Abstract
Intensive industrial and urban growth has led to the release of increasing amounts of environmental pollutants. Contamination by metals, in particular, deserves special attention due to their toxicity and potential to bioaccumulate via the food chain. Conventional techniques for the removal of toxic metals, radionuclides and precious metals from wastewater all have a number of drawbacks, such as incomplete metal extraction, high cost and risk of generating hazardous by‐products. Biosorption is a cost‐effective and environment‐friendly technology, an alternative to conventional wastewater treatment methods. Biosorption is a metabolically independent process, in which dead microbial biomass is capable of removal and concentrating metal ions from aqueous solutions. Free microbial biosorbents are of small size and low density, insufficient mechanical stability and low elasticity, which causes problems with metal ion desorption, separation of the sorbent from the medium and its regeneration. Hence, the possibilities for the implementation of continuous biosorbent processes for metal removal in flow‐type reactor systems are reduced and the practical application of biosorption in industrial conditions is limited. By immobilizing microbial biomass on suitable carriers the disadvantages of free biosorbents are eliminated and more opportunities for practical use of biosorption become available. This review examines different immobilization techniques and carriers, certain basic features and possibilities of using immobilized microbial biosorbents for the removal and concentration of metals from aqueous solutions.
Keywords: Biosorption, Heavy metals, Immobilization, Microbial biosorbents
Abbreviations
- CVAAS
Cold Vapor‐Atomic Absorption Spectrophotometry
- DSC
Differential Scanning Calorimetry
- EMC
Ethylenediamine‐modified Magnetic Chitosan Microparticle
- EYMC
Ethylenediamine‐modified Yeast Biomass coated with Magnetic Chitosan Microparticles
- FAAS
Flame Atomic Absorption Spectroscopy
- FTIR
Infrared IR Spectroscopy
- GO
Graphene Oxide
- ICP‐AES
Inductively Coupled Plasma Atomic Emission Spectroscopy
- MC
Magnetic Chitosan Microparticles
- NMR
Nuclear Magnetic Resonance
- PAA/HCl
Polyallylamine hydrochloride
- PEI
Polyethylenimene
- PVA
Polyvynyl alcohol
- SEM
Scanning Electron Microscopy
- SEM‐EDX
Scanning Electron Microscopy with Energy Dispersive X‐ray spectroscopy
- TEM
Transmission Electron Microscopy
- TGA
Thermogravimetry
- XPS
X Photoelectron Spectroscopy
- XRD
X‐ray difrracrion
- YMC
Yeast biomass coated with Magnetic Chitosan Microparticles
1. Introduction
Biosorption can be defined as a metabolically independent process, which involves the removal of inorganic and organic matter from a solution, by biological material 1, 2. Despite the vast diversity of target pollutants, most biosorption studies have focused on the removal of metals, which differ from other contaminants in that they are non‐biodegradable and easily accumulate in the food chain 3, 4, 5. Biosorption is an environmentally friendly and economical process for removal of metals, an alternative to conventional methods such as chemical precipitation 6, 7, 8, electrochemical processes 9, reverse osmosis 10, 11, ion exchange processes 12, 13, adsorption 14, 15. Along with the advantages, the methods used also have disadvantages like incomplete metal(s) removal, high energy and reagent requirements, generation of toxic sludge or other waste products, which makes careful disposal of waste requisite 16.
With the help of biosorption, toxic metals (Hg, Cr, Pb, Zn, Cu, Ni, Cd, As, Co and Sn), precious metals (Pd, Pt, Ag, Au and Ru), radio‐nuclides (U, Th, Ra, Am), dyes, fluorides, phthalates, and pharmaceuticals are removed from natural and industrial wastewaters 1.
Microbial biomass (bacteria, cyanobacteria, yeast, fungi), algae, waste biomass from the food industry and biotechnological processes, active sludge, plant and wood waste biomass, biomass containing chitin and chitosan may be used as biosorbents 17, 18. The biosorption mechanism is complex and, according to Volesky 19 and Fomina et al. 20, may involve physical adsorption, ion exchange, complex formation, reduction, precipitation.
Dead microbial biomass shows a number of advantages over living cells: low cost, absence of nutrient medium and maintenance of pure microbial cultures, high sorption and desorption rate, work over a wide pH range, use of simple equipment, rapid and easy regeneration of biomass used 21, 22.
The cell wall of microbial cells plays a key role in the removal of metal ions from aqueous solutions due to the presence of a great number of functional groups with different charge and geometry (carboxyl‐, hydroxide‐, amino‐, imidazole‐, sulfate‐, sulfhydryl‐, etc.) 1.
Biomass from: bacteria – Gram‐positive (Bacillus sp., Corynebacterium sp., etc.), Gram‐negative (Escherichia sp., Pseudomonas sp., etc.) and cyanobacteria (Anabaena sp., Sinechoestys sp., etc.), molds (Aspergillus sp., Rhizopus sp., etc.), basidiomycetes (Trichosporon sp., Trametes sp.), yeast (Saccharomyces sp., Candida sp., etc.), waste microbial biomass from the production of antibiotics, enzymes, amino acids and other biotechnological manufacturing processes can be use as biosorbents 1, 19.
Despite the fact that many types of biomass demonstrate higher sorption capacities than conventional sorbents, for example, ion‐exchange resins, their use is limited due to physical problems. Microbial biosorbents are characterized by small size and low density, insufficient mechanical stability and low elasticity. In dynamic flow mode biosorption, difficulties arise in liquid‐solid phase separation, sorbent swelling and clogging, low regeneration rate, etc. 23, 24.
Such problems can be solved by immobilizing microbial biomass onto a suitable carrier, whereby 0.5 to 1.5 mm particles with good porosity, physical and chemical stability can be reached. Immobilized biomass is easily regenerated, can be used repeatedly and incorporated into fixed and fluidized bed columns 23, 25. According to Wang and Chen, “the immobilization technique is one of the key elements for the practical application of biosorption” 19.
However, biosorption with immobilized biosorbents has certain disadvantages: extra cost, higher mechanical diffusion resistance, lower biosorbent capacity compared to free biomass, interaction between the carrier and the active sites of the biosorbent.
Despite the large number of research studies and patents, biosorption with immobilized microbial biomasses, as a method for removal of metals from natural and wastewater has not yet been sufficiently commercialized. The two major reasons may be are the above mentioned drawbacks of biosorption and the fact that the exact mechanism of the process is still not totally understood 26, 27.
The aim of this review is to analyze the main techniques and carriers used for immobilization of dead microbial biomasses, to summarize the researches conducted over the last decade on metal removal and concentration and to propose future directions for development and application of biosorption.
2. Immobilization techniques and carriers
The choice of carrier and method of immobilization of microbial biomass intended for biosorption of metal ions from natural and wastewaters is of essential importance as it determines the mechanical strength and chemical resistance of the final biosorbent particles utilized for successive sorption‐desorption cycles. The selected carriers and immobilization techniques should be inexpensive and should not raise the cost of the biosorption process.
The following classical methods, shown in Fig. 1, are used for immobilization of microbial biomass: adsorption, covalent bonds in vector compounds such as silica gel, entrapment/encapsulation in polymeric matrices and cross‐linking.
Figure 1.
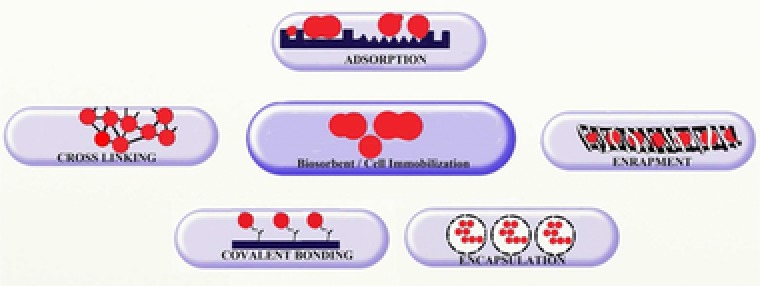
Methods of immobilization of microbial cells.
Adsorption immobilization involves retention of cellular biomass on the surface of the carrier at the expense of nonspecific Van der Waals interactions, hydrogen bonds, electrostatic and hydrophobic interactions. Silica gel, activated charcoal, zeolite, bentonite, diatomaceous earth can be used as carriers 28, 29, 30. In recent years, investigations have been conducted on nanoadsorbents, where microbial biomass is immobilized onto nano‐sized materials like silica, titania, etc. 31, 32. The carriers used in adsorption immobilization are characterized by high chemical, physical, and biological resistance. This immobilization technique is very popular because it is simple, fast and cheap, but a gradual wash‐out of the biomass from the carrier could occur 33.
In the so‐called covalent bonding immobilization, covalent bonds are formed between the carrier and the microbial biomass. For this purpose, the surface of the carrier has to be pre‐modified with appropriate reagents, which means that the additional operations make the process more expensive 34. The common vector compound (carrier) is silica gel. This technique is mainly used for immobilized algae 19, 35.
In entrapment in polymeric matrices, microbial cells are included in natural (agar, cellulose, alginate, chitosan, carrageenan) 36, 37 and synthetic carriers (polyacrylamide, polysulfone, polyvinyl alcohol, etc.) 27. One of the most commonly used natural carriers for immobilization is sodium alginate with crosslinking agent CaCl2 due to the high biocompatibility and simple gelatinization, although calcium alginate is sensitive to the presence of Na and/or K ions in the solution. Calcium alginate can swell and dissolve due to an ion exchange process between Na/K ions in the solution and the calcium ions in the beads, which limits its applicability 38, 39. Immobilization with natural polymeric carriers leads to the formation of gel particles, a major drawback being diffusion restrictions 23, 33. Synthetic carriers have higher stability than natural ones 40, 41. Encapsulation is an immobilization method, similar to entrapment, microbial cells are restricted by the membrane walls (usually in a bead forms) with free‐floating with the core space 33.
For mechanical stability, free or immobilized biomass can be cross‐linked: so‐called stapling reagents are added to the biomass and/or polymer suspension: phthalic anhydride, glutaraldehyde, epichlorhydrin, which lead to the formation of stable cell aggregates 42.
The advantages and disadvantages of commonly used classical immobilization techniques are shown in Table 1.
Table 1.
Techniques for biomass immobilization
| Technique | Carriers | Advantages | Disadvantages |
|---|---|---|---|
| Adsorption | Active charcoal, silica gel, nano‐silica, nano‐titania, etc. | Cheap, simple technique | Cell leakage |
| Еntrapment | Agar, cellulose, alginate, chitosan, carrageenan, polyacrylamide, polysulfone, PVA, etc. | Mechanical strength | Mass transfer resistance, abrasion of carrier material, cost |
| Cross‐linking | Glutaraldehyde, epichlorhydrin, etc. | Increased strength | Not universal |
| Encapsulation | Alginate, chitosan and/or crosslinking agent | Prevent cell leakage | Mass transfer resistance, fragile beads |
Microbial biomass immobilization can also be achieved with composite or carriers, a combination of inorganic and organic materials, whose joint performance is complementary, which renders composite materials with superior properties 43, 44.
To increase the biosorption capacity the primary biomass, before immobilization, can be chemically modified by introducing additional functional groups for metal ion binding 45, 46.
The so‐called biofunctional magnetic beads are noteworthy as they combine the advantages of biosorption and magnetic separation. After biosorption, the loaded biofunctional magnetic beads can be easily separated from the solution by using a weak external magnetic field. The addition of magnetite increases the mechanical strength of the biosorbent, and, therefore, its lifespan, which is important for its practical application 46, 47, 48.
3. Biosorption with immobilized microbial biomass
The efficiency of metal ion biosorption by immobilized microbial biomass depends on a number of factors: metal ion properties (ionic radius, degree of oxidation, covalent index), process conditions (medium acidity, initial metal ion concentration, biosorbent dosage and size), density of sorption centers, (depends on the biomass used and its pre‐treatment), the carrier and immobilization technique. Experimental data obtained from biosorption are modeled and simulated to elucidate the mechanism of the process, evaluate the change in operating parameters and optimization. Numerous models used in batch or continuous column modes are documented in the literature 19, 49.
To investigate the biosorption mechanism, and the stability, structure and morphology of biosorbents, a wide variety of analytical methods are used: infrared IR spectroscopy (FTIR), scanning electron microscopy (SEM), transmission electron microscopy (TEM), nuclear magnetic resonance (NMR), thermogravimetry (TGA), differential scanning calorimetry (DSC), x photoelectron spectroscopy (XPS), etc. 19, 49 see Table 2. It should be noted that some of the above‐mentioned techniques are extremely expensive for routine research, and the information they provide is not always necessary. Different techniques often carry different information, hence better interpretation of biosorption processes can be obtained by combining all research data.
Table 2.
Analytical techniques used in biosorption studies
| Analytical technique | Information |
|---|---|
| FTIR | Identification of functional groups |
| SЕМ | Confirmation and visualization of cell morphology |
| ТЕМ | Confirmation and visualization of internal cell morphology |
| NMR | Determination of active centers |
| TGA, DSC | Determination of thermal stability of sorbent |
| XPS | Determination of oxidation degree of bound metal ions |
3.1. Immobilized bacterial biosorbents
Bacteria have a complex cell wall that plays the role of a selective sorbent, combining a high surface/volume ratio and an abundance of potentially active sorption centers. The cell wall differs considerably from Gram‐negative to Gram‐positive bacteria, which is well documented in the literature 50, 51, 52. The higher sorption capacity of Gram‐positive bacteria is explained by the thicker peptidoglycan layer of the cell wall, in which teichoic and teichuronic acids are “encapsulated”, whereas the cell wall of Gram‐negative bacterial is composed of 10–20% peptidoglycan, but contains phospholipids and lipopolysaccharides 53.
Ahmad et al. 54 investigated the biosorption capacity of Bacillus subtilis biomass immobilized in calcium alginate, in batch mode toward Cd(II) ions. Under optimal conditions (pH 5.92, biosorbent mass 1 g/L, contact time 3 h, temperature 45 °C), the determined biosorbent capacity was 251.91 mg/g at an initial concentration of 496.23 mg/L. Five successful cycles of biosorption and desorption with 0.1 M HCl solution were performed.
Free heat‐inactived and polysulphone immobilized biomass of Arthrobacter sp. to remove Cu(II) ions from aqueous solution was studied in batch and continuous systems. The calculated maximum biosorption capacity, from the Langmuir isotherm model in batch mode, for the free biomass is 9.76% higher than the biosorption capacity for immobilized biomass. The immobilized biomass was used in continuous biosorption mode, a recovery rate of 89.56% and a biosorbent capacity of 32.64 mg/g were achieved, at a flow rate of 3.5 mL/min and 20 cm bed height. To evaluate the possibility of repeated use of the biosorbent, six consecutive adsorption/desorption cycles were carried out, whereat, after the sixth cycle, a decrease was observed in the biosorption capacity and Cu(II) removal 55.
Bacillus drentensis MG 21831T biomass was immobilized successfully in polysulfone +by Seo et al. 56. The resulting biosorbent had a highly porous structure, visualized by using SEM and TEM analyses. The specific surface area of the biosorbent determined by the Brunauer‐Emmett‐Teller's method was 2.65 m2/g, which is 250 times greater than the external surface area. SEM‐EDS analyses showed that Pb(II) and Cu(II) ions were successfully adsorbed as a form of plaque‐type solid crystal over the surface and inside pore walls. The estimated maximum biosorption capacity of the Langmuir model was 0.3332 mg/g for Pb(II) ions and 0.5598 mg/g for Cu(II) ions (initial metal ion concentration 0.01–100 mg/L, 20 °C, biosorbent dosage 40 g/L, contact time 440 min). In studies conducted in a continuous mode with a column (1 m in length and 0.02 m in diameter), more than 98% of Pb removal efficiency was maintained for 36 pore volumes and 1.553 g of Pb per g of biosorbent was removed. In the pilot scale studies, wastewater (with an acidity of less than 4) was treated for 40 days. At the end of the test, the biosorbent used was found to have retained its sorption capacity, the Cu, Cd, Zn, and Fe removal rates remained around 93%, a reason for the authors to claim that 1 kg of biosorbent can purify no less than 10 98 l of wastewater 57.
In a study of Xu et al., Bacillus strain CR‐7 isolated from soil was characterized by multiple metal and antibiotic resistance 58. To increase the biosorption capacity, the thermally deactivated biomass was treated with 0.1 M NaOH solution. Sodium alginate, gelatin and PVA were used for biomass immobilization. After analyzing the strength of immobilization beads and their mass transfer resistance, the immobilized biomass (1%) in 2% sodium alginate was found to be the most appropriate. Biosorption of two‐ and multi‐component solutions was performed. According to the authors, the biosorbent demonstrated an obvious “orderliness”, following the order of Cu(II) > Zn(II) in the solution containing these two metals, and following the order of Pb(II) > Cu(II) > Fe (III) > Zn(II) = Ni(II) > Cd(II) = Co(II) > Mn(II) in multi‐component solutions. The role of ‐NH groups in copper ion biosorption was established.
Waste biomass Escherichia coli, derived from monosodium glutamate fermentation industry, was modified with poly(allylamine hydrochloride) (PAA/HCl) and successfully immobilized as a chitosan fiber. SEM images showed that the modified biomass powder was totally embedded in the chitosan matrix. The biosorption capacity of the biocomposite was studied towards Pt(IV) removal in a highly acidic medium in both batch and column systems. In a column system, the biosorbent was regenerated after five sorption/desorption cycles with over 90.2% desorption efficiency, 0.005 M acidified thiourea solution was used as the eluent. The results obtained are promising for the recovery of precious metals from industrial effluents 45.
In a study conducted by Kim et al., a new biocomposite was manufactured by electrostatic attachment of polyethylenimene (PEI) to the surface of polysulfone‐biomass composite fiber, prepared through spinning of the mixture of waste Escherichia coli biomass/polysulfone in N,N‐dimethylformamide into water. The biocomposite was stable in real acetic acid industrial effluent from the Cavita process and could be used to recovery ruthenium in flow‐through column system, showing 120 beds of breakthrough volume 59. The same biosorbent can be used for recovery of Pd(II) ions from acidic solutions 60.
A biosorbent has been designed based on the reaction of iron oxide magnetic nanoparticles, n‐Fe3O4, with phthalic acid as a surface coating and protection material, and the obtained product was treated with Staphylococcus aureus for the formation of n‐Fe3O4‐ Phth‐S.aureus 61. The biosorption capacity of biosorbent and thermally deactivated biomass was studied in a batch mode for Pb(II), Ni(II) and Cu(II) ions. Under optimal conditions, the biocomposite had a higher maximum biosorption capacities for all metal ions than the thermally deactivated biomass, and where 1355, 985 and 795 μmol/g for Pb(II), Ni(II) and Cu(II) ions, respectively. The biosorbent exhibited high recovery rates of 99.4–100.0%, 92.6–97.5%, and 83.0–89.5% of Pb(II), Ni (II) and Cu(II) ions from model aqueous solutions and real contaminated water samples. The influence of concomitant ions was evaluated and the presence of Ca(II) and Mg(II) ions and was found to have an inhibitory effect. Using the FTIR analysis, functional groups located on the surface of thermally deactivated biomass of S. aureus and n‐Fe3O4‐Phth‐S. aureus were evaluated. The main functional groups on the surface of both biosorbents were found to be to some extent similar to each other. The functional groups of S. aureus and the core iron oxide nanoparticles have an important role in the sorption process of Pb(II), Ni(II) and Cu(II).
AMT‐BIOCLAIM ™ is a trademark of biosorbent derived from Bacillus subtilis waste biomass (pre‐treated with caustic soda to enhance metal‐binding), immobilized in extruded beads–PEI and glutaraldehyde. It is used to remove Cu(II), Cd(II), Pb(II), Zn(II) ions from water with greater than 99% efficiency. Economic analyses have revealed that the technology is 50% cheaper than chemical precipitation and 28% than ion‐exchange methods 49.
3.2. Immobilized yeast biosorbents
The yeast cell wall is made up of a number of organic compounds and their polymers, such as glucan (28%), mannan (31%), proteins (13%), lipids (8%), chitin and chitosan (2%) 62. The yeasts Saccharomyces cerevisiae, in free or immobilized form, are the most commonly studied biosorbents for biosorption metal removal, owing to the following four facts: yeasts are easily cultivated at a large scale; the biomass can be obtained from various food and beverage industries; S. cerevisiae is generally regarded as safe and it is an ideal model organism to identify the mechanism of biosorption in metal ion removal 63.
An interesting biosorbent is the one obtained by co‐immobilization of Jania rubens biomass and S. cerevisiae on mesoporous silica gel. It was found, in batch conditions, that the operating parameters and the presence of concomitant ions had an effect on biosorption efficiency of Th(IV) ions. For the concentration range from 50 to 500 mg/L, pH 4, sorbent dosage 10 g/L, 25°C and contact time 2 h, the maximum biosorption capacity was 26.95 mg/g 30.
Biosorption of Pb(II) ions from aqueous solutions has been studied in a batch system with ethylenediamine‐modified yeast biomass coated with magnetic chitosan microparticles (EYMC) 46. The biosorption capacity of the resulting biosorbent was compared with that of a control group of biosorbents–yeast biomass coated with magnetic chitosan microparticles (YMC), ethylenediamine‐modified magnetic chitosan microparticles (EMC) and magnetic chitosan microparticles (MC). It was found that in the pH range (2.5–6) investigated and in the specific experimental conditions (30°C, contact time 1 h), the adsorption capacity toward Pb(II) with an initial concentration of 50 mg/L decreased in the following order EYMC > EMC > YMC >MC. This fact can be explained by the introduction of additional functional groups after chemical modification with ethylenediamine. The Pb (II) ions adsorbed by the EYMC were successfully desorbed with 0.1 M EDTA solution. After four adsorption/desorption cycles, the biosorption capacity of EYMC decreased by 0.61 mg/g at an initial sorbate concentration of 50 mg/L.
Peng et al. immobilized waste biomass S. cerevisiae in Ca‐alginate (Ca‐SA), Ca‐alginate with graphene oxide (GO) and PVA‐Ca‐alginate‐graphene oxide in CaCl2‐boric acid solution 64. The obtained biosorbents were tested for U(VI) removal from aqueous solutions. Yeast gel beads prepared with 5% PVA‐1% SA‐2% yeast‐0.01% GO‐2% CaCl2 ‐saturated boric acid were observed to have the best biosorption properties and mechanical stability. The biosorption capacity determined from the Langmuir model was 32.8 mg/g. Using 0.1 M HNO3 the recovery of U(VI) ions was 91%. Comparison of the FTIR spectra of native and immobilized biomass with PVA and/or GO revealed changes in the molecular vibration of functional groups (carboxyl, amide and hydroxyl). The SEM‐EDX analysis indicates that U(VI) was adsorbed unevenly from the cell surface. Carboxyl and hydroxyl groups may be involved in U(VI) binding by yeast cells.
S. cerevisiae biomass immobilized onto the surface of chitosan‐coated magnetic nanoparticles was used to remove Cu(II) from aqueous solutions. The TEM analysis showed that the biomass had been successfully immobilized onto the surface of chitosan‐coated magnetic nanoparticles, and conglomerations were not observed. The XRD pictures confirmed that the nanoparticles were pure Fe3O4 with a spinel structure. At a concentration of copper ions of 60 mg/L and pH 4.5, a recovery rate of 96.8% was achieved 65.
A biosorbent of immobilized S. cerevisiae biomass in magnetic chitosan microspheres has been proposed for removal of Sr(II), Co(II), and Cs(I) from one‐ and two‐component solutions 66, 67. Initial pH values had significant effect on the removal of Sr(II), Co(II) and Cs(I). In the biosorption of two‐component solutions, the presence of second ions inhibited the biosorption of the first, the sorption capacity in one‐ and two‐component solutions changed as follows: Sr(II) > Co(II) > Cs(I).
Choudhury et al. immobilized dead S. cerevisiae biomass on titania nanopowder, and used glutaraldehyde as a cross‐linker. For the optimization of the process, a central composite design model was used, the design matrix was constructed by a two‐level three factor full factorial method. The results showed a 99.92% recovery rate at pH 1, an initial concentration of 100 mg/L, and 82.5 min contact time. The maximum biosorption capacity was determined to be 162.07 mg/g. The nanocomposite was further applied to spiked water and real effluent of tannery for effective sorption of Cr(VI) 32.
Bai et al. constructed a magnetic biocomposite for removal of uranium from aqueous solutions in a batch system. Rhodotorula glutinis biomass was supported on the nanoparticles of iron oxide. The biosorption capacity enhances with the increase in the initial concentration of the sorbate and temperature. The presence of concomitant potassium, sodium, calcium and magnesium ions did not show any interfering effect 68.
3.3. Immobilized fungus biosorbents
The fungal cell wall is made up of a microfibrillar layer and an amorphous layer. The cell wall is negatively charged due to the presence of carboxyl and phosphate groups while the amino groups are positively charged at low pH values of the medium. In addition to electrostatic attraction of metal ions to the above groups, formation of complexes containing N or O donor atoms (chitin and chitosan) is possible. Because of the low protein content in the cell wall, the involvement of amino groups in the metal‐ion retention is poor 19.
The most commonly used biosorbents, in the form of free and immobilized biomass, are the micromycetes of the Aspergillus, Rhizopus, Trichoderma, etc. genera 26. A characteristic feature of micromycetes is that they grow rapidly on inexpensive culture media, and the amount of biomass produced is abundant. Many of them are producers used in biotechnological industries to provide accessible and low cost waste biomass 69.
Biosorption of Cr(VI), Ni(II) and Zn(II) ions from model solutions and from electroplating effluent has been studied on Trichoderma viride biomass immobilized in calcium alginate into a continuous packed‐bed column. With the increase in the flow rate and initial metal ion concentration the breakthrough time and sorption capacity decreased. Increasing the bed height in the column increased the breakthrough time and sorption capacity. Five adsorption/desorption cycles were performed, the recovery efficiency for Cr(VI), Ni(II) and Zn(II) ions was determined to be 40.1, 75, and 53%, respectively 70.
Tan and Ting immobilized live and dead Trichoderma asperellum biomass in calcium alginate for Cu(II) biosorption. Plain alginate beads were used as a control. The order of copper uptake capacity of these biosorbents was: immobilized dead cells > immobilized living cells > blank Ca‐alginate. Adsorbed Cu(II) ions were successfully desorbed with 10 mM HCl solution 37.
Trichoderma harzianium biomass was immobilized onto Ca‐alginate and the resulting biosorbent was used for removal of uranium from aqueous solutions in continuous mode. The adsorption column containing 1.5 g dry weight of immobilized material purified 8.5 l of bacterial leach liquor (58 mg/L uranium concentration) before breakthrough occurred and the biosorbent became saturated after 25 L of influent volume. The biosorbed uranium was recovered in 200 mL of 0.1 M HCl resulting in 98.1–99.3% elution 71.
In the studies of Verma et al., Penicillium citrinum biomass, isolated from copper polluted sites, was easy cultivated and immobilized in calcium alginate and was used as a biosorbent for Cu(II) removal from model aqueous solutions. The maximum biosorption capacity for the immobilized biomass was 25 mg/g, and 22.7 mg/g for the free biomass at an initial concentration of copper ions of 20 to 90 mg/L 72.
For the removal of Ni(II) and Zn(II) ions, a biosorbent was designed by immobilizing thermally deactivated Pencillium fellutinum biomass in sodium bentonite 28. Under optimized process conditions, the maximum biosorption capacities determined by the Langmuir model were 111 mg/g for Ni(II) and 0.476 mg/g for Zn(II) ions. The presence of surfactant and salts (CaCl2, KCl, NaCl, CuSO4, FeCl3) was found to have no influence on the biosorption of the studied ions. Seven sorption/desorption cycles were performed using a 0.1 M solution of NaOH for ions desorption.
A low‐cost membrane‐type biosorbent was prepared by entrapping Penicillium waste biomass into the cross‐linked network of chitosan. Mycelium, сhitosan, and the membrane‐type biosorbent had similar FTIR spectra with almost the same peaks indicating that the main functional groups such as amide, carboxyl and hydroxyl groups were retained during the preparation process. The ‐NH and ‐OH groups of the membrane‐type biosorbent were involved in Cu(II) biosorption. The biosorbent was used for Cu(II) removal from wastewater in a plate column reactor, where it could be used successfully for 10 biosorption‐desorption‐regeneration cycles 73.
Aspergillus niger biomass was immobilized into Ca‐alginate gel 74. The biosorption capacity of the biosorbent was studied in batch mode for uranium(VI) ions in dependency of various operational factors, like initial pH of solution, contact time, initial concentration and biomass dosage. It was found that biosorption was strongly dependent from the pH of solution. The highest removal efficiency, for 50 mg/L U(VI) ions, was approximately 90% at pH 5, and the lowest – at pH 2. This phenomenon is due that the pH affect the availability of the binding sites on the biosorbent as also the speciation of uranium ions in solution. The recovery rate of the loaded U(VI) in 50 mL of 0.1 M HCl reached 93.09%.
Nanosilica (NSi) was used as a carrier for the immobilization of biomass of the genera Aspergillus, Fusarium and Penicillium. The biosorption capacities of the biosorbents NSi‐Asp, NSi‐Fus and NSi‐Pen toward Cr(III) and Cr(VI) ions from aqueous solutions were studied for different acidities of the medium, contact time, biosorbent amount and initial concentration of the sorbates. The maximum biosorption capacities for Cr(III) at pH 7 were 312.5, 357.1, and 212.8 mg/g for NSi‐Asp, NSi‐Fus, and NSi‐Pen, and for Cr(VI), at pH 2, were 526.3, 416.7 и 243.9 mg/g, respectively. The time to reach adsorption equilibrium under the experimental conditions was 15 min. A potential for speciation analysis and selective extraction of Cr(III) and Cr(VI) ions from aqueous effluents was indicated 75.
For removal of Cd(II) ions from model solutions, tap water, sea water and sea sediment samples, a biosorbent was designed by immobilizing thermally deactivated Aspergillus ustus biomass onto silicon dioxide‐nano‐powder. The biosorption capacity was estimated to be 204.08 μmol/g. The removal of Cd(II) ions from tap water was found to be 97.7%, established in two passage steps over multi‐stages micro‐column systems. In an analogous procedure with sea water samples, 97% extraction was obtained 76.
Mesoporous magnetic beads were prepared by entrapping powdered Rhizopus cohnii biomass and magnetic particles in the matrix of sodium alginate and PVA, which was formed by ionic polymerazition 47. The biofunctional magnetic beads show a good operational stability in wide pH (1 –12) and temperature (0–50°C) intervals. At pH 2.0 and 12 h contact time approximately 100% removal of Cr(VI) was observed at 40 mg/L initial concentration. By means of FTIR and SEM, it was established that the main groups involved in the Cr(VI) ions biosorption were NH3 +, NH2 +, –NH. A partial reduction of Cr(VI) to Cr(III) was observed during the biosorption. Five adsorption‐desorption cycles were performed using 0.1 M NaOH solution as the eluent. After the 5th cycle, the biosorption capacity decreased by 0.4 mg/g.
Akar et al. used montmorillonite type clay mineral as a carrier for immobilization of white rot fungi Trametes versicolor. SEM analysis shown that after immobilization, the biomass was uniformly distributed on the carrier. After Cu(II) biosorption, the pore size of the sorbent significantly decreased, due to the contribution of its macroporous structure. The biocomposite was successfully used to remove copper ions from real wastewater in continuous mode 29.
It is obvious that microbial biomasses are able to remove metal ions from model, natural and wastewater samples, but the biosorption capacity of each biosorbent depends on its pretreatment, chemical modification, method of immobilization and carrier. Table 3 shows maximum biosorption capacities of biosorbents, determined by Langmuir model, toward metal ions.
Table 3.
Maximum biosorption capacities for metal removal and operating conditions for various immobilized microbial biomass
| Biosorbent | Matrix | Metal ion | Concentration (mg/L) | Maximum capacity (mg/g) | pH | Dose (g/L) | Temperature (°C) | Time (h) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Arthrobacter sp. | Polysulfone | Cu(II) | 50–200 | 158.7 | 5 | n.a. | 30 | 1 | 55 |
| E.coli, PAA/HCl | Chitosan | Pt(IV) | 0–500 | 290.98 | 2 | 1 | 45 | 24 | 45 |
| E.coli | PEI/ polysulfone | Pd(II) | 0–500 | 216.9 | 2 | 0.66 | 25 | 24 | 60 |
| S.cerevisiae | Ca alginate | Cd(II) | 10–169 | 17.48 | 6.5 | n.a. | 20 | 3 | 84 |
| S.cerevisiae, modified | Chitosan, Fe3O4 | Pb(II) | 10–500 | 121.26 | 5.5 | 1 | 20 | 1 | 46 |
| S.cerevisiae, histidine | Chitosan | Ni(II) | 25–250 | 104.2 | 6 | 2 | 30 | 3 | 85 |
| S.cerevisiae | Chitosan, Fe3O4 | Cu(II) | 40–300 | 144.9 | 4.5 | 1.5 | 24 | 1 | 65 |
| S.cerevisiae | Chitosan, Fe3O4 | Sr(II) | 5–300 | 81.96 | 8 | 2 | n.a. | 5 | 66 |
| S.cerevisiae | Chitosan, Fe3O4 | Sr(II) | 5–300 | 36.97 | 6 | 2 | n.a. | 5 | 67 |
| Co(II) | 5–300 | 30.92 | 6 | 2 | n.a. | 5 | 67 | ||
| Cs(I) | 5–300 | 16.67 | 6 | 2 | n.a. | 5 | 67 | ||
| Rhodotorula glutinis | Nano iron oxide | U(VI) | 10–320 | 226 | 6 | 5.15 | 50 | 0.5 | 68 |
| Trichoderma asperellum | Ca alginate | Cu(II) | 50–600 | 140.85 | 5 | n.a. | 28 | 8 | 37 |
| Penicillium sp. | Chitosan | Cu(II) | 25–2000 | 126.58 | n.a. | 1 | 25 | 6 | 73 |
| Aspergillus niger | Ca alginate | U(VI) | 10–200 | 694.4 | 5 | 0.3 | 30 | 9 | 74 |
| Rhizopus cohnii, Fe3O4 | Na alginate/PVA | Cr(VI) | 5–200 | 6.97 | 1 | 10 | 37 | 8 | 47 |
| Trametes versicolor | Montmorillonite | Cu(II) | 25–200 | 62.80 | 5 | 1.2 | n.a. | 0.66 | 29 |
n.a. = not available.
In fact, researches of different authors cannot be compared: the maximum biosorption capacity is determined under different operating conditions and there is no standard procedure for determining the dry weight of the biomass and biosorbent used (temperature and time).
4. Immobilized microbial biomass used in solid phase extraction
Immobilized microbial biomass was successfully used as a sorbent in SPE, where the combination of pre‐concentration and metal ion separation by instrumental analysis (like flame atomic absorption spectroscopy–FAAS; cold vapor‐atomic absorption spectrophotometry–CVAAS; inductively coupled plasma atomic emission spectroscopy ‐ ICP‐AES) allowed the determination of very low concentrations (in some cases several orders of magnitude lower than the detection limits of the direct method).
In recent years, researches have been focused on nano‐carriers for biomass immobilization like TiO2 77, 78, 79, SiO2 80, multi‐walled carbon nanotubes 81, 82 and nanodiamond 83. They have been used as solid supports for biomasses, as a result highest preconcentration factors and lowest limit of detection were reached.
Solid phase extraction using biosorbents is an ecological method for pre‐concentrating and separating metal ions from different matrices and is characterized by a number of advantages over conventional sorbents: does not require the use of organic solvents, eliminates the need for chelate structures, a quick and easy conditioning stage, easy sorbent regeneration, etc.
Table 4 shows several types of immobilized microbial biomass used in SPE, such as sorbents for pre‐concentration and speciation analysis of metal ions from different matrices.
Table 4.
Immobilized microbial biomass – sorbents in solid phase extraction
| Biomass | Carrier | Metal ions | Sample for analysis | Method | Ref. |
|---|---|---|---|---|---|
| Anoxybacillus flavithermus | Amberlite XAD‐16 | Th(IV), Ce(III) | Bastnaesite ore sample | ICP‐OES | 86 |
| Bacillus altitudinis | Nanodiamond | Co(II), Cr(VI), Hg(II), Pb(II) | Food samples | ICP‐OES | 83 |
| Bacillus subtilis | Amberlite XAD‐4 | Cu(II), Cd(II) | Natural water | FAAS | 87 |
| Bacillus sphaericus | Chromosorb 106 | Ni(II), Ag (I) | Natural water, tobacco, soil | FAAS | 88 |
| Bacillus thuringiensis israelensis | Chromosorb 101 | Cu(II), Fe(III), Zn(II) | Vitamins, dialysis solutions, natural water | ICP‐OES | 89 |
| Geobacillus thermoleovorans subsp. stromboliensis | Chromosorb 106 | Ni(II), Cd(II) | Natural water, tobacco, soil | AAS | 90 |
| Staphylococcus aureus | Dowex Optipore V‐493 | Hg(II), CH3Hg+ | Natural water, fish | CVAAS | 91 |
| Streptomyces albus | Sepiolite | Cd(II), Zn(II), Ni(II) | Simulated fresh water | FAAS | 92 |
| Yamadazyma spartinae | TiO2 nanoparticles | Cr, Cu, Fe, Mn, Ni, Zn | Tap water and lake water | ICP‐AES | 79 |
| Aspergillus niger | Silica gel 60 | Fe(III), Pb(II), Ni(II) | Water and vegetable | FAAS | 93 |
| Boletus edulis | γ‐Fe2O3 nanoparticles | Co(II), Sn(II) | Spiked environmental, food | ICP‐OES | 94 |
| Penicillium digitatum | Pumice stone | Cr(VI), Cd(II), Mn(II) | Dam water, spring water, fish | FAAS | 95 |
5. Concluding remarks
-
‐
Microbial biomasses have different biosorptive abilities, which varied within each taxonomic group, and depend on their physical and/or chemical pretreatment, as well the operational conditions. The biosorption of metal ions from immobilized microbial biomass has been studied mainly at the laboratory scale (batch and column). The authors’ efforts are focused on structural and morphological analysis of immobilized biomass, clarification of the mechanism of biosorption, study of the effect of the process parameters on the biosorption efficiency, mainly of one‐ and two‐component aqueous solutions and mathematical modeling. There is few information for removal of metal ions by multi‐component solutions, which is of paramount importance for practical applications because of the multi‐component nature of wastewater. Future studies should be targeted at pilot‐scale studies, which are currently limited.
-
‐
The choice of immobilization technique and carrier are a key factors for the practical application of biosorption. There are no universal and perfect techniques and carriers that are appropriately suited for microbial biomass immobilization. It should be taken into account the mechanical strength, chemical resistance, regeneration, reuse of the immobilized biomass and the mass transfer limitations. Economic analyses are needed to evaluate the real cost of immobilized biosorbents and biosorption processes and their implementation into practice.
-
‐
Currently efforts should be directed to biosorption of valuable elements (precious metals) and radio‐nuclides from wastewaters onto immobilized bacterial biomasses. The studies that have been carried out at this time give encouraging results.
-
‐
Waste yeast and fungal biomasses (Saccharomyces sp., Rhizopus sp., Aspergillus sp.) from the food and biotechnological industries, should receive attention, due to their ability to bind both cations and anions to their cell wall, low cost, and availability.
-
‐
Types of immobilized microbial biomass are used as sorbents in solid phase extraction, where the combination of pre‐concentration and metal ion separation by means of instrumental analysis allows the determination of very low concentrations. Unfortunately, they are not widely used in analytical practice.
Present study was financially supported by the project FP17/FK002 granted by University of Plovdiv.
The authors have declared no conflicts of interest.
5 References
- 1. Gadd, G. M. , Biosorption: critical review of scientific rationale, environmental importance and significance for pollution treatment. J. Chem. Technol. Biotechnol. 2009, 84, 13–28. [Google Scholar]
- 2. Hansda, A. , Kumar, V. , Anshumali, A comparative review towards potential of microbial cells for heavy metal removal with emphasis on biosorption and bioaccumulation. World J. Microbiol. Biotechnol. 2016, 32,170. [DOI] [PubMed] [Google Scholar]
- 3. Abdi, O. , Kazemi, M. , A review study of biosorption of heavy metals and comparison between different biosorbents. J. Mater. Environ. Sci. 2015, 6, 1386–1399. [Google Scholar]
- 4. Rao, L. N. , Prabhakar, G. , Removal of heavy metals by biosorption – an overall review. Chem. Eng. Res. Stud. 2011, 2, 17–22. [Google Scholar]
- 5. Alluri, H. K. , Ronda, S. R. , Settalluri, V. S. , Bondili, J. S. et al., Biosorption: an eco‐friendly alternative for heavy metal removal. Afr. J. Biotechnol. 2007, 6, 2924–2931. [Google Scholar]
- 6. Ajmal, M. , Khan, A. , Nomani, A. A. , Ahmed, S. , Heavy metals: leaching from glazed surfaces of tea mugs. Sci. Total Environ. 1997, 207, 49–54. [DOI] [PubMed] [Google Scholar]
- 7. González‐Muñoza, M. J. , Rodríguez, M. A. , Luque S., Álvarez, J. R. , Recovery of heavy metals from metal industry waste waters by chemical precipitation and nanofiltration. Desalination 2006, 200, 742–744. [Google Scholar]
- 8. Macchi, G. , Marani, D. , Pagano M., Bagnuolo, G. , A bench study on lead removal from battery manufacturing wastewaters by carbonate precipitation. Water Res. 1996, 30, 3032–3036. [Google Scholar]
- 9. Hunsom, M. , Pruksathorn, K. , Damronglerd, S. , Vergnes H., Electrochemical treatment of heavy metals (Cu2+, Cr6+, Ni2+) from industrial effluent and modeling of copper reduction. Water Res. 2005, 39, 610–616. [DOI] [PubMed] [Google Scholar]
- 10. Atkinson, S. , World's largest desalination plant begins operating in Israel. Membr. Technol. 2005, 2005, 9–10. [Google Scholar]
- 11. Gholani, M. M. , Mokhtari, M. A. , Aameri A., Fard, M. R. A. , Application of reverse osmosis technology for arsenic removal from drinking water. Desalination 2006, 200, 725–727. [Google Scholar]
- 12. Ćurković, L. , Cerjan‐Stefanović Š., Filipan, T. , Metal ion exchange by natural and modified zeolites. Water Res. 1997, 31, 1379–1382. [Google Scholar]
- 13. Eom, T. H. , Lee, C. H. , Kim, J. H. , Lee, C. H. , Development of an ion exchange system for plating wastewater treatment. Desalinaton 2005, 180, 163–172. [Google Scholar]
- 14. Djukić, A. , Jovanović, U. , Tuvić, T. , Andrić V. et al., The potential of ball‐milled Serbian natural clay for removal of heavy metal contaminants from wastewaters: Simultaneous sorption of Ni, Cr, Cd and Pb ions. Ceram. Int. 2013, 39, 7173–7178. [Google Scholar]
- 15. Shavandi, M. A. , Haddadian, Z. , Ismail, M. H. S. , Abdullah, N. , Removal of Fe(III), Mn(II) and Zn(II) from palm oil mill effluent (POME) by natural zeolite. J. Taiwan Inst. Chem. Eng. 2012, 43, 750–759. [Google Scholar]
- 16. O'Connell, D. W. , Birkinshaw, C. , O'Dwyer T. F., Heavy metal adsorbents prepared from the modification of cellulose: a review. Bioresour. Technol. 2008, 99, 6709–6724. [DOI] [PubMed] [Google Scholar]
- 17. Wang, J. , Chen, C. , Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [DOI] [PubMed] [Google Scholar]
- 18. Park, D. , Yun, Y. S. , Park, J. M. , The past, present, and future trends in biosorption. Biotechnol. Bioproc. E. 2010, 15, 86–102. [Google Scholar]
- 19. Volesky, B. , Sorption and Biosorption, BV‐Sorbex, Montreal: 2003, pp. 97–100. [Google Scholar]
- 20. Fomina, M. , Gadd, G. M. , Biosorption: current perspectives on concept, definition and application. Bioresour. Technol. 2014, 160, 3–14. [DOI] [PubMed] [Google Scholar]
- 21. Modak, J. M. , Natarajan, K. A. , Biosorption of metals using nonliving biomass‐a review. Miner. Metall. Proc. 1995, 12, 189–196. [Google Scholar]
- 22. Vijayaraghavan, K. , Yun, Y. S. , Bacterial biosorbents and biosorption. Biotechnol. Adv. 2008, 26, 266–291. [DOI] [PubMed] [Google Scholar]
- 23. Ramrakhiani, L. , Ghosh, S. , Majumdar, S. , Surface modification of naturally available biomass for enhancement of heavy metal removal efficiency, upscaling prospects, and management aspects of spent biosorbents: a review. Appl. Biochem. Biotechnol. 2016, 180, 41–78. [DOI] [PubMed] [Google Scholar]
- 24. Veglio, F. , Beolchini, F. , Removal of metals by biosorption: a review. Hydrometallurgy 1997, 44, 301–316. [Google Scholar]
- 25. Andrès, Y. , Texier A. C., Le Cloirec, P. , Rare earth elements removal by microbial biosorption: a review. Environ. Technol. 2003, 24, 1367–1375. [DOI] [PubMed] [Google Scholar]
- 26. Dhankhar, R. , Hooda, A. , Fungal biosorption – an alternative to meet the challenges of heavy metal pollution in aqueous solutions, Environ. Technol. 2011, 32, 467–491. [DOI] [PubMed] [Google Scholar]
- 27. Fosso‐Kankeu, E. , Mulaba‐Bafubiandi A. F., Review of challenges in the escalation of metal‐biosorbing processes for wastewater treatment: applied and commercialized technologies. Afr. J. Biotechnol. 2014, 13, 1756–1771. [Google Scholar]
- 28. Rashid, A. , Bhatti, H. N. , Iqbal, M. , Noreen S., Fungal biomass composite with bentonite efficiency for nickel and zinc adsorption: a mechanistic study. Ecol. Eng. 2016, 91, 459–471. [Google Scholar]
- 29. Akar, S. T. , Akar, T. , Kaynak, Z. , Anilan, B. et al., Removal of copper(II) ions from synthetic solution and real wastewater by the combined action of dried Trametes versicolor cells and montmorillonite. Hydrometallurgy 2009, 97, 98–104. [Google Scholar]
- 30. Gok, C. , Turkozu, D. A. , Aytas, S. , Removal of Th(IV) ions from aqueous solution using bi‐functionalized algae‐yeast biosorbent. J. Radioanal. Nucl. Chem. 2011, 287, 533–541. [Google Scholar]
- 31. Mahmoud, M. E. , Yakout, A. A. , Abdel‐Aal, H. , Osman, M. M. , Immobilization of Fusarium verticillioides fungus on nano‐silica (NSi–Fus): a novel and efficient biosorbent for water treatment and solid phase extraction of Mg(II) and Ca(II). Bioresour. Technol. 2013, 134, 324–330. [DOI] [PubMed] [Google Scholar]
- 32. Choudhury, P. R. , Bhattacharya, P. , Ghosh, S. , Majumdar, S. et al., Removal of Cr(VI) by synthesized titania embedded dead yeast nanocomposite: optimization and modeling by response surface methodology. J. Environ. Chem. Eng. 2017, 5, 214–221. [Google Scholar]
- 33. Górecka, E. , Jastrzębska, M. , Immobilization techniques and biopolymer carriers. Biotechnol. Food Sci. 2011, 75, 65–86. [Google Scholar]
- 34. Cestari, A. R. , Airoldi, C. , Chemisorption on thiol‐silicas: divalent cations as a function of pH and primary amines on thiol–mercury adsorbed. J. Colloid. Interface. Sci. 1997, 195, 338–347. [DOI] [PubMed] [Google Scholar]
- 35. Ahalya, N. , Ramachandra, T. V. , Kanamadi, R. D. , Biosorption of heavy metals. Res. J. Chem. Environ. 2003, 7, 71–79. [Google Scholar]
- 36. Bishnoi, N. R. , Kumar, R. , Bishnoi, K. , Biosorption of Cr(VI) with Trichoderma viride immobilized fungal biomass and cell free Ca‐alginate beads. Indian J. Exp. Biol. 2007, 45, 657–664. [PubMed] [Google Scholar]
- 37. Tan, W. S. , Ting, A. S. Y. , Efficacy and reusability of alginate‐immobilized live and heat‐inactivated Trichoderma asperellum cells for Cu(II) removal from aqueous solution. Bioresour. Technol. 2012, 123, 290–295. [DOI] [PubMed] [Google Scholar]
- 38. Ivánová, D. , Horváthová, H. , Kaduková, J. , Kavuličová, J. , Stability of immobilized biosorbents and its influence on biosorption of copper. Nova Biotechnol. 2010, 10, 45–51. [Google Scholar]
- 39. Es, I. , Vieira, J. , Amaral, A. C. , Principles, techniques, and applications of biocatalyst immobilization for industrial application. Appl. Microbiol. Biotechnol. 2015, 99, 2065–2082. [DOI] [PubMed] [Google Scholar]
- 40. Stolarzewicz, I. , Bialecka‐Florjańczyk, E. , Majewska, E. , Krzyczkowska, J. , Immobilization of yeast on polymeric supports. Chem. Biochem. Eng. 2011, 25, 135–144. [Google Scholar]
- 41. Moreno‐Garrido, I. , Microalgae immobilization: current techniques and uses. Bioresour. Technol. 2008, 99, 3949–3964. [DOI] [PubMed] [Google Scholar]
- 42. Simeonova, A. , Godjevargova, T. , Ivanova, D. , Biosorption of heavy metals by dead Streptomyces fradiae . Environ. Eng. Sci. 2008, 25, 627–634. [Google Scholar]
- 43. Gochev, V. , Velkova, Z. , Stoytcheva, M. , Yemendzhiev, H. et al., Biosorption of Cu(II) from aqueous solutions by immobilized mycelium of Trametes versicolor . Biotechnol. Biotechnol. Equip. 2012, 26, 3365–3370. [Google Scholar]
- 44. Wang, C. , Huang, B. , Luo, H . Immobilized microorganism technology and its application. Yunnan Chem. Technol. 2007, 34, 79–82. [Google Scholar]
- 45. Mao, J. , Kim, S. , Wu, X. H. , Kwak, I‐S. et al., A sustainable cationic chitosan/E. coli fiber biosorbent for Pt(IV) removal and recovery in batch and column systems. Sep. Purif. Technol. 2015, 143, 32–39. [Google Scholar]
- 46. Li, T‐T. , Liu, Y‐G. , Peng, Q‐Q. , Hu, X‐J. et al., Removal of lead(II) from aqueous solution with ethylenediamine‐modified yeast biomass coated with magnetic chitosan microparticles: kinetic and equilibrium modeling. Chem. Eng. J. 2013, 214, 189–197. [Google Scholar]
- 47. Li, H. , Li, Z. , Liu, T. , Xiao, X. et al., A novel technology for biosorption and recovery hexavalent chromium in waste water by bio‐functional magnetic beads. Bioresour. Technol. 2008, 99, 6271–6279. [DOI] [PubMed] [Google Scholar]
- 48. Yin, Y. , Hu, J. , Wang, J. , Removal of Sr2+, Co2+, and Cs+ from aqueous solution by immobilized Saccharomyces cerevisiae with magnetic chitosan beads. Environ. Prog. Sustain. Energy 2017, 36, 989–996. [Google Scholar]
- 49. Michalak, I. , Chojnacka, K. , Witek‐krowiak, A. , State of the art for the biosorption process‐a review. Appl. Biochem. Biotechnol. 2013, 170, 1389–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sonnenfeld, E. M. , Beveridge, T. J. , Koch A. L., Doyle, R. J. , Asymmetric distribution of charge on the cell wall of Bacillus subtilis . J. Bacteriol. 1985, 163, 1167–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Urrutia, M. M. , General bacterial sorption processes, in: Wase J., Forster C., (Eds), Biosorbents for metal ions, CRC Press, London, 1997, pp. 39–66. [Google Scholar]
- 52. Eman, Z. G. , Production and characteristics of a heavy metals removing bioflocculant produced by Pseudomonas aeruginosa . Poli. J. Microbiol. 2012, 61, 281–289. [PubMed] [Google Scholar]
- 53. Beveridge, T. J. , Murray, R. G. E. , Sites of metal deposition in the cell wall of Bacillus subtilis . J. Bacteriol. 1980, 141, 876–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ahmad, M. F. , Haydar, S. , Bhatti, A. A. , Bari, A. J. , Application of artificial neural network for the prediction of biosorption capacity of immobilized Bacillus subtilis for the removal of cadmium ions from aqueous solution. Biochem. Eng. J. 2014, 84, 83–90. [Google Scholar]
- 55. Hasan S. H., Srivastava, P. , Batch and continuous biosorption of Cu(II) by immobilized biomass of Arthrobacter sp. J. Environ. Manage. 2009, 90, 3313–332. [DOI] [PubMed] [Google Scholar]
- 56. Seo, H. , Lee, M. , Wang S., Equilibrium and kinetic studies of the biosorption of dissolved metals on Bacillus drentensis immobilized in biocarrier beads. Environ. Eng. Res. 2013, 18, 45–53. [Google Scholar]
- 57. Kim, I. , Lee M., Wang, S. , Heavy metal removal in groundwater originating from acid mine drainage using dead Bacillus drentensis sp. immobilized in polysulfone polymer. J. Environ. Manage. 2014, 146, 568–574. [DOI] [PubMed] [Google Scholar]
- 58. Xu, J. , Song, X‐C. , Zhang, Q. , Pan, H. , et al., Characterization of metal removal of immobilized Bacillus strain CR‐7 biomass from aqueous solutions. J. Hazard. Mater. 2011. 187, 450–458. [DOI] [PubMed] [Google Scholar]
- 59. Kim, S. , Choi, Y. E. , Yun, Y. S. , Ruthenium recovery from acetic acid industrial effluent using chemically stable and high‐performance polyethylenimine‐coated polysulfone‐Escherichia coli biomass composite fibers. J. Hazard. Mater. 2016, 313, 29–36. [DOI] [PubMed] [Google Scholar]
- 60. Cho, C‐W. , Kang, S. B. , Kim, S. Yun, Y‐S. , et al., Reusable polyethylenimine‐coated polysulfone/bacterial biomass composite fiber biosorbent for recovery of Pd(II) from acidic solutions. Chem. Eng. J. 2016, 302, 545–551. [Google Scholar]
- 61. Mahmoud, M. E. , Abdou, A. E. H. , Mohamed, S. M. S. , Osman, M. M. , Engineered Staphylococcus aureus via immobilization on magnetic Fe3O4‐phthalate nanoparticles for biosorption of divalent ions from aqueous solutions. J. Environ. Chem. Eng. 2016, 4, 3810–3824. [Google Scholar]
- 62. Brady, D. , Stoll, A. D. , Starke, L. , Duncan, J. R. , Chemical and enzymatic extraction of heavy metal binding polymers from isolated cell walls of Saccharomyces cerevisiae. Biotechnol . Bioeng. 1994, 44, 297–302. [DOI] [PubMed] [Google Scholar]
- 63. Wang, J. , Chen, C. , Biosorption of heavy metals by Saccharomyces cerevisiae: a review. Biotechnol. Adv. 2006, 24, 427–451. [DOI] [PubMed] [Google Scholar]
- 64. Chen C., Wang J., Uranium removal by novel graphene oxide‐immobilized Saccharomyces cerevisiae gel beads. J. Environ. Radioact. 2016, 162–163, 134–145. [DOI] [PubMed] [Google Scholar]
- 65. Peng, Q. , Liu, Y. , Zeng, G. , Xu, W. , et al., Biosorption of copper(II) by immobilizing Saccharomyces cerevisiae on the surface of chitosan‐coated magnetic nanoparticles from aqueous solution. J. Hazard. Mater. 2010, 177, 676–682 [DOI] [PubMed] [Google Scholar]
- 66. Yin, Y. , Wang, J. , Yang, X. , Li, W. , Removal of strontium ions by immobilized Saccharomyces cerevisiae in magnetic chitosan microspheres. Nucl. Eng. Technol. 2017, 49, 172–177. [Google Scholar]
- 67. Yin, Y. , Hu, J. , Wang, J. , Removal of Sr2+, Co2+, and Cs+ from aqueous solution by immobilized Saccharomyces cerevisiae with magnetic chitosan beads. Environ. Prog. Sustain. Energy. 2017, 36, 989–996. [Google Scholar]
- 68. Bai, J. , Wu, X. , Fan, F. , Tian, W. , et al., Biosorption of uranium by magnetically modified Rhodotorula glutinis . Enzyme Microb. Technol. 2012. 51, 382–387. [DOI] [PubMed] [Google Scholar]
- 69. Sağ, Y. , Kutsal, T. , Recent trends in the biosorption of heavy metals: a review. Biotechnol. Bioprocess Eng. 2001, 6, 376–385. [Google Scholar]
- 70. Kumar, R. , Bhatia, D. , Singh, R. , Rani, S. , et al., Sorption of heavy metals from electroplating effluent using immobilized biomass Trichoderma viride in a continuous packed‐bed column. Int. Biodeterior. Biodegradation. 2011, 65, 1133–1139. [Google Scholar]
- 71. Akhtar, K. , Khalid, A. M. , Akhtar, M. W. , Ghauri, M. A. , Removal and recovery of uranium from aqueous solutions by Ca‐alginate immobilized Trichoderma harzianum . Bioresour. Technol. 2009, 100, 4551–4558. [DOI] [PubMed] [Google Scholar]
- 72. Verma, A. , Shalu, Singh, A. , Bishnoi, N. R. , Gupta A., Biosorption of Cu(II) using free and immobilized biomass of Penicillium citrinum . Ecol. Eng. 2013, 61, 486–490. [Google Scholar]
- 73. Xiao, G. , Zhang, X. , Su, H. , Tan, T. , Plate column biosorption of Cu(II) on membrane‐type biosorbent (MBS) of Penicillium biomass: optimization using statistical design methods. Bioresour. Technol. 2013, 143, 490–498. [DOI] [PubMed] [Google Scholar]
- 74. Ding, D‐X. , Tan, X. , Hu, N. , Li, G‐Y. , Removal and recovery of uranium(VI) from aqueous solutions by immobilized Aspergillus niger powder beads. Bioprocess Biosyst. Eng. 2012, 35, 1567–1576. [DOI] [PubMed] [Google Scholar]
- 75. Mahmoud, M. E. , Yakout, A. A. , Hany. A. A , Osman, M. M. , Speciation and selective biosorption of Cr(III) and Cr(VI) using nanosilica immobilized‐fungi biosorbents. J. Environ. Eng. 2015, 141, 04014079‐1–04014079‐9. [Google Scholar]
- 76. Mahmoud, M. E. , Yakout, A. A. , Abdel‐Aal, H. , Osman, M. M. , Enhanced biosorptive removal of cadmium from aqueous solutions by silicon dioxide nano‐powder, heat inactivated and immobilized Aspergillus ustus . Desalination 2011, 279, 291–297. [Google Scholar]
- 77. Bakircioglu, Y. , Bakircioglu, D. , Akman, S. , Biosorption of lead by filamentous fungal biomass‐loaded TiO2 nanoparticles. J. Hazard. Mater. 2010, 178, 1015–1020. [DOI] [PubMed] [Google Scholar]
- 78. Bakircioglu, D. , Ucar, G. , Kurtulus, Y. B. , Coliform bacteria immobilized on titanium dioxide nanoparticles as a biosorbent for trace lead preconcentration followed by atomic absorption spectrometric determination. Microchim. Acta. 2011, 174, 367–374. [Google Scholar]
- 79. Baytak, S. , Zereen, F. , Arslan, Z. , Preconcentration of trace elements from water samples on a minicolumn of yeast (Yamadazyma spartinae) immobilized TiO2 nanoparticles for determination by ICP‐AES. Talanta 2011, 84, 319–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mahmoud, M. E. , Yakout, A. A. , Abdel‐Aal, H. , Osman, M. M. , High performance SiO2‐nanoparticles‐immobilized‐Penicillium funiculosum for bioaccumulation and solid phase extraction of lead. Bioresour. Technol. 2012, 106, 125–132. [DOI] [PubMed] [Google Scholar]
- 81. Aydemir, N. , Tokman, N. , Akarsubasi, A. T. , Baysal, A. , et al., Determination of some trace elements by flame atomic absorption spectrometry after preconcentration and separation by Escherichia coli immobilized on multiwalled carbon nanotubes. Microchim. Acta. 2011, 175, 185–191. [Google Scholar]
- 82. Tuzen, M. , Saygi, K. O. , Usta, C. , Soylak, M. , Pseudomonas aeruginosa immobilized multiwalled carbon nanotubes as biosorbent for heavy metal ions. Bioresour. Technol. 2008, 99, 1563–1570. [DOI] [PubMed] [Google Scholar]
- 83. Özdemir, S. , Kilinc, E. , Celik, K. S. , Okumus, V. , et al., Simultaneous preconcentrations of Co2+, Cr6+, Hg2+ and Pb2+ ions by Bacillus altitudinis immobilized nanodiamond prior to their determinations in food samples by ICP‐OES. Food Chem. 2017, 215, 447–453. [DOI] [PubMed] [Google Scholar]
- 84. Tonk, S. , Măicăneanu, A. , Indolean, C. , Burca, S. , Madjik, C. , Application of immobilized waste brewery yeast cells for Cd2+ removal. Equilibrium and kinetics. J. Serb. Chem. Soc. 2011, 76, 363–373. [Google Scholar]
- 85. Nguyen, M. L. , Juang R.‐S., Modification of crosslinked chitosan beads with histidine and Saccharomyces cerevisiae for enhanced Ni(II) biosorption. J. Taiwan. Inst. Chem. Eng. 2015, 56, 96–102 [Google Scholar]
- 86. Yener, I. , Oral, E. V. , Dolak, I. , Ozdemir, S. , et al., A new method for preconcentration of Th(IV) and Ce(III) by thermophilic Anoxybacillus flavithermus immobilized on Amberlite XAD‐16 resin as a novel biosorbent. Ecol. Eng. 2017, 102, 43–49. [Google Scholar]
- 87. Dogru, M. , Gul‐Guven R., Erdogan, S. , The use of Bacillus subtilis immobilized on Amberlite XAD‐4 as a new biosorbent in trace metal determination. J. Hazard. Mater. 2007, 149, 166–173. [DOI] [PubMed] [Google Scholar]
- 88. Tuzen, M. , Soylak, M. , Column solid‐phase extraction of nickel and silver in environmental samples prior to their flame atomic absorption spectrometric determinations. J. Hazard. Mater. 2009, 164, 1428–1432. [DOI] [PubMed] [Google Scholar]
- 89. Mendil, D. , Tuzen, M. , Usta, U. , Soylak, M. , Bacillus thuringiensis var. israelensis immobilized on Chromosorb 101: a new solid phase extractant for preconcentration of heavy metal ions in environmental samples. J. Hazard. Mater. 2008, 150, 357–363. [DOI] [PubMed] [Google Scholar]
- 90. Özdemir, S. , Gul‐Guven, R. , Kilinc, E. , Dogru, M. , et al., Preconcentration of cadmium and nickel using the bioadsorbent Geobacillus thermoleovorans subsp. stromboliensis immobilized on Amberlite XAD‐4. Microchim. Acta 2010, 169, 79–85. [Google Scholar]
- 91. Tuzen, M. , Karaman, I. , Citak, D. , Soylak, M. , Mercury(II) and methyl mercury determinations in water and fish samples by using solid phase extraction and cold vapour atomic absorption spectrometry combination. Food Chem. Toxicol. 2009, 47, 1648–1652. [DOI] [PubMed] [Google Scholar]
- 92. Yildiz, D. , Kula, I. , Şahin, N. , Preconcentration and determination of Cd, Zn and Ni by flame atomic absorption spectrophotometry by using microorganism Streptomyces albus immobilized on sepiolite. Eurasian J. Anal. Chem. 2013, 8, 112–122. [Google Scholar]
- 93. Baytak, S. , Koçyiǧit, A. , Türker, A. R. , Determination of lead, iron and nickel in water and vegetable samples after preconcentration. Clean 2007, 35, 607–611. [Google Scholar]
- 94. Özdemir, S. , Yalcin, M. S. , Kilinc, E. , Soylak, M. , Boletus edulis loaded with γ‐Fe2O3 nanoparticles as a magnetic sorbent for preconcentration of Co(II) and Sn(II) prior to their determination by ICP‐OES. Microchim. Acta. 2018, 185, 1–9. [DOI] [PubMed] [Google Scholar]
- 95. Baytak, S. , Türker, A. R. , Determination of chromium, cadmium and manganese in water and fish samples after preconcentration using Penicillium digitatum immobilized on pumice stone. CLEAN 2009, 37, 314–318. [Google Scholar]


