Abstract
Background
Recently, use of nanotechnology in biomedical applications such as drug delivery and diagnostic and therapeutic tools has increased greatly. This study evaluated gold nanoparticle (GNPs)-induced nephrotoxic effects in rats in vivo, and examined protective effects of alpha-lipoic acid (α-Lip) and Vitamin E (Vit E) against nephrotoxicity, lipid peroxidation, and inflammatory kidney damage induced by GNPs.
Materials and Methods
Twenty-four male Wistar-Kyoto rats (220–240 g, 12 weeks old) were dosed with 50 μL of 10 nm GNPs administered intraperitoneally with or without 200 mg/kg/day Vit E or 200 mg/kg/day α-Lip. Serum was prepared for biochemical analyses. Kidney function was evaluated through measurement of creatinine (CR), uric acid (URIC), and blood urea nitrogen (BUN). Oxidative stress and lipid peroxidation were evaluated by measurement of reduced glutathione (GSH) and malondialdehyde (MDA) in kidney tissue homogenates.
Results and Conclusions
The results showed a significant rise in serum kidney function biomarkers including urea, URIC, CR, and BUN in GNP-treated rats compared to normal control rats. Furthermore, GNPs led to decreased GSH and elevated MDA levels. Vit E or α-Lip supplementation showed a beneficial effect against nephrotoxicity, lipid peroxidation, and inflammatory kidney damage induced by GNPs. This study suggests that use of natural antioxidants in combination with GNPs may be a useful tool in preventing GNPs toxicity.
Keywords: gold nanoparticles, Vitamin E, alpha-lipoic acid, nephrotoxicity, lipid peroxidation, natural antioxidants
Introduction
Nanoparticles (NPs) are potentially dangerous chemical substances that adhere to cell membranes and permeate animal organs. Nanoparticle surfaces can be changed and adjusted to the environmental transformation.1
Distribution of NPs in the liver, spleen, cerebrum, heart, and kidney may result in impaired function. A previous study showed that the dimensions and surface area of nanoparticles are important factors in increased reactive oxygen species (ROS) production2. Exposure to GNPs can result in inflammation, death of tubular epithelial cells, increased capillary permeability, blood loss, and inflammatory cell infiltration.3–8
Vit E is a critical fat-soluble antioxidant that supports mitosis, immune function, speeds of blood clotting, and serves as a natural defense against oxidative stress in tissues. Vit E also contributes to cell membrane function, elasticity, and integrity.9,10
Alpha-lipoic acid is a potent antioxidant and is a substrate for the pyruvate dehydrogenase complex found in mitochondria of hepatic, renal, and cardiac tissues. α-Lip can be reduced to dihydrolipoic acid, which has ROS scavenging activity, and maintains GSH and vitamins C and E, which are exogenous, in the reduced state.11 Prevention of toxicity induced by GNPs in vivo has not been reported. Therefore, this study aims to confirm nephrotoxicity, lipid peroxidation, and inflammatory kidney damage caused by GNPs, and to assess the role of Vit E and α-Lip acid in inhibiting or preventing alterations induced by GNPs in the kidney organ.
Materials and Methods
GNPs and Antioxidants
Size and morphology of 10nm GNPs (products MKN-Au-010; M K IPEX Corp; Divn MK Nano, CANADA) have shown spherical shape with mean size 9.45 ± 1.33 nm as calculated from the images taken by transmission electron microscope (TEM). The GNPs have been distributed in homogenous way through the solution. Vit E and α-Lip were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). The Vit E dose was 100mg/Kg BW/day, and the α-Lip acid dose was 200mg/Kg BW/day, according to Al-Rasheed et al., 2014.12
Animals
Twenty-four 12-week-old Wistar-Kyoto male rats (220–240 g) were acquired from the College of Pharmacy, Animal Centre, KSU. The rats were kept in cages under standard conditions (22±5°C temperature, 55±5% humidity, 12 hrs light/dark cycle). Rats were fed a regular rodent diet. The KSU Animal Use Committee approved the animal experiments, and all experiments were conducted in accordance with the committee’s guidelines approved by KSU Local Animal Care.
Experimental Design
Rats were acclimatized for 2 days, fasted for 24 hrs before injection, and randomly divided into four groups (n=6 each). The first group (G1; healthy rats) did not receive GNPs and served as a control. The second group (G2) was treated daily by intraperitoneal injection with 50 µL of 10 nm GNPs for 7 consecutive days. The third group (G3) was treated with 10 nm GNPs (50 µL) plus 100 mg/Kg BW/day of Vit E for 7 consecutive days. The fourth group (G4) was treated with 10 nm GNPs (50 µL) plus 200 mg/Kg BW/day of α-Lip for 7 consecutive days.
Blood Sampling and Tissue Preparation
Twenty-four hours after the final dose, rats were denied access to food for 12–14 hrs, then sacrificed. Blood samples were obtained from each rat and placed into Pasteurized tubes for serum separation. Samples were centrifuged at 3,000 rcf for 10 mins, and serum was stored at −80°C until use. Kidney tissues were collected and harvested by the midline incision technique, washed in ice-cold iso-osmotic saline, homogenized, and frozen at −80°C until use.
Serum Kidney Function Biomarkers
To assay kidney function, blood urea nitrogen (BUN), creatinine (CR), and uric acid (URIC) levels in serum were determined using a biochemical auto-analyser (Type 7170, Hitachi).
Oxidative Stress Biomarkers
Reduced Glutathione (GSH) Level Determination
The level of reduced glutathione (GSH) in the renal tissues was evaluated enzymatically according to Griffith, 1980.13 Tissues were homogenized in 0.2 M ice-cold perchloric acid containing EDTA (0.01%), then centrifuged at 10,000 rcf for 5 mins. One hundred microliters of supernatant was added to a spectrophotometer cuvette containing 100 µL of 6 mM 5,5-dithiobis-2-nitrobenzoic acid, 800 µL of 0.3 mM reduced NADPH, and 10 µL of 50 units/mL glutathione reductase prepared fresh in pH 7.5 phosphate buffer. Absorbance was determined at 412 nm for 120 mins at 30°C. Glutathione was assayed by comparing absorbance of the test solution to a glutathione standard.
Determination of Malondialdehyde (MDA)
Malondialdehyde (MDA) levels were measured as a marker of fatty acid peroxidation index in various organs using the spectrophotometric method described by Hochstein and Utley (1967). Tissues were weighed and homogenized (10% w/v) in 0.15 M frozen potassium chloride (KOH) with a potter homogenizer and motor-driven Teflon pestle. One milliliter of tissue homogenate was incubated at 37ºC for 2 hrs in a shaker. One milliliter of 10% (w/v) trichloroacetic acid added to the homogenate, and the mixture was centrifuged at 3000 rcf for 10 mins. One milliliter of the supernatant was mixed with one mL of 0.67% (w/v) 2-thiobarbituric acid and placed in a hot water bath for 10 mins, then allowed to cool. Following cooling, 1 mL of distilled water was added. The absorbance was determined at 535 nm, and malondialdehyde level was calculated using tetraethoxypropane (TEP) as a standard.
Statistical Analysis
Data obtained were presented as mean ± SE. To test for the difference between the means, one-way analysis of variance (ANOVA) was used, followed by Bonferroni multiple differences post hoc test, with significance determined by p≤0.05 and **p≤0.001.
Results
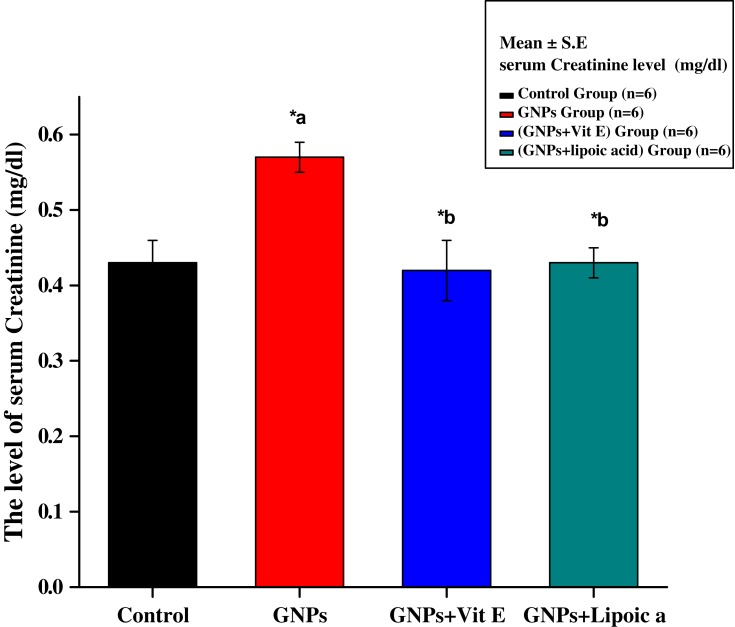
Figure 1 shows a major increase in serum creatinine level in the GNPs group (0.57 ± 0.02 mg/dL) compared to the control group (0.43 ± 0.03 mg/dL). Group 3 (GNPs + Vit E) and group 4 (GNPs + α-Lip) significantly reduced the level of creatinine to 0.42 ± 0.02 mg/dL and 0.43 ± 0.02 mg/dL, respectively. Administration of α-Lip or Vit E with GNPs blocked GNPs-induced increases in creatinine.
Figure 1.
Effect of GNPs, GNPs + Vit E, and GNPs + α-Lip on serum creatinine level in rats. *a represents significance between the control group and the GNPs groups (p<0.05). *b represents significance between the GNPs and the other groups (p<0.05).
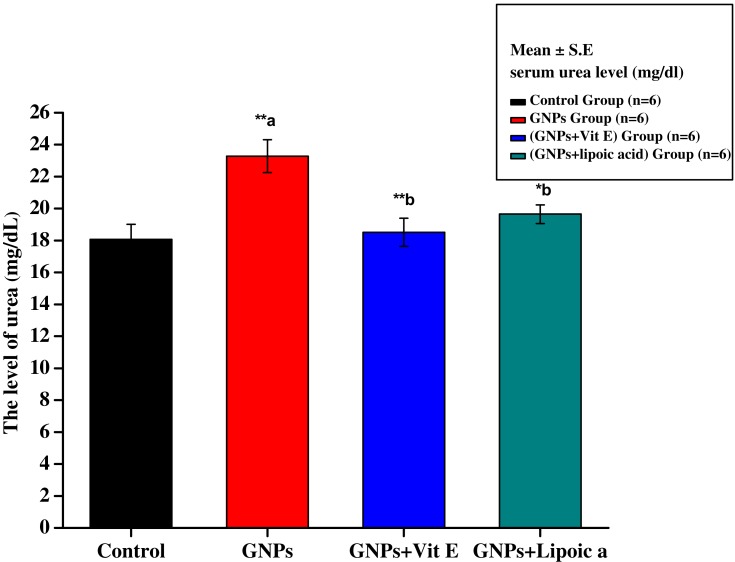
Figure 2 shows serum urea level of control (G1), GNPs (G2), G3, and G4 rats. Serum urea in G1 was 18.08 ± 0.93 mg/dL. After 7 days of intraperitoneal administration of GNPs, the mean level of urea increased significantly to 23.28 ± 1.03 mg/dL (**p<0.01), contributing to renal damage. Administration of Vit E + GNPs (G3) and α-Lip + GNPs (G4) significantly reduced the GNPs-induced elevation of urea to 19.52 ± 0.88 mg/dL, 20.22 ± 0.62 mg/dL, respectively.
Figure 2.
Effect of GNPs, GNPs + Vit E, and GNPs + α-Lip on serum urea level in rats. **a significant value (p˂0.001). *b represents significance between the GNPs and the other groups (p<0.05), **b significant value p˂0.001: the data were highly significant at this value.
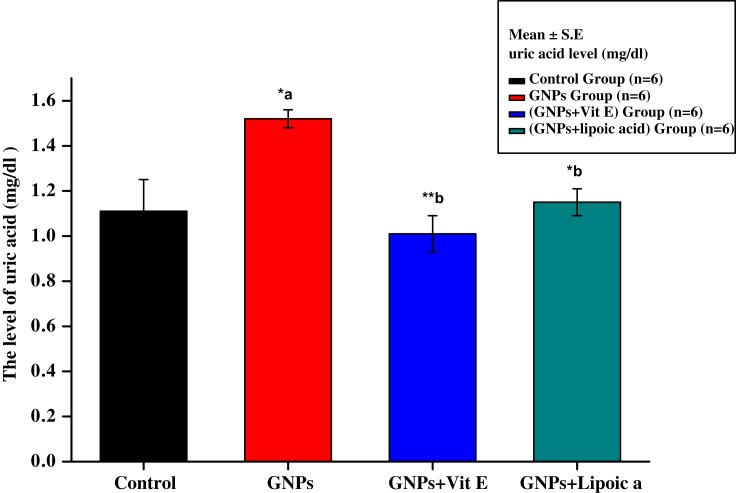
Figure 3 shows increased uric acid levels in the GNPs group (1.55 ± 0.75 mg/dL) compared with the control group (1.10 ± 0.98 mg/dL). Administration of Vit E + GNPs (G3) and α-Lip + GNPs (G4) significantly reduced kidney uric acid levels to 1.05 ± 0.85 mg/dL and 1.13 ± 0.65 mg/dL, respectively, compared to administration of GNPs alone.
Figure 3.
Effect of GNPs, GNPs + Vit E, and GNPs + α-Lip on serum uric acid level in rats. *a represents significance between the control group and the GNPs groups (p<0.05). *b represents significance between the GNPs and the other groups (p<0.05), **b significant value p˂0.001: the data were highly significant at this value.
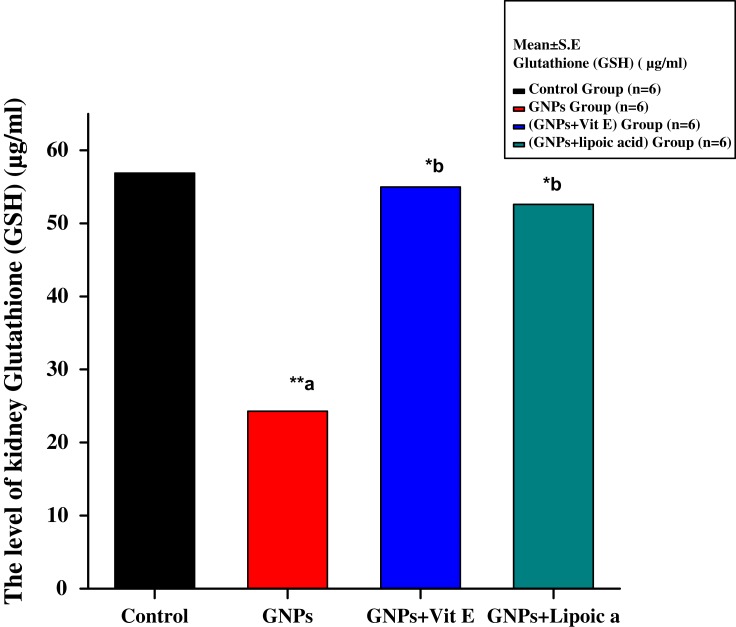
Figure 4 shows a significant decrease in kidney GSH level in the GNPs group to 24.20 ± 0.02 μg/mL compared to 56.85 ± 0.02 μg/mL in the control group. Administration of Vit E + GNPs (G3) and α-Lip + GNPs (G4) significantly elevated GSH levels to 56.12 ± 0.01 μg/mL and 53.15 ± 0.02 μg/mL, respectively, compared to GNPs alone.
Figure 4.
Effect of GNPs, GNPs + Vit E, and GNPs + α-Lip on kidney GSH level in rats.**a significant value (p˂0.001). *b represents significance between the GNPs and the other groups (p<0.05), **b significant value p˂0.001: the data were highly significant at this value.
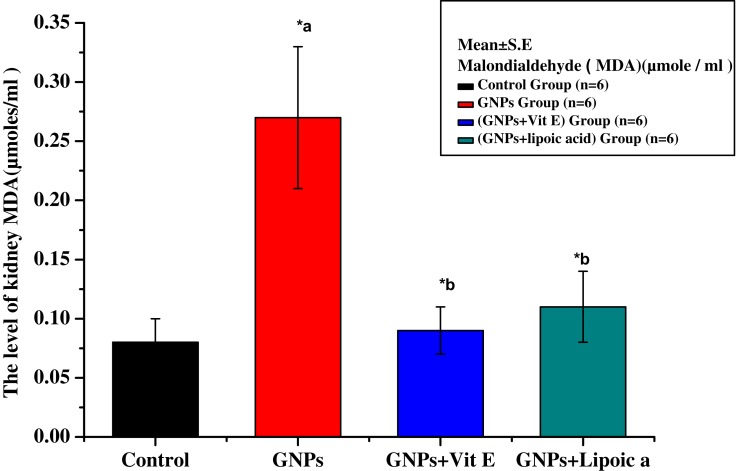
Figure 5 shows a significant increase in the lipid peroxidation product, malondialdehyde (MDA), in rat kidney tissues in response to GNP (0.27 ± 0.03 μmoles/mL) compared to control (0.07 ± 0.01 μmoles/mL). Co-administration of Vit E + GNPs (G3) and α-Lip + GNPs (G4) significantly reduced MDA levels to 0.09 ± 0.03 μmoles/mL and 0.12 ± 0.02 μmoles/mL, respectively.
Figure 5.
Effect of GNPs, GNPs + Vit E, and GNPs + α-Lip on kidney Malondialdehyde level in rats. *a represents significance between the control group and the GNPs groups (p<0.05). *b represents significance between the GNPs and the other groups (p<0.05).
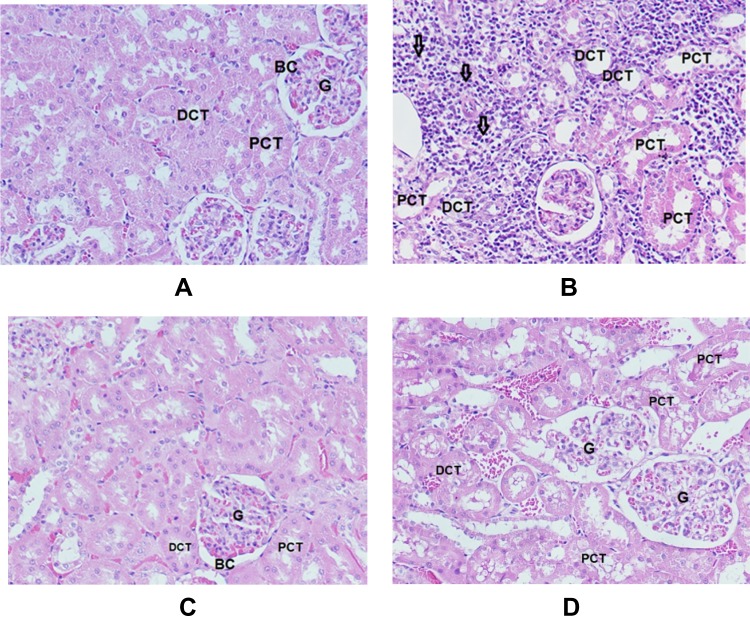
Figure 6 shows histopathological studies that give more evidences of the nephrotoxic effect of GNPs and the protective effect of Vit E and α-Lip.
Figure 6.
Sections in cortex of the kidney (H&E x 200). (A) Normal control rats (G1) show renal corpuscle consists of glomerulus (G) and a two-layered glomerular Bowman’s capsule (BC) that encloses glomerulus; the proximal convoluted tubules (PCT) and distal convoluted tubules (DCT). (B) GNPs-treated rats (G2) show inflammatory cells infiltration, mainly in the interstitial tissue (arrows), vacuolar degeneration surrounding PCT and DCT, tubule necrosis (star), dilation and vacuolar degeneration in PCT and DCT. (C) GNPs + Vitamin E (G3) shows normal renal corpuscle surrounded by normal tubules. (D) GNPs + α-lipoic acid (G4) shows vacuolar degeneration in PCT and DCT.
Discussion
NPs absorbed into the blood and are cleared by the kidney.14 This study confirmed nephrotoxic effects of GNPs. Our research shows that GNPs significantly elevated URIC, BUN, and CR levels compared to the control group. GSH levels in kidney tissues were significantly decreased, while MDA levels were significantly increased.
In serum from rats injected with GNPs, significant increases in CR and BUN levels compared to control confirmed nephrotoxicity (p<0.05). Vit E and α-Lip significantly reduced serum CR, URIC, and BUN levels compared to rats administered GNPs.
Vit E disrupts free radical reactions, resulting in formation of antioxidant radicals that are only moderately reactive. As a result, Vit E scavenges free radicals.15 Vit E quenches fatty acid peroxidation in membranes due to its radical scavenging ability.16 Thus, Vit E serves an essential role in renal tissues against peroxidative injury.17
Vit E supplementation may reduce oxidative stress, and also may be useful in reducing heart disease linked to chronic kidney failure. In vivo and in vitro studies have shown that Vit E provides a defense against fatty acid peroxidation and inhibits metal-mediated damage. α-Lip increases concentrations of hepatic cysteine and glutathione. α-Lip can reduce oxidized vitamin C, helping to maintain antioxidant status. α-Lip can boost production of glutathione, the foremost antioxidant in human cells.12,18,19
Decrease oxidative stress resulting α-Lip or Vit E may be responsible for their anti-inflammatory effects. GSH in kidney tissues decreased significantly after administering of GNPs. In contrast, GSH increased significantly after Vit E or α-Lip administration. GSH functions as a scavenger of poisons and free radical-generated species. GSH can also compensate for Vit E deficiency. Previous studies evaluated GSH levels in animals injected with Vit E or α–Lip after zinc nanoparticles injection.12,13,16–18
Conclusions
This study highlights the nephrotoxic effects induced by intraperitoneal administration of GNPs as confirmed by significant increases in serum kidney function markers such as URIC, CR, and BUN. In addition to a significant increase in the fatty acid peroxidation biomarker MDA, GNPs also resulted in significant reduction in the oxidative stress biomarker GSH. Administration of Vit E or α-Lip significantly reduced inflammatory kidney damage, lipid peroxidation, and oxidative stress, as determined by reduced serum markers, elevated tissue levels of GSH, and reduced tissue MDA. Our findings showed that Vit E and α-Lip are highly potent antioxidants that can protect against the kidney dysfunction, inflammatory damage, lipid peroxidation, and oxidative stress.
Acknowledgments
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding of this research through the research Group Project No. RGP-285
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Salata OV. Application of nanoparticles in biology and medicine. J Nanobiotechnol. 2004;2:3. doi: 10.1186/1477-3155-2-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moller P, Jacobsen NR, Folkmann JK, et al. Role of oxidative damage in toxicity of particulates. Free Radical Res. 2010;44(1):1–46. doi: 10.3109/10715760903300691 [DOI] [PubMed] [Google Scholar]
- 3.Abdelhalim MAK, BM J. Histological alterations in the liver of rats induced by different gold nanoparticles size, dose and exposure duration. J Nanobiotechnology. 2012;10:5. doi: 10.1186/1477-3155-10-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdelhalim MAK, Jarrar BM. Renal tissue alterations were size-dependent with smaller ones induced more effects and related to time exposure of gold nanoparticles. Lipids Health Dis. 2011;10:163. doi: 10.1186/1476-511X-10-163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdelhalim MAK. Gold nanoparticles administration induces disarray of heart muscle, hemorrhagic, chronic inflammatory cells infiltrated by small lymphocytes, cytoplasmic vacuolization and congested and dilated blood vessels. Lipids Health Dis. 2011;10:233. doi: 10.1186/1476-511X-10-233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moron MS, Depierre J, Mannervik B. Levels of glutathione, glutathione reductase and glutathione –S- transferase activities in rat lung and liver. Biochem Biophys Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7 [DOI] [PubMed] [Google Scholar]
- 7.Gupta A, Singh S, Jamal F, Nath MS, Sharma B. Synergistic effects of glutathione and Vit E on ROS mediated ethanol toxicity in isolated rat hepatocytes. Asian J Biol. 2011;6:347–356. [Google Scholar]
- 8.Lomaestro BM, Malone M. Glutathione in health and disease: pharmacotherapeutic issues. Ann Pharmacother. 1995;29:1263–1273. doi: 10.1177/106002809502901213 [DOI] [PubMed] [Google Scholar]
- 9.Skrzydlewska EI, IKI D, Figaszewski Z. Effect of Vit E derivative (U83836E) on membranes of rat liver cells after methanol intoxication. Pol J Environ Stud. 2001;10(2):95–100. [Google Scholar]
- 10.Coulter ID, Hardy ML, Morton SC, et al. Antioxidants vitamin C and Vit E for the prevention and treatment of cancer. J Gen Int Med. 2006;21:735–744. doi: 10.1111/j.1525-1497.2006.00483.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biewenga GP, Haenen GR, Bast A. The pharmacology of the antioxidant lipoic acid. Gen Pharmacol. 1997;29:315–331. doi: 10.1016/S0306-3623(96)00474-0 [DOI] [PubMed] [Google Scholar]
- 12.Al-Rasheed NM, Al-Rasheed NM, Abdel Baky NA, et al. Prophylactic role of α-lipoic acid and vitamin e against zinc oxide nanoparticles induced metabolic and immune disorders in rat’s liver. Eur Rev Med Pharmacol Sci. 2014;18:1813–1828. [PubMed] [Google Scholar]
- 13.Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106(1):207–212. doi: 10.1016/0003-2697(80)90139-6 [DOI] [PubMed] [Google Scholar]
- 14.Schipper ML, Iyer G, Koh AL, et al. Particle size, surface coating, and PEGylation influence the biodistribution of quantum dots in living mice. Small. 2009;5(1):126–134. doi: 10.1002/smll.v5:1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramos LF, Kane J, McMonagle E, et al. Effects of combination tocopherols and alpha lipoic acid therapy on oxidative stress and inflammatory biomarkers in chronic kidney disease. J Ren Nutr. 2011;21(3):211–218. doi: 10.1053/j.jrn.2010.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalender S, Kalender Y, Ogutcu A, Uzunhisarcikli M, Durak D, Açikgoz F. Endosulfan-induced cardiotoxicity and free radical metabolism in rats: the protective effect of Vit E. Toxicology. 2004;202(3):227–235. doi: 10.1016/j.tox.2004.05.010 [DOI] [PubMed] [Google Scholar]
- 17.Al-Attar AM. Antioxidant effect of Vit E treatment on some heavy metals induced renal and testicular injuries in male mice. Saudi J Biol Sci. 2011;18(1):63–72. doi: 10.1016/j.sjbs.2010.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Randle PJ, Kerbey AL, Espinal J. Mechanisms decreasing glucose oxidation in diabetes and starvation: role of lipid fuels and hormones. Diabetes Metab Rev. 1988;4:623–638. doi: 10.1002/dmr.v4:7 [DOI] [PubMed] [Google Scholar]
- 19.Vasdev S, Ford CA, Parai S, Longerrich L, Gadag V. Dietary alpha-lipoic acid supplementation lowers blood pressure in spontaneously hypertensive rats. J Hypertens. 2000;18:567–573. doi: 10.1097/00004872-200018050-00009 [DOI] [PubMed] [Google Scholar]