Abstract
Sustainable, ecological, and biocompatible materials are emerging for the development of novel components for tissue engineering. Microalgae being one of the unique organisms on Earth to provide various novel compounds with certain bioactivities are also a good source for the development of novel tissue scaffold materials. In this study, electrospinning technique was utilized to fabricate nanofibers from polycaprolactone loaded with microalgal extracts obtained from Haematococcus pluvialis (vegetative and carotenoid producing form) and Chlorella vulgaris. The FTIR results showed that, blending microalgae with polycaprolactone give unique bands rooted from microalgae and polycaprolactone structure. The samples were not diversified from each other, however stable bands were observed. SEM analysis revealed a uniform fiber fabrication with an average diameter of 810 ± 55 nm independent from microalgal extracts. MTT assay was done on HUVEC cell lines and results showed that nanofiber mats helped cell proliferation with extended time. Biodegradation resulted with mineral accumulation on the surface of same samples however the fiber degradation was uniform. With slow but stable biodegradation characteristics, microalgal extract loaded nanofiber mats holds great potential to be novel tissue scaffold material.
Keywords: electrospun, microalgae, PCL, tissue scaffolds
Abbreviations
- PCL
polycaprolactone
- ECM
extracellular matrix
- PBS
phosphate buffer saline
1. INTRODUCTION
Tissue engineering is a multidisciplinary branch of life sciences with strong correlation of cell biology, engineering, and material sciences 1. Demand on natural and synthetic supports for cells to grow as in natural conditions are urging. Scaffolds specifically designed for tissue regeneration represents a major component in tissue engineering to restore or regenerate, fully or particularly replace defective or malfunctioning tissues 2. Tissue scaffolds provide supports for cells to attach and proliferate in 3‐dimensions. The primary expectation from tissue scaffolds or scaffold materials is to help cells to grow as its original environment. Most of the scaffold materials are chosen from natural biomaterials or biocompatible synthetic origins 3. However, scaffolds providing cells condition to mimic their own nature is of great importance.
Human organs are naturally embedded in extracellular matrix (ECM) which is composed of nanofibers (proteoglycans, fibrous proteins etc.) 4. ECM provides a protective, nutritious environment for cells. Mimicking the natural organization of ECM is a challenging topic in tissue engineering in terms of functional and structural similarities 5. The ECM is not only the mechanical support to cells but also has a major role for cell growth and differentiation which is the challenge in tissue engineering. Novel techniques are developed to allow biomaterials to be fabricated as micro or nanofibers. Electrospinning technique is one of the simplest, cheapest, and efficient method for bio‐fabrication of certain tissue scaffolds used for this purpose 6.
Micro and nanofibers composed in random fiber mats are similar to the ECM structure of fibroblasts, osteoblasts, chondrocytes, and endothelium cells to provide rapid regeneration and differentiation 7. In electrospinning strategy, a mixture of a liquid polymer solution is injected into a syringe with varying needle diameter. When high voltage is applied, and polymer is continuously pumped through the tip of the needle; the droplets become to have a conical shape. After reaching a threshold, the conical shape of the polymer solution acts like a jet fluid flow and the polymer solution is casted on a collector plate by the application of electrical currents; while the solvent is evaporated enabling of stretching of the jet 8
Synthetic materials mostly face biocompatibility problems 9. Continuous search of natural biomaterials with strong biocompatibility is desired. Collagen, polysaccharides and some other proteins from biological sources hold great promise on this issue 3, 4. Microalgae, the primary sources of biological CO2/O2 balance on Earth, are one of the most valuable ecological organisms 10. Lately, bioactive metabolites obtained from microalgae are utilized in food, feed, agriculture, environmental sciences, pharmaceuticals, and cosmetics 11, 12, 13. Bioactive compounds in microalgal extract show antioxidant, proliferative, anticancer, antihypertensive, and antimicrobial activity 14, 15, 16. Microalgal extracts are also a promising candidate on regenerative tissue engineering for cardiovascular system 18. Recent developments in materials technology acknowledge the cell growth enhancers as support for tissue engineering. On this aspect utilization of microalgal extracts with defined bioactivities are emerging novel applications of phycology to tissue engineering. Microalgal extracts can alleviate the tissue like structure in nanofiber mats to provide a mimicking atmosphere for animal cells to proliferate. Microalgae is not only considered a form of aquaculture feed support but also gaining applications in biomedical research makes them an attractive source. Blending microalgae for nanofiber composition may open a new gate for novel smart and ecological materials coming from a natural source.
PRACTICAL APPLICATION
Tissue engineering is a growing field with the development of material sciences, novel techniques for scaffold construction, and utilization of bioactive compounds as main or supporting material for fabrication. Microalgae possess great advantage for life sciences application, being the sustainable and ecologically important life forms on Earth. Combination of microalgal bioprocesses with tissue engineering provides a renewable, green and reliable construction of novel compounds in terms of designing new biomaterials. Cardiovascular tissue engineering is of importance due to rapid increase in the related diseases. In this study, PCL nanofiber mats loaded with microalgal extracts obtained from Haematococcus pluvialis and Chlorella vulgaris were continuously fabricated using electrospinning technique. MTT cell proliferation analysis showed that nanofibers loaded with microalgal extracts enabled a great contribution for cellular proliferation enhancing the cellular growth of cardiovascular tissue cells. The results showed that microalgal compounds‐based tissue engineering is a promising field for the sustainable scaffold development.
Polycaprolactone (PCL) is one of the most preferred scaffold materials for hard and soft tissue applications 4. PCL is linear polyester and due to its good biodegradability and biocompatibility features, it is an interesting material for tissue engineering application 8.
In this study, it is aimed to use electrospinning technique to fabricate PCL based nanofiber mats blended with microalgal extracts. The originality of the research derived from the fact that, until now the existing literatures used algal proteins and algal biomass as blends for nanofiber characterization, however in this study Chlorella vulgaris and Haematococcus pluvialis solvent extracts were used as bioactive substances to enhance cell proliferation. Carotenoids are being claimed as one of the natural molecules which enhances the health of cardiac tissues. Carotenoids in cardiovascular systems acts as oxidative stress scavengers to prevent the damage obtained from the singlet oxygen species 17. The oxidative stress related mechanism of cardiac cells and responses to this stress by microalgal components shows that algae can boost new therapeutic applications in cardiovascular related diseases 13. However, the approach in this study to use microalgal carotenoids dense extracts as cell support materials are a new perspective of the development of the field. HUVEC cell line was chosen for cell culture experiments due to increasing demand on the development of cardiovascular tissue engineering materials 18. HUVEC cells could be utilized as a good starting cell line for the development of vascularized cardiac tissue engineering 19.
2. MATERIALS AND METHODS
2.1. Microalgae cultivation
Haematococcus pluvialis was a kind gift from Guiseppe Torzillo (CNR‐Italy). C. vulgaris (SAG 211–12) was bought from Experimentelle Phykologie und Sammlung von Algenkulturen der Universität Göttingen (EPSAG). All species were cultivated in BG‐11 medium. The agar slants were kept under 30 µε/m2s constant light. The cultures were renewed periodically using 5 mL liquid tube cultures, 25, 100, and 250 mL Erlenmeyer flasks, respectively. The cultures were shaken 120 rpm under constant light intensity of 50 µε/m2s. 250 mL Erlenmeyer cultures were used as inoculum.
2.2. Mass production of microalgal biomass
Microalgae cultures were cultivated in 500 mL bubble column photobioreactors (light path 6 cm). The PBR batches were inoculated from cultures at mid logarithmic phase, 10% v/v with 400 mL working volume and aerated 1 vvm. The light intensity was provided 70 µε/m2s intensity from both sides. C. vulgaris cultures were harvested after 14 days (late mid logarithmic phase). H. pluvialis cells reaching the stationary phase were exposed to 200 µε/m2s light intensity from both sides to induce carotenoid accumulation. The biomass was harvested with centrifugation 4000 rpm 10 min (Nüve NF048, Turkey), washed with distilled water twice and freeze dried. Dry biomass was kept in −20ºC before experiments.
2.3. Preparation of crude microalgal extracts
Chlorella vulgaris (CV_Green), H. pluvialis‐green (HP_Green), and H. pluvialis–carotenoid rich (HP_Red) biomasses were used for extraction. 0.2 g of biomass of each sample was weighted and mixed with 5 mL 3:1 v/v Acetone/MetOH. The samples were sonicated with a probe type sonicator (Bandelin Sonopuls, Germany) for 15 min two times (nine cycle, 65% power). The sonication was done in ice bath and in between two cycle samples were rested to cool down. 5 mL of solvent mixture was added and incubated for 2 h and centrifuged at 3500 rpm 10 min (Nüve NF048, Turkey). The supernatant and pellet were separated, and 10 mL solvent mixture was added on the pellet and incubated for 30 min and centrifuged. The solvent was evaporated at 45ºC (Stuart RE300, UK). The total extracts are weighted and kept at −20ºC for electrospinning experiments.
2.4. Electrospinning experiments
12% w/v PCL solution was prepared by dissolving PCL in dimethylformamide (DMF) and dichloromethane (DCM) (1:1) and stirred overnight. 10 mL of PCL solution was mixed with microalgae samples to prepare 4% of microalgal extracts‐PCL solution. The solutions were mixed with magnetic stirrers (MSH‐20A, Wise Stir) overnight at room temperature.
Each solution was loaded into a 10 mL syringe equipped with 29‐gauge stainless steel needle. Solutions were feed to the end of needle tip using a syringe pump (NE‐1000, New ERA Pump System, Inc., USA) at a flow rate of 2 mL/h. An aluminum sheet was used as the collector and placed at a distance of 15 cm from the needle tip with 28 circular lamellas (1 cm diameter) attached to the collector surface. Nanofibers were collected by applying a positive high voltage of 17.5 kV using high voltage power supply (Gamma High Voltage ES30).
2.5. Cell culture experiments
HUVEC cell lines were utilized for cell viability and cytotoxicity assays. Cell lines were cultured in DMEM‐HG medium supplemented with 15% FBS at 37°C in a 5% CO2 humidified incubator in 75 cm2 cell culture flasks. After a sub‐confluent monolayer was observed, cells were harvested with 0.25% trypsin/EDTA and suspended in fresh media before seeding onto nanofiber mats. The nanofibers are put in a Petri dish and UV sterilization was done for each side for 2 h.
24 well‐plates were covered with 1.5% agar to prevent cells to attach onto the surface of culture flask. The nanofiber mats were soaked into DMEM‐HG medium for 24 h. Each well was plated 1×105 cells/well and incubated at 37°C in a 5% CO2 humidified incubator for 30 min. Five hundred microliter of DMEM‐HG medium was added on each well and incubated. The medium was replaced in every 2 days. The PCL itself was utilized as control surface to see the effect of microalgal extracts.
2.6. MTT cell viability assay
Cell proliferation and viability of HUVEC cells on the nanofiber mats were determined by MTT assay. 1, 4, 7, and 10th days after cell seeding, culture medium from each cell was replaced with 500 µL of DMEM‐HG medium including MTT solution incubated for 240 min and dissolved formazan crystals was measured using a UV–visible spectrophotometer (MDS Molecular Devices, Versa Max,USA) at a wavelength of 570 nm, using 690 nm as reference. All the experiments were done 3 different sets with 5 replicates for each sample (PCL, CV_green, HP_green, and HP_red). DMSO was utilized as blank.
2.7. Biodegradation assay
Biodegradation assay was done in phosphate buffer saline (PBS) and followed for 7 days in 1X, 5X, and 10X concentrations. 2 × 1 cm nanofiber mats were cut from the aluminum foil surface and weighted and put in various PBS concentrations and incubated at 37ºC. The PBS solution was replaced in every 2 days. After 7 days, the samples were washed with distilled water and weighted. Also, the samples were analyzed with SEM for biodegradation characteristics and mineral accumulation.
2.8. Nanofiber characterization
The FTIR spectroscopy measurements in a 500–4000 cm−1 range were performed on a FTIR spectrometer (Frontier, Perkin‐Elmer) 4. The spectra were obtained at a 2 cm−1 resolution, 100 scans. Empty surface was used for blank analysis 20.
Morphological characterization of nanofiber mats was examined by scanning electron microscopy, SEM (QUANTA FEG 250). Dry fibers were coated with gold–palladium and imaged in secondary electron mode at 3 kV. Diameter of the fibers from each scaffold type was assessed quantitatively via NIH ImageJ software (http://rsb.info.nih.gov/ij/).
3. RESULTS AND DISCUSSION
3.1. Nanofiber fabrication
12% w/v PCL polymer solution was used as control experiment. Samples with 4% w/v algal extracts CV_Green, HP_Green, and HP_Red were prepared to see the effect of algal extracts blend in PCL nanofiber mats on the proliferation of vascular endothelial cell lines. The experimental conditions were optimized in previous study 4. Prior to any experiments, the surface characterization of the electrospun nanofibers was done. One lamellae of each sample were taken to observe under light microscopy with 4x magnification (Olympus) to have a first glance idea of the formation of the polymer matrices (Figure 1). Light microscopy observation shows that fibers were well formed with no traces of droplets due to the coating performance of the electrospinning device on the collector plate. Continuous and bead‐free fibers were produced however some traces of microalgal extracts could be observed with light microscopy (Figure 1B).
Figure 1.
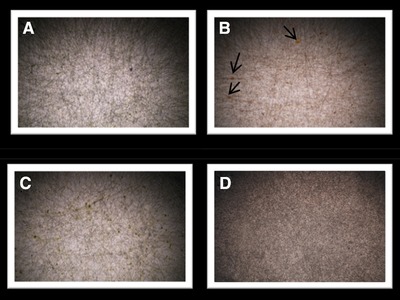
Optical microscopy image of fabricated PCL mats, (A) CV_Green, (B) HP_Red, (C) HP_Green, and (D) PCL, Black arrows show the traces of extracts
Morphology of the nanofiber mats was also determined via scanning electron microscopy operating at 3 kV (Figure 2). The average diameter distribution was measured with ImageJ application. The fibers were well oriented and distributed randomly. The overall morphology and pores of the samples were mostly distributed evenly, however samples containing microalgae extracts (Figure 2C and D) were covered with algal extracts. The variations in the samples with microalgal extracts (Figure 2B–D) showed little traces of microalgal extracts which was also confirmed with the FTIR experiments. The average diameter of the fibers was 810±55 nm. The range of diameters were in between 400–900 nm (Figure 3). We could observe fibers less than 200 and greater than 100 nm meter but their distribution was not abundant inside the mat. The changes in the microalgal extracts did not affect the average size distribution. The size distribution of the fibers was in accordance with previous studies using S. platensis as a blend for electrospinning experiments 21, 22. The size of the fiber diameter is mostly correlated with applied voltage, the polymer concentration in the solution and supporting materials inside the polymer solution. The uniform distribution of the fibers and their void sizes could be concluded as suitable for attachment of animal cells.
Figure 2.
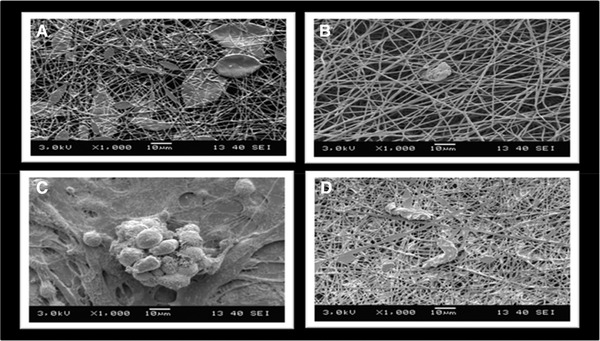
Nanofiber images on SEM (A) PCL, (B) HP_Green, (C) HP_Red, (D) CV_Green
Figure 3.
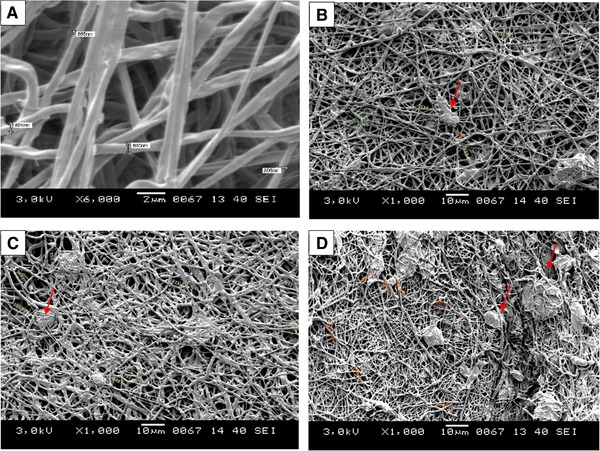
The diameter distribution of fibers; (A) PCL, (B) HP_Green, (C) HP_Red, and (D) CV_Green. Red arrows show the microalgal extracts
3.2. FTIR analysis results
Surface chemistry of the nanofiber mats (Figure 4) were analyzed using FTIR. Infrared bands of PCL were observed in all the samples; PCL (black line), PCL+CV_Green (green line), PCL+HP_Red (red line) and PCL+HP_Green (yellow line). CH2 stretching at 2943 cm−1 (asymmetric), CH2 stretching at 2866 cm−1 (symmetric), C=O stretching at 1721 cm−1, C–C and C–O stretching at 1721 cm−1, C–O–C stretching at 1239 cm−1, and O–C–O stretching at 1190 cm−1 4, 23. The results showed that existing of the microalgal compounds did not show great significance on the band spectrum related. However, in between 1500 and 1000 cm−1, specific groups related to microalgal IR band could be observed 20, 24, 25. FTIR results showed the incorporation of PCL and microalgal extracts yet no significant change was observed according to the IR spectrum.
Figure 4.
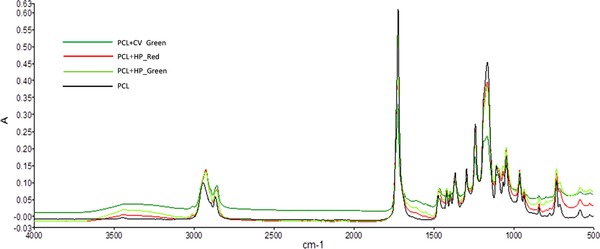
FTIR surface scan of Nanofiber mats loaded with microalgal extracts
3.3. Cell viability assays
The potential target of electrospun nanofibers supported with microalgal extracts was cardiovascular tissue engineering due to the various pigments in microalgal extracts 11, 26, 27. Microalgal extracts are known to have certain antioxidant activities which are emerging natural substances for cardiovascular health 17 The effect of carotenoids and chlorophyll based pigments in human physiology is a complex metabolism. Mostly pigments are obtained from nutrition and stored in adipose tissues due to their solubility in fats. However, the carotenoids in circulation system was observed as a protection chemical for cardiovascular health. HUVEC cell lines originated from umbilical vein/vascular endothelium cells which are a suitable cell line to investigate the potential utility of microalgal extract loaded nanofiber mat tissue scaffolds 26. HUVECs are good model cell lines for cardiac tissue engineering and their responses are valuable for the fate of the cells in vitro cardiovascular tissue engineering studies 19. Cytotoxicity and cell proliferation of the nanofiber mats were determined with MTT cell proliferation assay. MTT assay was performed with various time points of 1, 3, 7, and 10 days. Results of MTT assay was directly correlated with the cell proliferation and cell survival. The samples with high absorbance ratio was discussed as the scaffolds which enhanced cell growth. All the fibers loaded with algal extracts promoted cell proliferation through the timeline of MTT assay (Figure 5). CV_Green showed slightly better effect on cell proliferation however the results of all samples containing microalgal extracts didn't show significant variation on the proliferation. PCL polymer itself after 7 days showed a rapid decrease in cell proliferation (20% decrease). The decrease was only shown in PCL samples This result could be interpreted as the compounds in microalgal extracts helped HUVEC cells to proliferate and increase their life span. The results obtained from MTT assay showed that nanofiber mats loaded with CV_Green, HP_Green and HP_Red holds great contribution to cell proliferation with respect to time. PCL has a slow degradation kinetics and the surface is hydrophobic which encounters cell attachment as challenging. The algal extracts obtained in this study is mostly hydrophilic which could enhance cellular attachment on the surface so cells could proliferate easily. IR spectra clearly indicates the contribution of microalgal extracts on the formation of nanofiber mats. As it is clearly stated by the MTT assay, H. pluvialis, and C. vulgaris extracts does enhance cellular growth and cell survival which is critical in tissue scaffold applications. Non‐toxic nature of the algal compounds makes them a remarkable source to use 21. Kim et al. successfully addressed the PCL‐Spirulina nanofiber applications for neural cell tissue regeneration applications on primary astrocyte cells. The results showed that PCL and spirulina mix doesn't show cytotoxicity on the growth of neuronal cells which could be further developed neurodegenerative diseases engineering applications. The common findings with present study and previous studies agrees on the non‐cytotoxic nature of the microalgal extracts on animal cell growth. However, they don't concentrate on the bioactivity effects of the microalgae.
Figure 5.
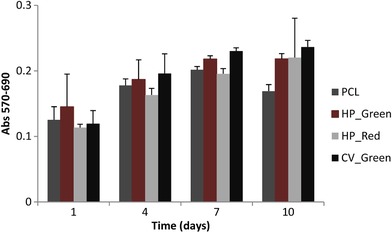
MTT cell viability assay
At another study, Spirulina is loaded with silk fibroin and PCL to see hemocompatibility and blood clotting activity of the scaffold materials. The results clearly indicated that microalgal extracts prevented platelet aggregation and showed anticoagulant activity with no cytotoxic effect. These results are in good accordance with our study and show the relevancy of algal extracts for tissue engineering applications 28. The authors also suggest testing various microalgae species which have been achieved in this study by the utilization of two important biotechnological microalgae species.
3.4. Biodegradation characteristics
Biodegradation of a tissue scaffold is highly important if the aim of the scaffold is to provide rapid and natural cell proliferation 18. It is known that PCL has good biodegradation kinetics depending on time and temperature as affecting parameters 29 which makes it as a suitable material for tissue engineering application. In this study, biodegradation of nanofiber mats was done in various concentration of PBS, 1X, 5X, and 10; respectively. The expected outcome of biodegradation assay was to see a decrease in the mass at the end of 7 days (Figure 6). There was an increase in the mass for 1X PCL sample and 10X samples. In 1X PCL sample the accumulation of minerals was obvious on the SEM imaging (Figure 7) however the reason to have a mineral accumulation was controversial to other outcomes of the biodegradation assay. It is thought to be due to the concentration of 10X PBS to allow mineral accumulation on the scaffold surface. However, other experiments showed decrease in the mass as expected. The decrease in the mass was not greater than 18.5 ± 0.25% for 10X CV_Green sample. Samples not showing mineral accumulation on the surface showed biodegradation in between 3–10%. The slow degradation characteristics can be a great advantage on the cell types that require longer regeneration and or proliferation characteristics. Since the biodegradation byproducts of PCL and microalgal compounds are not harmful to cells which are of great importance. SEM analysis of all the samples were done to visualize degraded mat surface (Figure 7). The SEM images showed that the fibers in 10X concentration got thinner and started to decay. However, the accumulation of the mineral traces can also be observed on the fibers covering as a mat 30. The conditions of the fibers for HP_Green and CV_Green remained well oriented even though they started to degrade. Algal compounds embedded inside the mats could helped to have a better consistency and due to their arrangement as fiber matrices it could show a slower degradation. The decomposition was not observed strongly which shows that they can be utilized for in vivo scaffolds applications with slower and stable fiber decomposition. Even though mineral accumulation couldn't be measured with HP_Red experiments, the images for biodegradation assay showed that a mat over the fibers was covered. It could be due to the ingredients of compounds existing in the extract which was rich in algal carotenoids. De Morais et al. used Spirulina to fabricate polyethylene oxide based nanofiber scaffolds 22. They managed to observe nanofiber mats however the challenge in this study was the stability of the nanofibers in aqueous environment due to the rapid hydrolysis of polyethylene oxide when contacts with water. Our study results in the stable and slow degradable nanofiber mat fabrication which could be used as a coating material for stents applications due to the stability in physiological environments. Slow degradation of PCL could be seen as a disadvantage, however for cells growing slowly and need to attach on scaffolds for a longer time the slow decomposition of the material could be seen as an advantage for cells to proliferate in three‐dimensional 28.
Figure 6.
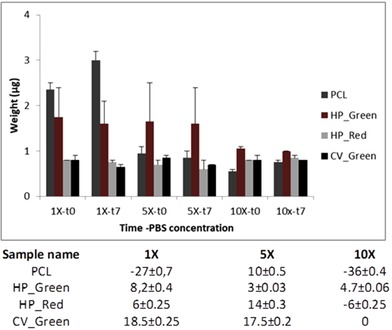
Biodegretadion results of the samples (graphic image), %biodegredation results of the sapmles after 7 days
Figure 7.
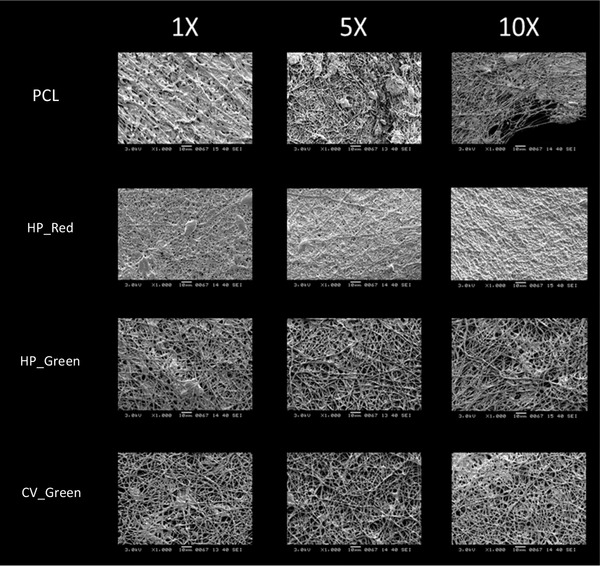
SEM images of PCL, HP_Red, HP_Green and CV_Green nanofiber mats degraded at 1X, 5X and 10X PBS solution after 7 days
4. CONCLUDING REMARKS
In this study, continuous nanofiber mats loaded with several microalgal extracts obtained from H. pluvialis and C. vulgaris were fabricated. The nanofibers were successfully produced using electrospinning technique. The continuous nanofiber mats were fabricated without any bead formation, the concentration of the PCL played a major role and the voltage applied served well for fiber generation 31. The average diameter was 810 ± 55 nm which was not affected from the differentiations in the composition of PCL blends with microalgal extracts. The distribution of the fibers was random but their size distribution range was consistent.
FTIR spectra was investigated to see the contribution of microalgal compounds to the nanofiber mats. The spectra clearly displayed that introduction of the microalgal extracts into PCL solution altered the composition of the mats in comparison to the PCL itself. MTT results showed that loading PCL solutions with either H. pluvialis or C. vulgaris extracts showed a great contribution the cell proliferation which could be due to the changes in hydrophobic‐hydrophilic compound ratio. The viability in PCL mats lowered at 10th day and the decrease in cell proliferation is only observed in PCL experiments. Biodegradation of the nanofibers were done in 1X, 5X, and 10X PBS solution and followed for 7 days. A clear degradation was observed which was expected for a tissue scaffold to degrade in time. However, some mineral accumulation could be observed on the surface of the fibers. PCL fibers lost their fiber morphology especially in 10X PBS solution however a good distribution of the fibers could still be observed fir samples containing microalgal extracts.
In conclusion, the results clearly show that, microalgal extracts could be a supporting material for the development of tissue scaffolds which could be strong candidates for utilization in cardiovascular experiments. However, the results are not only important for cardiovascular tissue engineering, but microalgae and bioactive compounds obtained from microalgal cells are strong candidates for the development of novel materials. The fabricated scaffolds could be further developed for artificial heart, stent and blood vessel applications where cardiac tissue engineering materials are needed.
CONFLICT OF INTEREST
The authors have declared no conflict of interest.
ACKNOWLEDGMENTS
The authors want to thank Assoc. Prof. Dr. Aylin Şendemir of Ege University Bioengineering department for their valuable contribution to share animal cell culture lines and laboratory infrastructure for MTT assays.
Cetmi SD, Renkler NZ, Kose A, Celik C, Oncel SS. Preparation of electrospun polycaprolactone nanofiber mats loaded with microalgal extracts. Eng Life Sci. 2019;19:691–699. 10.1002/elsc.201900009
REFERENCES
- 1. MacNeil, S. , Progress and opportunities for tissue‐engineered skin. Nature 2007, 445, 874–880. [DOI] [PubMed] [Google Scholar]
- 2. Chan, B. P. and Leong, K. W. , Scaffolding in tissue engineering: General approaches and tissue‐specific considerations. Eur. Spine J. 2008, 17, 467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. O'Brien, F.J. , Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- 4. Duman, M. , Şendemir Ürkmez, A. and Zeybek, B. , Electrospinning of nanofibrous polycaprolactone (PCL) and collagen‐blended polycaprolactone for wound dressing and tissue engineering. Usak Univ. J. Mater. Sci. 2015, 3, 121–121. [Google Scholar]
- 5. Alessandrino, A. , Marelli, B. , Arosio, C. , Fare, S. , et al., Electrospun silk fibroin mats for tissue engineering. Eng. Life Sci. 2008, 8, 219–225. [Google Scholar]
- 6. Park, J. S. , Electrospinning and its applications. Adv. Nat. Sci. Nanosci. Nanotechnol. 2010, 1, 043002. [Google Scholar]
- 7. Ekaputra, A. K. , Prestwich, G. D. , Cool, S. M. and Hutmacher, D. W. , The three‐dimensional vascularization of growth factor‐releasing hybrid scaffold of poly (ɛ‐caprolactone)/collagen fibers and hyaluronic acid hydrogel. Biomaterials 2011, 32, 8108–8117. [DOI] [PubMed] [Google Scholar]
- 8. Katsogiannis, K. A. G. , Vladisavljević, G. T. and Georgiadou, S. , Porous electrospun polycaprolactone (PCL) fibres by phase separation. Eur. Polym. J. 2015, 69, 284–295. [Google Scholar]
- 9. Liu, C.Z. and Czernuszka, J.T. , Development of biodegradable scaffolds for tissue engineering: a perspective on emerging technology. Mater. Sci. Technol. 2007, 23, 379–391. [Google Scholar]
- 10. Rasmussen, B. , Fletcher, I. R. , Brocks, J. J. and Kilburn, M. R. , Reassessing the first appearance of eukaryotes and cyanobacteria. Nature 2008, 455, 1101–1104. [DOI] [PubMed] [Google Scholar]
- 11. Pulz, M. O. and Gross, W. , Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004, 635–648. [DOI] [PubMed] [Google Scholar]
- 12. Duarte, K. , Justino, C. I. L. , Pereira, R. , Freitas, A. C. , et al., Green analytical methodologies for the discovery of bioactive compounds from marine sources. Trends Environ. Anal. Chem. 2014, 3–4, 43–52. [Google Scholar]
- 13. Koller, M. , Muhr, A. and Braunegg, G. , Microalgae as versatile cellular factories for valued products. Algal Res. 2014, 6, 52–63. [Google Scholar]
- 14. Skulberg, O. M. , Microalgae as a source of bioactive molecules ‐ Experience from cyanophyte research. J. Appl. Phycol. 2000, 12, 341–348. [Google Scholar]
- 15. Sheih, I.‐C. , Wu, T.‐K. and Fang, T. J. , Antioxidant properties of a new antioxidative peptide from algae protein waste hydrolysate in different oxidation systems. Bioresour. Technol. 2009, 100, 3419–3425. [DOI] [PubMed] [Google Scholar]
- 16. Kose, A. , Ozen, M. O. , Elibol, M. and Oncel, S. S. , Investigation of in vitro digestibility of dietary microalga Chlorella vulgaris and cyanobacterium Spirulina platensis as a nutritional supplement. 3 Biotech 2017, 7, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gammone, M. A. , Riccioni, G. and D'Orazio, N. , Carotenoids: Potential allies of cardiovascular health? Food Nutr. Res. 2015, 59, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rez, M. F. , Binobaid, A. , Alghosen, A. , Mirza, E. H. , et al., Tubular poly(ε ‐caprolactone)/chitosan nanofibrous scaffold prepared by electrospinning for vascular tissue engineering applications. J. Biomater. Tissue Eng. 2017, 7, 427–436. [Google Scholar]
- 19. Maiullari, F. , Costantini, M. , Milan, M. , Pace, V. , et al., A multi‐cellular 3D bioprinting approach for vascularized heart tissue engineering based on HUVECs and iPSC‐derived cardiomyocytes. Sci. Rep. 2018, 8, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kose, A. and Oncel, S. S. , Properties of microalgal enzymatic protein hydrolysates: Biochemical composition, protein distribution and FTIR characteristics. Biotechnol. Reports 2015, 6, 137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim, S. H. , Shin, C. , Min, S. K. , Jung, S. M. , et al., In vitro evaluation of the effects of electrospun PCL nanofiber mats containing the microalgae Spirulina (Arthrospira) extract on primary astrocytes. Colloids Surfaces B Biointerf. 2012, 90, 113–118. [DOI] [PubMed] [Google Scholar]
- 22. de Morais, M. G. , Stillings, C. , Dersch, R. , Rudisile, M. , et al., Preparation of nanofibers containing the microalga Spirulina (Arthrospira). Bioresour. Technol. 2010, 101, 2872–2876. [DOI] [PubMed] [Google Scholar]
- 23. Belbachir, K. , Noreen, R. , Gouspillou, G. and Petibois, C. , Collagen types analysis and differentiation by FTIR spectroscopy. Anal. Bioanal. Chem. 2009, 395, 829–837. [DOI] [PubMed] [Google Scholar]
- 24. Mayers, J. J. , Flynn, K. J. and Shields, R. J. , Rapid determination of bulk microalgal biochemical composition by Fourier‐Transform Infrared spectroscopy. Bioresour. Technol. 2013, 148, 215–220. [DOI] [PubMed] [Google Scholar]
- 25. Wagner, H. , Liu, Z. , Langner, U. , Stehfest, K. , et al., The use of FTIR spectroscopy to assess quantitative changes in the biochemical composition of microalgae. J. Biophotonics 2010, 3, 557–566. [DOI] [PubMed] [Google Scholar]
- 26. Yao, Y. , Wang, J. , Cui, Y. , Xu, R. , et al., Effect of sustained heparin release from PCL/chitosan hybrid small‐diameter vascular grafts on anti‐thrombogenic property and endothelialization. Acta Biomater. 2014, 10, 2739–2749. [DOI] [PubMed] [Google Scholar]
- 27. Park, S.‐A. , Ahn, J.‐B. , Choi, S.‐H. , Lee, J.‐S. , et al., The effects of particle size on the physicochemical properties of optimized astaxanthin‐rich Xanthophyllomyces dendrorhous‐loaded microparticles. LWT ‐ Food Sci. Technol. 2014, 55, 638–644. [Google Scholar]
- 28. Cha, B. G. , Kwak, H. W. , Park, A. R. , Kim, S. H. , et al., Structural characteristics and biological performance of silk fibroin nanofiber containing microalgae spirulina extract. Biopolymers 2014, 101, 307–318. [DOI] [PubMed] [Google Scholar]
- 29. Kweon, H. , Yoo, M. K. , Park, I. K. , Kim, T. H. , et al., A novel degradable polycaprolactone networks for tissue engineering. Biomaterials 2003, 24, 801–808. [DOI] [PubMed] [Google Scholar]
- 30. Unalan, I. , Colpankan, O. , Albayrak, A. Z. , Gorgun, C. , et al., Biocompatibility of plasma‐treated poly(3‐hydroxybutyrate‐co‐3‐hydroxyvalerate) nanofiber mats modified by silk fibroin for bone tissue regeneration. Mater. Sci. Eng. C 2016, 68, 842–850. [DOI] [PubMed] [Google Scholar]
- 31. Beachley, V. , Effect of electrospinning parameters on the nanofiber diameter and length. Mater. Sci. Eng. C 2009, 29, 663–668. [DOI] [PMC free article] [PubMed] [Google Scholar]


