Abstract
Nanoparticles of BSA and silk fibroin (SF) with entrapped α‐tocopherol were produced via ultrasonic emulsification. Populations with particle size of 200–300 nm and highly negatively charged were obtained for all the tested formulations. Entrapment efficiencies of around 99% revealed the effective encapsulation of α‐tocopherol into the produced nanoformulations. Generally, these nanodevices did not induce significant cytotoxicity to human skin keratinocytes for all the concentrations tested. The developed formulations showed free radical scavenging of ABTS.+ ability resulting from the synergistic effect between the proteins in formulation and the entrapped tocopherol. Overall, the results contribute for the establishment of BSA:VO and BSA:SF:VO as biodegradable and non‐toxic nanoformulations for the functionalization of textile devices and controlled delivery of tocopherol into the skin.
Keywords: α‐Tocopherol, Anti‐oxidant activity, Protein‐based nanoparticles, Skin treatment, Ultrasounds
Abbreviations
- ABTS
2,2'‐Azino‐bis(3‐ethylbenzothiazoline‐6‐sulfonic acid) diammonium salt
- BSA
bovine serum albumin
- DLS
dynamic light scattering
- DMEM
dubelcco's modified eagle's medium
- DMSO
dimethyl sulfoxide
- EtOH
ethanol
- FBS
fetal bovine serum
- MTT
3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide
- PBS
phosphate buffer solution
- PCS
photon correlation spectroscopy
- PDI
polydispersity
- SF
silk fibroin
- STEM
scanning transmission electron microscopy
- TOC
tocopherol
- UV‐Vis
ultraviolet visible
- VO
vegetable oil
1. Introduction
α‐Tocopherol, vitamin E, is the most active, lipid soluble, membrane‐bound antioxidant 1, 2, 3. It plays an important role in the prevention and treatment of some chronic and age‐related diseases, such as cardiovascular diseases, atherosclerosis, cancer, arthritis, Alzheimer's and Parkinson's 4. The supplementation of tocopherol offers also skin protection against harmful‐free radicals at which it is exposed 5. α‐Tocopherol acts as an antioxidant by donating one of its electrons to a free radical, and stabilizing it. The newly formed radical can bind to another radical, resulting in a non‐radical product, or it can reconvert to α‐tocopherol. This radical is quite stable due to the unpaired electron of the atom of oxygen which is delocalized in the aromatic ring 6.
The delivery of tocopherol has been provided in an emulsion or a nanoencapsulation form. Polymeric nanoparticles or liposomes have been achieved by a number of methodologies, including emulsion, microemulsion polymerization, interfacial or precipitation polymerization, emulsion evaporation, solvent displacement, etc. 7, 8. Despite their encapsulation ability, some drawbacks related with surface area, solubility, stability, controlled release, skin irritation, degradation and loading have hampered the efficient application 9. Nanocarriers with increased surface area and lipid carrier ability would present higher stability and higher drug loading capacity.
Herein, we explore the capacity of protein‐based nanocarriers to encapsulate tocopherol. The nanoformulations based on BSA and silk fibroin (SF) produced by ultrasonication would increase tocopherol entrapment. These two proteins were chosen due to their antioxidant ability and known application on the production of drug delivery carriers. The antioxidant properties of both proteins and tocopherol 10, 11 are expected to act in synergistically by increasing the overall scavenging oxidation of ABTS.+
The rationale of the study is to formulate tocopherol into protein‐based nanocarriers to produce non‐toxic and stable preparations which can be applied onto textile surfaces (gauzes, bandages, etc.) for medical and cosmetic purposes.
2. Materials and methods
Unless otherwise stated, all of the solvents and reagents used in this study were commercially supplied by Sigma−Aldrich and used as received. Cocoons were donated by Dr. Silvia Cappellozza from “Sezione Specializzata per la Bachicoltura” (Padova).
The experimental setup used for nanoparticles production was composed of a probe type ultrasound source (20 kHz Sonics & Materials Vibracell CV 33) fitted with a 3 mm diameter titanium micro tip. Power delivery was controlled as percentage amplitude (40%). The reaction vessel was an open glass cell (diameter 19 mm, and height, 75 mm) containing the sample solution (16 mL). The sonochemical reactor temperature was controlled via a thermostatted water bath with a freezer exchanger place within a thermojacket cell, which gave a steady operating temperature (10 ± 1°C).
The nanoparticles were prepared by an adaptation of Suslick's method and considering previous conditions reported by Silva et al. 12. Briefly, the bottom of a high‐intensity ultrasonic horn was positioned at the interface of the protein aqueous solution and vegetable oil/tocopherol at an amplitude of 40% at 10°C (±1) for 3 min. The ratio of aqueous/organic phase (%) used was 95/5 and the concentrations of proteins used were 10 gL−1 BSA solution in PBS pH 7.4 and 1 gL−1 SF solution (previously prepared as described by Rockwood et al. 13). The amount of α‐tocopherol used for encapsulation was 10 and 20% (v/v %), defined as the percentage related with the amount of oil in the final formulation ratio. Their characterization was made based on size distribution and zeta‐potential (ζ‐potential), at pH 7.4 (PBS buffer) and at 25°C, by using dynamic light scattering in a Malvern zetasizer NS, by photon correlation spectroscopy (PCS) and by electrophoretic laser Doppler anemometry, respectively. Each sample was measured in triplicate and results are presented as mean value ± standard deviation. Nanoemulsions, in suspension, were stored at 4°C for a period of 20 weeks. After predetermined storage times, the stability parameters, particles size, polydispersity and zeta‐potential, were determined as described above.
After synthesis, the nanoparticles‐containing phase was collected by centrifugation (1000 × g for 45 min) using centricon tubes (Amicon Ultra‐15, Millipore), a centrifugal filter unit containing a cellulose membrane with a molecular weight cut‐off of 100 kDa. The free proteins, BSA (66 kDa) and SF (˃25 kDa) in the aqueous phase after separation were quantified using the Bradford method 14 and the efficiency of nanoemulsions formation was determined by using the formula: where [C]i and [C]f is the initial and final concentration of the protein in the aqueous solution, respectively. Each sample was assayed in triplicate
The morphology of nanoparticles was evaluated by STEM analysis. The diluted nanoparticles suspensions were dropped on copper grids with a 400 mesh carbon film, 3 mm in diameter. The shape and morphology of the microspheres were observed by using a NOVA Nano SEM 200 FEI instrument.
After synthesis, the nanoparticles‐containing phase was collected by centrifugation (1000 × g for 45 min) using centricon tubes (Amicon Ultra‐15, Millipore), a centrifugal filter unit containing a cellulose membrane with a molecular weight cutoff of 30 kDa. The non‐encapsulated tocopherol was detected by UV‐Vis spectroscopy in the lower part of the centricon tubes. For this, spectral curves of stock solutions and of the solutions resulting from centrifugation were measured by UV‐Vis spectroscopy.
In order to evaluate the nanoparticles cytotoxicity, NCTC2544 cell line (human skin keratinocytes), was cultured in DMEM media, and supplemented with 7% FBS and 1% (v/v) penicillin/streptomycin solution. Cells were maintained in 75‐cm2 tissue culture flasks at 37°C in a humidified atmosphere with 5% CO2. The cell culture medium was renewed twice a week. Subculture was performed when confluence reached values near 80–90%.
Cells were seeded at a density of 7500 cells/well on a 96‐well tissue culture plate, the day before the experiments. NCTC 2544 cells were exposed to four concentrations (50, 100, 200, and 500 μg/mL) of nanoparticles and one concentration of free BSA, SF and TOC. The concentration of free BSA, SF and tocopherol correspond to the amount present in the highest concentration of nanoparticles tested. Cells incubated with DMSO (30% of the total volume) and cells without the addition of the compounds were used as positive and negative controls of cytotoxicity, respectively. Cells were incubated at 37°C in a humidified atmosphere with 5% CO2. At the end of 24 h of contact, cell metabolic activity was assessed by MTT viability assay 15. After incubation, the medium with nanoparticles was removed and 110 μL of medium with MTT (5 mg/mL) was added to each well, and cells were further incubated at 37°C for 2 h. The MTT solution was carefully decanted, and formazan crystals were dissolved in 110 μL of a DMSO/EtOH (1:1(v/v)) mixture. Colour was measured with 96‐well plate reader at 570 nm in a microplate reader SpectraMax Plus (Molecular Devices).
The antioxidant activity of nanoparticles was evaluated by ABTS radical cation (ABTS.+) scavenging activity according to the method described by Re et al. 16 with some modifications. ABTS.+ was produced by reacting 38.41 mg ABTS (at a final concentration 7 mM) and 6.623 mg potassium persulfate (at a final concentration 2.45 mM) in 10 mL demineralized H2O in an Erlenmeyer flask and keeping the mixture in the dark at 26 ± 3°C for 12–16 h with magnetic stirring. An aliquot of blue–green ABTS.+ solution was then diluted with 95% ethanol to give an absorbance of 0.45 ± 0.01 at 734 nm. For this, ABTS.+ adjusted solution (2.5 μL) and ethanol (197.5 μL) were mixed in a semi‐micro cuvette and the absorbance at 734 nm corresponding to the blank (E1) was recorded (Spectronic Genesys 5, Germany). For each sample test, five concentrations (10, 50, 100, 250 and 500 μg/mL) were analyzed. The reaction mixture was allowed to stand for exactly 6 min with the ABTS solution (E2). The ABTS.+ scavenge (%) was calculated as
Trolox (6‐hydroxy‐2,5,7,8‐tetramethychroman‐ 2‐carboxylic acid) was used an antioxidant standard. Trolox was prepared in ethanol for use as a stock standard and mixed with ABTS solution for scavenging determination.
3. Results and discussion
The developed nanoformulations were characterized by DLS and visualized by STEM. From Table 1 and Fig. 1 it is clear that both BSA and SF proteins were successfully converted into spherical and smooth nanoemulsions by using high‐intensity ultrasound (Fig. 1). This morphology is known to offer the highest encapsulation potential as well as high release performance and drug protection, providing the minimum contact with the aqueous environment 12. The mechanism of protein‐based nanoemulsions formation using ultrasounds was already reported by Silva et al. 12. They postulate that in an aqueous phase, proteins form stable 3D structures based on the balance between the outer hydrophilic segments covering the inner hydrophobic segments in a conformation of minimal energy. When high shear forces like ultrasound are applied to the biphasic system of water/oil, the proteins tend to adapt their structure and migrate to the interface. Depending on the protein structure, its adaptation may include a 3D modification of protein 12. The accommodation of SF to the water/oil interface tends to promote self‐assembly and β‐sheet formation 12. In all cases, the protein seems to form a shell with hydrophobic characteristics near the organic solvent and hydrophilic characteristics near water. Our results (Table 1) suggest that proteins are adsorbed at the interface in a multilayer fashion. Nanoemulsions formation is completely independent of the presence of cysteine, and the high shear forces are a driving force to achieve stable particles. The size of produced nanodevices was measured before and after filtration with 0.2 μm filters. All the formulations present narrow size distribution between 200–300 nm with low polydispersity. It can be depicted from Table 1 that depending on their composition the nanoparticle formulations present different particle size. Formulations containing only BSA in vegetable oil present lower particle size when compared with the formulations‐containing SF in their composition. SF behavior during particle formation is responsible for this difference. All the formulations produced are homogeneous with low values of PDI (from 0.1–0.2) which is a good indicator regarding future applications. The zeta potential is an important parameter in nanoparticles characterization which allows us to predict their stability over time. Long term stability of produced nanoparticles has been verified which is in accordance with the zeta potential data obtained. The nanoparticles are high negatively charged indicating high repulsive forces and low probability of agglomeration. All the developed formulations present similar zeta potential values (≈30 mV) even for the formulations with high amount of tocopherol encapsulated.
Table 1.
Size, PDI, zeta‐potential and storage stability of optimized nanoformulations as function of α‐tocopherol loading
| Formulation | Size (d.nm) | Pdi | Size (d.nm) | PDI | Zeta‐potential (mV) | Stability (days) | Nanoparticles formation | Encapsulated α‐tocopherol |
|---|---|---|---|---|---|---|---|---|
| Before filtration | Before filtration | After filtrationa | After filtrationa | |||||
| BSA:VO (0% TOC) | 242.1 | 0.049 | 179 | 0.138 | −36 | |||
| BSA:VO (10% TOC) | 279.9 | 0.092 | 173.6 | 0.135 | −36.1 | ≈99% | ≈99% | |
| BSA:VO (20% TOC) | 296.8 | 0.138 | 186.1 | 0.231 | −29.5 | 123 | ||
| BSA:SF:VO (0%TOC) | 319.4 | 0.065 | 165.4 | 0.113 | −36.8 | |||
| BSA:SF:VO (10% TOC) | 306.1 | 0.122 | 159.1 | 0.146 | −33.6 | |||
| BSA:SF:VO (20% TOC) | 302.2 | 0.095 | 159.2 | 0.208 | −40.4 |
Filtration means that nanoparticles were filtered with a 0.20 μm filter from Whatman for samples homogeneity
Figure 1.
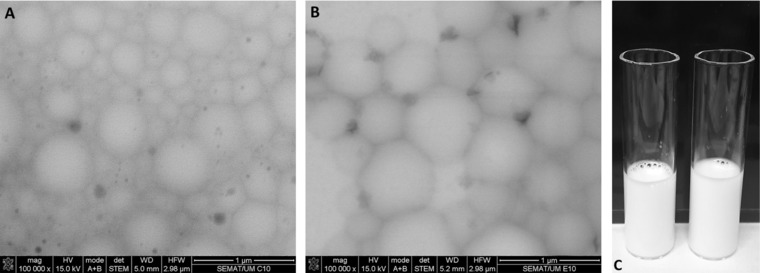
STEM microphotographs (100 000× magnification) of proteinaceous nanoparticles: (A) BSA and (B) BSA:SF obtained with a concentration of 10 g/L and 1 g/L of BSA and SF, respectively; (C) visual aspect of both formulations, left: BSA:VO (20%TOC), right: BSA:SF:VO (20%TOC).
As reported previously by Silva et al. 12, nanoemulsions produced with BSA and SF under ultrasonic field can reach formation yields very close to 100%. In this study, we verify that after centrifugation of the produced nanoemulsions with centricon tubes, the amount of material separated from nanoparticles was negligible. Thus all of the initial materials introduced in the formulation formed nanoemulsions. The entrapment efficiency indicates the amount of compound entrapped into the polymeric matrix. Herein, the theoretical entrapment efficiency is almost maximum. For both loading percentages of tocopherol, the entrapment efficiency was around 99% (data not shown). At the moment of nanoparticles formation, all the tocopherol is entrapped into the oil phase due to its lipophilic nature. These results are in accordance with the data reported in literature about encapsulation of hydrophobic compounds presenting entrapment efficiencies greater than 90% 7.
Considering future applications in skin, it was imperative to evaluate the potential cytotoxicity of the nanoformulations. To assess the potential cytotoxic effect of the nanoparticles, human skin keratinocytes (NCTC 2544) cells were used as model of general cytotoxicity. Analyzing Fig. 2, the tested nanoparticles did not show any significant toxic effect for any of the tested concentrations. The BSA:VO nanoparticles presented cell viability higher than 88.5 ± 11.5 % and the BSA:SF:VO nanoparticles presented cell viability higher than 90.6 ± 8.2 %.
Figure 2.
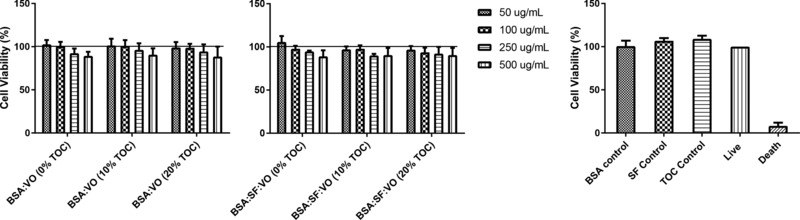
Relative viability of NCTC 2544 (human skin keratinocytes) evaluated with the MTT assay, after 24 h incubation in medium‐containing BSA:VO (0, 10 and 20% TOC), BSA:SF:VO (0, 10 and 20% TOC) and one concentration of free BSA, SF and TOC. The concentration of free BSA, SF and TOC correspond to the amount present in the highest concentration of nanoparticles tested. Cells incubated with culture medium were used as negative control and cells incubated with 30% DMSO as positive control of cytotoxicity. Data were determined in relation to the life control. Results are the mean ± SD of triplicate of three independent experiments.
There was an increase on the scavenging activity of trolox and nanoparticles‐containing tocopherol increasing the concentration of each sample. Trolox was used as a control to compare the antioxidant capacity of each formulation and the degree of inhibition was calculated as described in 2.9. The x‐axis represents the concentration of formulations (μM) and the y‐axis represents the scavenging effect (Fig. 3). Trolox and the nanoparticles with tocopherol display different plots, indicating significant differences in the scavenging activity of ABTS• + radical cations. The most similar behavior is observed for the formulation composed of BSA and SF with 20% tocopherol incorporated. The formulation‐containing BSA with 20% tocopherol present also a significant antioxidant activity however in a lower extent. The antioxidant nature of BSA 11, resulting from its ability to bind molecules such as metal ions, fatty acids, drugs and hormones, and of SF 10, 17, 18 acts synergistically enhancing the effect of tocopherol on the ABTS.+ scavenging. The mechanistic insights behind this may be explained by the diffusion of the entrapped tocopherol through the permeable nanoscaled matrix to the medium. The vitamin E which is close or attached to the nanoparticles surface can also be burst and act on the scavenging effect. The proteins present at the interface between medium and entrapped tocopherol contribute to the increased scavenging effect observed. The nanoscaled particles produced also contribute to tocopherol behavior by increase the surface area, solubility and stability of tocopherol. Thus, the concomitant antioxidant effect of BSA, SF and tocopherol in the new formulations is very promising for the development of antioxidant‐based applications for skin treatment.
Figure 3.
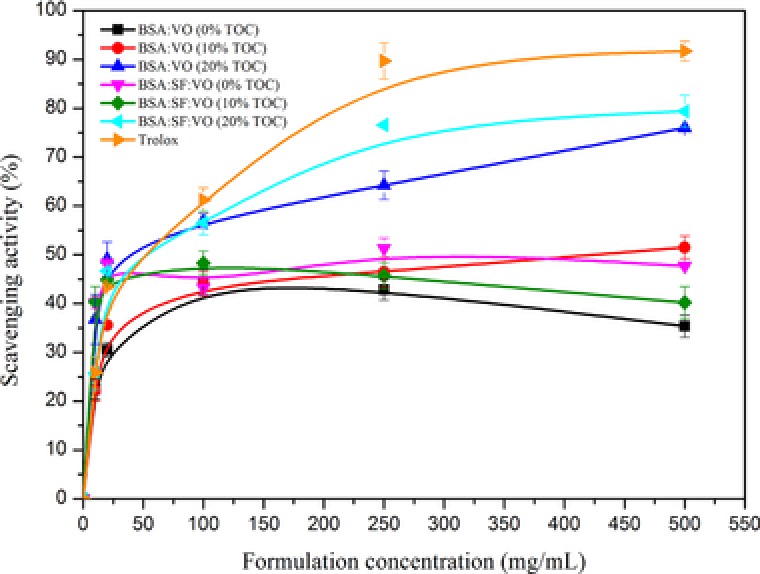
ABTS.+ radical scavenging activity of the various nanoformulations compared with Trolox. The values are the mean ± SD of three parallel measurements.
Practical application
α‐Tocopherol is an anti‐oxidant agent used in several medical and cosmetic applications. Due to its sensitivity it must be protected from light, heat and oxygen. Liposomes, polymeric and protein‐based nanodevices have been applied for its entrapment and delivery. The incorporation into liposomes or nanoparticles core is hindered by its lipophilicity. Nanoparticles of BSA and silk fibroin (SF) are produced by ultrasounds in a biphasic system consisting of an aqueous protein solution and vegetable oil (VO). The developed nanoparticles are promising vehicles to be used in a wide range of applications like textile supports for skin treatment. Cotton fabrics can be functionalized within these nanoparticles to produce textile devices for a continuous skin care. The release of Vitamin E from the textiles into the skin will turn into long life effect of detoxification of free radicals, anti‐inflammatory, repair and regeneration, and regulation of skin's moisture balance.
4. Concluding remarks
The protein‐based nanocarriers developed offer advantages over other conventional passive delivery devices. They have high surface area, high solubility, improved stability, no toxicity and drug loading improvement. These nanoemulsions are easy to produce and low time consuming. Their antioxidant power resulting from tocopherol and proteins action, make them promising devices for cosmetic applications.
Acknowledgments
This study was supported by the Portuguese Foundation for Science and Technology (FCT) under the scope of the strategic funding of UID/BIO/04469/2013 unit and COMPETE 2020 (POCI‐01‐0145‐FEDER‐006684) and BioTecNorte operation (NORTE‐01‐0145‐FEDER‐000004) funded by European Regional Development Fund under the scope of Norte2020 ‐ Programa Operacional Regional do Norte. Artur Ribeiro thanks FCT for the SFRH\BPD\98388\2013 grant. Fatemeh Shahmoradi would like to acknowledge the Iran Ministry of Science, Research and Technology (MSRT) for the monetary support.
5 References
- 1. Abla, M. J. , Banga, A. K. , Formulation of tocopherol nanocarriers and in vitro delivery into human skin. Int. J. Cosmet. Sci. 2014, 36, 239–246. [DOI] [PubMed] [Google Scholar]
- 2. Brigelius‐Flohé, R. , Traber, M. G. , Vitamin E: Function and metabolism. FASEB J. 1999, 13, 1145–1155. [PubMed] [Google Scholar]
- 3. Kamal‐Eldin, A. , Appelqvist, L.‐Å. , The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 1996, 31, 671–701. [DOI] [PubMed] [Google Scholar]
- 4. Usoro, O. B. , Mousa, S. A. , Vitamin E forms in Alzheimer's disease: A review of controversial and clinical experiences. Crit. Rev. Food Sci. Nutr. 2010, 50, 414–419. [DOI] [PubMed] [Google Scholar]
- 5. Oresajo, C. , Stephens, T. , Hino, P. D. , Law, R. M. et al., Protective effects of a topical antioxidant mixture containing vitamin C, ferulic acid, and phloretin against ultraviolet‐induced photodamage in human skin. J. Cosmet. Dermatol. 2008, 7, 290–297. [DOI] [PubMed] [Google Scholar]
- 6. Bramley, P. M. , Elmadfa, I. , Kafatos, A. , Kelly, F. J. et al., Vitamin E. J. Sci. Food Agric. 2000, 80, 913–938. [Google Scholar]
- 7. Imola Gabriela, Z. , Carlos Ernesto, A. , Cristina Mirela, S. , Nanoparticles with entrapped α‐tocopherol: Synthesis, characterization, and controlled release. Nanotechnology 2008, 19, 105606. [DOI] [PubMed] [Google Scholar]
- 8. Hategekimana, J. , Masamba, K. G. , Ma, J. , Zhong, F. , Encapsulation of vitamin E: Effect of physicochemical properties of wall material on retention and stability. Carbohydr. Polym. 2015, 124, 172–179. [DOI] [PubMed] [Google Scholar]
- 9. Gaba, B. , Fazil, M. , Khan, S. , Ali, A. et al., Nanostructured lipid carrier system for topical delivery of terbinafine hydrochloride. Bull. Faculty Pharm., Cairo Univ. 2015, 53, 147–159. [Google Scholar]
- 10. Joseph, B. , Raj, S. J. , Therapeutic applications and properties of silk proteins from Bombyx mori. Front. Life Sci. 2012, 6, 55–60. [Google Scholar]
- 11. Roche, M. , Rondeau, P. , Sinish, N. R. , Tarnus, E. et al., The antioxidant properties of serum albumin. FEBS Lett. 2008, 582, 1783–1787. [DOI] [PubMed] [Google Scholar]
- 12. Silva, R. , Ferreira, H. , Azoia, N. G. , Shimanovich, U. et al., Insights on the mechanism of formation of protein microspheres in a biphasic system. Mol. Pharm. 2012, 9, 3079–3088. [DOI] [PubMed] [Google Scholar]
- 13. Rockwood, D. N. , Preda, R. C. , Yücel, T. , Wang, X. et al., Materials fabrication from Bombyx mori silk fibroin. Nat. Protocols 2011, 6, 1612–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kruger, N. J. , The Bradford method for protein quantitation, in: J. M. Walker, (Ed.), Basic Protein and Peptide Protocols, Humana Press, Totowa, 1994, pp. 9–15. [Google Scholar]
- 15. Ribeiro, A. , Matamá, T. , Cruz, C. F. , Gomes, A. et al., Potential of human γD‐crystallin for hair damage repair: Insights into the mechanical properties and biocompatibility. Int. J. Cosmet. Sci. 2013, 35, 458–466. [DOI] [PubMed] [Google Scholar]
- 16. Re, R. , Pellegrini, N. , Proteggente, A. , Pannala, A. et al., Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [DOI] [PubMed] [Google Scholar]
- 17. Altman, G. H. , Diaz, F. , Jakuba, C. , Calabro, T. et al., Silk‐based biomaterials. Biomaterials 2003, 24, 401–416. [DOI] [PubMed] [Google Scholar]
- 18. Leal‐Egana, A. , Scheibel, T. Silk‐based materials for biomedical applications. Biotechnol. Appl. Biochem. 2010, 55, 155–167. [DOI] [PubMed] [Google Scholar]


