Abstract
During the last few years there is an increasing demand to the natural biologically active compounds. According to the World Health Organization (WHO) about 11% of the conventional medicines are of plant origin. Nowadays, plant biotechnologies are modern and reliable tool for producing valuable bioactive compounds. Recently, the potential of plant cells as foods also was confirmed. The advantages of plant in vitro systems over the intact plants are well known: growing under controlled and optimized laboratory conditions; independence of climatic and soil differences; preservation of rare and endangered plant species; cultivation in diverse bioreactor systems for increasing production yields of target metabolites.
There have been developed many in vitro systems for production of various plant bioactive compounds with potential application in food industries. But potential for industrial implementation of this technology depends on solving problems with the scale‐up of bioreactor cultivation, development of additional approaches for improving/modification of bioactivities of the target plant secondary metabolites, and to find way to exclude or replace in the culture media the carcinogenic plant growth regulator 2,4‐dichlorophenoxyacetic acid (2,4‐D) with its safety analogs, such as α‐naphtaleneacetic acid (NAA) and/or indole‐3‐butyric acid (IBA).
The aim of the current mini review is to summarize information about different in vitro systems of edible plants from the Balkan Peninsula with potential for producing food additives and biologically active substances and to describe prospects for successful industrial implementation of this technology.
Keywords: Biologically active substances, Edible plants, Natural food additives, Plant cultures
Abbreviations
- 2,4‐D
2,4‐Dichlorophenoxyacetic acid
- DW
dry weight
- FW
fresh weight
- IBA
Indole‐3‐butyric acid
- LS
Linsmaier and Skoog medium
- NAA
α‐Naphtaleneacetic acid
- RA
rosmarinic acid
1. Introduction
Since ancient times people used plants both as food and as sources of various valuable bioactive compounds. The extracts of medicinal and aromatic plants are used as food and cosmetic additives (e.g. essential oils, rosmarinic acid, vanillin), natural dyestuffs (e.g. betalains, anthocyanins), biopesticides (e.g. nicotine, rotenone, ryonidine), as well as phytotherapeutics (terpenic acids, phenolic acids, alkaloids: vinblastine, vincristine, caffeine, nicotine, ephedrine) 1, 2, 3.
Currently plants remain an essential source of biologically active compounds in spite of development of chemical or microbial technologies 2. According to the World Health Organization (WHO) 11% of the conventional medicines are exclusively of plant origin and a significant number are synthetic drugs obtained from natural precursors 4.
According to Turrill, 1929 5, Balkans are richer in flora than any comparable area in Europe. The flora in the region is characterized by high percentage of endemism. For example, in Bulgaria there are 270 higher plant species that are endemic to Balkan Peninsula, and other 174 species occur only on the territory of Bulgaria 6. These figures are of similar magnitude for the other Balkan countries, except for Greece, where about 750 species are endemics to the country 7.
The ethnobotanical studies in the recent decades revealed the peculiarities of traditional and contemporary plant use and the most popular wild plant species. Redzic, 2006 8 reported 308 plants used in nutrition and diet of local people in Bosnia and Herzegovina. Considerably lower number of species – 88 were noted by Nedelcheva, 2013 9 for Bulgaria. Luczaj et al., 2014 10 reviewed the wild food plants in Dalmatia (Southern Croatia) and concluded that the species used in the region were almost the same like in the other Mediterranean countries.
While many studies focused on the use of wild edible plants, relatively little is known about their cultivation. Currently, mostly aromatic and medicinal plants, having commercial value, are subjected to cultivation (for example, Mentha, Origanum, Melissa, Coriandrum), while the majority of wild edible plant species are collected from nature. This could cause disturbance of their natural populations and plant communities. Therefore, the cultivation could be a very promising tool for preventing the exhaustion of the natural resources and genepool of wild edible plants and could contribute to the conservation of their natural habitats 11, 12.
However, natural plant‐derived food additives and bioactive compounds extracted through conventional methods from intact plants are available only seasonally and are affected by environmental and geographical factors. Plant secondary metabolites are synthesized in minor amounts in specialized tissues (like trichomes) in variable yields year to year, that makes their extraction, isolation, and purification from intact plants difficult. Due to their very complex structure and/or chirality, in many cases organic synthesis is not possible or cost ineffective. These disadvantages can be overcome by plant in vitro cultures. Nowadays, plant biotechnology is modern and reliable tool for producing plant bioactive compounds. The advantages of plant in vitro systems over the intact plants are well known: year‐round growing under controlled and optimized laboratory conditions (pH, temperature, medium components, and others environmental factors); independence of climatic and soil differences; conservation of rare and endangered plant species; cultivation in diverse bioreactor systems for increasing production yields of target metabolites; separation of target compounds is much easier due to lower complexity of the cultured materials 3, 13, 14.
In the current mini review we summarize available information about in vitro plant systems of edible plants from the Balkan Peninsula producing food additives and biologically active substances.
2. In vitro systems of edible plants from the Balkan Peninsula producing food additives and bioactive substances
The capacity of plant cell, tissue, and organ cultures to produce and accumulate many valuable chemical compounds similar to the parent plant in nature has been highlighted since the inception of in vitro technology. The strong and growing demand in today's marketplace for natural, renewable products has refocused attention to in vitro plant materials as potential sources for secondary phytochemical products, and has paved the way for new research exploring secondary product expression in vitro 15.
According to the degree of differentiation of the cultivated plant tissues, plant in vitro systems could be classified as undifferentiated in vitro cultures (calli and cell suspensions) and differentiated in vitro cultures (shoots, hairy roots, adventitious roots, bulbs, somatic embryos) 3, 16.
2.1. Undifferentiated in vitro systems
Undifferentiated in vitro systems consist mainly of callus cultures and cell suspensions. Valuable bioactive substances from callus and cell suspension cultures have been successfully obtained from edible plants naturally grown in the Balkan Peninsula. Most of them belong to the Lamiaceae and Vitaceae families (Table 1).
Table 1.
In vitro systems of edible plants from the Balkan Peninsula producing food additives and bioactive substances
| Type of in vitro culture | Plant species (Family) | Bioactive compounds | Yield | References |
|---|---|---|---|---|
| Callus | Vitis vinifera L. cv. Öküzgözü (Vitaceae) | trans‐resveratrol | 62.23 μg/g FW | 17 |
| Cell suspension | Vitis vinifera L. cv. St. Laurent (Vitaceae) |
|
|
23 |
| Cell suspension | Vitis vinifera cv. Barbera (Vitaceae) | Free and mono glucosylated resveratrol | 32.72 μmol/g DW | 24 |
| Cell suspension | V. vinifera cv. Pinot Noir (Vitaceae) | trans‐resveratrol | 0.51 mg/L, 5.80 μg/g | 25 |
| Cell suspension | V. vinifera cv. Merzling (Vitaceae) | trans‐resveratrol | 4.31 mg/L, 3.91 μg/g | 25 |
| Cell suspension | V. amurensis (Vitaceae) | trans‐resveratrol | 225.22 mg/L, 187.35 μg/g | 25 |
| Cell suspension | V. riparia x V. berlandieri (Vitaceae) | trans‐resveratrol | 911.25 mg/L, 622.90 μg/g | 25 |
| Cell suspension | Ocimum basilicum L. (Lamiaceae) | Rosmarinic acid | 10 mg/g DW | 31 |
| Imobilized cell culture | Ocimum basilicum L. (Lamiaceae) | Rosmarinic acid | 20 mg/g DW | 32 |
| Callus | Satureja hortensis L. (Lamiaceae) | Rosmarinic acid | 82.3 4 ± 0.26 mg/g FW | 33 |
| Cell suspension | S. tomentosa Mill. (Lamiaceae) | Oleanolic acid,Ursolic acid |
|
35 |
| Rhizogenic callus | S. scabiosifolia Lam. (Lamiaceae) | Oleanolic acid | 829.14 mg/g DW | 37 |
| Cell suspension | Helianthus annuus L. (Asteraceae) | α ‐ tocopherol | 11.26 μg/g FW | 39 |
| Cell suspension | Arabidopsis thaliana L. (Brassicaceae) | α ‐ tocopherol | 9.00 μg/g FW | 39 |
| Cell suspension | Helianthus annuus L. (Asteraceae) | α ‐ tocopherol | 24 μg/g FW | 40 |
| Cell suspension | Helianthus annuus L. (Asteraceae) | α ‐ tocopherol | 77.00 μg/g DW | 41 |
| Cell suspension | Helianthus annuus L. (Asteraceae) | Linoleic acid | 14.1% of the identified total volatiles compound | 42 |
| Hairy roots | Salvia tomentosa Mill. (Lamiaceae) |
|
|
44 |
| Hairy roots | Beta vulgaris L. | Betanin | 53 mg/L | 45 |
| Shoots | Vitis vinifera cv. Feteasca Neagra, (Vitaceae) | trans‐resveratrol | 41.30 μg/g DW | 50 |
| Shoots | Vitis vinifera Cabernet Sauvignon (Vitaceae) | trans‐resveratrol | 7.94 μg/g DW | 50 |
| Shoots | S. officinalis L. (Lamiaceae) |
|
|
51 |
| Hairy roots | S. officinalis L. (Lamiaceae) | Rosmarinic acid | 30.9 ± 1.0 mg/g DW | 51 |
Keskin and Kunter, 2010 17 successfully obtained callus culture of Vitis vinifera L. cv. Öküzgözü, grown in Turkey that produce resveratrol ‐ a valuable stilbene. This secondary metabolite has been found to possess a number of health benefits: antiviral, antioxidant, anti‐inflammatory, cancer chemopreventive and therapeutic effects, prevent heart‐artery diseases by reducing cholesterol and harmful blot clots 18, 19, 20, 21. Due to these health benefits, there is an increasing demand for effective approaches to produce resveratrol. When 12‐day‐old culture of V. vinifera L. cv. Öküzgözü was exposed to UV light (254 nm) for 15 min. the amount of accumulated trans‐resveratrol was 26‐fold higher than the control calluses 17.
Except trans‐resveratrol, suspension cultures obtained of grapevine grown in Slovak Republic and Italy have been reported to accumulate a wide range of catechins, flavonoids and phenolic acids possessing multiple biological properties such as antioxidant, antibacterial, anticancer, estrogenic and heart‐protecting activities 22, 23, 24, 25. Most of these researches have been focused on improvement of in vitro production of the target compounds by adding elicitors, such as fungal pathogen Phaeomoniella chlamydospora, metyljasmonate 23, chitosan 24, dimethyl‐β‐cyclodextrin 25 or cultivation in batch and fed‐batch bioreactors 24. The elicited with dimethyl‐β‐cyclodextrin in vitro culture of V. riparia x V. berlandieri produced in the medium about 2400 times higher amount of trans‐resveratrol (911.25 mg/L) compared to untreated cells (0.37 mg/L) 25. Ferri et al., 2014 24 reported for 20‐fold higher production of free and mono glucosylated resveratrol (32.72 μmol/g DW or 7.47 μg/g DW) in fed‐batch cultivation of Vitis vinifera cv. Barbera treated with chitosan.
Beside by suspension cultures of grapevine, resveratrol was successfully obtained by many engineered microorganisms (Saccharomyces cerevisiae, Escherichia coli) 26, 27, 28, 29, 30. The highest yield of resveratrol was obtained by Mingji et al., 2016 30. They used metabolic engineering strategies, synthetic biology techniques and system biology approaches and constructed engineered strain of S. cerevisiae produced 811.50 and 754.70 mg/L resveratrol in fed‐batch fermentation on a minimal medium with glucose or ethanol, respectively. Besides, this strain produced resveratrol from low cost carbon sources in short process time with high purity that is a base for its commercial application. Additionally, this engineered strain of S. cerevisiae synthesized higher amount of resveratrol compared to the suspension cultures of grapevine 23, 25.
Cell suspension cultures of sweet basil (Ocimum basilicum L.), grown in Greece were successfully developed for production of rosmarinic acid (RA) – one of the widespread natural antioxidant in the Lamiaceae family 31, 32. Double volume of the immobilization matrix of cell suspension culture of sweet basil increased RA production hundred times. In addition, RA was secreted into the culture medium, where it was collected without terminating the culture of immobilized cells 31, 32.
Tepe and Sokmen, 2007 33 developed a callus culture of one of the most spread edible plant in the territory of Balkan Peninsula Satureja hortensis L. In this study, production and optimization of RA was investigated on Gamborg's B5 34 basal medium supplemented with different indole‐butyric acid (IBA) and N6‐benzyl aminopurine (6‐BA) combinations and sucrose concentrations.
Protocol for efficient cultivation of cell suspension culture of Salvia tomentosa Mill. (Lamiaceae), naturally grown in Bulgaria and production of valuable pentacyclic triterpenes was reported by Marchev et al., 2011 35. S. tomentosa Mill. suspension synthesized high amounts of oleanolic (71.89 μg/mL) and ursolic acid (256.28 μg/mL). However, the nutrient media used for its cultivation were supplemented with 2,4‐D, which makes the use of these valuable metabolites in food systems under discussion, concerning the safety of this plant growth regulator and possible cancer risk in humans 36.
It is well known that undifferentiated cell cultures have unstable growth and varying secondary metabolites yields because of the high level of somaclonal variation and unpredictable changes in endoreduplication pattern 37. To minimize those risks, the so called rhizogenic callus cultures ‐ fast growing undifferentiated transformed plant cells with a stable ploidy pattern have been developed and studied for secondary metabolites production 37. Such type of callus culture was initiated from S. scabiosifolia Lam. after genetic transformation with agropine‐type strain Agrobacterium rhizogenes ATCC 15834 and subculture of transformed roots on Linsmaier and Skoog (LS) medium 38. The obtained rhizogenic callus was used to initiate cell suspension culture with stable growth characteristics in liquid medium and predominant accumulation of oleanolic acid (829.14 μg/g DW) 37. The stable growth, the predictable yields, and cultivation in plant growth regulators free nutrient media of rhizogenic cell suspension culture of S. scabiosifolia Lam. seems to be a possible approach for producing oleanolic acid for foods 37.
After optimization of medium composition and culture conditions, cell suspension cultures of Helianthus annuus L. (Asteraceae) and Arabidopsis thaliana L. (Brassicaceae) were established for the production of the most active component of vitamin E, α‐tocopherol. A considerable increase (49 and 66%, respectively) of α‐tocopherol production was obtained in both cultures, after a treatment with jasmonic acid 39. Higher yeld of α‐tocopherol (24 μg/g FW) was obtained by standardized suspension of H. annuus L 40. Besides, Geipel et al., 2014 41 described an efficient protocol for α‐tocopherol production from photomixotrophic suspension culture of H. annus. Georgiev et al., 2010 42 also reported for cell suspension culture of Helianthus annuus L. (Asteraceae) that produced 14.1% linoleic acid of the identified total volatile compounds.
The homogeneity of an in vitro cell population, the large availability of material, the high rate of cell growth in controlled and reproducible bioreactor conditions make cell suspension cultures a valuable platform for the production of high‐value secondary metabolites and other substances of commercial interest 15. Further benefits include improved safety of the biosynthesized products based on the absence of environmental pollutants and agrochemicals, which is important for products legal registration as food ingredients 43.
2.2. Differentiated in vitro systems
Undifferentiated plant in vitro systems have several disadvantages as producers of food additives and bioactive metabolites. The main concern is that the biosynthesis of some metabolites requires a complex structural and physiological (cellular and tissues) compartments that only specialized and differentiated cells and organs can provide 3. Other important points are the low and variable yields and the high level of genetic instabilities of fast growing undifferentiated plant cells 16. The development of production processes based on differentiated plant in vitro systems could be a suitable solution to overcome those concerns.
Transformed hairy root cultures, obtained after genetic transformation with Agrobacterium rhizogenes strains are the preferable production systems for valuable bioactive substances among the differentiated plant in vitro systems. This is because transformed hairy roots usually have comparatively high growth rates, stable metabolite profiles, low level of somaclonal variability, high potential to accumulate secondary metabolites, as well as cultivation without exogenous growth regulators. These in vitro systems also can be easily cultivated in various bioreactor types and temporary immersion systems 3.
Marchev and co‐workers, 2011 44 developed hairy root culture of S. tomentosa Mill. by Agrobacterium transformation and cultivation in two‐phase temporary immersion RITA system with a presence of adsorbent resin Amberlite XAD‐4 as a second phase. The application of this approach had some important biotechnological advantages, such as an effective separation of the resin nets from the explants and removing of the biosynthesized phenolics from the cultivation medium. The authors 44 established that about 85% of released phenolic acids and 100% of released flavonoids were removed from the medium and 100% of the explants formed strong and healthy hairy roots for two weeks. This new transformation protocol was proved to be very effective and could be widely applied in other plant species, overproducing and releasing phytotoxic phenolics in high concentrations 44.
Production of the valuable food colorant betanin by Croatian Beta vulgaris L. hairy roots has been investigated by Kriznik and co‐workers, 2010 45. The authors reported volumetric yield of 53 mg/L betanin. This yield could be base for further industrially visible production process. Furthermore, there is available a huge amount of information about all bioprocess engineering aspects (media optimization, elicitation, bioreactor design improvement and etc.) for development of such process reported by Pavlov and co‐workers in the beginning of this century 46, 47, 48, 49.
It was established that shoot cultures of Vitis vinifera cv. Feteasca Neagra and Vitis vinifera cv Cabernet Sauvignon were suitable in vitro systems for trans‐resveratrol production. Elicitated cultures (with AlCl3) accumulated 41.30 μg/g DW and 97.94 μg/g DW trans‐resveratrol, respectively 50. These yields are higher than above described yields achieved by cell suspension culture of Vitis vinifera L. cv. Öküzgözü and Vitis vinifera cv. Barbera 17, 24, but lower than those reported from genetically modified yeasts 30.
It seems the differentiated in vitro cultures of S. officinalis L. (Lamiaceae) are suitable production systems for carnosic acid, carnosol and rosmarinic acid 51. Grzegorczyk and co‐workers, 2007 51 established that shoot culture produced carnosic acid, carnosol, rosmarinic acid, as the hairy root culture synthesized only rosmarinic acid. Reported volumetric yields for these compounds (Table 1) could be base for further development of economically sufficient processes, Furthermore hairy roots accumulated two fold higher quantities of rosmarinic acid than the suspension culture of the same edible plant 51.
Nevertheless achieved progress, still a challenge and a critical step that limits the industrial implementation of differentiated plant in vitro systems is their complicated scale up, as the main problem is transfer of biomasses at the inoculation processes 3. The growth of plant tissues or organs under controlled submerged conditions brings other engineering problems related to construction of vessels, realization of mass transfer, mixing and etc. 3. With the advance of bioprocess engineering, most of these technological issues have been solved in acceptable level and nowadays many bioreactor systems, as well as cultivation strategies have been developed and successfully applied for cultivation of various differentiated plant in vitro cultures including adventitious roots, transformed hairy roots, embryos, shoots and seedlings. Some of the processes have been implemented in commercial scale, as well 3, 52.
3. Conclusion and future prospects of bioprocess engineering aspects of production of bioactive compounds by plant in vitro systems
The objectives of many biotechnological industries are to develop plant in vitro culture techniques to the stage that the production of target bioactive compounds is cheaper either than extracting the naturally grown plants or the chemically synthesized product.
Conventional approaches for increasing the productivity of the plant in vitro cultures are based on media optimization, selection of a suitable plant tissue culture, elicitation by different biotic (microorganism‐derived elicitors; plant cell wall compounds; peptides, cyclodextrins) and abiotic elicitors (metal salts, UV light), as well as optimization of bioreactor design and environmental conditions of cultivation 53, 54, 55.
Despite a significant progress, the experience showed that the low yields and the complex scale‐up of bioreactor cultivation remain major limitations for industrial implementation of plant cell and tissue cultures. Therefore, there is an increasing necessity to find additional approaches for solving these limitations in yield, cost and bioprocessing conditions 56.
Biotransformation was proposed as a novel element of the integrated bioprocess approach for optimization of the production processes of food additives and bioactive compounds by plant in vitro systems (Fig. 1). It is based on modification bioactivity, bioavailability and toxicity of the produced secondary metabolites. Transformations of the structures of plant secondary metabolites are performed by bacteria and filamentous fungi as an adaptive response to their environment at physiological and/or biochemical level. They have ability to convert molecules at positions that are either difficult or impossible by chemical methods or are economically inexpedient. Besides, biotransformation proceeds at mild conditions regarding pH, temperature and pressure in contrast to the complicated steps required for their chemical synthesis. Another advantage of microbial transformations is that the obtained derivate is pure and with sufficient yield 57.
Figure 1.
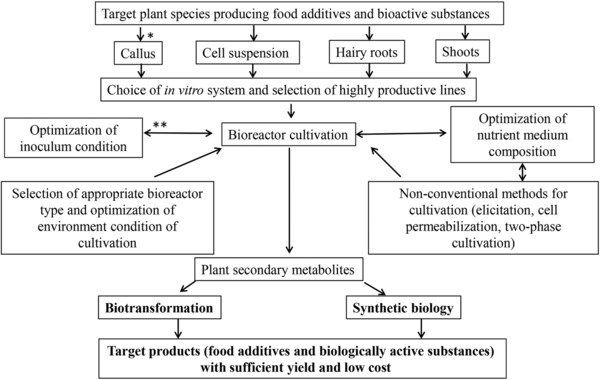
Integrated bioprocess approach for improving the production of food additives and bioactive compounds by plant in vitro systems (* consecutive steps; ** interconnected steps).
During the last years synthetic biology has been remarkably developed. So, it could be proposed as a second novel element in the integrated bioprocess approach for production of plant bioactive compounds with sufficient yields and low cost (Fig. 1). Synthetic biology is a promising engineering tool for controlling and programming cellular behavior and biosynthesis of target compounds 58. Most frequently, the yeasts are used as an appropriate biosynthetic matrix in this approach 59, but they also could be used for tailoring the metabolite pathways in the plant cells themselves. Omics studies and metabolic modeling enhance the understanding of yeast and plant cells metabolic networks. Nowadays, the challenges associated with synthetic biology are discovery or program of a biochemical pathway toward the desired compound, efficient and rapid assembly of this pathway in the host, optimization of enzymatic activity and expression levels of individual enzymes in the pathway, and optimization of the host cell for efficient supply of precursors, co‐factors, and energy for the process 60. Nevertheless of these limitations, the future development of the production of bioactive plant secondary metabolites will be based on the development of synthetic biological platforms – from one side for tailoring plant cell metabolism and from other for development of plant secondary metabolites production systems based on the yeasts.
Regardless of all described limitations of plant cells biotechnologies, the first commercial processes for production of cosmetics and food additives in Bulgaria are visible in the near future. Last year appeared the first Utility Model (Phytochemical profile of dedifferentiated cells of Calendula officinalis L.) developed by Prof. Atanas Pavlov and Dr. Vasil Georgiev, 2017 61 in collaboration with INOVA BM Ltd., on which base nowadays the development of the industrial processes is at the stage of the commercialization of the products.
The authors have declared no conflict of interest.
Acknowledgments
The authors are grateful for the financial support of this research by the Bulgarian Science Fund, Bulgarian Ministry of Education and Science by contract DM 06/1 ‐ 13.12.2016.
4 References
- 1. Mulabagal, V. , Tsay, H.‐S. , Plant cell cultures ‐ an alternative and efficient source for the production of biologically important secondary metabolites. Int. J. Appl. Sci. Eng. 2004. 2, 29–48. [Google Scholar]
- 2. Sheludko, Y. V. , Recent advances in plant biotechnology and genetic engineering for production of secondary metabolites. Cytology and Genetics. 2010, 44, 52–60. [PubMed] [Google Scholar]
- 3. Steingroewer, J. , Bley, T. , Georgiev, V. , Ivanov, I. , et al., Bioprocessing of differentiated plant in vitro systems. Eng. Life Sci. 2013, 13, 26–38. [Google Scholar]
- 4. Sahoo, N. , Manchikanti, P. , Dey, S. , Herbal drugs: Standards and regulation. Fitoterapia. 2010, 81, 462–471. [DOI] [PubMed] [Google Scholar]
- 5. Turrill, W. B. , The plant‐life of the Balkan peninsula: a phytogeographical study (Oxford Memoirs on Plant Geography). Geol. Mag. 2009, 66, 188–189. [Google Scholar]
- 6. Petrova, A., Vladimirov, V. , Balkan endemics in the Bulgarian flora. Phytol . Balcan. 2010, 16, 293–311. [Google Scholar]
- 7. Strid, A. , Andonovski, A. , Andonovski, V. , The high mountain vegetation of the Balkan peninsula, in: Nagy L., Grabherr G., Körner Ch., Thompson D.B.A. (Eds.), Alpine Biodiversity in Europe. Ecological Studies, Springer‐Verlag; Berlin Heidelberg: 2003, pp. 113–121. [Google Scholar]
- 8. Redzic, S. , Wild edible plants and their traditional use in the human nutrition in Bosnia and Herzegovina, Ecol. Food Nutr. 2006, 45, 189–232. [Google Scholar]
- 9. Nedelcheva, A. , An ethnobotanical study of wild edible plants in Bulgaria, Eurasia J. Biosci. 2013, 7, 77–94. [Google Scholar]
- 10. Łuczaj, Ł. , Dolina, K. , Fressel, N. , Perković, S. , Wild food plants of Dalmatia (Croatia), in: Pieroni A., Quave C. (Eds), Ethnobotany and Biocultural Diversities in the Balkans, Springer, New York, NY: 2014, pp. 137–148. [Google Scholar]
- 11. Hardalova, R. , Evstatieva, L. , Gussev, Ch. , Wild medicinal plant resources in Bulgaria and recommendation for their long‐term development, in: Meine C. (Ed.), National Strategy for Biodiversity Conservation. Pensoft, Sofia: 1998, pp. 527–561. [Google Scholar]
- 12. Turner, N. , Łuczaj, Ł. , Migliorini, P. , Pieroni, A. , et al., Edible and tended wild plants, traditional ecological knowledge and agroecology. Crit. Rev. Plant Sci. 2011, 30, 198–225. [Google Scholar]
- 13. Kim, Y. , Wyslouzil, B. E. , Weathers, P. J. , Secondary metabolism of hairy root cultures in bioreactors. In vitro Cell Dev‐Pl. 2002, 38, 1–10. [Google Scholar]
- 14. Srivastava, S. , Srivastava, A. K. , Hairy root culture for massproduction of high‐value secondary metabolites. Crit. Rev. Biotechnol. 2007, 27, 29–43. [DOI] [PubMed] [Google Scholar]
- 15. Bharati, A. J. , Bansal, Y. K. , In vitro production of flavonoids: a review. World J. Pharm. Pharm. Sci. 2014, 3, 508–533. [Google Scholar]
- 16. Georgiev, V. , Schumann, A. , Pavlov A., Bley, T. , Temporary immersion systems in plant biotechnology. Eng. Life Sci. 2014, 14, 607–621. [Google Scholar]
- 17. Keskin, N. , Kunter, B. , Production of trans‐resveratrol in callus tissue of Őkűzgőzű (Vitis vinifera L.) in response to ultraviolet‐C irradiation. J. Anim. Plant. Sci. 2010, 20, 197–200. [Google Scholar]
- 18. Bradamante, S. , Barenghi, L. , Villa, A. , Cardiovascular protective effects of resveratrol. Cardiovasc. Drug Rev. 2004, 22, 169–188. [DOI] [PubMed] [Google Scholar]
- 19. Szekeres, T. , Saiko, P. , Fritzer‐Szekeres, M. , Djavan, B. , Jager, W. , Chemopreventive effects of resveratrol and resveratrol derivatives. Ann. N. Y. Acad. Sci. 2011, 1215, 89–95. [DOI] [PubMed] [Google Scholar]
- 20. Huang, T. C. , Lu, K. T. , Wo, Y. Y. , Wu, Y. J. , et al., Resveratrol protects rats from A‐induced neurotoxicity by the reduction of iNOS expression and lipid per‐oxidation, PLoS One. 2011, 6, e29102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baur, J. A. , Sinclair, D. A. , Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [DOI] [PubMed] [Google Scholar]
- 22. Guerrero, R. F. , Garcia‐Parrilla, M. C. , Puertas, B. , Cantos‐Villar, E. , Wine, resveratrol and health: a review. Nat. Prod. Commun. 2009, 4, 635–658. [PubMed] [Google Scholar]
- 23. Sak, M. , Dokupilova, I. , Mihalik, D. , Lakatošova, J. , Gubišova, M. et al., Elicitation of phenolic compounds in cell culture of Vitis vinifera L. by Phaeomoniella chlamydospora. Nova Biotechnol. Chim. 2014, 13, 162–171. [Google Scholar]
- 24. Ferri, M. , Dipalo, S. C. , Bagni, N. , Tassoni, A. , Chitosan elicits mono‐glucosylated stilbene production and release in fed‐batch bioreactor cultures of grape cells. Food Chem.. 2014, 124, 1473–1479. [Google Scholar]
- 25. Zamboni, A. , Vrhovsek, U. , Kassemeyer, H. H. , Mattivi, F. U. , Elicitor‐induced resveratrol production in cell cultures of different grape genotypes (Vitis spp.). Vitis. 2006, 45, 63–68. [Google Scholar]
- 26. Becker, J. V. , Armstrong, G. O. , van der Merwe, M. J. , Lambrechts, M. G. , et al., Metabolic engineering of Saccharomyces cerevisiae for the synthesis of the wine‐related antioxidant resveratrol. FEMS Yeast Res.. 2003, 4, 79–85. [DOI] [PubMed] [Google Scholar]
- 27. Beekwilder, J. , Wolswinkel, R. , Jonker, H. , Hall, R. , et al., Production of resveratrol in recombinant microorganisms. Appl. Environ. Microbiol. 2006, 72, 5670–5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shin, S. Y. , Han, N. S. , Park, Y. C. , Kim, M. D. , et al., Production of resveratrol from p‐coumaric acid in recombinant Saccharomyces cerevisiae expressing 4‐coumarate: coenzyme A ligase and stilbene synthase genes. Enzyme Microb. Technol. 2011, 48, 48–53. [DOI] [PubMed] [Google Scholar]
- 29. Watts, K. T. , Lee, P. C and Schmidt‐Dannert, C. , Biosynthesis of plant‐specific stilbene polyketides in metabolically engineered Escherichia coli. BMC Biotechnol.. 2006. 6, 10.1186/1472-6750-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mingji, L. , Engineering of Saccharomyces cerevisiae for production of resveratrol and its derivatives, The Novo Nordisk Foundation Center for Biosustainability, Technical University of Denmark, 2016, PhD thesis.</bib>
- 31. Kintzios, S. , Makri, O. , Panagiotopoulos, E. , Scapeti, M. , In vitro rosmarinic acid accumulation in sweet basil (Ocimum basilicum L.). Biotechnol. Lett. 2003, 25, 405–408. [DOI] [PubMed] [Google Scholar]
- 32. Moschopoulou, G. , Kintzios, S. , Achievement of thousand‐fold accumulation of rosmarinic acid in immobilized cells of sweet basil (Ocimum basilicum L.) by ten‐fold increase of the volume of the immobilization matrix. J. Biol. Res. 2011, 15, 59–65. [Google Scholar]
- 33. Tepe, B. , Sokmen, A. , Production and optimisation of rosmarinic acid by Satureja hortensis L. callus cultures. Nat. Prod. Res. 2007, 21, 1133–1144. [DOI] [PubMed] [Google Scholar]
- 34. Gamborg, O. L , Miller R. A., Ojima, K. , Nutrient requirements of suspension culture of soybean root cells. Ex. Cell. Res. 1968, 50, 15–158. [DOI] [PubMed] [Google Scholar]
- 35. Marchev, A. , Ivanov, I. , Georgiev, V. , Pavlov, A. , Determination of di‐ and triterpenes in Salvia tomentosa Mill. cell suspension culture by high performance liquid chromatography. Scientific works of University of Food Technologies “Food science, engineering and technologies ”, 2012, 59, 229–233. [Google Scholar]
- 36. Loomis, D. , Guyton, K. , Grosse, Y. , El Ghissasi, F. et al., Carcinogenicity of lindane, DDT, and 2,4‐dichlorophenoxyacetic acid. Lancet Oncol.. 2015, 16, 891–892. [DOI] [PubMed] [Google Scholar]
- 37. Marchev, A. , Georgiev, V. , Badjakov, I. , Kondakova, V. , et al., Triterpenes production by rhizogenic callus of Salvia scabiosifolia Lam. obtained via Agrobacterium rhizogenes mediated genetic transformation. Biotechnol. Biotechnol. Eq. 2011, 25, 30–33. [Google Scholar]
- 38. Linsmaier, E. M. , Skoog, F. , Organic growth factor requirements of tobacco tissue cultures. Physiol. Plant. 1965, 18, 100–127. [Google Scholar]
- 39. Gala, R. , Mita, G. , Caretto, S. , Improving α‐tocopherol production in plant cell cultures. J. Plant Physiol.. 2005, 162, 782–784 [DOI] [PubMed] [Google Scholar]
- 40. Caretto, S. , Nisi, R. , Paradiso, A. , Laura, D. , Tocopherol production in plant cell cultures. Mol. Nutr. Food Res. 2010, 54, 726–730. [DOI] [PubMed] [Google Scholar]
- 41. Geipel, K. , Song, X. , Socher, M. L. , Kümmritz, S. , et al., Induction of a photomixotrophic plant cell culture of Helianthus annuus and optimization of culture conditions for improved α‐tocopherol production. Appl. Microbiol. Biotechnol. 2014, 98, 2029–2040. [DOI] [PubMed] [Google Scholar]
- 42. Georgiev, M. , Georgiev, V. , Penchev, P. , Antonova, D. , et al., Volatile metabolic profiles of cell suspension cultures of Lavandula vera, Nicotiana tabacum and Helianthus annuus, cultivated under different regimes. Eng . Life Sci.. 2010, 10, 148–157. [Google Scholar]
- 43. Angelov, A. , Gotcheva, V. , Safety assessment and regulations or food ingredients derived from plant in vitro systems, in; Pavlov A., Bley T. (Eds.), Bioprocessing of Plant In vitro Systems, Reference Series in Phytochemistry, Springer International Publishing; AG 2016, pp. 1–14. [Google Scholar]
- 44. Marchev, A. , Georgiev, V. , Ivanov, I. , Badjakov, I. , et al., Two phase temporary immersion system for Agrobacterium rhizogenes genetic transformation of sage (Salvia tomentosa Mill.). Biotechnol. Lett. 2011, 33, 1873–1878. [DOI] [PubMed] [Google Scholar]
- 45. Kriznik, B. , Pavokovic, D. , Enhancement of betanin yield in transformed cells of sugar beet (Beta vulgaris L.). Acta Bot. Croat. 2010, 69, 173–182. [Google Scholar]
- 46. Pavlov, A. , Georgiev, V. , Ilieva, M. , Betalain biosynthesis by red beet (Beta vulgaris L.) hairy root culture. Process Biochem. 2005, 40, 1531–1533. [Google Scholar]
- 47. Pavlov, A. , Georgiev, V. , Kovatcheva, P. , Relationship between type and age of the inoculum cultures and betalains biosynthesis by Beta vulgaris hairy root culture. Biotechnol. Lett. 2003, 25, 307–309, [DOI] [PubMed] [Google Scholar]
- 48. Pavlov, A. , Georgiev, M. , Bley, T. , Batch and fed‐batch production of betalains by red beet (Beta vulgaris) hairy roots in a bubble column reactor. Z. Naturforsch. 2007, 62c, 439–446. [DOI] [PubMed] [Google Scholar]
- 49. Georgiev, V. , Ilieva, M. , Bley, T. , Pavlov, A. , Betalain production in plant in vitro systems. Acta Physiol. Plant. 2008, 30, 581–593. [Google Scholar]
- 50. Bejan, C. , Visoiu, E. , Resveratrol biosynthesis on in vitro culture conditions in grapevine (cv. Feteasca Neagra and Cabernet Sauvignon) under the action of AlCl3 as elicitor agent. Rom. Biotechnol. Lett, 2011, 16, 97–101. [Google Scholar]
- 51. Grzegorczyk, I. , Matkowski, A. , Wysokin´ska, H. , Antioxidant activity of extracts from in vitro cultures of Salvia officinalis L. Food Chem.. 2007, 104, 536–54. [Google Scholar]
- 52. Georgiev, M.G. , Weber, J. , Bioreactors for plant cells: hardware configuration and internal environment optimization as tools for wider commercialization. Biotechnol. Lett. 2014, 36, 1359–1367. [DOI] [PubMed] [Google Scholar]
- 53. Zhao, J. , Davis, L.C. , Verpoorte, R. , Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol. Adv. 2005, 23, 283–333. [DOI] [PubMed] [Google Scholar]
- 54. Georgiev, M. , Weber, J. , Maciuk, A. , Bioprocessing of plant cell cultures for mass production of targeted compounds. Appl. Microbiol. Biotechnol. 2009, 83, 809–823. [DOI] [PubMed] [Google Scholar]
- 55. Hussain, M. , Fareed, S. , Ansari, S. , Rahman, M. A. , et al., Current approaches toward production of secondary plant metabolites. J. Pharm . Bioallied Sci. 2012, 4, 10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pavlov, A. , Plant cells and algae in bioreactors. Eng. Life Sci. 2014, 14, 548–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mutafova, B. , Fernandes, P. , Mutafov, S. , Berkov, S. , et al., Microbial transformations of plant secondary metabolites, in: Pavlov A., Bley T. (eds.), Bioprocessing of Plant In vitro Systems, Reference Series in Phytochemistry, Springer International Publishing; AG 2016, pp. 1–41. [Google Scholar]
- 58. Derek, J. M. , Valiante, V. , Unkles, S. E. , Brakhage, A. A. , Synthetic biology of fungal natural products. Front Microbiol. 2015, 6, 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sharma, K.K. , Exploiting fungi in synthetic biology: commentary. Curr. Synthetic Sys. Biol. 2015, 3, 123–124. [Google Scholar]
- 60. Mingji, L. , Borodina, I. , Application of synthetic biology for production of chemicals in yeast Saccharomyces cerevisiae. FEMS Yeast Res.. 2015, 15, 1–12. [DOI] [PubMed] [Google Scholar]
- 61. Pavlov, A. , Georgiev, V. , Phytochemical profile of dedifferentiated cells of Calendula officinalis L. BG Patent 2773, 2017.


