Abstract
There is a great interest in increasing the levels of production of nanocellulose, either by adjusting production systems or by improving the raw material. Despite all the advantages and applications, nanocellulose still has a high cost compared to common fibers and to reverse this scenario the development of new, cheaper, and more efficient means of production is required. The market trend is to have an increase in the mass production of nanocellulose; there is a great expectation of world trade. In this sense, research in this sector is on the rise, because once the cost is not an obstacle to production, this material will have more and more market. Production of the cellulose fibers is determinant for the production of nanocellulose by a hydrolyzing agent with a reasonable yield. This work presents several aspects of this new material, mainly addressing the enzymatic pathway, presenting the hydrolysis conditions such as pH, biomass concentration, enzymatic loading, temperature, and time. Also, the commonly used characterization methods are presented, as well as aspects of the nanocellulose production market.
Keywords: characterization methods, endoglucanase, enzymatic hydrolysis, market trend, nanocellulose
Abbreviations
- AFM
atomic force microscopy
- CBH
cellobiohydrolases
- CNF
cellulose nanofibre
- CNC
cellulose nanocrystal
- CD
catalytic domain
- CrI
crystallinity index
- EG
endoglucanases
- XRD
X‐ray diffraction
- ZP
zeta potential
1. INTRODUCTION
There is an increasing demand for more sustainable materials that can replace petroleum‐based polymers in technological applications. More economically and environmentally friendly processes and raw materials are being developed to fulfill these demands 1. Therefore, cellulose‐based materials, such as nanocellulose composites, have great potential to assume this role in a near future because of its natural availability, biodegradability, and outstanding mechanical properties 2.
Cellulose is the most abundant renewable polymer on the earth and it is present mainly in plants and some species of bacteria. It is commonly present in nature in lignocellulosic biomass, as a microfibril cellulose form, wrapped by other biopolymers such as lignin and hemicellulose. These two components act as a mechanical reinforcement and microbiological protection in plant cells 3, 4.
As the demand for renewable fuels, such as bioethanol, increases, more and more biorefined residues are generated. The exploitation of these residues to produce second‐generation ethanol with coproduction of nanocelluloses may represent a valuable increase in the profitability of the biorefinery industry and also provides a great solution to the environmental impact of the waste that would be generatedbrk 5, 6.
Nanocellulose has a broad variety of applications, depending on the range of the generated product physical properties. These properties are affected mainly by the production process utilized and by the composition of the lignocellulosic biomass 7.
Nanocellulose is defined as cellulosic materials with at least one of its dimensions in the nanometric scale (1 nm = 10−9 m) 8. Its important features, such as high surface area, hydrophilicity, and high crystallinity, are due to intrinsic characteristics of cellulose and nanoparticles. Also, it presents high axial stiffness (up to 130 GPa), high aspect ratio (varying from 10 to 100), low density (approximately 1.5 g/cm3), and low coefficient of thermal expansion (approximately 1 ppm/K). These features make it a promising material for mechanical reinforcements in polymer composites 9.
Cellulose fibers are divided into two domains: amorphous and crystalline. The first domain is the most susceptible for an enzyme or acid hydrolysis. The second one presents lower accessibility to those attacks, owing to the shield effect of lignin and hemicellulose. Amorphous regions are imperfections on the crystalline matrix and the proportion between them and crystalline domains depends strictly on the lignocellulosic biomass origin 10.
There are three types of nanocellulose morphologies that can be obtained: (a) Cellulose Nanofibre (CNF), which is normally obtained by an enzymatic and/or mechanical disintegration process; (b) Cellulose Nanocrystal (CNC), which is obtained by a treatment with concentrated mineral acids, mainly HCl or H2SO4 11; and (c) Bacterial Nanocellulose, synthesized exclusively by a family of bacteria, known as Gluconoacetobacter xylinius 12. However, the focus of this work is to present plant‐derived nanocellulose.
The nanocellulose physical properties and biocompatibility are objects of interest for applications of these particles on drug delivery systems 13, 14 and also food additives 15. These features, along with the high surface area, make nanocellulose a strong candidate for biocompatible scaffolds for cell culture and tissue engineering applications 14, 16.
Most nanocellulose application studies aim the design and development of biopolymer composites. This can be explained by the outstanding mechanical properties and high surface area of these nanoparticles. Both features can provide composites with higher mechanical resistance and better particle aggregation 17.
Environmentally friendly packaging polymers and even antimicrobial packaging materials containing nanocellulose in its matrix have been reported 18. Many different polymers functionalization with nanocellulose can be carried out, depending on the aimed purpose 19. The broad range of nanocellulose technical applications, that may be positively impacted by its development, reinforces the need for extensive studies to achieve plenitude of its potential.
The growing number of publications related to enzymatic production of nanocellulose between 2007 and 2018 highlights the academic and commercial potential of this study. Technological discoveries and developments about nanocellulose are in their maturation period, which means that is the right moment to invest resources and intellectual activity on nanocellulose research. Medical and dental patents started to have a light decrease in registered documents. On the other hand, publications focusing on the improvement of the production process and on material applications is presenting a consistent increase during the last few years, especially the ones aiming at the development of nanocellulose composites 20.
PRACTICAL APPLICATION
The research shows various contributions and advantages that the production of nanocellulose provides from a scientific and economic point of view, focusing on its production through enzymatic hydrolysis. The study compiles several examples of production and techniques of characterization of nanocellulose found in the literature.
As can be seen in Figure 1, publications related to enzymatic production of nanocellulose have dramatically increased over the last 10 years. This tendency illustrates that this subject is at the early stages of development, with potential of developing novel and more efficient production methods, characterization, and innovative applications during the next few years.
Figure 1.
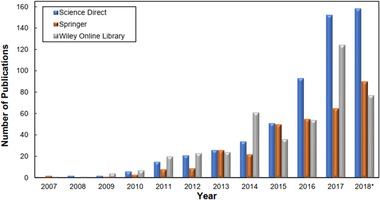
Number of publications related to enzymatic production of nanocellulose from 2007 to 2018* (specifically on December 8, 2018)
The increasing number of patents related to the production of nanocellulose is a response to its high market value, as shown in Figure 2.
Figure 2.
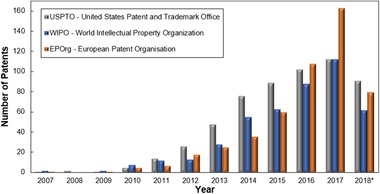
Patent publications over the years from different sources (WIPO, EPOrg, and USPTO), from 2007 to 2018* (specifically on December 8, 2018)
2. NANOCELLULOSE PRODUCTION
2.1. Current production methods
Concentrated mineral acid hydrolysis (mainly HCl or H2SO4) is the most common method used for CNCs production. Although it provides higher yields when compared with enzyme hydrolysis, it requires higher capital investment, because more expensive materials for reactors, tanks, and piping are required, on account of the corrosive nature of reactants. Besides, the effluent produced is harmful to the environment and operational costs may increase regarding the necessity of intensive effluent treatments 21.
Cellulose fibers, transformed into smaller microfibrils by mechanical disintegration, are produced in many different ranges of width (in nanoscale) and length for more than three decades 22. Even though mechanical disintegration with homogenization can be easily upscaled and operated continuously, this method requires high energy consumption, providing an expensive product 23.
As an emerging method, enzymatic hydrolysis may be an interesting pathway to nanocellulose fibrils production. In contrast, with both methods previously cited, enzyme hydrolysis does not generate toxic residues as mineral acid hydrolysis does. Moreover, enzymatic hydrolysis conditions are normally held in mild thermal and pressure conditions, resulting in a lower energy‐intensive process. Also, nanocellulose produced by enzymes normally has more high‐valued applications, because of its morphologies 24.
2.2. Commercialization of nanocellulose
The market trend points to an increase in mass production of nanocellulose; there is a great expectation of worldwide trade. The consulting company, Future Markets Inc, estimated the data presented in Figure 3 that better analyze this tendency. According to this source, it is believed that there will be a production of up to more than 9000 tons of nanocellulose per year.
Figure 3.
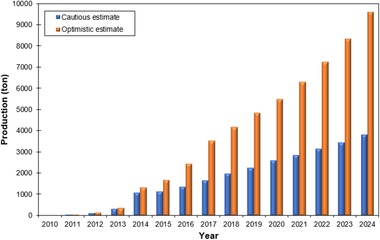
Estimates of nanocellulose production (adapted from Future Markets Inc, The Global Market for Nanocellulose to 2024) http://www.vtt.fi/inf/pdf/technology/2014/t199.pdf
The bacterial nanocellulose production is very limited compared to the large‐scale production of CNCs and cellulose nanofibrils because of the high cost to support bacterial growth and low yield 14. Tables 1 and 2 present some companies that elaborate and have been developing technology to produce nanocellulose (nanocrystals or nanofibrils) in the large scale.
Table 1.
International Company involved with production of cellulose nanocrystals (Adapted from Tappinano, “Summary of International Activities on Cellulosic Nanomaterials“) http://www.tappinano.org/media/1096/tc6-world-cnm-activities-summary-july-29-2015.pdf)
| Cellulose nanocrystals (CNCs) | |||||
|---|---|---|---|---|---|
| Country | Company | Product/trade name | Production capacity | Source | Method |
| USA | American Process Inc. | BioPlus; CNCs | 0.5 ton/day (estimated) | Wood chips; Agricultural residues (bamboo, grasses) | Sulfur dioxide and ethanol pretreatment |
| USDA Forest Service Forest Products Laboratory (FPL) | CNCs | 50 kg/week | Wood pulp | Sulfuric acid hydrolysis | |
| Sweden | MoRe Research | Nanocrystalline cellulose | 0.1 ton/day (pilot plant in place during first half of 2016) | Paper industry sludge | Controlled sulfuric acid hydrolysis, washing, and sonication |
| China | Tianjin Haojia Cellulose Co., Ltd. | NCC | – | Dissolving pulp; cotton; bleached kraft pulp | Mechanical shearing combined with enzymatic and acid hydrolysis |
| India | Indian Council of Agricultural Research–Central Institute for Research on Cotton Technology (ICAR‐CIRCOT) | Nanowhiskers | 10 kg/8 h (pilot plant) | Cotton and cotton linters | Novel microbial, enzymatic and chemomechanical processes |
Table 2.
International Company involved with production of cellulose nanofibrils (adapted from Tappinano, “Summary of International Activities on Cellulosic Nanomaterials“) http://www.tappinano.org/media/1096/tc6-world-cnm-activities-summary-july-29-2015.pdf)
| Cellulose nanofibrils (CNFs) | |||||
|---|---|---|---|---|---|
| Country | Company | Product/trade name | Production capacity | Source | Method |
| Canada | Kruger Bioproducts Inc. | FILOCELL; Cellulose filaments | 5 tons/day | Wood pulp fiber | Mechanical treatment |
| USA | American Process Inc. (AVAPCO) | BioPlus CNFs | 0.5 ton/day (estimated) | low cost woody and non‐woody biomass (forest and agricultural residues, bamboo, and grasses) | SO2 and ethanol to fractione biomass; Mechanical treatment |
| USDA Forest Service Forest Products Laboratory (FPL) | CNFs | 5 kg/week (pilot plant in place during 2012) | Wood pulp | TEMPO oxidation and mechanical treatment | |
| Sweden | Innventia AB | CMFs | 100 kg/day (pilot plant in place during first half of 2014) | Wood fibers | Chemical and/or enzyme pretreatment, Mechanical treatment (homogenization) |
| Finland | UPM‐Kymmene Ltd. | Biofibrils | – | Wood fibers | Mechanical treatment |
| UK | Zelfo Technology GmbH | MFC | – | Cellulose fibres, fibre‐based waste (recycled) | Cellulose Optimization Resource Efficient (CORE). “Technology that allows a modification of cellulose fibres using minimum energy and water” |
| Imerys | FibreLean; MFC | 1000 to > 10,000 tons/year (pilot plant) | Range of (wood) pulp species | No fiber pretreatment; co‐grinding mineral with fiber | |
| China | Tianjin Haojia Cellulose Co., Ltd. | NFC | – | Dissolving cotton pulp; Bleached sulfate pulp (soft‐ and hardwood) | TEMPO oxidation and mechanical treatment |
| Japan | Daicel | Microfibrillated Cellulose; Celish™ | – | Purified pulp | Mechanical treatment |
| Nippon Paper Industries | CNFs; Cellenpia | 30 ton/year | Wood pulp | TEMPO oxidation; carboxymethylation; mechanical treatment | |
3. NANOCELLULOSE CHARACTERIZATION METHODS
There are several available techniques used for nanocellulose particle characterization. They provide highly relevant information about particle morphology and their behavior in other matrices such as suspensions and polymer composites. There are many different types of analysis that could provide valuable information from the experiments. Some of them are briefly described next.
3.1. Atomic force microscopy (AFM)
This technique is largely used to study a 3D surface morphology. In terms of nanocellulose, it can be used to measure the particle dimensions 25 and also patterns of aggregation or dispersion on the surface 26.
3.2. Transmission electron microscopy and scanning electron microscopy (SEM)
Both techniques provide high‐resolution images in nanometric scales. It gives a clear view of nanocellulose, size, shape, and uniformity 27. When used to compare pretreated particles with the nanocellulose obtained, it can give valuable information about changes occurred during the treatment 28.
3.3. X‐ray diffraction (XRD)
The ratio between the crystalline and amorphous regions determines the crystallinity index (CrI) of the cellulose, which together with the orientation of the crystalline and amorphous domains in the fibers affect the mechanical properties of the cellulose fibers. The standard technique used to measure the CrI is XRD 29, which consists of the incidence of an electron beam that, when being diffracted, contains information about the structure of the sample. The decrease in the degree of crystallinity is evidenced by XRD by the decrease of the intensity and increase of the width at half‐height of the diffraction peaks, the same can be observed for crystallites less than 1 μm 30. It also can help to the evaluate efficiency of the enzymatic reaction by monitoring the increase of crystallinity between feedstock and product; the reduction of the amorphous region by enzymatic action leads to an increase of crystallinity.
3.4. Zeta potential (ZP)
This technique is a useful indicator of the effective electrical charge on its surface in the particles, it correlates with the attractiveness or repulsion electrostatic intensity of particles in colloidal suspensions. ZP can be measured by an instrument in which a small sample of the suspension is inserted, providing readability in mV, which can be a positive or negative number. The higher the ZP, the more likely the suspension to be stable as the charged particles repel each other and this force overcomes the natural tendency to aggregate. It can provide valuable information about behavior of particles in emulsions, foams, and also an aggregation of nanocellulose, which should be low for good results as a reinforcement agent 31.
3.5. Thermal analysis
The thermal analysis of nanomaterials is becoming more common as the manipulation of its surface expands and new ideas for its use are diffused. The main techniques used widely to provide rich information on thermal decomposition and impurities of nanocellulose are the thermogravimetric analysis, differential thermogravimetry, and differential scanning calorimetry 11. It is known that thermogravimetric analysis thermograms show that the degradation temperatures for all nanocrystals, which are constituted essentially of three events. The first major decomposition event occurs at temperatures below 100°C due to the evaporation of adsorbed and bound water and/or low molecular weight compounds adsorbed on the surface of the nanocrystals and represents on average of approximately10% of the total weight loss of the samples. For all CNCs, the initial weight loss is followed by a plateau that extends to the beginning of the second major decomposition event. The second prevailing decomposition event corresponds to the degradation of cellulose, which consists of several competing processes, such as depolymerization, dehydration, and decomposition of glycosidic units. The third major decomposition event is attributed to the oxidation and decomposition of carbonized residues to form gaseous products of low molecular weight. The thermal stability of nanocrystalline cellulose is mainly due to the sulphate groups on the surface of them. If these are modified or sodium is used as counter ion, the thermal stability is increased 58, 59.
3.6. Dynamic light scattering
This is a light scattering technique used to determine the hydrodynamic diameter of nanoparticles in suspension. The diffusion coefficient of the particles is measured by the light scattering characteristics applied by the device. The coefficient obtained is used to calculate the hydrodynamic diameter (Hd) of particles using the Stokes‐Einstein equation. This technique presents a simple and fast operation; however, it has limitations in solutions that have polydispersed samples, due to the fact that clustered or very large particles can overlap and trap in the reading of smaller particles 32, 33, 34.
Table 3 shows examples of nanocellulose characterization that were obtained by enzymatic techniques mentioned.
Table 3.
Examples of nanocellulose characterization found in the literature
| Method | Source | Techniques of analysis | Responses | References |
|---|---|---|---|---|
| Enzymatic | Bleached Kraft eucalyptus pulp | SEMTEM | Cellulose nanofibril image | 27 |
| Recycled pulp | SEMTEMDLSZP | Cellulose nanofibril imageHd: 100 to 3.5 mm−31.37 mV | 30 | |
| Commercial MFC;Eucalyptus holocellulose;Sugarcane bagasse;Wood Pulp from Pine | AFMSEMXRD | Cellulose nanofibril imageCommercial MFCCrI: 72.76 to 81.02%Eucalyptus holocelluloseCrI: 58.42 to 65.94%Sugarcane bagasseCrI: 59.60 to 63.95%Wood Pulp from PineCrI: 59.33 to 68.48% | 26 | |
| Wood fiber pulp | AFMSEM | Cellulose nanofibril image | 25 | |
| Orange waste residue | SEM | Cellulose nanofibril image | 28 | |
| Sugarcane bagasse | AFMSEMTEMXRD | Cellulose nanocrystal imageCrI: 52 to 83.7% | 31 | |
| Soybean straw | TEMXRDZP | Cellulose nanofibril imageCrI: 45 to 68%−20.8 mV to −24.5 mV | 35 | |
| Bleached Kraft maple pulp | SEMTEM | Cellulose nanofibril image | 6 | |
| Non‐ezymatic | Bleached aspen kraft pulp | AFMXRD | Cellulose nanocrystal imageCrI: 80.42 to 84.86% | 36 |
| Banana rachis;Sisal;Kapok;Pineapple leaf;Coir | AFMSEMTEMXRD | Cellulose nanofibril imageBanana rachisCrI: 80.9%Sisal plantCrI: 91.3%Kapok treeCrI: 86.5%Pineapple leafCrI: 92.3%CoirCrI: 84.5% | 37 | |
| Bleached sulfite chemical;Pulp;Cotton;Microcrystalline cellulose | SEMDLS | Cellulose nanocrystal imageHd: 50 nm to 5μm | 38 | |
| Rice straw | AFMSEMTEMXRD | Cellulose nanocrystal imageCrI: 61.8 to 91.2% | 39 | |
| Oil Palm FibersXianxing | SEMTEMXRD | Cellulose nanofibril imageCrI: 59.35% | 40 | |
| Sugarcane Bagass | AFMSEMTEMXRD | Cellulose nanocrystal imageCrI: 72.5% | 41 |
3.7. Crystallinity index
The cellulosic materials are characterized by having in their molecular structure ordered, considered crystalline, and disordered, considered amorphous, regions 42. In addition, many nanocellulose production researches focus on the analysis of the CrIs of cellulosic materials 43. The measurement of the CrI is important, because it is related to the tenacity of the fibers and the absorption of the enzyme by the cellulose in enzymatic hydrolysis processes 30, 44.
The techniques generally used to measure the CrI are: XRD, nuclear magnetic resonance, Fourier transform Infrared and Raman spectroscopy 45. However, most research use XRD in the analyzes, through the semiempirical method of the Segal et al., due to its simpler method of analysis 46. Equation (1) and Figure 4 illustrate and explain the method of measuring the CrI.
| (1) |
where:
Figure 4.
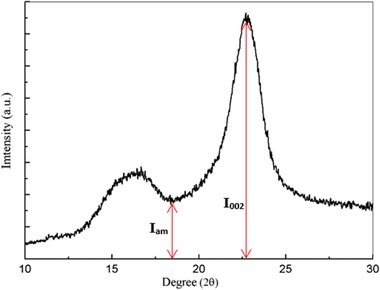
X‐ray diffractogram of cellulosic material
CrI (%) is the CrI;
I 002 is the maximum diffraction intensity (the 002 plane), located around 2θ = 22.5° and corresponds to the crystalline material;
I am is the minimum diffraction intensity located around 2θ = 18° and corresponds to the amorphous material.
4. ENZYMATIC PRODUCTION OF NANOCELLULOSE
The transformation of cellulose into monosaccharides can be achieved in a chemical process carried out by a special group of enzymes known as cellulases. Cellulases are a group of enzymes that catalyze the breakdown of cellulose polymer into smaller polymer branches or even to cellobiose and glucose. Traditionally, these enzymes are divided into three groups: endoglucanases, exoglucanases, and cellobiohydrolases (CBH) 47, 48, 49.
It is widely accepted by the scientific literature that the complete hydrolysis of cellulose into glucose requires the synergistic action of at least two of the three enzymes: First, the amorphous regions of the cellulose chain are more accessible by endoglucanases (EG, endo‐1,4‐β‐d‐glucanase, EC 3.2.1.4), and randomly separates the internal bonds of the glycan chains, thus resulting, nonreducing ends of cello‐oligosaccharides, for subsequent action of CBH or exoglucanase (1,4‐β‐d‐glucan‐cellobiohydrolase, EC 3.2.1.91). The CBH then hydrolyzes these ends of the chain progressively, producing cellobiose as the main product. Finally, ß‐glucosidase (BG, cellobiase, ß‐d‐glucoside glucanohydrolase, EC 3.2.1.21) hydrolyses cellobiose to glucose and also releases glucose from the nonreducing ends of the soluble cellooligosaccharides. There is a high degree of synergy between CBH (exoglucanases) and endoglucanases, which is necessary for better hydrolysis of cellulose. Although this division is clear and well described, the role of proteins involved in the process is still poorly characterized 50, 51, 52.
4.1. Catalytic mechanism of endoglucanase
In terms of enzymatic production of nanocellulose, endoglucanases are the ones with the highest interest, because their action is focused in the amorphous region of the cellulose, breaking the polymer into smaller length polymers 48.
Current studies on enzymatic hydrolysis of cellulose focus their attention to the synergetic effect of the different enzymes, allied with mechanical treatments or acid reactants. A broader comprehension of the synergetic effects of those mechanisms can lead to higher product properties predictability, which is essential to the development of reliable industrial products ready for commercialization. Also, the effect of enzyme hydrolysis can help to reduce energy costs during the mechanical treatment (the next step) 53.
Once cellulose crystals are produced from the crystalline region, the selectivity of endoglucanase enzyme is used to explore the hydrolysis of the amorphous regions, which yield CNCs, while the crystalline region is fragmented into cellulose nanofibrils by mechanical treatments such as ultrasonication.
Orłowski et al. used atomistic molecular dynamics simulations to study one of the endoglucanases of the T‐fungus reesei, known as endoglucanase II (EG II or Cel5A), which is one of the proteins of this group. Based on the results of the atomistic simulation, the authors discuss how the enzyme Cel5A interacts with cellulose fibrils composed of crystalline and amorphous regions. Cel5A and many related proteins contain a carbohydrate binding modulus, a catalytic domain (CD), and a linker that unifies them. Cel5A is one of the most abundant endoglucanases with its highest activity at pH 4.6–4.8. In essence, this work shows that the carbohydrate binding domain of the enzyme prefers to interact with crystalline regions of cellulose, while the CD has a high affinity for the amorphous regions of the fibrils. Particularly, by electrostatic interactions, the CD attracts loose glucose chains to its catalytic slit 54.
5. PRODUCTION TECHNOLOGY
Cellulose fibers exposure to a hydrolyzing agent is determinant to the nanocellulose crystals production, with a reasonable yield. Thus, lignin and hemicellulose, which induce a shield effect to the cellulose, must be removed from the lignocellulosic biomass. There are several methods of lignin and hemicellulose removal, by chemical pretreatments such as sodium carbonate treatment, periodate‐chlorite oxidation, sulfonation, and many others 8. Each lignocellulosic matrix composition would require different pretreatments steps 19. Table 4 presents some relevant works of enzymatic production of nanocellulose.
Table 4.
Summary of some reports of enzymatic production of nanocellulose
| Conditions of hydrolysis | Dimensions | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Material | Description of the treatment | Enzymes | pH | Concentration of biomass | Enzyme concentration | Temperature —Time | Diameter | Length | References |
| Bleached Kraft eucalyptus pulp | Enzymatic hydrolysis followed by microfluidization | EG and CBH | 4.8 | 10% pulp | 15 FPU/gram of substrate | 50°—48 h | 20 nm | Above 500 nm | 27 |
| Recycled pulp | Enzymatic hydrolysis with microwave and conventional heating | EG | 6.8 | 0.8% (0.2 g/ 25 ml) | 420 EGU/gram of substrate | 50°C—1 h | 30–80 nm | 100 nm–1.8 μm | 30 |
| Commercial MFC; eucalyptus holocellulose; sugarcane bagasse; Wood Pulp from Pine | Wet disk milling and Enzymatic hydrolysis | EG and BG | 5 | 1% biomass | 345 EGU/g of substrate | 45°C—72 h | 15‐30 nm | Several micrometers | 26 |
| Wood fiber pulp | Enzymatic hydrolysis followed by mechanical shearing | EG | 7 | 3% pulp | 0.02%–3% enzyme concentration | 50°C—2 h | 5–30 nm | Several micrometers | 25 |
| Orange waste residue | Enzymatic hydrolysis followed by bleaching | BG and Comercial Enzymes | 4 | 17% orange waste residue | 3.4 mg of pulpzyme HA, 3.4 mg of celluclast, and 0.6 mg of β‐galactosidase per gram of substrate | 50°C—48 h | 180 nm | 1.3 mm | 28 |
| Sugarcane bagasse | Pretreatment with liquid hot water (90°C) and ten minutes follows by enzymatic hydrolysis | EG and BG | 5 | 10% bagasse | 7, 12, and 22 mg of protein per gram of cellulose | 50°C—24 h | 14–18 nm | 195–250 nm | 31 |
| Soybean straw | Enzymatic hydrolysis followed by pretreatment with NaOH and H2O2 | BG and CBH | 4 | 0.02% (3 g/150 mL) | 93 μL of Optimash VR per gram of substrate | 50°C—42 h | 15 nm | ∼300 nm | 35 |
| Bleached Kraft maple pulp | Enzymatic hydrolysis followed by ultrasound | Cellic CTec 2 and Cellic HTec 2 (comercial enzymes) | 4.8 | 5% (5 g/100 mL) | 0.04 mL CTec 2 + 0.02 mL HTec2 per gram of substrate | 50°C—72 h | 5–10 nm | 1 μm | 6 |
Combined treatment with endoglucanases and mechanical shearing of wood fiber pulps provided nanofibers with higher aspect ratio and molecular mass than nanofibers resulting from acidic treatment 25. Besides producing microfibrils with interesting properties for reinforcement polymers, this method gives an environmentally friendly method, free of toxic and corrosive chemicals that are commonly used in acidic treatments.
The intensification of enzymatic hydrolysis time of bleached Kraft eucalyptus pulp has shown to significantly reduce solid cellulose degree of polymerization. Such depolymerization helped the formation of cellulose nanofibers by mechanical treatments 27.
It was shown that nanocellulose is a potential high‐valued coproduct of 2G ethanol production chain. The fibers could be obtained by enzyme hydrolysis of sugarcane bagasse pretreated with liquid hot water. For this source of biomass, more efficient disruption of the lignocellulosic materials can lead to better results in overcoming cellulose recalcitrance 31.
The combination of wet disk milling with enzymatic hydrolysis has been shown as an alternative to coproduce CNCs during the saccharification process in biorefineries. This method has been proved efficient not only for sugarcane bagasse, but also for other lignocellulosic biomasses 26. Soybean straws pretreated with NaOH or H2O2 have been reported also as a good source of cellulose nanofibers by enzymatic treatment 35. Depending on the pretreatment employed, different properties may be yield, leading to different possibilities of applications. The use of this kind of processes also produces sugars, which can be used for fermentation processes such as ethanol production.
Another interesting case report was the production of nanocellulose from orange waste residue. The use of this residue to produce nanocellulose could be economically feasible by integration with the orange juice industry, d‐limonene, and ethanol, once throughout the process, all these components can be extracted from the orange fruits. These integrations would not only help the economic balance of the process, but also would help to give an end to solid residues that could generate a negative environmental impact 28.
Microwave heating has been shown to be an interesting alternative to conventional heating to provide higher yields of nanocellulose crystals using endoglucanase as the hydrolysis agent. Besides, high‐negative ZPs were also obtained, suggesting the suitability for those particles as nanofillers in reinforced polymer composites 30.
A high aspect ratio is highly desirable when nanocellulose production is directed toward the development of reinforced, because it enables critical length for stress transfer to the reinforcing phase 55.
6. PRODUCTION VIA ENZYMATIC HYDROLYSIS
6.1. Characteristic of production via enzymatic hydrolysis
The method of production of nanocellulose through enzymatic hydrolysis is less explored in the literature than nonenzymatic, which is often alkaline or acidic processes 12. This methodology presents some peculiarities and characteristics that may be advantageous than the other. Because of this, there is a necessity to develop and invest production through enzymatic methods.
The preparation applied via enzymatic hydrolysis in their procedures, usually, a temperature close to 50°C and pH 5, different from the nonenzymatic procedures that present less harmful conditions due to the fact of applying temperatures commonly around 100ºC with very high or very low pH. These differences make the first procedure produces less toxic waste and less corrosive. This feature facilitates the application of nanocellulose via enzymatic hydrolysis in possible cosmetic and pharmaceutical products 40, 56.
However, the enzymatic process requires more time to prepare the particles than the nonenzymatic. The first preparation usually takes on the scale of days, while the other takes on the scale of hours 4, 40.
Generally, the processes of nanocellulose production through enzymatic hydrolysis generate cellulose nanofibrils while nonenzymatic processes generate CNCs 12. One of the characteristics of CNCs is that it generally has a higher CrI than the other, due to the fact that it has more hydrolyzed amorphous regions, and has a shorter length. While the cellulose nanofibrils present greater flexibility due to the presence of a longer structure with some amorphous regions, this characteristic allows this type of nanocellulose to have a greater mechanical resistance 9, 12.
6.2. Nanocellulose production route via enzymatic hydrolysis
The disadvantages of the enzymatic hydrolysis technology are still the high costs of the enzymes and the process is slower. However, the reduction of these costs has attracted the interest of research centers and companies in recent years, due to the high potential of high saccharification efficiency and high penetration, as well as the low level of pollution. With the use of enzymatic hydrolysis, high yields and almost pure cellulose material are favored, in addition to, reducing environmental impact and obtaining products suitable for biomedical applications [61].
The processes of nanocellulose production via enzymatic hydrolysis have several steps. Figure 5 shows, in a simplified way, the main procedures that allow the production of nanocellulose. This whole route of production was based on the literature and summarizes some production procedures discussed to follow.
Figure 5.
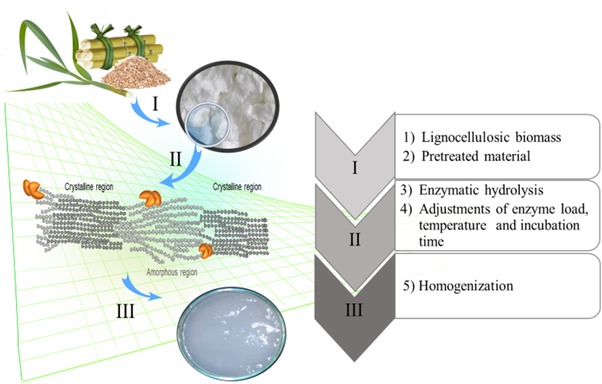
Nanocellulose production diagram via enzymatic hydrolysis
The process between the steps, according to the enumerations: The first stage (I), consists of the pretreatment of lignocellulosic biomass. The objective of this pretreatment applied in lignocellulose is to break the interconnecting structure of its composition. This exposure raises the surface area of the polymers and facilitates the effectiveness of the enzymatic treatment 56. The main techniques applied as pretreatment are the mechanical processes combined with a washing process 25, 26, 31. The second step (II) consists of the controlled enzymatic hydrolysis of fiber samples and it is the main step for the production of nanocellulose. In this phase, the pretreated material is solubilized in the buffer solution with an enzymatic cocktail. The enzymatic cocktail has the function of breaking the polymer of the cellulose into smaller polymers. The endoglucanases have a relatively moderate activity, which is suitable for the hydrolysis of amorphous cellulose regions without complete hydrolysis for glucose, but preserving the crystalline cellulose domain accordingly 47, 48, 49. The added buffer ensures a more constant enzymatic activity. The pH range that is used varies from 4 to 7, according to the literature 6, 25, 26, 27, 28, 30, 31, 35. The reagents are mixed constantly, usually at a constant temperature range from 45 to 50ºC. The mixing time, can range from 1 to 72 h. This time discrepancy is probably due to the different types and conditions of the lignocellulosic materials, the mixing speed, the distinct enzymatic cocktails and pH, which are presented in the literature. All these variations result in different times allowing the material to be hydrolyzed to obtain nanocellulose 4, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 30, 31, 35; Finally, the third step (III) is the homogenization process of the enzymatically treated material by a simple washing, suspension, or mechanical homogenization. These final procedures allow the production of nanocellulose with homogeneous consistency 6, 25, 26, 27, 28, 30, 35.
7. FINAL REMARKS
7.1. Challenges and future perspectives
One major issue involving the use of nanocellulose in composites is the fact that most polymers used are hydrophobic and nanocellulose is hydrophilic. This can be a problem during the mixture of the components, increasing the tendency of nanocellulose to form aggregates that would not only give nondispersed matrices, but would attenuate the nanoscale properties to a reinforcement agent. The hydrophobicity present in the nanocellulose can be resolved with the application of some organic solvents, for example, N,N‐dimethyl formamide, dimethyl sulfoxide, and N‐methyl pyrrolidone, as reported in the literature 2, 57.
Current methods provide typically a low yield and products with a quite broad range of sizes. In order to turn the product more viable to commercialization or industrial applications, these results need to be improved. The most common pathways to achieve better yields are concentrated on pretreatment improvements. One possible way to financially overcome the lower yield provided by the enzymes is the integration of nanocellulose production with other industries such as biorefineries, food and juice manufacturing processes. The residual biomass of these industries can be used in the production of nanocellulose, as a way to improve profitability. Other pathways to achieve better yields are concentrated on pretreatment improvements.
The growth tendency of studies related to the production of nanocellulose using enzyme treatments highlights the potential of this method. Despite some pointed difficulties that have to be overcome, enzymatic production of nanocellulose shows itself as an environmental friendly pathway to give a product with properties that meet the physical requirements for many valuable applications, specially reinforced polymer composites.
CONFLICT OF INTEREST
The authors have declared no conflict of interest.
ACKNOWLEDGMENT
This study was financed in part by the Coordination for the Improvement of Higher Education Personnel (CAPES)—Brazil—Finance Code 001, National Council for Scientific and Technological Development (CNPq), and Agir‐Innovation Agency‐UFF—Brazil.
Ribeiro RSA, Pohlmann BC, Calado V, Bojorge N, Pereira N Jr.. Production of nanocellulose by enzymatic hydrolysis: Trends and challenges. Eng Life Sci 2019;19:279–291. 10.1002/elsc.201800158
REFERENCES
- 1. Barcelos, C. A. , Maeda, R. N. , Santa Anna, L. M. M. , Pereira, N. , Sweet sorghum as a whole‐crop feedstock for ethanol production. Biomass Bioenergy. 2016, 94, 46–56. [Google Scholar]
- 2. Dufresne, A. , Nanocellulose: a new ageless bionanomaterial. Mater. Today. 2013, 16, 220–227. [Google Scholar]
- 3. Durán, N. , Lemes, A. P. , Durán, M. , Freer, J. , Baeza, J. , A minireview of cellulose nanocrystals and its potential integration as co‐product in bioethanol production. J. Chil. Chem. Soc. 2011, 56, 672–677. [Google Scholar]
- 4. Lee, H. V. , Hamid, S. B. A. , Zain, S. K. , Conversion of lignocellulosic biomass to nanocellulose: structure and chemical process. Sci. World J. 2014, 2014, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Philippidis, G. P. , Smith, T. K. , Wyman, C. E. , Study of the enzymatic hydrolysis of cellulose for production of fuel ethanol by the simultaneous saccharification and fermentation process. Biotechnol. Bioeng. 1993, 41, 846–853. [DOI] [PubMed] [Google Scholar]
- 6. Song, Q. , Winter, W. T. , Bujanovic, B. M. , Amidon, T. E. , Nanofibrillated cellulose (NFC): a high‐value co‐product that improves the economics of cellulosic ethanol production. Energies. 2014, 7, 607–618. [Google Scholar]
- 7. Siqueira, G. , Bras, J. , Dufresne, A. , New process of chemical grafting of cellulose nanoparticles with a long chain isocyanate. Langmuir.2010, 26, 402–411. [DOI] [PubMed] [Google Scholar]
- 8. Nechyporchuk, O. , Belgacem, M. N. , Bras, J. , Production of cellulose nanofibrils: a review of recent advances. Ind. Crops Prod. 2016, 93, 2–25. [Google Scholar]
- 9. Börjesson, M. , Westman, G. , Crystalline nanocellulose—preparation, modification, and properties, in: Poletto M. (Ed.), Cellulose—Fundamental Aspects and Current Trends. UK: InTech Open; 2015. pp. 159–191. [Google Scholar]
- 10. Silva, R. , Haraguchi, S. K. , Muniz, E. C. , Rubira, A. F. , Application of lignocellulosic fiber in polymer chemistry and in composites. Quim. Nova. 2009, 32, 661–671. [Google Scholar]
- 11. Klemm, D. , et al., Nanocelluloses: a new family of nature‐based materials. Angew. Chemie—Int. Ed. 2011, 50, 5438–5466. [DOI] [PubMed] [Google Scholar]
- 12. Brinchi, L. , Cotana, F. , Fortunati, E. , Kenny, J. M. , Production of nanocrystalline cellulose from lignocellulosic biomass: technology and applications. Carbohydr. Polym. 2013, 94, 154–169. [DOI] [PubMed] [Google Scholar]
- 13. Moon, R. , Beck, S. , Rudie, A. , Cellulose nanocrystals—a material with unique properties and many potential applications, in: Postek; M.T., Moon; R.J., Rudie; A.W., Bilodeau M.A. (Eds.), Production and Applications of Cellulose Nanomaterials. Pakistan: Tappi Press; 2013. pp. 9–12. [Google Scholar]
- 14. Lin, N. , Dufresne, A. , Nanocellulose in biomedicine: current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar]
- 15. Roman, M. , Dong, S. , Hirani, A. , Lee, Y. W. , Cellulose nanocrystals for drug delivery. Polysacch. Mater. Perform. by Des. 2009, 1017, 4–81. [Google Scholar]
- 16. Klemm, D. , et al., Nanocellulose materials—different cellulose, different functionality. Macromol. Symp. 2009, 280, 60–71. [Google Scholar]
- 17. Peng, B. L. , Dhar, N. , Liu, H. L. , Tam, K. C. , Chemistry and applications of nanocrystalline cellulose and its derivatives: a nanotechnology perspective. Can. J. Chem. Eng. 2011, 9999, 1191–1206. [Google Scholar]
- 18. Machado, B. A. S. , Nunes, I. L. , Pereira, F. V. , Druzian, J. I. , Development and evaluation of the effectiveness of biodegradable films of cassava starch with nanocelulose as reinforcement and yerba mate extract as an additive antioxidant. Ciência Rural. 2012, 42, 2085–2091. [Google Scholar]
- 19. Azeredo, H. M. C. , Rosa, M. F. , Mattoso, L. H. C. , Nanocellulose in bio‐based food packaging applications. Ind. Crops Prod. 2017, 97, 664–671. [Google Scholar]
- 20. Milanez, D. H. , Amaral, R. M. do , Faria, L. I. L. de , Gregolin, J. A. R. , Assessing nanocellulose developments using science and technology indicators. Mater. Res. 2013, 16, 635–641. [Google Scholar]
- 21. Zain, N. F. M. , Yusop, S. M. , Ahmad, I. , Preparation and characterization of cellulose and nanocellulose from pomelo (citrus grandis). albedo. J. Nutr. Food Sci. 2014, 5, 10–13. [Google Scholar]
- 22. Turbak, A. , Snyder, F. , Sandberg, K. , Microfibrillated cellulose, a new cellulose product: properties, uses, and commercial potential. J. Appl. Polym. Sci., 1983, 37, 815–827. [Google Scholar]
- 23. Spence, K.L. , Venditti, R. A. , Rojas, O. J. , Habibi, Y. , Pawlak, J. J. , A comparative study of energy consumption and physical properties of microfibrillated cellulose produced by different processing methods. Cellulose 2011, 18, 1097–1111. [Google Scholar]
- 24. Fritz, C. , et al., Nanocellulose and proteins: exploiting their interactions for production, immobilization, and synthesis of biocompatible materials. Adv. Polym. Sci. 2015, 271, 207–224. [Google Scholar]
- 25. Henriksson, M. , Henriksson, G. , Berglund, L. A. , Lindström, T. , An environmentally friendly method for enzyme‐assisted preparation of microfibrillated cellulose (MFC) nanofibers. Eur. Polym. J. 2007, 43, 3434–3441. [Google Scholar]
- 26. Teixeira, R. S. S. , et al., Combining biomass wet disk milling and endoglucanase/β ‐glucosidase hydrolysis for the production of cellulose nanocrystals. Carbohydr. Polym. 2015, 128, 75–81. [DOI] [PubMed] [Google Scholar]
- 27. Zhu, J. Y. , Sabo, R. , Luo, X. , Integrated production of nano‐fibrillated cellulose and cellulosic biofuel (ethanol) by enzymatic fractionation of wood fibers. Green Chem.2011, 13, 1339–1344. [Google Scholar]
- 28. Tsukamoto, J. , Durán, N. , Tasic, L. , Nanocellulose and bioethanol production from orange waste using isolated microorganisms. J. Braz. Chem. Soc. 2013, 24, 1537–1543. [Google Scholar]
- 29. Zugenmaier P., (Ed.), Crystalline Cellulose and Cellulose Derivatives: Characterization and Structures. Berlin: Springer; 2008. [Google Scholar]
- 30. Filson, P. B. , Dawson‐Andoh, B. E. , Schwegler‐Berry, D. , Enzymatic‐mediated production of cellulose nanocrystals from recycled pulp. Green Chem., 2009, 11, 1808–1814. [Google Scholar]
- 31. Camargo, L. A. , et al., Feasibility of manufacturing cellulose nanocrystals from the solid residues of second‐generation ethanol production from sugarcane bagasse. Bioenergy Res., 2016, 9, 894–906. [Google Scholar]
- 32. Hoo, C. M. , Starostin, N. , West, P. , Mecartney, M. L. , A comparison of atomic force microscopy (AFM) and dynamic light scattering (DLS) methods to characterize nanoparticle size distributions. J. Nanoparticle Res. 2008, 10, 89–96. [Google Scholar]
- 33. Kaszuba, M. , McKnight, D. , Connah, M. T. , McNeil‐Watson, F. K. , Nobbmann, U. , Measuring sub nanometre sizes using dynamic light scattering. J. Nanoparticle Res., 2008, 10, 823–829. [Google Scholar]
- 34. Fissan, H. , Ristig, S. , Kaminski, H. , Asbach, C. , Epple, M. , Comparison of different characterization methods for nanoparticle dispersions before and after aerosolization. Anal. Methods. 2014, 6, 7324‐7334. [Google Scholar]
- 35. Martelli‐Tosi, M. , Torricillas, M. da S. , Martins, M. A. , Assis, O. B. G. de , Tapia‐Blácido, D. R. , Using commercial enzymes to produce cellulose nanofibers from soybean straw. J. Nanomater. 2016, 2016, 1–10. [Google Scholar]
- 36. Xu, Q. , et al., Nanocrystalline cellulose from aspen kraft pulp and its application in deinked pulp. Int. J. Biol. Macromol. 2013, 60, 241–247. [DOI] [PubMed] [Google Scholar]
- 37. Deepa, B. , et al., Utilization of various lignocellulosic biomass for the production of nanocellulose: a comparative study. Cellulose.2015, 22, 1075–1090. [Google Scholar]
- 38. Hettrich, K. , Pinnow, M. , Volkert, B. , Passauer, L. , Fischer, S. , Novel aspects of nanocellulose. Cellulose. 2014, 21, 2479–2488. [Google Scholar]
- 39. Lu, P. , Hsieh, Y. Lo , 2012. Preparation and characterization of cellulose nanocrystals from rice straw. Carbohydr. Polym. 87, 564–573. [DOI] [PubMed] [Google Scholar]
- 40. Luo, X. , Wang, X. , Preparation and characterization of nanocellulose fibers from naoh/urea pretreatment of oil palm fibers. BioResources 2017, 12, 5826–5837. [Google Scholar]
- 41. Kumar, A. , Negi, Y. S. , Choudhary, V. , Bhardwaj, N. K. , Characterization of cellulose nanocrystals produced by acid‐hydrolysis from sugarcane bagasse as agro‐waste. J. Mater. Phys. Chem. 2014, 2, 1–8. [Google Scholar]
- 42. Dufresne A. (Ed.) Nanocellulose—From Nature to High Performance Tailored Materials. Berlin: De Gruyter; 2012. [Google Scholar]
- 43. Lee K.Y. (Ed.) Nanocellulose and Sustainability Production, Properties, Applications, and Case Studies. FL: CRC Press; 2018. [Google Scholar]
- 44. Tomaz, R. M. A. G. , Bittencourt, E. , Sabino, N. P. , Kondo, J. I , Crystallinity index determination on cellulosic fibers, Bragantia 1994, 53, 121–126. [Google Scholar]
- 45. Lee, C. , et al., Correlations of apparent cellulose crystallinity determined by xrd, nmr, ir, raman, and sfg methods. Adv Polym Sci. 2016, 271, 115–131. [Google Scholar]
- 46. Segal, L. , Creely, J. J. , Martin, A. E. , Conrad, C. M . An empirical method for estimating the degree of crystallinity of native cellulose using the X‐Ray diffractometer. Textile Res. J. 1959, 29(10), 786–794. [Google Scholar]
- 47. Bhat, M. K. , Bhat, S. , Cellulose degrading enzymes and their potential industrial applications. Biotechnol. Adv. 1997, 15, 583–620. [DOI] [PubMed] [Google Scholar]
- 48. Josefsson, P. , Henriksson, G. , Wågberg, L. , The physical action of cellulases revealed by a quartz crystal microbalance study using ultrathin cellulose films and pure cellulases. Biomacromolecules 2008, 9, 249–254. [DOI] [PubMed] [Google Scholar]
- 49. Barcelos, C. A. , Rocha, V. A. , Groposo, C. , Castro, A. M. , Pereira, N. Jr , Enzymes and accessory proteins involved in the hydrolysis of lignocellulosic biomass for bioethanol production. Mycol. Curr. Futur. Dev. 2015, 1, 23–56. [Google Scholar]
- 50. Yeoman, C. J. , Han, Y. , Dodd, D. , Schroeder, C. M. et al., Thermostable enzymes as biocatalysts in the biofuel industry. Adv. Appl. Microbiol. 2010, 70, 1–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kostylev, M. , Wilson, D. , Synergistic interactions in cellulose hydrolysis. Biofuels 2014, 3, 61–70. [Google Scholar]
- 52. Knott, B. C. , et al., The mechanism of cellulose hydrolysis by a two‐step, retaining cellobiohydrolase elucidated by structural and transition path sampling studies. J. Am. Chem. Soc. 2014, 136, 321–329. [DOI] [PubMed] [Google Scholar]
- 53. Bharimalla, A. K. , Deshmukh, S. P. , Patil, P. G. , Vigneshwaran, N. , Energy efficient manufacturing of nanocellulose by chemo‐ and bio‐mechanical processes : a review. Would J. Nano Sci. Eng. 2015, 5, 204–212. [Google Scholar]
- 54. Orłowski, A. , et al., How endoglucanase enzymes act on cellulose nanofibrils: role of amorphous regions revealed by atomistic simulations. Cellulose 2015, 22, 2911–2925. [Google Scholar]
- 55. Eichhorn, S. J. , et al., Review: current international research into cellulose nanofibres and nanocomposites, J Mater Sci. 2010, 45, 1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kumar, P. , Barrett, D. M. , Delwiche, M. J. , Stroeve, P. , Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar]
- 57. Okita, Y. , Fujisawa, S. , Saito, T. , Isogai, A. , TEMPO‐oxidized cellulose nanofibrils dispersed in organic solvents. Biomacromolecules 2011, 12, 518–522. [DOI] [PubMed] [Google Scholar]
- 58. Henrique, M. A , et al., Kinetic study of the thermal decomposition of cellulose nanocrystals with different polymorphs, cellulose I and II, extracted from different sources and using different types of acids. Ind. Crops Prod. 2015, 76, 128–140. [Google Scholar]
- 59. Börjesson, M. , Sahlin, K. , Bernin, D. , Westman, G. , Increased thermal stability of nanocellulose composites by functionalization of the sulfate groups on cellulose nanocrystals with azetidinium ions. J. Appl. Polym. Sci., 2017, 135, 45963. [Google Scholar]
- 60. Dai, J. , Chae, M. , Beyene, D. , Danumah, C. , Tosto, F. , & Bressler, D. C. , Co‐production of cellulose nanocrystals and fermentable sugars assisted by endoglucanase treatment of wood pulp. Materials (Basel, Switzerland: ), 2018, 11, 1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Karim, Z. , Afrin, S. , Husain, Q. , Danish, R. , Necessity of enzymatic hydrolysis for production and functionalization of nanocelluloses. Crit Rev Biotechnol. 2017, 37, 355–370. [DOI] [PubMed] [Google Scholar]


