Abstract
Recently, bacterial cellulose (BC) based wound dressing have raised significant interests in medical fields. However, to our best knowledge, it is apparent that the BC itself has no antibacterial activity. In this study, we optimized graphene oxide‐silver (GO‐Ag) nanohybrid synthesis using Response Surface Methodology and impregnate it to BC and carefully investigate their antibacterial activities against both the Gram‐negative bacteria Escherichia coli and the Gram‐positive bacteria Staphylococcus aureus. We discover that, compared to silver nanoparticles, GO‐Ag nanohybrid with an optimal GO suspension's pH and ratio is much more effective and shows synergistically enhanced, strong antibacterial activities at rather low dose. The GO‐Ag nanohybrid is more toxic to E. coli than that to S. aureus. The antibacterial and mechanical properties of BC/GO‐Ag composite are further investigated.
Keywords: Antibacterial effects, Bacterial cellulose, Graphene oxide/Silver nanohybrid, Nanocomposite, Wound dressing
Abbreviations
- BC
Bacterial cellulose
- GO
Graphene oxide
- NPs
Nanoparticles
1. Introduction
Advanced approaches to wound healing have attracted much attention in the last decades due to the use of novel types of dressing that takes an active part in wound protection. The use of bacterial cellulose (BC) is attractive for advanced wound management because of favorable characteristics of BC such as high purity, biocompatibility, structural crystallinity, oxygen permeability, high water absorption capacity and extremely hydrophilic surface, high tensile strength, homologous structure, excellent biodegradability, biological properties and non‐toxicity. However, BC itself does not have antibacterial activity and this may reduce its effectiveness as a treatment for wounds 1.
The deficiencies mentioned above limit the application of BC in different fields; thus, there is a need for a synthesis of its composites. Owing to its structural features, BC has shown tremendous potential as a matrix in the synthesis of various composite materials. BC composites have been formed using a broad range of materials ranging from nanoparticles to polymers, many of which have been synthesized to enhancing antibacterial properties of BC 2, 3.
In general, inorganic nanomaterials play an important role in antibacterial applications due to their large surface area and the properties imparted by their particle shapes 4. Metal and metal oxide nanoparticles, well known for their highly potent antibacterial effect, include silver (Ag), titanium oxide (TiO2), copper oxide (CuO), and zinc oxide (ZnO). Like other polymeric materials, BC can be used to make composites with metals and metallic oxides through a variety of synthetic approaches 5, 6.
A wide variety of BC composites has also been explored as potential wound dressing materials. Several studies have attempted to found and investigate matrices impregnated with antibacterial active nanoparticles 7. Numerous studies have shown that BC/Ag composite has antibacterial activity against both Gram‐positive and Gram‐negative bacteria 8, 9, 10. Katepetch et al. (2013) found BC composite with ZnO nanoparticles has antibacterial activity against E. coli and S. aureus. The composite was fabricated by ultrasonic assisted in situ precipitation of zinc oxide onto the BC surface, resulting in the formation of nanocrystalline particles without affecting the 3D structure of the BC substrate 11. Recent studies report the effectiveness of BC‐based antibacterial dressings with impregnated TiO2 nanoparticles against some strains of Gram‐positive and Gram‐negative bacteria as well as against yeast colonies. In addition to antibacterial properties, the RBC‐TiO2 nanocomposites showed impressive cell adhesion and proliferation capabilities with animal fibroblast cells without showing any toxic effects 12. In another major study, Shao et al. (2015) found that BC composites with graphene oxide (GO) proved excellent antibacterial activity against E. coli and S. aureus 13.
Graphene oxide, a two‐dimensional carbon material, has attracted a great deal of attention 14. Apart from the layered structure with a large theoretical specific surface area, GO nanosheets bear abundant oxygen‐containing surface groups, such as hydroxyl, epoxide, carbonyl and carboxyl groups 15. The presence of such groups not only allows the GO sheets to be well dispersed in water to yield a colloidally stable suspension, but also offers potential application as nanoscale substrates for the fabrication of flexible GO based composite materials. For instance, GO has been employed as a support to anchor gold nanoparticles for catalytic applications 16. Very recently, it has been reported that GO could inhibit the growth of E. coli. It has also been found that graphene and GO could damage the cell membrane by direct contact with the bacteria. This material has been used as a promising building block for preparing new composites. On the other hand, it is well‐known that AgNPs possess antimicrobial activity and have been used as biocide agents in health, food and textile applications 17. Accordingly, silver nanoparticles assembled on graphene oxide sheets have been exploited as novel antibacterial systems 18.
As mentioned above, some previous studies have reported the synthesis of GO‐Ag nanocomposite and its antibacterial activity 18, 19. To the best of our knowledge, no previous study has investigated effect of GO‐Ag nanocomposite impregnation on BC. In this research, synthesis process of GO‐Ag nanohybrid was optimized using Response Surface Methodology (RSM) and impregnate on BC and investigate their antibacterial activities and mechanical properties.
2. Materials and methods
Gluconacetobacter xylinus (strain PTCCC 1734), Escherichia coli and Staphylococcus aureus were purchased from Microbiological Resources Centre, Iranian Research Organization for Science and Technology. All materials were analytical grade and used without further purification.
2.1. Production of bacterial cellulose
The Hestrin and Schramm (HS) medium used for growing G. xylinus contains 20 g/L glucose, 5 g/L peptone, 5 g/L yeast extract, 1.15 g/L citric acid, 2.7 g/L Na2HPO4, 10 mL ethanol . HS medium was adjusted to pH 6.0 and autoclaved at 120°C for 20 min. After cooling, inoculum culture was prepared by transferring G. xylinus cell suspension stored at –70°C into 100 mL HS medium and statically cultivated at 30°C for 2 days, when the cell number reached to 3.2 × 106 cell per mL without any cellulose production. The statically grown culture was then inoculated into a HS media with 10 vol. % inoculation size and Incubated at 30°C for 7 days to produce bacterial cellulose. After incubation, bacterial cellulose pellicles produced on the surface of each liquid culture medium were harvested. The residue was then washed with abundant distilled water and purified by boiling them in 1.0% NaOH for 2 h, finally thoroughly washed in distilled water 20. Then BC dewatered by the hot pressing device and dried at 40°C for 72 h.
2.2. Synthesis of nanoparticles
2.2.1. Synthesis of graphene oxide (GO)
Graphene oxide was synthesized from graphite powder using modified Hummer's method. In brief, 1 g of graphite and 0.5 g of sodium nitrate were mixed together followed by the addition of 23 mL of concentrated sulphuric acid under constant stirring. After 1 h, 3 g of potassium permanganate (KMnO4) was added gradually to the above solution while keeping the temperature less than 20°C to prevent overheating and explosion. The mixture was stirred at 35°C for 12 h and the resulting solution was diluted by adding 500 mL of water under vigorous stirring. To ensure the completion of reaction with KMnO4, the suspension was further treated with 30% H2O2 solution (5 mL). The resulting mixture was washed with HCl and H2O respectively, followed by filtration and drying, graphene oxide sheets were thus obtained 21.
2.2.2. Synthesis and optimization of graphene oxide‐silver nanohybrid
For the preparation of GO‐Ag nanohybrid, 10.0 mL of a homogeneous aqueous suspension containing 0.07 mg/mL of GO (with pHs: 5.4, 7.4, 9.4) was mixed with a defined mass of AgNO3 (by fixing the amount of GO and varying the AgNO3 weight, that provided ratio: 0.05, 0.15 and 0.25), and this volume was maintained under strong stirring (in dark room) for 90 min before adding the reducing agent. After this period, an amount of 40 mg of NaBH4 for each mL of GO dispersion was added to the system. The reaction mixture was stirred for another 30 min. For isolation of nanohybrid, the suspension centrifuged at 4000 rpm for 10 min and then the supernatant was replaced with deionized water. This process repeated for two times 22. In the aim of measuring amount of silver loaded on GO, Mohr's method was performed 23.
Optimization of GO‐Ag nanohybrid formation parameters was performed by employing the response surface methodology (RSM). Based on the literature reviews, the significant independent variables that affecting GO‐Ag nanohybrid formation are GO suspension's pH and ratio. The silver loaded on GO sheets was considered as the response. The central values (zero level) chosen for experimental design were pH = 7.4 for GO suspension and ratio = 0.15. Table 1 shows factors and levels used in the experimental design. Table 2 shows the designed experiments totally.
Table 1.
Experimental range and levels of independent factors
| Independent factors | Levels | |||
|---|---|---|---|---|
| –1 | 0 | +1 | ||
| pH of GO suspension | 5.4 | 7.4 | 9.4 | |
|
|
0.05 | 0.15 | 0.25 | |
Table 2.
Design of experimental process based on RSM model for optimization of GO‐Ag nanohybrid synthesis
| Number | Experimental design | |
|---|---|---|
| pH | ratio | |
| 1 | −1 | −1 |
| 2 | −1 | +1 |
| 3 | +1 | −1 |
| 4 | +1 | +1 |
| 5 | 0 | −1 |
| 6 | 0 | +1 |
| 7 | −1 | 0 |
| 8 | +1 | 0 |
| 9 | 0 | 0 |
| 10 | 0 | 0 |
| 11 | 0 | 0 |
| 12 | 0 | 0 |
| 13 | 0 | 0 |
2.3. Impregnation of GO‐Ag nanohybrid into bacterial cellulose
According to information obtained from experimental design, optimized GO‐Ag nanohybrid at optimal conditions of pH and] ratio was synthesized. To impregnate GO‐Ag nanohybrid into BC, the optimized GO‐Ag nanohybrid solution sprayed on wet BC at room temperature. The resulting composite dewatered using the heat press machine significantly and then dried at 40°C for 3 days.
2.4. Tensile strength and elongation tests
The mechanical properties were evaluated by conducting tensile strength and elongation‐at‐break using a Texture Analyzer according to the ASTM standard method 24. In these tests, pre‐conditioned BC/GO‐Ag composite cut into 1.0 cm (W) × 5.0 cm (L) strips and mounted between the grips of the machine with initial grip gap of 50 mm. Sample was uniaxially pulled until breaking at a cross‐head speed of 2.0 mm/min. Each test trial consisted of five replicate measurements to obtain the average values. The results of tensile and elongation tests were expressed by MPa and percentage (%), respectively.
2.5. Characterization
The characterization of the prepared nanoparticles and composites were determined by UV absorption spectrum, Scanning Electron Microscope (SEM) and Transmission Electron Microscope (TEM) analysis. Further, characteristic was determined by X‐ray Diffraction (XRD), Raman spectra, Fourier Transform Infrared Spectroscopy (FTIR) and Atomic force microscopy (AFM).
2.6. Evaluation of antibacterial activity
The antibacterial activity of GO and GO‐Ag was evaluated by microdilution assays, as described by the Clinical and Laboratory Standards Institute (CLSI). To determine the minimal inhibitory concentration (MIC) E. coli and S. aureus were used as model microorganisms. The strains were cultivated in Mueller‐Hinton broth incubated at 37°C for 24 h. The bacterial suspensions were diluted with Mueller‐Hinton broth to OD570 = 0.2 and were distributed (100 μL) in a 96‐well plate. Each well was exposed to different concentrations of GO and GO‐Ag (from 1.0 to 100.0 μg/mL). The plates were incubated at 37°C, and the bacterial growth was observed after 18 h. The test was repeated three times. The MIC was considered as the lowest nanomaterial concentration where no bacterial growth was visualized.
To investigate the antibacterial properties of BC/GO‐Ag composite, a similar procedure was followed and each well was exposed to the bacteria suspension cultured on equal parts of pure BC and BC/GO‐Ag. After incubation for 20 h at 37°C, the MIC was determined by visual examinations on the basis of the lowest concentration of sample solution in cells with no bacterial growth 19.
3. Results and discussion
3.1. Characterization
3.1.1. Bacterial cellulose
The results obtained from the SEM and FTIR analysis of BC produced by G. xylinus are shown in Figs. 1 and 2, respectively. As shown in Fig. 1 BC fibers are well‐organized 3‐D network structures and porous with interconnecting pores. The pore size varies in a certain range. The structures are nearly identical to those obtained by Wen et al. 25.
Figure 1.
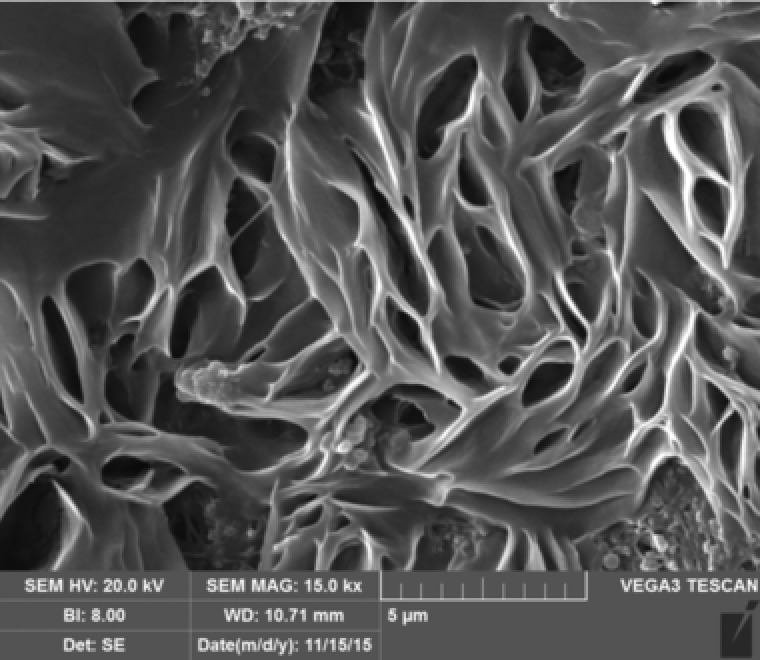
SEM micrograph of bacterial cellulose.
Figure 2.
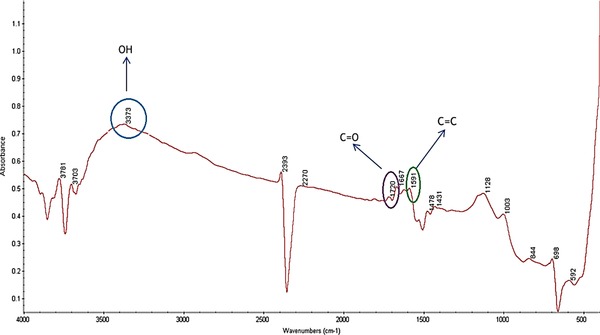
FTIR spectrum of bacterial cellulose.
Figure 2 represents the FTIR spectra of the BC. The absorption band assigned to the hydroxyl and carboxyl groups is observed at 3373 and 1720 cm−1, respectively. Furthermore, the peaks at 1591 cm−1 is derived from aromatic skeletal vibrations (C=C). Also the carbonyl amide absorption band in 1667 cm−1 may be the result of unwashed bacteria after treating with NaOH in BC purification process.
3.1.2. Graphene oxide
The results obtained from the preliminary analysis of GO are shown in Figs. 3, 4, 5. As shown in Fig. 3A synthesized GO was mostly single‐layered with a topographic height of 2 nm according to AFM characterization (Fig. 3B). Raman spectroscopy is a nondestructive method that is widely used to obtain information from the carbon‐based nanostructures. G and D are two main characteristic peaks in Raman spectroscopy. The peak G represents ordered organization of carbon atoms with sp 2 hybrid arrangement, while the peak D indicates the presence of incomplete sites on the graphene oxide (as well as functional groups bonded to the surface). As shown in Fig. 4 peak D and G appeared in the range of 1600 and 1300 cm−1, respectively. Low intensity of peak D compared to peak G indicates that graphene has been generated with little defects. Graphene produced by Li and colleagues in 2013 have peak G in 1597 cm−1 and peak D in 1358 cm−1 26. In Ding and colleagues study, peak G and D was in the range of 1600 and 1340 cm−1, respectively 27. SEM and TEM micrographs in Figs. 5A and B confirmed AFM results as single‐layered GO nanoparticles.
Figure 3.
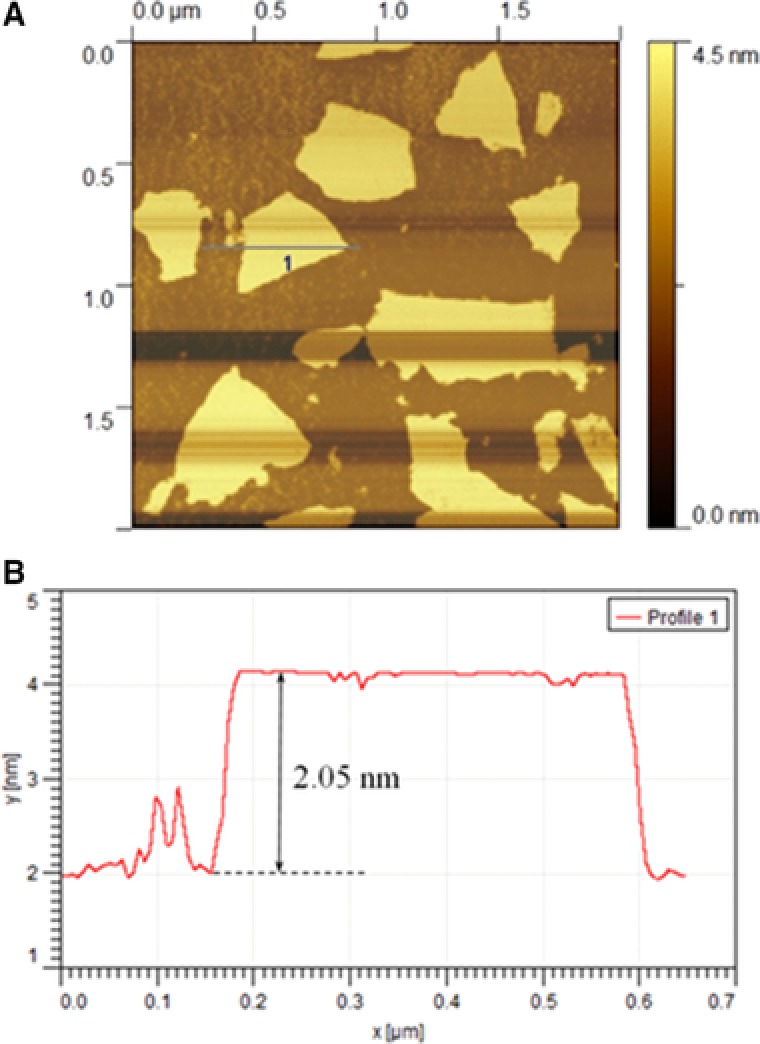
(A) AFM image and (B) height profile of GO.
Figure 4.
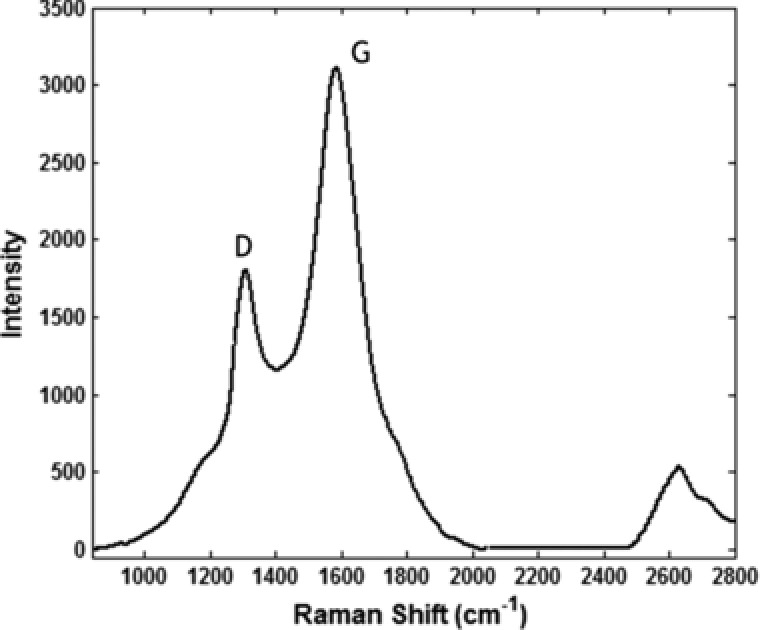
Raman spectra of GO.
Figure 5.
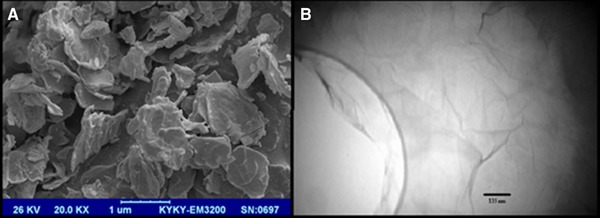
(A) TEM and (B) SEM micrograph of GO.
3.1.3. Graphene oxide‐silver nanohybrid
The results obtained from the UV absorption analysis of GO‐Ag nanohybrid are shown in Fig. 6. The absorption intensity of the peak at 420 nm increases gradually with an increase in Ag NPs concentration (Fig. 6B) that indicates higher levels of Ag NPs loaded on GO sheets, at optimized GO‐Ag nanohybrid.
Figure 6.
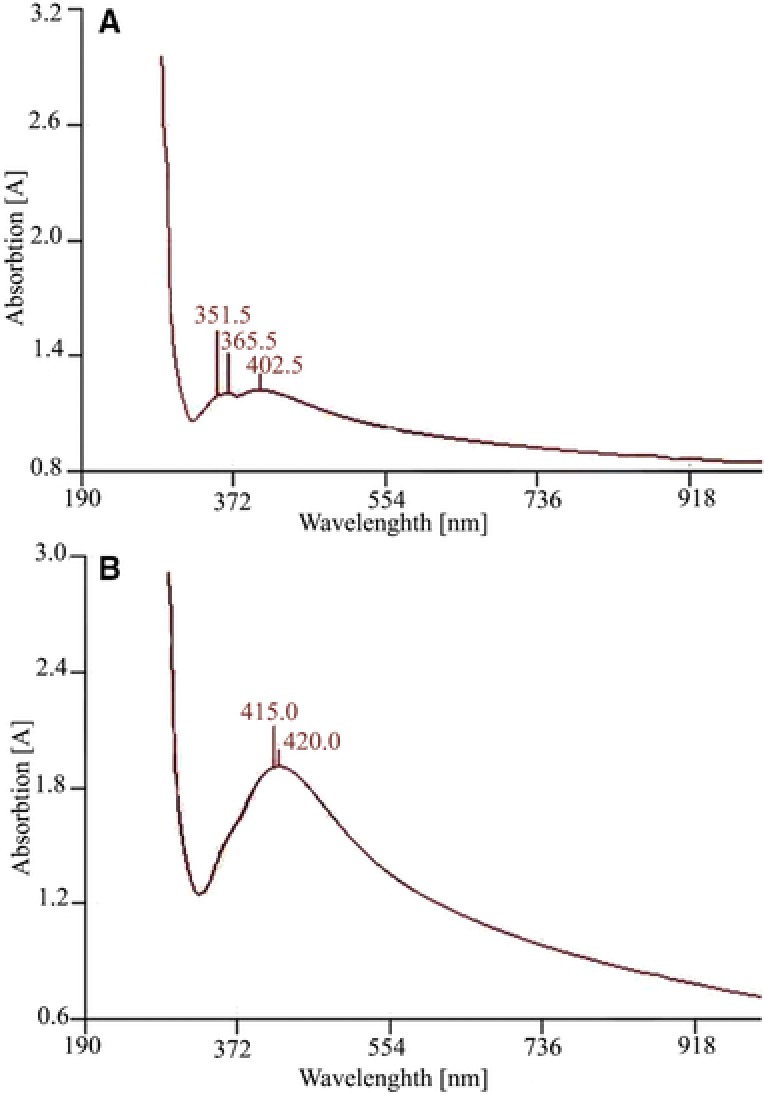
The absorption spectra of GO‐Ag nanohybrid (A) non‐optimized conditions (B) Optimized conditions.
The FTIR spectra of GO and GO‐Ag nanohybrid are shown in Fig. 7C. In GO, absorption peaks at 1050, 1200 and 1600 cm−1 were attributed to the C–O stretching, C–O–C stretching and C–O stretching, respectively. Oxygen related peaks demonstrated well oxidation of graphite by oxidation agents. In Fig. 7C (b), some peaks shifted and became lower ascribing to the reduction of GO. Resulted peaks are similar to data of Tian et al. that includes peaks in 1050 cm−1 (C–O), 1218 cm−1 (C–O–C) and 1725 cm−1 (C=O) 28.
Figure 7.
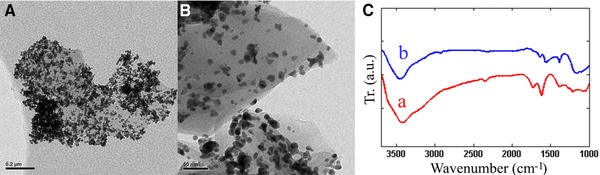
TEM images of GO‐Ag nanohybrid (A) optimized conditions (B) non‐optimized conditions and (C) FTIR spectrum of the GO (a) and GO‐Ag nanohybrid (b).
As shown in the TEM images (Figs. 7A and B), AgNPs uniformly distributed on the optimized GO‐Ag surface in comparison to non‐optimized GO‐Ag.
3.1.4. Bacterial cellulose/graphene oxide‐silver composite
As shown in Figs. 8A and B nanoparticles impregnated in porous fibers of cellulose and made a smaller pore size and formed tangled and stronger structure that caused more mechanical resistance. Figs. 8C and D represents the X‐ray diffraction (XRD) patterns of BC and BC/GO‐Ag composite. As shown in Fig. 8C, BC shows a typical diffraction peak at around 39.58o and 26.64o which confirmed BC synthesis that with the combination of GO and BC the peaks become weak. Figure 5D around 17° corresponding to the GO indicates its uniform distribution on BC matrices.
Figure 8.
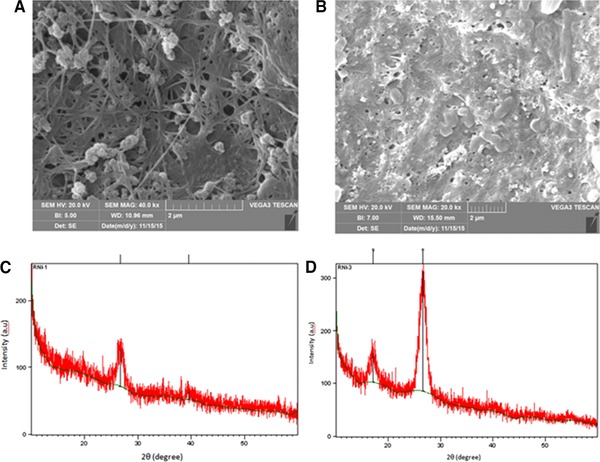
SEM images of (A) BC and (B) BC/GO‐Ag nanocomposite and the XRD spectrum of the (C) BC and (D) BC/GO‐Ag composite.
3.2. Tensile strength and elongation tests
The tensile elongation values of the BC (as control) and reinforced BC with different weight percentage of GO‐Ag nanohybrid (0.125, 0.25, 0.5 and 0.75) are shown in Fig. 9.
Figure 9.
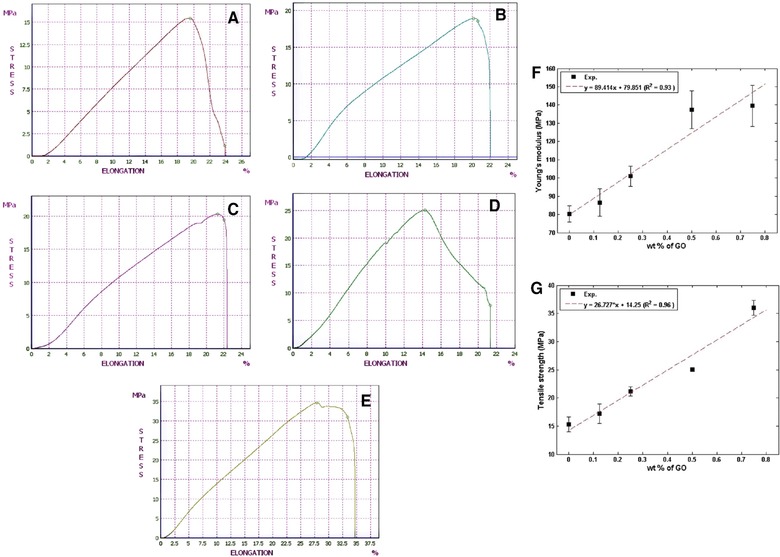
The tensile elongation of (A) BC, (B) BC with 0.125 wt.% nanohybrid, (C) BC with 0.25 wt.% of nanohybrid, (D) BC with 0.5 wt.% nanohybrid and (E) BC with 0.75 wt.% nanohybrid (F) Young's modulus and (G) Tensile strength of BC reinforced with different weight percentage.
Tensile strength and Young's module of BC/GO‐Ag composite with different weight percentage of GO‐Ag nanohybrid are shown in Figs. 9F and G. Obviously, the addition of nanohybrid reinforced the ultimate tensile strength and increased the Young's Modulus. Remarkably, this improvement was achieved at a very low nanohybrid loading (0.1–0.2 wt.%). for instance, the incorporation of 0.2 wt.% nanohybrid increased the Young's Modulus 23% and TS 43% that is similar to the results of Tian et al. (2014) 28. The increment of tensile strength should be attributed to the good coherence between the regenerated cellulose matrix and nanohybrid fillers, and the presence of strong interactions, which was confirmed by FTIR and XRD patterns.
3.3. Data analysis and evaluation of RSM model
The experiments were conducted based on the design matrix under the defined conditions. Responses obtained from the experimental runs are listed in Table 3. Thirteen experiments were conducted using the CCD method. Four experiments were repeated to estimate experimental errors.
Table 3.
Experimental design matrix and results
| RUN | Experimental design | |||
|---|---|---|---|---|
| pH |
|
Loaded silver | ||
| 1 | −1 | −1 | 3.42 | |
| 2 | −1 | +1 | 4.52 | |
| 3 | +1 | −1 | 1.90 | |
| 4 | +1 | +1 | 3.25 | |
| 5 | 0 | −1 | 2.00 | |
| 6 | 0 | +1 | 2.51 | |
| 7 | −1 | 0 | 3.92 | |
| 8 | +1 | 0 | 4.31 | |
| 9 | 0 | 0 | 5.27 | |
| 10 | 0 | 0 | 5.07 | |
| 11 | 0 | 0 | 4.97 | |
| 12 | 0 | 0 | 5.17 | |
| 13 | 0 | 0 | 5.00 | |
The obtained responses were analyzed using response surface methodology. The GO suspension's pH and ratio are variable A and B, respectively. R 1 is the amount of silver loaded onto the GO sheets. Table 4 shows Analysis of variance.
Table 4.
Analysis of variance for experimental responses at different factor levels
| Term | Sum of Sq. | Mean Sq. | F value | p‐value |
|---|---|---|---|---|
| A | 1.46 | 1.46 | 2.28 | 0.105 |
| B | 0.96 | 0.96 | 1.5 | 0.2606 |
| AB | 0.016 | 0.016 | 0.024 | 0.8803 |
| A2 | 10.68 | 10.68 | 16.66 | 0.0047 |
| B2 | 0.031 | 0.031 | 0.049 | 0.8318 |
R2 = 0.8052
According to the p‐value (p < 0.05), variables A and A2 are the important and effective factors. As a result, the final equation obtained for the R1 response is as follows:
The optimum conditions for R1 response surface based on variable code was A = 0.04 and B = –0.82 that is equal to 7.48 and 0.068 for GO suspension's pH and ratio, respectively.
Figure 10 shows a 3‐D Response surfaces for the CCD. As shown in Fig. 10, when the GO suspension's pH have a tendency to level 0 (pH = 7.4) and the ratio reduced to level −1 (0.05), the amount of R1 response increases, which is equivalent to increasing the amount of silver particles loaded on graphene sheets.
Figure 10.
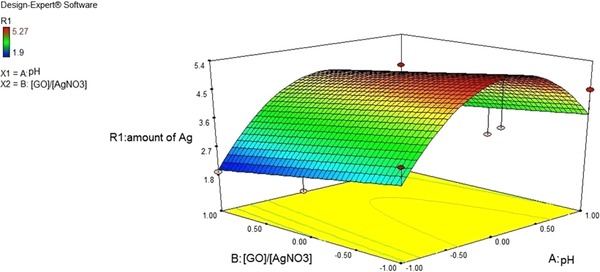
Effect of GO suspension's pH and on loaded silver on the surface of GO sheets.
3.4. Antibacterial assay
The antibacterial properties of GO‐Ag nanohybrid were investigated against E. coli and S. aureus. The MIC values for these bacterial strains are shown in Table 5. The MIC of GO‐Ag nanohybrid for E. coli and S. aureus were 0.01 and 0.17 mg/mL, respectively, which were lower than AgNO3 suspension MIC values and in the case of gram‐negative bacteria its bactericide effect was equal to the conventional antibiotic tetracycline. Taken together, the data from previous reports and the results presented here demonstrate that GO‐Ag nanohybrid impart high bactericidal activities. Consequently, this will improve their potential applications in the medical field.
Table 5.
MIC values of the GO‐Ag nanohybrid
| Bacteria | MIC | ||
|---|---|---|---|
| GO‐Ag nanohybrid | AgNO3 suspansion | Tetracycline | |
| S. aureus | 0.17 | 0.5 | 0.01 |
| E. coli | 0.01 | 0.09 | 0.01 |
The antibacterial properties of BC/GO‐Ag composite investigated by measuring OD of the bacteria cultured on pure BC and nanocomposite. Escherichia coli shows full growth on BC, while it was stopped at OD = 0.09 on BC/GO‐Ag composite.
The antibacterial activity of Ag nanoparticles was investigated against Gram‐negative bacterial strains E. coli and P. aeruginosa in Das et al. study. It was observed that P. aeruginosa was more sensitive to the Ag nanoparticles which produced maximum growth inhibition zone. Additionally, the variation of Ag concentration during the synthesis of Ag nanoparticles affects bacterial growth 29. In another study the antibacterial activity of freeze‐dried silver nanoparticle‐impregnated bacterial cellulose for was measured by the disc diffusion method. No inhibition zone was observed with the pure bacterial cellulose as control which shows the effect of Ag nanoparticles on bacteria 30. Wu et al. compared the antibacterial effect of Ag nanoparticles/bacterial cellulose composite against E. coli, S. aureus and P. aeruginosa. The results showed that the antibacterial activity against E. coli and P. aeruginosa were lower than that against S. aureus. This observation is due to the difference in cell walls between Gram‐positive and Gram‐negative bacteria 10. More studies about antibacterial activity of BC in combination with different materials is shown on Table 6.
Table 6.
The result of antibacterial activity for different composites of BC
| Composite | Bacteria | Result | Reference | Year |
|---|---|---|---|---|
| Bacterial cellulose (BC) and montmorillonite (MMT) MMT, Na‐MMT, Ca‐MMT and Cu‐MMT | Escherichia coli and Staphylococcus aureus | BC‐Cu‐MMT composites prepared with 2 and 4% MMT displayed clear zones of inhibition against E. coli and S. aureus. | 31 | 2013 |
| Silver nanoparticles into bacterial cellulose (BC) | Escherichia coli | Nearly 100% antibacterial activity | 32 | 2010 |
| Bacterial cellulose‐Chitosan | Escherichia coli and Staphylococcus aureus | In the antibacterial test, the addition of chitosan in BC showed significant growth inhibition against Escherichia coli and Staphylococcus aureus | 33 | 2013 |
| BC‐Ag nanocomposites | Escherichia coli, Staphylococcus aureus, Bacillus subtilis and Candida albicans | The experimental results showed BC‐Ag nanocomposites have excellent anti‐bacterial activities | 34 | 2015 |
| BC/sodium alginate (SA)‐ Silver sulfadiazine (AgSD) composites | Escherichia coli, Staphylococcus aureus and Candida albicans | The composite has excellent antibacterial activities and good biocompatibility | 35 | 2015 |
| SiO2 coated Cu nanoparticles (Cu@SiO2/BC) | Staphylococcus aureus and Escherichia coli | Excellent antibacterial activity | 36 | 2016 |
| ZnO‐deposited BC composites | Staphylococcus aureus and Escherichia coli | Strong antibacterial activity without a photocatalytic reaction | 37 | 2016 |
| Bacterial cellulose‐zinc oxide nanocomposites | Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus and Citrobacter freundii | The composite exhibited 90, 87.4, 94.3 and 90.9% activity against Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus and Citrobacter freundii, respectively | 38 | 2017 |
4. Concluding remarks
The GO‐Ag nanohybrid was synthesized. The effect of important parameters including GO suspension's pH and was studied. The response surface methodology was employed to determine the most suitable processing conditions to attain optimized conditions. The GO‐Ag nanohybrid impregnated to bacterial cellulose and its mechanical and antibacterial properties investigated. The presence of GO‐Ag nanohybrid increased the mechanical strength and antibacterial activity of bacterial cellulose. Taken together, the present study indicates that BC impregnated with GO‐Ag nanohybrid is a potential wound dressing material. However, more work on clinical wounds of smaller and larger animals will need to be done before its application on to humans.
Practical application
The present work describes the synthesis processes and characterization of a potential wound dressing material based on graphene oxide/silver nanocomposite‐coated bacterial cellulose. The prepared nanocomposite was characterized for their morphological properties, mechanical properties and antibacterial activity. It shows very good antibacterial properties and mechanical strength that made it a good choice for medical applications and future use.
The authors have declared no conflict of interest.
Contributor Information
Javad Mohammadnejad, Email: mohamadnejad@ut.ac.ir.
Meisam Omidi, Email: maysam.omidi@gmail.com.
5 References
- 1. Sulaeva, I. , Henniges, U. , Rosenau, T. , Potthast, A. , Bacterial cellulose as a material for wound treatment: properties and modifications: a review. Biotechnol. Adv. 2015, 33, 1547–1571. [DOI] [PubMed] [Google Scholar]
- 2. Shah, N. , Ul‐Islam, M. , Khattak W. A., Park J. K., Overview of bacterial cellulose composites: a multipurpose advanced material. Carbohydr. Polym. 2013, 98, 1585–1598. [DOI] [PubMed] [Google Scholar]
- 3. Zhu, W. , Li, W. , He, Y. , Duan, T. , In‐situ biopreparation of biocompatible bacterial cellulose/graphene oxide composites pellets. Appl. Surf. Sci. 2015, 338, 22–26. [Google Scholar]
- 4. Moghimi S. M., Farhangrazi Z. S., Just so stories: the random acts of anti‐cancer nanomedicine performance. Nanomedicine 2014, 10, 1661–1666. [DOI] [PubMed] [Google Scholar]
- 5. Beyth, N. , Houri‐Haddad, Y. , Domb, A. , Khan, W. et al., Alternative antimicrobial approach: nano‐antimicrobial materials. Evid. Based Complementary Altern Med. 2015, 2015, 246012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ravishankar Rai, V. , Jamuna Bai, A. , Nanoparticles and their potential application as antimicrobials. Formatex 2011, 197–209. [Google Scholar]
- 7. Galdiero, S. , Falanga, A. , Vitiello, M. , Cantisani, M. et al., Silver nanoparticles as potential antiviral agents. Molecules 2011, 16, 8894–8918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maneerung, T. , Tokura, S. , Rujiravanit, R. , Impregnation of silver nanoparticles into bacterial cellulose for antimicrobial wound dressing. Carbohydr. Polym. 2008, 72, 43–51. [Google Scholar]
- 9. de Santa Maria, L.C. , Santos, A. L. C. , Oliveira, P. C. , Barud, H. S. et al., Synthesis and characterization of silver nanoparticles impregnated into bacterial cellulose. Mater. Lett. 2009, 63, 797–799. [Google Scholar]
- 10. Wu, J. , Zheng, Y. , Song, W. , Luan, J. et al., In situ synthesis of silver‐nanoparticles/bacterial cellulose composites for slow‐released antimicrobial wound dressing. Carbohydr. Polym. 2014, 102, 762–771. [DOI] [PubMed] [Google Scholar]
- 11. Katepetch, C. , Rujiravanit, R. , Tamura, H. , Formation of nanocrystalline ZnO particles into bacterial cellulose pellicle by ultrasonic‐assisted in situ synthesis. Cellulose 2013, 20, 1275–1292. [Google Scholar]
- 12. Khan, S. , Ul‐Islam, M. , Khattak W. A., Ullah M. W. et al., Bacterial cellulose‐titanium dioxide nanocomposites: nanostructural characteristics, antibacterial mechanism, and biocompatibility. Cellulose 2015, 22, 565–579. [Google Scholar]
- 13. Shao, W. , Liu, H. , Liu, X. , Wang, S. et al., Anti‐bacterial performances and biocompatibility of bacterial cellulose/graphene oxide composites. RSC Adv. 2015, 5, 4795–4803. [Google Scholar]
- 14. Ma, J. , Zhang, J. , Xiong, Z. , Yong, Y. et al., Preparation, characterization and antibacterial properties of silver‐modified graphene oxide. J. Mater. Chem. 2011, 21, 3350. [Google Scholar]
- 15. Hur, J. , Shin, J. , Yoo, J. , Seo Y. S., Competitive adsorption of metals onto magnetic graphene oxide: comparison with other carbonaceous adsorbents. Sci. World J. 2015, 2015, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Puértolas, B. , Mayoral, Á. , Arenal, R. , Solsona, B. et al., High‐temperature stable gold nanoparticle catalysts for application under severe conditions: the role of TiO2 nanodomains in structure and activity. ACS Catal. 2015, 5, 1078–1086. [Google Scholar]
- 17. Das, M. R. , Sarma, R. K. , Borah, S. C. , Kumari, R. et al., The synthesis of citrate‐modified silver nanoparticles in an aqueous suspension of graphene oxide nanosheets and their antibacterial activity. Colloids Surf. B Biointerfaces 2013, 105, 128–136. [DOI] [PubMed] [Google Scholar]
- 18. De Faria, A. F. , Martinez, D. S. T. , Meira, S. M. M. , de Moraes, A. C. M. et al., Anti‐adhesion and antibacterial activity of silver nanoparticles supported on graphene oxide sheets. Colloids Surf. B Biointerfaces 2014, 113, 115–124. [DOI] [PubMed] [Google Scholar]
- 19. Yun, H. , Kim, J. D. , Choi, H. C. , Lee, C. W. , Antibacterial activity of CNT‐Ag and GO‐Ag nanocomposites against gram‐negative and gram‐positive bacteria. Bull. Korean Chem. Soc. 2013, 34, 3261–3264. [Google Scholar]
- 20. Santos, S. M. , Carbajo, J. M. , Quintana, E. , Ibarra, D. et al., Characterization of purified bacterial cellulose focused on its use on paper restoration. Carbohydr. Polym. 2015, 116, 173–181. [DOI] [PubMed] [Google Scholar]
- 21. Santosh, S. , Bhanureka, Graphene based piezo resistive sensor fabrication and its characterization, in: Second Internationa Conference on Recent Advances in Science & Engineering‐2015, 2015.
- 22. Mehl, H. , Oliveira, M. M. , Zarbin, A. J. G. , Thin and transparent films of graphene/silver nanoparticles obtained at liquid‐liquid interfaces: preparation, characterization and application as SERS substrates. J. Colloid Interface Sci. 2015, 438, 29–38. [DOI] [PubMed] [Google Scholar]
- 23. Jo, D. H. , Lee, C. H. , Jung, H. , Jeon, S. et al., Effect of amine surface density on CO2 adsorption behaviors of amine‐functionalized polystyrene. Bull. Chem. Soc. Japan 2015, 88, 1317–1322. [Google Scholar]
- 24. ASTM , D 882: standard test method for tensile properties of thin plastic sheeting. Astm 2002, 14, 1–10. [Google Scholar]
- 25. Wen, X. , Zheng, Y. , Wu, J. , Yue, L. et al., In vitro and in vivo investigation of bacterial cellulose dressing containing uniform silver sulfadiazine nanoparticles for burn wound healing. Prog. Natural Sci. Mater. Int. 2015, 25, 197–203. [Google Scholar]
- 26. Li, C. , Wang, X. , Chen, F. , Zhang, C. et al., The antifungal activity of graphene oxide‐silver nanocomposites. Biomaterials 2013, 34, 3882–3890. [DOI] [PubMed] [Google Scholar]
- 27. Ding, G. , Xie, S. , Liu, Y. , Wang, L. et al., Graphene oxide‐silver nanocomposite as SERS substrate for dye detection: effects of silver loading amount and composite dosage. Appl. Surf. Sci. 2015, 345, 310–318. [Google Scholar]
- 28. Tian, M. , Qu, L. , Zhang, X. , Zhang, K. et al., Enhanced mechanical and thermal properties of regenerated cellulose/graphene composite fibers. Carbohydr. Polym. 2014, 111, 456–462. [DOI] [PubMed] [Google Scholar]
- 29. Das, M. R. , Sarma, R. K. , Saikia, R. , Kale, V. S. et al., Synthesis of silver nanoparticles in an aqueous suspension of graphene oxide sheets and its antimicrobial activity. Colloids Surf. B Biointerfaces 2011, 83, 16–22. [DOI] [PubMed] [Google Scholar]
- 30. Maneerung, T. , Tokura, S. , Rujiravanit, R. , Impregnation of silver nanoparticles into bacterial cellulose for antimicrobial wound dressing. Carbohydr. Polym. 2008, 72, 43–51. [Google Scholar]
- 31. Ul‐Islam, M. , Khan, T. , Khattak, W. A. , Park, J. K. , Bacterial cellulose‐MMTs nanoreinforced composite films: novel wound dressing material with antibacterial properties. Cellulose 2013, 20, 589–596. [Google Scholar]
- 32. Maria, L. , Santos, A. L. , Oliveira, P. C. , Valle, A. S. et al., Preparation and antibacterial activity of silver nanoparticles impregnated in bacterial cellulose. Polimeros. 2010, 20, 72–77. [Google Scholar]
- 33. Lin, W.‐C. , Lien, C.‐C. , Yeh, H.‐J. , Yu, C.‐M. et al., Bacterial cellulose and bacterial cellulose–chitosan membranes for wound dressing applications. Carbohydr. Polym. 2013, 94, 603–611. [DOI] [PubMed] [Google Scholar]
- 34. Shao, W. , Liu, H. , Liu, X. , Sun, H. et al., pH‐responsive release behavior and anti‐bacterial activity of bacterial cellulose‐silver nanocomposites. Int. J. Biol. Macromol. 2015, 76, 209–217. [DOI] [PubMed] [Google Scholar]
- 35. Shao, W. , Liu, H. , Liu, X. , Wang, S. et al., Development of silver sulfadiazine loaded bacterial cellulose/sodium alginate composite films with enhanced antibacterial property. Carbohydr. Polym. 2015, 132, 351–358. [DOI] [PubMed] [Google Scholar]
- 36. Ma, B. , Huang, Y. , Zhu, C. , Chen, C. et al., Novel Cu@SiO2/bacterial cellulose nanofibers: Preparation and excellent performance in antibacterial activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 62, 656–661. [DOI] [PubMed] [Google Scholar]
- 37. Janpetch, N. , Saito, N. , Rujiravanit, R. , Fabrication of bacterial cellulose‐ZnO composite via solution plasma process for antibacterial applications. Carbohydr. Polym. 2016, 148, 335–344. [DOI] [PubMed] [Google Scholar]
- 38. Khalid, A. , Khan, R. , Ul‐Islam, M. , Khan, T. et al., Bacterial cellulose‐zinc oxide nanocomposites as a novel dressing system for burn wounds. Carbohydr. Polym. 2017, 164, 214–221. [DOI] [PubMed] [Google Scholar]


