Abstract
Anaerobic conversion of carbohydrates can generate various end‐products. Besides physical parameters such as pH and temperature, the types of carbohydrate being fermented influences the fermentation pattern. Under uncontrolled pH, microbial mixed cultures from activated sludge and anaerobic digester sludge anaerobically produced ethanol from glucose while producing lactic acid from starch conversion. This trend was not only observed in batch trials. Also, continuous chemostat operation of anaerobic digester sludge resulted in the reproducible predominance of ethanol fermentation from glucose solution and lactic acid production from starch. Different feeding regimes and substrate availability (shock load versus continuous feeding) in glucose fermentation under non‐controlled pH did not affect the ethanol production as the major end product. Shifts in feed composition from glucose to starch and vice versa result in an immediate change of fermentation end products formation.
Keywords: Anaerobic acidification, Ethanol, Glucose, Lactic acid, Starch
Abbreviations
- FW
food wastes
- LA
lactic acid
- VFA
volatile fatty acids
1. Introduction
The anaerobic conversion of carbohydrates is important in a number of different industries for example alcohol and biochemical production, food preservation and food waste conversion to bioenergy and bio‐products. The formation of end‐products generated from carbohydrate fermentation is highly dependent on several factors including the microorganisms present in the fermentation reaction, the types of substrate being fermented, and the environmental conditions applied such as temperature and alkalinity 1, 2. The carbohydrates in our daily meals typically consist of starch such as in rice, wheat and potato, and soluble sugars such as glucose and fructose in fruit. Knowledge about the effect of the type of glucose versus starch on the outcome of microbial fermentations can hence be potentially useful for industrial fermentation systems.
The food wastes (FW) are considered as the main contributors for municipal solid wastes in the developed as well as developing worlds, and they reflect a challenge regarding disposal problems. Since FW typically contains a significant amount of carbon and high moisture content, some biological waste treatment technologies can be applied to deal with the issues. The biological conversion of FW can be done by either composting or anaerobic processes. Because of the costs of oxygen transfer, odour emission and other problems, anaerobic digestion is the preferred process for food waste conversion. Anaerobic digestion can be carried out as a single process but for food waste conversion an acid stage step is typically used before the anaerobic digestion step 3, 4.
Since FW contains a significant amount of polysaccharides including starch and certain oligosaccharides, it can be a promising raw material for producing valuable products (bioenergy and bio‐products) via fermentation process 5, 6. The anaerobic acid stage fermentation can produce organic acids, alcohols and hydrogen gas as typical fermentation products 6, 7. Some of these products are considered useful for the subsequent anaerobic digestion while others (e.g. propionic acid) are less desired 8. However, fermentation end products from anaerobic acid stage fermentation of food waste carbohydrates can also be useful end products in their own right, for example ethanol and hydrogen as potential fuels, or lactic acid (LA) as a precursor for bioplastic production 9, 10, 11.
In anaerobic acid stage fermentation, complex organic materials are firstly converted to soluble substances such as sugars, amino acids and fatty acids via hydrolysis process. This process is carried out and accelerated by extracellular enzymes. The major end products generated from the anaerobic acid stage fermentation are volatile fatty acids (VFA), alcohols, and gases (e.g. hydrogen and carbon dioxide) 12, 13, 14. Two further types of metabolic pathways that can occur in anaerobic acid stage fermentation include alcohols and mixed acid types 15. In two‐phase anaerobic digestion process, lactic acid is an intermediate product that could be generated from the acid stage fermentation or acidogenesis phase, and it would be converted into VFA (e.g. acetate and propionate) prior to the formation methane as the end product 16. In the anaerobic acid stage fermentation, 2 mol of lactic acid could be produced from the oxidation of 1 mol of glucose. Also, fermentation of 1 mol glucose could produce 2 mol of ethanol as the end‐product.
Since both ethanol and lactic acid are valuable products that can be generated from fermentation of carbohydrates, exploring conditions that favour the production of either of the product is of potential industrial significance. For example the development of ethanol producers during lactic acid production 17, and the contamination of ethanolic fermentations by lactic acid bacteria are well known problems in the industry 18, 19. Thus, the questions addressed in this study are clearly relevant for processes such as anaerobic acid stage fermentation of wastes, dairy fermentation, technical lactic acid or ethanol production, and food and beverage fermentations.
The aim of the current paper is to study the effect of operating conditions and carbohydrate composition on the fermentation end products in both batch and chemostat trials using generic aerobic (activated sludge) and anaerobic (anaerobic sewage sludge digester) under uncontrolled pH.
2. Materials and methods
2.1. Anaerobic inocula
The inocula used for this experiment were anaerobic digestion and activated sludge derived from the Woodman Point and Subiaco Wastewater Treatment Plant in Perth city, Western Australia. These inocula were stored overnight in the fridge at 4°C prior to the start of the experiments in order to avoid evaporation of organics 20, and to slow down as well as inactivate microbial activity in the sludge 21.
2.2. Medium composition
Each substrate loaded into the reactor was mixed with the medium solution. The medium solution used was composed of 125 mg/L NaHCO3, 44 mg/L KH2PO4, 160 mg/L NH4Cl, 25 mg/L MgSO4·7H2O and 1 g/L Bacto‐yeast extract, 1 g/L Bacto‐peptone, 1.25 ml/L of trace element solution, which contained (g/L): ethylene‐diamine‐tetra‐acetic acid (EDTA) 15, ZnSO4·7H2O 0.43, CoCl2·6H2O 0.24, MnCl2·4H2O 0.99, CuSO4·5H2O 0.25, NaMoO4·2H2O 0.22, NiCl2·6H2O 0.19, NaSeO4·10H2O 0.21, H3BO4 0.014 and NaWO4·2H2O 0.050.
2.3. Reactor operation and experimental design
2.3.1. Batch experiment
For batch tests, glass reactors with 80 mL working volume were used as anaerobic completely mixed batch reactors. The reactors were sealed with rubber stoppers fitted with sample ports. The temperature was maintained at 35 ± 0.5°C using automatic thermostatic water bath, and the reactors were completely mixed using magnetic stirrer at a constant 120 rpm.
2.3.2. Investigation of glucose and starch fermentation in the mixed culture
To assess as well as compare the nature of anaerobic acid stage fermentation process of glucose and starch at non‐sterile mixed cultures, investigations were carried out in batch tests. Two reactors inoculated with 10% anaerobic sludge, were added with 40 g/L of starch and 40 g/L of glucose. Other two reactors inoculated with 10% activated sludge, were also added with 40 g/L of starch and 40 g/L of glucose. The starch used for all tests in this study was a boiled rice flour starch. The rice flour starch was a commercial rice flour product (88% TS) produced by the Rice Product Refining Company under the trade name of Erawan Rice Flour. It consisted of untreated starch with no preservative and was sold by Erawan Marketing Co., Ltd. (Bangkok, Thailand). Calculation of the end product results was based on the glucose equivalent of starch as each gram of starch theoretically can be converted into 1.11 g of glucose 22.
2.3.3. Fermentation of the mixtures of glucose and starch
To evaluate the effects of different composition of starch and glucose on the organic end product formation, fermentation of the mixtures of glucose and starch was carried out in batch system. In this test, each reactor was inoculated with 10% anaerobic sludge. The first reactor was added with the mixture of 50% glucose (20 g/L) and 50% starch (20 g/L), and the second reactor was loaded with the mixture of 75% glucose (30 g/L) and 25% starch (10 g/L). The total carbohydrate concentration applied in was 40 g/L.
2.3.4. Chemostat system
Two cylindrical 300 mL computer controlled glass reactors were used for the chemostat system. The total working volume of each reactor was 200 mL. The dimensions of each reactor were 9.5 cm height and 6 cm diameter. Each reactor was continuously stirred with 120 rpm and maintained at the temperature of 35 ± 0.5°C. ORP and pH probes were installed to the reactors. The data measured was recorded into a spreadsheet using a LabJack U12 data acquisition card and the process control software LabVIEW™ (version7.1 National Instrument). Feeding and wasting was set up based on the feeding type applied using peristaltic pump (Master Flex, Console Drive, Model 77201–62, Cole‐Parmer Instrument Company), meaning that feeding as well as wasting was carried out at the same time once the conditions of the culture meet the particular logic applied in the computer program.
2.3.5. Chemostat process on the acid stage fermentation of starch and glucose
Two identical reactors were operated in the chemostat system for acid stage fermentation process with hydraulic retention time (HRT) of 4 days. The first reactor was fed with 12.5 mL every 6 h indicating that substrate loaded into the bioreactor was four times a day. The second reactor was fed continuously with the total flow rate of each reactor at 50 mL/day. The flow rate of 50 mL/day was based on the culture volume of 200 mL divided by 4 day of HRT. The dilution rate applied in this chemostat experiment was 0.25 day−1 that was based on the medium flow rate of 50 mL/day divided by the culture volume of 200 mL. The main substrate used for starch fermentation in the chemostat system was starch with the concentration of 40 g/L. Medium solution along with a 10% anaerobic sludge as inoculum was used for enhancing the start‐up process. No alkaline as well as acid solution used for controlling pH.
For glucose fermentation in chemostat, the first step carried out was to feed each reactor with 100 g/L glucose along with medium solution. Then, the experiment was repeated by reducing glucose concentration to 5 g/L in order to confirm whether the same trends also occurred at the low feed glucose concentration. During this process, pH culture was not maintained; thus, no alkaline as well as acid solution was used.
2.3.6. Investigation of the swapped feeding method in the chemostat
Effects of changing substrates feeding on the on‐going anaerobic acid stage fermentation of glucose and starch were investigated via chemostat tests. Two identical reactors were run at chemostat system with hydraulic retention time (HRT) of 4 days. Total flow rate of each reactor was 50 mL/day. The first step, reactor 1 was fed with 100% starch (40 g/L), and reactor 2 was fed with the mixture of a 50% starch (20 g/L) and 50% glucose (20 g/L). After two cycle of HRT, reactor 1 was loaded with 100% glucose (40 g/L), and reactor 2 was fed with 100% starch (40 g/L). Then, after the next 2 cycle of HRT, reactor 1 was fed again with 100% starch (40 g/L), and reactor 2 was fed with 100% glucose (40 g/L). Prior to closing the sample gas port and running the process, each reactor was purged with the nitrogen gas for 5 min to get rid of the oxygen traces. During the process, pH was not controlled.
2.4. Analytical methods
Samples taken on a daily basis were immediately centrifuged at 15 000 rpm for 5 min. The centrates were then filtrated through a Millex GP with filter unit 0.22 μm Millipore express PES membranes, 150 psi (10 bars) of housing limit. The filtrates were transferred into 1.5 mL Eppendorf tubes and stored at 5°C up to 48 h prior to analysis. The fermentation product composition including VFAs (acetate, propionate, and butyrate), acetone, ethanol, butanol and lactic acid was analyzed by gas chromatography. To 1.2 mL of the standards/samples 300 μL formic acid (99%) was added. The lactic acid measurement was carried out by adding 480 μL of periodic acid (100 mM), and 300 μL formic acid (99%) to 720 μL of the standard as well as the sample 23.
An Agilent 7820A gas chromatograph (GC) with auto‐sampler, stainless steel injection block, and flame‐ionization detector was utilized. Injector and detector (FID) temperatures were set at 250 and 300°C. Hydrogen and air flow rates at the FID were 30 and 400 mL/min, respectively. The signal source from front signal FID generates data with the peak width of 5 Hz/0.04 min. A fused silica capillary column of an Altech ECONOCAP™ EC‐1 was used with the length of 30 m, inside diameter of 0.25 mm and film thickness of 0.25 μm. Nitrogen gas was used as the carrier gas with a flow rate of 1.2 mL/min and at the inlet sample was split 10:1. The injection volume was set at 0.4 μL. The run time was programmed at 11.667 min. The oven temperature was set as follows: initial temperature 50°C; held for 2 min; temperature ramp 75°C/min to 130°C; held for 5.0 min; temperature ramp 75°C/min to 250°C; held for 2 min 23.
3. Results
3.1. Characteristics of glucose and starch fermentation in the mixed culture
In order to test to what extend the type of carbohydrate influences the behavior of mixed microbial activities, two types of carbohydrates, sugar (glucose) and starch was added to anaerobic and aerobic sludge biomass, respectively (Supporting Information Fig. 1, Table 1). For both microbial cultures the main end‐product from glucose fermentation was ethanol, while starch fermentation led to lactic acid as the main fermentation end‐product. Because of the relatively low buffer capacity of 1.5 mM NaHCO3 (as alkalinity) the pH in all cultures dropped from around 7 to 3 within 2 days of incubation (Supporting Information Fig. 1). Ethanol accumulated to up to 330 mM from glucose fermentation, while lactic acid never reached more than 200 mM from starch fermentation. This suggested that conversion of starch was incomplete, possibly due to only partial hydrolysis of starch or inhibition due to acidification. In batch culture, independent of the origin of the microbial culture glucose led to ethanolic and starch to lactic fermentation. This finding is quite different to the study by Weaver et al. 24 revealing that glucose or starch fermentation inoculated with faecal suspension, produced VFAs (acetate, propionate and butyrate) as the major end product. However, they did not measure ethanol as well as lactic acid that could be possibly found in their tests.
Table 1.
Anaerobic acidification results among different carbohydrates
| Type of substrates | Substrate concentration (g/L) | Type of inoculum | Type of feeding | pH (After 48‐h incubation) | Main end‐product | Main end‐product concentration (mmol/L) |
|---|---|---|---|---|---|---|
| Glucose | 100 | Anaerobic sludge | Continuous | 4.78 | Ethanol | 96 |
| 100 | Anaerobic sludge | Shock load | 4.99 | Ethanol | 168.03 | |
| 5 | Anaerobic sludge | Continuous | 4.88 | Acetate | 11.58 | |
| 5 | Anaerobic sludge | Shock load | 5.52 | Ethanol | 16.51 | |
| 40 | Anaerobic sludge | Batch | 2.96 | Ethanol | 297 | |
| 40 | Activated sludge | Batch | 3.06 | Ethanol | 289.43 | |
| Starch | 40 | Anaerobic sludge | Batch | 3.64 | Lactic acid | 55.77 |
| 40 | Activated sludge | Batch | 3.53 | Lactic acid | 34.2 | |
| 40 | Anaerobic sludge | Continuous | 4.13 | Lactic acid | 56 | |
| 40 | Anaerobic sludge | Shock load | 4.37 | Lactic acid | 70 |
3.2. Fermentation of the mixtures of glucose and starch
In general substrates in food waste fermentation comprise a mixture of glucose and starch. To test whether the above described trends also occur in the mixtures, a series of batch fermentation trials using different ratios of glucose and starch were tested (Fig. 1A and B). Mixtures of 50% starch and 50% glucose led to lactic acid as the main end product (57%) with significant amounts of ethanol (30%) produced as the second most abundant end product (Fig. 1). A ratio of 75% glucose and 25% starch, resulted in approximately equal amounts of lactic acid and ethanol produced (Fig. 1B). Results suggest that high glucose concentration in the broth stimulate ethanol producers despite the fact that lactic acid bacteria also can use glucose to form lactic acid.
Figure 1.
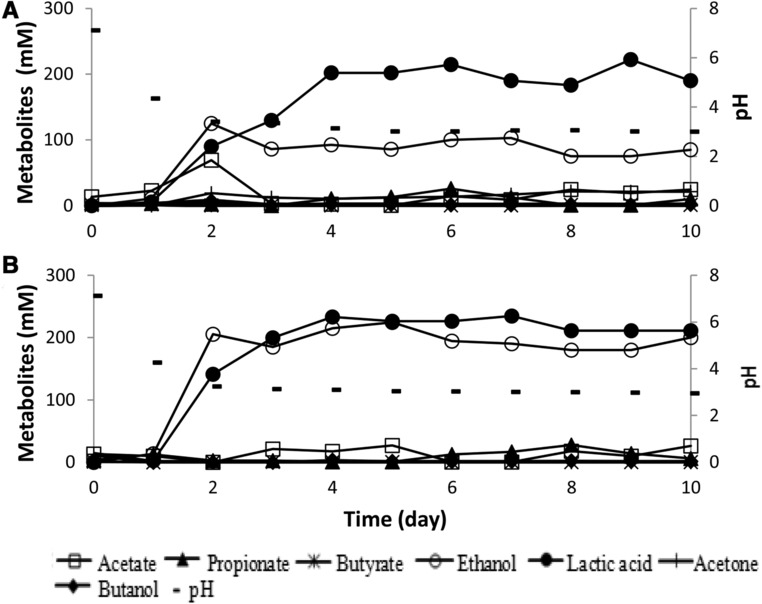
Organic end product formation of batch digester under the different composition of substrates inoculated with anaerobic sludge as a mixed culture: (A) 50% glucose and 50% starch, (B) 75% glucose and 25% starch. Total carbohydrates used were 40 g/L (222 mM glucose equivalent).
3.3. Chemostat tests for starch fermentation
The results from batch test showed the tendency of lactic fermentation from starch and ethanolic fermentation from glucose, even if the substrates were presented as mixtures. However, in many real world carbohydrates fermenting systems, such as anaerobic digesters, and acid stage reactors there is a continuous supply of food. In order to test to what extend the tested substrates also encourage the development of a stable culture in continuously fed operation, the substrates were tested in computer controlled chemostats inoculated with 10% of anaerobic digester sludge. Chemostats were operated at hydraulic retention times of 4 days and operated for 16 days to allow the development of a steady state enrichment culture.
To test whether the type of feeding regime influences the development of mixed microbial activities during starch fermentation, continuous feeding, and 6 hourly shocks loads was applied to the anaerobic digester inoculum (Supporting Information Fig. 2, Table 1). Both reactors selected primarily for lactic acid formation, leading to acidification and pH drops from 7 to 3 (Supporting Information Fig. 2). The lactic acid production yields were 0.53 and 0.48 mmol/mmol glucose equivalent (0.27 and 0.25 g/g starch) for of shock loads and continuous cultures respectively. This shows only 25% of starch was converted to the lactic acid. The low conversion yield could be due to the low pH in the broth that is known to become inhibitory already at a pH of around 5 25, 26. As revealed in batch and chemostat experiments (Supporting Information Fig. 1B, D and Supporting Information Fig. 2), the hydrolysis of starch was inhibited at low pH, and thereby would stop fermentation to form metabolites. This occurred as under acidic condition (pH < 5.0) the activity of enzymes involved in starch fermentation was limited 26, 27 in which at low pH the activity of amylase to hydrolyse polymeric carbohydrate into soluble carbohydrate was inhibited, and also hydrolysis of maltose (the sugar obtained from the digestion of starch) into glucose by maltase enzyme was hindered under acidic culture (pH < 5.0) [27, 28].
These chemostat trials reaffirmed our batch experimental results confirming that lactic acid was the main product from starch fermentation, independent of the different type of feeding regimes. Previous work showed that fermentation of starch produced VFA and alcohols 29. The difference could lie in the pH control (around 4.5) used by the authors. Further, the study also did not conduct lactic acid analysis that could possibly be present in their starch fermentation broth as well.
3.4. Chemostat tests for glucose fermentation
The results from batch test showed ethanol as the main end‐product from glucose fermentation. To investigate the effect of glucose feed on the medium term selection of fermentative organisms computer controlled chemostats were established. Long‐term glucose fermentation was tested for different feeding regimes (continuous and 6 hourly shock loads). Results showed that both feeding regimes selected for the development of ethanol fermenting microbes with ethanol representing more than 95% of end products. Despite the fact that the pH was even lower than during lactic fermentation from starch the conversion of carbohydrate to ethanol was more complete. Ethanol production yields were higher than for starch with about 1.5 mol ethanol/mol glucose and productivity was 520 and 400 mmol/(L d) for shock and continuous loading respectively (Supporting Information Fig. 3A and B, Table 1).
Results showed that the pH for ethanol fermentation was lower than lactic acid fermentation. This was due to the fact that the ethanol producing culture fed with glucose was not inhibited at low pH (pH<4.0), and the low pH could trigger the production of ethanol 30. Therefore, when the ethanol was continuously produced from the glucose fermenting culture, other acids such as acetic acid and carbonic acid would continuously be generated during the anaerobic process in the closed or batch system as well as continuous operation (Supporting Information Fig. 1, 2B and 3B). Those acids would acidify the culture and contribute to increase proton concentration in the fermentation broth that may lower pH culture deeper. This phenomenon was different from the lactic acid fermentation in which the lactic acid producing culture fed with starch was inhibited when pH culture dropped from 5.0 to 4.0. This occurred as starch may not be completely degraded when pH culture was too acidic 27. The low pH may prevent the formation of other metabolites or organic acids that may also contribute to increase proton concentration and/or lower pH more deeply.
A repeat experiment with lower feed glucose (5 g/L) confirmed that glucose feed selected for ethanol producing microbes (Table 1, Supporting Information Fig. 4A and B). The change of soluble carbohydrate concentration during the fermentation was depicted in Supporting Information Fig. 5. Results showed that the soluble carbohydrate (5 g/L) was converted into ethanol (39.3 mM) as the main metabolite, and the leftover of soluble carbohydrate within 12 h of incubation was 2.44 mM (Supporting Information Fig. S5). Further, results also revealed that during the process, pH in both cultures dropped from 7 to 3 (Supporting Information Fig. S4A and B). This indicated that ethanol producers were not suppressed by an extreme sour condition in the reactor. This is also in agreement with the study by Arroyo‐López, et al. 31 finding that yeast as ethanol producers still can grow at low pH between 2.8 and 3.2 as long as the cells adaptation to the media was accomplished 32.
3.5. Effect of glucose and starch feeding on the shift of the end product formation
In order to determine whether the preferred end products formed in continuous operation was due to the long term development of a particular culture (e.g. Lactic acid bacteria versus yeasts) or due to the carbohydrate type triggering a specific fermentation pathway, step changes in the chemostat operation were carried out. Two reactors were set up, the first reactor was initially fed with 100% starch (40 g/L) and the second reactor initially fed with a 50% starch and 50% glucose (20 g/L each).
As observed before, the trial initially fed with 100% starch (Fig. 2A), resulted in lactic acid as the major end product accounting for approximately 60% of the total identified organic compounds. A sudden switch from starch (40 g/L) to glucose (40 g/L) feed on day 9 caused an immediate change in end product formation. Lactic acid dropped significantly from 100 to 40 mM while at the same time ethanol increased from 17 to a 108 mM (Fig. 2A). The lactic acid concentration went back to over 100 mM on day 13 (Fig. 2A) since lactic acid producers also can use glucose as a readily substrate (monomer or soluble sugar) to form lactic acid as the main end‐product. This phenomenon also could be seen in batch experiment (Fig. 1) in which the mixture of starch and glucose could stimulate the lactic acid producers to form lactic acid as the main metabolite. However, when glucose was fed to the reactor, it would stimulate the ethanol producers to dominate the fermentation process and form ethanol as the main metabolite (Fig. 2A). An increase of glucose concentration in the fermentation broth also could drive the ethanol producers to outcompete the lactic acid producers (Fig. 1B) to form ethanol as the end‐product.
Figure 2.
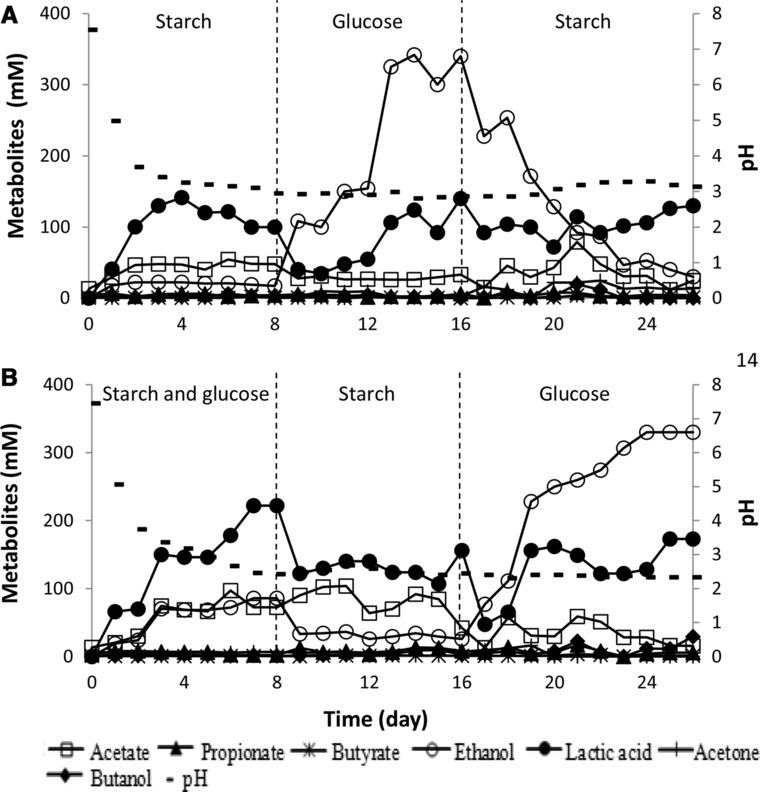
Profile of the metabolic shifts under conditions of uncontrolled pH and HRT 4 day with different substrate feeding: (A) started with starch feed and (B) started with a mixture of starch and glucose feed.
As depicted in Fig. 2A, when the feed was changed back to starch only on day 17, ethanol production immediately ceased and the fermentation pattern return to lactic acid dominated fermentation. In the chemostat that was initially fed with a mixture of 50% starch and 50% glucose (20 g/L each), the reactor produced a mixture of lactic acid, ethanol, and acetate. Again, switches to full starch feed and full glucose feed caused an immediate shift towards lactic or ethanolic fermentation, respectively (Fig. 2B).
The decrease of lactic acid production on day 9 when the feed was changed from the mixture of starch and glucose into pure starch was due to the fact that the starch cannot be converted and fermented completely under the acidic culture (Fig. 2B) in which at this stage pH culture has been lower than 3.0. This result reaffirmed the previous batch and continuous experiments, which revealed that starch fermentation was inhibited under the acidic condition (pH < 4.0) (Supporting Information Fig. 1B and D, Supporting Information Fig. 2A and B). Even if the concentration of lactic acid dropped on day 9, ethanol formation was still lower than the lactic acid production. This indicated that the starch fermentation induced the formation of lactic acid as the main metabolite rather than the ethanol production. These results suggest that the change in feed triggers a sudden change in metabolism rather than a gradual change in population.
4. Discussion
Results from this study suggest that the readily available monosaccharide glucose is effectively used by ethanolic fermenting microbes that possibly out‐compete lactic acid bacteria, in particular at low pH values. Interestingly the trend is reversed with starch as the feed. It is established that lactic acid bacteria are effective starch degraders with the essential enzymes amylase and maltase 33, 34, 35, while many yeasts such as Saccharomyces are not effective starch degraders 36. Some previous studies also found that many types of yeast are not able to degrade starch due to the fact that they are not able to produce extracellular amylase 37. The results suggest that the extracellular hydrolysis of starch initiated by lactic bacteria does not free sugars at sufficiently high concentration in the bulk solution to become a substrate for ethanolic yeasts. Hence the starch hydrolysis by bacteria must be in the juxtaposition between cells and insoluble starch, such that hydrolysed sugars are principally available to the hydrolysing cells.
Some previous studies reported that lactic acid can be dominant in the undefined mixed culture when high concentrations of an easily degradable substrate are available 38, 39. The authors found that once the lactic acid is produced it will inhibit all microorganisms including ethanol and VFA producers since lactic acid bacteria can drive the pH into an extremely low pH and suppress the growth of other anaerobic microorganisms. However, our current results revealed that ethanol producers also can survive at an extremely low pH as long as a high amount of glucose was available in the fermentation broth. A possible reason for differences in results obtained could be the fact that the authors provided only low concentrations glucose (2–3.5 g/L) and that pH was controlled between 4.7 to 6.7 38, 39.
5. Concluding remarks
The anaerobic acid stage fermentation under uncontrolled pH using natural undefined mixed inocula revealed that substrate containing glucose tended to form ethanol while lactic acid tended to be formed from starch fermentation. Different feeding regimes and substrate availability (shock load versus continuous feeding) in glucose fermentation under non‐controlled pH did not affect the ethanol production as the major end product. Shifts in feed composition from glucose to starch and vice versa result in an immediate change of fermentation patterns. This is of significance in industrial fermentation systems.
The authors have declared no conflict of interest.
Supporting information
Supporting material
Acknowledgments
The authors acknowledge financial support received from the Directorate General of Higher Education, Ministry of Research, Technology and Higher Education of Indonesia (RISTEKDIKTI) (Guarantee Letter No: 403/E4.4/K/2014).
6 References
- 1. Hwang, M. H. , Jang, N. J. , Hyun, S. H. , Kim, I. S. , Anaerobic bio‐hydrogen production from ethanol fermentation: the role of pH. J. Biotechnol. 2004, 111, 297–309. [DOI] [PubMed] [Google Scholar]
- 2. Kim, J. K. , Oh, B. R. , Chun, Y. N. , Kim, S. W. , Effects of temperature and hydraulic retention time on anaerobic digestion of food waste. J. Biosci. Bioeng. 2006, 102, 328–332 [DOI] [PubMed] [Google Scholar]
- 3. Liu, D. , Liu, D. , Zeng, R. J. , Angelidaki, I. , Hydrogen and methane production from household solid waste in the two‐stage fermentation process. Water Res. 2006, 40, 2230–2236. [DOI] [PubMed] [Google Scholar]
- 4. Bo, Z. , Pin‐jing, H. , Performance assessment of two‐stage anaerobic digestion of kitchen wastes. Environ. Technol. 2014, 35, 1277–1285. [DOI] [PubMed] [Google Scholar]
- 5. Ohkouchi, Y. , Inoue, Y. , Direct production of L (+)‐lactic acid from starch and food wastes using Lactobacillus manihotivorans LMG18011. Bioresour. Technol. 2006, 97, 1554–1562. [DOI] [PubMed] [Google Scholar]
- 6. Zhang, C. , Su, H. , Baeyens, J. , Tan, T. , Reviewing the anaerobic digestion of food waste for biogas production. Renew Sus. Energy Rev. 2014, 38, 383–392. [Google Scholar]
- 7. Panichnumsin, P. , Nopharatana, A. , Ahring, B. , Chaiprasert, P. , Enhanced biomethanation in co‐digestion of Cassava pulp and pig manure using a two‐phase anaerobic system. J. Sus. Energy Environ. 2012, 3, 73–79. [Google Scholar]
- 8. Ren, N. , Wang, B. , Huang, J. C. , Ethanol‐type fermentation from carbohydrate in high rate acidogenic reactor. Biotechnol. Bioeng. 1997, 54, 428–433. [DOI] [PubMed] [Google Scholar]
- 9. RedCorn, R. , Engelberth, A. S. , Identifying conditions to optimize lactic acid production from food waste co‐digested with primary sludge. Biochem. Eng. J. 2016, 105, 205–213. [Google Scholar]
- 10. Datta, R. , Henry, M. , Lactic acid: recent advances in products, processes and technologies—a review. J. Chem. Technol. Biotechnol. 2006, 81, 1119–1129. [Google Scholar]
- 11. Liang, S. , McDonald, A. G. , Coats, E. R. , Lactic acid production from potato peel waste by anaerobic sequencing batch fermentation using undefined mixed culture. Waste Manage. 2015, 45, 51–56. [DOI] [PubMed] [Google Scholar]
- 12. Yu, H. G. , Fang, H. H. , Acidogenesis of dairy wastewater at various pH levels. Water Sci. Technol. 2002, 45, 201–206. [PubMed] [Google Scholar]
- 13. Ahring, B. K. , Sandberg, M. , Angelidaki, I. , Volatile fatty acids as indicators of process imbalance in anaerobic digestors. Appl. Microbiol. Biotechnol. 1995, 43, 559–565. [Google Scholar]
- 14. Lin, C. Y. , Chang, R. C. , Hydrogen production during the anaerobic acidogenic conversion of glucose. J. Chem. Technol. Biotechnol. 1999, 74, 498–500. [Google Scholar]
- 15. Thauer, R. K. , Jungermann, K. , Decker, K. , Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977, 41, 100–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pipyn, P. , Verstraete, W. , Lactate and ethanol as intermediates in two‐phase anaerobic digestion. Biotechnol. Bioeng. 1981, 23, 1145–1154. [Google Scholar]
- 17. Gobbetti, M , Corsetti, A , Rossi, J. , The sourdough microflora. Interactions between lactic acid bacteria and yeasts: metabolism of carbohydrates. Appl. Microbiol. Biotechnol. 1994, 41, 456–460. [DOI] [PubMed] [Google Scholar]
- 18. Makanjuola, D. B. , Tymon, A. , Springham, D. G. , Some effects of lactic acid bacteria on laboratory‐scale yeast fermentations. Enzyme Microbial. Technol. 1992, 14, 350–357. [Google Scholar]
- 19. Bayrock, D. P. , Ingledew, W. M. , Inhibition of yeast by lactic acid bacteria in continuous culture: nutrient depletion and/or acid toxicity?. J. Ind. Microbiol. Biotechnol. 2004, 31, 362–368. [DOI] [PubMed] [Google Scholar]
- 20. Eskicioglu, C. , Kennedy, K. J. , Droste, R. L. , Enhanced disinfection and methane production from sewage sludge by microwave irradiation. Desalination 2009, 248, 279–285. [Google Scholar]
- 21. Simpson, C. A. , Geornaras, I. , Yoon, Y. , Scanga, J. A. , Kendall, P. A. , Sofos, J. N. , Effect of inoculum preparation procedure and storage time and temperature on the fate of Listeria monocytogenes on inoculated salami. J. Food Protection 2008, 71, 494–501. [DOI] [PubMed] [Google Scholar]
- 22. Li, J. , Danao, M. G. C. , Chen, S. F. , Li, S. , Singh, V. , Brown, P. J. , Prediction of starch content and ethanol yields of sorghum grain using near infrared spectroscopy. J. Near Inf. Spectrosc. 2015, 23, 85–92. [Google Scholar]
- 23. Darwin, Charles W. , Cord‐Ruwisch R. Concurrent lactic and volatile fatty acid analysis of microbial fermentation samples by gas chromatography with heat pre‐treatment. J. Chromatogr. Sci. 2018, 56, 1–5. [DOI] [PubMed] [Google Scholar]
- 24. Weaver, G. A. , Krause, J. A. , Miller, T. L. , Wolin, M. J . Constancy of glucose and starch fermentations by two different human faecal microbial communities. Gut 1989, 30, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bertoft, E. , Andtfolk, C. , Kulp, S. E. , Effect of pH, temperature, and calcium ions on barley malt α‐amylase isoenzymes. J. Inst. Brew. 1984, 90, 298–302. [Google Scholar]
- 26. Stark, I. E. , Somogyi, M. , Note on the fermentation of maltose and glucose in alkaline solutions. J. Biol. Chem. 1942, 142, 579–584. [Google Scholar]
- 27. Santoyo, M. C. , Loiseau, G. , Sanoja, R. R. , Guyot, J. P. , Study of starch fermentation at low pH by Lactobacillus fermentum Ogi E1 reveals uncoupling between growth and α‐amylase production at pH 4.0. Int. J. Food Microbiol. 2003, 80, 77–87. [DOI] [PubMed] [Google Scholar]
- 28. Russell, J. R. , Young, A. W. , Jorgensen, N. A. , Effect of dietary corn starch intake on pancreatic amylase and intestinal maltase and pH in cattle. J. Anim. Sci. 1981, 52, 1177–1182. [DOI] [PubMed] [Google Scholar]
- 29. Guo, W. Q. , Ren, N. Q. , Chen, Z. B. , Liu, B. F. et al., Simultaneous biohydrogen production and starch wastewater treatment in an acidogenic expanded granular sludge bed reactor by mixed culture for long‐term operation. Int. J. Hydrog. Energy 2008, 33, 7397–7404. [Google Scholar]
- 30. Ganigué, R. , Sánchez‐Paredes, P. , Bañeras, L. , Colprim, J. , Low fermentation pH is a trigger to alcohol production, but a killer to chain elongation. Front. Microbiol. 2016, 7, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Arroyo‐López, F. N. , Orlić, S. , Querol, A. , Barrio, E. , Effects of temperature, pH and sugar concentration on the growth parameters of Saccharomyces cerevisiae, S. kudriavzevii and their interspecific hybrid. Int. J. Food Microbiol. 2009, 131, 120–127. [DOI] [PubMed] [Google Scholar]
- 32. López, F. N. A. , Quintana, M. D. , Fernández, G. , Use of the generalized z‐value concept to study the effects of temperature, NaCl concentration and pH on Pichia anomala, a yeast related to table olive fermentation. Int. J. Food Microbiol. 2006, 106, 45–51. [DOI] [PubMed] [Google Scholar]
- 33. Vishnu, C. , Naveena, B. J. , Altaf, M. D. , Venkateshwar, M. et al., Amylopullulanase A novel enzyme of L. amylophilus GV6 in direct fermentation of starch to L (+) lactic acid. Enz. Microb. Technol. 2006, 38, 545–550. [Google Scholar]
- 34. Ehrmann, M. A. , Vogel, R. F. , Maltose metabolism of Lactobacillus sanfranciscensis: cloning and heterologous expression of the key enzymes, maltose phosphorylase and phosphoglucomutase. FEMS Microbiol. Lett. 1998, 169, 81–86. [DOI] [PubMed] [Google Scholar]
- 35. Darwin, Barnes, A. , Cord‐Ruwisch, R. , In vitro rumen fermentation of soluble and non-soluble polymeric carbohydrates in relation to ruminal acidosis. Ann. Microbiol. 2018, 68, 1–8. [Google Scholar]
- 36. Lacerda, I. C. , Miranda, R. L. , Borelli, B. M. , Nunes, Á. C. et al., Lactic acid bacteria and yeasts associated with spontaneous fermentations during the production of sour cassava starch in Brazil. Int. J. Food Microbiol. 2005, 105, 213–219. [DOI] [PubMed] [Google Scholar]
- 37. Ampe, F. , ben Omar, N. , Moizan, C. , Wacher, C. et al., Polyphasic study of the spatial distribution of microorganisms in Mexican pozol, a fermented maize dough, demonstrates the need for cultivation‐independent methods to investigate traditional fermentations. Appl. Environ. Microbiol. 1999, 65, 5464–5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Russell, J. B. , Hino, T. , Regulation of lactate production in Streptococcus bovis: a spiraling effect that contributes to rumen acidosis. J. Dairy Sci. 1985, 68, 1712–1721. [DOI] [PubMed] [Google Scholar]
- 39. Owens, F. N. , Secrist, D. S. , Hill, W. J. , Gill, D. R. , Acidosis in cattle: a review. J. Anim. Sci. 1998, 76, 275–286. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting material


