Abstract
Highland barley is one of the most important industrial crops in Tibetan plateau. Previous research indicated that highland barley has many medical functions. In this work, the antibacterial abilities of highland barley were investigated. The protein solutions hydrolyzed by trypsin for 4 h exhibited the highest antibacterial activity. An antibacterial peptide, barleycin, was screened and purified by magnetic liposome extraction combining with the protein profiles of reversed‐phase high‐performance liquid chromatography (RP‐HPLC). Structure, characterization, and safety evaluation of barleycin were further investigated. Amino acids sequence was determined as Lys‐Ile‐Ile‐Ile‐Pro‐Pro‐Leu‐Phe‐His by N‐sequencing. Circular dichroism spectra indicated the a‐helix conformation of barleycin. The activity spectrum included Bacillus subtilis, Staphylcoccus aureus, Listeria innocua and Escherichia coli and the MICs were from 4 to 16 μg/mL. Safety evaluations with cytotoxicity and hemolytic suggested this antibacterial peptide could be considered as safe at MICs. Finally, mode of action of barleycin on sensitive cells was primarily studied. The results suggested the damage of cell membrane.
Keywords: Antibacterial peptide, Characterization, Highland barley, Magnetic liposome, Purification
1. Introduction
As the results of excessive use of antibiotics, there are more and more antibiotic bacteria emerging. The growing problem has resulted in a search for novel antibacterial alternatives. Antibacterial peptides mostly have the advantages of nontoxin, high antibacterial activity and good selectivity 1. Thus, they may be used as a kind of new anti‐microbial agents in food or medical industry 2.
Highland barley or Tibetan barley is the principal cereal cultivated on the Tibetan plateau. It is rich in starch and protein and is used mainly to make milk tea, tsampa, and alcohol. Many ancient Chinese medicine books recorded that the highland barley has many medical functions including antidiarrheal, antioxidants, anticancer and inhibiting cholesterol level and anti‐inflammatory 3, 4. Although antibacterial peptides have been reported in many resources 5, 6, there are no reports about isolation of antibacterial peptide from highland barley. As highland barley is one of the most important industrial crops in Tibetan plateau, deep developments of highland barley paly the significant role on the improvement of local economy.
The screening and isolation of antibacterial peptides from natural sources is a challenging task because these complex samples can contain varied ingredients 7, 8. Rapid and efficient methods to screen and separate antibacterial peptides from natural sources for safe application are thus needed. Selective interaction between antibacterial peptides and sensitive microbial cell membrane is the precondition and basis of antibacterial peptides to activate against sensitive cells 9, 10, 11. This principle could be used to isolate and purify antibacterial peptides. In this study, highland barley proteins were subjected to different enzymes to obtain the antibacterial fragments. Further, antibacterial peptide, barley, was screened and purified by an efficient two‐step method. Another essential objective of this study is characterization of this antibacterial peptide.
2. Materials and methods
2.1. Enzymatic hydrolysis of highland barley protein
After grounding to achieve the uniformity, 20 g of highland barley were dissolved in 100 mL HCl solution (0.01 M). The protein section of highland barley was extracted with 3‐fold volume ethanol solution as described in 12 and then subjected to hydrolysis by pepsin, trypsin, neutrase, papain, and alcalase, respectively, as described in 3. Each hydrolysis was performed for 1, 2, 3, 4, and 5 h. The hydrolysis was stopped by heating at 100°C for 10 min. The hydrolysate was clarified by centrifugation (5000 × g, 4°C, 15 min), and then the supernatant was 10‐fold concentrated to get the hydrolysis of highland barley protein. The spot‐on‐lawn method was applied to determine the antibacterial activity 13 with E.coli CICC 10300 as indicator stains. The concentration of proteins was determined by Bradford method 13.
2.2. Preparation of the magnetic nanoliposomes
Ferromagnetic Fe3O4 nanoparticles were prepared by hydrothermal method with 3.5 g FeCl3·6H2O, 2.3 g sodium acetate, 40 mL glycol and 40 mL oleic acid 14. Lipid of E. coli CICC 10300 was extracted with 1:2 (v/v) chloroform‐methanol, chloroform, and ultra‐pure water 15. Nanomagnetic liposome particles were prepared by the thin film dispersion method 16. Size, polydispersity index (PDI), and zeta potential were measured using Zeta sizer analysis equipment (DelsaMax PRO, Beckman, USA). Magnetic characterization was tested by an alternating gradient magnetometer (MicroMagTM Model 2900, Princeton Measurements Corporation, USA).
2.3. Screen and purification of antibacterial peptide from highland barley
The hydrolysate of highland barley protein was first filtered through 10 kDa cut‐off membranes (Millipore, Los Angeles, CA, USA) and then was dialyzed with 100 Da cut‐off membranes (Millipore). The magnetic liposome was equilibrated with PBS buffer (10 mM, pH 7.2) containing 50 mM NaCl. The hydrolysis of highland barley protein was shacked with the magnetic liposome. After 24 h of co‐culture, the mixture was centrifuged (10 000, 4°C, 20 min) and the supernatant was adjusted to the original volume. RP‐HPLC was used to detect the liquid chromatographic fingerprint of the supernatants of hydrolysate of highland barley protein solution before and after incubation with magnetic liposome. Chromatographic conditions: column: reversed‐phase C18 250 × 4.6 mM; column temperature: 25°C; sample volume: 10 μL; flow rate of mobile phase: 1 mL/min; mobile phase: solvent A containing aqueous solution of 0.1% (V/V) TFA; solvent B is the 80% (V/V) acetonitrile solution containing 0.1% (V/V) TFA; procedures: 0–30 min, 0–100% solvent B linear gradient elution. Detection wavelength: 215 nm. The different elution was collected and adjusted to the same concentration to test for the antibacterial activity with E.coli CICC 10300 as the indicator strain. The concentration of proteins was determined by Bradford method 13. The elution with the highest activity was further analyzed and purified by C18 reverse phase column (0.5 mL/min, min 0–40, 10–100% B).
2.4. Structural characterization of barleycin
Purified antibacterial peptide was tested by N‐amino acids sequence (Procise491, ABI, USA) in Bejing Huada Protein innovation Co. Ltd. Homology search of amino acid sequence was searched in NCBI (http://www.ncbi.nlm.nih.gov/). Physicochemical properties of barleycin were predicted by bioinformatics tool (ProtParam in Expasy ProtParam). CD spectrum was used to analyze the structure of barleycin. Software Hyperchem 8.0 was used to calculate and predict the 3D model of barleycin structure under the lowest energy state.
2.5. Antibacterial activity of barleycin
Activity spectrum of barleycin (100 μg/mL) was determined with the selected indicator strains within Table 1. Minimum inhibitory concentrations (MICs) were defined as the lowest barleycin concentration that completely inhibited the growth of bacteria 13 and determined by testing the OD600 of bacteria suspensions treated with different dilutions of barleycin.
Table 1.
Entrapment encapsulation (EE), size, polydispersity (PDI) and zeta potential of the magnetic liposomes
| EE% | Size (nm) | PDI | Zeta potential (mV) | MS (emu/g) |
|---|---|---|---|---|
| 94.28 | 271.5 ± 8.9 | 0.33 ± 0.04 | ‐49.2 ± 3.2 | 21.7 ± 2.8 |
2.6. Hemolytic test n
Hemolytic test of barleycin on the human red blood cells (provided by Shaanxi human hospital, Shaanxi, China) with PBS (10 mM, pH 7.2) as negative control and 0.1% Triton X‐100 used as the positive control. Cytotoxicity test (MTT test) on hepatocyte and renal cells of human beings (HL7702 and HREpiC from ATCC) was performed.
2.7. Leakages of cytoplasmic contents of Escherichia coli
Mid‐exponential phrase cells of E.coli CICC 10300 were washed and re‐suspended in sterile saline water. Barleycin at 2 MIC was added in. Changes in K+ out, OD260 and OD 280 were recorded as previous study 13. Sample without the barleycin was used as the control.
2.8. Statistics
SPSS 18.0 software was used to analyze date. All data were averagely represented for three times and are shown as mean ± standard deviation.
3. Results and discussion
3.1. Antibacterial activity of highland barley hydrolysates
Fragments hydrolyzed by trypsin for 4 h exhibited the highest antibacterial activity (Fig. 1). Hydrolysis time influences the cleavage degree of the peptide chain. The fact that the pepsin 4‐h treatment of hydrolysate suggested the highest antibacterial activity was a cooperative result of amino acid composition and structures. Similar results were reported by other researchers 3, 12, 17. In the following experiments, highland barley fragments hydrolyzed by pepsin for 4 h were used for antibacterial peptide purification in the following experiments.
Figure 1.
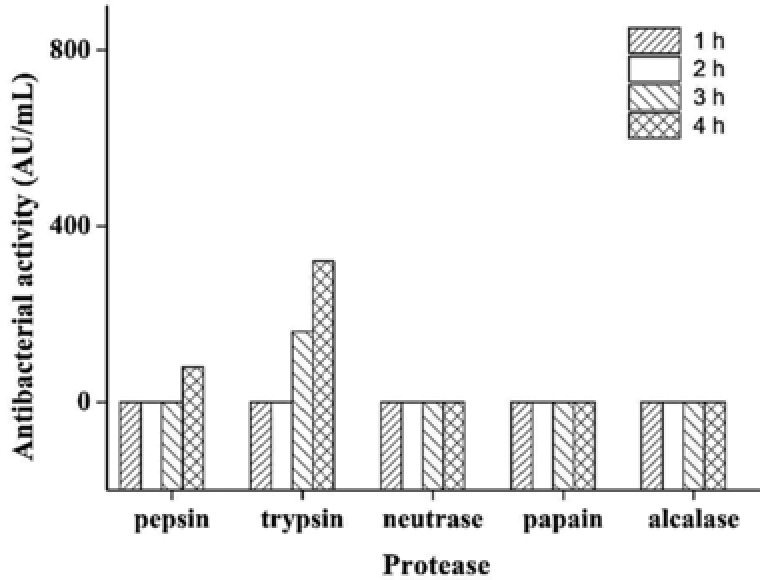
Antibacterial activities of highland barley fragments hydrolyzed by different enzymes for 4 h.
3.2. Characterization of magnetic nanoliposome
The characterizations of magnetic nanoliposome were investigated (Table 1). The encapsulation efficiency was 96.73%. The encapsulation efficiency normally ranged from 80 to 100% 16, 17, 18. The average diameter of magnetic liposome was 271.5 nm and a polydispersity index of 0.33, indicating a low liposome polydispersity 18. Nanoliposome showed a zeta potential of ‐49.2 mV. High zeta‐potential means more stability of liposome because the repulsive interactions would increase, reducing the frequency of liposome collisions 18. The saturation magnetization (MS) was 21.7 emu/g and the superparamagnetism was observed. This means that when the external magnetic field was removed, the magnetization becomes 0. It is very important for application in magnetic separation process 19.
3.3. Purification of barleycin
After adsorption, the peaks 1, 2, 3, and 4 almost disappeared. The peak area and retention time of other peaks before and after adsorption were consistent (Fig. 2A). Peak 4 showed the highest antibacterial activity. In order to verify the method of target direction, experiments also detected the antibacterial effect of the peak which had no change in peak shape and peak area and these peaks showed no antibacterial activity, indicating that this method had better selection and targeting. It was able to quickly locate the potential antibacterial components in chromatographic fingerprints.
Figure 2.
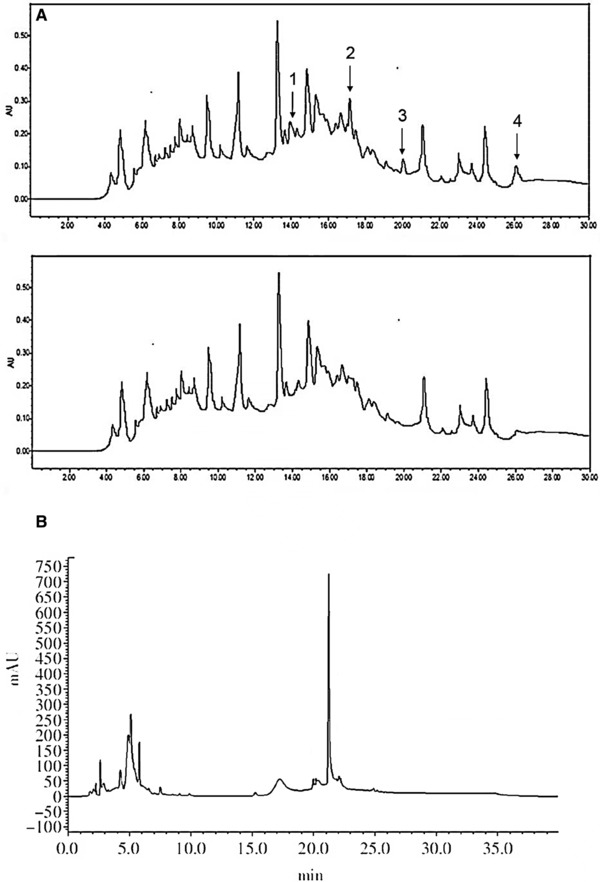
RP‐HPLC fingerprint profiles of highland barley hydrolysates (A) before and (B) after extraction by magnetic liposome solid‐phase.
Traditional methods of peptide purification are always more than three steps including organic solvent extraction, gel filtration chromatography, and RP‐HPLC etc. 20, 21, 22, 23. For example, Martinez et al. used n‐propanol extraction, ion‐exchange chromatography, and HPLC to get two antibacterial peptides 24. The drawbacks are too complex with loss of the activities. In this study, the magnetic liposome adsorptions combined with protein profiles of RP‐HPLC are more convenient and efficient.
3.4. Structural characterization of barleycin
The amino acid sequence of the nine amino acids was successfully identified as Lys‐Ile‐Ile‐Ile‐Pro‐Pro‐Leu‐Phe‐His. The molecular mass is calculated as 1077.38Da. A similarity search was performed for comparing the sequence of amino acids. The result suggested “no hits found” (http://web.expasy.org/blast/). The antibacterial peptide hence is safely assigned as a novel peptide and named barleycin. The theoretical isoelectric point is 8.76. Total number of positively charged residues is 1. The positive charge of antibacterial peptide played an important role on the mode of action 5, 25. Bovicin HC5 was reported to two positive charges, which played an important role in interacting with the cell membrane of the sensitive bacteria 10. Aliphatic index is 173.33. Grand average of hydropathicity (GRAVY) is 1.089 > 0, indicating it is a hydrophobic peptide. Antibacterial peptides usually have one or more hydrophobic centers, and the mechanism of antibacterial peptides is related to its hydrophobic nature 24, 26, 27. The minimum energy state structure (Energy = ‐9.843087; Gradient = 0.096196) of barleycin indicated that it is in a‐helix conformation (Fig. 3A).This conformation was consistent with the results of CD spectrum (Fig. 3B).This well‐defined conformation is good in protecting against some adverse conditions in the environment 10, 12. Tang et al. reported that MDpep9 is in a‐helix which is very stable at the acid environment as well as Pediocin 34 which is also in the a‐helix conformation 2, 15.
Figure 3.
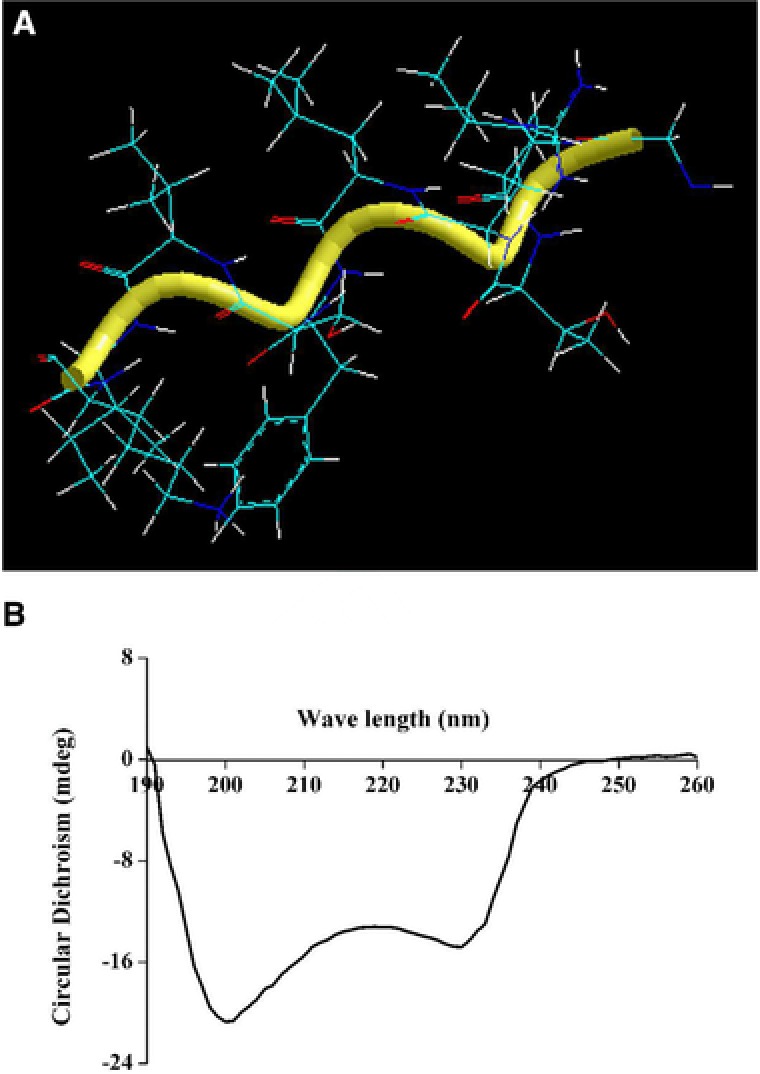
Structure of barleycin. (A) The three‐dimensional structure of barleycin predicated with Hyperchem 8.0 software; (B) CD spectrum of barleycin.
3.5. Antibacterial activities and characterizations of barleycin
The activity spectrum and MIC of barleycin were listed in Table 2. Barleycin had inhibitory activities toward Bacillus subtilis, Staphylcoccus aureus, Listeria innocua, E. coli, and Shigella dysenteriae. However, barleycin was not able to inhibit the growth of fungus, such as Penicillium italicum and Saccharomyces cerevisiae. The MICs were from 4 to 16 μg/mL which were low. Heat and freeze‐thaw treatment did not affect the activity of barleycin and it was effective within the pH range 3–10.
Table 2.
Characterizations of barleycin
| Conditions | Activity | MIC (μg/mL) |
|---|---|---|
| Bacillus subtilis (CICC 10034,CICC 10732, CICC 10012) | 3/3 | 8 |
| Staphylcoccus aureus (CICC 10384, CICC 10307, CICC 10786, CICC 10201) | 4/4 | 4–8 |
| Listeria innocua (CICC 10416, CICC 21529) | 2/2 | 16 |
| Escherichia coli (CICC 10003, CICC 10032, CICC 10004, CICC 10361) | 4/4 | 4 |
| Shigella dysenteriae ATCC 51302 | 1/1 | 8 |
| Penicillium italicum (CICC 40659, CICC 40946) | 0/2 | |
| Saccharomyces cerevisiae (CICC 1002, CICC 1010, CICC 1016, CICC 1023, CICC 1043) | 0/5 | |
| 60°C, 80°C, 100°C | + | |
| Freeze‐thaw | + | |
| pH3‐10 | + |
Compared with other antibacterial peptides reported recently 5, 25, for example, JCpep8 b and MDpep9, barleycin MICs against E.coli were found to have higher potency. This might be because the liposomes used to screen barleycin were scoured from E.coli cell membranes. Negatively charged phospholipids on the outer surface of the negatively charged lipid A of lipopolysacharide (in Gram‐negative bacteria) might also contribute to this 5, 12, 25.
3.6. Safety evaluation
Cytotoxicity of barleycin by MTT assay showed that the viability of HL7702 and HREpiC cells was 100.2 and 100.3%, respectively in the presence of 256 μg/mL of barleycin (Table 3). The results indicated that barleycin had no cytotoxicity. When barleycin was less than 256 μg/mL, the human blood cell did not show hemolysis phenomenon. Combined with the MIC of tested bacteria, the barleycin at the concentration to inhibit spoilage bacteria was primarily considered as safe. Therefore, it has potential application value. These data indicated that barleycin may be a promising candidate for antibacterial drug and food preservative. Normally, the antibacterial peptides from animals are not active against animal cells (safe to human cells) because they are secreted by animals to protect from harmful microorganisms in the environment 12, 13.
Table 3.
Safety evaluation of barleycin
| Viability | ||
|---|---|---|
| MTT | HL7702 | 100.2% ± 0.4% |
| HREpiC | 100.4% ± 0.7% | |
| Hemolysis | ||
| Hemolytic assay | 64 μg/mL | 0 |
| 128 μg/mL | 0 | |
| 256 μg/mL | 3.2% ± 1.7% | |
| 512 μg/mL | 19.5% ± 3.5% |
3.7. Leakage of E.coli cytoplasmic content
Escherichia coli CICC 10300 cells treated with 2 MIC barleycin displayed obviously changes in the leakage of K+. About 1.1 μmol K+ were lost in 2 h (Fig 4A).The leakages of cytoplasmic proteins and nucleic acid were increased as the treatment time elongated (Fig. 4B and C). These results indicated that barleycin would damage the permeability of cell membrane and cause the leak out of cytoplasmic content and even the death of sensitive bacteria. Other researchers also reported that membrane permeability played a very important role on the mode of action of cationic antibacterial peptides 5, 9, 11.
Figure 4.
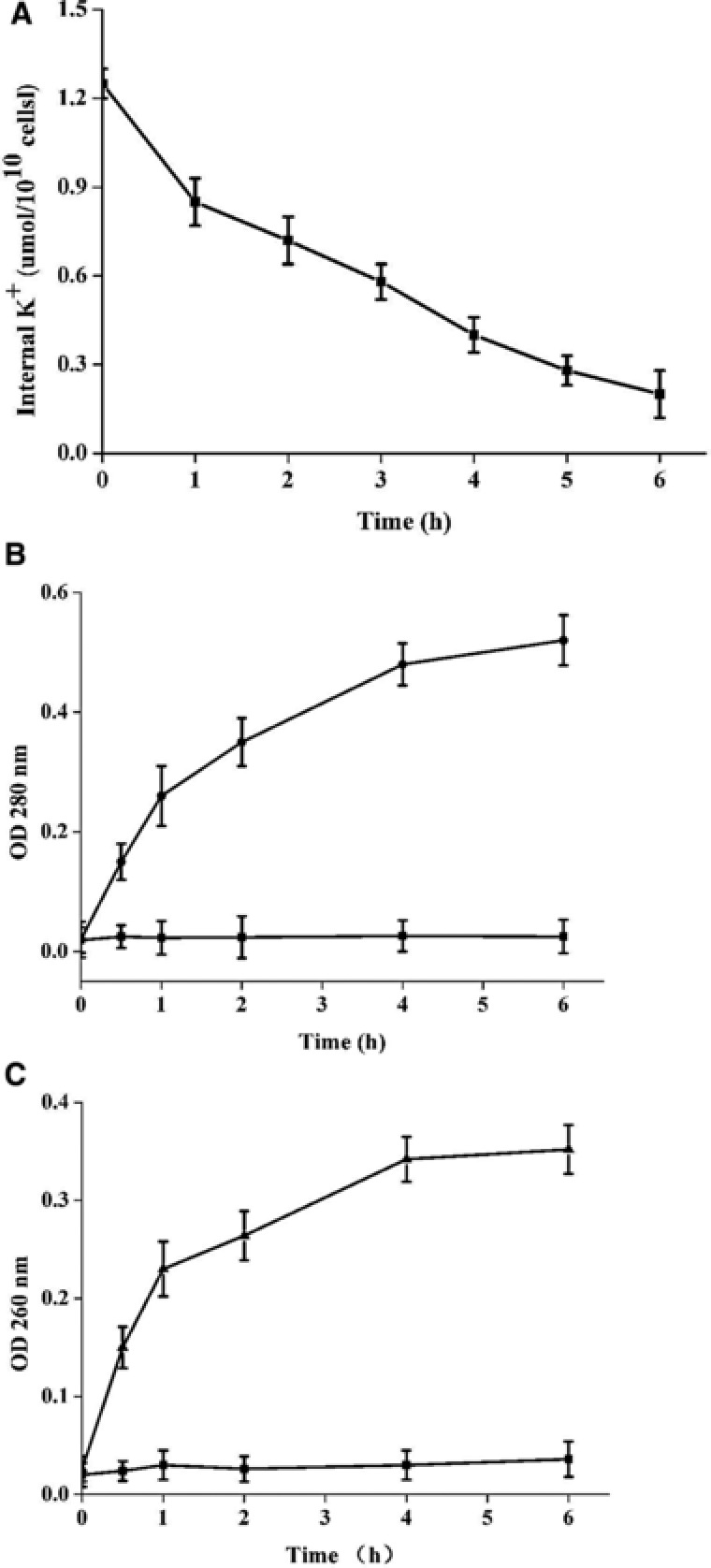
Leakage of of E.coli CICC 10300 cytoplasmic content after treatment of barleycin. (A) Leakage of K+; (B) leakages of enzymes; (C) leakages of nucleic acids.
4. Concluding remarks
Fragments hydrolyzed by trypsin for 4 h exhibited the highest antibacterial activity. A novel antibacterial peptide named as barleycin was successfully purified from the hydrolysate of highland barley proteins by magnetic liposome adsorption combined with the protein profiles of RP‐HPCL. The average diameter of magnetic liposomes was 271.5 nm and the saturation magnetization (MS) is 21.7 emu/g with superparamagnetism. Amino acids sequence of barleycin was determined as Lys‐Ile‐Ile‐Ile‐Pro‐Pro‐Leu‐Phe‐His by N‐sequencing. The CD spectra suggested that the barleycin is in a‐helix conformation. Barleycin exhibits the inhibitory activities toward Bacillus subtilis, Staphylcoccus aureus, Listeria innocua, E. coli, and Shigella dysenteriae. The MICs were from 4 to 16 μg/mL which were low. E.coli is very sensitive to barleycin, this might be because the liposomes used to screen barleycin were scoured from E. coli. Heat and freeze‐thaw treatment did not affect the activity of barleycin and it was effective within the pH range 3–10. Barleycin was estimated as safe by cytotoxicity and hemolytic test. The primary mode of action of barleycin is to change the permeability of the bacterial cell membrane and cause the leak out of cytoplasmic content and even the death of sensitive bacteria. In summary, barleycin can be considered to have the potential value for application against E. coli in medical industry or food preservatives.
Practical application
An innovative method, magnetic liposome adsorptions combining the protein profiles of RP‐HPLC, was developed to target screen and efficiently purify the antibacterial peptide; the antibacterial peptide purified in this study, barleycin, has the great potential for application in food/medical industry.
The authors have declared no conflict of interest.
Acknowledgments
This study was funded by Research Foundation of Science and Technology Bureau of Shaanxi, China (2015SZS‐15‐05), Collaborative Innovation Center Research Foundation (QBXT‐Z(P)‐15‐28), the China Scholarship Council (CSC) Foundation (201608615024), Special Scientists’ Foundations of Shaanxi University of Technology (SLGKYQD2014‐2‐18), and Research Foundations of Shaanxi University of Technology (SLGQD13‐13).
5 References
- 1. Zhang, Y. , Guo, B. Y. , Cao, Y. , Huang, Q. R. , Antibacterial effects of a cell‐penetrating peptide isolated from Kefir. J. Agri. Food Chem. 2016, 64, 3234–3242. [DOI] [PubMed] [Google Scholar]
- 2. Kaur, G. , Singh, T. P. , Malik, R. K. , Antibacterial efficacy of Nisin, Pediocin 34 and Enterocin FH99 against Listeria monocytogenes and cross resistance of its bacteriocin resistant variants to common food preservatives. Brazil J. Microbiol. 2013, 14, 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tomasinsig, L. , Skerlavaj, B. , Scarsini, M. , Guida, F. et al., Comparative activity and mechanism of action of three types of bovine antibacterial peptides against pathogenic Prototheca spp. J. Peptide Sci. 2012, 18, 105–113. [DOI] [PubMed] [Google Scholar]
- 4. Lin, M. C. , Hui, C. F. , Chen, J. Y. , Wu, J. L. , Truncated antibacterial peptides from marine organisms retain anticancer activity and antibacterial activity against multidrug‐resistant Staphylococcus aureus . Peptides 2013, 44, 139–148. [DOI] [PubMed] [Google Scholar]
- 5. Goh, H.F. , Philip, K. , Isolation and mode of action of bacteriocin BacC1 produced by nonpathogenic Enterococcus faecium C1. J. Dairy Sci. 2015, 98, 5080–5090. [DOI] [PubMed] [Google Scholar]
- 6. Xiao, J. H. , Zhang, H. , Antibacterial peptide isolated from ovalbumin hydrolysate by immobilized liposome‐binding extraction. J. Biomel. Screen. 2012, 17, 752–760. [Google Scholar]
- 7. Fiori, S. , Urgeghe, P. P. , Hammami, W. , Razzu, S. et al., Biocontrol activity of four non‐ and low‐fermenting yeast strains against Aspergillus carbonarius and their ability to remove ochratoxin A from grape juice. Int. J. Food Microbiol. 2014, 189, 45–50. [DOI] [PubMed] [Google Scholar]
- 8. Zhao, S. G. , Han, J.Z. , Bie, X. M. , Lu, Z. X. et al., Purification and characterization of Plantaricin JLA‐9: a novel bacteriocin against Bacillus spp. produced by Lactobacillus plantarum JLA‐9 from Suan‐Tsai, a Traditional Chinese fermented cabbage. J. Agri. Food Chem. 2016, 64, 2754–2764. [DOI] [PubMed] [Google Scholar]
- 9. Hawrani, A. , Howe, R. A. , Walsh, T. R. , Dempsey, C. E. , Thermodynamics of RTA3 peptide binding to membranes and consequences for antibacterial activity. Biochim. Biophys. acta‐ Biomembrane 2010, 1798, 1254–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paiva, A. D. , Irving, N. , Breukink, E. , Mantovani, H. C. , Interaction with lipid II induces conformational changes in bovicin HC5 Structure. Antimicrob. Agents Chemother. 2012, 56, 4586–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Panagiota, K. , Kyriakou, E. B. , Kristianse, n P. E. , Kaznessis, Y. N. , Interactions of a class IIb bacteriocin with a model lipid bilayer, investigated through molecular dynamics simulations. 2016. Biochim. Biophys. Acta (BBA) – Biomembranes, 2016, 1858, 824–835. [DOI] [PubMed] [Google Scholar]
- 12. Tang, W. T. , Zhang, H. , Wang, L. , New cationic antimicrobial peptide screened from boiled‐dried anchovies by immobilized bacterial membrane liposome chromatography. J. Agr. Food Chem. 2014, 62, 1564‐1571. [DOI] [PubMed] [Google Scholar]
- 13. Yue T. L., Pei J. J., Yuan Y. H., Purification and Characterization of anti‐Alicyclobacillus bacteriocin produced by Lactobacillus rhamnosus . J. Food Protect. 2013, 76, 1575–1581. [DOI] [PubMed] [Google Scholar]
- 14. Duan, L. , Jia, S. , Wang, Y. Lianfeng Duan, S. et al., Synthesis of Fe3O4 polyhedra by hydrothermal method: using l‐arginine as precipitator. J. Mater. Sci. 2009, 44, 4407. [Google Scholar]
- 15. Tang, Y. L. , Shi, Y. H. , Zhao, W. , Hao, G. et al., Interaction of MDpep9, a novel antimicrobial peptide from Chinese traditional edible larvae of housefly, with Escherichia coli genomic DNA. Food Chem., 2009, 115, 867–872. [Google Scholar]
- 16. Malheiros, P. S. , Micheletto, Y. M. S. , Silveira, N. P. , Brandelli, A. , Development and characterization of phosphatidylcholine nanovesicles containing the antibacterial peptide nisin. Food Res. Int. 2010. 43, 1198–1203. [Google Scholar]
- 17. Xiao, J. H. , Zhang, H. , Ding, S. D. , Thermodynamics of antimicrobial peptide JCpep8 binding to living staphylococcus aureus as pseudostationary phase in capillary electrochromatography and consequences for antimicrobial activity. J. Agri. Food Chem. 2012, 60, 4535–4541. [DOI] [PubMed] [Google Scholar]
- 18. Boelter, J. F. , Brandelli, A. , Innovative bionanocomposite films of edible proteins containing liposome‐encapsulated nisin and halloysite nanoclay, Colloid Surface B, 2016,145, 740‐747. [DOI] [PubMed] [Google Scholar]
- 19. Qin, Z. , Du, S. , Luo, Y. , Liao, Z. et al., Hydrothermal synthesis of superparamagnetic and red luminescent bifunctional Fe3O4@Mn2+‐doped NaYF4:Yb/Er core@shell monodisperse nanoparticles and their subsequent ligand exchange in water, Appli. Surface Sci. 2016, 378, 15, 174–180. [Google Scholar]
- 20. Jeong, H. Y. , Deok, H. W. , Mi‐Sook, C. , Myung‐Suk, C. , Microfluidic based biosensing for Escherichia coli detection by embedding antibacterial peptide‐labeled beads. Sens. Actuators, B: Chemical, 2014, 191, 211–218. [Google Scholar]
- 21. Ma, Y. , Liu, C. , Liu, X. , Wu, J. et al., Peptidomics and genomics analysis of novel antibacterial peptides from the frog, Rana nigrovittata, Genomics 2010, 95, 66–71. [DOI] [PubMed] [Google Scholar]
- 22. Zhang, S. , Guo, H. , Shi, F. , Wang, H. et al., Hainanenins: A novel family of antibacterial peptides with strong activity from Hainan cascade‐frog, Amolops Hainanensis. Peptides, 2012. 33, 251–257. [DOI] [PubMed] [Google Scholar]
- 23. Wang, Y. , Zhang, Y. , Lee, W. H. , Zhang, Y. et al., Novel Peptides from skins of amphibians showed broad‐spectrum antibacterial activities. Chem. Biol. & Drug Des., 2016, 87, 419–424. [DOI] [PubMed] [Google Scholar]
- 24. Martinez, R. C. R. , Staliano, C. D. , Vieira, A. D. S. , Villarreal, M. L. M. et al., Gombossy de Melo Franco, B. D., Bacteriocin production and inhibition of Listeria monocytogenes by Lactobacillus sakei subsp. sakei 2a in a potentially symbiotic cheese spread. Food Microbiol. 2015, 48, 143–152. [DOI] [PubMed] [Google Scholar]
- 25. Miao, J. Y. , Guo, H. X. , Chen, F. L. , Zhao, L. C. et al., Antibacterial effects of a cell‐penetrating peptide isolated from Kefir. J. Agri. Food Chem. 2016, 64, 3234–3242. [DOI] [PubMed] [Google Scholar]
- 26. Chamorro, C. , Boerman, M.A. , Arnusch, C.J. , Breukink, E. , Pieters, R.J. Enhancing membrane disruption by targeting and multivalent presentation of antimicrobial peptides. Biochim. Biophys. Acta. 2012, 18, 2171‐2174. [DOI] [PubMed] [Google Scholar]
- 27. Li, L. , Shi, Y. , Cheserek, M.J. , Su, G. et al., Antibacterial activity and dual mechanisms of peptide analog derived from cell‐penetrating peptide against Salmonella typhimurium and Streptococcus pyogenes . Appli. Microbiol. Biotechnol. 2013, 97, 1711‐1723. [DOI] [PubMed] [Google Scholar]


