attempt much bigger inoculum size for its big amount of second stage seed. To all inoculum size, the initial ratio of two microorganisms ranged from 10:1 to 70:1, when cultivated for 24 h, the ratio of yeast to E. coli was ranging from 4 to 12. For instance, according to OD600 = 2, the ratio was from 3.7 to 10.0 increasing by sequence to the initial ratio at 24 h. However, the ratio of yeast to E. coli showed less difference since 48 h. The ratio was approximately 4 at the end of the fermentation. It was demonstrated that the ratio of the two engineered microorganisms influenced the metabolism most at first 48 hours most caused of the carbon source flux.
Figure 4.
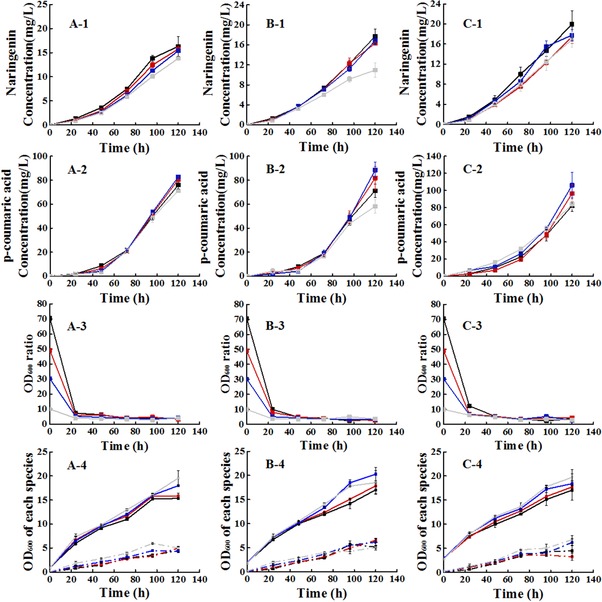
Effect of inoculum size and inoculum ratio on naringenin and p‐coumaric acid fermentation. Series A represented the inoculum size OD600 = 1; Series B represented the inoculum size OD600 = 2; Series C represented the inoculum size OD600 = 3. 1 represented the naringenin yield; 2 represented the p‐coumaric acid yield; 3 represented the OD600 ratio of two microorganisms; 4 represented the OD600 of each species involved in the co‐culture system. In series 4, the solid line represented the OD600 of S. cerevisiae, and the dash dot represented the OD600 of E. coli. Line black represented the S. cerevisiae: E. coli = 70:1; Line red represented the S. cerevisiae: E. coli = 50:1; Line blue represented the S. cerevisiae: E. coli = 30:1; Line grey represented the S. cerevisiae: E. coli = 10:1. All error bars indicate ± SD, n = 3.
Different ratios showed almost the same assumption rate of xylose. Only inoculated with a ratio of 70:1, no acetate was detected during the fermentation in the co‐culture system. And the highest yield of naringenin 19.94 mg/L was achieved at the inoculation ratio of 70:1 in the 3 inoculum size. It was demonstrated that the big inoculum size really caused an enhancement at a fixed inoculation ratio. For instance, when the inoculum ratio was fixed at 70:1, the production of naringenin increased with the increase of the inoculation. When the inoculum size was fixed at 3, the amount of E. coli decreased with the increase of the inoculation ratio. Although it caused a low biomass in the end, it achieved 18% increase compared with that of 10:1. The results indicated that the low inoculation of E. coli not only eliminated inhibiting effect of acetate to cells but also limited the population of yeast by the acetate concentration. When it comes to the p‐coumaric acid production, there was still a problem laying in the conversion to naringenin. The p‐coumaric acid concentration was high and showed no rules due to the different ratios. As a consequence, to drive the metabolic flux from p‐coumaric acid to naringenin is the key point to improve the naringenin production in our future work.
4. Concluding remarks
For the first time, a co‐culture system was developed for the production of an important value‐added product naringenin based on the relationship of E. coli and S. cerevisiae when utilizing D‐xylose as the sole carbon source. In order to streamline metabolite flux for the production of naringenin from xylose, modifications of the wild type E. coli and overexpression of the critical enzyme to S. cerevisiae were applied. Furthermore, we optimized the synthetic medium by orthogonal design, aiming to seek the optimal medium condition to the production of naringenin. Moreover, the inoculum size and inoculum ratio were also being considered to improve the product concentration. The results of our study have indicated that the microbial co‐cultivation with combination of pathway and optimization of synthetic medium has a great potential for the future flavonoids production.
Practical application
A co‐culture system of Saccharomyces cerevisiae and Escherichia coli was developed for production of naringenin, which is a valued‐added natural product. D‐xylose created a harmonious cultivated condition for the two engineered microorganisms as the sole carbon source without competition and no more by‐product ethanol produced. First of all, a plenty of optimizations of cultivated medium were presented to improve the naringenin production. Furthermore, the inoculum size and inoculum ratio of S. cerevisiae and E. coli were explored to seek an appropriate inoculation. This work not only provided a method for co‐culture of S. cerevisiae and E. coli from D‐xylose, but also displayed a new way to produce the valuable natural products with a long pathway.
The authors have declared no conflict of interest.
Supporting information
Supplementary Table 1 The HPLC gradient eluted conditions for naringenin and p‐coumaric acid
Supplementary Table 2 The synthesized sequences of the heterologous genes were listed
Acknowledgments
This work was supported by the Ministry of Science and Technology of China (‘‘973’’ Program: 2014CB745102, 2013CB733601), the National Natural Science Foundation of China (31470610), Science and Technology Program of Tianjin (13RCGFSY19800).
5 References
- 1. Jeffries, T. W. , Utilization of xylose by bacteria, yeasts, and fungi. Adv. Biochem. Eng. Biotechnol. 1983, 27, 1–32. [DOI] [PubMed] [Google Scholar]
- 2. Olofsson, K. , Bertilsson, M. , Liden, G. , A short review on SSF‐an interesting process option for ethanol production from lignocellulosic feedstocks. Biotechnol. Biofuels 2008, 1, 7–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aristidou, A. , Penttilä, M. , Metabolic engineering applications to renewable resource utilization. Curr. Opin. Biotechnol. 2000, 11, 187–198. [DOI] [PubMed] [Google Scholar]
- 4. Li, R. , Chen, Q. , Wang, P. G. , Qi, Q. , A novel‐designed Escherichia coli for the production of various polyhydroxyalkanoates from inexpensive substrate mixture. Appl. Microbiol. Biotechnol. 2007, 75, 1103–1109. [DOI] [PubMed] [Google Scholar]
- 5. Stephanopoulos, G. , Challenges in Engineering Microbes for Biofuels Production. Science 2007, 315, 801–804. [DOI] [PubMed] [Google Scholar]
- 6. Sarris, D. , Papanikolaou, S. , Biotechnological production of ethanol: biochemistry, processes and technologies. Eng. Life Sci. 2016, 16, 307–329. [Google Scholar]
- 7. Yomano, L. P. , York, S. W. , Shanmugam, K. T. , Ingram, L. O. , Deletion of methylglyoxal synthase gene (mgsA) increased sugar co‐metabolism in ethanol‐producing Escherichia coli . Biotechnol. Lett. 2009, 31, 1389–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vinuselvi, P. , Jungmin, P. , Jaemyung, L. , Kikwang, O. et al., Engineering microorganisms for biofuel production. Biofuels 2011, 2, 153–166. [Google Scholar]
- 9. Nijveldt, R. J. , Nood, E. V. , Hoorn, D. E. V. , Boelens, P. G. et al., Flavonoids: a review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [DOI] [PubMed] [Google Scholar]
- 10. Limem, I. , Guedon, E. , Hehn, A. , Bourgaud, F. et al., Production of phenylpropanoid compounds by recombinant microorganisms expressing plant‐specific biosynthesis genes. Process Biochem. 2008, 43, 463–479. [Google Scholar]
- 11. Fowler, Z. L. , Koffas, M. A. , Biosynthesis and biotechnological production of flavanones: current state and perspectives. Appl. Microbiol. Biotechnol. 2009, 83, 799–808. [DOI] [PubMed] [Google Scholar]
- 12. Wang, B. , Zhang, X. , Inhibitory effects of Broccolini leaf flavonoids on human cancer cells. Scanning 2012, 34, 1–5. [DOI] [PubMed] [Google Scholar]
- 13. Wang, Y. , Chen, S. , Yu, O. , Metabolic engineering of flavonoids in plants and microorganisms. Appl. Microbiol. Biotechnol. 2011, 91, 949–956. [DOI] [PubMed] [Google Scholar]
- 14. Yan, Y. , Kohli, A. , Koffas, M. A. , Biosynthesis of natural flavanones in Saccharomyces cerevisiae . Appl. Environ. Microbiol. 2005, 71, 5610–5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhao, S. , Andrew, J. J. , Lachance, D. M. , Bhan, N. et al., Improvement of catechin production in Escherichia coli through combinatorial metabolic engineering. Metab. Eng. 2015, 28, 43–53. [DOI] [PubMed] [Google Scholar]
- 16. Zhu, S. , Wu, J. , Du, G. , Zhou, J. et al., Efficient synthesis of eriodictyol from L‐tyrosine in Escherichia coli . Appl. Environ. Microbiol. 2014, 80, 3072–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Winkel‐Shirley, B. , Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001, 126, 485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koopmann, E. , Hahlbrock, K. , Differentially regulated NADPH:cytochrome P450 oxidoreductases in parsley. Proc. Natl. Acad. Sci. U. S. A. 1997, 94, 14954–14959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Costa, M. A. , Bedgar, D. L. , Moinuddin, S. G. , Kim, K. W. et al., Characterization in vitro and in vivo of the putative multigene 4‐coumarate:CoA ligase network in Arabidopsis: syringyl lignin and sinapate/sinapyl alcohol derivative formation. Phytochemistry 2005, 66, 2072–2091. [DOI] [PubMed] [Google Scholar]
- 20. Zhou, K. , Qiao, K. , Edgar, S. , Stephanopoulos, G. , Distributing a metabolic pathway among a microbial consortium enhances production of natural products. Nat. Biotechnol. 2015, 33, 377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Song, H. , Ding, M. Z. , Jia, X. Q. , Ma, Q. et al., Synthetic microbial consortia: from systematic analysis to construction and applications. Chem. Soc. Rev. 2014, 43, 6954–6981. [DOI] [PubMed] [Google Scholar]
- 22. Wu, J. , Du, G. , Chen, J. , Zhou, J. , Enhancing flavonoid production by systematically tuning the central metabolic pathways based on a CRISPR interference system in Escherichia coli . Sci. Rep. 2015, 5, 13477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Santos, C. N. , Koffas, M. , Stephanopoulos, G. , Optimization of a heterologous pathway for the production of flavonoids from glucose. Metab. Eng. 2011, 13, 392–400. [DOI] [PubMed] [Google Scholar]
- 24. Wu, J. , Zhou, T. , Du, G. , Zhou, J. et al., Modular optimization of heterologous pathways for de novo synthesis of (2S)‐naringenin in Escherichia coli . PLoS One 2014, 9, e101492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang, Y. , Lin, Y. , Li, L. , Linhardt, R. J. et al., Regulating malonyl‐CoA metabolism via synthetic antisense RNAs for enhanced biosynthesis of natural products. Metab. Eng. 2015, 29, 217–226. [DOI] [PubMed] [Google Scholar]
- 26. Koopman, F. , Beekwilder, J. , Crimi, B. , Houwelingen, A. V. et al., De novo production of the flavonoid naringenin in engineered Saccharomyces cerevisiae . Microb. Cell Fact. 2012, 11, 155–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rodriguez, A. , Kildegaard, K. R. , Li, M. , Borodina, I. et al., Establishment of a yeast platform strain for production of p‐coumaric acid through metabolic engineering of aromatic amino acid biosynthesis. Metab. Eng. 2015, 31, 181–188. [DOI] [PubMed] [Google Scholar]
- 28. Minty, J. J. , Singer, M. E. , Scholz, S. A. , Bae, C. H. et al., Design and characterization of synthetic fungal‐bacterial consortia for direct production of isobutanol from cellulosic biomass. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 14592–14597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xia, T. , Altman, E. , Eiteman, M. A. , Succinate production from xylose‐glucose mixtures using a consortium of engineered Escherichia coli . Eng. Life Sci., 2015, 15, 65–72. [Google Scholar]
- 30. Katsuyama, Y. , Miyahisa, I. , Funa, N. , Horinouchi, S. , One‐pot synthesis of genistein from tyrosine by coincubation of genetically engineered Escherichia coli and Saccharomyces cerevisiae cells. Appl. Microbiol. Biotechnol. 2007, 73, 1143–1149. [DOI] [PubMed] [Google Scholar]
- 31. Bayer, T. S. , Widmaier, D. M. , Temme, K. , Mirsky, E. A. et al., Synthesis of methyl halides from biomass using engineered microbes. J. Am. Chem. Soc. 2009, 131, 6508–6515. [DOI] [PubMed] [Google Scholar]
- 32. Sun, J. , Shao, Z. , Zhao, H. , Nair, N. et al., Cloning and characterization of a panel of constitutive promoters for applications in pathway engineering in Saccharomyces cerevisiae . Biotechnol. Bioeng. 2012, 109, 2082–2092. [DOI] [PubMed] [Google Scholar]
- 33. Engler, C. , Gruetzner, R. , Kandzia, R. , Marillonnet, S. , Golden gate shuffling: a one‐pot DNA shuffling method based on type IIs restriction enzymes. PLoS One 2009, 4, e5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gietz, R. D. , Schiestl, R. H. , High‐efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat. Protoc. 2007, 2, 31–34. [DOI] [PubMed] [Google Scholar]
- 35. Liu, D. , Li, B. , Liu, H. , Guo, X. et al., Profiling influences of gene overexpression on heterologous resveratrol production in Saccharomyces cerevisiae . Front Chem Sci Eng. 2016, 1–9. [Google Scholar]
- 36. Yao, Y. F. , Wang, C. S. , Qiao, J. , Zhao, G. R. , Metabolic engineering of Escherichia coli for production of salvianic acid A via an artificial biosynthetic pathway. Metab. Eng. 2013, 19, 79–87. [DOI] [PubMed] [Google Scholar]
- 37. Trantas, E. , Panopoulos, N. , Ververidis, F. , Metabolic engineering of the complete pathway leading to heterologous biosynthesis of various flavonoids and stilbenoids in Saccharomyces cerevisiae . Metab. Eng. 2009, 11, 355–366. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1 The HPLC gradient eluted conditions for naringenin and p‐coumaric acid
Supplementary Table 2 The synthesized sequences of the heterologous genes were listed


