Abstract
A new isolated Bacillus mojavensis strain I4 was found as producer of biosurfactants by different screening methods, such as parafilm M test, hemolytic activity, oil displacement test, emulsification index, surface tension, and lipase production assay. Enhanced biosurfactants production was obtained using glucose and glutamic acid as carbon and nitrogen sources, respectively. The optimal production of the biosurfactants was obtained by using a C/N ratio of 17, pH of 7.0, and temperature of 37°C. The surface tension was reduced to 29 mN/m and the emulsification index E24 of 62% was achieved after 72 h of culture. The purified biosurfactants showed stability with regard to surface tension reduction and emulsification in a wide range of temperatures (4–120°C), pH (4–10), and salinity (2–12% of NaCl). The thin‐layer chromatography showed that the produced biosurfactants were lipopeptides. The biosurfactants were characterized as a group of anionic lipopeptides with zeta potential measurement. Chromatographic characterization using HPLC revealed that I4 lipopeptides contained numerous isoforms and surfactin was the major component. Moreover, the I4 lipopeptides showed interesting angiotensin‐converting enzyme‐inhibitory activity.
Keywords: ACE‐inhibitory activity, Bacillus mojavensis, Lipopeptides biosufactants
Abbreviation
- ACE
angiotensin‐converting enzyme
1. Introduction
Biosurfactants are amphiphilic compounds with hydrophilic heads and hydrophobic tails normally hydrocarbon chains that reduce the surface tension between liquid phases such as at oil–water and air–water interfaces. Biosurfactants are highly valued for their multipurpose therapeutic properties, including their immunomodulatory, antitumor, antiinflamatory, antioxidant, and antipathogenic activities 1. As compared to their chemical equivalents they harbor high surface activities with low CMC. Biosurfactants are biodegradable and non‐toxic which makes them applicable in detergency, soaping, emulsification, foaming, and resource recovering 2. Furthermore, biosurfactants are classified according to their molecular structure into mainly glycolipids (e.g., rhamnolipids), lipopeptides (e.g., surfactin), polymeric biosurfactants (e.g., emulsan), fatty acids (e.g., alkanoic acids), and phospholipids 3. Lipopeptides are constituted by a fatty acid in combination with a peptide moiety and correspond to an isoform group that differs by the composition of the peptide moiety, the length of the fatty acid chain and the link between the two parts 4. Lipopeptides are mainly synthesized by many bacilli and other species. Otherwise, glycolipids are constituted by a fatty acid in combination with a carbohydrate moiety and correspond to a group of compounds that differs by the nature of the lipid and carbohydrate moiety 4. They are synthesized by Pseudomonas and Candida species 5. Among lipopeptides, surfactin is one of the most powerful biosurfactant that was first reported for Bacillus subtilis 6. Surfactin is considered as an extracellular or cell membrane‐associated secondary metabolite, its structure depends on production medium composition, such as the ratio of carbon and nitrogen sources 7. Indeed, at low concentrations (20 μmol/L), surfactin could decrease the surface tension of water from 72 to 27 mN/m which is considerably lower than the surface tensions of rhamnolipids 8. Moreover, several Bacillus strains, such as Bacillus pumilus, Bacillus mojavensis, Bacillus licheniformis, and Bacillus amyloliquefaciens, also synthesize surfactin 9. Indeed, to improve biosurfactants production yield, several methods are employed, such as medium optimization, agro‐industrial waste fermentation, and strain improvement by mutagenesis or recombination 10. Furthermore, to achieve optimal biosurfactants production, the isolation of appropriate microorganism and the optimization of the culture medium are critical parameters. Moreover, carbon and nitrogen sources and operational conditions significantly affect the nature and the yield of biosurfactants produced by the bacterium 11. Thus, the objective of the present study was to enhance the production of biosurfactants from B. mojavensis I4 strain by the optimization of the production parameters, such as carbon and nitrogen sources, C/N ratio, pH, and temperature. Moreover, the physical proprieties and HPLC analysis of the partially purified biosurfactants were carried. The potential use of I4 biosurfactants as antihypertensive agent was evaluated using angiotensin‐converting enzyme (ACE) inhibitory activity determination.
2. Materials and methods
2.1. Reagents
All chemicals and solvents used in the present study were purchased at the analytical grade or highest level of purity available. For HPLC analysis, acetonitrile, trifluoro acetic acid, and methanol used were of HPLC grade. Surfactin powder (with a purity of 98%) used as a standard for HPLC, ACE from rabbit lung, and the ACE synthetic substrate hippuryl‐l‐histidyl‐l‐leucine were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
2.2. Bacterial strain
The bacterial strain, previously isolated and identified as B. mojavensis I4 12 was maintained on nutrient agar at 4°C and also stored as glycerol stocks at −80°C.
2.3. Screening assays for biosurfacatants production
Biosurfactants activity was detected in the culture broth of B. mojavensis I4 by different methods, such as parafilm M test, oil displacement test, hemolytic activity, lipase production, emulsification index, and surface tension. All the experiments were carried in triplicates.
2.3.1. Parafilm M test
A 25 μL aliquot of culture supernatant was dropped on a strip of parafilm M (4 in × 125 ft, Sigma) as a hydrophobic surface and then the diameter of the droplet was evaluated as previously described 13. Triton X‐100 and sterile medium were used as positive and negative control, respectively.
2.3.2. Oil displacement test
The oil displacement test was used to measure the diameter of the clear zone, which occurs after dropping a surfactant containing solution on an oil–water interface. Forty microliters of distilled water was dropped onto 15‐cm‐diameter Petri dish and 20 μL of diesel oil was dropped onto the water surface, and then 10 μL of culture broth supernatant was added 14. The diameter of the clear halo on the oil surface was measured and compared to distillated water and Triton X‐100 was used as control as above.
2.3.3. Hemolytic activity determination
The hemolytic activity of B. mojavensis I4 was tested on blood agar plate containing 5% v/v of human blood, plate was incubated at 37°C for 24–48 h. Hemolytic activity was detected as the presence of a defined clear zone around the streaks.
2.3.4. Lipase production assay
Rhodamine B plate assay was used for the detection of lipolytic activity as previously described 15. The rhodamine B plate was streaked with freshly prepared I4 strain. After incubation for 24 h at 37°C, the plate was exposed to UV light. The presence of orange fluorescence around the colonies was used as an index for lipolytic activity.
2.3.5. Determination of emulsification index
The emulsification index was determined as previously described 16. Indeed, 2 mL of cell‐free broth was supplemented with equal volume of vegetable oil and then mixed by vortex at high speed for 2 min. The mixture was left undisturbed for the next 24 h. The emulsification index (E24) was calculated according to the following formula:
where He is the height of emulsion, and Ht is the total height of the mixture.
2.3.6. Surface tension determination
The surface tensions of the culture broth supernatant and chemical surfactants (SDS;10% w/v and Triton X‐100) were measured according to the De Nouy methodology using a tensiometer TD1 (Lauda‐Königshofen, Germany).
2.4. Inoculum preparation and culture condition
Twenty‐five microliters of Lauria–Bertani medium, in a 250 mL shake flask, was inoculated with the I4 strain and incubated overnight at 37°C with shaking at 180 rpm. Three percent v/v of inoculum was transferred into 1 L flask containing 100 mL of basal salts medium (pH 7) containing in g/L: KH2PO4 (0.5), K2HPO4 (1), KCl (0.1), MgSO4 (0.5), FeSO4 (0.008), CaCl2 (0.05), NH4Cl (2), and 1 mL/L trace elements solution (4.4 mg/L ZnSO4, 3.3 mg/L MnSO4, 0.1 mg/L CuSO4, and 1 mg/L NaBr). The culture was carried for 48 h at 37°C and 180 rpm.
2.5. Medium optimization for biosurfactants production
2.5.1. Effect of carbon and nitrogen sources on biosurfactants production
The medium optimization was conducted in a series of experiments where only one factor was changed while others were fixed. Three factors carbon sources, nitrogen sources, and C/N ratio were selected. For carbon sources selection fructose, maltose, glucose, mannitol, lactose, and sorbitol were used. Ammonium chloride (2 g/L) was used as the nitrogen source. The different carbon sources were added at a final concentration of 10 g/L. After the selection of the carbon source, to determine its optimal concentration, different glucose concentrations ranging from 10 to 45 g/L were tested. For the selection of adequate nitrogen source, urea, peptone, yeast extract, ammonium sulfate, glutamic acid, and ammonium chloride, were tested at a concentration of 2 g/L while glucose concentration was fixed at 30 g/L. After the selection of the carbon and nitrogen sources, the C/N ratio was also investigated. For this purpose, several C/N ratios from 8 to 122 were tested. Subsequently, samples were collected after 24 h of culture and the effect of C/N ratios was evaluated by the determination of surface tension and production yield. All experiments were performed in triplicate.
2.5.2. Effect of pH and temperature on the biosurfactants production
The effect of pH and temperature on the biosurfactants production was investigated. Indeed, different range of pH (4–10) and temperature (30, 37, and 40°C) were tested. The effect of these parameters was assessed by the determination of the emulsification index (E24).
2.5.3. Growth kinetics and biosurfactants production
After the optimization of both medium composition and cultivation conditions, kinetics of biosurfactants production were monitored for a duration of 72 h by measuring the pH, bacterial growth, biosurfactants production, glucose consumption, and surface tension of the culture supernatant.
2.6. Extraction and partial purification of the biosurfactants
The bacterial cells were removed from 48 h grown culture by centrifugation at 10 000 rpm at 4°C for 20 min. The biosurfactants precipitated from the cell‐free supernatant overnight at 4°C by adding HCl (6N) to a final pH of 2. Grey‐white pellet formed after precipitation was recovered by centrifugation at 10 000 rpm for 20 min at 4°C and then dissolved in distillated water and neutralized by NaOH to a final pH of 8 to ensure total dissolving of the biosurfactants 17. The biosurfactants solution was dialyzed against demineralized water at 4°C in a Cellu‐Sep© membrane (Membrane Filtration Products, Seguin, USA) for 48 h 18. Finally, the partially purified biosurfactants was lyophilized and stored at −20°C until use.
2.7. Effect of environmental factors on biosurfactants stability
The lyophilized biosurfactants were dissolved in distilled water at the concentration of 2 mg/mL. The stability against pH was assessed by varying the pH of biosurfactants solution from 2 to 12 with 6N HCl or 6N NaOH solutions. Moreover, the stability against salinity was tested at different concentration of NaCl (2–12% w/v). For the effect of temperature, the biosurfactants solution was incubated at different temperatures (4–120°C) for 30 min 19. After each treatment, the samples were allowed to stand at room temperature before surface tension and emulsification index (E24) determination. All the experiments were carried out in triplicate.
2.8. Determination of substrate specificity and emulsifying activity
The ability of the partially purified biosurfactants to emulsify liquid hydrocarbons, such as crude oil, corn oil, olive oil, paraffin oil, kerosene, n‐hexane, and toluene, was investigated. An aliquot of 2 mL of the purified biosurfactants solution (2 mg/mL) was mixed with equal volume of each hydrocarbon. The mixture was vortexed vigorously for 2 min and left to stand for 24 h before emulsion index (E24) determination.
2.9. Biosurfactants characterization by TLC
The partially purified biosurfactants from I4 were dissolved in methanol and analyzed by TLC on silica gel plates (G60; Merck, Germany). Chromatograms were developed with chloroform:methanol:acetic acid (65:15:2, v/v). The plates were then exposed to iodine vapors, Molisch reagent, and ninhydrin (1%) for the detection of lipid, sugar, and free amino groups, respectively. After corresponding reagent spray, the plates were heated at 110°C until the appearance of the respective color.
2.10. Determination of biosurfactants ionic character and zeta potential analysis
The ionic charge of the partially purified I4 biosurfactants was determined using the agar double diffusion method 20. Two rows of wells were mined in 1% agar and the biosurfactants were filled into the wells of the first row, while the remaining ones were filled with 20 mM solution of SDS or CTAB as anionic and cationic compound, respectively. The appearance of precipitation lines between the wells, indicative of the ionic character of the biosurfactants. The result was obtained after 48 h incubation at room temperature.
The zeta potential of the biosurfactants was determined using a Zetasizer Software for the nano, APS, and μV (Version 7.01).The sample was diluted (0.5% w/w) with distilled water at pH 7. The mean f‐potential (ZP) values (±SD) were obtained from the instrument.
2.11. HPLC analysis
The purified biosurfactants were concentrated on a C18 cartridge. This later was packed with 20 mL methanol (100%) and then rinsed with 10 mL of ultra‐pure water. One milliliter of sample, filtered with 0.22 μm cellulose filter, was deposited and then washed with 8 mL of ultra‐pure water. The biosurfactants retained by the cartridge were eluted with 8 mL methanol (100%). The eluate was totally dried under vacuum (Speed vac), resuspended in 200 mL of methanol, and then injected in RP‐HPLC.
For HPLC analysis, an aliquot of 20 μL each of surfactin, used standard (1 g/L), and the biosurfactants solutions (1 g/L) were injected automatically in an RP C18 column (Vydac, 5 μm, 250 × 3.0 mm, 218 TP). The mobile phase was held for 20 min in isocratic mode, and then the samples were eluted using a linear gradient mobile phase composed of acetonitrile:water:trifluoro acetic acid (80:20:0.1) at a flow rate of 0.6 mL/min. The retention time and the second derivative of the spectrum between 200 and 400 nm (Diode Array PDA 996, Waters Corp.) were used to identify the eluted molecules (Millenium Software, Waters Corp.). The purity of test surfactin was calculated as follow:
2.12. Determination of the ACE inhibition activity
The ACE‐inhibition activity was measured in triplicate. A sample solution (80 μL) at different concentrations (0.2–3 mg/mL) of I4 biosurfactants was mixed with 200 μL of 5 mM hippuryl‐l‐histidyl‐l‐leucine, and then incubated for 3 min at 37°C. The biosurfactants and hippuryl‐l‐histidyl‐l‐leucine were prepared in 100 mM borate buffer (pH 8.3) containing 300 mM NaCl. The reactions were then initiated by adding 20 μL of 0.1 U/mL ACE from rabbit lung prepared in the same buffer. After incubation for 30 min at 37°C, the reactions were stopped by the addition of 250 μL of 0.05 M HCl. The liberated hippuric acid (HA) was extracted with ethyl acetate (1.7 mL) and then evaporated at 90°C for 10 min. The residue was dissolved in 1 mL of distilled water, and the absorbance of the extract at 228 nm was determined using an UV–Visible spectrophotometer (UV mini 1240; UV/VIS spectrophotometer, SHIMADZU, Japan). The average value from three determinations at each concentration was used to calculate the ACE inhibition rate as follows:
where A is the absorbance of HA generated in the presence of ACE inhibitor, B is the absorbance of HA generated without ACE inhibitors, and C is the absorbance of HA generated without ACE. The concentration of ACE inhibitor required to inhibit 50% of ACE activity under the above conditions was defined as IC50.
2.13. Statistic analysis
All the experiments were carried in triplicate. Data were reported as mean ± standard error. Statistics were done using JMP software 10 (SAS Institute). Tukey's HSD tests were used to determine the significant differences between samples means that a 95% confidence level was considered significant (p < 0.05).
3. Results and discussion
3.1. Screening assays for biosurfactants production
Different screening methods were employed to investigate the potential of biosurfactants production from microorganism 13. Thus, in this study, parafilm M test, lipase activity, hemolytic activity, oil displacement test, emulsification index, and surface tension were used to investigate the production of biosurfactants by B. mojavensis strain I4.
The parafilm M test (Supporting Information, Fig. S1A) showed that the drop diameter of cell‐free supernatant (9 mm) was larger than the drop diameter of Triton X‐100 (7 mm) and sterile medium (3 mm). This finding indicated that the strain I4 could produce biosurfactants. Moreover, from the results illustrated in Table 1, biosurfactants from I4 showed the largest area in the oil displacement test toward diesel (110 cm2) compared to Triton X‐100 (80 cm2). This result confirmed that B. mojavensis I4 could produce surface‐active molecules. Furthermore, the production of a clear zone around the bacterial streaks on blood agar plate (Supporting Information, Fig. S1B) suggested that the I4 strain possessed hemolytic activity. The result corroborated those reported by Carrillo et al. 21 which showed that hemolytic activity is an efficient screening criterion for surfactant‐producing strain. The strain I4 produced an orange fluorescence zone around the grown colonies under UV light in the lipase activity assay (Supporting Information, Fig. S1C). This result showed that I4 strain possessed lipolytic activity which is highly correlated with bioemulsifiers production capacity in bacteria 22.
Table 1.
Results for primary screening of biosurfactants‐producing strain
| Tests | Oil displacement test (cm) | Surface tension (mN/m) | E24 (%) |
|---|---|---|---|
| I4 Biosurfactant | 11 ± 0.5 | 31.5 ± 0.8 | 60 ± 0.4 |
| Triton X‐100 | 8 ± 0.9 | 32 ± 0.9 | nd |
| SDS (10%) | nd | 34.8 ± 1.3 | nd |
nd: non‐determined.
The different screening tests carried above showed that B. mojavensis I4 could produce biosurfactants. Moreover, a microorganism is considered promising biosurfactant producer, if it could reduce the surface tension to less than 40 mN/m and maintained at least 50% of the original emulsion volume after 24 h of emulsification 23. The surface tension and the emulsification index of cell‐free broth were determined. Results (Table 1) showed that I4 cell‐free broth reduced the surface tension until 31.5 ± 0.8 mN/m which was lower than SDS (34.8 ± 1.3 mN/m) used as chemical surfactant. Moreover, higher emulsification index (60%) was found to be stable for more than 1 week at room temperature (Table 1).
3.2. Optimization of cultivation medium
It was reported that medium composition strongly influenced cell growth and the accumulation of metabolic products, thus the optimization of these parameters can improve the bacterial efficiency 24. The production yield of biosurfactants is highly correlated with the cultivation conditions and also with medium composition. In this study, several parameters were investigated in order to optimize the biosurfactants' production from B. mojavensis I4.
3.2.1. Effect of carbon sources on the biosurfactants production
Different carbon sources, such as glucose, mannitol, lactose, fructose, maltose, and sorbitol, were used as carbon sources for the biosurfactants' production. Results (Fig. 1A) showed that biosurfactants' production was significantly affected by the nature of the carbon source used. Indeed, lactose and sorbitol led to the lowest biosurfactants' yield compared to other sources. In contrary, higher biosurfactants production was observed when using fructose, maltose, and glucose as substrate. Moreover, glucose showed the highest biosurfactants yield with surface tension reduction from 60 to 30 mN/m. Abdel‐Mawgoud et al. 25 reported that the minimal biosurfactants' production by B. subtilis isolate BS5 was obtained when using lactose as the sole carbon source and the production was significantly enhanced when glucose was used as carbon source.
Figure 1.
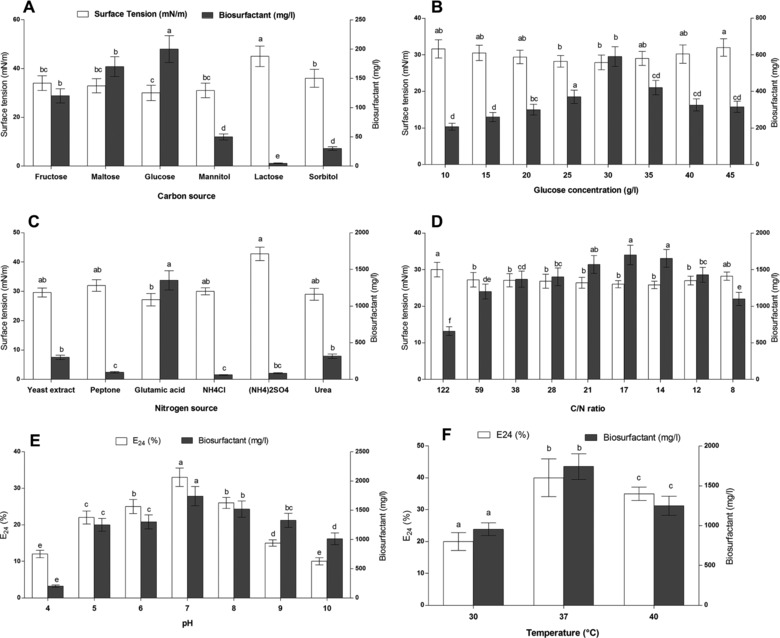
Effect of different culture conditions on biosurfactants production from Bacillus mojavensis I4
(A) carbon sources, (B) glucose concentration, (C) nitrogen sources, (D) C/N ratios, (E) pH, and (F) temperature.
3.2.2. Effect of glucose concentration on the biosurfactants' production
Previous analysis showed that glucose was the most efficient carbon source (Fig. 1A). Therefore, different concentrations of glucose, ranging from 10 to 45 g/L, were tested in order to define the optimal one. Results (Fig. 1B) showed that the highest biosurfactants' production was obtained when glucose was used at 3%. At this concentration, biosurfactants' production was increased to 600 mg/L with a surface tension of 27 mN/m. Coronel‐León et al. 26 reported that the maximum biosurfactants' production by B. licheniformis AL1.1 was obtained using 1.5% glucose. Moreover, Ghribi and Ellouze‐Chaabouni 27 also showed that the maximum biosurfactants' production by B. subtilis SPB1 was achieved by using glucose as carbon source at a concentration of 4%.
3.2.3. Effect of nitrogen sources on the biosurfactants' production
Concerning nitrogen sources, as for carbon source, the biosurfactants' production was modulated by nature of nitrogen source used in the medium. As shown in Fig. 1C, B. mojavensis I4 could use nitrogen sources, such as yeast extract, urea, and ammonium chloride, to produce biosurfactants. Moreover, the highest production yield was obtained when glutamic acid was used as the sole nitrogen source (1350 mg/L).
3.2.4. Determination of the optimal C/N ratio
Previous results (Fig. 1A and B) showed that glucose and glutamic acid were the best carbon and nitrogen sources for biosurfactants' production, respectively. Indeed, the C/N ratio is also a critical parameter which affects biosurfactants' production. In this study, different C/N ratios were tested. Results showed that the optimal biosurfactants' production was achieved when using 0.6% of glutamic acid which corresponded to a C/N ratio of 17 (Fig. 1D). The increase of the C/N ratio over to 122 seemed not improving the production of the biosurfactants. Similarly, Ghribi and Ellouze‐Chaabouni 27 reported that the production of biosurfactants by B. subtilis SPB1 increases by adjusting the C/N ratio and found that a ratio of over seven could decrease the production yield.
3.2.5. Effect of pH and temperature on the biosurfactants' production
The strain I4 showed gradual increase in E24 and biosurfactants production until pH 7 which was the optimal one (Fig. 1E). Several studies have shown that the pH of the medium affects significantly the biosynthesis of lipopeptides by Bacillus strains. Gancel et al. 28 reported that enhanced production of lipopeptides, such as fengycin and surfactin by B. subtilis strain ATCC 21332 was obtained when the pH of the medium was adjusted to 7. Similarly, Abdel‐Mawgoud et al. 25 showed that the slightly acidic pH ranging from 6.5 to 6.8 favored the production of lipopeptides by B. subtilis strain BS5. The effect of cultivation temperature showed that it affected the biosurfactants production and also the E24 (Fig. 1F). Indeed, the highest biosurfactants production (2100 mg/L) and E24 were obtained at 37°C. Similarly, the maximum surfactin production was achieved at 37.4°C by B. subtilis DSM 3256 29 and B. subtilis SPB1 4. Contrarily, enhanced biosurfactants' production was obtained at 40°C by Bacillus subtilis HOB2 30 and at 30°C from Bacillus mojavensis A21 31. Indeed, optimal temperature and pH could enhance the efficiency of the biosurfactants production whereas these parameters may have negative effect on biosurfactants production depending on the nature of the bacterium.
3.3. Growth kinetics and biosurfactants' production
The growth kinetics of strain I4 in the optimal culture medium was carried out to study biosurfactants' production dynamics. Figure 2 shows the growth kinetics of I4 until 72 h at 37°C and 180 rpm. The kinetics growth curve showed a parallel relationship between biosurfactants production, biomass (OD at 600 nm), surface tension, pH, and glucose consumption, suggesting a growth associated biosurfactants' production. Maximum growth and the highest biosurfactants' production were observed after 48 h of incubation, and thereafter the biomass decreased slightly (Fig. 2). The growth associated production of biosurfactants has been reported for several other microorganisms 32. The surface tension of culture broth decreased rapidly after inoculation and reached its lowest value (30 mN/m) after about 48 h and then remained nearly constant until the end of cultivation (72 h).
Figure 2.
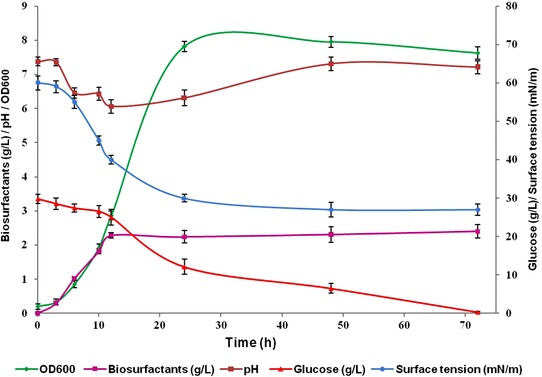
Time course profiles of pH, surface tension, biosurfactant production, OD600, and glucose consumption of B. mojavensis I4 grown in mineral supplemented with 3% glucose and 0.6% glutamic acid during 72 h at 37°C and 180 rpm.
The evolution of the cultivation medium pH showed that it decreased to 6.0 during the first 12 h and seemed to increase gradually reaching the highest value of 8 after 72 h. In fermentation with pH control, its variation was due to the microorganism metabolism. Moreover, similar pH variation was observed for B. licheniformis AL1.1 cultivated on glucose 26.
3.4. Stability related to surface tension and emulsification
The stability of the partially purified biosurfactants was tested over a wide temperature range. The biosurfactants were stable during 30 min at temperatures ranging from 4 to 121°C (Table 2). The emulsification activity and the surface tension reducing activity seemed to be stable at these temperatures. This result corroborate those of lipopeptides produced by B. subtilis SPB1 which preserved 80% of its emulsifying activity for 1 h at 60°C 27 and those reported for B. subtilis BS5 which showed to be stable after 1 h treatment at 100°C 25.
Table 2.
Influences of temperature, salt concentration, and pH on the surface tension reducing activity and on the Emulsification index of partially purified biosurfactants from B. mojavensis I4
| Surface tension reduction (mN/m) | E24 (%) | |
|---|---|---|
| Temperature (°C) | ||
| 4 | 27.20 ± 0.16 | 59.23 ± 2.13 |
| 30 | 27.62 ± 0.21 | 60.10 ± 3.20 |
| 50 | 28.21 ± 0.43 | 61.62 ± 2.45 |
| 70 | 27.50 ± 0.18 | 55.06 ± 2.52 |
| 100 | 29.21 ± 0.62 | 53.23 ± 2.40 |
| 121 | 29.56 ± 0.84 | 52.21 ± 2.05 |
| NaCl (%) | ||
| 2 | 28.20 ± 0.12 | 60.17 ± 2.14 |
| 4 | 28.35 ± 0.32 | 58.02 ± 3.62 |
| 6 | 27.42 ± 0.85 | 55.27 ± 4.12 |
| 8 | 27.10 ± 0.36 | 50.30 ± 3.47 |
| 10 | 27.10 ± 0.25 | 58.02 ± 2.89 |
| 12 | 28.12 ± 0.52 | 56.32 ± 2.57 |
| pH | ||
| 2 | 29.52 ± 0.21 | 39.49 ± 2.31 |
| 4 | 28.92 ± 0.31 | 45.20 ± 2.14 |
| 6 | 28.05 ± 0.74 | 57.72 ± 3.21 |
| 8 | 27.23 ± 0.62 | 60.17 ± 2.00 |
| 10 | 27.06 ± 0.28 | 57.10 ± 3.10 |
| 12 | 28.06 ± 0.14 | 51.70 ± 3.45 |
The stability of the biosurfactants toward NaCl was investigated (Table 2). The biosurfactants maintained its capacity of reducing surface tension in the presence of NaCl up to 12%. The emulsification activity against corn oil also remained stable over the tested salt concentrations. The application of the biosurfactants in the bioremediation of contaminated marines required high stability in saline medium. Considering that the highest sea salinity of the world is of 3%, the biosurfactants from B. mojavensis I4 could be applied in saline environments. Stability of emulsion in saline condition was also reported for biosurfactants produced by B. licheniformis strain JF‐2 33.
Concerning I4 biosurfactants' stability toward pH, its surface activity was stable at pH ranging between 6 and 12 suggesting its stability at alkaline pH compared to acidic ones (Table 2). It was reported that lichenysin, a lipopeptide produced by B. licheniformis JF‐2, retained its emulsifying activity at pH ranging from 4.5 to 9 34. Moreover, Gong et al. 35 also reported that the surface activity of the biosurfactants from B. subtilis strain was stable at pH between 6 and 12 while its activity decreases at pH below 5. These results suggested that high pH could improve the emulsification activity which could be due to a better stability of fatty acid surfactant micelles in alkaline medium and the precipitation of secondary metabolites at higher pH 36.
3.5. Substrate specificity of biosurfactants and determination of emulsifying activity
The emulsification capacity is an important propriety of a surfactant. The Emulsification index of I4 biosurfactants tested on different substrates is reported in Table 3. Hexane showed the highest E24 value of 63 while diesel showed the lowest one. Emulsification of hydrocarbons by the culture I4 was in the order of hexane > corn oil > olive oil > toluene > kerosene > paraffin oil > diesel. The results showed that I4 biosurfactants could emulsify different hydrocarbons. The emulsion of corn oil produced by the isolate was stable for a month compared to other tested hydrocarbons. The ability of biosurfactants to form stable emulsions with vegetable oils and fats suggests its potential application as cleaning and emulsifying agent in the food industry.
Table 3.
Emulsification index (E24) of hydrophobic substrates by the biosurfactants from B. mojavensis I4
| Substrates | E24 (%) |
|---|---|
| Corn oil | 62.21 ± 2.21 |
| Olive oil | 58.62 ± 2.54 |
| Paraffin oil | 34.31 ± 3.12 |
| Diesel | 12.31 ± 1.23 |
| Kerosene | 45.20 ± 2.98 |
| Hexane | 63.70 ± 2.78 |
| Toluene | 51.72± 2.12 |
3.6. Biosurfactants' characterization by TLC
The biosurfactants analysis by TLC (Fig. 3A) using specific staining reagents produced several spots with different retention factors (Rf). Three pink spots with Rf value of 0.02, 0.09, and 0.16 showed positive reactions when sprayed with ninhydrin reagent suggesting the presence of amino acids. One spot with Rf value of 0.09 for lipids was detected with iodine vapors. Moreover, negative reaction for sugars detection was obtained with Molish reagents. The presence of both peptide units and lipid moieties on the same spots suggested that B. mojavensis I4 produced lipopeptides type biosurfactants in medium supplemented with glucose as the sole carbon source.
Figure 3.
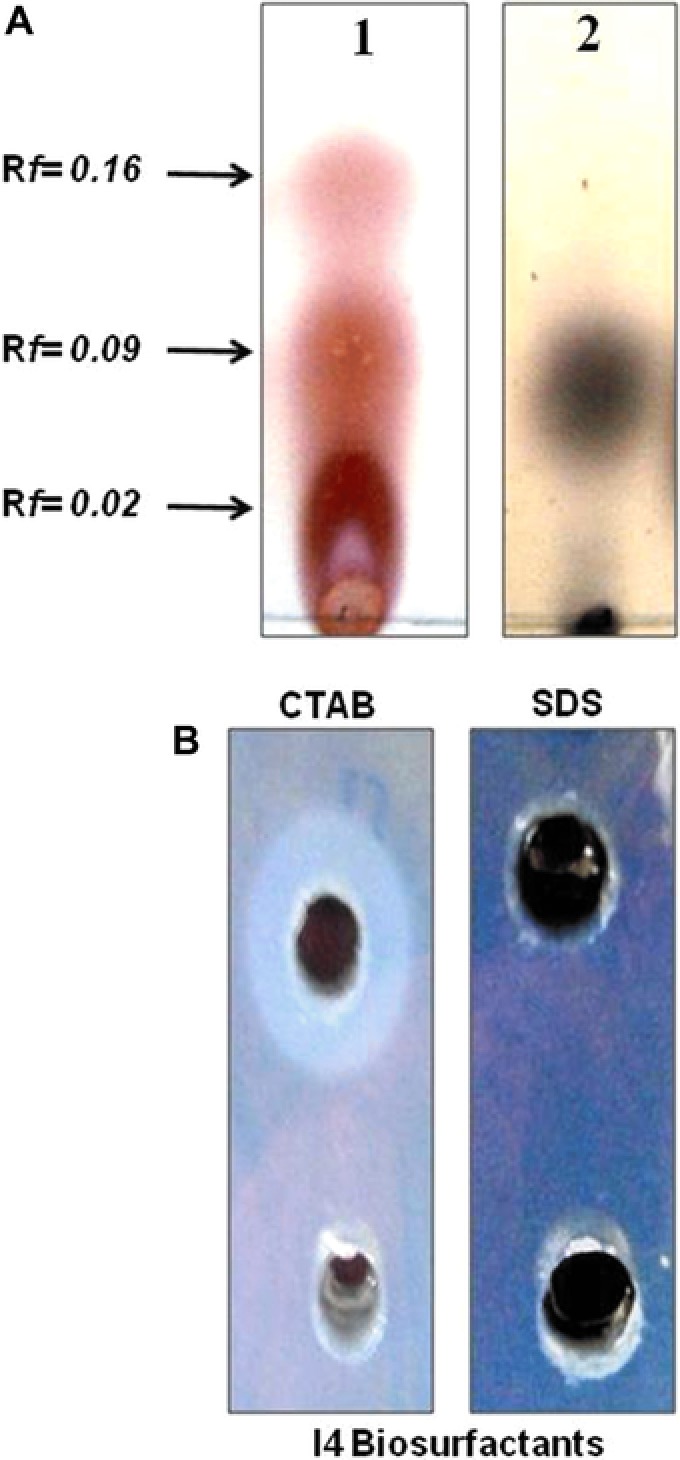
(A) TLC analysis of purified biosurfactants: (1) spots stained with ninhydrin (for detection of peptides) and (2) spots stained with iodine vapor staining for lipid. The Rf values represent the retention factors for the different spots. (B) Double diffusion on agar of an anionic biosurfactant produced by B. mojavensis I4.
3.7. Determination of biosurfactants' ionic character
Agar double diffusion tests (Fig. 3B), based on the passive diffusion of two compounds bearing charges of the same or opposite types in a weakly concentrated gel, revealed the appearance of precipitation lines between the biosurfactants and the cationic compound (CTAB) compared to anionic one (SDS) suggesting that the produced biosurfactants were anionic. This result was confirmed by the zeta potential analysis which revealed that the particles formed have negatively charged surfaces at pH 7 ( −40 mV). The high zeta potential value (either positive or negative), typically more than 30 mV maintains a stable system.
3.8. Reverse phase HPLC analysis of lipopeptides
The HPLC analysis showed that the standard surfactin contained ten isoforms, which eluted between the retention time of 15 and 33 min (Fig. 4A). Five of these isoforms were major, namely, peaks 4, 6, 8, 9, and 10, and the other isoforms were minor, namely, peaks 1, 2, 3, 5, and 7. Furthermore, Fig. 4A shows that all surfactin isoforms are well separated except four isoforms (peaks 5 and 6; peaks 8 and 9), which differ in their retention times by 1 min. Figure 4B shows that the HPLC spectrogram of the biosurfactants from B. mojavensis I4 shows similar retention peaks (designated by letters) to those of surfactin (designated by numbers). The purity of surfactin in the dried sample was 71%. This result suggested that the I4 produced surfactin was major biosurfactant in the lipopeptides mixture. Although HPLC analysis revealed the major compound present in the biosurfacants' mixture, the determination of detailed structural information and peptide sequence of I4 biosurfactants by MALDI–TOF‐MS/MS and NMR techniques will provide useful instruction for adequate applications of I4 biosurfactants. Indeed, surfactin is a bacterial cyclic lipopeptide which is produced by various Bacillus strains and is primarily recognized as one of the most effective biosurfactants 18. Surfactin has a wide range of environmental and therapeutic applications, such as in environmental bioremediation and antibacterial treatments. Moreover, surfactin exhibiting mainly antibacterial activity act to destabilize the phospholipid membrane of the target phytopathogen 18. This antibacterial activity of surfactin made it a very interesting candidate in therapeutics, in agriculture as plant pathogen biocontrol agent, and also as an inducer of plant defense since it induces plant immunity leading to better resistance to phytopathogen 18.
Figure 4.
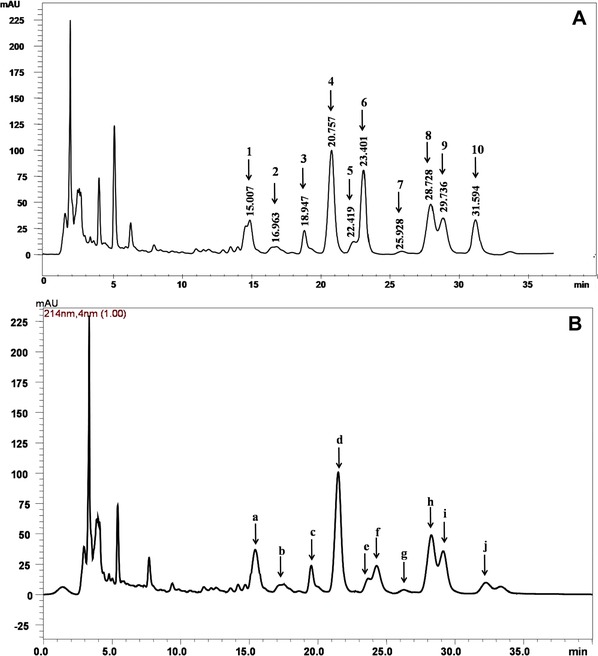
Analytical reversed‐phase HPLC absorbance spectrogram of surfactin (A) and partially purified biosurfactants from B. mojavensis (B).
3.9. Determination of the ACE‐inhibition activity
The inhibition of ACE using dietary antihypertensive agents presents a good strategy to control hypertension. Since synthetic ACE inhibitors may be a source of adverse side effects 37, natural molecule could be alternative ACE inhibitors. Thus, ACE‐inhibitory activity of I4 lipopeptide biosurfactants was investigated. Results (Supporting Information, Fig. S2) showed that I4 lipopeptides exhibited concentration‐dependent ACE‐inhibitory activity. The highest activity (81.23 ± 1.2) was observed at 3 mg/mL in a dose‐dependent pattern. The IC50 value for the ACE inhibition of lipopeptides was 0.7 mg/mL.
4. Concluding remarks
This study showed that the isolate I4 could produce a biosurfactant, using glucose and glutamic acid as carbon and nitrogen sources, respectively. The maximum biosurfactants' yield of 2 g/L was obtained after 48 h of culture. The produced lipopeptides were stable at high temperature and in a wide range of pH and salt concentrations. The TLC analysis revealed that B. mojavensis I4 produced lipopeptide‐type biosurfactants. Moreover, zeta potential measurement showed that the lipopeptides were anionic. HPLC analysis showed that surfactin was the major compound in the lipopeptides mixture. Furthermore, the produced lipopeptides exhibited interesting ACE‐inhibitory activity suggesting its potential usage in pharmaceutical industries.
Practical application
Different screening methods revealed that Bacillus mojavensis strain I4 produced biosurfactants. The purified biosurfactants were stable in a wide range of temperatures, pH, and salinity. Furthermore, HPLC analysis revealed that surfactin was the major lipopeptide. Indeed, the purified lipopeptides showed interesting ACE‐inhibitory activity and could be useful for therapeutic or pharmaceutical purposes as effective antihypertensive agent.
The authors have declared no conflict of interest.
Supporting information
Supporting information
Acknowledgements
This work was funded by the Ministry of Higher Education and Scientific Research‐Tunisia.
5 References
- 1. Rodrigues, L. , van der Mei, H. C. , Teixeira, J. , Oliveira, R. , Influence of biosurfactants from probiotic bacteria on formation of biofilms on voice prostheses. Appl. Environ. Microbiol. 2004, 70, 4408–4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thavasi, R. , Jayalakshmi, S. , Banat, I. M. , Application of biosurfactant produced from peanut oil cake by Lactobacillus delbrueckii in biodegradation of crude oil. Bioresour. Technol. 2011, 102, 3366–3372. [DOI] [PubMed] [Google Scholar]
- 3. Lang, S. , Wullbrandt, D. , Rhamnose lipids—Biosynthesis, microbial production and application potential. Appl. Microbiol. Biotechnol. 1999, 51, 22–32. [DOI] [PubMed] [Google Scholar]
- 4. Mnif, I. , Hammami, I. , Triki, M. A. , Azabou, M. C. et al., Antifungal efficiency of a lipopeptide biosurfactant derived from Bacillus subtilis SPB1 versus the phytopathogenic fungus, Fusarium solani . Environ. Sci. Pollut. Res. Int. 2015, 22, 18137–18147. [DOI] [PubMed] [Google Scholar]
- 5. Bodour, A. A. , Drees, K. P. , Maier, R. M. , Distribution of biosurfactant‐producing bacteria in undisturbed and contaminated arid Southwestern soils. Appl. Environ. Microbiol. 2003, 69, 3280–3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yeh, M. S. , Wei, Y. H. , Chang, J. S. , Enhanced production of surfactin from Bacillus subtilis by addition of solid carriers. Biotechnol. Prog. 2005, 21, 1329–1334. [DOI] [PubMed] [Google Scholar]
- 7. Janek, T. , Lukaszewicz, M. , Krasowska, A. , Identification and characterization of biosurfactants produced by the Arctic bacterium Pseudomonas putida BD2. Colloids. Surf. B Biointerfaces 2013, 110, 379–386. [DOI] [PubMed] [Google Scholar]
- 8. Li, Y.‐M. , Haddad, N. I. A. , Yang, S.‐Z. , Mu, B.‐Z. , Variants of lipopeptides produced by Bacillus licheniformis HSN221 in different medium components evaluated by a rapid method ESI–MS. Int. J. Pept. Res. Ther. 2008, 14, 229–235. [Google Scholar]
- 9. Alvarez, F. , Castro, M. , Principe, A. , Borioli, G. et al., The plant‐associated Bacillus amyloliquefaciens strains MEP2 18 and ARP2 3 capable of producing the cyclic lipopeptides iturin or surfactin and fengycin are effective in biocontrol of sclerotinia stem rot disease. J. Appl. Microbiol. 2012, 112, 159–174. [DOI] [PubMed] [Google Scholar]
- 10. Muthusamy, K. , Gopalakrishnan, S. , Ravi, T. K. , Sivachidambaram, P., Biosurfactants: Properties, commercial production and application. Curr. Sci. 2008, 94, 736–747. [Google Scholar]
- 11. Mnif, I. , Chaabouni‐Ellouze, S. , Ghribi, D . Optimization of the nutritional parameters for enhanced production of B. subtilis SPB1 biosurfactant in submerged culture using response surface methodology. Biotechnol. Res. Int. 2012, 2012, 795430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ghazala, I. , Sayari, N. , Romdhane, M. B. , Ellouz‐Chaabouni, S. et al., Assessment of pectinase production by Bacillus mojavensis I4 using an economical substrate and its potential application in oil sesame extraction. J. Food Sci. Technol. 2015, 52, 7710–7722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Satpute, S. K. , Banpurkar, A. G. , Dhakephalkar, P. K. , Banat, I. M. et al., Methods for investigating biosurfactants and bioemulsifiers: A review. Crit. Rev. Biotechnol. 2010, 30, 127–144. [DOI] [PubMed] [Google Scholar]
- 14. Rodrigues, L. R. , Teixeira, J. A. , van der Mei, H. C. , Oliveira, R. , Physicochemical and functional characterization of a biosurfactant produced by Lactococcus lactis 53. Colloids Surf. B Biointerfaces 2006, 49, 79–86. [DOI] [PubMed] [Google Scholar]
- 15. Kouker, G. , Jaeger, K. E. , Specific and sensitive plate assay for bacterial lipases. Appl. Environ. Microbiol. 1987, 53, 211–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cooper, D. G. , Goldenberg, B. G. , Surface‐active agents from two bacillus species. Appl. Environ. Microbiol. 1987, 53, 224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu, X. Y. , Yang, S. Z. , Mu, B. Z. , Isolation and characterization of a C12‐lipopeptide produced by Bacillus subtilis HSO 121. J. Pept. Sci. 2008, 14, 864–875. [DOI] [PubMed] [Google Scholar]
- 18. Shaligram, N. S. , Singhal, R. S , Surfactin: A review on biosynthesis, fermentation, purification and applications. Food Technol. Biotechnol. 2010, 48, 119–134. [Google Scholar]
- 19. Dubey, K. V. , Charde, P. N. , Meshram, S. U. , Shendre, L. P. et al., Surface‐active potential of biosurfactants produced in curd whey by Pseudomonas aeruginosa strain‐PP2 and Kocuria turfanesis strain‐J at extreme environmental conditions. Bioresour. Technol. 2012, 126, 368–374. [DOI] [PubMed] [Google Scholar]
- 20. Meylheuc, T. , van Oss, C. J. , Bellon‐Fontaine, M. N. , Adsorption of biosurfactant on solid surfaces and consequences regarding the bioadhesion of Listeria monocytogenes LO28. J. Appl. Microbiol. 2001, 91, 822–832. [DOI] [PubMed] [Google Scholar]
- 21. Carrillo, P. G. , Mardaraz, C. , Pitta‐Alvarez, S. I. , Giulietti, A. M. , Isolation and selection of biosurfactant‐producing bacteria. World J. Microbiol. Biotechnol. 1996, 12, 82–84. [DOI] [PubMed] [Google Scholar]
- 22. Sriram, M. I. , Kalishwaralal, K. , Deepak, V. , Gracerosepat, R. et al., Biofilm inhibition and antimicrobial action of lipopeptide biosurfactant produced by heavy metal tolerant strain Bacillus cereus NK1. Colloids Surf. B Biointerfaces 2011, 85, 174–181. [DOI] [PubMed] [Google Scholar]
- 23. Shete, A. M. , Wadhawa, G. , Banat, I. M. , Chopade, B. A. , Mapping of patents on bioemulsifier and biosurfactant: A review. J. Sci. Ind. Res. 2006, 65, 91–115. [Google Scholar]
- 24. Najafi, A. R. , Rahimpour, M. R. , Jahanmiri, A. H. , Roostaazad, R. et al., Enhancing biosurfactant production from an indigenous strain of Bacillus mycoides by optimizing the growth conditions using a response surface methodology. Chem. Eng. J. 2010, 163, 188–194. [Google Scholar]
- 25. Abdel‐Mawgoud, A. M. , Aboulwafa, M. M. , Hassouna, N. A. , Optimization of surfactin production by Bacillus subtilis isolate BS5. Appl. Biochem. Biotechnol. 2008, 150, 305–325. [DOI] [PubMed] [Google Scholar]
- 26. Coronel‐León, J. , Grau, G. , Grau‐Campistany, A. , Farfan, M. et al., Biosurfactant production by AL 1.1, a Bacillus licheniformis strain isolated from Antarctica: Production, chemical characterization and properties. Ann. Microbiol. 2015, 65, 2065–2078. [Google Scholar]
- 27. Ghribi, D. , Ellouze‐Chaabouni, S. , Enhancement of Bacillus subtilis lipopeptide biosurfactants production through optimization of medium composition and adequate control of aeration. Biotechnol. Res. Int. 2011, 2011, 653654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gancel, F. , Montastruc, L. , Liu, T. , Zhao, L. et al., Lipopeptide overproduction by cell immobilization on iron‐enriched light polymer particles. Process Biochem. 2009, 44, 975–978. [Google Scholar]
- 29. Sivapathasekaran, C. , Sen, R . Origin, properties, production and purification of microbial surfactants as molecules with immense commercial potential. Tenside Surfact. Det. 2017, 54, 92–107. [Google Scholar]
- 30. Haddad, N. I. , Wang, J. , Mu, B. , Identification of a biosurfactant producing strain: Bacillus subtilis HOB2. Protein Pept. Lett. 2009, 16, 7–13. [DOI] [PubMed] [Google Scholar]
- 31. Ben Ayed, H. , Hmidet, N. , Bechet, M. , Chollet, M. et al., Identification and biochemical characteristics of lipopeptides from Bacillus mojavensis A21. Process Biochem. 2014, 49, 1699–1707. [Google Scholar]
- 32. Burgos‐Diaz, C. , Pons, R. , Teruel, J. A. , Aranda, F. J. et al., The production and physicochemical properties of a biosurfactant mixture obtained from Sphingobacterium detergens . J. Colloid. Interface Sci. 2013, 394, 368–379. [DOI] [PubMed] [Google Scholar]
- 33. Ilori, M. O. , Amobi, C. J. , Odocha, A. C. , Factors affecting biosurfactant production by oil degrading aeromonas spp. isolated from a tropical environment. Chemosphere 2005, 61, 985–992. [DOI] [PubMed] [Google Scholar]
- 34. McInerney, M. J. , Javaheri, M. , Nagle, D. P. , Jr. Properties of the biosurfactant produced by Bacillus licheniformis strain JF‐2. J. Ind. Microbiol. 1990, 5, 95–101. [DOI] [PubMed] [Google Scholar]
- 35. Gong, M. , Wang, J. D. , Zhang, J. , Yang, H. et al., Study of the antifungal ability of Bacillus subtilis strain PY‐1 in vitro and identification of its antifungal substance (iturin A). Acta Biochim. Biophys. Sin. (Shanghai) 2006, 38, 233–240. [DOI] [PubMed] [Google Scholar]
- 36. Abouseoud, M. , Maachi, R. , Amrane, A. , Boudergua, S . et al., Evaluation of different carbon and nitrogen sources in production of biosurfactant by Pseudomonas fluorescens . Desalination 2008, 223, 143–151. [Google Scholar]
- 37. Segura‐Campos, M. , Chel‐Guerrero, L. , Betancur‐Ancona, D. , Hernandez‐Escalante, V. M. , Bioavailability of bioactive peptides. Food Rev. Int. 2011, 27, 213–226. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


