Abstract
Volatile fatty acids (VFA) represent short‐chain fatty acids consisting of six or fewer carbon atoms that can be distilled at atmospheric pressure. In anaerobic digestion processes VFAs are of central importance for maintaining stable reactor performance and biogas production, are used as indicators for arising problems and are important process monitoring parameters. In the present study, sludge derived form a full‐scale anaerobic digester of a wastewater treatment plant was spiked with formate, acetate, propionate, and butyrate in order to evaluate various commonly used techniques for VFA extraction, preservation, and storage. It was shown that VFA extraction after centrifugation warranted the highest recovery rates for spiked VFAs. Moreover, experiments clearly indicated the importance of a fast sample handling, including the necessity of immediate cooling of the samples. Chemical sample preservation within a narrow time frame or deep freezing emerged as an alternative to instant VFA extraction. Short‐time storage of extracted VFA samples at + 4°C is an option for up to 7 days, for longer periods storage at –20°C was found to be applicable.
Keywords: Acetate, Biogas, conservation, Extraction, VFA
Abbreviations
- PA
phenoxyacetic acid
- VFA
volatile fatty acid
1. Introduction
The anaerobic decomposition process is characterized by the complex interaction of various microorganisms form the domain of Bacteria as well as the domain of Archaea and can be divided into different phases represented by hydrolysis, acidogenesis, acetogenesis, and methanogenesis 1, 2, 3, during which volatile fatty acids (VFAs) are representing intermediate products from the degradation of complex substances on the one hand and the end products of the microbial decomposition process H2, CO2, and CH4 on the other hand. VFAs are defined as short‐chain fatty acids consisting of six or fewer carbon atoms that can be distilled at atmospheric pressure 4. In anaerobic digestion processes these acids, in particular formic, acetic, propionic, and butyric acid, are of central importance for maintaining stable reactor performance and biogas production, therefore, they are important process monitoring parameters indicating arising problems 5, 6, 7. Which VFA in whatever ratio to select and what control strategies to apply in order to maintain an efficient microbial community achieving best digestion results and biogas yields has been discussed controversially 8. However, the reliable qualitative and quantitative analysis of reactor VFAs is crucial in order to provide a meaningful measure for the process status 9, 10.
Commonly, the determination of VFAs is performed using GC 11, headspace GC 12, HPLC 13, or spectroscopic methods 14 in accordingly equipped analytical laboratories. Titrimetric methods represent low cost techniques resulting in a VFA sum parameter 15, but do not consider the composition of the single VFAs making ratio calculations impossible. In any case an important aspect of reliable VFA qualification and in particular quantification is the choice of extraction and sample preservation procedures. Various filtrations 13, dialysis 16, and centrifugation techniques 17 have been applied so far but were validated rarely.
In the present study, frequently used techniques for VFA extraction, preservation, and storage were adopted and evaluated by spiking experiments in order to determine the extractable concentrations of formate, acetate, propionate, and butyrate via HPLC using sludge of a full‐scale anaerobic digester of a wastewater treatment plant.
2. Material and methods
2.1. Matrix
Anaerobically digested sludge of the wastewater treatment plant Zirl, Austria, was used for all analysis as the basic matrix. Sludge characteristics and some basic parameters of the digestion plant in Zirl can be found in Table 1. Sludge samples were taken in prewarmed 1000 mL screw cap glass bottles and transported into the lab within 15 min maintaining reactor temperature by warming pads and using an insulated box. In the lab the sample masses were determined and the sludge kept at 38°C (original digester temperature).
Table 1.
Sludge characteristics and some basic parameters of the waste water treatment plant in Zirl, Austria
| Parameter | |
|---|---|
| Mode of digestion | Cosubstrate digestion |
| Reactor capacity (population equivalents) | 61 500 |
| Electrical conductivity [mS cm−1] | 10.1 |
| pH | 7.9 |
| Specific weight (38°C) | 0.972 ± 0.004 kg/L |
| Osmotic strength [mOsmol L−1] | 177 |
| TS [g 100 g−1 fresh weight] | 3.1 |
| VS [g 100 g−1 total solids] | 61.0 |
| Alkalinity (pH 4.3) [mmol L−1] | 100 |
| NH4‐N [mg N L−1]a) | 1 385 |
| Phosphate [mg P L−1]b) | 34 |
| Proteins [mg L−1]c) | 41 |
2.2. Spiking and sampling
Depending on the respective experiment samples were spiked with 3.0–5.0 mmol kg−1 fresh weight each of C1 – C4 VFAs (formate, acetate, propionate, and butyrate, sodium from each) and 1.8–3.0 mmol kg−1 fresh weight of phenoxyacetic acid sodium salt (PA). The spiking was carried out per mass unit since a reproducible addition per volume was not possible. In order to guarantee homogenous distribution, spiked sludge was shaken head‐over‐head for 10 min. Prior to spiking a sample was taken and analyzed for the sludge's native VFA concentrations. The de facto concentrations were analyzed by HPLC immediately after spiking and used as the starting concentrations in all calculations. For the evaluation of the extraction procedure the theoretically added VFA concentration was used.
2.3. Volatile fatty acid analysis
HPLC analysis of VFAs was conducted as described before 18 but using a Fast Fruit Column (Phenomenex, Germany) at 25°C and a time program starting at a flow rate of 0.25 mL min−1 for 20 min, than ramping to 1.0 mL min−1 within 10 min, and finally keeping this flow rate until method stop at 45 min. For qualification and quantitative calibration the Volatile Acid Standard Mix from Sigma, Germany (46975‐U, Lot: LB 98693) was used containing formate, acetate, propionate, iso‐butyrate, butyrate, iso‐valerate, valerate, iso‐capronate, capronate, and heptanoate 10 mM each. Phenoxyacetic acid (PA) (Sigma, 158518) was used as an internal standard. PA resists microbial degradation, has low volatility under the experimental conditions (pK a = 3.17) and was therefore used as a tracer for the evaluation of extraction efficiency, liquid volume availability, and HPLC performance, independent of microbial VFA decomposition. All samples were passed through a 0.2 μm RC (regenerated cellulose) filter prior to HPLC analysis.
2.4. Experimental setup and procedure
Four different factors were evaluated:
Effect of different separation techniques on VFA recovery from spiked sludge.
Decomposition of spiked VFAs under original digester temperature and at +4°C.
Effect of thermic and chemical sample preservation and precipitation/coagulation agents on VFA recovery from spiked sludge.
Effect of sample storage at +4°C and –20°C, respectively, on the VFA recovery.
2.4.1. Separation techniques
Techniques for solids removal comprised gravity filtration, vacuum filtration, centrifugation, and dialysis. Gravity filtration: fluted filters (MN 615 ¼ x Ø150 mm) were placed in a funnel and flask in triplicate, and filled with 15 g of spiked sludge. It took approx. 5 min to obtain the filtrate volume necessary for subsequent VFA analysis. Vacuum filtration: bottle‐top system filters (Thermo Scientific, Nalgene™ Rapid‐Flow™, Ø75 mm, PES 0.45 μm) were placed on 250 mL flasks in triplicate, 20 g sludge were added and vacuum was applied. It took approx. 5 min to obtain the filtrate volume necessary for subsequent VFA analysis. Centrifugation: representative 1.0 g aliquots of native sludge were centrifuged in 1.5 mL Eppendorf tubes at 15 000 × g for 15 min. Dialysis was performed for 24 h on ice using Visking #44114 dialysis tubing (Ø 21 mm, 15 cm) filled with 10 mL bidistilled water. In triplicate, approx. 200 g of spiked sludge were transferred into a 250 mL screw cap glass bottle, the filled dialysis tubing was added, and the flask closed and placed on ice. After 24 h liquid was taken from the tubing by a syringe and cannula.
2.4.2. VFA decompostion
Sludge spiked with (5.0 mmol kg−1 of each fatty acid) was incubated at 38 and 4°C, respectively, and sampled over a period of 150 min in 30 min intervals in order to generate a time‐dependent degradation pattern for the different VFAs. Attention was paid to cool the sludge designated for incubation at 4°C even before VFA addition. Sample preparation was carried out by centrifugation technique as described above.
2.4.3. Deep freezing
Deep freezing was carried out in order to test a method conserving a sample by fast cooling and therefore inhibiting microbial activity. After spiking, 1 g aliquots of sample were immediately frozen at –20°C in 1.5 mL Eppendorf tubes. The samples were thawed after 24 h in a water bath at 30°C and subsequently centrifuged at 15 000 x g for 15 min prior to further analysis.
2.4.4. Chemical sample preservation
Moreover, chemical preservation and precipitation/coagulation, respectively, was tested using ZnCl2, a commercial preservation kit bought from an engineering consultant office (Germany, exact formulation unknown but containing copper), CuCl2‐3Cu(OH)2, CuSO4, and CuCl2. For the evaluation of ZnCl2 125 μL of a 58 mM ZnCl2 solution were transferred to 750 μL of sludge centrifugation supernatant, mixed, and 125 μL of a 70 mM NaOH solution was added. Subsequently, the mixture was subjected to sample preparation with centrifugation technique as described above. For testing the commercial preservation kit for reactor VFA sampling, 130 mg of a provided (unknown, but obviously hardly water soluble) preservation substance, obtained in 15 mL tubes, was intensively mixed with 10 g of spiked sludge. Subsequently, 2 mL of sample were centrifuged as described above. Copper, a strong microbial toxin, was tested in various compounds and concentrations. CuSO4 was added to the spiked sludge to a final concentration of 1 mM, CuCl2, and CuCl2‐3Cu(OH)2 to give 50 mM. The solutions were stirred until the arisen foam collapsed (especially with CuCl2). Throughout the different chemical sample preservation steps the pH was monitored continuously and kept > 6.3 with 70 mM NaOH. In this way, conserved samples were kept at room temperature and VFA analyses were conducted after 1, 3, and 5 days of storage.
2.4.5. Storage of extracted samples
The possibility to store extracted samples at +4°C and –20°C, respectively, was tested and the VFA recovery rates were evaluated. For this purpose, sludge was cooled down to 4°C, subsequently spiked with 4.0 mmol kg−1 VFAs and 2 mmol kg−1 phenoxyacetic acid and shaken (head‐over‐head) for 5 min to distribute the added VFAs evenly. Subsequently, samples were taken, centrifuged as described above and passed through a 0.20 μm RC filter (Phenomenex, Germany). Immediately, triplicate analyses of VFA concentrations were conducted in order to obtain a starting value. Remaining samples were stored at +4°C and –20°C, respectively, until analysis (1, 2, 5, 7, 15, 22, 32 days), thawed and centrifuged (15 000 x g, 15 min). Six hundred microliters of supernatants were subjected to HPLC analysis.
2.5. Statistical analyses
Statistical analyses were performed by using the Software packages Statistica 9.0 (StatSoft®) and SigmaPlot 12.0 (Systat Software Inc.). Results are given as means ± SD out of three replicates. Significant differences were ascertained by one‐way or multifactorial ANOVA. A significance level of 0.05 was used to assess differences between treatments. Fisher's Least Significant Difference Test was used to discriminate between single variants.
3. Results and discussion
3.1. Effect of different sample preparation techniques
Sludge derived from a wastewater treatment plant was spiked with formate, acetate, propionate, butyrate, and PA. In Fig. 1 the sum of recovered VFAs (formate, acetate, propionate, and butyrate) is depicted for the different sample preparation techniques. Significant differences were found for dialysis only, the technique that yielded the lowest total VFA concentration and resulted in the highest standard deviations. In particular, formate and butyrate recovery rates were poor (Fig. 2) with formate completely failing to be recovered by dialysis. Although the dialysis tubes were placed in closed bottles and on ice, this could not prevent the formate loss, probably due to microbial decomposition and/or outgassing over the 24 h period. The highest VFA recovery was found for the centrifugation technique yielding 94.9% of the theoretically added sum of VFAs (4 × 4.13 mmol kg−1). For acetate (99%), propionate (103%), and butyrate (105%) of the theoretically added values were found not differing significantly between the applied methods (data not shown). However, significant differences were found for formate, where the centrifugation technique resulted in a recovery of 62% being the highest of all tested methods. Looking at the applied tracer PA revealed a recovery of 107%, where a theoretical concentration of 1.80 mmol kg−1 PA was added to the sludge and a mean of 1.92 mM (± 0.016) recovered. Similar to the recovery rates of propionate and butyrate the values >100% were likely due to the diffusible volume of PA, propionate, and butyrate into the sludge matrix. The measured surplus of the tracer demonstrated clearly, that there is a nondiffusible volume fraction in the anaerobic sludge, in which the added acid amount could not dissolve. Such fraction could include larger solids particles or nondiffusible microbial biomass. Although the extraction of VFAs was done on a mass basis (centrifugation of 1.0 g), the chromatographic quantification had to be carried out in the resulting liquid and therefore was on a volume basis resulting in this overestimation of VFA concentrations.
Figure 1.
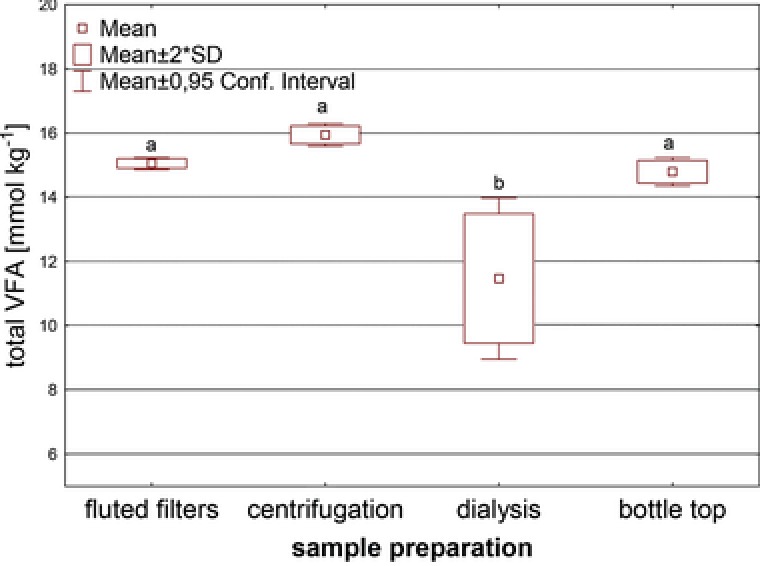
Recovered total VFA concentrations [mM] (sum of formate, acetate, propionate, and butyrate) for the different sample preparation techniques. A total of 16.5 mmol VFAs kg−1 fresh weight were added. Different letters are indicating significant differences (p < 0.05).
Figure 2.
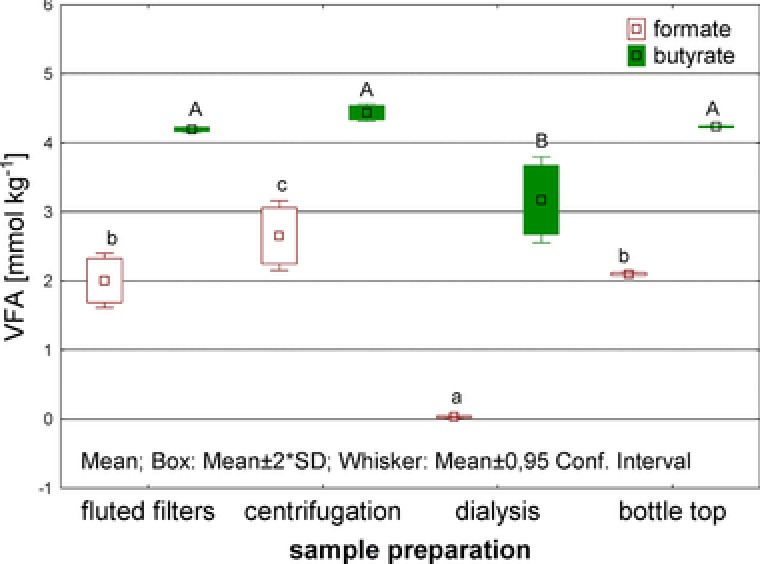
Recovered VFA concentrations [mM] of formate and butyrate for the different sample preparation techniques. Different characters are indicating significant differences (p < 0.05).
In conclusion, centrifugation technique allowed the significantly highest recovery rates for formate, is fast, cheap, and easy applicable. Therefore, this sample preparation technique was selected in the subsequent experiments as the best‐working sample preparation method.
3.2. Decomposition of spiked VFAs
In order to evaluate the time course of VFA degradation, interesting in particular for the time from taking a sludge sample until its further processing, spiked sludge was incubated at 38°C and sampled over a period of 150 min in 30 min intervals. To test sample cooling immediately after taking it, the experiment was repeated at 4°C.
Maintaining the reactor temperature of 38°C strongly affected the recovery rates of the spiked VFAs as demonstrated in Fig. 3. A strong impact could be ascertained for formate and butyrate, less strong but also present for propionate, whereas acetate has to be reviewed very cautiously. As can be seen in Fig. 3A starting concentrations of formate were already very low (1.61 mM ± 0.075). On the one hand formate is highly volatile at 38°C–one of the reasons why it was included in this study–and although the samples were mixed in tightly closed screw cap glass flasks for only 10 min prior to the first sampling, this was enough to dramatically decrease the recovery of formate. On the other hand formate is a direct substrate for methanogenic Archaea 19–another reason to be included in this study. This additionally might have reduced the extractable concentration of this VFA. The fast decrease in the observed concentration of formate indicates the importance of a very fast and accurate sample handling right after sampling. After 30 min only minute amounts of formate were detectable and after 60 min formate could not be detected anymore. In contrast, propionate concentrations decreased during the 150 min incubation period from a starting value of 4.62 mM (± 0.022) to 4.10 mM (± 0.104) indicating a recovery of 89%. For butyrate the extracted concentration decreased from 4.11 mM (± 0.017) at the beginning to 2.75 mM (± 0.040) after 150 min reflecting a recovery of only 55% when the samples were maintained at reactor temperatures. This decrease may be attributed to microbial decomposition by syntrophic consortia of acetogenic bacteria and methanogens 20 resulting in acetate. Therefore, the interpretation of acetate concentrations has to be carried out very cautiously. Acetate is the most important intermediate and a direct precursor of methane in anaerobic digestion processes and so fast turn‐over rates were expected at the beginning of the experiment. However, acetate concentrations decreased slowly and after 150 min still 91.4% of the starting concentration could be detected. Likely this slow decrease was not due to reduced turn‐over of acetate, but as mentioned above, to the fact, that the decomposition of butyrate yielded acetate (1 mol butyrate → 2 mol acetate) and superimposed the degradation of the latter. Additionally, this assumption is corroborated by the slightly increasing values of acetate after 90 and 120 min at 38°C.
Figure 3.
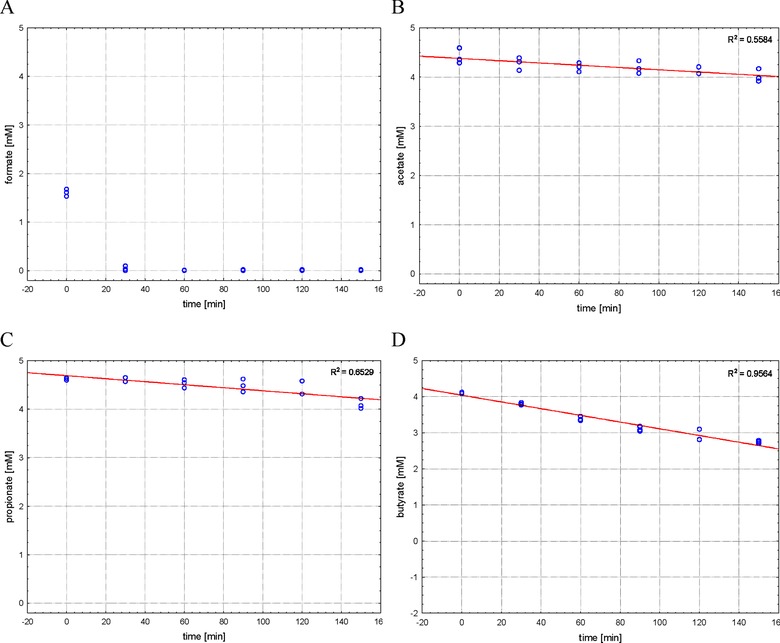
Recovery of VFAs in spiked sludge. Samples were not cooled but kept at original reactor temperature after spiking. (A) Formate; (B) acetate; (C) proprionate; (D) butyrate.
When the spiked sludge was incubated at 4°C the results were different. Although the degradation as seen for 38°C samples could not be stopped completely, at least it could be reduced (Fig. 4). Most acutely this can be seen for formate and butyrate with recoveries of 75.1 and 93.4%, respectively, after 150 min. A list of recovery rates [%] during 38 and 4°C incubation can be found in Table 2 for 30, 60, and 150 min as well as the calculated loss per hour. It can be seen that an immediate cooling down restricted losses to < 2.5% for acetate, propionate, and butyrate during the first 60 min, which is acceptable for most applications. However, after 150 min losses were found to be as high as 7%.
Figure 4.
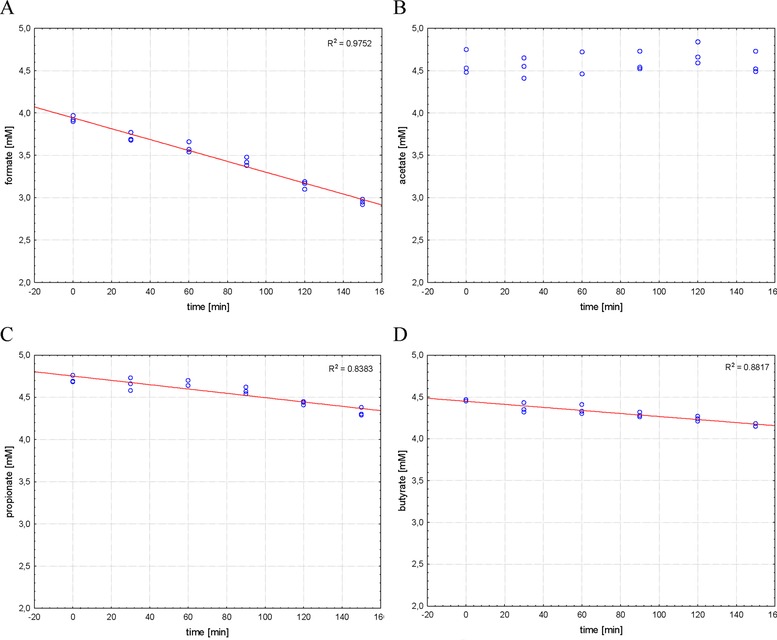
Recovery of VFAs in spiked sludge. Samples were cooled and subsequently spiked. (A) Formate; (B) acetate; (C) proprionate; (D) butyrate.
Table 2.
Recovery [%] from initial concentrations of spiked VFAs and loss of VFA h−1 at 4 and 38°C, respectively
| Min | 38°C | 4°C | ||||||
|---|---|---|---|---|---|---|---|---|
| Formate | Acetate | Propionate | Butyrate | Formate | Acetate | Propionate | Butyrate | |
| 30 | 2.7 | 97.0 | 99.7 | 92.5 | 94.5 | 98.9 | 98.9 | 97.8 |
| 60 | 0.2 | 95.2 | 98.0 | 82.5 | 91.3 | 99.1 | 98.9 | 97.4 |
| 150 | 0.4 | 91.2 | 88.7 | 66.9 | 75.1 | 99.9 | 91.8 | 93.4 |
| Loss [mmol L−1 h−1]a | Completely lost | 0.172 | 0.118 | 0.626 | 0.372 | 0.114 | 0.123 | |
In conclusion, experiments clearly indicate the importance of a fast sample handling for reliable reactor VFA analysis, showing that an immediate chilling of reactor samples is essential.
3.3. Effect of sample preservation
Sample preservation prior to VFA extraction is aiming at retarding microbial degradation in order to keep the VFA concentration in a sample unchanged until the extraction step. Therefore different preservation methods were tested. From the beginning it became evident that ZnCl2 and CuSO4 were inappropriate; depending on the investigated VFA, ZnCl2 reduced the extractable VFA concentration by 5–15% directly after addition, similar effects were found for CuSO4 (data not shown) and therefore both were not used any further. Also, a commercial preservation kit (based on Cu toxicity) did not conserve the sample sufficiently. Only 7.5% of the total initially added VFAs could be detected after 3 days of storage. CuCl2‐3Cu(OH)2, in contrast, showed quite a promising preservation effect during the first 3 days of storage (Table 3), when added at a concentration level of 50 mM. However, at day 5 the extractable VFA concentration was significantly reduced, possibly indicating an insufficient concentration of the poorly soluble CuCl2‐3Cu(OH)2 allowing an onset of microbial activity. A better preservation effect was found for CuCl2, allowing recovery rates of > 95% after 5 days of storage (Table 3). The loss of approx. 5% within the first 24 h may be attributed to a lag phase for CuCl2 toxicity.
Table 3.
Recovery [%] of spiked total VFAs (calculated from the sum of masses of formate, acetate, propionate, and butyrate) after addition of preservation agents or deep freezing
| day | CuCl2‐3Cu(OH)2 | Commercial kit | CuCl2 | −20°C |
|---|---|---|---|---|
| 1 | 101 | 60.8 | 95.1 | nd |
| 3 | 102 | 7.51 | 95.5 | nd |
| 5 | 69.7 | nd | 95.8 | 92.7 |
nd: not determined.
Deep freezing of the freshly spiked sample resulted in a recovery of > 92% of the added VFAs. Probably, losses were mainly attributed to formate volatility or direct microbial activity during the time necessary to reach the target temperature of –20°C. If the recovery of acetate, propionate, and butyrate are taken as a basis approx. 98% of spiked VFAs could be conserved by deep freezing.
3.4. Effect of storage
The effect of storage on the recovery of extracted and filtered (0.2 μm) VFA spiked sludge samples was evaluated at temperatures of +4°C and –20°C. For 7 days VFA concentrations remained stable at both storage temperatures (Fig. 5). However, at day 15 a dramatic decrease in VFA concentrations in samples stored at +4°C was observed, along with reproducibility as reflected by an increased standard deviation. This is particularly important since practically samples have to be stored prior VFA analysis, e.g. samples typically await HPLC analysis using an autosampler at 4°C. Therefore, it has to be taken care not to enqueue more samples than possible to be analyzed within 7 days.
Figure 5.
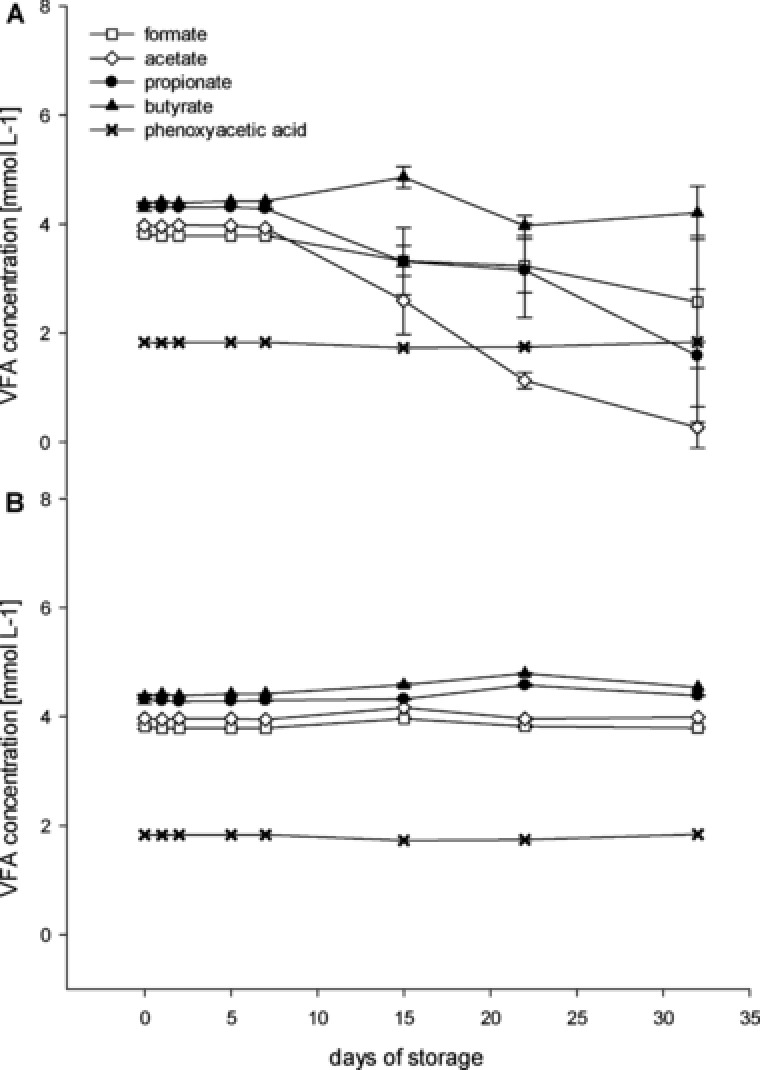
Concentrations of extracted VFAs and phenoxyacetic acid (for details please refer to text) during 32 days of storage at (A) +4°C and (B) –20°C.
At –20°C, however, even prolonged storage resulted in stable VFA concentrations. The observed decrease of VFA concentrations at 4°C supposably can be attributed to microbial growth and activity shown by the metabolization of mainly acetate (with coeval butyrate generation), the most important intermediate in methanogenic habitats. The increased variance in samples stored more than 7 days can therefore be explained by different microbial communities or microbial activity in general, which led to the observed reduction of VFAs. Another indication for a microbiologically induced VFA decrease is backed by the fact that the measured values for the microbial inert tracer PA stayed constant (this is additionally proofing stable HPLC performance).
Another aspect that is scarcely described in scientific articles is a change in the sample matrix after freezing and thawing 21. In the present study this change was observed and resulted in a precipitate that was found for both, samples that underwent freezing and thawing and those stored at 4°C. For freeze/thawed samples the effect was stronger, however, an impact on the recovery of VFAs could not be found. Ibrahim and coworkers 21 attributed those sedimented particles to higher molecular weight molecules, which did not impact VFA concentrations but total (organic) carbon contents.
4. Concluding remarks
It was shown that VFA extraction after centrifugation warranted the highest recovery rates for spiked VFAs. This technique is reproducible, convenient, fast, and cheap and appropriate for most applications aiming at determining reactor VFA composition and concentration. Experiments clearly indicated the importance of a fast sample handling, including the necessity of immediate cooling of samples. Chemical sample preservation within a narrow time frame or deep freezing is also an alternative to instant VFA extraction. Short‐time storage of extracted VFA samples at +4°C is an option for up to 7 days, for longer periods storage at –20°C is suggested.
Practical application
In anaerobic digestion processes VFAs are of central importance for maintaining stable reactor performance and biogas production, are used as indicators for arising problems and are important process monitoring parameters. Many different extraction, preservation, and storage procedures with different expenditures for time, consumables, and technical equipment are applied for VFA qualification and quantification in practical use–often without any validation of the procedure partly due to technical equipment circumstances. This study presents data on these issues, suggests a very simple and cost effective procedure for VFA extraction from anaerobic digestion systems securing reliable results, shows a simple way to store extracted samples safely, and therefore might help the experimenter–in science or industry, academic, or nonacademic–to avoid costs, time, and/or anger derived from inappropriate procedures and/or equipment.
The authors declare no financial or commercial conflict of interest.
Acknowledgments
The study was supported by the COMET program within the alpS‐Centre for Climate Change Adaptation.
5 References
- 1. Gerardi M. H. (Ed.), The Microbiology of Anaerobic Digesters. John Wiley & Sons, Hoboken: 2003, pp. 1–177. [Google Scholar]
- 2. Mackie, R. I. , White, B. A. , Bryant, M. P ., Methanogenesis, biochemistry, in: Lederberg J. (Ed.), Encyclopedia of Microbiology, Academic Press, San Diego: 1992, pp. 97–109. [Google Scholar]
- 3. Schink, B. , Anaerobic digestion: Concepts, limits and perspectives. Water Sci. Technol. 2002, 45, 1–8. [PubMed] [Google Scholar]
- 4. APHO , Standard Methods for the Examination of Water and Wastewater, 18th edn., APHO American Public Health Organization, USA: 1992. [Google Scholar]
- 5. Björnsson, L. , Murto, M. , Mattiasson, B. , Evaluation of parameters for monitoring an anaerobic co‐digestion process. Appl. Microbiol. Biotechnol. 2000, 54, 844–849. [DOI] [PubMed] [Google Scholar]
- 6. Nielsen, H. B. , Uellendahl, H. , Ahring, B. K. , Regulation and optimization of the biogas process: Proprionate as a key parameter. Biomass Bioenergy 2011, 3, 820–830. [Google Scholar]
- 7. Wagner, A. O. , Reitschuler, C. , Illmer, P. , Effect of different acetate:propionate ratios on the methanogenic community during thermophilic anaerobic digestion in batch experiements. Biochem. Eng. J. 2014, 90, 154–161. [Google Scholar]
- 8. Wagner, A. O. , Malin, C. , Lins, P. , Gstraunthaler, G. , Illmer, P. , Reactor performance of a 750 m(3) anaerobic digestion plant: Varied substrate input conditions impacting methanogenic community. Anaerobe 2014, 29, 29–33. [DOI] [PubMed] [Google Scholar]
- 9. Boe, K. , Batstone, D. J. , Steyer, J.‐P. , Angelidaki, I. , State indicators for monitoring the anaerobic digestion process. Water Res. 2010, 44, 5973–5980. [DOI] [PubMed] [Google Scholar]
- 10. Pind, P. F. , Angelidaki, I. , Ahring, B. K. , Dynamics of the anaerobic process: Effects of volatile fatty acids. Biotechnol. Bioeng. 2003, 82, 791–801. [DOI] [PubMed] [Google Scholar]
- 11. Angelidaki, I. , Petersen, S. P. , Ahring, B. K. , Effects of lipids on thermophilic anaerobic‐digestion and reduction of lipid inhibition upon addition of bentonite. Appl. Microbiol. Biotechnol. 1990, 33, 469–472. [DOI] [PubMed] [Google Scholar]
- 12. Cruwys, J. , Dinsdale, R. , Hawkes, F. , Hawkes, D. , Development of a static headspace gas chromatographic procedure for the routine analysis of volatile fatty acids in wastewaters. J. Chromatogr. A 2002, 945, 195–209. [DOI] [PubMed] [Google Scholar]
- 13. Canale, A. , Valente, M. E. , Ciotti, A. , Determination of volatile carboxylic acids (C1–C5i) and lactic acid in aqueous acid extracts of silage by high performance liquid chromatography. J. Sci. Food Agric. 1984, 35, 1178–1182. [Google Scholar]
- 14. Falk, H. M. , Reichling, P. , Andersen, C. , Benz, R. , Online monitoring of concentration and dynamics of volatile fatty acids in anaerobic digestion processes with mid‐infrared spectroscopy. Bioprocess. Biosys. Eng. 2015, 38, 237–249. [DOI] [PubMed] [Google Scholar]
- 15. Lahav, O. , Morgan, B. E. , Loewenthal, R. E. , Rapid, simple, and accurate method for measurement of VFA and carbonate alkalinity in anaerobic reactors. Environ. Sci. Technol. 2002, 36, 2736–2741. [DOI] [PubMed] [Google Scholar]
- 16. Probst, M. , Walde, J. , Pümpel, T. , Wagner, A. O. , Insam, H. , A closed loop for municipal organic solid waste by lactic acid fermentation. Bioresour. Technol. 2014, 175, 142–151. [DOI] [PubMed] [Google Scholar]
- 17. Lins, P. , Malin, C. , Wagner, A. O. , Illmer, P. , Reduction of accumulated volatile fatty acids by an acetate‐degrading enrichment culture. FEMS Microbiol. Ecol. 2010, 71, 469–478. [DOI] [PubMed] [Google Scholar]
- 18. Wagner, A. O. , Hohlbrugger, P. , Lins, P. , Illmer, P. , Effects of different nitrogen sources on the biogas production—a lab‐scale investigation. Microbiol. Res. 2012, 167, 630–636. [DOI] [PubMed] [Google Scholar]
- 19. Lins, P. , Schwarzenauer, T. , Reitschuler, C. , Wagner, A. O. , Illmer, P. , Methanogenic potential of formate in thermophilic anaerobic digestion. Waste Manag. Res. 2012, 30, 1031–1040. [DOI] [PubMed] [Google Scholar]
- 20. Schink, B. , Stams, A. J. M. , Synthrophism among procaryotes, in: Dworkin M., Falkow S., Rosenberg E., Schleifer K.‐H., Stackebrandt E. (eds.), The Prokaryotes. Springer, New York, Berlin, Heidelberg: 2006. [Google Scholar]
- 21. Ibrahim, V. , Hey, T. , Jönsson, K. , Determining short chain fatty acids in sewage sludge hydrolysate: A comparison of three analytical methods and investigation of sample storage effects. J. Environ. Sci. 2014, 26, 926–933. [DOI] [PubMed] [Google Scholar]
- 22. Lloret, S. M. , Andrés, J. V. , Legua, C. M. , Falcó, P. C. , Determination of ammonia and primary amine compounds and Kjeldahl nitrogen in water samples with a modified Roth's fluorimetric method. Talanta 2005, 65, 869–875. [DOI] [PubMed] [Google Scholar]
- 23. Schinner F., Öhlinger R., Kandeler E., Margesin R. (Eds.), Methods in Soil Biology. Springer, Berlin Heidelberg: 1996. [Google Scholar]
- 24. Bradford, M. M. , A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Anal. Biochem. 1976, 72, 248–254. [DOI] [PubMed] [Google Scholar]


