Abstract
Salvia miltiorrhiza Bunge is an important herb for the treatment of cerebrovascular and cardiovascular diseases with bioactive compounds (phenolic acids and tanshinones). Abundant studies showed that tanshinones could be stimulated by biotic and abiotic stresses, but limited information is available on biosynthesis of phenolic acids promoted by biotic stresses. The aim of the present work was to isolate and identify rhizosphere bacteria which stimulated phenolic compound in Salvia miltiorrhiza hairy roots and investigated the internal mechanism, providing a potential means to enhance content of pharmaceuticals in S. miltiorrhiza. The results showed that six bacteria, namely, HYR1, HYR26, SCR22, 14DSR23, DS6, and LNHR13, belonging to the genus Pseudomonas and Pantoea, significantly promoted the growth and content of major phenolic acids, RA and SAB. Bacteria LNHR13 was the most effective one, with the contents of RA and SAB reaching ∼2.5‐fold (30.1 mg/g DW) and ∼2.3‐fold (48.3 mg/g DW) as those of the control, respectively. Phytohormones and polysaccharides produced by bacteria showed potential responsibility for the growth and biosynthesis of secondary metabolites of S. miltiorrhiza. Meanwhile, we found that the more abundant the types and contents of phytohormones, the stronger their stimulating effect on the content of salvianolic acids.
Keywords: Phenolic acids, Phytohormones, Polysaccharides, Rhizosphere bacteria, Salvia miltiorrhiza hairy root
Abbreviations
- CT
cryptotanshinone
- DT‐I
dihydrotanshinone I
- DW
dry weight
- ES
ethanol sediments
- GA
gibberellic acid
- IAA
indole‐3‐acetic acid
- iP
N6‐isopentenyladenine
- LSCSF
lipo‐soluble constituents of supernatant fluid
- PSF
polysaccharide fraction
- RA
rosmarinic acid
- SA
salicylic acid
- SAB
salvianolic acid B
- SF
supernatant fluid
- T‐IIA
tanshinone IIA
- T‐I
tanshinone I
- t‐Z7G
trans‐zeatin‐7‐glucoside
- t‐ZOG
trans‐zeatin‐O‐glucoside
- WSCSF
water‐soluble constituents of supernatant fluid
1. Introduction
Salvia miltiorrhiza Bunge (Lamiaceae), commonly known as Danshen, is an important herb in China 1. Its rhizomes, having been widely used in the treatment of cerebrovascular and cardiovascular diseases in China as well as other countries, contain two types of bioactive substances, namely, phenolic acids and tanshinones 2. SAB is a quality indicator of Danshen, and RA is the vital precursor for SAB biosynthesis, and they are two major phenolic acids in Danshen 3. Phenolic compounds have been shown to have significant bioactivities such as antioxidant, anti‐ischemia reperfusion, and antithrombotic effects 5. Tanshinones mainly include T‐I, T‐IIA, CT, DT‐I 4. Cardiovascular disease is one of major threats to the health of people around the world, accounting for approximately 30% of all deaths in United States in 2011 reported by the World Health Organization 6 Thus, demand for good quality Danshen is increasing, while quality degradation, high levels of pesticides and heavy metals are all detrimental to cultivated Danshen 7. Therefore, it is necessary to find efficient ways to enhance the content of medicinal substances in S. miltiorrhiza and adopt in vitro culturing of this plant.
Recently, S. miltiorrhiza hairy roots have been considered as a potential substitute for extracting medicinal components and are widely used in academic research 8. In addition, accumulation of secondary metabolites often occurs in many plants subjected to abiotic and biotic stresses, especially in defense responses of plants triggered by microbial pathogens and beneficial microbes 9. Rhizosphere microorganisms adhering to plant roots are directly affected by the activity of the corresponding plant. Different plants release different types and contents of organic carbon 10, thereby shaping the associated microbial communities, and these microbes in turn influence the host 11. Thus, rhizosphere microorganisms have the potential to induce the accumulation of secondary metabolites of S. miltiorrhiza. Moreover, most studies have shown that bacterial phytohormones play a significant role in these microorganism–plant interactions 12, 13, 14.
Recently, abundant evidence showed that tanshinones could be stimulated by biotic and abiotic elicitors, but so far only a few studies have been done on the biosynthesis of phenolic acids 15. In particularly, limited information is available on phenolic acids promoted by biotic elicitors. Moreover, most research in recent years has been carried out on enzyme‐molecular‐mechanisms in the promotion of secondary metabolite biosynthesis by microbes, rarely considering the role of metabolites produced by bacteria in this condition. Therefore, the objectives of the present study are to isolate rhizosphere microorganisms from S. miltiorrhiza to determine whether any of these microorganisms can stimulate phenolic compounds of S. miltiorrhiza, and also to investigate the role of metabolites produced by bacteria in such cases.
2. Materials and methods
2.1. Isolation and identification of rhizosphere microbes from the roots of S. miltiorrhiza
Fresh roots of biennial wild‐type S. miltiorrhiza Bunge were collected from their main natural habitat (Shaanxi Province, China) and transported to our laboratory for processing within 24 h of collection. The roots of S. miltiorrhiza were washed thoroughly in sterile distilled water to remove the soil and detrital material attached to the roots and then shaken on a mechanical gyrator shaker in sterile phosphate‐buffered saline 16. Finally, the samples were spread on Luria–Bertani (LB) 17 plates and placed on incubator at 28°C until the appearance of visible bacterial growth. The single colonies on LB plates were selected and subcultured onto a fresh LB medium until pure culture formed. The bacterial strains were identified by sequencing the 16S rRNA gene, which was carried out by GENEWIZ, Inc. (Suzhou, China). The phylogenetic tree based on the 16S rRNA gene sequences was constructed by the neighbor‐joining method using MEGA‐5 software.
2.2. Preparation of bacterial elicitor and hairy root cultures
The strains isolated from the roots of S. miltiorrhiza were kept in our microbial culture storage facility. The bacteria used for the experiments was prepared in a liquid beef extract peptone medium (pH 7.2) at 28°C in shaker flasks at 220 rpm for 3 d, reaching an optical density value of 1.0. After concentrated by centrifuge at 12 400 × g (RCF) for 10 min, the culture broth was filtered through a 0.22 μm sterile membrane to remove bacteria and the filtrate used as elicitors.
The S. miltiorrhiza hairy roots were obtained by infecting the aseptic plantlets with a Ri T‐DNA‐bearing Agrobacterium rhizogenes bacterium (ATCC15834). All experiments were performed in 250 mL Erlenmeyer flasks, which were placed on an orbital shaker at 25°C 110 rpm in the dark. Each flask was filled with 100 mL of the hormone‐free liquid 6, 7‐V medium (with 30 g/L sucrose) 18 and was inoculated with 0.3 g fresh weight of roots for 18 d in the middle or late growth phase of hairy roots as preculture. The preculture of hairy roots were treated by the bacterial elicitors at 1.5% (v/v) on the day 18. The control treatments were added to the same volume liquid beef extract peptone medium. The hairy roots were harvested on the 6th day after the treatment and cultured for an overall period of 24 d 19.
2.3. Measurements of root weight and metabolite content
The hairy roots were collected from culture flasks, washed with sterile distilled water, blotted dry by a paper towel, and dried in an oven at 45°C for 3 d to measure the dry weight. The dried root samples were ground into powder and extracted ultrasonically with methanol–water solution (10 mg roots mL−1; 7:3 v/v) for 45 min. The extract was filtered through a 0.45 μm membrane, and the filtrate was introduced to a high‐performance liquid chromatography (HPLC) system for the analysis of the secondary metabolites. Analysis followed the methods described by Xing 4. In short, the analysis was performed on a Waters HPLC system (Waters, Milford, MA, USA) using a ZORBAX SB‐C18 chromatographic column at 30°C, with solvent A (acetonitrile)/solvent B (0.02% phosphoric acid solution) gradient as the mobile phase with a flow rate of 1 mL/min, and with UV detection at 270 nm for the lipid‐soluble diterpenoids and 288 nm for the water–soluble phenolic acids. The quantification of secondary metabolites was performed by building of calibration curves. Empower 2 software was used for data acquisition and processing. The authentic standards were obtained from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China).
2.4. Isolation of the components in fermentation broth of strain LNHR13
SF, ES, WSCSF, LSCSF, PSF, were separated. As shown in Fig. 1, 60 mL fermentation broth of strain LNHR13 was concentrated under reduced pressure at 60°C to an appropriate volume, adding 4 volumes of 95% ethanol, precipitating at ‐20°C for one day. Then, the solution was centrifuged at 8600 × g (RCF) for 10 min, and the precipitate and supernatant fluid was collected, obtaining ES and SF. A part of SF was further extracted by ethyl acetate, obtaining WSCSF and LSCSF. A part of precipitate was further subjected to deproteinization with Sevage reagent (chloroform–n‐butanol 5:1, v/v), and small molecule impurities were removed by dialysis with a dialysis tubing whose differing molecular‐weight cutoffs is 2000 Da, obtaining PSF. The concentration of components in isolated fraction were regulated as same as that in fermentation broth. The components were inoculated to hairy root at 1.5% (v/v) on day 18 of the S. miltiorrhiza hairy root culture. Control treatments were added to the same volume of sterile distilled water.
Figure 1.
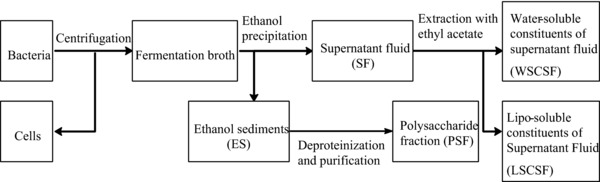
The way of isolating the components of fermentation broth of strain LNHR13.
2.5. LC–MS/MS analysis of the phytohormones in selected bacteria
The fermentation broth was concentrated and filtered through a 0.22 μm sterile membrane prior to liquid chromatography‐triple quadrupole mass spectrometry (LC–MS/MS) analysis. About 25 acidic and 18 alkaline phytohormones and their major metabolites were analyzed in this work, including auxins, abscisic acid, jasmonic acid, SA, cytokinins and GA; details are described by Cao et al. 20. The 43 standards of phytohormones and their metabolites were purchased from Olchemim Ltd. (Olomouc, Czech Republic). LC–MS/MS analysis followed the methods described by Cao et al. 20. In brief, the mass spectrometer was equipped with Surveyor HPLC system (Thermo Fisher Scientific, San Jose, CA, USA) consisting of a Surveyor LC pump, a solvent degasser and a Surveyor autosampler (injection volume 5 μL). The separations were performed by using a ZORBAX Extend‐C18 column (100 × 2.1 mm, 1.8 m; Agilent, Shanghai, China) at 30°C with a H2O (+5 mM ammonium formate) (A)/ MeOH (B) gradient (flow rate 150 μL/min). Samples were analyzed by using a gradient program as follows: 0–30 min, 10–45% MeOH; 30–35 min, 45–95% MeOH; and 35.1 min, 10% MeOH. The quantification and confirmation were performed on a TSQ Quantum Access Max (Thermo Fisher Scientific, Bremen, Germany), equipped with an HESI‐II heated electrospray ionization source. The optimized electrospray ion source (ESI) operating conditions were: needle spray voltage, (−) 2.2 kV/(+) 2.8 kV; skimmer offset, 3 V; sheath N2 gas pressure, 35 arbitrary units; auxiliary N2 gas pressure, 5 arbitrary units; ion transfer capillary temperature, 300°C; vaporizer temperature, 350°C, and collision Ar gas pressure, 1.5 mTorr. All of the analytes were monitored using the selected reaction monitoring mode. Xcal‐ibur (version 2.2, Thermo Fisher Scientific) and LCQuan software (version 2.6, Thermo Fisher Scientific) were used for instrument control, and data acquisition and processing.
2.6. Validation the effect of SA and IAA on the biomass and phenolic compounds synthesis in S. miltiorrhiza
Salicylic acid and indole‐3‐acetic acid were purchased from Sangon Biotech (Shanghai) Co., Ltd. (Shanghai, China). Concentrations of phytohormone treatment solutions were designed based on that of bacterial phytohormones. The concentrations of IAA were set as following: 0, 700, 3150, 4450, 12150, 71550 ng/mL, and that of SA were set as following: 0, 28, 35, 100, 200, 315 ng/mL. The treatment methods on S. miltiorrhiza and measurements of root weight and metabolite content were the same as described above.
2.7. Data analysis
All experiments, including controls and different treatments of hairy root cultures, HPLC analysis, LC–MS/MS analysis and validation were performed in triplicate. The data were processed using SPSS software ver. 22 (SPSS Inc., Chicago, IL). Significant differences were analyzed by one‐way analysis of variance (ANOVA). Differences were considered significant at p < 0.05, p < 0.01, and p < 0.001.
3. Results
3.1. Isolation and identification of rhizosphere bacteria with the ability to stimulate phenolic acids
Ninety‐six bacterial strains were isolated from the roots of S. miltiorrhiza plants on the basis of morphology roughly. The measurement of root weight and metabolite contents of S. miltiorrhiza hairy roots treated with the fermentation broth of these strains showed that strains HYR1, HYR26, SCR22, 14DSR23, DS6, and LNHR13 could significantly promote the production of phenolic acids.
Then, these strains were identified by sequencing 16S rRNA. As shown in Fig. 2, the strains HYR1, HYR26, SCR22, 14DSR23, DS6, and LNHR13 exhibited high homology (100, 99.93, 99.84, 98.66, 99.93, and 99.86%) with Pseudomonas chlororaphis subsp. aurantiaca NCIB 10068T, Pseudomonas chlororaphis subsp. aurantiaca NCIB 10068T, Pseudomonas chlororaphis subsp. piscium JF3835T, Pantoea rodasii LMG 26273T, Pseudomonas vancouverensis ATCC 700688T and Pseudomonas chlororaphis subsp. aureofaciens NBRC 3521T in the terms of 16S rRNA sequence, respectively. We deposited the corresponding sequences in the GenBank database with accession numbers of KX272886, KX272965, KX273059, KX273064, KX273060 and KY458549, respectively.
Figure 2.
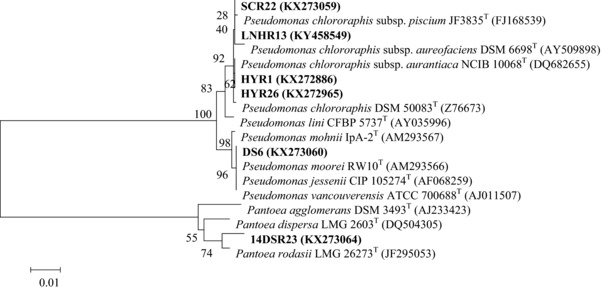
Phylogenetic tree based on the results of 16S rRNA sequence comparison.
P. rodasii and Pseudomonas groups have recently been considered as plant growth‐promoting rhizobacteria 21, 22.
3.2. Effects of rhizosphere microbes on the biomass and secondary metabolites of S. miltiorrhiza hairy roots
As shown in Fig. 3A, the selected strains significantly increased the dry weight of the hairy roots compared with the control, with the exception of DS6. The most effective strain was HYR1, which increased the biomass by ∼44.2% compared to that of the control.
Figure 3.
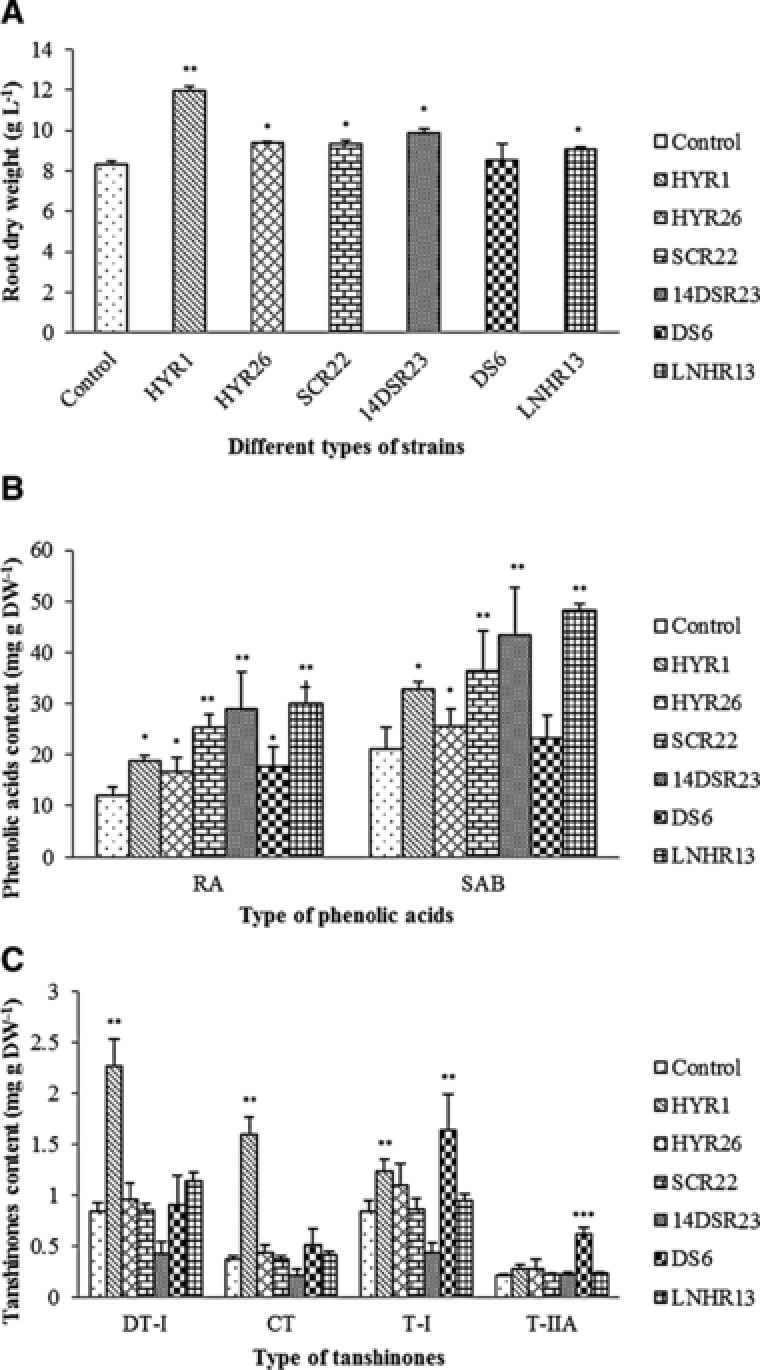
Effects of control and sterile fermentation broth of strains HYR1, HYR26, SCR22, 14DSR23, DS6, and LNHR13 on the growth (A) as well as accumulation of phenolic acids (B) and tanshinones (C) in S. miltiorrhiza hairy roots in 6 d. Values are presented as mean ± SD, n = 3. The asterisks indicate significant differences at p < 0.05, p < 0.01, and p < 0.001, marked as *, **, and ***, respectively.
The effects of these bacteria on the accumulation of phenolic acids in S. miltiorrhiza hairy roots are shown in Fig. 3B. In all strain treatments, the accumulation of RA and SAB in hairy roots was stimulated. The contents of RA and SAB subjected to strain HYR1 were 18.9 mg g DW–1 and 32.7 mg g DW–1, respectively, which were ∼57.0% and ∼54.0% higher than the corresponding levels of the control. Hairy roots treated with SCR22, 14DSR23 and LNHR13 showed ∼111%, ∼140% and ∼150% increases in RA content than the control, respectively, and ∼71.9%, ∼104.2% and 128% increases in SAB content than the control, respectively. In addition, both HYR26 and DS6 exhibited a slightly significant stimulating effect on RA and SAB accumulation. When considering the dry weight, it was evident that the yields of both RA and SAB increased. Furthermore, we performed an additional experiment to detect the relevant genes expression in biosynthesis pathway of the phenolic compounds in S. miltiorrhiza with the treatment of the most effective strain LNHR13, and the methods and results was shown in Supporting Information Fig. 1.
Tanshinones are another group of bioactive constituents in S. miltiorrhiza. Thus, another indicator of application of these bacteria is to determine their effects on the accumulation of tanshinones in S. miltiorrhiza hairy roots. As shown in Fig. 3C, DT‐I, T‐I, CT, and T‐IIA were stimulated by the treatment with strain HYR1, with contents reaching 2.7‐, 4.3‐, 1.5‐, and 1.3‐fold levels of those of the control, respectively. Strain DS6 exerted a significantly positive effect on stimulating the contents of T‐I and T‐IIA in hairy roots, with levels 1.9‐ and 2.9‐fold that of the control, respectively; strains HYR26, SCR22 and LNHR13 showed a slight effect on content of tanshinones. However, strain 14DSR23 significantly decreased the contents of T‐I, CT, and DT‐I in hairy roots.
3.3. Effects of isolated components of strain LNHR13 on the biomass and secondary metabolites of S. miltiorrhiza hairy roots
Recently, some previous researches have already study to improve tanshinones contents by microbes, including one rhizosphere bacteria (Bacillus cereus)[23], while limited information is available on phenolic acids promoted by biotic elicitors, so we treated S. miltiorrhiza hairy roots with isolated components of strain LNHR13 to evaluate its active component.
As shown in Fig. 4, the biomass of hairy roots was increased with treatment of SF, ES, WSCSF, LSCSF, and PSF, with the dry weight increasing by 8.1, 3.7, 5.9, 1.0, and 4.9%. Both SF and WSCSF exhibited a significant stimulating effect on RA and SAB accumulation, with the content of RA increasing 23.0% and 29.7% that of control, respectively, and the content of SAB increasing 46.8%, 48.4% that of control, respectively. PSF only significantly stimulated the content of SAB, with the increase rate reaching 49.1%. Other fractions had no significant effect on phenolic acids biosynthesis. In addition, all isolated fractions stimulated the biosynthesis of tanshinones. When taking the biomass into consideration, the effects of PSF and WSCSF were better than others on the increase of tanshinone contents per unit volume. The DT‐I, CT, T‐I and T‐IIA content under PSF treatment, reaching 4.7‐, 1.6‐, 2.6‐, and 2.7‐fold per unit volume that of control, respectively. The content of DT‐I, CT, T‐I, and T‐IIA in hairy roots with treatment of WSCSF were 3.4‐, 1.6‐, 2.6‐, 2.3 per unit volume fold, respectively, compared with the control.
Figure 4.
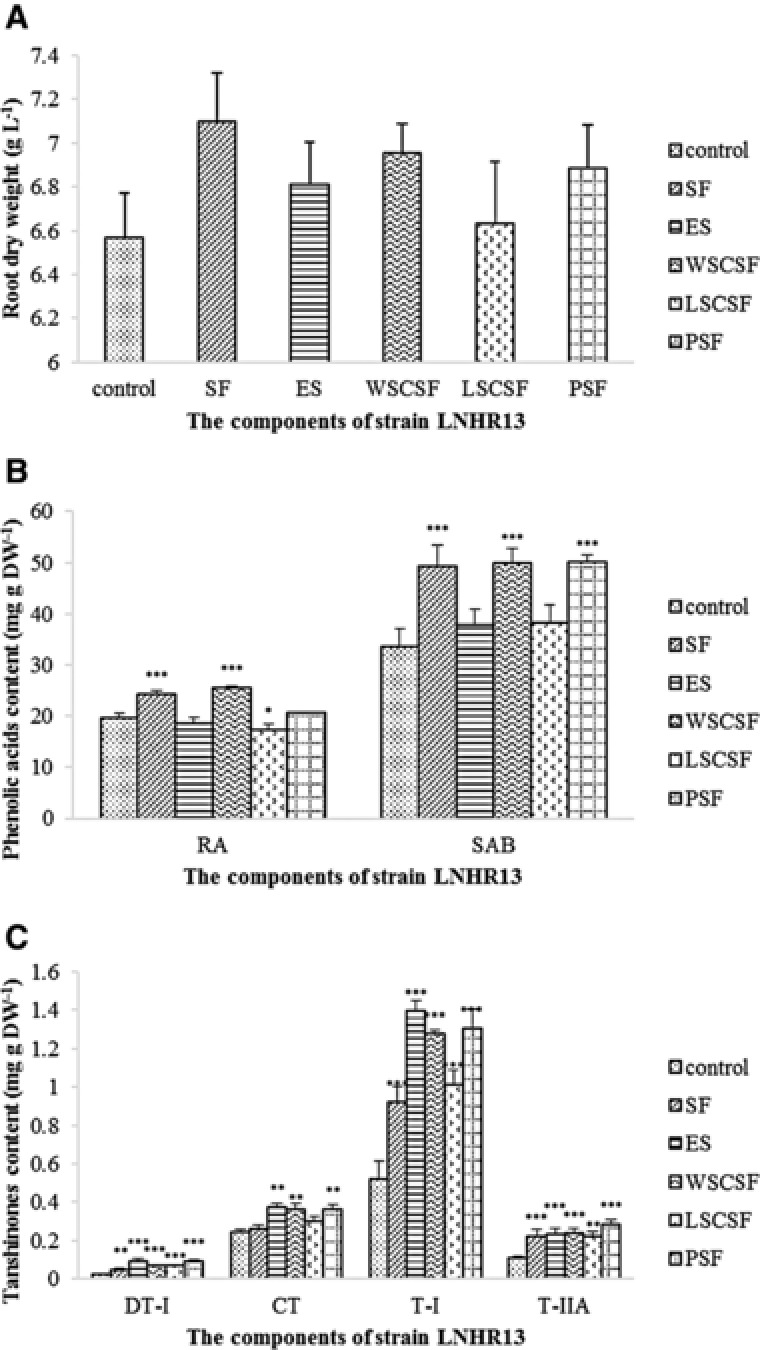
Effect of components in LNHR13 on the growth (A) as well as accumulation of phenolic acids (B) and tanshinones (C) in S. miltiorrhiza hairy roots in 6 d. Values are presented as mean ± SD, n = 3. The asterisks indicate significant differences at p < 0.05, p < 0.01, and p < 0.001, marked as *, **, and ***, respectively.
3.4. Identification and quantification of phytohormones in selected bacteria by LC‐MS/MS
In general, the substances in water‐soluble fraction were small molecular substances, and phytohormone which plays an outstanding role in regulation of plants growth and development was potential one among these substances. Taking this reason into consideration, we further investigated the phytohormones in fermentation broth of LNHR13 and its isolated components. The results are shown in Table 1. There were various phytohormones in fermentation broth, including IAA, iP, SA, t‐Z7G, t‐ZOG, GA12 and N 6‐isopentenyladenine‐7‐glucoside. The contents of IAA, SA and t‐ZOG were relatively higher than others, whose contents were 677.524, 27.313, and 88.027 ng/mL, respectively. The species of phytohormones in each isolated fraction were relatively less and content relatively lower than that in fermentation broth. In addition, the active fraction (fermentation broth, SF and WSCSF) for stimulating phenolic acids biosynthesis contained t‐ZOG, which does not exist in the non‐active one (LSCSF).
Table 1.
The phytohormones in components of fermentation broth of bacteria LNHR13 (ng/mL)
| Phytohormone | Fermentation broth | SF | WSCSF | LSCSF |
|---|---|---|---|---|
| IAA | 677.5 ± 52.1 | 27.4 ± 1.7 | 11.6 ± 1.8 | 11.4 ± 0.4 |
| iP | 10.0 ± 0.8 | N/Da | N/D | N/D |
| SA | 27.3 ± 0.7 | N/D | N/D | N/D |
| t‐Z7G | 4.1 ± 0.2 | N/D | N/D | N/D |
| t‐ZOG | 88.0 ± 5.9 | 6.0 ± 0.1 | 4.7 ± 0.3 | N/D |
| GA12 | 0.8 ± 0.1 | N/D | N/D | N/D |
| IP7Gb | 0.5 ± 0.1 | N/D | N/D | N/D |
Not detected;
N 6‐isopentenyladenine‐7‐glucoside.
To verify the role of phytohormones in phenolic acids biosynthesis, we investigated phytohormones in the fermentation broth of all selected strains, and the results are shown in Table 2. The phytohormones in selected bacteria included both acidic and alkaline phytohormones and their major metabolites. The common phytohormones were IAA, SA, t‐ZOG, indole‐3‐acetyl‐l‐phenylalanine, iP, t‐Z7G. IAA was found to be the most abundant of these phytohormones in all strains, ranging from 780.92 to 71543.05 ng/mL, with SA and t‐ZOG being the next most abundant phytohormones. The content of SA ranged from 33.619 to 88.027 ng/mL, and that of t‐ZOG ranged from 28.86 to 379.21 ng/mL.
Table 2.
The phytohormones in selected bacteria (ng/mL)
| Phytohormone | SCR22 | HYR1 | HYR26 | 14DSR23 | DS6 |
|---|---|---|---|---|---|
| dihydrozeatin‐9‐glucoside (DZ9G) | N/Da | N/D | N/D | N/D | 0.2 ± 0.0 |
| dihydrozeatin‐O‐glucoside (DZOG) | 0.1 ± 0.0 | N/D | N/D | N/D | N/D |
| IAA | 4433.3 ± 150.9 | 780.9 ± 20.2 | 3149.0 ± 107.8 | 12 140.9 ± 218.9 | 71 543.1 ± 472.2 |
| dindole‐3‐acetyl‐L‐alanine (IAA‐Ala) | N/D | N/D | N/D | N/D | 1.4 ± 0.1 |
| indole‐3‐acetyl‐L‐asparticacid (IAA‐Asp) | N/D | 4.5 ± 0.3 | N/D | N/D | 9.3 ± 0.3 |
| indole‐3‐acetyl‐L‐phenylalanine (IAA‐Phe) | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 1.8 ± 0.1 | 0.5 ± 0.0 |
| indole‐3‐acetyl‐L‐valine (IAA‐Val) | N/D | N/D | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.3 ± 0.0 |
| iP | 4.8 ± 0.2 | 3.8 ± 0.1 | 4.2 ± 0.2 | 6.2 ± 0.2 | 9.9 ± 0.4 |
| N 6‐isopentenyladenosine (iPR) | N/D | N/D | N/D | 0.5 ± 0.0 | 3.1 ± 0.1 |
| SA | 34.3 ± 1.3 | 28.9 ± 1.0 | 34.4 ± 1.3 | 379.2 ± 8.1 | 310.8 ± 12.9 |
| trans‐zeatin riboside (t‐ZR) | N/D | 0.1 ± 0.0 | N/D | N/D | N/D |
| t‐Z7G | 3.5 ± 0.2 | 2.8 ± 0.1 | 1.3 ± 0.1 | 18.5 ± 1.1 | 25.9 ± 1.0 |
| t‐ZOG | 59.1 ± 2.9 | 33.9 ± 1.7 | 45.7 ± 2.4 | 51.5 ± 2.8 | 58.8 ± 3.4 |
Not detected.
3.5. Effects of phytohormones on the biomass and phenolic acids of S. miltiorrhiza hairy roots
Considering IAA and SA with high concentration may play an important role in promotion of phenolic acids, while there were various substances in bacterial broth which may interfere the effect of IAA and SA, we performed further experiments for testing their effect alone. As shown in Fig. 5A, the biomass was increased by low concentrations of IAA (700‐12150 ng/mL) and its peak appeared at application of 700 ng/mL of IAA, while the biomass was inhibited by the high concentrations of IAA (71550 ng/mL). The effects of RA and SAB biosynthesis were shown in Fig. 5B and Fig. 5C. The applications of SA at the concentration of 28 to 315 ng/mL had an obvious effect on the accumulations of RA and SAB compared to the control, and the peak of phenolic acids appeared at 28 and 35 ng/mL.
Figure 5.
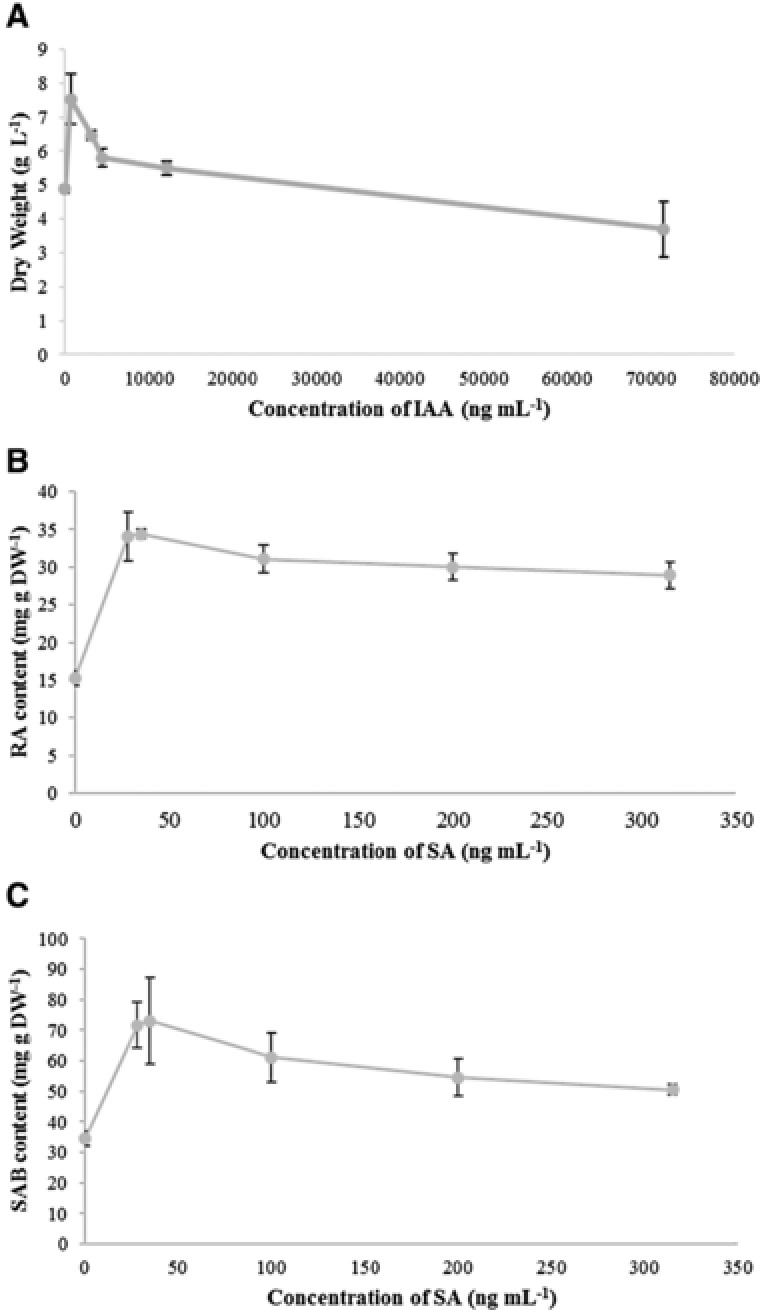
The effect of IAA and SA on the growth (A) as well as accumulation of RA and SAB in S. miltiorrhiza hairy roots. Values are presented as mean ± SD, n = 3.
4. Discussion
These results show that, with the exception of DS6, all strains could significantly promote the growth of S. miltiorrhiza hairy roots. A series of papers studied the possible mechanism of growth promotion. One of the main approaches to promoting growth by P. chlororaphis, P. rodasii, and P. vancouverensis is through production of IAA 24, 25, 26, 27. The detection of phytohormones in strain LNHR13 and its isolated components showed that the content of IAA was related the increase rate of biomass, the higher the content of IAA, the higher the increment of biomass. In addition, all these strains produced IAA. The contents of IAA in these strains were HYR1< HYR26< SCR22<14DSR23< DS6. With the increase of IAA concentration in these strains, the accordingly biomasses of hairy roots (except treated by14DSR23) increased at first and decreased at last, while all more than that of control. In general, IAA promotes plant growth at low concentrations and inhibits plant growth in high concentrations 28, therefore, the results above approximately fit the dose–response curve for IAA. And the result in the Fig. 5A also showed that IAA played an important role in the growth promotion. The content of IAA in DS6 was the highest among these strains, and it was putative that the content of IAA in DS6 reached the level of inhibiting the growth of hairy root, which was verified in Fig. 5A. In addition, there were no significant differences in weight between the SA treated group and the control, which was the same as in a previous study of S. miltiorrhiza hairy root 5. Meanwhile, iP and zeatin had positive activities on the growth of other plants 29, 30, so it was possible that they contributed to the growth of S. miltiorrhiza hairy root.
Meanwhile, the results showed that all six strains were able to increase the contents of RA and SAB in the hairy roots of S. miltiorrhiza. The increase rate of phenolic acids production induced by strain LNHR13 (the best strain) was less significantly than that by some signal molecules, such as ethylene 32 and SA 5, whereas better than other elicitors, such as heavy metal salts (Ag+) 4, nonionic surfactants (Tween 20 and Triton X‐100) 33 and yeast extract 34. Above all, the evidence about phenolic acids content promoted by biotic elicitors is rarely available, and even most of the biotic elicitors (both live microbes and constituents of microbial cells) inhibit the biosynthesis of phenolic acids 2, 17, 23, 31. So, it's reasonable to isolate and investigate the bioactive component in these bacteria. Regarding the results in the isolated components of strain LHNR13 and their effect on the biosynthesis of second metabolism in S. miltiorrhiza hairy root, it was speculated that the active ingredients which stimulated the content of phenolic acids were in WSCSF. In general, the substances in water‐soluble fraction were small molecular substances, including water‐soluble acid and phytohormones. In previous studies, GA 32, SA 5, ABA 32 and methyl jasmonate 35 had positive effects on the growth and secondary metabolism of Salvia miltiorrhiza, so we speculated that phytohormones are ones of the important active substances in WSCSF. In addition, in this study, comparing the relationship between the contents and species of phytohormones in fermentation broth of strain LNHR13 and its isolated components and their effect on the biomass and secondary metabolites of S. miltiorrhiza hairy roots, we found that the more abundant the types and contents of phytohormones, the stronger their stimulating effect on the content of salvianolic acids. One review summarized that the most likely reason of this phenomenon was that due to a synergistic or potentiating effect, combined using of different elicitors can be more effective than using single elicitor only 36. In addition, the active fraction for stimulating phenolic acids biosynthesis contained t‐ZOG, which was not exist in the non‐active one, indicating that t‐ZOG should play an important role in phenolic acids biosynthesis. As shown in Table 2, abundant phytohormones were detected in the selected strains and these strains produced SA at different concentrations. In previous studies, the application of 22.5 mg/L of SA had an obvious effect on the accumulations of SAB and RA 5, 37, 38, 39. Thus, we speculated that SA in selected strains plays an important role in the biosynthesis of phenolic compounds. The results in further experiment showed that SA treatment solutions at the concentration of 28 to 315 ng/mL had a greatly stimulation on biosynthesis of RA and SAB.
In addition to phytohormones, rhizosphere bacteria shall produce other compounds that influence the biosynthesis of pharmaceutical compounds or bioactive ingredients in S. miltiorrhiza hairy roots. Some studies reported that the polysaccharide fraction from Bacillus cereus 23 and Trichoderma atroviride 31 was the main active constituent of these microbes responsible for stimulating the biosynthesis of tanshinones in S. miltiorrhiza hairy roots. In this study, we found the PSF of LNHR13 significantly stimulated both phenolic acids and tanshinones in S. miltiorrhiza hairy roots. Obviously, polysaccharide fraction from LNHR13 was another active component to influence the biosynthesis of pharmaceutical compounds in S. miltiorrhiza hairy roots.
In conclusion, this paper reported biotic elicitors from bacteria that significantly promote the biosynthesis of phenolic acids. Most of the effective elicitors belong to the genus Pseudomonas, and different strains of the same species were found to exert varied effects on secondary metabolite production. Strain LNHR13 was the most effective stimulator of phenolic acids and strain HYR1 could significantly stimulate both the growth and secondary metabolite contents, so we can choose the suitable or specific elicitor dependent on the particular target compounds for promotion. For example, we can apply LNHR13 to induce hairy roots when RA or SAB is the target one, while choose HYR1 to increase the content of DT‐I. Significantly, limited information is available on phenolic acids promoted by biotic elicitors, so we explored the underlying mechanism, finding that bacterial phytohormones and polysaccharide fraction were two major active components to influence the biosynthesis of pharmaceutical compounds in S. miltiorrhiza hairy roots.
Practical application
Biotic stresses exhibit an outstanding effect on biosynthesis of secondary metabolites in medicinal plants. The present work was intended to isolate and identify rhizosphere bacteria (biotic stress) which was applied to stimulate biosynthesis of phenolic compounds in Salvia miltiorrhiza hairy roots and also to investigate the internal mechanism, providing a potential means to enhance content of medicinal substances in S. miltiorrhiza.
The authors have declared no conflict of interest.
Supporting information
Supporting Information
Acknowledgments
This research is supported by the Science Foundation of Zhejiang Sci‐Tech University (14042217‐Y), the Zhejiang Natural Science Foundation of China (LY15C010005 and LY16C030002) and the Zhejiang Province Public Agricultural Project (2014C32117).
5 References
- 1. Siu, K. C. , Wu, J. Y. Enhanced release of tanshinones and phenolics by nonionic surfactants from Salvia miltiorrhiza hairy roots, Eng Life Sci, 2014, 14, 685–690. [Google Scholar]
- 2. Yan, Y. , Zhang, S. C. , Zhang, J. Y. , Ma, P. D. , et al. Effect and mechanism of endophytic bacteria on growth and secondary metabolite synthesis in Salvia miltiorrhiza hairy roots, Acta Physiologiae Plantarum, 2014, 36, 1095–1105. [Google Scholar]
- 3. Zhang, Y. , Yan, Y. P. , Wu, Y. C. , Hua, W. P. , et al. Pathway engineering for phenolic acid accumulations in Salvia miltiorrhiza by combinational genetic manipulation, Metab Eng, 2014, 21, 71–80. [DOI] [PubMed] [Google Scholar]
- 4. Xing, B. , Yang, D. , Guo, W. , Liang, Z. , et al. Ag+ as a more effective elicitor for production of tanshinones than phenolic acids in Salvia miltiorrhiza hairy roots, Molecules, 2015, 20, 309–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dong, J. E. , Wan, G. W. , Liang, Z. S. Accumulation of salicylic acid‐induced phenolic compounds and raised activities of secondary metabolic and antioxidative enzymes in Salvia miltiorrhiza cell culture, J Biotechnol, 2010, 148, 99–104. [DOI] [PubMed] [Google Scholar]
- 6. Santulli, G. Epidemiology of Cardiovascular Disease in the 21st Century: Updated Numbers and Updated Facts, J Cardiovasc Dis Res, 2013, 1, 1. [Google Scholar]
- 7. Qiu, D. Y. , Song, J. Y. , Danshen's In Vitro Culture, In: Yan X. (Ed.). Dan Shen (Salvia miltiorrhiza) in Medicine Springer, Dordrecht: 2015. pp. 69–87. [Google Scholar]
- 8. Wang, C. L. , Liang, Z. S. , Li, D. R. , Yang, J. L. Salvianolic acids production in Salvia miltiorrhiza Bunge was regulated by both soluble sugars accumulation and growth substances salicylic acid and methyl jasmonate, J. Medicinal Plants Res., 2012, 6, 2666–2673. [Google Scholar]
- 9. Bennett, R. N. , Wallsgrove, R. M. Secondary metabolites in plant defence mechanisms, New Phytologist, 1994, 127, 617–633. [DOI] [PubMed] [Google Scholar]
- 10. Jones, D. L. , Nguyen, C. , Finlay, R. D. Carbon flow in the rhizosphere: carbon trading at the soil‐root interface, Plant Soil, 2009, 321, 5–33. [Google Scholar]
- 11. Haney, C. H. , Samuel, B. S. , Bush, J. , Ausubel, F. M. Associations with rhizosphere bacteria can confer an adaptive advantage to plants, Nature Plants, 2015, 1, 15051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Karadeniz, A. , Topcuoğlu, Ş. , Inan, S. Auxin, gibberellin, cytokinin and abscisic acid production in some bacteria, World J Microbiol Biotechnol, 2006, 22, 1061–1064. [Google Scholar]
- 13. Spaepen, S. , Vanderleyden, J. , Remans, R. Indole‐3‐acetic acid in microbial and microorganism‐plant signaling, FEMS Microbiol Rev, 2007, 31, 425–448. [DOI] [PubMed] [Google Scholar]
- 14. Yang, S. J. , Zhang, X. H. , Cao, Z. Y. , Zhao, K. P. , et al. Growth‐promoting Sphingomonas paucimobilis ZJSH1 associated with Dendrobium officinale through phytohormone production and nitrogen fixation, Microb. Biotechnol., 2014, 7, 611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang, J. W. , Wu, J. Y. , Effective elicitors and process strategies for enhancement of secondary metabolite production in hairy root cultures, Biotechnology of Hairy Root Systems Springer; 2013: pp. 55–89. [DOI] [PubMed] [Google Scholar]
- 16. Bashan, Y. , Holguin, G. , Lifshitz, R. Isolation and characterization of plant growth‐promoting rhizobacteria, Methods in Plant Molecular Biology and Biotechnology CRC Press, Boca Raton, 1993, 331–345. [Google Scholar]
- 17. Wu, J. Y. , Ng, J. , Shi, M. , Wu, S. J. Enhanced secondary metabolite (tanshinone) production of Salvia miltiorrhiza hairy roots in a novel root‐bacteria coculture process, Appl Microbiol Biotechnol, 2007, 77, 543–550. [DOI] [PubMed] [Google Scholar]
- 18. Veliky, I. A. , Martin, S. M. A fermenter for plant cell suspension cultures, Can J Microbiol, 1970, 16, 223–226. [DOI] [PubMed] [Google Scholar]
- 19. Zhao, J. L. , Zhou, L. G. , Wu, J. Y. Effects of biotic and abiotic elicitors on cell growth and tanshinone accumulation in Salvia miltiorrhiza cell cultures, Appl Microbiol Biotechnol, 2010, 87, 137–144. [DOI] [PubMed] [Google Scholar]
- 20. Cao, Z. Y. , Sun, L. H. , Mou, R. X. , Zhang, L. P. , et al. Profiling of phytohormones and their major metabolites in rice using binary solid‐phase extraction and liquid chromatography‐triple quadrupole mass spectrometry, J Chromatogr, 2016, 1451, 67–74. [DOI] [PubMed] [Google Scholar]
- 21. Egamberdieva, D. , Lugtenberg, B. , Use of plant growth‐promoting rhizobacteria to alleviate salinity stress in plants, In: Miransari M. (Ed.). Use of Microbes for the Alleviation of Soil Stresses, Volume 1 Springer, New York: 2014. pp. 73–96. [Google Scholar]
- 22. Walpola, B. C. , Noh, J. G. , Kim, C. K. , Kyung, K. C. , et al. Optimization of indole‐3‐acetic production by phosphate solubilization bacteria isolated from waste mushroom bed of Agaricus bisporus , J Mushrooms, 2013, 11, 53–62. [Google Scholar]
- 23. Zhao, J. L. , Zhou, L. G. , Wu, J. Y. Promotion of Salvia miltiorrhiza hairy root growth and tanshinone production by polysaccharide–protein fractions of plant growth‐promoting rhizobacterium Bacillus cereus , Process Biochem, 2010, 45, 1517–1522. [Google Scholar]
- 24. Mishra, P. K. , Mishra, S. , Selvakumar, G. , Bisht, S. C. , et al. Characterisation of a psychrotolerant plant growth promoting Pseudomonas sp. strain PGERs17 (MTCC 9000) isolated from North Western Indian Himalayas, Ann. Microbiol., 2008, 58, 561–568. [Google Scholar]
- 25. Ahemad, M. , Khan, M. S. Plant growth promoting activities of phosphate‐solubilizing Enterobacter asburiae as influenced by fungicides, EurAsian J. BioSci. 2010, 4, 88–95. [Google Scholar]
- 26. Reddy, P. P. (Ed.) Plant Growth Promoting Rhizobacteria for Horticultural Crop Protection, Springer, India: 2014. [Google Scholar]
- 27. Liu, H. M. , He, Y. J. , Jiang, H. X. , Peng, H. S. , et al. Characterization of a phenazine‐producing strain Pseudomonas chlororaphis GP72 with broad‐spectrum antifungal activity from green pepper rhizosphere, Curr. Microbiol., 2007, 54, 302–306. [DOI] [PubMed] [Google Scholar]
- 28. Lessard, R. J. , Wolff, K. , Winkelmann, R. K. The dose‐response curves for IAA induced elongation growth and acidification of the incubation medium of Zea mays coleoptile segments, Physiol Plant, 1990, 80, 257–261. [Google Scholar]
- 29. Jaakola, L. , Tolvanen, A. , Laine, K. , Hohtola, A. Effect of N6‐isopentenyladenine concentration on growth initiation in vitro and rooting of bilberry and lingonberry microshoots, Plant Cell Tiss Org Cult, 2001, 66, 73–77. [Google Scholar]
- 30. Reed, B. M. , Abdelnour‐Esquivel, A. The use of zeatin to initiate in vitro cultures of Vaccinium species and cultivars, HortScience, 1991, 26, 1320–1322. [Google Scholar]
- 31. Ming, Q. , Su, C. , Zheng, C. , Jia, M. , et al. Elicitors from the endophytic fungus Trichoderma atroviride promote Salvia miltiorrhiza hairy root growth and tanshinone biosynthesis, J Exp Bot, 2013, 64, 5687–5694. [DOI] [PubMed] [Google Scholar]
- 32. Liang, Z. S. , Ma, Y. N. , Xu, T. , Cui, B. M. , et al. Effects of abscisic acid, gibberellin, ethylene and their interactions on production of phenolic acids in Salvia miltiorrhiza bunge hairy roots, PLoS One, 2013, 8, e72806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Siu, K. C. , Wu, J. Y. Enhanced release of tanshinones and phenolics by nonionic surfactants from Salvia miltiorrhiza hairy roots, Eng Life Sci, 2015, 14, 685–690. [Google Scholar]
- 34. Yan, Q. , Shi, M. , Ng, J. , Wu, J. Y. Elicitor‐induced rosmarinic acid accumulation and secondary metabolism enzyme activities in Salvia miltiorrhiza hairy roots, Plant Sci, 2006, 170, 853–858. [Google Scholar]
- 35. Xiao, Y. , Gao, S. H. , Di, P. , Chen, J. F. , et al. Methyl jasmonate dramatically enhances the accumulation of phenolic acids in Salvia miltiorrhiza hairy root cultures, Physiol. Plantar., 2009, 137, 1–9. [DOI] [PubMed] [Google Scholar]
- 36. Wang, J. W. , Wu, J. Y. Tanshinone biosynthesis in Salvia miltiorrhiza and production in plant tissue cultures, Appl Microbiol Biotechnol, 2010, 88, 437–449. [DOI] [PubMed] [Google Scholar]
- 37. Hao, W. F. , Guo, H. B. , Zhang, J. Y. , Hu, G. G. , et al. Hydrogen peroxide is involved in salicylic acid‐elicited rosmarinic acid production in Salvia miltiorrhiza cell cultures, Scientific World J., 2014, 2014, 843764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guo, H. B. , Dang, X. L. , Dong, J. E. Hydrogen peroxide and nitric oxide are involved in salicylic acid‐induced salvianolic acid B production in Salvia miltiorrhiza cell cultures, Molecules, 2014, 19, 5913–5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guo, H. , Zhu, N. , Deyholos, M. K. , Liu, J. , et al. Calcium mobilization in salicylic acid‐induced Salvia miltiorrhiza cell cultures and its effect on the accumulation of rosmarinic acid, Appl. Biochem. Biotechnol., 2015, 175, 2689–2702. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


