Abstract
While the methylotrophic yeast Pichia pastoris enables the industrial‐scale biosynthesis of many recombinant products, large amount of nutrient‐rich biomass emerged along this process. Polysaccharides, especially glucans that are abundant in the cell wall of P. pastoris, are yet to be better utilized owing to their various biological activities. However, the isolation and purification of cell wall glucan from P. pastoris has not been reported. In this study, we established an environment‐friendly approach, including induced autolysis, hot‐water treatment, ultrasonication, isopropanol extraction, and protease treatment, to isolate and purify glucan from the cell wall of P. pastoris. We achieved a purity of 85.3% and a yield of 11.7% for the purified glucan. Proteins, nucleic acids, lipids, and ash were efficiently removed during the purification. The activities of the purified glucan were investigated in mice fed with a high‐fat diet. The purified glucan decreased the level of total cholesterol and triglycerides by 30.3 and 29.7%, respectively. This result suggested that the cell wall glucan of P. pastoris could be developed to a therapeutic agent for dyslipidemia. Our study proposed an environment‐friendly and effective method to isolate and purify the glucan from P. pastoris, providing solid foundation for the high‐value utilization of this yeast.
Keywords: Biological activity, Dyslipidemia, Glucan, Isolation and purification, Pichia pastoris
Abbreviations
- HBsAg
hepatitis B surface antigen
- HSA
human serum albumin
- TNF‐α
tumor necrotizing factor‐α
1. Introduction
Pichia pastoris, a methylotrophic yeast, has long been utilized as a highly efficient eukaryotic expression system 1. Genetically engineered P. pastoris strains have been developed for the biosynthesis of a wide variety of recombinant proteins, including tumor necrotizing factor‐α (TNF‐α), hepatitis B surface antigen (HBsAg), and human serum albumin (HSA) 2. Multiple advantages, including the convenience of genetic manipulation, the capability of this yeast to perform eukaryotic post‐translational modifications, and the ability of this yeast to express exogenous proteins to high levels, together made P. pastoris an ideal and popular eukaryotic expression system 3. Another key advantage of P. pastoris as a host for the biosynthesis of exogenous proteins is that this yeast can grow to high densities. With proper culture medium and growth conditions, P. pastoris can grow to a cell density that is comparable to an OD600 value of 500, with the yeast cells occupying about 40% of the volume of the culture suspension 2. Most of the recombinant proteins in P. pastoris are designed to be secreted into the culture medium and harvested by filtration, centrifugation, or aqueous two‐phase extraction 4. The spent cell bodies consequently become a waste by‐product. Currently, the yeast cells are heat‐inactivated and used as fertilizers or feed for domesticated animals, which is an energy‐consuming process leading to low‐value products 5. Thus, developing a cost‐effective approach to convert the cell bodies of P. pastoris to high‐value products is an urgent need in protein industry.
Yeast cells are coated by a thick and rigid cell wall. The major component of the cell wall is polysaccharides, occupying 85% of the total biomass 6. The other components include proteins and chitin, which together contribute to 15% of the total biomass 6. Previous structural studies have discovered that the yeast cell wall is composed of three layers 7. Immediately outside the cell membrane is a layer of β‐glucan, which occupies around 50% of the dry weight of the cell wall 7. The structure of yeast derived β‐glucan has been determined in the baker's yeast Saccharomyces cerevisiae. About 85% of the glucans are long‐chain molecules with around 1500 glucose moieties. The other 15% of the glucans are short‐chain molecules composed of around 150 glucose moieties 7. The majority of the glucose moieties are connected by β‐(1→3)‐D‐linkages, with the other moieties connected through β‐(1→6)‐D‐linkages and branches linked through C‐1, C‐3, and C‐6 7. Immediately outside the β‐glucan layer is a layer of proteins. Mannan and phosphorylated mannan together form the outmost layer of the cell 6. These yeast cell wall polysaccharides, similar to other polysaccharides, exhibit multiple biological activities including immunity enhancement, anti‐tumor, anti‐infection, and resistance to radiation 8, 9, 10, 11. However, to our knowledge, the composition of the cell wall has not been well characterized in P. pastoris. Nor are there reports on the isolation and bioactivities of β‐glucan from P. pastoris, especially, from recombinant strains. Therefore, developing a process to isolate and purify polysaccharides from P. pastoris, especially β‐glucans, will provide a favorable approach for the high‐value utilization of the spent P. pastoris cells.
Breaking the cell wall is the key step for the isolation of glucan; it also plays an important role for the inactivation of P. pastoris. However, it is challenging to disintegrate the cell wall of yeasts due to its thickness and rigidity. In the past decades, polysaccharides of yeast cell wall were often isolated by the treatment of chemicals including acid, alkali, sodium hypochlorite, and enzymes 12. The rationale of acid‐ or alkali‐treatment is that part of the water‐insoluble glucan has a higher solubility in acids or alkali solutions. The solubilized glucan are subsequently precipitated by ethanol 13. Sodium hypochlorite can oxidize β‐glucan and disintegrate the cell wall; thus, it has also been widely utilized for the isolation of cell wall polysaccharide 14. However, these methods share noticeable disadvantages: a major proportion of the polysaccharides was degraded by the acids, alkali, or oxidative agents during the isolation and purification process, leading to poor yields of the product. Besides, the acids or alkali may also be carried over into the polysaccharide product, leading to unsatisfactory purities and impacting the quality of the product. Moreover, the corrosive reagents utilized in the isolation and purification process are a major contaminant to the environment. Recently, multi‐step environment‐friendly procedures have been developed to isolate and purify polysaccharides from the cell wall of S. cerevisiae 13, 15. The central procedures in the process are induced autolysis and hot‐water treatment, followed by homogenization, through which the rigid cell wall is disintegrated. These procedures avoided the utilization of corrosive chemicals such as strong acids and alkali, thus are more favorable for the environment. Besides, induced autolysis inactivates the cells in a mild condition. Therefore, applying this approach in P. pastoris may lead us to a cost‐effective approach to inactivate the recombinant microorganism and to better utilize the by‐products of protein expression.
In this study, we developed a multi‐step environment‐friendly method to isolate and purify the glucan from the spent cells of P. pastoris. The schematic diagram of our approach is shown in Fig. 1. We characterized the removal rate of the impurities during each step of the isolation process and determined the purity and yield of the product. We also evaluated the biological activities of the purified glucan in mice fed with a high‐fat diet.
Figure 1.
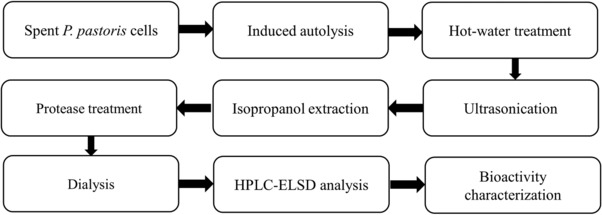
Schematic diagram of the isolation, preparation, and characterization of glucan from the cell wall of P. pastoris.
2. Materials and methods
2.1. Materials
Spent P. pastoris cells, a by‐product from the biosynthesis of recombinant human serum albumin, was kindly provided by North China Pharmaceutical Group Co., Ltd. (Hebei, China). The yeast cells were washed repeatedly with distilled water until the supernatant became colorless to eliminate the impurities. The cells were then collected and lyophilized for further steps.
The kit for Lawry assay was purchased from Solarbio Co. (Beijing, China). The Gram staining kit was purchased from Beijing Land Bridge Technology Co. (Beijing, China). Porcine pepsin and bromelain from pineapple was purchased from Hufeng Co. (Shanghai, China). Bovine trypsin, papain from cariepapaya, thermolysin from Bacillus, and flavoenzyme from yeast were purchased from Xuemei Co. (Jiangsu, China). The assay kits for total cholesterol and triglycerides were purchased from Nanjing Jiancheng Bioengineering Institute (Jiangsu, China). The other chemicals used in this study were of analytical grade.
Male Kunming mice (16 to 20 g) were purchased from the SPF Laboratory Animal Center of Dalian Medical University. The mice were maintained under standard laboratory conditions. The current study protocol was approved by international ethical guidelines and the Institutional Animal Care and Use Committee of Dalian University of Technology with the permission No. SCXK‐2013‐0003.
2.2. Chemical analysis
The content of the carbohydrates (β‐glucan and mannan) was determined by HPLC‐ELSD (detailed in Section 2.8), followed by standard curve fitting using the area under the curve generated from ELSD. Glucose solution (1 M) and mannose solution (1 M) were used as the standards for the generation of standard curves. The content of total protein in the sample was determined by Lawry method, using bovine serum albumin (250 mg/L) as the standard. The content of total lipid was determined by acid‐hydrolysis method. Briefly, yeast was treated with HCl and the mixture was extracted by ethanol and ether. The extract was collected and the solvent was evaporated. The mass of the total lipids was then precisely measured. The content of ash was determined by incinerating the dried yeast sample in a furnace at 600°C for 5 h and the weight of the residual ash was measured.
2.3. Induced autolysis
The parameters of induced autolysis, including the solid‐liquid ratio, the concentration of NaCl as an autolysis promoter, pH, temperature, and the duration of treatment, were evaluated. A response surface analysis was performed subsequently to further determine the interaction between the parameters and the effect of the parameters to the ratio of autolysis. The ratio of the autolysis was defined as the total loss of dry biomass during the autolysis, and was calculated by Eq. (1):
| (1) |
R: ratio of autolysis
in which W0 represents the weight of yeast before autolysis and W represents the weight after autolysis. After the induced autolysis, the cell suspension was heated at 85°C for 10 min and the cells were collected by centrifugation at 5000 × g for 10 min.
2.4. High‐pressure hot‐water treatment
Parameters including the solid‐liquid ratio, pH, and the duration of the treatment were evaluated. The concentration of protein in the supernatant was determined by Lawry assay and used as an indicator to evaluate the efficiency of hot‐water treatment. Hot‐water treatment was then performed with the parameters determined. After hot‐water treatment, the cells were collected by centrifugation at 8000 × g for 10 min and washed three times with distilled water.
2.5. Ultrasonication
For ultrasonication, the parameters including the solid‐liquid ratio, the power of operation and the cycle‐index were determined. The ultrasonication was performed at a frequency of 20 kHz for 10 s, with an interval of 2 s in a 30‐mL system. After the ultrasonication, the supernatant was collected for the determination of protein content. The insoluble fraction containing raw β‐glucan was collected for further purification steps.
2.6. Isopropanol extraction and protease treatment
The raw glucan was washed for 5 times with isopropanol and acetone with a volume ratio of 1:11. Subsequently, the cells were treated with isopropanol at 85°C for 2 h to remove the residual lipids. After the isopropanol extraction, the raw glucan was collected by centrifugation at 8000 × g for 10 min.
Six proteases, including pepsin, papain, flavoenzyme, trypsin, bromelain, and thermolysin, were tested for the proteolysis of the cell wall glucan. The raw glucan was treated with each enzyme at the corresponding optimal pH and temperature of each enzyme for 4 h. The enzyme generating polysaccharides with the highest purity and yield were selected and tested at its optimal conditions to determine the appropriate dose and the duration of treatment. After the proteolysis, the reaction mixture was heated at 85°C for 15 min to inactivate the enzyme. After the protease treatment, dialysis was performed with a 13,000 kDa cut‐off dialysis tubing for 36 h at 4°C against distilled water with frequent replacement of water to remove the amino acids in the glucan. The purified β‐glucan was lyophilized at –40°C after dialysis.
2.7. Microscopy of the P. pastoris cells at each stage of glucan purification
Scanning electron microscopy was performed at each stage of the purification process according to the procedures described in the published literature, to visualize the change of cell morphology during the isolation and purification process 15. Samples were prepared, examined and the images were recorded with a Hitachi S‐4800 scanning electron microscope (Hitachi, Japan).
Gram staining and methylene blue staining of the yeast cells was performed before and after the induced autolysis to display the lysis and the inactivation of the cells by induced autolysis, respectively. Gram staining was also performed after hot‐water treatment and ultrasonication, respectively, to determine the lysis percentage of the yeast cells at each step. The stained cells were observed by a Nikon optical microscope (Nikon, Japan) at 1000 × magnification. The yeast cells with intact cell wall were stained violet and the lysed cells were stained red as the ruptured cell wall could not retain the crystal violet. The total yeast cells in each sample were enumerated before autolysis. The violet cells and red cells in each sample were enumerated respectively after the induced autolysis. The lysis percentage of the yeast cells were calculated by Eq. (2) 15:
| (2) |
in which CT indicates the number of yeast cells before autolysis in a representative field. indicates the number of yeast cells stained violet by Gram staining after induced autolysis in a representative field. The concentration of the cells in the samples were normalized before Gram staining.
2.8. Analysis of the purified glucan
The high‐performance liquid chromatography (HPLC) analysis of the purified glucan was performed using a Kromasil NH2 (250 × 4.6 mm, 5 μm) HPLC column. The sample was eluted by a mobile phase composed of 75% acetonitrile and 25% distilled water, with a constant flow rate of 1 mL/min. For evaporative light scattering detection (ELSD), nitrogen was used as the vehicle gas and the pressure was set at 25 psi. The temperature for the drift tube was set at 40°C. Glucose solution (1 M) and mannose solution (1 M) were used as the standards for the analysis. Standard curves were generated using the corresponding standard solution to determine the relationship between the absorbance and the concentration of the glucose and mannose in the solution. The content of glucan and mannan were determined by fitting the peak area to the equation of the standard curve.
2.9. Characterization of the hypolipidemic bioactivities of purified glucan
Thirty‐two (32) Kunming Mice (16 to 25 g) was fed with a high‐fat diet for 28 consecutive days. The mice were then randomized into 4 groups, with 8 mice in each group. Mice in each group was administered with 0.5% CMC‐Na (CG), 50 mg/kg/d glucan in 0.5% CMC‐Na (LDG), 150 mg/kg/d glucan in 0.5% CMC‐Na (MDG), or 250 mg/kg/d glucan in 0.5% CMC‐Na (HDG), respectively, by oral gavage for 30 consecutive days. The mice were then sacrificed on day 31 and the body weight of each mouse was measured. The blood samples of each mouse were collected and centrifuged at 2000 × g for 10 min at 4°C. The plasma was collected and used as substrates for the following assays. The plasma level of total cholesterol and triglycerides were measured following the protocol provided along with the corresponding assay kit.
2.10. Statistical analysis
The results of the bioactivity analyses were expressed as the mean ± standard deviation, with a p value <0.05 indicating differences of statistically significance. One‐way ANOVA was performed to determine the optimal conditions for each step of the procedure. All experimental validations were performed in triplicates.
3. Results
3.1. Induced autolysis
Induced autolysis was performed to induce the degradation and the subsequent release of cellular macromolecules of P. pastoris in a mild condition. The parameters that led to the highest ratio of induced autolysis was determined and shown in Supporting Information Fig. 1, including a solid‐liquid ratio of 1:15, a pH of 6.0 and an incubation at 50°C for 30 h. Interestingly, the ratio of induced autolysis was the highest without the addition of NaCl, which is contrary to the result from S. cerevisiae 15. The most relevant factor for the ratio of induced autolysis is the duration of treatment (Factor A), followed by the pH (Factor B) and the temperature (Factor C) of the system. A notable interaction between Factor A and Factor B was observed and characterized by Eq. (3):
| (3) |
R = ratio of autolysis.
The corresponding 2D contour plot and 3D response surface plot are shown in Fig. 2A and B, respectively, to illustrate the relationship between the response (ratio of autolysis) and the parameters. The ratio of autolysis determined by the subsequent verification was close to the predicted data (40.4 vs 39.4%), confirming the validity of the predicted model.
Figure 2.
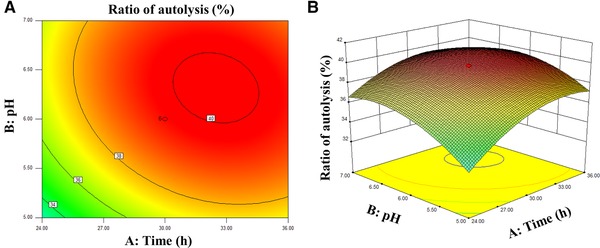
The effect of time, pH, and their interaction on the ratio of autolysis. (A) 2D contour plot showing the effect of A: time (h) and B: pH on the ratio of autolysis. (B) 3D response surface plot showing the effect of A: time (h) and B: pH on the ratio of autolysis.
We further investigated the kinetics of the induced autolysis with the parameters predicted by the 3D response surface plot (duration of treatment = 33 h, pH = 6.4, and temperature = 47°C). The profile of the carbohydrates and proteins in the supernatant of the induced autolysis was shown in Fig. 3A. The content of polysaccharides and proteins in the supernatant and the ratio of induced autolysis kept increasing till 33 h (Fig. 3A). The concentration of polysaccharides and proteins at 33 h were 5.84 mg/mL and 1.22 mg/mL, respectively (Fig. 3A). Whole wavelength scanning was performed to analyze the components in the supernatant. A notable peak of absorbance was observed at 260 nm, suggesting that nucleic acids were efficiently released from the yeast cells during induced autolysis (Fig. 3B). This result was further confirmed by a step‐wise analysis of the content of nucleic acids and proteins in the product of each step (Table 1). The content of protein and nucleic acid decreased dramatically from 45.1 to 26.7% and from 8.9 to 1.4% after induced autolysis (Table 1), with a mean removal ratio of 64.1 and 90.1%, respectively (Supporting Information Table 1). The content of lipids and ash were also dramatically decreased during induced autolysis, with a mean cumulative removal ratio of 48.1 and 49.1%, respectively (Supporting Information Table 1).
Figure 3.
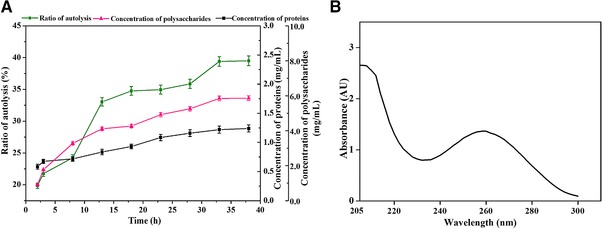
Kinetic analysis of induced autolysis. (A) Variation of the concentrations of protein and polysaccharide during induced autolysis of P. pastoris. The content of protein and polysaccharide during the autolysis process was determined and presented. (B) Whole wavelength scanning of the lysate from induced autolysis. The lysate from the induced autolysis under optimal conditions were centrifuged and harvested. The absorbance of the lysate was determined by whole‐wavelength scanning.
Table 1.
Step‐wise analysis of the content of each component during the purification
| Procedure | Crude protein (%) | Nucleic acids (%) | Lipid (%) | Ash (%) | Carbohydrate (%) | Yield (%) |
|---|---|---|---|---|---|---|
| Untreated cells | 45.1 ± 0.24 | 8.9 ± 0.12 | 11.8 ± 0.13 | 7.4 ± 0.41 | 36.8 ± 0.35 | 100 ± 0 |
| Induced autolysis | 26.7 ± 0.076 | 1.4 ± 0.09 | 10.2 ± 0.15 | 6.2 ± 0.12 | 52.7 ± 1.74 | 60.5 ± 0.13 |
| Hot‐water treatment | 28.7 ± 0.18 | 0.3 ± 0.056 | 8.5 ± 0.12 | 3.4 ± 0.14 | 64.5 ± 1.73 | 25.9 ± 0.08 |
| Ultrasonication | 5.3 ± 0.17 | 0.3 ± 0.035 | 5.6 ± 0.08 | 3.9 ± 0.13 | 83.7 ± 1.92 | 17.5 ± 0.05 |
| Isopropanol extraction | 5.0 ± 0.33 | 0.2 ± 0.045 | 0.7 ± 0.05 | 3.3 ± 0.12 | 84.0 ± 2.22 | 15.8 ± 0.11 |
| Protease treatment | 3.3 ± 0.13 | 0.08 ± 0.004 | 0.5 ± 0.03 | 2.7 ± 0.08 | 85.3 ± 2.06 | 11.7 ± 0.04 |
The data were presented as the mean of 3 replicates ± standard deviation.
3.2. High‐pressure hot‐water treatment
Hot‐water treatment was performed subsequently at 121°C to remove the mannoproteins and to reduce the rigidity of the cell wall. According to Supporting Information Fig. 2, the parameters leading to the highest efficiency included a solid‐liquid ratio of 1:30, a pH of 7.5, and a duration of 4 h. Hot‐water treatment efficiently reduced the content of lipid (from 10.2 to 8.5%, mean removal ratio = 64.2%), ash (from 6.2 to 3.4%, mean removal ratio = 76.6%) and nucleic acid (from 1.4 to 0.3%, mean removal ratio = 89.7%) in the product (Table 1 and Supporting Information Table 1).
3.3. Ultrasonication
Ultrasonication was performed to disrupt the cell wall of the yeast. According to Supporting Information Fig. 3, among the conditions tested, a solid‐liquid ratio of 1:40 led to the highest efficiency of ultrasonication. The power was determined to be 400 W and the number of cycles was determined to be 60, to balance the energy consumption and yield. Ultrasonication efficiently reduced the content of residual proteins in the product (from 28.7 to 5.3%, mean removal ratio = 87.4%), leading to an increase of the purity of polysaccharide in the sample (from 64.5 to 83.7%) (Table 1 and Supporting Information Table 1).
3.4. Isopropanol extraction and protease treatment
The cell wall disrupted by ultrasonication was treated by isopropanol at a solid‐liquid ratio of 1:4 to remove the residual lipids. Isopropanol extraction efficiently decreased the content of the residual lipids from 5.6 to 0.7%, with a mean removal ratio of 87.5% (Table 1 and Supporting Information Table 1). The product was then treated with proteases to remove the residual proteins. According to Supporting Information Fig. 4, papain exhibited the highest activity for the degradation of the residual proteins, with a removal ratio of 51.1%. Bromelain exhibited the second highest activity, with a removal ratio of 50.0%. Papain was then selected for the protease treatment due to its relative higher activity and cost‐effectiveness. After the protease treatment, the content of residual proteins decreased from 5.0 to 3.3%, with a mean removal ratio of 52.1% (Table 1 and Supporting Information Table 1).
3.5. Biochemical analysis of the purified β‐glucan
The purified polysaccharide was dialyzed, treated with sulfuric acid and analyzed by high‐performance liquid chromatography‐evaporative light scattering detection (HPLC‐ELSD). The sample generated a major peak at 15.79 min corresponding to glucose and a minor peak at 13.02 min corresponding to mannose (Fig. 4). The area of the peak was fitted to the standard curve equation generated by mannose standard (Eq. (4)) as follows:
| (4) |
in which A represents the absorbance of the sample detected by ELSD and c represents the concentration of mannose. Results from the standard curve equation and peak area analysis showed that the content of the residual mannose in the product was less than 2%, suggesting that glucan was the main component in the purified polysaccharide.
Figure 4.
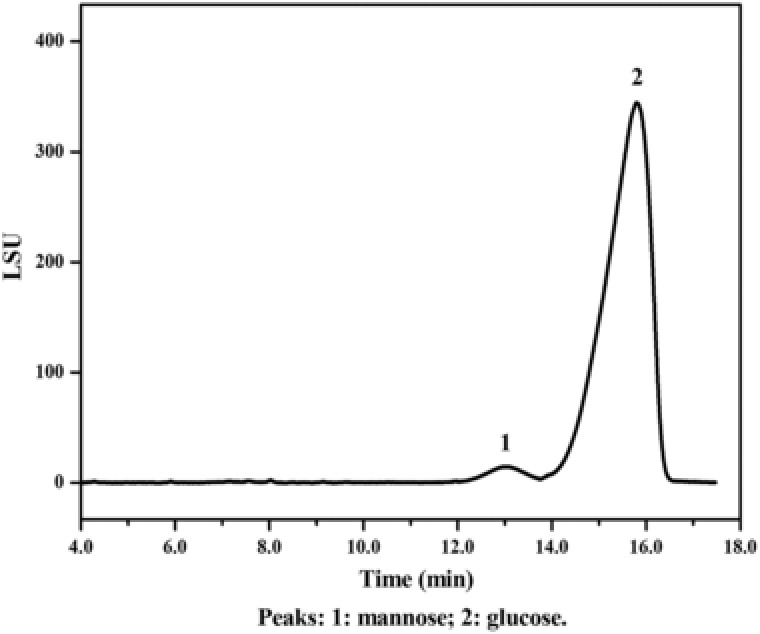
HPLC‐ELSD chromatograph of the polysaccharides isolated and purified from the cell wall of P. pastoris. The yeast cells were treated by induced autolysis, high‐pressure hot‐water treatment, and sonication. The isolated polysaccharide was further purified by isopropanol and papain to remove the residual lipids and proteins, respectively. The purified polysaccharide was treated by sulfuric acid, neutralized by BaCO3 and the content of glucan in the purified product was separated by high‐performance liquid chromatography (HPLC) using a Kromasil NH2 (250 × 4.6 mm, 5 μm) HPLC column, with a mobile phase (acetonitrile:H2O = 3:1) and a constant flow rate of 1 mL/min. For ELSD analysis, nitrogen was used as the vehicle gas and the pressure was set at 25 psi. The temperature for the drift tube was set at 40°C. Glucose solution (1 M) and mannose solution (1 M) was used as standards for the analysis.
3.6. Microscopic analysis of the P. pastoris cells
Scanning electron microscopy was performed to examine the morphology of P. pastoris cells at each step of the purification procedure. According to Fig. 5A, untreated cells had intact spherical structures and smooth surfaces, without notable folds or holes. Notable holes appeared on the surface of the cells after induced autolysis (Fig. 5B). After hot‐water treatment, the cell wall became convoluted, with apparent folds (Fig. 5C). After ultrasonication, the cell wall started to disintegrate, with layers peeling off from the cells (Fig. 5D). The cells were completely disintegrated after isopropanol extraction and protease treatment, leaving cell wall fragments in the field (Fig. 5E). In comparison, heat inactivated P. pastoris cells had intact structures, with few observable holes on the cell surface (Fig. 5F).
Figure 5.
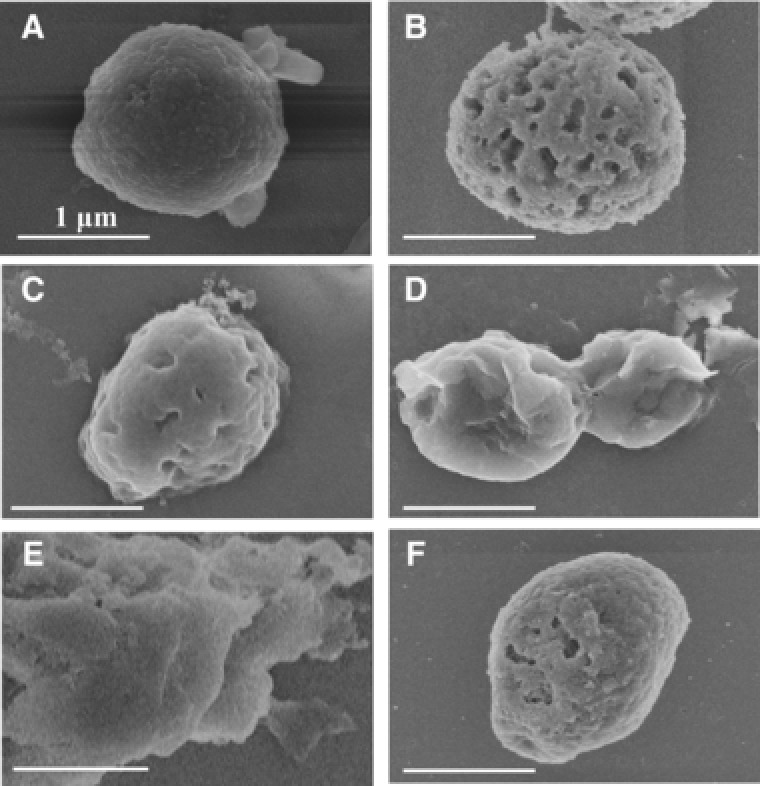
Step‐wise scanning electron micrograph of P. pastoris cells at each stage of polysaccharide isolation. P. pastoris cells at each stage of the isolation were prepared and imaged by a Hitachi S‐4800 scanning electron microscope. The images were recorded at 5.0 kV. (A) Intact cells of P. pastoris before polysaccharide isolation; (B) P. pastoris cells after induced autolysis; (C) P. pastoris cells after high‐pressure hot‐water treatment; (C) P. pastoris cells after ultrasonication; (E) P. pastoris cells after isopropanol extraction; (F) P. pastoris cells inactivated by autoclaving. Bar: 1 μm.
We also performed Gram staining and methylene blue staining to examine the effect of autolysis on the cell wall integrity of the yeast. As is shown in Supporting Information Fig. 5, prior to autolysis, the cells were stained violet by Gram staining and were colorless after methylene blue staining, which suggests that the cell wall was intact and the cells are viable (Supporting Information Fig. 5A and B). In contrast, after the autolysis, a proportion of yeast cells stained red by Gram staining could be observed (Supporting Information Fig. 5C). The lysis percentage of the cells after induced autolysis were calculated to be 21.3% by Eq. (2), suggesting that induced autolysis efficiently disrupted the cell wall in a mild condition. The lysis percentage increased to 27.7% after hot‐water treatment and dramatically increased to 89.2% after ultrasonication, suggesting that the cell wall of the yeasts was further disintegrated along with the purification. Consistently, the proportion of the cells stained blue after methylene blue staining also dramatically increased after induced autolysis, suggesting that the cells were inactivated during induced autolysis (Supporting Information Fig. 5).
3.7. Characterization of the biological activities of the purified glucan
We further characterized the bioactivities of glucans purified from the cell wall of P. pastoris on the regulation of lipid profile, specifically, total cholesterol and triglycerides, in mice fed with a high‐fat diet. According to Fig. 6, purified glucans significantly improved the level of both total cholesterol and triglycerides in a dose dependent manner. Glucan at low‐dose (50 mg/kg/d), medium‐dose (150 mg/kg/d), and high‐dose (250 mg/kg/d) all led to statistically significant improvements for the level of total cholesterol (Fig. 6A) and triglycerides (Fig. 6B). These results indicate that β‐glucan from P. pastoris exhibited a lipid‐lowering effect on mice fed with a high‐fat diet.
Figure 6.
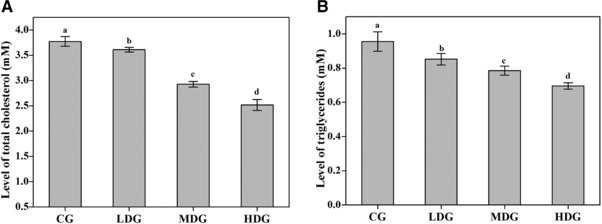
The effect of the purified glucan from the cell wall of P. pastoris on plasma total cholesterol and triglyceride. Thirty‐two (32) Kunming mice (16–25 g) fed with a high‐fat diet were randomized into 4 groups. Mice in each group was administered with 0.5% CMC‐Na (CG), 50 mg/kg/d β‐glucan in 0.5% CMC‐Na (LDG), 150 mg/kg/d β‐glucan in 0.5% CMC‐Na (MDG), or 250 mg/kg/d β‐glucan in 0.5% CMC‐Na (HDG), respectively, by oral gavage for 30 days. The plasma of each mice was collected on Day 31 and the plasma level of total cholesterol and triglyceride were measured following the protocol of the corresponding kit. Different superscript letters associated with the values (a, b, c, and d) represent significant differences between each comparison.
4. Discussion
Many approaches for the isolation of cell wall polysaccharides were performed in harsh conditions with the application of strong acids and alkali, as the water‐insoluble glucan tend to be better solubilized in these chemicals 12. Thammakiti et al. treated S. cerevisiae cells with sodium hydroxide followed by acetic acid to isolate glucans from the cell wall, achieving a yield of 8.4% and a purity of 55% 13. Hunter Jr. et al. treated S. cerevisiae with 0.1 M sodium hydroxide followed by 0.1 M acetic acid and 3% H2O2, achieving a purity of 80.5% 16. Ohno et al. used sodium hypochlorite followed by DMSO treatment to isolate glucans, achieving a yield of 3.7% 14. These unsatisfactory yields or purities were attributed to the acids or alkali added, which remained in the final product and led to the degradation of long‐chain glucans. Moreover, the acids and alkali utilized, if not handled and disposed properly, are also hazardous to the environment. To overcome these potential pitfalls, Liu et al. utilized a multi‐step approach including induced autolysis, hot‐water treatment, high‐pressure homogenization, isopropanol extraction, and protease treatment, to isolate the polysaccharides from S. cerevisiae, with a yield of 11.18% and a purity of 93.12% 15. Magnani et al. utilized a similar approach, replacing the high‐pressure homogenization with ultrasonication homogenization, and achieved a yield of 11.08%, which was comparable to that achieved by Liu et al. 15, 17. In this study, we developed an environment‐friendly and effective approach to isolate and purify glucan from the cell wall of P. pastoris. We achieved a yield and purity of the glucan in P. pastoris that is comparable with those from the studies in S. cerevisiae. Notably, the content of residual nucleic acid was as low as 0.08%, suggesting that our approach efficiently removed the exogenous DNA engineered into P. pastoris, which could be a potential biohazard if uptaken by surrounding environmental bacteria. Besides, our approach efficiently inactivated the recombinant yeast cells in a mild condition. Thus, our method provided a cost‐effective approach for the inactivation of the recombinant yeast and the high‐value utilization of the spent cells of P. pastoris.
Induced autolysis is the key step for polysaccharide isolation and inactivation of yeast cells. When the activity of yeast cells is impacted, for instance, by stressed conditions or environmental factors, endogenous enzymes are activated to degrade proteins and other macromolecules in the cells 18. NaCl is often used as an autolysis promoter to induce osmotic pressure. The addition of 3% NaCl yielded the highest ratio of autolysis in S. cerevisiae, suggesting that osmotic pressure is a key factor for the autolysis of this yeast 15. However, in our research, we found the ratio of autolysis is in negative correlation to the concentration of NaCl. These results suggested that the cell wall structure and component of the two yeasts might be different, thus leading to different optimal conditions of induced autolysis. The study on the relationship between cell wall structure, composition, and optimal conditions of autolysis in yeasts is an interesting topic for future studies.
Hot‐water treatment efficiently removed ash, genetic material, water‐soluble mannan, and part of the mannoproteins from the cell wall. The content of ash, nucleic acids, lipids, and crude proteins were effectively decreased during hot‐water treatment. The removal ratios of these impurities were comparable to the results of the study by Liu et al. 15. The apparent loss of carbohydrate during hot‐water treatment was also partially attributed to the removal of glycogen residues 15. Another benefit of performing hot‐water treatment is that this procedure caused the cell wall to swell, thus decreasing the rigidity of the cell wall and making the cells easier to be disrupted in the subsequent homogenization 15.
Two approaches have been adopted in the previous researches for the homogenization of the yeast cells, high‐pressure homogenization and ultrasonication 15, 17. High‐pressure homogenization has been utilized in multiple previous researches owing to its high efficiency 13, 15. However, high‐pressure homogenization usually requires up to six passes before a high lysis percentage is achieved, which may require extra time and energy consumption. Recently, ultrasonication has also appealed attraction due to its relative simplicity, a comparable efficiency to high‐pressure homogenization, and its ability to remove the amorphous proportions of (1→6)‐β‐D‐glucan that was trapped between the fibrils of the glucan in the cell wall 19. Magnani et al. used ultrasonication to disrupt the cell wall for the isolation of β‐glucan and a comparable yield was achieved (11.08 vs 11.18% by Liu et al.) 17. Protein was efficiently removed and the purity of carbohydrates dramatically increased through ultrasonication. The lysis percentage also dramatically increased, which was consistent to the results generated by Magnani et al. 17.
Multiple biological activities of yeast polysaccharides, including immune enhancement, anti‐cancer, and anti‐oxidation, have been discovered 20, 21, 22. Especially, yeast‐derived β‐glucan was discovered to regulate the level of lipid 23. Multiple reports have shown that diets and drinks enriched with β‐glucan lowered the level of total cholesterol, possibly by increasing the level of bile acid synthesis and decreasing the absorption of cholesterols 24. In an in vivo study, Vetvicka et al. found that yeast‐derived β‐glucan lowered the level of cholesterol in Balb/c mice in a dose‐dependent manner 23. Consistent with this research, we found that β‐glucan isolated from the cell wall of P. pastoris also exhibited hypolipidemic activity to a comparable level. Therefore, the glucan purified from P. pastoris is a promising component in the cell wall that can be developed to diet supplements with hypolipidemic activity. Our research provides an effective approach to convert the waste biomass of P. pastoris to high‐value products, which may further improve the cost‐effectiveness of P. pastoris as a host for the biosynthesis of recombinant proteins.
Practical application
Pichia pastoris has been widely used for the industry‐scale biosynthesis of multiple recombinant proteins. After the exogenous proteins are harvested, the yeast cells become a by‐product yet to be better utilized. In this study, we established a multi‐step, environment‐friendly approach, including induced autolysis, hot‐water treatment, ultrasonication, isopropanol extraction, and protease treatment, to isolate and purify cell wall glucan from P. pastoris. Following our method, glucan was effectively isolated and purified from P. pastoris, with a purity of 85.3% and a yield of 11.7%. Meanwhile, P. pastoris cells were efficiently inactivated during induced autolysis. Besides, we found that the purified glucan lowered the levels of total cholesterol and triglycerides in mice fed with a high‐fat diet, suggesting a potential role of the glucan for the treatment of dyslipidemia. Our approach enabled the high‐value utilization of P. pastoris in an environmental‐friendly and energy‐conservative manner that can be widely applied in protein‐engineering industry.
The authors have declared no conflict of interest.
Supporting information
Supporting Information
Acknowledgments
This study was supported by the grant from National Natural Science Foundation of China (81172966). We would like to thank Dr. Shicong Wang from North China Pharmaceutics Group Co., Ltd. for the thoughtful discussion on the industrial processing of spent P. pastoris.
5 References
- 1. Ahmad, M. , Hirz, M. , Pichler, H. , Schwab, H. , Protein expression in Pichia pastoris: recent achievements and perspectives for heterologous protein production. Appl. Microbiol. Biot. 2014, 98, 5301–5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cereghino, J. L. , Cregg, J. M. , Heterologous protein expression in the methylotrophic yeast Pichia pastoris . FEMS Microbiol. Rev. 2000, 24, 45–66. [DOI] [PubMed] [Google Scholar]
- 3. Ahn, J. , Jang, M.‐J. , Ang, K. S. , Lee, H. et al. Codon optimization of Saccharomyces cerevisiae mating factor alpha prepro‐leader to improve recombinant protein production in Pichia pastoris . Biotechnol. Lett. 2016, 38, 2137–2143. [DOI] [PubMed] [Google Scholar]
- 4. Dong, Y. , Zhang, F. , Wang, Z. , Du, L. et al., Extraction and purification of recombinant human serum albumin from Pichia pastoris broths using aqueous two‐phase system combined with hydrophobic interaction chromatography. J. Chromatogr. A. 2012, 1245, 143–149. [DOI] [PubMed] [Google Scholar]
- 5. Wang, Y. , Hu, Y. , Lei, Y. , Lv, Y. et al., Survey of intracellular protein extraction methods from Pichia pastoris . World. J. Eng. Technol. 2015, 3, 1–6. [Google Scholar]
- 6. Aguilar‐Uscanga, B. , Francois, J. M. , A study of the yeast cell wall composition and structure in response to growth conditions and mode of cultivation. Lett. Appl. Microbiol. 2003, 37, 268–274. [DOI] [PubMed] [Google Scholar]
- 7. Free, S. J. , Fungal cell wall organization and biosynthesis. Adv. Genet. 2013, 81, 33–82. [DOI] [PubMed] [Google Scholar]
- 8. Kogan, G. , Kocher, A. , Role of yeast cell wall polysaccharides in pig nutrition and health protection. Livest. Sci. 2007, 109, 161–165. [Google Scholar]
- 9. Toklu, H. Z. , Sener, G. , Jahovic, N. , Uslu, B. et al., β‐glucan protects against burn‐induced oxidative organ damage in rats. Int. Immunopharmacol. 2006, 6, 156–169. [DOI] [PubMed] [Google Scholar]
- 10. Vinogradov, E. , Bent, O. , Duus, J. , Isolation and characterization of non‐labeled and 13C‐labeled mannans from Pichia pastoris yeast. Carbohydr. Res. 2000, 325, 216–221. [DOI] [PubMed] [Google Scholar]
- 11. Sier, C. F. , Gelderman, K. A. , Prins, F. A. , Gorter, A. , Beta‐glucan enhanced killing of renal cell carcinoma micrometastases by monoclonal antibody G250 directed complement activation. Int. J. Cancer. 2004, 109, 900–908. [DOI] [PubMed] [Google Scholar]
- 12. Shi, L. , Bioactivities, isolation and purification methods of polysaccharides from natural products: A review. Int. J. Biol. Macromol. 2016, 92, 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thammakiti, S. , Suphantharika, M. , Phaesuwan, T. , Verduyn, C. , Preparation of spent brewer's yeast β‐glucans for potential applications in the food industry. Int. J. Food. Microbiol. 2004, 39, 21–29. [Google Scholar]
- 14. Ohno, N. , Miura, T. , Miura, N. N. , Adachi, Y. et al., Structure and biological activities of hypochlorite oxidized zymosan. Carbohyd. Polym. 2001, 70, 339–349. [Google Scholar]
- 15. Liu, X. , Wang, Q. , Cui, S. , Liu, H. , A new isolation method of β‐D‐glucans from spent yeast Saccharomyces cerevisiae . Food. Hydrocolloid. 2008, 22, 239–247. [Google Scholar]
- 16. Hunter, K. W., Jr. , Gault, R. A. , Berner, M. D. , Preparation of microparticularte β‐glucan from Saccharomyces cerevisiae for use in immune potentiation. Lett. Appl. Microbiol. 2002, 35, 267–271. [DOI] [PubMed] [Google Scholar]
- 17. Magnani, M. , Calliari, C. M. , Macedo, F. , Mori, M. P. et al., Optimized methodology for extraction of (1→3)(1→6)‐β‐D‐glucan from Saccharomyces cerevisiae and in vitro evaluation of the cytotoxicity and genotoxicity of the corresponding carboxymethyl derivative. Carbohyd. Polym. 2009, 78, 658–665. [Google Scholar]
- 18. Xu, W. , Wang, J. , Li, Q. , Microarray studies on lager brewer's yeasts reveal cell status in the process of autolysis. FEMS. Yeast. Res. 2014, 14, 714–728. [DOI] [PubMed] [Google Scholar]
- 19. Šandula, J. , Kogan G., Kačuráková, M. , Machová, E. , Microbial (1→3)‐β‐D‐glucans, their preparation, physio‐chemical characterization and immunomodulatory activity. Carbohyd. Polym. 1999, 38, 247–253. [Google Scholar]
- 20. Yang, T. , Zhang, S. , Wang, R. , Li, D. et al., Polysaccharides from Rhizoma Panacis Majoris and its anti‐oxidant activity. Int. J. Biol. Macromol. 2016, 86, 756–763. [DOI] [PubMed] [Google Scholar]
- 21. Yang, X. , Wang, R. , Zhang, S. , Zhu, W. et al., Polysaccharides from Panax japonicus C.A. Meyer and their antioxidant activities. Carbohyd. Polym. 2014, 101, 386–391. [DOI] [PubMed] [Google Scholar]
- 22. Zeng, Z. , Xu, Y. , Zhang, B. , Antidiabetic activity of a lotus leaf selenium (Se)‐polysaccharide in rats with gestational diabetes mellitus. Biol. Trace. Elem. Res. 2017, 176, 321–327. [DOI] [PubMed] [Google Scholar]
- 23. Vetvicka, V. , Vetvickova, J. , Effects of yeast‐derived β‐glucans on blood cholesterol and macrophage functionality. J. Immunotoxicol. 2009, 6, 30–35. [DOI] [PubMed] [Google Scholar]
- 24. Whitehead, A. , Beck, E. J. , Tosh, S. , Wolever, T. M. , Cholesterol‐lowering effects of oat β‐glucan: a meta‐analysis of randomized controlled trials. Am. J. Clin. Nutr. 2014, 100, 1413–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


