Abstract
Microbial oil production has received significant attention as a potential precursor for the production of biofuels, oleochemicals and food products. In this study, six oleaginous yeasts, isolated from fruits, were selected based on their ability to accumulate high intracellular content of microbial oil (20–48% w/w of total dry weight). The highest content of saturated fatty acids was 68.7% (w/w), whereas the highest content of oleic acid was 62.7% (w/w). Furthermore, nutrient‐rich hydrolysates produced via enzymatic hydrolysis of flour‐rich waste streams generated by a confectionery industry were evaluated as fermentation media for microbial oil production via fed‐batch bioreactor cultures using one of the most promising isolates, namely VV_D4. A total dry weight of 40 g/L with a microbial oil content of 39% (w/w) was produced by isolate VV_D4. Critical biodiesel properties were estimated based on the fatty acid composition and correlated with the international standards. The microbial oil produced by the new isolates could be potentially used for biodiesel production.
Keywords: Biodiesel, Biorefineries, Fermentation, Microbial oil production, Yeast isolation
Abbreviations
- BLAST
basic local alignment search tool
- CFPP
cold filter plugging point
- CN
cetane number
- FAMEs
fatty acid methyl esters
- FAN
Free amino nitrogen
- FID
flame ionization detector
- FRW
flour‐rich waste
- GenBank
genetic sequence database
- HMF
5‐hydroxy‐methyl‐furfural
- HPLC
high performance liquid chromatography
- IPS
intra‐cellular polysaccharides
- LC
length of chain
- LCV
low calorific value
- PCR
polymerase chain reaction
- rRNA
ribosomal ribonucleic acid
- TDW
total dry weight
- YPD
yeast extract peptone dextrose
1. Introduction
Microbial oil is commonly defined as lipids that are accumulated by oleaginous microorganisms. In general, oleaginous microorganisms are able to accumulate intracellular oil at more than 20% of their cell dry weight. Many microorganisms that belong to the genera of yeast, fungi, bacteria and algae have been reported to accumulate significant amounts of lipids during fermentation on various renewable resources 1. Among these, oleaginous yeasts are desirable industrial microorganisms due to their ability to accumulate high lipid content (up to 70% w/w), achieve high growth rates, tolerate high sugar concentrations, and utilize a wide range of carbon sources. Several strains belonging to the genera Rhodosporidium sp., Rhodotorula sp., Yarrowia sp., Cryptococcus sp., Lipomyces sp., have been reported to accumulate lipids between 40 and 70% of their dry cell weight under nutrient‐limiting conditions 2.
Microbial oil accumulation in yeasts fulfills energy storage purposes and it consists of triaglycerols (TAGs) which are produced in the endoplasmic reticulum and lipid bodies in the cytosol. Oleaginous yeasts are able to utilize a wide range of carbon sources for de novo lipid biosynthesis. Microbial oil production is triggered under nutrient limiting conditions leading to accumulation of citric acid in the mitochondrion that is subsequently secreted into the cytoplasm where it is converted to acetyl‐CoA (the precursor for fatty acid synthesis) and oxaloacetate by ATP citrate lyase 1. Various substrates can be valorized by oleaginous yeasts for microbial oil production such as pure sugars, sugar‐rich wastes (molasses and cheese whey), lignocellulosic materials, biodiesel derived glycerol, starch and food‐processing wastes. The food industrial sector produces huge amounts of by‐product streams. Confectionary industrial residues mainly consist of starch and other nutrients that are required for microbial growth (e.g. proteins, minerals) 3. Lignocellulosic biomass could be also used for microbial oil production. Complete hydrolysis of lignocellulosic materials produce a variety of monosaccharides that consist of pentoses (xylose, arabinose) and hexoses (glucose, galactose, and mannose), but also inhibitors (furfural, HMF, acetic acid) are generated 4. The utilization of all sugars derived from agri‐industrial waste streams is essential in order to enhance the microbial oil production via fermentation 1.
Microbial oil production by oleaginous yeasts can be used for biodiesel production and as a raw material for oleochemical production such as detergents, soaps, plastics, surfactants, lubricants, paints, and additives for the food and cosmetic industry among others 1, 5. Biodiesel is a renewable, biodegradable and non‐toxic energy resource that can be produced by transesterification of triacylglycerols (from edible and non‐edible oil‐rich feedstocks) with methanol to produce fatty acid methyl esters. However, due to the continuously increasing demand for first generation biodiesel and the fact that biofuel production should depend on non‐edible feedstocks, researchers focus on the production of microbial oil as an alternative feedstock for biodiesel production.
Microbial oil produced by oleaginous microorganisms has unique fatty acid composition which is affected by the type of carbon source, nature of microorganism and the cultivation conditions. In most cases, the fatty acid profile is similar to plant and animal oils, containing mainly C16 and C18 fatty acids. To evaluate the suitability of biodiesel production from microbial oil, several properties should be analyzed. Cetane number, kinematic viscosity, flash point, low calorific value, cold filter plugging point are some of the physicochemical biodiesel properties that must conform to the international standards (American Society for Testing and Materials ASTM D6751 and EN 14214 in Europe). Many researchers have developed mathematical models in order to predict the dependence between the fatty acid composition and biodiesel properties 6, 7.
Another potential application of the microbial oil produced by yeast strains in the food industrial sector could be the production of exotic fats such as cocoa butter substitutes. Cocoa butter has high saturated fatty acid content (approximately 60% and particularly 35% stearic acid and 25% palmitic acid). Papanikolaou et al. evaluated single cell oil production by Yarrowia lipoytica that could be used as substitute for cocoa butter 8.
In this study, several yeast strains were isolated from different fruits and studied for lipid accumulation utilizing sucrose as carbon source. Six isolates that indicated high level of intracellular lipid content were selected and identified. Subsequently, the utilization of various carbon sources for lipid production by the selected yeast strains was studied. The most promising isolate, VV_D4, was used in order to conduct a fed‐batch bioreactor fermentation using hydrolysates from flour‐rich waste streams as fermentation media. The fatty acid composition was determined and the physicochemical properties of each microbial oil were estimated using literature‐cited mathematical models in order to evaluate the potential of these oils for biodiesel production.
2. Materials and methods
2.1. Isolation
The yeast strains were isolated from the surface and the body of red and white grapes (Vitis vinifera), apples (Malus domestica), prickly pears (Opuntia ficus‐indica), pears (Pyrus communis and Pyrus pyrifolia), damsons (Prunus domestica subsp, insititia), plums (Prunus domestica), melons (Cumunis melo), pomegranates (Punica granatum) and figs (Ficus carica). 10 g of the samples were suspended in 90 mL of sterile saline solution and shaken vigorously in a stomacher apparatus (Laboratory Blender 400, Seward Medical, London) for 60 seconds. Serial dilutions of the sample suspension were performed in sterile saline solution. An amount of 0.5 mL was spread on Yeast Extract Dextrose Chloramphenicol agar (LAB M, Lancashire, UK) and incubated for 24 –48 h at 28°C. After incubation, morphologically different colonies were selected and isolated by successive subcultures on Yeast Extract Dextrose Chloramphenicol agar. Then, each colony was transferred with a wire loop into liquid media containing (in g/L): 20, sucrose; 0.3, yeast extract; 0.5, corn steep liquor; 11.7, NaH2PO4·2H2O; 1.5, K2HPO4; 1, NaCl; 1, CaCl2·2H2O; 0.04, MgSO4·H2O; 0.044, ZnSO4·7H2O; 0.016, FeCl3·6H2O in a 100 mL Erlenmeyer flask with 20 mL total fermentation volume and incubated for 24–48 h at 180 rpm and 28°C. The yeast strains which showed lipid globules within the cell were selected for the screening study.
2.2. Screening of oleaginous yeasts
For the selection of the oleaginous yeasts, isolates that indicated lipid accumulation were initially cultivated in 250 mL Erlenmeyer flasks, containing 50 mL of the liquid medium presented above. Cultures were inoculated with 10% (v/v) of 24‐h pre‐culture inoculum in YPD medium (10 g/L glucose, 10 g/L peptone, 10 g/L yeast extract). Flasks were incubated in an orbital shaker at an agitation speed of 180 rpm for 48 h and 28°C. The initial pH was adjusted to 5.5 before autoclaving.
2.3. Screening of the selected strains
The selected yeast strains were further cultivated in 500 mL Erlenmeyer flasks containing 100 mL of the growth medium presented above. The carbon sources used were glucose, xylose, mannose, arabinose and galactose with initial concentration of 20 g/L. Also a control without the carbon source was used so as to determine the total dry weight (TDW) produced from nitrogen sources and minerals only. Cultures were inoculated with 10% (v/v) of 24‐h pre‐culture inocula. The pre‐culture used was the same as described above. The shake flask fermentations have been carried out in triplicates.
2.4. Identification of oleaginous yeast strains
DNA extraction and amplification of the D1/D2 region of 26S‐rRNA gene were performed according to Pateraki et al. 9. PCR amplification products were sent to a commercial facility for sequencing. The results were aligned with yeast type strains in GenBank using the BLAST program to determine the closest known relatives based on the partial 26S‐rRNA gene sequence.
2.5. Production of flour‐rich waste hydrolysate
A generic feedstock from flour‐rich waste (FRW) hydrolysate was produced using the optimized method reported by Tsakona et al. 3. In brief, crude enzymes produced by solid state fermentation of Aspergillus awamori were mixed with a suspension of flour‐rich waste streams for the production of fermentation feedstock that was subsequently evaluated in fed‐batch experiment for microbial oil production by the isolate with code name VV_D4. The initial flour concentration was 100 g/L. The solid state fermentation was carried out in 250 mL Erlenmeyer flasks using 5 g of wheat milling by‐products. This process is described in detail by Tsakona et al. 3.
2.6. Bioreactor fermentation
The fed‐batch fermentation was conducted in a 3.4 L bioreactor (Labfors 4, Infors HT) with a working volume of 2 L using FRW hydrolysate as a carbon and nutrient source supplemented with yeast extract and corn steep liquor in order to achieve an initial FAN concentration of 260 mg/L. The mineral content added in the fermentation medium was (in g/L): 11.7, NaH2PO4·2H2O; 1.5, K2HPO4; 1, NaCl; 1, CaCl2·2H2O; 0.04, MgSO4·H2O; 0.044, ZnSO4·7H2O; 0.016, FeCl3·6H2O. The pH was maintained at 5.7 by automatic addition of 5 M NaOH and 10% (v/v) H2SO4. The air flow rate was maintained at 1 vvm and the temperature at 27°C. The agitation rate was controlled in the range of 150–400 rpm aiming to maintain the dissolved oxygen (DO) concentration at 20% of saturation. A 10% (v/v) of inoculum cultivated for 24 h was used. The initial glucose concentration was 10 g/L and feeding addition began when glucose concentration reached 5 g/L. A concentrated glucose solution (600 g/L) was added in the bioreactor in order to maintain the concentration of glucose at the range of 5–25 g/L. Fermentation samples were taken periodically for the analysis of glucose, free amino nitrogen (FAN), total dry weight, total intra‐cellular polysaccharides (IPS) and intracellular lipids.
2.7. Analytical methods
2.7.1. Determination of total dry weight
Each sample of fermentation broth (10 mL) was harvested by centrifugation at 9000 × g for 10 min (Hettich Universal Centrifuge, model 320‐R, United Kingdom) and washed twice with distilled water. The total dry weight was determined gravimetrically after drying at 102°C for 24 h.
2.7.2. Determination of sugars, intracellular polysaccharides and free amino nitrogen
Sugar concentration was determined by an HPLC SHIMADZU UFLC XR system equipped with a Phenomenex Rezex ROA column with size 300 mm × 7.8 mm coupled to a differential refractometer. Operating conditions were as follows: sample volume 20 μL; mobile phase 10 mM H2SO4; flow rate 0.6 mL/min; column temperature 45°C for the determination of sucrose and glucose, while 70°C was used for galactose, mannose, arabinose and xylose determination.
Total IPS was determined by the method reported by Tsakona et al. 3 with slight modifications. Briefly, 2 mL of hydrochloric acid (2 M) were mixed with 50 mg of dry weight of yeast cell mass followed by boiling (100˚C) for 30 min. Cellular debris was removed by centrifugation and the supernatant was analyzed by HPLC for IPS determination. FAN concentration was quantified according to the ninhydrin colorimetric method as reported by Lie 10.
2.7.3. Determination of lipid content
The lipid content was extracted and determined according to Folch et al. 11 with few modifications. An acid pretreatment method was performed by adding 4 mL of hydrochloric acid (2 M) for each 300 mg of dry cell mass weight and incubating at 80°C for 1 h. Then, the acid hydrolyzed mass was centrifuged (6000 × g for 10 min) to separate the aqueous upper phase from the organic lower phase. The lower phase containing the lipids was stirred with chloroform: methanol mixture (2:1). Total cellular lipid was recovered and gravimetrically quantified by Folch et al. 11.
2.7.4. Fatty acid methyl‐esters (FAMEs)
Lipids were converted to methyl‐esters according to the analytical method described by Tsakona et al. 3. The lipid composition was determined using a gas chromatograph (Fisons 8060 unit) equipped with FID detector and using a Chrompack column (60 m × 0.25 mm, film thickness 0.25 mm, J&W Scientific). Helium was used as carrier gas, at a flow rate of 2 mL/min. Oven temperature was started from 200°C and held for 13 min, increased to 220°C at a rate of 2°C/min and then with a rate of 20°C/min at 240°C and held for 3 min. The injector and the detector temperature were adjusted at 250°C.
2.8. Calculation of biodiesel properties
The properties of FAME namely length of chain (LC), cetane number (CN), low calorific value (LCV), cold filter plugging point (CFPP), flash point (FP) and kinematic viscosity (μ) were estimated based on the equations of Pinzi et al. 12 and Ramos et al. 7 (Table 1).
Table 1.
Mathematical models to predict the properties of microbial oil derived biodiesel
| Properties | Unit | Equation model | R2 | References |
|---|---|---|---|---|
| Unsaturation degree | – | UD = (1%MU + 2%DU + 3%TU)/100 | nr | 12 |
| Chain length | – | LC = R(nCn cn), | nr | |
| Cetane number | – | CN = Σ XME CNME | nr | 7 |
| Cold filter plugging point | °C | CFPP = –0.4880X+36.0548 (0<X≤88) | nr | |
| CFPP = –2.7043X+232.0036 (88<X≤100) | ||||
| Low calorific value | kJ/kg | LCV = 29385.4 + 486.866 LC – 387.766 UD | 98.22 | 12 |
| Flash point | °C | FP = 1008.48 – 136.166 LC + 142.578 UD +5.14811 LC2 –10.6906 LC UD +9.26352 UD2 | 95.94 | |
| Viscosity | mm2/s | μ = –1.8327 + 0.209794 LC + 0.738911 UD + 0.0166791 LC2–0.16336 LC UD + 0.335547 UD2 | 96.69 |
nr: not reported
Where nCn is the number of carbon atoms of each fatty acid, cn is the percentage of weight of each methyl ester containing this fatty acid, %MU is the percentage of weight of monounsaturated methyl esters, %DU is the percentage of weight of di‐unsaturated methyl esters and %TU is the percentage of weight of tri‐unsaturated methyl esters, XME is the weight percentage of each methyl ester and CNME is the cetane number of individual methyl ester, X is the content of the unsaturated fatty acid (wt %)
3. Results and discussion
3.1. Isolation and screening
Several species such as Rhodosporidium sp., Rhodotorula sp., Yarrowia sp., Cryptococcus sp., Lipomyces sp. are well known for intracellular lipid accumulation when cultivated on various carbon sources. Recent studies focus on the isolation of newly oleaginous yeasts from different environments (e.g. soil, plant materials, waste of palm oil production processes and biodiesel plants, flowers, glacial environments) with the ability to accumulate microbial oil under nutrient limiting conditions 13, 14, 15, 16. In this study, a total of 88 yeast strains were isolated from nine species of fruits. Yeast isolates were cultivated in shake flasks with sucrose as carbon source in nitrogen‐limited media to direct the microbial metabolism towards the synthesis of secondary metabolites. Fifty two strains which showed lipid bodies inside the cells were selected and evaluated for their ability to accumulate lipids. The total lipid content of twenty one isolates was more than 20% (w/w) of the total dry cell weight indicating their oleaginous nature.
Among the lipid producing strains, the isolates PD_D2, VV_D4, PC_A2, PD_F1, PD_D3 and MD_F1 that showed high lipid content, were selected for further investigation. The total dry weight, lipid concentration and lipid content are shown in Table 2. The selected yeast strains showed high total dry weight (5.6–8.4 g/L), while the lipid content was in the range of 25–48.6 % (w/w).
Table 2.
Total dry weight, lipid concentration and lipid content achieved by the selected oleaginous yeast strains in shake flask fermentations using sucrose as carbon source
| Isolation source | Code name | Time (h) | Total dry weight (g/L) | Lipid concentration (g/L) | Lipid content (% w/w) |
|---|---|---|---|---|---|
| Prunus domestica | PD_D2 | 48 | 8.0 ± 0.30 | 2.8 ± 0.31 | 34.8 ± 1.57 |
| Prunus domestica | PD_F1 | 48 | 7.3 ± 0.10 | 2.0 ± 0.11 | 27.3 ± 1.88 |
| Vitis vinifera | VV_D4 | 48 | 8.0 ± 0.17 | 2.7 ± 0.06 | 33.2 ± 0.12 |
| Pyrus communis | PC_A2 | 24 | 5.6 ± 0.17 | 2.7 ± 0.28 | 48.6 ± 1.48 |
| Pyrus pyrifolia | PP_D3 | 48 | 8.4 ± 0.31 | 2.1 ± 0.09 | 25.0 ± 0.10 |
| Malus domestica | MD_F1 | 48 | 8.0 ± 0.06 | 2.5 ± 0.20 | 31.9 ± 2.27 |
The utilization of sucrose as a carbon source for microbial oil production has been reported in a few studies. Candida curvata and Rhodotorula glutinis have been studied for microbial oil production using sucrose as a carbon source, however only Candida curvata could accumulate high lipid contents (34%, w/w) in batch cultures 17, 18, 19. Several yeast strains (e.g. Rhodotula 110, Cryptococcus podzolicus, Trichosporon porosum, Pichia segobiensis, Trichosporonoides spathulata, Kodamaea ohmeri, Cryptococcus sp. and Cryptococcus musci) have been isolated and studied for microbial oil production using various carbon sources as well as agricultural residues 15, 16, 20. Promising results have been reported by Enshaeieh et al. 20, and Kitcha and Cheirsilp 15 using Rhodotula 110 and Trichosporonoides spathulata, respectively. Rhodotula 110, isolated from soil, exhibited the highest lipid content (58.2%, w/w) in optimized conditions with glucose as carbon source, while the use of lignocellulosic material (corn stalk and wheat straw hydrolysate) was also studied and the results indicated high potential for lipid accumulation (38.9 and 43.4%, respectively) 15, 20. Moreover, the isolated oleaginous yeast Trichosporonoides spathulata could produce lipids (42.8%, w/w) using crude glycerol as carbon source 15.
3.2. Identification of oleaginous yeast strains
The six yeast isolates that were selected for further investigation were identified to their closest known relatives based on the partial 26S‐rRNA gene sequence. Figure 1 represents the phylogenetic relationship of six newly isolated yeasts with oleaginous and non‐oleaginous yeast type strains and Table 3 represents the phylogenetic position of the six isolates based on the D1/D2 region of 26S‐rRNA gene sequence. All strains appeared to belong to the genus Metschnikowia and the identity percentage to their closest relatives was 97–99%. Species that belong to the genus Metschnikowia have been mainly studied for ethanol production during the first stages of alcoholic fermentation in wine making 9. There has been one report for the production of microbial oil by Metschnikowia pulcherrima 21. Open air stirred tank reactors of 500 L were used for the cultivation of Metschnikowia pulcherrima in a temperature controlled glasshouse, under non‐sterile conditions, at low pH using glycerol as a carbon source. The total dry weight after 15 days was around 2 g/L with a lipid content of 34% (w/w).
Figure 1.
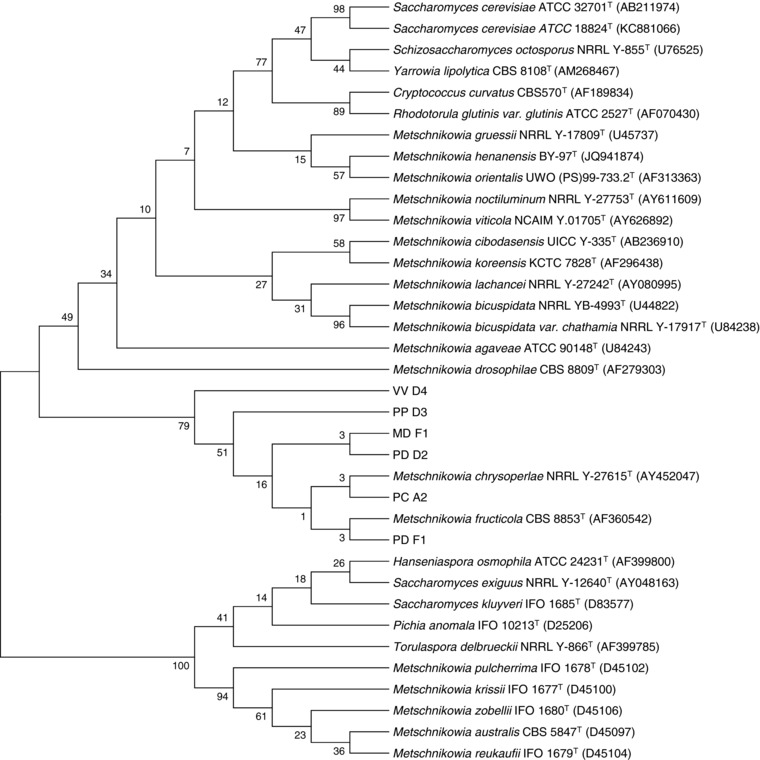
Phylogenetic position of the newly yeast isolates and comparison with type yeast strains based on Neighbor‐Joining distance analysis of 26S rRNA gene sequences. Bootstrap values at the node were calculated from 1000 samplings. GenBank/EMBL/DDBJ accession numbers are given in parenthesis.
Table 3.
Phylogenetic position of the isolates based on sequencing of the D1/D2 region of 26S‐rRNA gene
| Strain | Closest relative | Identity (%) | Accession number |
|---|---|---|---|
| PD_D2 | Metschnikowia sp. 11–1088 clone e2 | 98% | KM275357 |
| VV_D4 | Metschnikowia aff. pulcherrima P01A016 | 98% | JX188181 |
| PC_A2 | Metschnikowia pulcherrima strain B‐NC‐13‐F08 | 99% | KJ794649 |
| PD_F1 | Metschnikowia pulcherrima isolate Qch1 | 99% | HM067867 |
| PP_D3 | Metschnikowia pulcherrima strain BZYXYL‐1 | 97% | JX041890 |
| MD_F1 | Metschnikowia aff. pulcherrima P01A016 | 99% | JX188181 |
3.3. Microbial oil production from different carbon sources
The ability of the selected isolates to accumulate lipids on a wide range of carbon sources, including glucose, xylose, galactose, mannose and arabinose, was evaluated. Figure 2 presents the total dry weight and lipid concentration achieved by the selected strains in media with initial carbon source of 20 g/L when maximum lipid accumulation was achieved. All strains have the ability to consume all C5 and C6 sugars and accumulate microbial oil. The consumption of different carbon sources led to varying lipid accumulation and fatty acid composition. Xylose consumption led to the highest total dry weight for isolates PD_F1 and PP_D3. In the case of PC_A2 and MD_F1, the total dry weight reached the highest values when arabinose was used as a carbon source, while isolates PD_D2 and VV_D4 presented the highest total dry weight production in mannose and galactose, respectively. The utilization of glucose resulted in high lipid accumulation for isolates PD_F1 and PP_D3, where the lipid concentrations achieved were 2.4 g/L and 2.7 g/L corresponding to intracellular lipid contents of 37% (w/w) and 32.8% (w/w), respectively. Galactose consumption led to the highest intracellular lipid contents for strains PP_D3 (27.3%) and MD_F1 (29.4%). Furthermore, PD_D2 and PC_A2 have shown higher lipid content in xylose, despite the low total dry weight produced. Among the carbon sources tested, arabinose, glucose, galactose and xylose resulted in the highest lipid concentration for PD_F1, PP_D3, MD_F1 and PP_D3, respectively. The consumption of mannose led to relatively high lipid accumulation (21%, w/w) only in the case of PP_D3. Comparing the isolates, PP_D3 reached the maximum lipid concentration in all substrates ranging from 2 to 2.7 g/L, except for arabinose.
Figure 2.
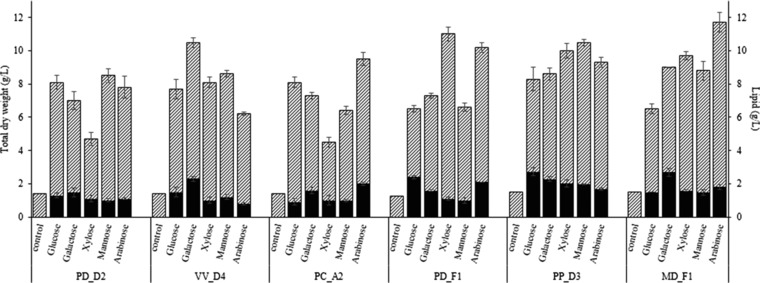
Total dry weight (grey bar) and lipid concentration (black bar) achieved by selected oleaginous yeasts using a range of carbon sources at an initial sucrose concentration of 20 g/L.
Table 4 presents lipid production reported in various literature‐cited publications using different oleaginous yeasts cultivated mainly in shake flasks on C5 and C6 sugars. Although glucose has been extensively studied as carbon source for microbial oil production, many oleaginous yeasts show high efficiency on lipid production using xylose as carbon source. Lipomyces starkeyi and Candida curvata have been reported to accumulate lipids up to around 50% of their total dry weight 22, 23. Even though arabinose contributes 1 to 18% of the total carbohydrates in hydrolysates there are limited publications reporting lipid production from arabinose, where one of the highest lipid contents (25%, w/w) was achieved by Lipomyces starkeyi 2.
Table 4.
Microbial oil production by oleaginous yeasts cultivated on various carbon sources in shake flask cultures
| Carbon source | Strain | Initial sugar concentration (g/L) | Total dry weight (g/L) | Lipid (g/L) | Lipid content (%, w/w) | Lipid yield (g/g) | References |
|---|---|---|---|---|---|---|---|
| Glucose | Rhodotorula glutinis 2.107 | 50 | 4 | 0.5 | 13 | 0.05 | 35 |
| Lipomyces starkeyi 2.1390 | 6.2 | 2.3 | 37.2 | 0.11 | |||
| Lipomyces starkeyi 2.1608 | 9.4 | 2 | 21.8 | 0.11 | |||
| Rhodosporidium toruloides 2.1389 | 4.3 | 1.7 | 39.3 | 0.13 | |||
| Rhodotorula glutinis 2.704 | 5.5 | 0.9 | 16.7 | 0.03 | |||
| Rhodotorula glutinis | 100 | 15.2 | 5.6 | 36.6 | 0.06 | 14 | |
| 29.8 | 14.7 | 49.3 | 0.15 | ||||
| Candida curvata a | 30 | 10.2 | 3.4 | 33.2 | 0.12 | 22 | |
| Lipomyces starkeyi AS 2.1560 | 39 | 23.8 | nr | 53 | 0.18 | 23 | |
| Rhodotorula glutinis IIP‐30a | 30 | 5.1 | 0.4 | 7.5 | 0.03 | 19 | |
| PD_D2 | 20 | 8.1 ± 0.40 | 1.3 ± 0.15 | 15.4 ± 1.09 | 0.06 | This study | |
| VV_D4 | 20 | 7.7 ± 0.60 | 1.5 ± 0.30 | 19.3 ± 1.39 | 0.07 | ||
| PC_A2 | 20 | 8.1 ± 0.30 | 0.9 ± 0.20 | 11.0 ± 1.06 | 0.04 | ||
| PD_F1 | 20 | 6.5 ± 0.20 | 2.4 ± 0.10 | 37.0 ± 1.68 | 0.11 | ||
| PP_D3 | 20 | 8.3 ± 0.70 | 2.7 ± 0.25 | 32.8 ± 1.75 | 0.12 | ||
| MD_F1 | 20 | 6.5 ± 0.30 | 1.5 ± 0.01 | 23.1 ± 1.07 | 0.07 | ||
| Xylose | Candida curvata a | 30 | 9.9 | 4.8 | 48.6 | 0.17 | 18 |
| Lipomyces starkeyi AS 2.1560 | 70 | 22.3 | 12.7 | 57 | 0.18 | 23 | |
| Lipomyces starkeyi AS2. 1390 | nr | 20.9 | 4.3 | 20.5 | nr | 2 | |
| Rhodosporidium toruloides AS 2. 1389 | nr | 7.2 | 1.9 | 26.8 | nr | ||
| Rhodotorula glutinis AS 2. 703 | nr | 6.9 | 0.8 | 12 | nr | ||
| Rhodosporidium toruloides | 40 | 12.5 | 4.9 | 38.9 | 0.15 | 36 | |
| Lipomyces starkeyi | 30 | 12.3 | 4.3 | 35 | nr | 37 | |
| PD_D2 | 20 | 4.7 ± 0.40 | 1.1 ± 0.20 | 23.2 ± 1.28 | 0.09 | This study | |
| VV_D4 | 20 | 8.1 ± 0.30 | 1.0 ± 0.20 | 12.5 ± 1.93 | 0.06 | ||
| PC_A2 | 20 | 4.5 ± 0.30 | 1.0 ± 0.30 | 22.8 ± 0.18 | 0.13 | ||
| PD_F1 | 20 | 11 ± 0.40 | 1.1 ± 0.10 | 10.0 ± 0.55 | 0.06 | ||
| PP_D3 | 20 | 10 ± 0.43 | 2.0 ± 0.21 | 19.9 ± 1.26 | 0.1 | ||
| MD_F1 | 20 | 9.7 ± 0.25 | 1.6 ± 0.05 | 16.6 ± 0.95 | 0.08 | ||
| Galactose | Trichosporon fermentans | 100 | 23.6 | 13.9 | 59 | 0.15 | 38 |
| PD_D2 | 20 | 7.0 ± 0.55 | 1.5 ± 0.27 | 21.4 ± 1.19 | 0.11 | This study | |
| VV_D4 | 20 | 10.5 ± 0.30 | 2.3 ± 0.15 | 21.5 ± 1.04 | 0.1 | ||
| PC_A2 | 20 | 7.3 ± 0.18 | 1.6 ± 0.19 | 21.8 ± 1.11 | 0.13 | ||
| PD_F1 | 20 | 7.3 ± 0.13 | 1.6 ± 0.06 | 21.9 ± 0.51 | 0.13 | ||
| PP_D3 | 20 | 8.6 ± 0.35 | 2.3 ± 0.15 | 27.3 ± 0.58 | 0.15 | ||
| MD_F1 | 20 | 9.0 ± 0.03 | 2.7 ± 0.25 | 29.4 ± 1.85 | 0.13 | ||
| Mannose | Trichosporon fermentans | 100 | 22.7 | 11.5 | 50.4 | 0.14 | 38 |
| PD_D2 | 20 | 8.5 ± 0.40 | 1.0 ± 0.10 | 11.7 ± 0.62 | 0.05 | This study | |
| VV_D4 | 20 | 8.6 ± 0.20 | 1.2 ± 0.14 | 14.4 ± 1.35 | 0.06 | ||
| PC_A2 | 20 | 6.4 ± 0.25 | 1.0 ± 0.08 | 15.0 ± 0.67 | 0.05 | ||
| PD_F1 | 20 | 6.5 ± 0.24 | 1.0 ± 0.17 | 14.4 ± 1.08 | 0.05 | ||
| PP_D3 | 20 | 9.5 ± 0.20 | 2.0 ± 0.01 | 21.0 ± 0.34 | 0.1 | ||
| MD_F1 | 20 | 8.8 ± 0.55 | 1.5 ± 0.17 | 16.8 ± 0.94 | 0.06 | ||
| Arabinose | Lipomyces starkeyi AS2. 1390 | nr | 14 | 3.5 | 24.9 | nr | 2 |
| Rhodosporidium toruloides AS 2. 1389 | nr | 4.8 | 0.8 | 16.8 | nr | ||
| Rhodotorula glutinis AS 2. 703 | nr | 4.3 | 0.2 | 4.9 | nr | ||
| PD_D2 | 20 | 7.8 ± 0.65 | 1.1 ± 0.05 | 14.2 ± 0.55 | 0.07 | This study | |
| VV_D4 | 20 | 6.2 ± 0.10 | 0.8 ± 0.08 | 13.5 ± 1.07 | 0.07 | ||
| PC_A2 | 20 | 9.5 ± 0.40 | 2.0 ± 0.09 | 21.1 ± 0.08 | 0.09 | ||
| PD_F1 | 20 | 10.2 ± 0.30 | 2.0 ± 0.01 | 20.6 ± 0.49 | 0.1 | ||
| PP_D3 | 20 | 9.3 ± 0.30 | 1.7 ± 0.06 | 17.8 ± 1.27 | 0.08 | ||
| MD_F1 | 20 | 11.7 ± 0.60 | 1.8 ± 0.15 | 15.7 ± 0.48 | 0.08 |
nr: not reported
batch bioreactor
3.4. Fed‐batch fermentation using hydrolysates from flour‐rich waste streams
Following the promising results of the screening study, a waste stream from the confectionary industry was used for the production of fermentation media in order to evaluate the potential for microbial oil production by the isolate VV_D4. Specifically, flour‐rich waste hydrolysate was used as carbon source. An important parameter that influences the microbial oil accumulation in oleaginous yeasts is the nitrogen content of the respective cultivation media. A nutrient complete medium was used at the beginning of the fermentation in order to achieve a high cell density. The initial FAN concentration was 260 mg/L. The accumulation of lipids was achieved via continuous supply of a glucose‐rich feeding solution when nitrogen limitation was attained. Figure 3 presents the glucose, FAN, TDW, and lipid concentration obtained during cultivation of VV_D4 on FRW hydrolysate. The TDW reached was 40 g/L with an intracellular lipid content of 39% (w/w) after 240 h. Lipid accumulation started when FAN was depleted from the fermentation broth. The accumulation of endo‐polysaccharides was also observed after FAN was depleted along with lipid production. From 50 to 120 h endo‐polysaccharide formation was observed to increase gradually from 0.21 to 0.5 g‐IPS/g‐TDW. Until the end of the fermentation, the IPS content remained stable at 0.5 g‐IPS/g‐TDW.
Figure 3.
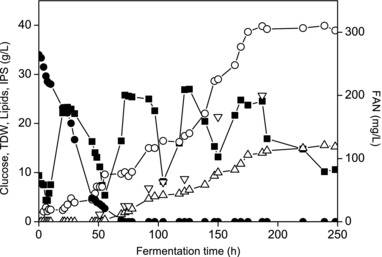
Consumption of glucose (■), FAN (●) and production of TDW (○) and lipids (△) during fed‐batch fermentation of VV_D4 using FRW hydrolysate.
The valorization of waste from the food industry has been rarely used as raw material for microbial oil production by oleaginous yeasts. Tsakona et al. 3 has reported the utilization of flour rich hydrolysate for microbial oil production by Lipomyces starkeyi reaching a TDW of 109.8 g/L with lipid content of 57.8% (w/w) in fed‐batch fermentation. Moreover, fish wastewater supplemented with glucose has been used by Lipomyces starkeyi in shake flask culture where the highest lipid concentration achieved was 2.7 g/L 24.
3.5. Lipid composition
The fatty acid composition of microbial oil is significantly affected by the microorganism, the substrate and the culture conditions employed. In most oleaginous microorganisms, the fatty acid profile of microbial oil contains mainly C16 and C18 fatty acids. Based on the fatty acid profile, microbial oils could have various applications for the production of biodiesel, surfactants, waxes, lubricants and chemical feedstocks 6, 25. In this study, the fatty acid composition of lipids produced in the different carbon sources was determined in order to evaluate the potential use of lipids as raw material for biodiesel production. Table 5 presents the fatty acid content produced by the selected yeast isolates when cultivated on different carbon sources when maximum lipid accumulation was achieved. Table 5 also presents the fatty acid profile of common vegetable oils and animal fats. The produced lipids mainly contain fatty acids with 16 and 18 carbon atoms. Specifically, the predominant fatty acids were oleic acid (18:1) followed by mainly palmitic acid (C16:0), stearic acid (18:0) and linoleic acid (18:2). The fatty acid profiles are similar to the common vegetable oil feedstocks used for biodiesel production. In particular, a total content of saturated fatty acids of about 68.7% was produced by PD_F1 when cultivated on glucose, whereas the highest content of oleic acid (62.7%) was observed by VV_D4 using mannose as substrate (Fig. 4). The lipids that contain more than 50% (w/w) of mono‐unsaturated fatty acids have similar fatty acid composition to rapeseed oil (Table 5). The microbial lipids that contain more than 50% (w/w) of saturated fatty acids have similar fatty acid composition to most animal fats and in some cases palm oil. The fatty acid profile of the microbial oil produced by isolate VV_D4 in fed‐batch fermentation was similar to most animal fats.
Table 5.
Fatty acid composition (%) of microbial oils produced by the selected strains on various carbon sources (shake flask fermentations have been carried out in triplicate) and range of fatty acid composition (%) of common vegetable oils and animal fats
| Strain | Substrate | C16:0 | C16:1 | C18:0 | C18:1 | C18:2 | C18:3 | Others |
|---|---|---|---|---|---|---|---|---|
| PD_D2 | Glucose | 12.5 | 7.3 | 3.9 | 54.2 | 15.5 | 1.6 | 5.1 |
| Galactose | 19.0 | 4.9 | 24.9 | 36.0 | 13.7 | 0.0 | 1.5 | |
| Xylose | 16.9 | 8.6 | 2.7 | 53.5 | 11.1 | 1.8 | 5.2 | |
| Mannose | 12.3 | 7.0 | 0.0 | 58.8 | 17.8 | 0.0 | 4.1 | |
| Arabinose | 15.2 | 6.0 | 28.0 | 44.9 | 0.0 | 1.5 | 4.4 | |
| VV_D4 | Glucose | 13.9 | 7.9 | 9.6 | 53.4 | 13.1 | 1.5 | 0.6 |
| Galactose | 14.1 | 9.5 | 10.4 | 51.9 | 11.4 | 1.0 | 1.6 | |
| Xylose | 15.6 | 8.6 | 2.8 | 53.8 | 13.8 | 1.4 | 3.9 | |
| Mannose | 13.4 | 7.1 | 0.0 | 62.7 | 14.2 | 0.0 | 2.7 | |
| Arabinose | 16.9 | 9.8 | 2.9 | 61.2 | 9.2 | 0.0 | 0.0 | |
| PC_A2 | Glucose | 13.6 | 8.4 | 1.4 | 58.3 | 14.1 | 0.3 | 3.9 |
| Galactose | 15.7 | 8.1 | 7.6 | 52.1 | 12.9 | 1.3 | 2.3 | |
| Xylose | 24.3 | 2.1 | 9.9 | 44.7 | 8.6 | 1.2 | 9.2 | |
| Mannose | 13.2 | 7.7 | 0.0 | 59.6 | 19.5 | 0.0 | 0.0 | |
| Arabinose | 19.7 | 7.9 | 17.9 | 48.9 | 5.3 | 0.0 | 0.3 | |
| PD_F1 | Glucose | 13.8 | 2.4 | 54.9 | 21.1 | 7.9 | 0.0 | 0.0 |
| Galactose | 26.1 | 3.5 | 6.7 | 52.0 | 11.6 | 0.0 | 0.0 | |
| Xylose | 17.6 | 6.3 | 3.0 | 54.5 | 13.1 | 1.2 | 4.3 | |
| Mannose | 15.8 | 5.6 | 0.0 | 60.5 | 18.1 | 0.0 | 0.0 | |
| Arabinose | 16.3 | 6.0 | 10.9 | 55.3 | 9.9 | 1.0 | 0.5 | |
| PP_D3 | Glucose | 16.5 | 1.8 | 41.7 | 28.3 | 11.7 | 0.0 | 0.0 |
| Galactose | 18.5 | 7.0 | 6.6 | 53.1 | 11.1 | 1.0 | 2.8 | |
| Xylose | 20.0 | 7.8 | 7.8 | 48.8 | 11.1 | 1.3 | 3.2 | |
| Mannose | 18.0 | 5.9 | 3.2 | 59.5 | 11.5 | 0.0 | 1.7 | |
| Arabinose | 15.4 | 6.8 | 9.0 | 55.5 | 11.4 | 1.2 | 0.7 | |
| MD_F1 | Glucose | 14.3 | 7.4 | 3.1 | 57.6 | 14.6 | 0.0 | 3.0 |
| Galactose | 14.9 | 6.0 | 10.9 | 55.3 | 9.9 | 1.0 | 1.9 | |
| Xylose | 16.4 | 6.8 | 9.0 | 51.2 | 11.5 | 1.2 | 3.8 | |
| Mannose | 18.0 | 5.9 | 3.2 | 59.5 | 11.5 | 0.0 | 1.7 | |
| Arabinose | 17.2 | 0.0 | 0.0 | 60.1 | 22.8 | 0.0 | 0.0 | |
| VV_D4 | RFW | 28.8 | 0.0 | 15.9 | 41.6 | 13.7 | 0.0 | 0.0 |
| Oils and fats | C16:0 | C16:1 | C18:0 | C18:1 | C18:2 | C18:3 | References | |
| Sunflower oil | 5–7 | – | 3‐6 | 14–40 | 48–74 | – | 28 | |
| Soybean oil | 10–12 | – | 3–5 | 18–26 | 49–57 | 6–9 | ||
| Cottonseed oil | 22–26 | – | 2–3 | 15–22 | 47–58 | traces | ||
| Rapeseed oil | 2–5 | – | 0–4 | 51–68 | 18‐25 | 7–11 | 39 | |
| Palm oil | 39–50 | – | 3–5 | 38–45 | 8–12 | traces | ||
| Tallow | 24–32 | – | 20–25 | 37–43 | 2–3 | – | 40 | |
| Lard | 28–30 | – | 12–18 | 40–50 | 7–13 | 0–1 | ||
| Yellow grease | 23.24 | – | 12.96 | 44.32 | 6.97 | 0.67 | ||
| Brown grease | 22.83 | – | 12.54 | 42.36 | 12.09 | 0.82 | ||
Figure 4.
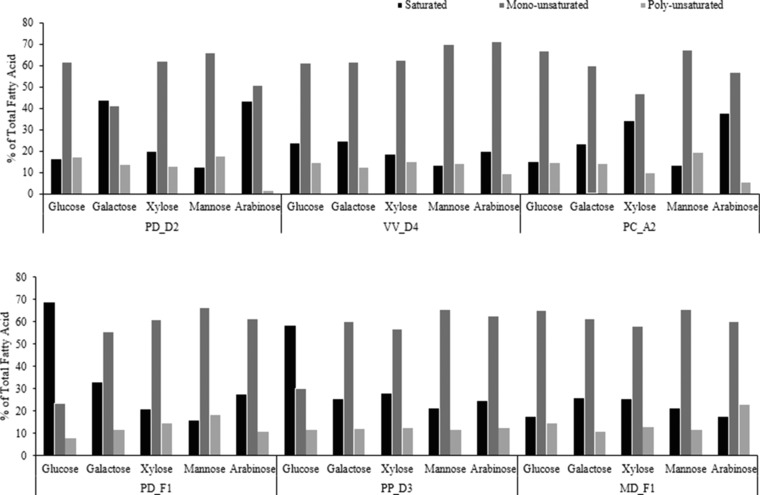
Effect of carbon source on saturated, mono‐unsaturated and poly‐unsaturated fatty acid composition.
In some cases, the fatty acid composition was significantly affected by the type of carbon source provided and also by the yeast strain used. For instance, the fatty acid profiles produced by PD_D2 in arabinose and galactose were similar to tallow, while the microbial oil produced by PP_D3 and PC_A2 on xylose and PD_F1 on galactose primarily consisted of palmitic and oleic acids. Moreover, when the isolates PD_F1 and PP_D3 were cultivated on glucose, a high content of saturated fatty acid (13.8% and 16.5% of palmitic acid and 54.9% and 41.7% of stearic acid, respectively) was produced. Similar compositions of fatty acids have been reported by Easterling et al. 26 with Rhodotorula glutinis cultivated on xylose and glycerol, where the fraction of saturated FAMEs were 69 and 68%, respectively 26. Furthermore, the fatty acid composition of the lipids produced by Candida tropicalis using molasses as carbon source contained a high content of palmitic acid (29.7%) and stearic acid (56.2%) 27.
3.6. Biodiesel properties
Fuel properties depend on the chemical structure of the fatty acid methyl esters. Predictive equations and mathematical models have been developed and used to predict some parameters in the biodiesel based on the FAME composition 7. In this study, the different physicochemical biodiesel properties were determined using mathematical equations based on the FAME profile and compared with the international standards. Table 6 presents the properties of oil produced by yeast isolates cultivated on different carbon sources and also the USA and European specifications regarding the accepted biodiesel properties.
Table 6.
Biodiesel properties based on the fatty acid composition of the microbial oil produced by selective isolates in different carbon sources
| Strain | Substrate | CN | UD | LC | LCV (kJ/kg) | FP (˚C) | Μ (mm2/s) | CFPP (°C) |
|---|---|---|---|---|---|---|---|---|
| PD_D2 | Glucose | 53 | 0.97 | 16.69 | 37133.18 | 143.76 | 4.69 | −2.28 |
| Galactose | 61 | 0.68 | 17.25 | 37519.28 | 167.27 | 5.48 | 9.4 | |
| Xylose | 54 | 0.9 | 16.56 | 37097.08 | 141.75 | 4.71 | −0.62 | |
| Mannose | 53 | 1.01 | 16.88 | 37211.4 | 148.04 | 4.75 | −4.75 | |
| Arabinose | 62 | 0.55 | 16.78 | 37342.25 | 155.74 | 5.38 | 10.48 | |
| VV_D4 | Glucose | 57 | 0.92 | 17.45 | 37525.1 | 167.43 | 5.25 | −0.96 |
| Galactose | 57 | 0.87 | 17.24 | 37438.54 | 161.59 | 5.18 | −0.01 | |
| Xylose | 54 | 0.94 | 16.81 | 37202.65 | 147.41 | 4.81 | −1.82 | |
| Mannose | 55 | 0.98 | 17.11 | 37336.6 | 155.22 | 4.94 | −4.93 | |
| Arabinose | 58 | 0.89 | 17.47 | 37542.34 | 168.63 | 5.29 | −3.08 | |
| PC_A2 | Glucose | 54 | 0.96 | 16.86 | 37222.97 | 148.57 | 4.82 | −3.56 |
| Galactose | 56 | 0.9 | 17.1 | 37364.36 | 156.85 | 5.05 | −0.26 | |
| Xylose | 56 | 0.68 | 15.81 | 36821.66 | 128.9 | 4.56 | 8.43 | |
| Mannose | 55 | 1.06 | 17.58 | 37533.73 | 168.08 | 5.12 | −6.32 | |
| Arabinose | 62 | 0.67 | 17.39 | 37592.59 | 172.55 | 5.59 | 5.75 | |
| PD_F1 | Glucose | 68 | 0.39 | 17.68 | 37839.33 | 193.33 | 6.29 | 20.75 |
| Galactose | 60 | 0.79 | 17.41 | 37555.17 | 169.63 | 5.42 | 3.27 | |
| Xylose | 55 | 0.91 | 16.74 | 37184.99 | 146.39 | 4.82 | −0.6 | |
| Mannose | 56 | 1.02 | 17.57 | 37544.33 | 168.78 | 5.17 | −5.04 | |
| Arabinose | 59 | 0.84 | 17.46 | 37560.85 | 169.97 | 5.37 | 0.8 | |
| PP_D3 | Glucose | 66 | 0.53 | 17.63 | 37763.6 | 186.27 | 6 | 15.68 |
| Galactose | 57 | 0.85 | 16.99 | 37326.92 | 154.53 | 5.05 | 0.86 | |
| Xylose | 57 | 0.83 | 16.87 | 37278.75 | 151.63 | 5.01 | 2.39 | |
| Mannose | 57 | 0.89 | 17.21 | 37419.41 | 160.35 | 5.14 | −1.53 | |
| Arabinose | 58 | 0.89 | 17.43 | 37528.32 | 167.66 | 5.28 | −0.5 | |
| MD_F1 | Glucose | 55 | 0.94 | 17.03 | 37311.13 | 153.64 | 4.95 | −2.79 |
| Galactose | 58 | 0.84 | 17.24 | 37453.6 | 162.59 | 5.23 | 0.8 | |
| Xylose | 56 | 0.85 | 16.86 | 37265.2 | 150.85 | 4.97 | 1.51 | |
| Mannose | 57 | 0.89 | 17.21 | 37419.47 | 160.35 | 5.14 | −1.53 | |
| Arabinose | 56 | 1.06 | 17.66 | 37572.3 | 170.76 | 5.17 | −4.36 | |
| VV_D4 | FRW | 62 | 0.69 | 17.42 | 37600.73 | 173.11 | 5.59 | 9.08 |
| EN 14214 | >51 | >120 | 3.5‐5 | |||||
| ASTM D6751 | >47 | >93 | 1.9‐6 |
In general, saturated long chain fatty acids excel in oxidation stability, cetane number and flash point, while exhibit poor cold flow properties, higher kinematic viscosity and lower calorific value 28. The biodiesel generated from microbial oils produced by the isolates cultivated in all carbon sources are within the limits set by EN 14214 and ASTM D6751 regarding cetane number and flash point. Cetane number is a primary indicator of fuel quality which is related to the ignition delay time. The predicted cetane number of microbial oil produced by the isolates were in all cases high, due to the high content of methyl esters of saturated fatty acids (stearic and palmitic acids). Regarding flash point the values of the microbial oil produced by the yeast strains are more than 128°C making the biodiesel safe to handle, store and transport.
Significant differences in terms of CFPP and kinematic viscosity were found for yeast oil derived biodiesel. CFPP is an important parameter that describes the fluidity in low temperature and depends on the regulations of each country. Saturated and long chain fatty acids have an adverse effect on cold flow properties of biodiesel. Oils with high saturated degree have higher melting points causing crystallization of the esters in low temperatures 29.
Several methods have been employed to improve the cold flow properties of biodiesel. Blending biodiesel with petroleum diesel or use of cold flow improvers could result in better value regarding cold flow properties 30. Winterization is another method to improve cold flow properties by removing saturated methyl ester 31. Wang et al. 32 investigated the addition of surfactants for reduction of CFPP where the addition of poly‐glycerol ester has the greatest effect to the CFPP 32.
Concerning the kinematic viscosity calculated by the predictive model showed that only some microbial oils fulfill the limit set by EN 14214, while in the case of the limit set by ASTM D6751 the vast majority of microbial oils lead to acceptable values of kinematic viscosity. High kinematic viscosity could affect the atomization of fuel on injection which has negative effects on the performance of the engine. Several techniques could be employed in order to reduce the kinematic viscosity such as preheating the oils, blending or dilution with other fuels in appropriate ratios, thermal cracking / pyrolysis, ultrasonic treatment and supercritical methanol trans‐esterification 33, 34.
4. Concluding remarks
Transition to the bio‐economy era from the petroleum‐based economy is a challenging process while the demand for renewable feedstocks has increased. Industrial waste and by‐product streams constitute valuable renewable resources for the production of commodity chemicals, bio‐polymers and fuels. The six new isolates identified in this study are promising for the production of microbial oil and biodiesel using various carbon sources. Waste streams from the confectionary industry have been used in this study for the production of 15 g/L microbial oil via fed‐batch bioreactor cultures.
The authors declare no conflict of interest.
Acknowledgments
The work presented in this study has been funded by the National Council for Scientific and Technological Development of the Ministry of Science, Technology and Innovation (CNPq/MCTI) through the Special Visiting Researcher fellowship (Process number: 313772/2013‐4). The authors also wish to acknowledge the COST Action TD1203 entitled “Food waste valorisation for sustainable chemicals, materials & fuels (EUBis)”
Contributor Information
Chrysanthi Pateraki, Email: paterakichr@aua.gr.
Apostolis Koutinas, Email: akoutinas@aua.gr.
5 References
- 1. Papanikolaou, S. , Aggelis, G. , Lipids of oleaginous yeasts. Part I: Biochemistry of single cell oil production. Eur. J. Lipid Sci. Technol. 2011, 113, 1031–1051. [Google Scholar]
- 2. Ageitos, J. M. , Vallejo, J. A. , Veiga‐Crespo, P. , Villa, T. G. , Oily yeasts as oleaginous cell factories. Appl. Microbiol. Biotechnol. 2011, 90, 1219–1227. [DOI] [PubMed] [Google Scholar]
- 3. Tsakona, S. , Kopsahelis, N. , Chatzifragkou, A. , Papanikolaou, S. et al., Formulation of fermentation media from flour‐rich waste streams for microbial lipid production by Lipomyces starkeyi . J. Biotechnol. 2014, 189, 36–45. [DOI] [PubMed] [Google Scholar]
- 4. Sitepu, I. , Selby, T. , Lin, T. , Zhu, S. et al., Carbon source utilization and inhibitor tolerance of 45 oleaginous yeast species. J. Ind. Microbiol. Biotechnol. 2015, 41, 1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koutinas, A. A. , Vlysidis, A. , Pleissner, D. , Kopsahelis, N. et al., Valorization of industrial waste and by‐product streams via fermentation for the production of chemicals and biopolymers. Chem. Soc. Rev. 2014, 43, 2587–2627. [DOI] [PubMed] [Google Scholar]
- 6. Leiva‐Candia, D. E. , Pinzi, S. , Redel‐Macías, M. D. , Koutinas, A. et al., The potential for agro‐industrial waste utilization using oleaginous yeast for the production of biodiesel. Fuel 2014, 123, 33–42. [Google Scholar]
- 7. Ramos, M. J. , Fernández, C. M. , Casas, A. , Rodríguez, L. et al., Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour. Technol. 2009, 100, 261–268. [DOI] [PubMed] [Google Scholar]
- 8. Papanikolaou, S. , Chevalot, I. , Komaitis, M. , Aggelis, G. et al., Kinetic profile of the cellular lipid composition in an oleaginous Yarrowia lipolytica capable of producing a cocoa‐butter substitute from industrial fats. Antonie Van Leeuwenhoek 2001, 80, 215–224. [DOI] [PubMed] [Google Scholar]
- 9. Pateraki, C. , Paramithiotis, S. , Doulgeraki, A. I. , Kallithraka, S. et al., Effect of sulfur dioxide addition in wild yeast population dynamics and polyphenolic composition during spontaneous red wine fermentation from Vitis vinifera cultivar Agiorgitiko. Eur. Food Res. Technol. 2014, 239, 1067–1075. [Google Scholar]
- 10. Lie, S. , The EBC‐Ninhydrin method for determination of free alpha amino nitrogen. Inst. Brew. 1973, 79, 37–41. [Google Scholar]
- 11. Folch, J. , Lees, M. , Stanley, G. H. S. , A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [PubMed] [Google Scholar]
- 12. Pinzi, S. , Leiva, D. , Arzamendi, G. , Gandia, L. M. et al., Multiple response optimization of vegetable oils fatty acid composition to improve biodiesel physical properties. Bioresour. Technol. 2011, 102, 7280–7288. [DOI] [PubMed] [Google Scholar]
- 13. Amaretti, A. , Raimondi, S. , Leonardi, A. , Rossi, M. , Candida freyschussii: An oleaginous yeast producing lipids from glycerol. Chem. Eng. Trans. 2012, 27, 139–144. [Google Scholar]
- 14. Dai, C. , Tao, J. , Xie, F. , Dai, Y. et al., Biodiesel generation from oleaginous yeast Rhodotorula glutinis with xylose assimilating capacity. African J. Biotechnol. 2007, 6, 2130–2134. [Google Scholar]
- 15. Kitcha, S. , Cheirsilp, B. , Screening of Oleaginous yeasts and optimization for lipid Production using crude glycerol as a carbon source. Energy Procedia 2011, 9, 274–282. [Google Scholar]
- 16. Tanimura, A. , Takashima, M. , Sugita, T. , Endoh, R. et al., Cryptococcus terricola is a promising oleaginous yeast for biodiesel production from starch through consolidated bioprocessing. Sci. Rep. 2014, 4, 4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fei, Q. , Chang, H. N. , Shang, L. , Choi, J. et al., The effect of volatile fatty acids as a sole carbon source on lipid accumulation by Cryptococcus albidus for biodiesel production. Bioresour. Technol. 2011, 102, 2695–2701. [DOI] [PubMed] [Google Scholar]
- 18. Evans, C. T. , Ratledge, C. , A comparison of the oleaginous yeast,Candida curvata, grown on different carbon sources in continuous and batch culture. Lipids 1983, 18, 623–629. [DOI] [PubMed] [Google Scholar]
- 19. Johnson, V. W. , Singht, M. , Saini, V. S. , Adhikari, D. K. et al., Utilization of molasses for the production of fat by an oleaginous yeast , Rhodotorula glutinis IIP‐30. J. Ind. Microbiol. 1995, 14, 1–4. [Google Scholar]
- 20. Enshaeieh, M. , Abdoli, A. , Nahvi, I. , Madani, M. , Selection and optimization of single cell oil production from Rodotorula 110 using environmental waste as substrate. J. Cell Mol. Res. 2013, 4, 68–75. [Google Scholar]
- 21. Santamauro, F. , Whiffin, F. M. , Scott, R. J. , Chuck, C. J. , Low‐cost lipid production by an oleaginous yeast cultured in non‐sterile conditions using model waste resources. Biotechnol. Biofuels 2014, 7, 34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Evans, C. T. , Scragg, A. H. , Ratledge, C. , Candida, D. , A Comparative study of citrate efflux from mitochondria of oleaginous an non‐oleaginous yeasts. Eur. J. Biochem. 1983, 130, 195–204. [DOI] [PubMed] [Google Scholar]
- 23. Gong, Z. , Wang, Q. , Shen, H. , Hu, C. et al., Co‐fermentation of cellobiose and xylose by Lipomyces starkeyi for lipid production. Bioresour. Technol. 2012, 117, 20–24. [DOI] [PubMed] [Google Scholar]
- 24. Huang, L. , Zhang, B. , Gao, B. , Sun, G. , Application of fishmeal wastewater as a potential low‐cost medium for lipid production by Lipomyces starkeyi HL. Environ. Technol. 2011, 32, 1975–1981. [DOI] [PubMed] [Google Scholar]
- 25. Huang, C. , Chen, X.‐F. , Xiong, L. , Chen, X. et al., Oil production by the yeast Trichosporon dermatis cultured in enzymatic hydrolysates of corncobs. Bioresour. Technol. 2012, 110, 711–714. [DOI] [PubMed] [Google Scholar]
- 26. Easterling, E. R. , French, W. T. , Hernandez, R. , Licha, M. , The effect of glycerol as a sole and secondary substrate on the growth and fatty acid composition of Rhodotorula glutinis . Bioresour. Technol. 2009, 100, 356–361. [DOI] [PubMed] [Google Scholar]
- 27. Karatay, S. E. , Dönmez, G. , Improving the lipid accumulation properties of the yeast cells for biodiesel production using molasses. Bioresour. Technol. 2010, 101, 7988–7990. [DOI] [PubMed] [Google Scholar]
- 28. Knothe, G. , “Designer” biodiesel: Optimizing fatty ester composition to improve fuel properties. Energy Fuels 2008, 22, 1358–1364. [Google Scholar]
- 29. Edith, O. , Janius, R. B. , Yunus, R. , Factors affecting the cold flow behaviour of biodiesel and methods for improvement–A review. J. Sci. Technol. 2012, 20, 1–14. [Google Scholar]
- 30. Dunn, R. O. , Shoi, M. W. , Bagby, M. O. , Improving the low‐temperature properties of alternative diesel fuels: Vegetable oil‐derived methyl esters. J. Am. Oil Chem. Soc. 1996, 73, 1719–1728. [Google Scholar]
- 31. Pérez, Á. , Casas, A. , Fernández, C. M. , Ramos, M. J. et al., Winterization of peanut biodiesel to improve the cold flow properties. Bioresour. Technol. 2010, 101, 7375–7381. [DOI] [PubMed] [Google Scholar]
- 32. Wang, Y. , Ma, S. , Zhao, M. , Kuang, L. et al., Improving the cold flow properties of biodiesel from waste cooking oil by surfactants and detergent fractionation. Fuel 2011, 90, 1036–1040. [Google Scholar]
- 33. Knothe, G. , Steidley, K. R. , Kinematic viscosity of biodiesel fuel components and related compounds. Influence of compound structure and comparison to petrodiesel fuel components. Fuel 2005, 84, 1059–1065. [Google Scholar]
- 34. Isioma, N. , Muhammad, Y. , Sylvester, O. D. , Innocent, D. et al., Cold flow properties and kinematic viscosity of biodiesel. Univers. J. Chem. 2013, 1, 135–141. [Google Scholar]
- 35. Chen, X. , Li, Z. , Zhang, X. , Hu, F. et al., Screening of oleaginous yeast strains tolerant to lignocellulose degradation compounds. Appl. Biochem. Biotechnol. 2009, 159, 591–604. [DOI] [PubMed] [Google Scholar]
- 36. Pan, L. , Yang, D. , Shao, L. , Li, W. et al., Isolation of the oleaginous yeasts from the soil and studies of their lipid‐producing capacities. Food Technol. Biotechnol. 2009, 47, 215–220. [Google Scholar]
- 37. Tapia V, E. , Anschau, A. , Coradini, A. L. , Franco, T. et al., Optimization of lipid production by the oleaginous yeast Lipomyces starkeyi by random mutagenesis coupled to cerulenin screening. AMB Express 2012, 2, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Huang, C. , Zong, M. , Wu, H. , Liu, Q. , Microbial oil production from rice straw hydrolysate by Trichosporon fermentans. Bioresour. Technol. 2009, 100, 4535–4538. [DOI] [PubMed] [Google Scholar]
- 39. Sargeant, La. , Chuck, C. J. , Donnelly, J. , Bannister, C. D. et al., Optimizing the lipid profile, to produce either a palm oil or biodiesel substitute, by manipulation of the culture conditions for Rhodotorula glutinis. Biofuels 2014, 5, 33–43. [Google Scholar]
- 40. Canakci, M. , Van Gerpen, J. , Biodiesel production from oils and fats with high free fatty acids. Am. Soc. Agric. Eng. 2001, 44, 1429–1436. [Google Scholar]


