Abstract
Bienzymatic production of laminaribiose from sucrose and glucose was combined with adsorption on zeolite BEA to introduce a first capture and purification step. Downstream processing including washing and desorption steps was characterized and optimized on a milliliter scale in batch mode. Results were then transferred to a packed bed system for enzymatic production and adsorption where the influence of adsorbent particle diameter on purity and productivity was evaluated. Finally, a continuous enzymatic production of laminaribiose was conducted over 10 days. The subsequent downstream processing of the loaded zeolites led to purities of over 0.5 gLaminaribiose gsugar −1 in the desorbate with a total productivity of 5.6 mgLaminaribiose Lenzyme bed −1 h−1 without the use of recycles.
Keywords: Adsorption, Continuous packed bed reactor, Downstream processing, Laminaribiose, Zeolite BEA
Abbreviations
- DSP
downstream processing
- Fru
fructose
- GI
glucose isomerase
- Glc
glucose
- Lam
laminaribiose
- LP
laminaribiose phosphorylase
- RB
reaction buffer
- SB
Sørensen buffer
- SP
sucrose phosphorylase
- Suc
sucrose
1. Introduction
Laminaribiose, a 3‐β‐D‐Glucosyl‐D‐glucose disaccharide, has application in medicine, food, and agriculture fields and is considered as a building block for new pharmaceuticals 1, 2. The conventional synthesis method of laminaribiose is chemical hydrolysis of laminarin 2 through which disadvantages such as low production yields, high consumption of energy and water, demand for several organic solvents as well as need for more sophisticated regulating systems are encountered. Therefore, an enzymatic approach that provides a more cost efficient and biodegradable alternative is desirable 3. A trienzymatic synthesis method has been suggested by Kitaoka to produce laminaribiose from sucrose using native sucrose phosphorylase (SP), laminaribiose phosphorylase (LP) and glucose isomerase (GI) 4. However, GI could not be used in this multienzymatic system because it needed much higher temperature compared to the other two enzymes (SP and LP). More specifically, GI as a main enzyme in production of high fructose corn syrup (HFCS) is used at much higher temperatures. Furthermore, the required amount of glucose for this reaction system (which was initially supposed to be produced by GI) is produced via partial hydrolysis action of SP. But glucose is needed for start of the reaction and kinetics requirements and for that reason, glucose is added at the beginning of the reaction 5. Therefore, a bienzymatic reaction system using LP and SP immobilized on Sepabeads and encapsulated in chitosan with sucrose and glucose as substrates was applied to produce laminaribiose under mild conditions 6, 7. Although diffusion in chitosan is significantly slower than free diffusion 8, the impact on overall reaction rate is small 9 and thermal stability of chitosan‐based hybrid immobilizates is increased by a factor of 10 compared to native enzymes 7. Hence, in a previous work this biocatalyst system was used to set‐up an enzymatic synthesis in a continuous packed bed production system with an operational time of 10 days 10 but higher productivity in continuous operation comes with a lower titer and purity of laminaribiose compared to batch mode 7. Establishing an efficient and selective product recovery system is thus essential for industrial application of this process.
Adsorption is a well‐suited unit operation for this first product removal or capture step here due to high selectivity especially in multi‐component mixtures and the low operational temperature, thus avoiding hydrolysis of laminaribiose. Besides the use of zeolites in chromatographic columns for sugar separation 11, type BEA zeolites have shown a high selectivity between different carbohydrates 12 and have already been used to capture the disaccharide isomaltose in enzymatic reactions 13, 14, 15, 16. Moreover, commercially available BEA 150 zeolite extrudates have shown advantageous adsorption isotherms for the separation of laminaribiose against fructose and glucose as needed here 17, 18 whereas sucrose adsorbs even weaker than the solvent water 19. Successful combination of enzymatic production and adsorption with subsequent downstream processing has only been shown for a monoenzymatic production system where enzymatic catalysis and adsorption were integrated in a set‐up comprising of fluidized bed of biocatalysts and a slurry of zeolite particles 16, 20. In here, a bienzymatic production of laminaribiose is combined with adsorption in a system comprising of two solid phases (biocatalyst pearls and zeolite particles) and one fluid phase of reaction solution implemented in two consecutive fixed beds.
In this study, the combination of a continuous bienzymatic production of laminaribiose with an adsorption of laminaribiose in zeolite BEA using two consecutive packed bed columns is investigated. A feasible combination of washing and desorption steps to balance yield and purity in downstream processing of the loaded zeolites is implemented in batch experiments. After that, the results are transferred to packed bed columns of larger scale and the occurring challenges like the influence of zeolite particle size are addressed. Finally, a continuous production system is implemented to demonstrate the strengths of the proposed production system.
2. Materials and methods
2.1. Materials and buffers
All chemicals were of analytical grade and purchased from Carl Roth GmbH + Co. KG, Germany unless otherwise stated. Sodium hydroxide, sodium chloride and Chitosan were purchased from Sigma‐Aldrich Corporation. Sepabeads (Resindion type: EC‐EP/S) was purchased from Biokal, Netherlands.
Either double distilled water (for batch experiments) or deionized water (MilliQ‐Water) (for all other experiments) was used. 0.05 M phosphate buffer, referred to as reaction buffer (RB) in the following, was prepared by dissolving 7.1 g of sodium dihydrogen phosphate in 1 L of pure water and adjusting the pH value to 6 with sodium hydroxide. 0.3 M Sørensen phosphate buffer (SB) was prepared by dissolving 21.3 g of sodium hydrogen phosphate and 21 g of potassium dihydrogen phosphate in 1 L of water resulting in a pH of 7. Solutions with a concentration of 5 and 20% (v/v) ethanol in water were prepared for desorption. Laminaribiose phosphorylase (LP) was produced in our lab from Euglena gracilis based on the cultivation method described before 21. Sucrose phosphorylase (SP) was kindly donated by Bitop AG.
2.2. Immobilized enzymes
LP and SP were immobilized through a hybrid‐immobilization procedure developed in previous studies following a two‐pearl concept 6, 7, 10. In this procedure, a covalent linkage on Sepabeads (EC‐EP/S with 0.1–0.3 mm particle size) was performed first. For that purpose, 1 mL of native enzyme solution in 1 M sodium phosphate buffer at pH 7 was incubated with 0.1 g Sepabeads at room temperature for 24 h and then washed three times with reaction buffer (RB). Secondly, encapsulation in chitosan was achieved by suspending 0.1 g Sepabead‐enzyme in 30 mL of an acetic acid chitosan solution (1.5%) at pH 4.75 and mixing at 30°C in a water bath shaker. This homogenous slurry was then poured down into a pressurized cylinder where chitosan pearls were dropped out from nozzles at the bottom and stirred in sodium polyphosphate buffer (10%) at pH 6.5 for 20 min on ice. In the two‐pearl concept, the hybrid immobilizates of LP‐ and SP‐Sepabeads were encapsulated in separate chitosan pearls.
2.3. Zeolite preparation
Zeolite H‐BEA 150 extrudate with a module of 150 from Clariant International Ltd. were used (trade name CZB 150). To avoid a pH‐shift during use of protonated zeolites, a cation exchange was performed. For this purpose, 100 g of the protonated zeolites were mixed in 500 mL of a 0.2 M sodium chloride solution at 70°C and sodium hydroxide was added frequently until a constant pH of 6 was reached. Afterward, zeolites were washed twice with 1 L pure water to remove chloride ions. The first washing step was done at 4°C for 5 min and the second step at 70°C for 1 h. Zeolites were then dried at 80°C for 16 h and activated at 400°C in ambient atmosphere for 4 h.
The rod‐shaped extrudates had a uniform width of 1.4 mm and a mean length of (4.1 ± 1.4) mm. As crushing has no influence on the adsorption equilibrium of BEA 150 22, extrudates were crushed with mortar and pestle and classified in five fractions from 200 to 400 μm, 400 to 650 μm, 650 to 800 μm, 800 to 1000 μm and 1000 to 1600 μm by sieving. These particles were then applied consecutively during a continuous operation in five different beds. The maximum particle size of each fraction was used to calculate the ratio of column diameter (30 mm) to particle diameter (d c/d p) resulting in 75, 50, 37.5, 30, and 18.8, respectively. The wall effect in packed beds can be neglected when the ratio d c/d p is above 7 23, which is the case here for all five fractions.
Purity, P of laminaribiose was calculated as gram of laminaribiose per gram of all sugars:
| (1) |
with the concentration of the respective sugar, c. Purity ratio was then calculated based on the purity of laminaribiose in the fraction from last desorption step compared to the purity of laminaribiose in the reaction solution.
In order to measure the total porosity of the packed zeolite bed in the columns, a volumetric and weighing test was performed for 30 g of zeolites in the glass column as used in the continuous experiments. Water was pumped slowly to the column until the surface of zeolite bed was covered and all zeolite particles were completely soaked. Afterwards, the column outlet was opened to let the excessive water exit the column while collected in measuring cylinder. By deducting this eluted volume from pumped volume, the water holdup by this 30 g zeolite was determined to be (22 ± 1) mL. Furthermore, measuring dry and wet zeolite after this test showed 21.1 g difference. Therefore, a mean volume of 22 mL was considered resulting in 0.73 mL liquid per gram zeolite.
2.4. Washing and desorption procedure
In batch experiments, washing and desorption were performed in 5 mL micro reaction tubes (Eppendorf AG). Solid/liquid separation was done by decanting before every washing and desorption step. Zeolites were then mixed manually with 2 mL washing solution per gram zeolite (dry mass). Desorption steps were conducted with 1 mL eluent per gram zeolite in a shaking incubator (3032 from GFL Gesellschaft für Labortechnik mbH). Samples of 300 μL were regularly taken.
In fixed bed experiments, washing was done directly in the column. Therefore, 2 mL washing solution per gram zeolite was pumped through the column with a washing time of 5 or 20 min. Then, desorption was performed in 500 mL laboratory glass bottles (DWK Life Sciences) using a TER 2 heating bath with magnetic stirrer (IKA Werke GmbH & Co. KG). The specifications of the different downstream processing (DSP) procedures are listed in Table 1.
Table 1.
Overview of downstream processing procedures
| DSP procedure | A | B | C | D |
|---|---|---|---|---|
| Scale | Batch | Batch | Packed bed | Packed bed |
| Washing | Two times | Five times | Three times | Three times |
| SB / RB | SB | SB | SB | |
| 2 mL g−1 for 1 min | 2 mL g−1 for 1 min | 2 mL g−1 for 5 min / 20 min | 2 mL g−1 for 20 min | |
| Pre‐desorption 1 | Pure water | Pure water | Pure water | Pure water |
| 1 mL g−1 for 22 h | 1 mL g−1 for 22 h | 2 mL g−1 for 1 h | 1 mL g−1 for 1 h | |
| Pre‐desorption 2 | See above | See above | Pure water | Pure water |
| 2 mL g−1 for 2 h | 1 mL g−1 for 2 h | |||
| Final desorption | 5% ethanol | 5% ethanol | ‐ | 20% ethanol |
| 1 mL g−1 for 22 h | 1 mL g−1 for 22 h | 1 mL g−1 for 18 h |
All washing steps were carried out at 4°C, pre‐desorption and final desorption at 50°C.
The yield, Yi in each step i of DSP was calculated as mass of laminaribiose exiting this and all following streams until the last step p divided by the mass of laminaribiose exiting with all streams:
| (2) |
with the mass calculated from the concentration c of laminaribiose and the volume Vk of an exiting stream k. Assuming that all sugars were desorbed in the last desorption step p, a theoretical purity P theo, i of a washing or desorption step i was defined as sum of laminaribiose mass exiting with all elution solutions from i to p divided by the sum of all sugar masses exiting those streams:
| (3) |
2.5. Batch production of laminaribiose
Batch production and adsorption of laminaribiose was conducted in 5 mL cylindrical micro‐reaction tubes with L × ID = 55 mm × 15 mm. To prepare the substrate solution, sucrose and glucose were added to the reaction buffer with final concentrations of 0.25 M and 0.15 M respectively. 0.8 g of LP immobilizates together with 0.8 g of SP immobilizates were incubated with 0.65 g of zeolites in 3.2 mL of substrate solution up to 160 h at 35°C in a shaking incubator. For desorption, zeolites were manually separated from the enzyme immobilizates after solid/liquid separation.
2.6. Continuous production and adsorption of laminaribiose in packed bed reactors
A cylindrical glass column packed bed reactor with dimensions L × ID = 51 mm × 30 mm packed with 25 g of immobilizate (36 mL bed volume, packing density 0.7 g mL−1) and LP:SP ratio of 2 was used to continuously produce laminaribiose. Substrate solution comprising of sucrose 0.2 M and glucose 0.04 M in reaction buffer was pumped upward through the packed bed using a peristaltic pump (ISMATEC model: ISM831C) at a flow rate of 0.1 mL min−1 resulting in a superficial bed velocity of 0.14 mm min−1. The whole set up was operated in a temperated chamber at 35°C 10.
A glass column with dimensions L × ID = 70 mm × 30 mm packed with 30 g zeolite BEA of different particle size (packing density 0.6 g mL−1) was used to continuously adsorb the produced laminaribiose (Fig. 1).
Figure 1.
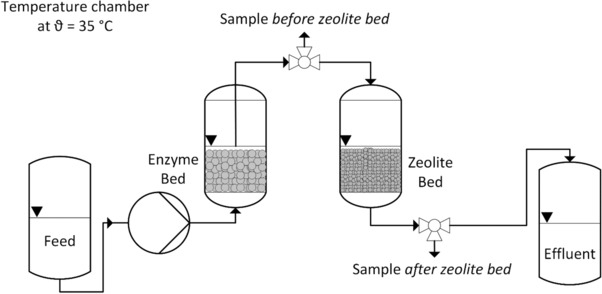
Schematic of the serial set up for production and adsorption of laminaribiose in a packed bed reactor.
Operation started with only enzymatic reactor in line and continued this way for 24 h until steady state was reached. The first zeolite bed was then implemented in line and after specified durations of time, a new freshly packed zeolite column replaced this zeolite column. These exchanges continued until end of operation while in the meantime, used zeolite batches were processed further through washing and desorption procedure (Section 2.4).
The normalized concentrations after zeolite bed was calculated using concentrations upstream the zeolite bed at process time t as inlet concentrations and after the bed at process time t + 4 h as outlet concentration since the residence time in the zeolite bed was approximately 4 h:
| (4) |
2.7. Analysis
For batch experiments, concentrations of sucrose, glucose, fructose and laminaribiose were determined using an YMC‐Pack Polyamine II column at 35°C with acetonitrile/water (75/25 (v/v)) as eluent and a Shodex RI‐101 detector.
Concentration of sucrose, glucose, fructose and laminaribiose in continuous experiments were measured by anion exchange chromatography using pulsed amperometric detection (HPAEC‐PAD). Carbopac PA1 (4 mm × 250 mm) column with a guard column Carbopac PA1 (4 mm × 50 mm) were used to perform the chromatography. A 41 min‐long gradient program comprising of 100 mM sodium hydroxide as the main eluent (A) and 1 M sodium acetate in 100 mM sodium hydroxide as co‐eluent (B) was used to elute sugars at 25°C using a gradient pump (SYKAM, model S2100) at a flow rate of 1 mL min−1. Gradient elution started with 100% (A) and after 1 min, (B) was increased to 20% over 6.3 min and stayed so until minute 16. Over the next 20 min, (B) was lowered to zero and 100% (A) was applied again until the end. Samples were filtered using 0.22 μm filters before injection.
3. Results and discussion
First, zeolites are loaded in a bienzymatic batch process to gain a representative mixture of laminaribiose, fructose, glucose and sucrose adsorbed on zeolite BEA and the subsequent downstream processing of these loaded zeolites is investigated. Second, the established protocol is transferred to the larger scale of fixed bed adsorption. Finally, continuous enzymatic production of laminaribiose is combined with adsorption on zeolite BEA as first capture and purification step.
3.1. Optimization of washing and desorption procedure
Laminaribiose was produced in batch experiments to gain a representative mixture of sugars adsorbed on zeolite BEA, see 7 for details. The loaded zeolites were then washed and desorbed with DSP A (two washing steps, two pre‐desorption steps and one final desorption) to evaluate the influence of the washing buffer.
The yield, Yi and theoretical purity P theo, i of a washing or desorption step i of DSP are calculated based on Eqs. (2) and (3) respectively. These values are shown in Supporting Information Fig. 1 together with the concentrations of glucose, fructose, sucrose and laminaribiose in both washing solutions. Initially, theoretical purities of 0.29 gLam gsugar −1 and 0.25 gLam gsugar −1 were achieved for zeolites washed with reaction buffer (RB) and Sørensen buffer (SB), respectively. The use of RB increased the theoretical purity by 26% to 0.37 gLam gsugar −1 in two washing steps. SB led to a higher relative increase in theoretical purity of 32% to 0.33 gLam gsugar −1. This is in line with the observation that ions, which strengthen hydrophobic interactions like Na+, K+ or PO3 2− lead to an increased adsorption of carbohydrates on zeolites 24 as those ions are present in a higher concentration in SB. Hence, SB was used for further downstream processing (DSP B, DSP C and DSP D).
Since high concentrations of sucrose, glucose and fructose remained in the eluent after two washing steps, washing procedure was extended to five washing steps. Corresponding concentrations of sugars in eluent solutions using DSP B (five washing steps, two pre‐desorption steps and one final desorption) are shown in Fig. 2 A and B. Here, theoretical purity increases from initially 0.48 gLam gsugar −1 to 0.70 gLam gsugar −1. The eluted quantity of sucrose, glucose, fructose and laminaribiose decreases with every washing step. Concurrently, increase in theoretical purity decreases, too. With the first three washing steps, theoretical purity increased by approximately 10% each step, but with fourth and fifth washing step, purity increased less than 5% each. As the first desorption step leads to significantly higher concentrations of glucose, fructose, sucrose and laminaribiose than the fourth washing step and increases theoretical purity again by more than 10%, three washing steps were found to be sufficient for the following packed bed experiments (DSP C and DSP D).
Figure 2.
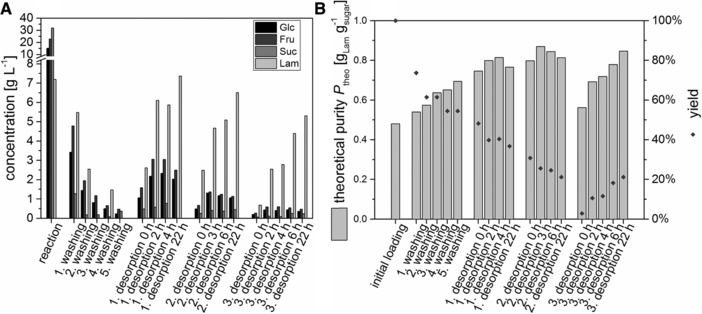
(A) Concentration of glucose, fructose, sucrose and laminaribiose in eluent solutions during five times washing with 2 mL SB per gram zeolite, two desorption steps with 1 mL pure water per gram zeolite and final desorption with 1 mL 5% ethanol per gram zeolite. (B) Corresponding yields and theoretical purity of laminaribiose.
Concentrations in different desorption steps show that purity of laminaribiose is strongly influenced by co‐desorption of fructose and glucose especially for the first two desorption steps in pure water (Fig. 2B). Hence, it seems reasonable to evaluate the first two steps within the meaning of pre‐desorption steps to improve the final purity in the last desorption step. Then, it can be seen that laminaribiose desorbs slower than the other sugars with the highest concentration of laminaribiose measured after 22 h. Hence, theoretical purity is 0.81 gLam gsugar −1 after 2 h of the first desorption step and decreases to 0.77 gLam gsugar −1 after 22 h as more laminaribiose is lost in the eluent of the pre‐desorption with increasing desorption time. The same is true for the second desorption step where theoretical purity is increased to 0.87 gLam gsugar −1 after 3 h of desorption and then decreases to 0.81 gLam gsugar −1 after 22 h (Note that the starting point of the second pre‐desorption is a theoretical purity of 0.77 gLam gsugar −1 after 22 h of desorption in the first pre‐desorption.) Hence, pre‐desorption to remove sucrose, fructose and glucose is carried out for approximately 2 h in the packed bed experiments (DSP C and DSP D). The final desorption step with 5% ethanol solution desorbed remaining sugars. Here again 22 h were needed for complete desorption equilibrium of laminaribiose.
While most findings from batch experiments like the ratio of eluent to zeolite or desorption times and temperatures can readily be transferred to downstream processing of packed beds, the proper implementation of washing needs special attention. This is due to two facts. First, the concentrations of glucose and especially sucrose in the reaction solution are much higher than in batch experiments whereas the concentration of laminaribiose is much smaller (by a factor of app. 25). This increases the influence of washing on final purity. Second, the hydrodynamic mixing behavior is different in packed beds while the objective to provide sufficient contact time for the exchange between washing solution and interstitial liquid but as short as possible to avoid desorption stays the same. Hence, DSP C (three washing steps, two desorption steps) uses two different washing times of 5 and 20 min for two packed beds each. Sucrose is used as marker for dilution during different steps since it showed no measurable adsorption. A contact time of 5 min is not enough as the concentration of sucrose even increases by a factor of 4 in the first desorption step (Supporting Information Fig. 2). In contrast, a contact time of 20 min enables a smooth dilution by a factor (cSuc,i/cSuc, i ‐1) of 0.33 ± 0.09 over all washing and desorption steps which is in line with batch experiments (dilution factor of 0.49 ± 0.26 in five washing steps) and the expected dilution factor of 0.26 (V holdup/(V holdup + V wash)). Note that the dilution factor in batch shows a higher standard deviation due to the limit of quantification for sucrose in the HPLC system used. As the required contact time for washing is larger and desorption in batch already took 22 h, ethanol concentration was increased to 20% in the final desorption step of DSP D, to increase the driving force. In conclusion, the final downstream processing procedure DSP D (three washing steps, two pre‐desorption steps and one final desorption) is specified for downstream processing of packed beds and used in the following.
3.2. Influence of particle size on adsorption of laminaribiose in packed beds
At the first 24 h of continuous operation, only the enzymatic reactor is operated until steady state and the maximum concentration of laminaribiose are reached. However, there is an average decline of 32% in laminaribiose production by the bienzymatic systems presented here (0.37 g/L) compared to the previous study (0.55 g/L) by Abi et al. because of partial decay in enzyme activity due to storage 10.
After establishing the enzymatic reaction and the DSP of the zeolites, columns packed with zeolite extrudates (4 mm) were used in several batches for different operating times in continuous production and adsorption. The breakthrough curves of first packed beds with mean zeolite particle size of 4 mm showed clear dispersion as identified by observing high concentration in the initial effluent of most beds (Supporting Information Fig. 3). This was considered to be due to inefficient contact between liquid and zeolite particles since at the same time, obvious channeling through these beds was observed too. To address this problem and to evaluate the influence of zeolite particle size on adsorption efficiency, different zeolite particle sizes were tested, see Section 2.3.
DSP D was used for washing and desorption of these beds. As depicted in Fig. 3, the highest increase in purity (calculated based on Eq. (1)) of laminaribiose is achieved using mean zeolite particle size of 1300 μm with a 78.2 fold improvement in laminaribiose purity.
Figure 3.
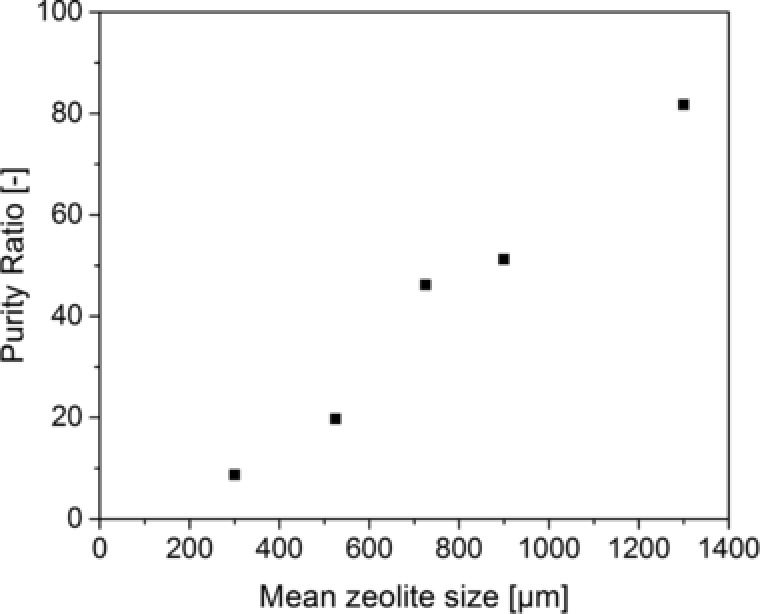
Ratio of purity of laminaribiose in last eluent and reaction solution for different particle sizes of zeolite using DSP D.
Better results achieved for bigger particle sizes can be explained as follows. Using small particles with a size distribution could cause blocking of channels inside the bed or macropores in the zeolite extrudates whereas this effect is expected to be not very pronounced when using larger particles. This is supported by the fact that relatively high masses of glucose and sucrose are carried over from the washing steps, that are conducted in the packed beds, into the desorption steps for small particles. This leads to relatively high concentrations of glucose and sucrose in the last eluent and thus to poor purities of laminaribiose. Hence, optimum conditions can be defined using the 1300 μm fraction zeolite and DSP D. A stable use of these conditions with six beds in a 10‐day‐long continuous production and adsorption of laminaribiose is shown in the next section.
3.3. Continuous production of laminaribiose and adsorption in packed beds using optimal conditions over 10 days
Figure 4 presents results of the optimized continuous production over 10 days with six zeolite beds.
Figure 4.
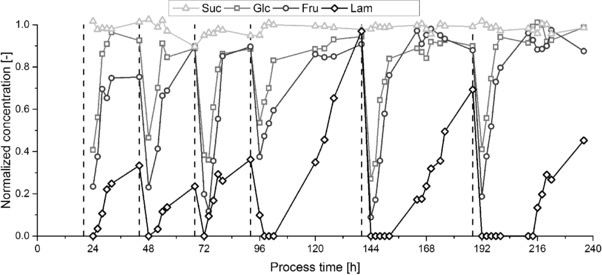
Normalized concentration of sucrose (light grey open triangles and line), glucose (gray open squares and line), fructose (dark gray open circles and line) and laminaribiose (black open diamonds and line) after the zeolite bed over process time (lines as guide to the eye). Dashed vertical lines indicate integration of a fresh zeolite bed. ϑ = 35°C, c suc,inlet = 200 mM, c glc,inlet = 40 mM, d p = 1300 μm.
A good stability of the process and the expected order of breakthrough with an immediate breakthrough of sucrose followed by glucose, fructose and laminaribiose can be seen.
Similar behavior throughout the adsorption process is visible for all of adsorption fractions in Fig. 4. The very good stability of the process is also represented in Fig. 5 where the first three beds with a total bed‐operating time of 24 h each and the last three beds with a maximum bed‐operating time of 48 h each are averaged. The differences in breakthrough behavior become more visible, too. Normalized concentration of each component, c*i, after zeolite bed is calculated based on Eq. (4). Glucose shows a normalized concentration of about 0.4 in the first effluent after 4 h and reaches a plateau after 12 h. Normalized fructose concentration is about 0.2 in the first effluent. The breakthrough curve is approximately 2 h shifted to longer bed‐operating times compared to glucose but otherwise similar. The breakthrough of laminaribiose is more complex and differs for the two different groups of zeolite beds. For the first 96 h (comprising of three beds), the normalized concentration is zero after 4 h bed‐operating time and increases to about 0.2 after 10 h. Then, the increase is slower until a concentration of 0.3 is reached after 24 h. For the second group of beds, no laminaribiose is detectable at the zeolite column effluent up to 12 h of bed‐operating time. Then, normalized concentration is about 0.1 after 24 h and increases steadily to 0.6 after 36 h. One reason for this different behavior for laminaribiose is the decrease in the inlet concentration of laminaribiose due the loss of activity of the enzymes. The inlet concentration after 24 h bed‐operating time is rather constant for the first three beds (c = 0.29 ± 0.02 g L−1) and decreases afterward to 0.22, 0.15 and 0.07 g L−1for the fourth, fifth, and sixth bed, respectively. However, no effect of inlet concentration of laminaribiose on normalized outlet concentration is expected as the concentration is still in the linear range of the isotherm according to 15, 25. Moreover, the changes in concentration of the other adsorbing components are too small to influence the adsorption of laminaribiose significantly. This may be attributed to the existence of a high‐energy binding site that was already detected for fructose and sorbitol on this zeolite extrudate 19. For fructose, the high‐energy binding site showed a maximum loading of q max = 1.2 mg g−1 with b ≈ 3 L g−1. If this binding site had similar properties for laminaribiose, it would explain the differences in breakthrough behavior for different inlet concentrations of laminaribiose as the linear range of this binding site is already left. With this limitation, all beds show a similar behavior and are thus treated similar in the following using DSP D.
Figure 5.
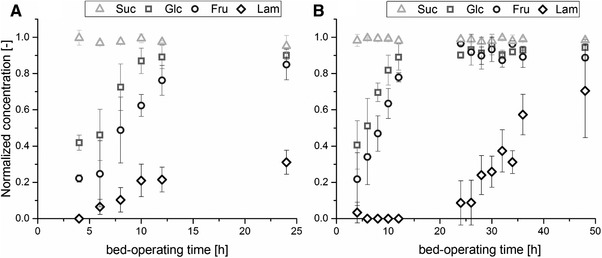
Normalized concentration of sucrose (light gray open triangles), glucose (gray open squares), fructose (dark gray open circles) and laminaribiose (black open diamonds) after the zeolite bed over bed‐operating time. First three beds (A) and last three beds (B) were averaged.
This subsequent downstream processing is shown exemplarily in Fig. 6 for the first zeolite bed. Obviously, the major impurities are sucrose and glucose in all steps whereas fructose does not account substantially. Hence, the effects of the different steps of downstream processing are discussed with a focus on sucrose, glucose and laminaribiose.
Figure 6.
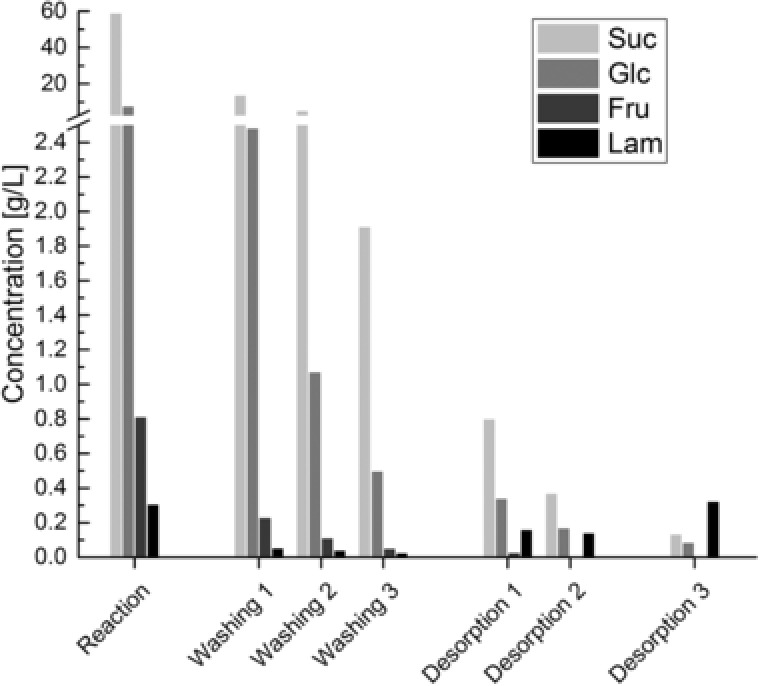
Concentration of sucrose, glucose, fructose, and laminaribiose in the column inlet (reaction) and after different steps of downstream processing following DSP D. Note the break of the concentration‐axis.
For that purpose, Table 2 additionally states the concentration ratio of sucrose to laminaribiose and glucose in each step as well as the concentration ratio of sucrose in each step to the previous step (dilution). As stated above, sucrose shows no measurable adsorption and can thus be used as marker for dilution during different steps. The three washing steps show an average dilution of 0.33 ± 0.08 which is in line with the expected value of 0.27. However, standard deviation is rather high, possibly due to difficulties in liquid handling, mixing and uncertainties in measurement of concentrations. Desorption steps show an average ratio of 0.41 ± 0.05 what is also in line with the expected value of 0.42. Overall, the results of sucrose fit well to the expected values for dilution and can be used as benchmark for the other substances that are additionally influenced by adsorption. The ratio of sucrose to laminaribiose decreases steadily from the first washing step to the last desorption. On the one hand, the decrease shows that laminaribiose is adsorbed and the washing procedure preferentially removes sucrose in terms of total mass thus increasing the purity in the last step. On the other hand, the steady decrease even in the washing steps indicates that laminaribiose already desorbs at these steps underlining the necessity to limit washing steps and to use high molar buffers at low temperatures. The ratio of sucrose concentration to glucose concentration decreases similarly. This indicates that the adsorption of glucose cannot be neglected and that the desorption steps in water (desorption 1 and 2) are necessary to remove adsorbed glucose and increase the purity in the last desorption step. However, this increase in purity comes with a decrease in total yield. Fig. 7 shows this relationship for the average of all six beds.
Table 2.
Ratios of sucrose concentration in a downstream processing step to the sucrose concentration in the previous step as well as to the laminaribiose and glucose concentrations in the same step for the different steps
| Step | c Suc, i +1/c Suc, i [‐] | c Suc, i/c Lam, i [‐] | c Suc, i/c Glc, i [‐] |
|---|---|---|---|
| Reaction | – | 191.88 | 7.43 |
| Washing 1 | 0.24 | 252.02 | 5.65 |
| Washing 2 | 0.39 | 130.82 | 5.10 |
| Washing 3 | 0.35 | 70.82 | 3.82 |
| Desorption 1 | 0.42 | 4.96 | 2.34 |
| Desorption 2 | 0.46 | 2.61 | 2.17 |
| Desorption 3 | 0.36 | 0.41 | 1.54 |
Figure 7.
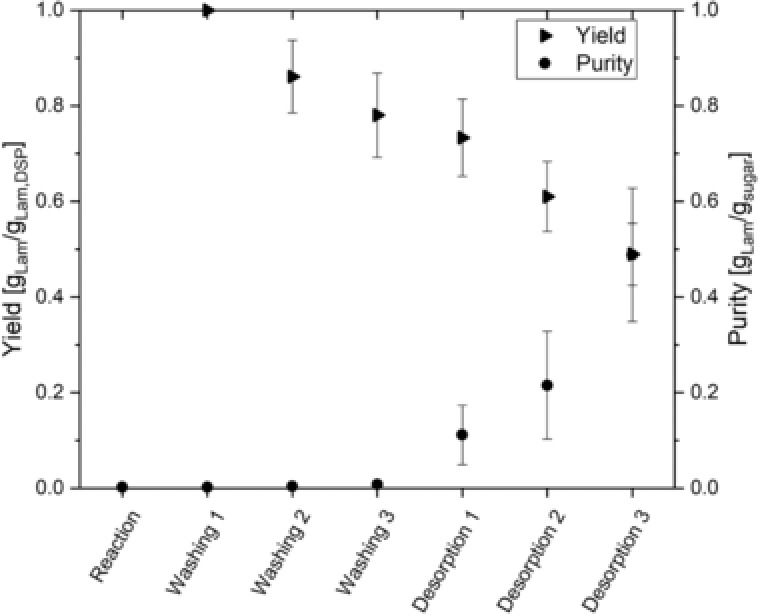
Yield (triangles) and purity (circles) in different steps of the downstream processing.
First, it is worth noting again that all six beds show a similar behavior in terms of yield and purity although the inlet concentration of laminaribiose changes by a factor of six from the first to the last bed. Second, the usual trade‐off between yield and purity can be seen and with the implemented downstream processing, about 45% of all laminaribiose exiting the downstream processing is in the last eluent and interstitial liquid with a purity of approximately 0.5 . With respect to the overall process, 58% of all laminaribiose produced is found in exiting streams of the downstream processing. This brings the total yield of downstream processing to 26%. This is a significant improvement considering the similar titer in reaction mixture and last desorbate and the increase of purity from approximately 0.0025 gLam gsugar −1 in the reaction mixture by a factor of 200 to 0.5 in the last desorbate. Moreover, an additional share of 10% of total laminaribiose is available for a rather simple recycle as it exists with the first and second desorbate with average purities of 0.11 and 0.22 , respectively and titer between 0.03 gLam L−1 and 0.15 gLam L−1 depending on zeolite bed. The total productivity of the process shows a maximum of 19.6 mgLam (Lenzyme bed h)‐1 for the first zeolite bed and an average of 5.6 mgLam (Lenzyme bed h)‐1 for all six beds.
4. Concluding remarks
Adsorption‐integrated production of laminaribiose from sucrose and glucose in a bienzymatic system of LP and SP was implemented in batch and continuous processes. Zeolite BEA was used to adsorb laminaribiose as the first capture and purification step. Particle sizes of zeolite and downstream processing were optimized. Continuous production and adsorption of laminaribiose in packed beds over 10 days showed acceptable stability with similar adsorption behavior for six beds. Downstream processing led to a 200‐fold increase in laminaribiose purity with a similar titer compared to the reaction solution and a yield of 26%. In summary, 1.3 g laminaribiose per liter enzyme bed were produced with a purity of 0.5 in 10 days.
Future work will investigate the implementation of recycle streams. Especially, the first two desorption steps yield rather high titer and purities of laminaribiose and are therefore predestinated for recycle. With these recycles, a higher number of washing steps will allow for high purities of laminaribiose already in the first desorbate.
Nomenclature
| b | [L g−1] | Langmuir parameter |
| c | [g L−1] | concentration |
| c* | [‐] | normalized concentration |
| d | [mm] | diameter |
| ID | [mm] | inner diameter |
| L | [mm] | length |
| P | [g g−1] | purity |
| q | [mg g−1] | loading |
| V | [mL] | volume |
| Y | [‐] | yield |
Greek symbols
| ϑ | [°C] | temperature |
Indices
| i,m | step | |
| k | exiting stream | |
| c | column | |
| p | particle | |
| Fru | fructose | |
| Glc | glucose | |
| Lam | laminaribiose | |
| max | maximum | |
| Suc | sucrose | |
| theo | theoretical |
Practical application
Laminaribiose is a high value oligosaccharide with versatile applications, e.g. in pharmaceutical industry. Conventional chemical synthesis of this sugar has many disadvantages and leads to a current market price for laminaribiose of 3200 Euros per gram. Therefore, an alternative, sustainable production without current disadvantages is of interest. This is achieved by a continuous bienzymatic reaction in packed bed reactors in combination with an adsorption on zeolite BEA to selectively recover laminaribiose from the sugar mixture in the reactor outlet. Development and optimization of downstream processing of loaded zeolites is crucial to reach high purities and balance the trade‐off between purity and yield. Downstream processing steps including washing and desorption are characterized and optimized. As a result, an optimum and operationally stable continuous production and adsorption system for laminaribiose is developed from which laminaribiose solutions with a purity of 50% are achieved in a scale of several hundred milligrams per day.
The authors have declared no conflict of interest.
Supporting information
Supporting Information
Acknowledgments
This work was supported by the German Research Foundation (DFG) under grant no. SCHO 842/9‐2 and JO 355/3‐2.
5 References
- 1. Ten Bruggencate, S. J. , Bovee‐Oudenhoven, I. M. , Feitsma, A. L. , van Hoffen, E. , Schoterman, M. H. , Functional role and mechanisms of sialyllactose and other sialylated milk oligosaccharides. Nutr. Res. 2014, 72, 377–389. [DOI] [PubMed] [Google Scholar]
- 2. Giese, E. C. , Covizzi, L. G. , Dekker, R. F. H. , Monteiro, N. K. et al., Enzymatic hydrolysis of botryosphaeran and laminarin by β‐1,3‐glucanases produced by Botryosphaeria rhodina and Trichoderma harzianum Rifai. Process Biochem. 2006, 41, 1265–1271. [Google Scholar]
- 3. Ogawa, Y. , Noda, K. , Kimura, S. , Kitaoka, M. , Wada, M. , Facile preparation of highly crystalline lamellae of (1→3)‐β‐d‐glucan using an extract of euglena gracilis. Int. J. Biol. Macromol. 2014, 64, 415–419. [DOI] [PubMed] [Google Scholar]
- 4. Kitaoka, M. , Sasaki, T. , Taniguchi, H. , Conversion of sucrose into cellobiose using sucrose phosphorylase, xylose isomerase and cellobiose phosphorylase. Denpun Kagaku 1992, 39, 281–283. [Google Scholar]
- 5. Müller, C. , Immobilisierung von Enzymen für die multienzymatische Synthese von Laminaribiose. Dissertation, 2018.
- 6. Müller, C. , Ortmann, T. , Abi, A. , Hartig, D. et al., Immobilization and characterization of E. gracilis extract with enriched laminaribiose phosphorylase activity for bienzymatic production of laminaribiose. Appl. Biochem. Biotechnol. 2016, 182, 1–19. [DOI] [PubMed] [Google Scholar]
- 7. Müller, C. , Hartig, D. , Vorländer, K. , Sass, A.‐C. et al., Chitosan‐based hybrid immobilization in bienzymatic reactions and its application to the production of laminaribiose. Bioprocess Biosyst. Eng. 2017. [DOI] [PubMed] [Google Scholar]
- 8. Hartig, D. , Hacke, S. , Scholl, S. , Concentration‐dependent diffusion coefficients for fructose in highly permeable chitosan polymers. Chem. Eng.Technol. 2018a, 41, 454–460. [Google Scholar]
- 9. Hartig, D. , Hacke, S. , Ott, L. , Gabrielczyk, J. et al., Diffusion studies of glucose and sucrose in chitosan membranes and beads for enzymatic production processes. Chem. Eng. Technol. 2018b, 41, 1433–1440. [Google Scholar]
- 10. Abi, A. , Wang, A. , Jördening, H.‐J. , Continuous laminaribiose production using an immobilized bienzymatic system in a packed bed reactor. App. Biochem. Biotechnol. 2018, 10.1007/s12010-018-2779-2. [DOI] [PubMed] [Google Scholar]
- 11. Kuhn, R. C. , Filho, F. M. , Separation of fructooligosaccharides using zeolite fixed bed columns. J. Chromatogr. B 2010, 878, 2023–2028. [DOI] [PubMed] [Google Scholar]
- 12. Buttersack, C. , Fornefett, I. , Mahrholz, J. , Buchholz, K. , Specific adsorption from aqueous phase on apolar zeolites. Stud. Surf. Sci. Catal. 1997, 105, 1723–1730. [Google Scholar]
- 13. Berensmeier, S. and Buchholz, K. , Separation of isomaltose from high sugar concentrated enzyme reaction mixture by dealuminated β‐zeolite. Sep. Purif. Technol. 2004, 38, 129–138. [Google Scholar]
- 14. Ergezinger, M. , Bohnet, M. , Berensmeier, S. , Bucholz, K. , Integrated enzymatic synthesis and adsorption of isomaltose in a multiphase fluidized bed reactor. Eng. Life Sci. 2006, 6, 481–487. [Google Scholar]
- 15. Holtkamp, M. , Erhardt, F. A. , Jördening, H. J. , Scholl, S. , Reaction‐integrated separation of isomaltose by ad‐ and desorption on zeolite. Chem. Eng. Process. 2009, 48, 852–858. [Google Scholar]
- 16. Holtkamp, M. , Scholl, S. , Adsorption properties of BEA zeolites and their aluminum phosphate extrudates for purification of isomaltose. Adsorption 2011a, 17, 801–811. [Google Scholar]
- 17. Waluga, T. , Scholl, S. , Adsorption of laminaribiose in an in‐situ product recovery process. In AIP Conf. Proc. 2011, 1453, 271–275. [Google Scholar]
- 18. Waluga, T. , Scholl, S. , Process design aspects for reaction‐integrated adsorption in multi‐enzymatic catalysis. Chem. Eng. Technol. 2015, 38, 1817–1826. [Google Scholar]
- 19. Hartig, D. , Schwindt, N. , Scholl, S. , Using the local adsorption equilibrium distribution based on a Langmuir type adsorption model to investigate liquid phase adsorption of sugars on zeolite BEA. Adsorption 2017, 23, 433–441. [Google Scholar]
- 20. Holtkamp, M. , Scholl, S. , Downstream processing for isomaltose following a reaction integrated adsorption. Chem.‐Ing.‐Tech. 2011b, 83, 191–194. [Google Scholar]
- 21. Abi, A. , Müller, C. , Jördening, H.‐J. , Improved laminaribiose phosphorylase production by Euglena gracilis in a bioreactor: a comparative study of different cultivation methods. Biotechnol. Bioprocess Eng. 2017, 22, 272–280. [Google Scholar]
- 22. Hartig, D. , Waluga, T. , Scholl, S. , Expanding the elution by characteristic point method to columns with a finite number of theoretical plates. J. Chromatogr. A 2015, 1413, 77–84. [DOI] [PubMed] [Google Scholar]
- 23. Mehta, D. and Hawley, M. C. , Wall effect in packed columns. Ind. Eng. Chem. Process Design Dev. 1969, 8, 280–282. [Google Scholar]
- 24. Wach, W. , Wechselwirkung von Kohlenhydraten mit Zeolithen vom Faujasit‐Typ. Dissertation, 1994.
- 25. Hartig, D. , Kluitmann, J. , Scholl, S. , An expanded Markham and Benton approach to describe multicomponent adsorption of sugars on zeolite beta. Microporous Mesoporous Mater. 2018, 273, 171–176. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


