Abstract
The atmospheric CO2 increase is considered the main cause of global warming. Microalgae are photosynthetic microorganisms that can help in CO2 mitigation and at the same time produce value‐added compounds. In this study, Scenedesmus obliquus, Chlorella vulgaris, and Chlorella protothecoides were cultivated under 0.035 (air), 5 and 10% (v/v) of CO2 concentrations in air to evaluate the performance of the microalgae in terms of kinetic growth parameters, theoretical CO2 biofixation rate, and biomass composition. Among the microalgae studied, S. obliquus presented the highest values of specific growth rate (μ = 1.28 d−1), maximum productivities (P max = 0.28 g L−1d−1), and theoretical CO2 biofixation rates (0.56 g L−1d−1) at 10% CO2. The highest oil content was found at 5% CO2, and the fatty acid profile was not influenced by the concentration of CO2 in the inflow gas mixture and was in compliance with EN 14214, being suitable for biodiesel purposes. The impact of the CO2 on S. obliquus cells’ viability/cell membrane integrity evaluated by the in‐line flow cytometry is quite innovative and fast, and revealed that 86.4% of the cells were damaged/permeabilized in cultures without the addition of CO2.
Keywords: Chlorella protothecoides, Chlorella vulgaris, CO2 biofixation, Flow cytometry, Scenedesmus obliquus
Abbreviations
- FAMEs
fatty acid methyl esters
- Pavg
average biomass productivity
- PBR
photobioreactor
- PCO2
theoretical CO2 fixation rate
- PI
propidium iodide
- PUFAs
polyunsaturated fatty acids
1. Introduction
The increasing levels of CO2 in the atmosphere are considered the main cause of global warming and climate change 1, 2. In 2015, CO2 concentrations surpassed 400 mg L−1, which could start being “dangerous” and highly destructive to the world's global climate 3. In order to reduce the CO2 load from the atmosphere, several technologies of carbon sequestration and storage have been developed (physicochemical, adsorption, membrane technology, cryogenic fraction, injection into deep oceans/geological formations, etc.) 4. However, most of these methods require considerable storage space and have high operational, monitoring, and maintenance costs and raise serious concerns about possible CO2 leakage over time 5.
Phototrophic carbon biofixation through microalgae cultivation is a potential and sustainable alternative to mitigate CO2 in the atmosphere and, at the same time, produce high value‐added biomass that could be of environmental and economic importance 6. Photosynthetic microorganisms, such as microalgae and cyanobacteria, use photosynthesis to harness sunlight and fix the inorganic carbon from the atmosphere or another source, which is then converted into biomass, and assimilated in the form of carbohydrate, lipids, and/or proteins 7. When compared to terrestrial plants, microalgae/cyanobacteria have a higher photosynthetic efficiency, as they can grow 10–50 times faster 8, meaning a much higher CO2 mitigation. Furthermore, microalgae are valuable source of high‐value products, such as biopolymers, natural colorants (pigments), polyunsaturated fatty acids (PUFAs), therapeutic substances, and oils/sugars to produce biofuels 8, 9, 10, 11, 12. The combination of CO2 biofixation with high‐value products and/or biofuel production is seen as a sustainable process and very promising alternative to other CO2 mitigation strategies. According to the literature (e.g., 13, 14, 15), Scenedesmus obliquus, Chlorella vulgaris, and Chlorella protothecoides have been identified as promising candidates to mitigate significant quantities of CO2 concomitantly with the accumulation of considerable amount of lipids and value‐added compounds. Since CO2 assimilation by microalgae is related to cell growth, the CO2 biofixation ability is species dependent 16. However, some authors claimed that CO2 aeration above 5% could be harmful to microalgae cells 17, while others showed an exceptional tolerance to high CO2 concentrations up to 40% 18, 19.
In this work, S. obliquus, C. vulgaris, and C. protothecoides were cultivated under different CO2 concentrations: 0.035 (air), 5 and 10% (v/v) in air, in order to choose the best microalga in terms of specific growth rate, average biomass productivity, theoretical CO2 biofixation, as well as oil and fatty acid (content and profile), to be used for biodiesel production. The pigment accumulation (total chlorophyll, beta‐carotene, and lutein) was also evaluated for potential economic valorization of the whole CO2 biomitigation system.
A more detailed study was performed with S. obliquus including the use of lower (2.5%) and higher (15%) CO2 concentrations, since this microalga had the best performance. Flow cytometry was used to evaluate the impact of different CO2 percentages on S. obliquus cell viability/membrane integrity.
2. Materials and methods
2.1. Microorganism and growth medium
The microalgae species used for this study were S. obliquus, strain ACOI 204/07, obtained from the Algae Collection from Coimbra's University; C. vulgaris, strain 58, isolated by INETI (Lisbon, Portugal), and C. protothecoides, strain 25, provided by UTEX Collection (Texas University of Austin, USA).
Bristol's medium was used to grow S. obliquus and Chlorella medium was used to grow Chlorella sp. and C. protothecoides 20. The media were previously sterilized by autoclaving at 121°C, 2 bar over 20 min.
The inoculum volume was 1/10 of the total volume of the photobioreactor (PBR).
2.2. Photobioreactor and operation
All experiments were carried out in glass bubble column PBR (1 L). Each PBR was operated on batch mode, over 15 days, under ≈28°C, and with an artificial light source (Photosynthetic Photon Flux Density‐PPFD ≈74 μmol m−2 s−1 [photosynthetically active radiation—PAR]) provided by three fluorescent lamps (TL‐D 18 w/54‐765, Philips Co.) of 18 W placed on two opposite sides of the PBR. Different CO2 concentrations (5 and 10% (v/v)) were supplied to the PBR, at 1 vvm feeding rate. The gas flow rate was regulated by the flow meter and the gas composition, according to the CO2 percent chosen, was regulated by a gas mixer (Map Mixer 9001 ME, PBI Dansensor) coupled to 10 L pressure tank connected to a CO2 bottle (99.9% purity). The inlet and outlet air was measured by a self‐contained nondispersive infrared CO2 sensor complete with infrared source and dual wavelength filter (model Checkmate II, PBI Dansensor).
For S. obliquus 2.5 (v/v) and 15% (v/v) CO2 were also tested.
All the experiments were performed in duplicate and the results are expressed as average ± SD. Control assays were carried out using air (0.035% CO2) without any CO2 supplementation.
2.3. Determination of microalgae biomass growth
The microalgal biomass growth was evaluated by measuring OD (λ = 540 nm; Hitachi U‐2000) and dry weight using glass fiber filters (GF/C, 47 mm, Whatman) dried at 80°C overnight.
2.4. Determination of growth kinetic parameters and CO2 biofixation rate
The growth kinetic parameters evaluated were the specific growth rate (μ), the average biomass productivity (P avg), and the theoretical CO2 fixation rate (PCO2).
The specific growth rate (d−1) was calculated according to Eq. (1):
| (1) |
where X 0 and X t, are the dry weight cell concentrations (g L−1), at the beginning, t 0, (d) and at the end of the exponential growth phase, t x, (d), respectively.
To calculate the average biomass productivity (P avg) Eq. (2) was used:
| (2) |
where X max is the maximum dry weight cell concentration (g L−1) at the time t t (time when the dry weight cell concentration was maximum) and X 0 is the dry weight cell concentration at the beginning of cultivation time (t 0, d−1).
The average theoretical CO2 biofixation rate (PCO2) was calculated based on Eq.(3):
| (3) |
or,
| (4) |
where P avg denotes the average biomass productivity (g L−1 d−1) that was calculated based on Eq. (2). The value 1.88 represents the theoretical value of CO2 biofixated (g g−1), assuming general CO0.48H1.83N0.11P0.01 21.
The percentage of CO2 utilization efficiency is calculated based on Eq. (4), where PCO2 is the average theoretical CO2 biofixation rate (g L−1 d−1) and input CO2, is the amount of CO2 present in the air‐mixture supplied to the culture (g L−1 d1).
2.5. Analytical methods
2.5.1. Cellular viability
Flow cytometry (FACScalibur, Becton Dickinson, USA) was used to evaluate the cellular viability/cell membrane integrity during S. obliquus cultivations at different CO2 levels. Propidium iodide (PI) was used to distinguish permeable from nonpermeable cells. PI is known to stain nucleic acids in cells characterized by defective membrane integrity 22 and was read in the FL2 channel (585/42 nm). Before staining S. obliquus samples with PI, cells were run in the flow cytometer for autofluorescence analysis. A region was established to define the cell autofluorescence population. After staining with PI, the cells that increased their fluorescence in the FL2 channel, moving away from the autofluorescence region, were considered as stained with PI (PI+), thus having injured membrane (permeabilized cells). Cells that remained in the autofluorescence region were considered as not stained with PI (PI−), thus having intact membrane (intact cells).
A number of positive controls were carried out, inducing membrane permeabilization, by ethanol (75%, v/v) and heat (boiling water) treatments. For ethanol treatment, 1 mL ethanol (75%, v/v) was added to centrifuge S. obliquus cells (9503 × g Heraeus Biofuge 15 centrifuge, Hong‐Kong). After 1‐min incubation, cells were washed, resuspended in PBS (pH 7.0) and analyzed in the flow cytometer, after PI staining. For heat treatment, cells were incubated in a water boiling bath for 15 min. Afterwards they were diluted in PBS solution, stained with PI, and analyzed. These controls were then used for further comparison with data obtained from the microalgal cultivations.
Fresh samples were collected from the PBR in each experiment and subjected to an ultrasound bath (Transsonic T660/H, Elma, Spain) for 10 s. Before analyzing, cells were diluted in PBS to attain 800–1000 events/s. 2.5 μL of PI (stock solution 1 mg mL−1) were added to 497.5 μL of the diluted sample, totaling a 500 μL volume.
2.5.2. Lipid extraction and analysis of fatty acid profile
After the experiments were completed, each biomass was recovered by centrifugation at 10 000 rpm and 4°C for over 10 min (Centrifuge model Heraeus Multifuge 3S R+, Thermo Scientific, Whaltman), followed by freeze drying for over 24 h. Lipid extraction was performed by direct transesterification to fatty acid methyl esters (FAMEs) using the Lepage and Roy method 23, using heptadecanoic acid (C17:0) as internal standard. The lipid profile was analyzed by GC (Scion GC‐436, Bruker, Germany) equipped with SUPELCOWAX 10 (Supelcowax, Bellafonte, Palo Alto, CA) 30‐m capillary column (0.32 mm of internal diameter and 0.25 μm of film thickness). The carrier gas (Helium) was kept at a constant rate of 1.6 mL min−1. The column was programmed at an initial temperature of 200°C for over 20 min, then increased by 2°C min−1 to 220°C and held over 14 min. The injector and detector temperatures were 250 and 280°C, respectively, and split ratio was 1:20 for 5 min and 1:10 for the remaining time. The column pressure was 13.5 psi.
FAMEs were identified by comparison of the retention times of the samples peaks with that of standard FAMEs mixture (Sigma). Fatty acid composition was calculated as a percentage of the total fatty acids present in the sample, determined from the peak areas proportional to total area. The biomass total oil content was calculated according to Eq. (5):
| (5) |
where Ɛ A is the sum of the areas of all peaks; A pi the area corresponding to the internal standard (C17:0); m pi (mg) the mass of internal standard, and m sample (mg) is the mass of the microalgae sample.
2.5.3. Pigment extraction and quantification
In a test tube was weighted 10 mg of microalgae dried sample and was added roughly 0.7 g of glass beads and 2 mL of acetone (90%). The tube was covered by aluminum foil, then was vigorously homogenized in a vortex over 2 min, cooled with an ice bath (2 min), and centrifuged (Sigma Sartorius 2–6E) at 3900 rpm over 10 min. The extract was poured into another tube. The whole process was repeated until the extract and the biomass become colorless.
The quantification of total chlorophyll content was estimated based on Ritchie's equations 24 (Eqs. (6) and (7)):
| (6) |
| (7) |
where A 630, A 647, A 664, A 691 is the absorbance at 630, 647, 664, and 691 of wavelength (nm), respectively.
The identification and quantification of the pigments on the extracts were performed on a Hewlett‐Packard HP‐1100 series liquid chromatograph (Hewlett‐Packard, Waldrom, Germany). The HPLC was equipped with a Vydac C18 TP54 reversed‐phase column (250 × 4.6 mm μ‐bondapack) and a UV–Vis detector, set on the wavelength relative to the maximum of absorbance found for each pigment (λ = 450 nm for lutein and beta‐carotene). The eluent used was methanol (100%). The pigments were eluted over 20 min with a flow rate of 1 mL min−1 and column pressure of 52 bars. Before the injection, all extract samples were filtered through a PTFE filter syringe (membrane solutions) of 0.22 μm of pore size and 13 mm of diameter to a vial.
2.5.4. Statistical analysis
Statistical analysis was conducted by analysis of variance (ANOVA), post hoc comparisons, Scheffé test, run by the program Statistica 8.0 (Statsoft). The statistical significance was evaluated by estimation of the descriptive level (p), being the results considered statistically significant when p < 0.05 (confidence level >95%).
3. Results and discussion
3.1. Microalgae (S. obliquus, C. vulgaris, C. protothecoides) growth in response to CO2 supply
Figure 1 presents the growth curves (ODλ = 540 nm) for the three microalgae under the three CO2 concentrations (air, 5 and 10% CO2 addition), at an aeration rate of 1.0 vvm. The cultures showed a better performance under CO2‐enriched air conditions, to an extent which is species dependent.
Figure 1.
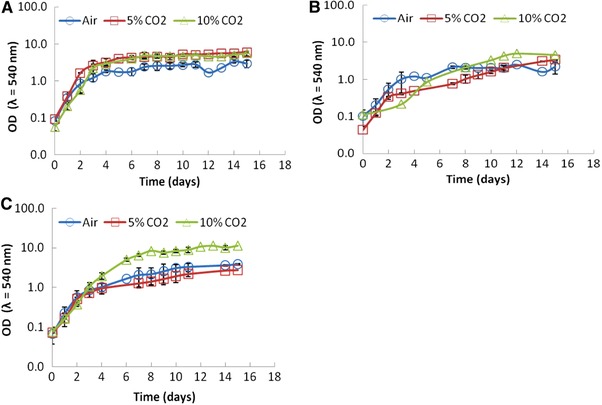
Growth curves of the cultures of (A) Scenedesmus obliquus, (B) Chlorella vulgaris, and (C) Chlorella protothecoides (≈ 28°C; 74 μmol photons m−2 s−1; 1 vvm) subjected to different CO2 concentrations tested: (Air (2); 5% CO2 (2) 10% CO2 (2)). Vertical bars represent SDs between the average of two duplicates.
Previous researches showed that the concentration of CO2 aeration above 5% could be harmful to microalgal cells and inhibit the microalgal growth 17. On the other hand, Iwasaki et al. 18 and Murakami and Ikenouchi 19 showed an exceptional tolerance to high CO2 concentration of up to 40% by a marine Chlorococcum littorale alga. De Morais and Costa 13 also reported the microalgae S. obliquus, C. kessleri, and Spirulina sp. also exhibited good tolerance up to 18% CO2, indicating their great potentials for CO2 fixation from CO2‐rich streams. However, it is well documented that preadapting cells at a low concentration of CO2 is an alternative approach to increase CO2 tolerance without effects during microalgal growth 14.
Figure 2 shows the influence of CO2 on specific growth rate (μ), average biomass productivity (P avg), and theoretical CO2 biofixation (PCO2).
Figure 2.
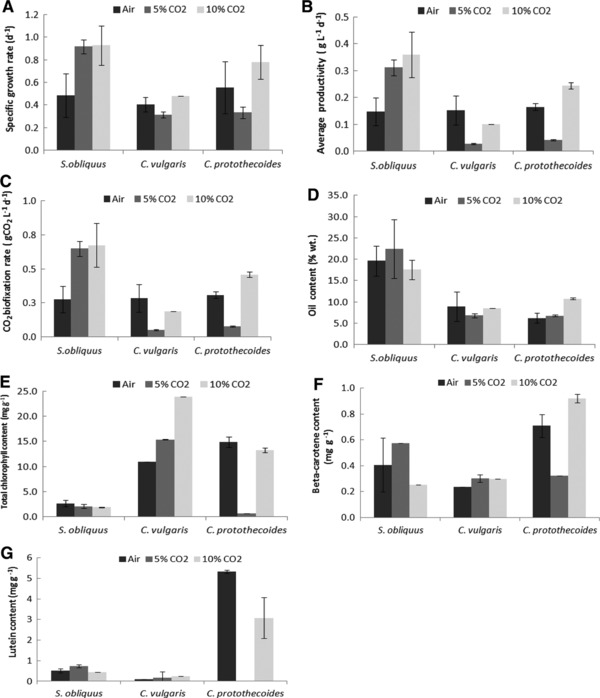
Representation of (A) specific growth rate (d−1), (B) average productivity (g L−1 d−1), (C) CO2 biofixation rate (gCO2 L−1 d−1), (D) oil content (%, w/w), (E) total chlorophyll content (μg/mg), (F) beta‐carotene content (mg/mg), (G) lutein content (mg/mg) from the cultures of Scenedesmus obliquus, Chlorella vulgaris, and Chlorella protothecoides (≈28°C; 74 μmol photons m−2 s−1; 1 vvm), subjected to the tested CO2 concentrations: Air (2); 5% (v/v) CO2 (2); 10% (v/v) CO2 (2). Vertical bars represent SDs.
Considering the specific growth rate (Fig. 2A), it was observed that S. obliquus led to the highest result for 10% (v/v) CO2‐enriched air, with 1.28 d−1. This good performance was in tandem with the increased average productivities (0.28 g L−1 d−1) (Fig. 2B), and theoretical CO2 biofixation rates (0.53 g CO2 L−1 d−1) (Fig. 2C) for this microalga.
De Morais and Costa 25 reported that S. obliquus could grow with up to 18% CO2, with a μ max obtained of 0.26 and 0.25 d−1 for both 6% and 12% (v/v) CO2‐enriched air, while the maximum cell concentration was 1.14 and 1.12 g dry cell weight L−1, respectively. In these conditions, the P max was 0.09 and 0.08 g−1 d−1 with 6 and 12% CO2, respectively, which is much lower compared to the results obtained in this work (Table 1).
Table 1.
Scenedesmus obliquus kinetic parameters cultivated at different CO2 concentrations
| CO2 (v/v) | μa (d−1) | X max b (g L−1) | P avg c (g L−1 d−1) | PCO2 d (g CO2 L−1 d−1) |
|---|---|---|---|---|
| Control | 0.98 ± 0.26 | 1.99 ± 0.05 | 0.14 ± 0.05 | 0.26 ± 0.05 |
| 2.5% CO2 | 1.39 ± 0.02 | 3.23 ± 0.00 | 0.21 ± 0.00 | 0.40 ± 0.00 |
| 5% CO2 | 1.01 ± 0.32 | 3.21 ± 0.21 | 0.27 ± 0.02 | 0.50 ± 0.02 |
| 10% CO2 | 1.28 ± 0.11 | 2.80 ± 0.47 | 0.28 ± 0.04 | 0.53 ± 0.04 |
| 15% CO2 | 0.93 ± 0.00 | 2.51 ± 0.04 | 0.25 ± 0.04 | 0.47 ± 0.04 |
μ = specific growth rate.
X max = maximum biomass concentration.
P avg = average biomass productivity.
PCO2 = theoretical CO2 biofixation rate.
Under 5% CO2 (v/v) S. obliquus reached the highest values of either average productivity, carbon dioxide biofixation, or oil content, proving its robustness in the presence of CO2 concentrations higher than 0.035% (Fig. 2B–D).
In general, S. obliquus had a better performance in terms of specific growth rates, maximum biomass concentration, average biomass productivity, and CO2 biofixation rate with CO2‐enriched air, thus this microalga was selected for further studies (2.5 and 15% CO2‐enriched air).
The increase of specific growth rate (μ) with CO2 enrichment is in agreement with Lam and Lee 26 who found that C. vulgaris biomass increased by 44.9%, from air (0.035%) to air enriched with 5% (v/v) CO2. This result is corroborated by Zheng et al. 27 and Nayak et al. 28 who also found a higher biomass productivity and lipid productivity. The decrease of the μ registered for C. vulgaris under 10% CO2 concentration (μ = 0.41 d−1) could be explained by the CO2 saturation present in the medium. In fact, the Chlorella media were supplemented with 0.5 g L−1 NaHCO3 (∼0.36 g L−1 CO2), besides the additional CO2‐enriched air. Possibly, under these conditions, C. vulgaris can suppress its CO2 needs and the CO2 uptake can decrease, leading to a decline on microalgal growth rate 29.
The values of kinetics parameters presented in Table 1 are higher than those reported by Ho et al. 30 who studied S. obliquus CNW‐N growth under various CO2 feeding concentrations and found a maximum μ = 1.19 d−1 (achieved at 10% CO2). De Morais 13, 25 also reported for S. obliquus a specific growth rate about seven times lower, when the microalgae was grown under 6 and 12% CO2.
The maximum biomass concentrations were observed for the experiments at 2.5 and 5% (∼3.2 g L−1; Table 1) and the maximum average productivity was achieved under 10% (v/v) CO2 concentration (0.28 g L−1 d−1, Table 1). According to Ho et al. 30, S. obliquus CNW‐N growth under 10% CO2 attained the maximum biomass concentration of 3.51 g L−1 and biomass productivity of 0.29 g L−1 d−1, slightly higher than those here reported for S. obliquus ACOI 204/07.
3.2. Microalgae biomass oil and pigment content
The contents of oil, total chlorophyll, beta‐carotene, lutein, as well as, the fatty acid profile were evaluated, in order to analyze the influence of the CO2 on the microalga composition. This study aimed the economical valorization of the produced biomass.
Oils are the most desirable component for biodiesel production (e.g., 8, 30, 31).
Scenedesmus obliquus presented the higher oil content especially when 5% CO2‐enriched air was supplied (22.5% oil wt.) (Fig. 2D). However, the lipid productivity seems to not have a direct correlation with CO2 concentration (p > 0.05).
Chlorella protothecoides exhibited a value of 10.8% (w w−1) for lipids under 10% (v/v) CO2‐enriched air. It is well reported that C. protothecoides is a mixotrophic microalga, that can grow photoautotrophically or heterotrophically. Under heterotrophic conditions, this microalga can reach up to 55.2% (w w−1) dry weight lipid content as compared to 14.6% (w w−1) in autotrophic cells 32.
The effect of CO2 concentrations on the total chlorophyll and their major carotenoids (beta‐carotene and lutein) contents, for the three microalgae, was investigated (Fig. 2E, 2F and 2G, respectively). The total chlorophyll content ranged between 0.7 (C. protothecoides (5% (v/v) CO2)) and 23.9 mg g−1 (C. vulgaris (10%, v/v, CO2)). Scenedesmus obliquus showed the lower values. This microalga, generally does not present great amounts of chlorophyll, despite displaying the highest light harvesting performance (Fig. 2E) that are typical values for these microalgae 31, 32, 33.
Thereafter, for C. vulgaris it was verified a slightly increasing trend on the total chlorophyll content, with the increase of CO2. Apparently, this strain was more capable of using carbon for pigment accumulation instead of the production of storage compounds, such as lipids, in the tested conditions. In fact, the C. vulgaris chlorophyll content is one of the highest in nature 34.
Beta‐carotene stood out with low values (Fig. 2F) and lutein ranged between 0.01 (C. vulgaris (air)) and 5.3 mg g−1 (C. protothecoides [air]). The lutein concentration barely overcame 1 mg g−1 for S. obliquus and C. vulgaris and the influence of CO2 seems to be negligible. The CO2 supplementation on C. protothecoides had a positive influence on the lutein content; however, the control culture presented the best value (5.33 mg g−1).
The low content of pigments in the different trials of this work is an expected result as it is well documented that high salinity, stress temperature, high light intensity, and nutrient limitation (mainly nitrogen) trigger carotenoids accumulation (e.g., 31, 33). These induction factors were not imposed in the present work.
Regarding beta‐carotene, it was not reported, so far, an influence of CO2 on its accumulation. The lutein content in this work ranged from 0.01 to 5.33 mg g−1, and literature, indicates values of 3.4 to 4.6 mg g−1 31, 35. The value of 4.10 mg g−1 is the best value obtained in autotrophic growth at laboratory scale for C. protothecoides 31 slightly lower compared to the result obtained in this study.
In an overall, S. obliquus seems to be more efficient in accumulating lipids, whereas C. vulgaris is better to synthetize chlorophylls and beta‐carotene, and C. protothecoides to synthetize lutein.
3.3. Scenedesmus obliquus—Effect of CO2 level on growth kinetics and biomass profile. Cell viability/membrane integrity
As S. obliquus showed the best results, a more detailed study was performed also with 2.5 and 15% CO2. The growth and biomass average productivity, cell viability (accessorized by flow cytometry), oil content, and fatty acid profile were also evaluated.
3.3.1. CO2 level
Figure 3 presents S. obliquus biomass growth and medium pH along the time under the different tested CO2 concentrations and Table 1 shows the corresponding kinetic parameters.
Figure 3.
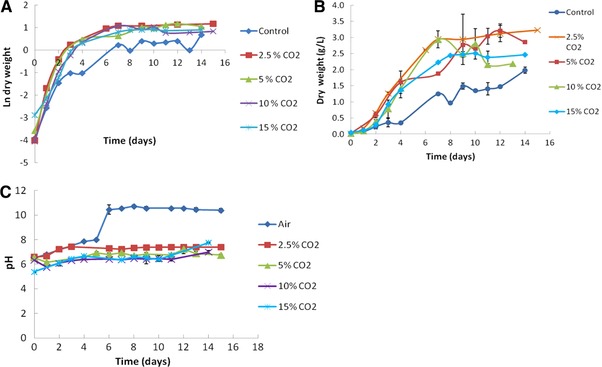
Natural logarithm of (A) dry weight, (B) dry weight, and (C) medium pH of Scenedesmus obliquus cultivations at different CO2 concentrations.
S. obliquus cultures presented a similar growth pattern when CO2‐enriched streams were applied. However, a slightly better performance was detected for cultures under 2.5 and 5% (v/v) CO2 concentrations. It can also be noticed that S. obliquus seems to have good tolerance under air supplemented with 15% CO2, do not demanding any sort of pre‐adaptation, which can be important toward future applications of this microalga for CO2 biofixation in real flue gases.
All the assays attained the stationary phase at t = 3 days (Fig. 3A). The experiment at 15% CO2 and the control assay showed the lowest specific growth rates (0.93 d−1 and 0.98 d−1, respectively, Table 1). The experiments at 2.5% attained the highest specific growth rate (1.39 d−1) followed by 5 and 10% CO2 (1.01 d−1 and 1.28 d−1, respectively, Table 1).
The maximum biomass concentrations were observed for the experiments at 2.5 and 5% (∼3.2 g L−1, Table 1) and the maximum average productivity was achieved fewer than 10% CO2 concentration (0.28 g L−1 d−1, Table 1).
The lowest biomass concentration and biomass productivity were observed for the control experiment (1.99 g L−1 and 0.14 g L−1 d−1, respectively). This could be due to the medium pH variation during the cultivations (Fig. 3C). In fact, while the medium pH of all the cultivations supplied with CO2‐enriched air varied between 5.75 and 7.77, the control medium pH varied between 6.54 (t = 0 days) and 10.45 (at t = 6 days), occurring the biggest increase at t = 5 days (from pH 8.0–10.4), when the culture reached the stationary phase.
The high increase of the pH of the control culture could be due to a carbon limitation. Only a slightly increase during the assays with CO2‐enriched air was observed, indicating that the available carbon was sufficient to control the pH. In fact, most algal growth occurs in the region near neutral pH, which could explain the highest specific growth rate observed for the trials conducted at 2.5% CO2 as the medium pH was almost constant (∼7.30) during most of the cultivation time course (Fig. 3C).
The low specific growth rate and maximum biomass concentration found for the experiment at 15% CO2 could be due to the carbon excess that could have inhibited the microalgal growth.
Since microalgae use CO2 as a nutrient source, it was expected that growth with higher CO2 concentration in the gas mixture, would increase their growth performance. However, S. obliquus seems to prefer CO2 intermediate levels: 2.5 and 5% (v/v) to grow but also had a good development under higher CO2 concentrations. This was reported by several researchers who sorted out that Chlorella sp., Nannochloropsis oculata, Dunaliella terticlecta, and S. obliquus had optimal growth potential in the range of 2–6% CO2, and the growth was decreased by increasing CO2 levels 1, 36.
The theoretical CO2 biofixation rate ranged between 0.26 and 0.53 g CO2 L−1d−1 for all the experiments. Such results are within those reported in various related studies 13, 37, although Ho et al. 30 have reported a higher CO2 removal rate for S. obliquus CNW‐N (5.5 g CO2 L−1d−1).
The authors also tried different aeration rates (0.25, 0.5, 0.75 and 1 vvm) at 5% CO2 (v/v) resulting specific growth rates, biomass productivities, and CO2 biofixation in the range of the ones obtained previously, with a slightly decrease on biofixation when the aeration rates increased (results not showed).
3.3.2. Biomass profile at different CO2 levels
The oil content (w w−1) for S. obliquus was accessed (Table 2), as well as its fatty acid profile, under the different CO2 tested concentrations (Table 2). In this study, the higher final oil values were obtained for 2.5 and 5% CO2 tested concentrations, with 26.3 and 22.4%, w w−1, respectively, although no significant differences (p > 0.05) were detected.
Table 2.
Fatty acid profile percentage (w w−1) and total oil content percentage (w w−1) of Scenedesmus obliquus biomass for the different CO2 concentrations percentage (v v−1). (Bold values: the more important fatty acids for each air/CO2 concentration)
| CO2 concentration % (v/v) | ||||||
|---|---|---|---|---|---|---|
| Fatty acid profile % (w/w) | Air | 2.5 | 5 | 7.5 | 10 | 15 |
| C14:0 | 0.5 ± 0.1 | 0.3 ± 0.0 | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.0 |
| C16:0 | 24.7 ± 1.3 | 24.9 ± 0.1 | 23.6 ± 0.1 | 22.0 ± 0.4 | 24.3 ± 1.4 | 25.8 ± 0.1 |
| C16:1 | 1.4 ± 0.1 | 1.4 ± 0.1 | 1.3 ± 0.3 | 1.9 ± 0.0 | 1.5 ± 0.3 | 1.1 ± 0.0 |
| C18:0 | 2.2 ± 0.4 | 4.4 ± 0.1 | 3.4 ± 0.2 | 3.3 ± 0.1 | 2.8 ± 0.4 | 3.3 ± 0.0 |
| C18:1 | 42.8 ± 3.8 | 40.1 ± 0.3 | 42.8 ± 1.5 | 42.0 ± 0.5 | 41.6 ± 2.6 | 41.2 ± 0.2 |
| C18:2 | 9.9 ± 1.3 | 12.6 ± 0.1 | 10.3 ± 0.2 | 10.3 ± 0.0 | 9.0 ± 1.4 | 9.1 ± 0.1 |
| C18:3 | 8.7 ± 3.1 | 7.1 ± 0.2 | 9.4 ± 1.0 | 9.3 ± 0.1 | 9.7 ± 2.4 | 7.9 ± 0.1 |
| C20:0 | 0.2 ± 0.1 | 0.6 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.1 | 0.7 ± 0.0 |
| C20:1 | 0.1 ± 0.0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| C22:0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 |
| C22:1 | ‐ | 0.2 ± 0.1 | ‐ | ‐ | ‐ | 0.8 ± 0.0 |
| C24:0 | 0.1 ± 0.0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Other | 9.3 ± 1.3 | 8.2 ± 0.2 | 8.5 ± 0.1 | 10.5± 0.7 | 10.3± 0.9 | 9.5 ± 0.1 |
| Total oil content % (w/w) | 19.6 ± 3.4 | 26.3 ± 0.3 | 22.4 ± 6.9 | ‐ | 17.6 ± 2.3 | 20.3 ± 2.1 |
In addition to establish the oil content from microalgae cultures, it is equally important to obtain an appropriate composition of fatty acid profiles, as they have a strong effect on the quality of the biodiesel produced. Table 2 depicted the composition of the major fatty acids from the cultures with different CO2 concentrations.
Gas chromatography revealed that C16:0 (palmitic acid), C18:1 (oleic acid), and C18:2 (linoleic acid) were the main known fatty acids present in the S. obliquus along the tested CO2 concentrations. The results showed that there were no significant changes in the fatty acid profiles, neither a perceptible trend, under the different tested CO2 concentrations.
Among the unsaturated fatty acids, special attention should be paid in relation to the α‐linolenic methyl ester (C18:3) and polyunsaturated methyl esters (PUFAs, ≥4 double bonds) content due to the EN 14214 38, that specifies limits of 12 and 1%, respectively. α‐Linoleic acid (C18:3), for all CO2 concentrations, assumed values under 12%, and do not present PUFAs. The unsaturated fatty acids were the main components of the total fatty acids for S. obliquus. The highest amount of unsaturated fatty acids was obtained under 5% CO2 (63.8%). Regarding EN 14214 38, S. obliquus evidenced a good profile composition for suitable biodiesel production, regardless CO2 concentrations, and the biodiesel produced would have low viscosity due to the unsaturated fatty acids present.
Prior studies showed that high levels of CO2 enhance the production of polyunsaturated fatty acids such as C18:2 and C18:3 39, 40.
However, it is essential to note that the low oil content obtained in the studied conditions (often below 20%) was expected, since microalgae CO2 biofixation/supplementation leads to low oil accumulation/synthesis. Several studies report that higher lipid synthesis occurs when the productivity is low, due to (some) stress(es) factor(s) (e.g., 8, 31, 41). A two‐stage production is a common resource (beyond the scope of this study) 15.
3.3.3. Cell's viability/membrane integrity at different CO2 levels
PI in association with flow cytometry was used to detect S. obliquus cell's viability/membrane integrity during the microalgal cultivations supplied with air streams enriched with different CO2 concentrations. A number of previous flow cytometric controls were carried out using exponential growing (healthy) cells and heat‐treated cells with injured membrane (Table 3), in order to ensure that S. obliquus stress response could be monitored by flow cytometry in association with PI. These flow cytometric controls were then used for further comparison with data obtained from the microalgal cultivations.
Table 3.
Percentage of Scenedesmus obliquus cells with permeabilized membrane, collected from exponential growing and heat‐treated cultures (for flow cytometric controls) and from cultivations conducted at different CO2 concentrations, at the end of the experiments
| Samples | Cells with permeabilized membrane (%) | |
|---|---|---|
| Flow cytometric controls | Exponential growing culture | 4.3 ± 0.2 |
| Heat‐treated cells | 97.0 ± 4.8 | |
| Experiments | 0% CO2 | 86.4 ± 4.3 |
| 2.5% CO2 | 6.7 ± 0.3 | |
| 5% CO2 | 2.2 ± 0.1 | |
| 10% CO2 | 1.5 ± 0.1 | |
| 15% CO2 | 5.4 ± 0.3 |
Scenedesmus obliquus exponential growing culture showed only 4.3% of cells with injured membrane. As expected, the heat treatment induced massive S. obliquus cell membrane disruption. These preliminary flow cytometric control tests demonstrated that flow cytometry in association with PI was able to detect membrane integrity changes as a result of environmental stress conditions. Table 3 and Fig. 4 also show the percentage of permeabilized cells at the end of the experiments and along the microalgal cultivations, respectively. The proportion of damaged cells increased significantly for the control assay (0% CO2), attaining 86.4% at the end of the cultivation (13 days).
Figure 4.
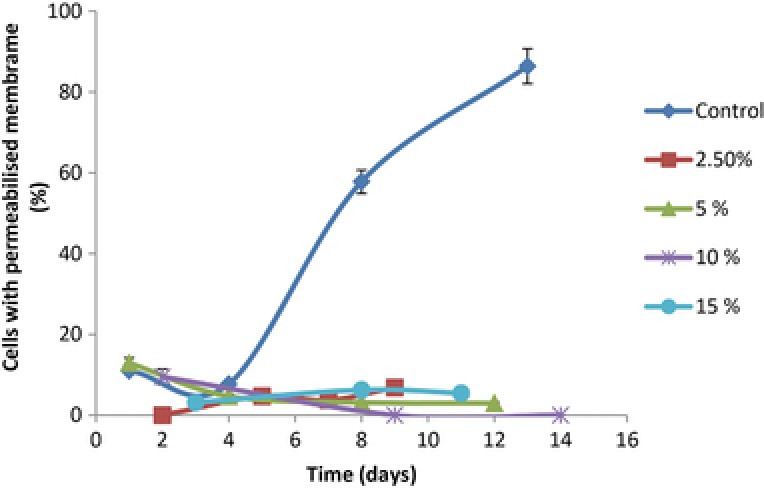
Percentage of Scenedesmus obliquus cells with permeabilized membrane grown at different CO2 concentrations. Results represent the average value of two independent replicate with a SD lower than 10%.
The experiments supplied with CO2‐enriched air displayed, at the end of the cultivations, a percentage of permeabilized cells that varied between 1.5 and 6.7%.
Despite the specific growth rate and maximum biomass concentration were lower for the tested 15% CO2 (Table 1), the proportion of stressed cells was similar for all these experiments, indicating that at 15 % CO2 the cells did not experience stress or adverse environment.
The difference between the proportion of permeabilized cells found in the control and experiments supplied with CO2‐enriched air (Fig. 3) was attributed to the carbon limitation that might have occurred in the control experience, resulting in an extreme high pH medium (10.7) that induced a massive membrane permeabilization. According to Vonshak 42, the pH medium affects both gas absorption and nutrient availability which, in turn, affects the cell metabolism. Lane and Burris 43 reported that S. quadricauda could maintain its intracellular pH close to neutrality at external pH values between 3.0 and 9.0. In this work, the pH medium control attained values higher than 10.0 as a result of carbon depletion. At such pH, and without carbon, it is likely that the cells could not generate enough energy to maintain their homeostasis, leading to cell membrane damage and, eventually, to cell death.
4. Concluding remarks
Among the three microalga tested in this work, Scenedesmus obliquus seems to be the most appropriate in terms of kinetic growth parameters in addition to having a better oil accumulation, which is economically important for biodiesel production purposes.
Cultures supplemented with CO2‐enriched air enhanced growth kinetics (growth rates and productivities) and produced considerably high oil yields (>20% w w−1, particularly under 5% (v/v) CO2 concentrations).
Flow cytometry—an innovative approach to CO2 mitigation studies—revealed a high proportion of permeabilized cells in the control assay, which could be due to the environmental pH increase as a result of carbon depletion. The cultures supplied with CO2‐enriched air, revealed a low level of permeabilized cells (below 7%). This highlighted the benefits of using CO2‐enriched air both at the cellular level and for biomass production. The fact that S. obliquus did not show any need for a preadaptation to 15% (v/v) CO2 is important for future applications of this microalga in real flue gases.
This work also highlighted the benefits of using flow cytometry as a technique that could successfully differentiate cells that were intact from those that had an injured membrane, allowing for better microalgae screening for tolerance to high levels of CO2.
Practical application
Microalgae have a great potential to mitigate CO2, contributing for the diminishing of the gases with greenhouse effect, thus climate change and global warming. Besides, their biomass could be used as a source of important high‐value compounds, namely pigments, to be used in several industries, as well as a feedstock for the bioenergy industry. The screening of microalgae in order to choose the best one in terms of CO2 mitigation concomitant with an interesting biomass composition is fundamental. Flow cytometry is an innovative technique to evaluate the cell viability/membrane integrity allowing to a good and fast in‐line microalgae screening for tolerance to high levels of CO2.
The authors have declared no conflict of interest.
Supporting information
supporting information
Acknowledgments
Batista acknowledges postdoctoral grant from Fundação para a Ciência e a Tecnologia (SFRH/BPD/84812/2012). COST Action 1408 EUALGAE‐ European network for algal bioproducts (2015‐2018). The authors thank Dr. Cristina Oliveira for fatty acids analysis by gas chromatography, Dr. Paula Passarinho for support in HPLC pigment analysis, Graça Gomes and Natércia Santos for technical support. The authors would also like to thank the Editor and the reviewers for their valuable comments that have improved the manuscript quality.
5 References
- 1. Chiu, S. , Kaob, C. Y. , Tsai, M. T. , Ong, S. C. et al., Lipid accumulation and CO2 utilization of Nannochloropsis oculata in response to CO2 aeration. Bioresour. Technol. 2009, 100(2), 833–838. [DOI] [PubMed] [Google Scholar]
- 2. Cheah, W. Y. , Show, P. L. , Chang, J‐S. , Ling, T. C. et al ., Biosequestration of atmospheric CO2 and flue gas‐containing CO2 by microalgae. Bioresour. Technol. 2015, 184, 190–201. [DOI] [PubMed] [Google Scholar]
- 3. Hansen, J. , Sato, M. , Ruedy, R. , Kharecha, P. et al ., Dangerous human‐made interference with climate: A GISS modelE study. Atmos. Chem. Phys. 2007, 7, 2287–2312. [Google Scholar]
- 4. Kumar, A. , Ergas, S. , Yuan, X. , Sahu, A. et al., Enhanced CO2 fixation and biofuel production via microalgae: Recent developments and future directions. Trends Biotechnol. 2010, 28, 371–380. [DOI] [PubMed] [Google Scholar]
- 5. Bilanovic, D. , Andargatchew, A. , Kroeger, T. , Shelef, G. , Freshwater and marine microalgae sequestering of CO2 at different C and N concentrations – Response surface methodology analysis. Energ. Convers. Manage. 2009, 50, 262–267. [Google Scholar]
- 6. Razzak, S. A. , Al‐Aslani, I. , Hossain, M. M. , Hydrodynamics and mass transfer of CO2 in water in a tubular photobioreactor. Eng. Life Sci. 2016, 16, 355–363. 10.1002/elsc1040.201500063. [DOI] [Google Scholar]
- 7. Madigan, M. T. , Martinko, J. M. , Bender, K. S. , Buckley, D. H. et al., Brock Biology of Microorganisms, Benjamin Cummings, Boston, MA: 2014, . [Google Scholar]
- 8. Gouveia, L. , Oliveira, A. C. , Microalgae as a raw material for biofuels production. J. Ind. Microbiol. Biot. 2009, 36, 269–274. [DOI] [PubMed] [Google Scholar]
- 9. Pacheco, R. , Ferreira, A. F. , Pinto, T. , Nobre, B. P. et al., The production of pigments & hydrogen through a Spirogyra sp. biorefinery. Energ. Convers. Manage. 2015, 89, 789–797. [Google Scholar]
- 10. Batista, A. P. , Moura, P. , Marques, P. A. S. S. , Ortigueira, J. et al., Scenedesmus obliquus as feedstock for biohydrogen production by Enterobacter aerogenes and Clostridium butyricum . Fuel 2014, 117, 537–543. [Google Scholar]
- 11. Toledo‐Cervantes, A. , Morales, M. , Novelo, E. , Revah, S. , Carbon dioxide fixation and lipid storage by Scenedesmus obtusiusculus . Bioresour. Technol. 2013, 130, 652–658. [DOI] [PubMed] [Google Scholar]
- 12. Miranda, J. R. , Passarinho, P. C. , Gouveia, L. , Pre‐treatment optimization of Scenedesmus obliquus microalga for bioethanol production. Bioresour. Technol. 2012, 104, 342–348. [DOI] [PubMed] [Google Scholar]
- 13. De Morais, M. G. , Costa, J. A. V. , Carbon dioxide fixation by Chlorella kessleri, C. vulgaris, Scenedesmus obliquus and Spirulina sp. cultivated in flasks and vertical tubular photobioreactors. Biotechnol. Lett. 2007, 29, 1349–1352. [DOI] [PubMed] [Google Scholar]
- 14. Lee, J. , Kim, D. , Lee, J. , Park, S. et al., Effects of SO2 and NO on growth of Chlorella sp. KR‐1. Biosour. Technol. 2002, 82, 2–5. [DOI] [PubMed] [Google Scholar]
- 15. Ho, S. H. , Chen, C. Y. , Yeh, K. L. , Chen, W. M. et al., Characterization of photosynthetic carbon dioxide fixation ability of indigenous Scenedesmus obliquus isolates. Biochem. Eng. J. 2010, 53, 57–62. [Google Scholar]
- 16. Yoo, C. , Jun, S. Y. , Lee, J. Y. , Ahn, C. Y. et al., Selection of microalgae for lipid production under high levels carbon dioxide. Bioresour. Technol. 2010, 101, 171–74. [DOI] [PubMed] [Google Scholar]
- 17. Chiu, S. Y. , Kaob, C. Y. , Chen, C. H. , Kuan, T. C. et al., Reduction of CO2 by a high‐density culture of Chlorella sp. in a semicontinuous photobioreactor. Bioresour. Technol. 2008, 99, 3389–3396. [DOI] [PubMed] [Google Scholar]
- 18. Iwasaki, I. , Hu, Q. , Kurano, N. , Miyachi, S. , Effect of extremely high‐CO2 stress on energy distribution between photosystem I and photosystem II in a “high‐CO2” tolerant green alga, Chlorococcum littorale and the intolerant green alga Stichococcus bacillaris . J. Photochem. Photobiol. B 1998, 44, 184–190. [Google Scholar]
- 19. Murakami, M. , Ikenouchi, M. , The biological CO2 fixation and utilization project by rite (2)—Screening and breeding of microalgae with high capability in fixing CO2 . Energ. Convers. Manage. 1997, 38, S493–S497. [Google Scholar]
- 20. Vonshak, A. , Laboratory techniques for the cultivation of microalgae, in Richmond, A. (Ed.), Handbook of Microalgal Mass Culture, 1st ed, Balckwell, Boca Raton, FL: 1986, pp. 117–145. [Google Scholar]
- 21. Chisti, Y. , Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [DOI] [PubMed] [Google Scholar]
- 22. Da Silva, T. L. , Feijão, D. , Roseiro, J. C. , Reis, A. , Monitoring Rhodotorula glutinis CCMI 145 physiological response and oil production growing on xylose and glucose using multi‐parameter flow cytometry. Bioresour. Technol. 2011, 102, 2998–3006. [DOI] [PubMed] [Google Scholar]
- 23. Lepage, G. , Roy, C. C. , Direct transesterification of all classes of lipids in a one‐step reaction. J. Lipid Res. 1986, 27, 114–120. [PubMed] [Google Scholar]
- 24. Ritchie, R. J. , Universal chlorophyll equations for estimating chlorophylls a, b, c, and d and total chlorophylls in natural assemblages of photosynthetic organisms using acetone, methanol, or ethanol solvents. Photosynthetica 2008, 46, 115–126. [Google Scholar]
- 25. De Morais, M. G. , Costa, J. A. V. , Isolation and selection of microalgae from coal fired thermoelectric power plant for biofixation of carbon dioxide. Energ. Conver. Manage. 2007, 48, 2169–2173. [Google Scholar]
- 26. Lam, M. K. , Lee, K. T. , Effect of carbon source towards the growth of Chlorella vulgaris for CO2 bio‐mitigation and biodiesel production. Int. J. Greenh. Gas Control 2013, 14, 169–176. [Google Scholar]
- 27. Zheng, H. , Gao, Z. , Yin, F. , Ji, X. et al., Effect of CO2 supply conditions on lipid production of Chlorella vulgaris from enzymatic hydrolysates of lipid‐extracted microalgal biomass residues. Bioresour. Technol. 2012, 126, 124–30. [DOI] [PubMed] [Google Scholar]
- 28. Nayak, M. , Karemore, A. , Sen, R. , Performance evaluation of microalgae for concomitant wastewater bioremediation, CO2 biofixation and lipid biosynthesis for biodiesel application. Algal Res. 2016, 16, 216–223. [Google Scholar]
- 29. Salih, F. M. , Microalgae tolerance to high concentrations of carbon dioxide: A review. J. Environ. Protect. 2011, 2, 648–654. [Google Scholar]
- 30. Ho, S. H. , Chen, W. M. , Chang, J. S. , Scenedesmus obliquus CNW‐N as a potential candidate for CO2 mitigation and biodiesel production. Bioresour. Technol. 2010, 101, 8725–8730. [DOI] [PubMed] [Google Scholar]
- 31. Campenni’, L. , Nobre, B. P. , Santos, A. C. , Oliveira, A. C. et al., Carotenoid and lipid production by the autotrophic microalga Chlorella protothecoides under nutritional, salinity, and luminosity stress conditions. Appl. Microbiol. Biotech. 2013, 97, 1383–1393. [DOI] [PubMed] [Google Scholar]
- 32. Miao, X. , Wu, Q. , High yield bio‐oil production from fast pyrolysis by metabolic controlling of Chlorella protothecoides . J. Biotechnol. 2004, 110, 85–93. [DOI] [PubMed] [Google Scholar]
- 33. Gouveia, L. , Veloso, V. , Reis, A. , Fernandes, H. L. et al., Evolution of pigment composition in Chlorella vulgaris during carotenogenesis. Bioresour. Technol. 1996, 57, 157–159. [Google Scholar]
- 34. Seyfabadi, J. , Ramezanpour, Z. , Khoeyi, Z. A. , Protein, fatty acid, and pigment content of Chlorella vulgaris under different light regimes. J. Appl. Phycol. 2011, 23, 721–726. [Google Scholar]
- 35. Del Campo, J. A. , García‐González, M. , Guerrero, M. G. , Outdoor cultivation of microalgae for carotenoid production: Current state and perspectives. Appl. Microb. Biotechnol. 2007, 74, 1163–1174. [DOI] [PubMed] [Google Scholar]
- 36. Fulke, A. B. , Mudliar, S. N. , Yadav, R. , Shekh, A. et al., Bio‐mitigation of CO2, calcite formation and simultaneous biodiesel precursors production using Chlorella sp. Bioresour. Technol. 2010, 101, 8473–8476. [DOI] [PubMed] [Google Scholar]
- 37. De Morais, M. G. , Costa, J. A. V. , Biofixation of carbon dioxide by Spirulina sp. and Scenedesmus obliquus cultivated in a three‐stage serial tubular photobioreactor. J. Biotechnol. 2007, 129, 439–445. [DOI] [PubMed] [Google Scholar]
- 38.EN 14214, Liquid petroleum products. Fatty acid methyl esters (FAME) for use in diesel engines and heating applications. Requirements and test methods. 2012.
- 39. Tang, D. , Han, W. , Li, P. , Miao, X. et al., CO2 biofixation and fatty acid composition of Scenedesmus obliquus and Chlorella pyrenoidosa in response to different CO2 levels. Bioresour. Technol. 2011, 102, 3071–3076. [DOI] [PubMed] [Google Scholar]
- 40. Ota, M. , Kato, Y. , Watanabe, H. , Watanabe, M. et al., Fatty acid production from a highly CO2 tolerant alga, Chlorocuccum littorale, in the presence of inorganic carbon and nitrate. Bioresour. Technol. 2009, 100, 5237–5242. [DOI] [PubMed] [Google Scholar]
- 41. Gouveia, L. , Marques, A. E. , Da Silva, T. L. , Reis, A. , Neochloris oleabundans UTEX #1185: A suitable renewable lipid source for biofuel production. J. Ind. Microbiol. Biot. 2009, 36, 821–826. [DOI] [PubMed] [Google Scholar]
- 42. Vonshak, A. , Spirulina platensis (Arthrospira): Physiology, cell biology and biotechnologym. J. Appl. Phycol. 1997, 9, 295–296. [Google Scholar]
- 43. Lane, A. E. , Burris, J. E. , Effects of environmental pH on the Internal pH of Chlorella pyrenoidosa, Scenedesmus quadricauda, and Euglena mutabilis . Plant Physiol. 1981, 68, 439–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supporting information


